Abstract
Diabetic foot (DF) is one of the most common complications of diabetes and is associated with high morbidity, disability, lethality and low cure-rate. The clinical diagnosis and treatment of DF need to be standardized. The Chinese Diabetic Foot Cell and Interventional Therapy Technology Alliance has released six editions of guidelines and standards for clinical diagnosis and interventional treatment of DF, which filled the gap in the domestic DF treatment standard and played an important role in improving the level of diagnosis and treatment in China. In line with the latest developments in diagnosis and treatment, the Alliance, along with other 89 institutions, developed and issued the new edition based on the sixth edition to help standardize the clinical diagnosis and treatment of DF in China.
Keywords: Diabetic foot/diabetes, Interventional comprehensive diagnosis and treatment, Guidelines of clinical
Diabetic foot is one of the serious complications of diabetes and is associated with a high risk of amputation and death. It is important to carry out standardized clinical diagnosis and treatment. The customization and use of diabetic foot guidelines/consensus can effectively improve this standardization. In January 2016, the American Association of Foot Medicine (APMA), in conjunction with the Society for Vascular Surgery and the Society for Vascular Medicine jointly issued a practice guideline for the management of diabetic foot in the Journal of Vascular Surgery1 to standardize the screening, diagnosis, treatment, and prevention of diabetic foot disease. The Chinese Diabetic Foot Cell and Interventional Therapy Technology Alliance released this guideline in accordance with the latest international consensus and after taking into consideration the actual situation in China. This guideline is intended to be used as a reference by the majority of scholars in the field.
1. Pathological basis of diabetic foot
Diabetic foot was first described by Oakley in 1956.2 In 1972, Catterall3 defined it as the loss of sensation due to neuropathy and loss of vitality due to ischemia, along with an infection in the feet. Diabetic foot is a complex lesion caused by multiple factors. Tissue ischemia, peripheral neuropathy, and infection are the pathological basis of diabetic foot, and are usually combined. Peripheral neuropathy and tissue ischemia act as initiating factors in the pathogenesis, while infection is often secondary.
1.1. Peripheral neuropathy in patients with diabetic foot
Diabetic peripheral neuropathy (DPN) is common in clinical practice and mostly coexists with vascular disease, involving motor, sensory, and autonomic nerves. In the initial stages, sensory neuropathy can lead to dysesthesia, increasing the susceptibility of the foot to pressure and mechanical and thermal injuries; thereafter, motor neuropathy alters foot biomechanics and leads to variations in anatomy, causing foot deformity, limited joint mobility, and changes in foot load.4
Simple DPN is not included in the scope of this guideline. This change of peripheral neuropathy is further explained on the basis of combined lower limb vascular disease. Based on the writing objectives of this guideline, the category of diabetic foot referred to below has been narrowed to diabetic foot lesions associated with vascular lesions of the lower extremities causing tissue ischemia, with or without leg ulcers.
1.2. Ischemic or neuro-ischemic lesions in patients with diabetic foot
For every 1% increase in glycosylated hemoglobin (HbA1c), the risk of peripheral vascular disease (PAD) increases by 25%–28%.5 According to the results of a large cohort study in Europe,6 approximately half of the cases of diabetic foot originate from neuroischemic or ischemic lesions. Ischemia is the most important factor that prevents the healing of the lesions.7 Ischemia is therefore the root cause of diabetic foot that must be screened for first unless there is conclusive evidence.
Neuroischemic lesions result from the synergistic effect of DPN and tissue ischemia, which reduces oxygen delivery to metabolic tissues.7 Macroangiopathy and microvascular dysfunction impair blood perfusion in diabetic foot.8 An important feature of diabetic macroangiopathy is that calcification of the arteries of the lower limb causes a significant decrease in vascular elasticity, resulting in false negative results in the ankle-brachial index (ABI) and toe-brachial index (TBI). From a clinical point of view, ischemic and neuroischemic lesions can be considered to be associated with the same pathogenic factor, which may require vascular recanalization treatment.
1.3. Diabetic foot infection
Neuroischemic ulcers in diabetic foot are easily infected; however, infection is rarely the cause of the ulcers. Moreover, the occurrence of infection is closely related to the probability of amputation, especially in patients with PAD (7). Deep infection is characterized by osteomyelitis or soft tissue infection disseminated along the tendon, which is a direct factor related to the probability of amputation and fatality. Patient outcomes are associated with the extent of infection, comorbidities, and the presence or absence of PAD.6,9
2. Diagnosis and evaluation of diabetic foot
2.1. Clinical manifestations
2.1.1. Intermittent claudication; rest pain
Diabetic patients with intermittent claudication complain of difficulty in walking, which may recover after rest, but recurs during walking or exercise, and is classified as mild, moderate, or severe according to the degree of difficulty and walking distance. Resting pain refers to the different degrees of pain in the lower limbs of patients under non-exercise conditions.
The symptoms of intermittent claudication and rest pain caused by ischemia mainly occur in the toe or the metatarsal head to foot. The symptoms, which are otherwise manageable, are aggravated on elevation of the lower limbs.
2.1.2. Ulcers and gangrene
The majority of ulcers occur in the presence of severe ischemia, with the most common sites being the heel and the first and fifth metatarsals. The typical ulcer presents with nonviable marginal tissue, a pale necrotic base, and may be covered with fibrous tissue. The earliest site of gangrene is the toes, which can gradually extend to the ankle joint and may even involve the level above the ankle joint in severe cases. Gangrene is a serious consequence of severe ischemia and nerve injury in the diabetic foot, which develops due to infection, often endangering the patient's life and affecting vital organ function.
Intermittent claudication, rest pain, ulceration, and gangrene are the basis for assessing the degree of ischemia in diabetic foot. Refer to the Rutherford classification (Table 1) for the classification criteria.
Table 1.
Rutherford classification.
| Classification | Symptoms |
|---|---|
| Grade 0 | Asymptomatic |
| Grade 1 | Mild intermittent claudication ∗ |
| Grade 2 | Moderate intermittent claudication ∗∗ |
| Grade 3 | Severe intermittent claudication ∗∗∗ |
| Grade 4 | Pain at rest |
| Grade 5 | Limited ulcer, necrosis |
| Grade 6 | Extensive ulceration, necrosis |
Note:∗: With the help of treadmill, the slope is 12%, the speed is 60–70 m/min, the pain-free walking distance (PFWD) is > 300–500 m, ∗: PFWD is > 100–300 m, ∗: PFWD ≤ 100 m.
2.1.3. Paresthesia of the lower limb
Skin paresthesia is a clinical manifestation in patients with DPN. The most common symptoms are numbness and irregular tingling sensation in the lower limbs, more common at night, which may be accompanied by varying degrees of decrease in the skin sensations of temperature, touch, and deep vibration in the lower limbs. This paresthesia can be judged by a simple physical examination.
2.1.4. Nutritional changes in the skin
Nutritional changes in the skin are the result of a combination of peripheral neuropathy and ischemia, mainly manifested as dryness and desquamation of the skin of the lower extremities, decreased skin elasticity, decreased subcutaneous fat layer, and skin pigmentation.
2.1.5. Foot deformity
Foot deformities in patients with diabetic foot are mainly characterized by progressive Charcot joint disease, damage to weight-bearing joints, as well as claw toes and hammer toes.
2.2. Medical history
The purpose of taking medical history is the assessment of risk factors for the patient's prognosis and collection of the necessary information to guide the patient's out-of-hospital treatment. The information collected must include but is not limited to the following contents: 1. disease and drug treatment, 2. cardiovascular risk factors, 3. occupation and hobbies, 4. lifestyle, 5. smoking, drinking, or drug use, 6. diabetes-related diseases, such as diabetic nephropathy, retinopathy, and neuropathy, and 7. family history or genetic history.
2.3. Examination of peripheral neuropathy
The diagnosis of DPN is divided into four layers: layer 1 refers to the presence of symptoms or signs of DPN (any one sign from ankle reflex, pressure sensation, vibration sensation, pinprick sensation, or temperature sensation is positive), along with abnormal nerve conduction, which can confirm the diagnosis; layer 2 refers to the presence of symptoms and one positive sign for DPN, or absence of symptoms but ≥ two positive signs which can be clinically diagnosed; layer 3 refers to the presence of symptoms but no signs of DPN, or absence of symptoms but one positive sign, which leads to a suspected diagnosis; and layer 4 refers to the absence of symptoms and signs, with only the presence of abnormal nerve conduction, which is a subclinical diagnosis.
2.3.1. Pressure perception
A 10 g Semmes-Weinstein monofilament is used to pressurize the skin at the base of the first toe and at the base of the first and fifth metatarsal heads twice in 2 s to bend the monofilament, and a simulation test is performed to determine sensation by the patient. If there are ulcers, gangrenes, cocoons, or scars on the testing, the test is performed on the surrounding skin. A negative response twice represents protective skin paresthesia.
2.3.2. Vibration sensation
Two tests and one simulation test are performed using a 128 Hz tuning fork, vertically touching the dorsal aspect of the distal phalanx of the first toe, and the patient is asked if they felt it. A negative response twice represents abnormal vibration sensation. If the patient cannot feel the vibration in the 1st distal phalanx, it is necessary to move the test position to the medial and lateral malleolus and the tibial tubercle.
2.3.3. Tactile sensation
Two tests and a simulation test are performed on the dorsum of the patient's foot using a piece of sterile cotton and the patient is enquired whether they felt it. Two negative responses represent tactile abnormalities.
2.3.4. Achilles tendon reflex
The normal response is gastrocnemius contraction and plantar flexion. The reflex is considered abnormal if the above reaction is significantly enhanced, weakened, or absent. Patients with diabetic foot present and ipsilateral tibial nerve palsy under the premise of excluding sciatic nerve damage, lumbar disc herniation, and sciatica, presenting with symptoms such as diminished or absent Achilles tendon reflexes.
2.3.5. Electromyogram
Electromyography is more objective than clinical examination and can identify whether sensory and motor nerve fiber conduction is abnormal.
2.4. Hemodynamic examination
Palpation of the superficial arterial pulse site is the basis for all vascular examinations, and significant PAD can often be initially diagnosed by judging the condition of the arterial flow by palpation. Further diagnosis needs to be made based on the following parameters:
2.4.1. ABI
The normal ABI ranges from >0.90 to 1.10, with >0.40 to 0.90 categorized as mild to moderate ischemia and ≤0.40 as severe ischemia. Patients with an ABI ≤0.40 have a significantly greater risk of rest pain and ulceration. However, ABI in patients with diabetic foot may also be within the normal range (cut-off value 1.0 to 1.1)10; therefore, more reliable detection methods are needed to support the diagnosis.
2.4.2. TBI
TBI >0.75 is generally considered normal, and TBI <0.25 represents critical limb ischemia (CLI). Toe pressure <30 mmHg in patients with rest pain can indicate a diagnosis of CLI, while toe pressure <50 mmHg in patients with ulcers or gangrene can be considered as CLI. TBI has the similar drawback to ABI, that is, its judgment criteria are less reliable in patients with diabetic foot. With regard to TASC II (TransAtlantic Inter-Society Consensus, TASC), toe pressure < 50 mmHg in a patient with diabetic foot can be used as a cut-off value for preliminary judgment of combined CLI.11
2.4.3. Toe/finger oxygen saturation index (TFI)
TFI is the ratio of the oxygen saturation of the hallux of a foot to that of the ipsilateral thumb. TFI <0.9 indicates a certain degree of ischemia of the hallux; however, TFI is affected by many factors, and other relevant examinations are needed for further diagnosis.
2.4.4. Segmental blood pressure (SBP)
Measurement of SBP can be used to localize the arterial lesions in patients with diabetic foot with CLI; however, its results are affected by various factors such as severe arteriosclerosis, preventing its use as a solitary basis for localizing arterial lesions.
2.5. Evaluation of tissue perfusion
2.5.1. Transcutaneous oxygen pressure (TcPO2)
The partial pressure of oxygen reflects the status of lower limb oxygen metabolism in patients with diabetic foot or CLI and is currently the most commonly used method to detect the level of tissue blood perfusion. TcPO2 can be used to assess the severity of macroangiopathy and microvascular perfusion disorders, determine the need for recanalization in patients, and predict the treatment outcome and probability of ulcer healing.
TcPO2 is generally measured on the anterolateral aspect of the leg at 10 cm above the dorsum of the foot, below the knee, and above the knee, with a normal value of approximately 60 mmHg. According to TASC II, TcPO2 < 30 mmHg can be used as a cut-off value for diagnosing diabetic foot with CLI and predicting unhealed ulcers.
2.5.2. Skin perfusion pressure (SPP) and hyperspectral tissue oxygenation measurements
SPP is also an examination method for assessing microcirculation and can be used to predict ulcer prognosis. It is evaluated using the laser Doppler technique, and its measurement represents the target blood pressure required to restore microcirculation and capillary blood flow, with a cut-off value of 30 mmHg; however, the accuracy of SPP in predicting ulcer healing is lower than that of TcPO2. Hyperspectral tissue oxygenation measurement is also a method to predict ulcer healing and determine microcirculatory abnormalities in diabetic foot, although it is used mainly as a research tool.
2.5.3. Five-point system of the Tongji University
In this system of evaluation, the patient lies still for 5 min, the room temperature is 21 °C, the measurement point is exposed for 30 s, and an infrared thermometer is used to detect the skin temperature at five points: the inferior border of patella, medial malleolus, lateral malleolus, and back and sole of the foot. The frontal temperature is used as the reference skin temperature. Considering the measurement of dermatoglyphic changes before treatment, and compared to the constant rated temperature, this method is simple and convenient to determine and detect changes in the therapeutic effect after treatment.
2.6. Physical or imaging examinations
Assessment of the anatomical location, morphology, and extent of vascular lesions allows decision-making regarding their treatment. At present, the commonly used imaging examination methods include color Doppler ultrasound (CDUS), magnetic resonance angiography (MRA), computed tomography angiography (CTA), and digital subtraction angiography (DSA). Different examination techniques have their own advantages and disadvantages, and choice of examination method should be based on the actual situation of patients and their treatment needs.
2.6.1. Vascular ultrasound (VUS)
VUS has many advantages, such as cost-effectiveness and convenience; however, the accuracy of its results depends more on operator experience, and may demonstrate poor imaging of iliac arteries, distal arterioles and collaterals, and low sensitivity to severe vascular calcifications and multi-segment PAD.
2.6.2. MRA
In contrast to CDUS and CTA, MRA is not affected by vascular calcification; however, it tends to overestimate the degree of stenosis due to the presence of turbulent blood flow at the site of stenosis. In addition, below-knee vascular imaging is vulnerable to interference from the veins. The presence of metal implants may lead to false images of vascular obstruction. It cannot be used for examination of patients with contraindications such as presence of metal implants, implantable electronic equipment, and claustrophobia.
2.6.3. CTA
CTA allows assessment of stented vessels and provides fast imaging with a high spatial resolution. Patient acceptance is higher for CTA compared to MRA; however, severe vessel wall calcification can interfere with the imaging quality.
2.6.4. DSA
At present, CTA is still considered the “gold standard” for angiography. Its main drawback is an invasive nature and possibility of catheter-related complications in the target vessel and puncture site. Under normal circumstances, DSA is only used for confirmation of lesion condition and guidance of endovascular treatment when other imaging examinations such as VUS, CTA, and MRA cannot provide sufficient information on the anatomical location and shape of the vascular lesions.
2.7. Evaluation and grading of ulcers and infections
Ulcer area, depth of the involved tissue, co-infection, and tissue necrosis generally need to be considered in cases of diabetic foot ulcers. Although there is no uniform standard of evaluation, the Wagner classification is commonly used for the assessment of these factors at present (Table 2).
Table 2.
Wagner classification of diabetic foot ulcer.
| Grading | Symptoms |
|---|---|
| Grade 0 | No ulcer |
| Grade 1 | Superficial ulceration, involving the entire thickness of the skin but not the subcutaneous tissue |
| Grade 2 | Deep ulcers penetrating into muscle layers and ligaments without bone involvement and without abscess |
| Grade 3 | Deep ulcers with cellulitis or abscess formation, often accompanied by osteomyelitis |
| Grade 4 | Local small gangrene |
| Grade 5 | Extensive gangrene involving entire foot |
The diagnosis of diabetic foot infection can be made based on the symptoms and signs of local inflammation, including typical manifestations such as exudate; local redness; swelling and heat; pain; systemic symptoms of fever, leukocytosis, accelerated erythrocyte sedimentation rate; and elevated CRP. Infections occur mostly secondary to ulcers and may not be associated with them. The extent and degree of infection are important factors that affect the prognosis. A wide range of infections and significant systemic inflammatory response often predict the risk of amputation and death.
2.8. Classification criteria of Tongji University
A new grading standard was established, based on the vascular anatomy, function detection and effective microcirculation of patients. Diabetic vascular disease was divided into three stages and six grades: stage 0, normal; stage I: < 4 points, mild; stage Ⅱa: 4–6 points; stage Ⅱb: 7–9 points; stage Ⅱc: 10–12 points; stage III: > 12 points. This system was used to evaluate 481 patients with diabetic peripheral vascular disease before and after treatment. The normality test, using SAS 9.4 software, revealed that the difference in the scores before and after treatment did not meet the normal distribution. Therefore, the Wilcoxon signed-rank test was performed to determine the differences. The test statistic was T = 4726.5, p < 0.0001. According to the α = 0.05 test level, the difference was statistically significant. The scores before and after treatment were different, and the median decrease in scores after treatment compared to that before treatment was 7.56, 7, 8, 9, 10.
3. Treatment of diabetic foot
The pathological basis of the occurrence and development of diabetic foot is complex, and its treatment is based on a variety of methods of comprehensive treatment and multidisciplinary cooperation. The treatment of lower limb ischemia and infection is most essential.
3.1. Interventions for PAD risk factors
3.1.1. Smoking
Smoking is the most important risk factor for PAD and is equally responsible for the development of PAD in patients with diabetes. Smoking cessation is advised for all patients with diabetic foot. Smoking cessation success can be improved by adjunctive means, such as nicotine replacement therapy, nicotine receptor antagonist varenicline, and the antidepressant drug bupropion.
3.1.2. Hypertension
Hypertension is one of the independent risk factors of PAD. The target of antihypertensive treatment recommended in this guideline for diabetic patients is a blood pressure <130 mmHg/80 mmHg12; however, blood pressure often reaches 140 mmHg/90 mmHg in elderly and critically ill patients. A combination of multiple antihypertensive drugs may be required to effectively reduce blood pressure, and an individual specialist should be responsible for the administration of the antihypertensive treatment.
Control of blood pressure reduces the risk of PAD; however, it is not known whether lowering the blood pressure can delay PAD progression. It is generally believed that patients with PAD and hypertension should undergo antihypertensive therapy to reduce the risk of cardiovascular and cerebrovascular events. In the process of lowering the blood pressure, blood flow may be decreased, which is tolerable to most patients; however, the blood flow may already be decreased in patients with severe ischemia, resulting in aggravated symptoms.
3.1.3. Hyperlipidemia
Elevated cholesterol, low-density lipoprotein (LDL), triglycerides, and lipoprotein (a) are all independent risk factors for PAD. The ideal target level of blood lipids is LDL <1.8 mmol/L. Administration of statins is a contemporary first-line regimen for the treatment of hyperlipidemia as it has the effect of stabilizing vascular plaques as well as reducing the incidence of vascular embolism. Administration of atorvastatin 40 mg daily reduced cardiovascular disease mortality and non-coronary revascularization requirements by approximately 17% and 16%, respectively. The recommended criterion in this guideline is LDL-C ≤ 2.6 mmol/L (100 mg/dl) in patients with PAD; statin therapy is required if diet and exercise interventions fail to achieve the target lipid level.
3.2. Glucose control
Glycemic control is the basic treatment for diabetic foot, and hyperglycemia itself is associated with the development and progression of PAD to CLI. Patients with diabetes and CLI have lower rates of limb preservation than non-diabetic patients with the same degree of vascular disease. Glycemic control is also an important prognostic factor for diabetic foot ulcer infections.
Suggested controlled blood glucose levels are HbA1c < 7.0%, fasting blood glucose <7.8 mmol/L, and random blood glucose <10.0 mmol/L. However, these criteria can be relaxed in elderly patients or those who experience hypoglycemic reactions.13
Endocrinologists are responsible for the development of hypoglycemic therapy regimens. Insulin injection should be mainly used to control blood glucose, with oral hypoglycemic agents as an adjunct, during PAD endovascular or open surgery and in patients with protracted ulcer healing or infection.
3.3. Medical treatment of peripheral neuropathy
At present, there is a lack of effective treatment for DPN, and the existing methods, including neurotrophic drugs and metabolic therapy, can only delay the progression of peripheral neuropathy. Patients with diabetic foot often require long-term treatment in order to improve diabetic neuropathy, and the efficacy of the treatment reduces with delayed or shorter course of treatment. Arthrodesis can be considered for management of Charcot arthropathy in young patients. In addition, reduced mobility and protective braces are effective methods for controlling symptoms associated with foot deformity caused by neuropathy in such patients.
3.4. Management of vascular lesions
3.4.1. Antiplatelet therapy
Patients with diabetic foot require long-term antiplatelet therapy. The recommended dose is 75–325 mg daily for aspirin and 75 mg daily for clopidogrel. Monoantiplatelet therapy is generally used; however, a combination of aspirin and clopidogrel may be considered in patients undergoing endovascular treatment of the femoropopliteal and infrapopliteal arteries or infrapopliteal vascular bypass surgery.14
Although both aspirin and clopidogrel can reduce the risk of cardiovascular and cerebrovascular events in patients with PAD, their combination may increase the risk of bleeding. Therefore, long-term use is not recommended. Beraprost sodium has less side effects, and its combination with aspirin can enhance the antiplatelet effect without increasing the risk of bleeding. The use of this combination therapy is recommended.
3.4.2. Vasoactive drug therapy
Prostaglandins are effective vasoactive drugs which act by dilating microvasculature, inhibiting platelet aggregation, and increasing tissue perfusion. Diabetic foot angiopathy also includes occlusion of large vessels, sclerosis, and microvascular dysfunction; therefore, prostaglandins (e.g., beraprost sodium) also aid in successful arterial recanalization. Alternative drugs for vasoactive drug therapy include cilostazol and ginkgo biloba.
3.4.3. Treatment of CLI
CLI vascular lesions are characterized by multiple, multilevel lesions, and often involve the tibiofibular artery below the knee. The decision regarding the necessity of recanalization treatment is based on the signs and symptoms of the patient. Imaging can be used as a basis for decision-making and selection of recanalization treatment options when considering the Rutherford classification, Wagner classification of ulcers, lower limb infection, hemodynamics, and tissue perfusion.
Once recanalization treatment is performed, TASC II guidelines can be referred for the selection of treatment options for aortoiliac and femoropopliteal artery lesions based on imaging findings. As diabetic foot is often associated with complications such as multiple vascular diseases involving the carotid and coronary arteries, endovascular treatment can be considered even for long-segment lower limb arterial occlusion to avoid the risks of general anesthesia and open surgery as far as possible. The evaluation of the carotid and renal arteries is the basis of and key to appropriate treatment.
Endovascular treatment is the first-line regimen for infrapopliteal artery disease. The basic goal is the restoration of at least one patent feeding artery to the affected foot. The effect of selecting target vessels according to the area of ischemic pain and ulcer necrosis lesions and restoring direct blood supply was significantly higher compared to that of indirect blood supply. If direct recovery fails, the PPL (pedal-plantar loop) technique can be attempted to establish an indirect loop.
Before BTK (below the knee) and CLI treatment, the renal artery and its function should be evaluated by angiography, and treatment for abnormal renal function should take first priority.
3.5. Management of ulcers and infections
3.5.1. Anti-infective drug therapy
Diabetic foot infection is clinically diagnosed based on the symptoms and signs of local and systemic inflammation. For all clinically infected ulcers, tissue samples should be collected for culture to identify the causative bacteria. Antimicrobial drug therapy is not mandatory for Wagner grade 1 patients. The effect of antimicrobial agents is related to the success of recanalization therapy. In emergency cases, broad-spectrum antibacterial drugs should be administered along with adequate decompression and drainage of the infectious foci for severe deep infections, and timely adjustments should be made after obtaining the results of secretion culture of the infectious foci.
3.5.2. Debridement
In principle, all macroscopically infected necrotic and nonviable tissue is removed from the ulcer until healthy, bleeding soft tissue or bone tissue is exposed. The epidermal hyperkeratosis surrounding the ulcer must also be removed. If associated with osteomyelitis or joint infection and gangrene, amputation is considered. Repeated debridement may be required with change in the extent of necrosis. For superficial infections or dry gangrene, where there is no life-threatening condition or risk of amputation, revascularization should be performed first to ensure the preservation of maximal tissue with survival potential during late debridement. Therefore, vascular reconstruction (except in cases of disappearance of the femoral or popliteal pulse) should be considered in the presence of cardiovascular risk factors when surgical benefits outweigh the risks. The patient and family members should be intimated in detail regarding the surgical plans, risks, and precautions, preoperatively. Amputation should be performed only if the surgical risks far outweigh the benefits and there is no possibility of wound healing or revascularization.
3.5.3. Timing of treatment for infection, debridement, and recanalization
For deep infections, emergency incision and drainage of the abscess with thorough debridement is of utmost importance. Debridement should be performed first, followed by vascular recanalization. In the absence of sepsis, simultaneous debridement and endovascular recanalization may be performed. Distal bypass grafting should be performed 2–5 days after the debridement and systemic sepsis control.
3.5.4. Angiography and revascularization
Angiography and revascularization should be considered when a patient with diabetic foot presents with the following conditions: (1) toe pressure < 30 mmHg or TcPO2 < 25 mmHg (2) foot ulcers that do not improve after 4–6 weeks of active treatment, regardless of bedside test results or when microangiopathy cannot be regarded as the cause of foot ulcer nonunion (3)ankle pressure < 50 mmHg or ABI < 0.5. Of these, the last condition is considered for emergency angiography and revascularization.15, 16, 17
Revascularization methods for ischemic limbs include bypass surgery and endovascular treatment. TASC II states that revascularization should be performed in CLI populations presenting with ischemic rest pain, ulcers, or gangrene; however, there is still a lack of evidence regarding the method of selecting the best revascularization modality. Only one clinical randomized controlled trial has compared the efficacy of bypass surgery and percutaneous transluminal angioplasty for CLI, and it did not reveal any differences between the two treatment modalities regarding amputation-free survival, treatment cost, and quality of life. There is a growing trend towards endovascular treatment first; however, retrospective literature suggests an increased probability of late reintervention after endovascular treatment, especially in patients with long-segment vessel occlusions.18
3.5.5. Adjunctive therapy to promote open wound healing
After debridement of the infected necrotic tissue, open wounds are left to heal without grafting due to excessive skin defects. Due to peripheral autonomic neuropathy and microcirculation disturbance, the growth of granulation tissue in the wound is slow and there is a risk of secondary infection; adjuvant therapy is required to promote granulation tissue growth and facilitate optimal conditions for secondary wound healing or secondary skin grafting. Hyperbaric oxygen therapy and continuous negative pressure drainage of the wound are both effective adjuvant therapies. Hyperbaric oxygen therapy is effective in the healing of moderate ischemic ulcer wounds.19
Under the condition that the wound surface is rich in granulation tissue and the infected tissue has been completely removed, continuous negative pressure drainage of the wound surface can be the choice of treatment, with its effects being superior to hyperbaric oxygen therapy; however, it must be based on reconstruction of adequate blood flow without residual significant infection and gangrene.20
3.6. Amputation
Amputation is the only method of preventing a severe infection from becoming life-threatening. It is a disabling surgery; therefore, the indications must be strictly understood. Clear communication with the patient and their family is essential, along with consent from both. The indications for amputation are as follows:
-
(1)
Wagner grade 4 and above gangrene.
-
(2)
Wagner grade 3 with severe infection, accompanied by life-threatening systemic symptoms.
-
(3)
Severe limb ischemia presenting with intolerable pain, limb necrosis, or dissemination of infection after active medical conservative treatment.
-
(4)
Diabetic Charcot neuroosteoarthropathy with infection that is refractory to comprehensive treatment and severely affects function. The use of prostheses after amputation can improve function and quality of life, which is a relative indication.
Evaluation of amputation level: The correct amputation level can not only ensure the primary wound healing, but also reduce the disability level.21 The ideal amputation level is the most distal end to ensure complete wound healing. The following examination methods are often used as evaluation criteria for the selection of the amputation level:22, 23, 24, 25
-
1.
Clinical signs: On the basis of limb color, skin temperature, peripheral arterial pulse, arteriographic results, and intraoperative skin edge bleeding, a lower plane of amputation is selected for younger patients, while a relatively higher plane is selected for older patients.
-
2.
Blood flow doppler arterial manometry: Doppler arterial manometry is considered accurate in judging the requirement for above-the-knee or below-the-knee amputations and is the basic screening test for evaluation of limb amputation and amputation plane.
-
3.
Determination of oxygen partial pressure: It has a good predictive effect on the healing ability of the amputation level. An oxygen partial pressure less than 2.67 kPa (20 mmHg) indicates poor healing ability of the amputation plane, while oxygen partial pressure more than 5.33 kPa (40 mmHg) indicates good prognosis of the amputation plane.
-
4.
Arteriography: Arteriography, including CTA, MRA, and DSA, is the most intuitive method for predicting the prognosis of lower limb ischemia. Of these, DSA is the most accurate.
-
5.
Other imaging studies: Radiographic examinations are preferred; however, the findings may be normal in the first 14 days in cases of osteomyelitis; therefore, multiple examinations should be performed when osteomyelitis is suspected and the initial radiographic findings are negative. Computed tomography can show skeletal involvement, and magnetic resonance imaging is helpful for early detection of osteomyelitis and its differentiation from Charcot neuroarthropathy. Radionuclide scanning can differentiate between infectious and non-infectious nature of soft tissue inflammation.
4. Cutting-edge diagnostic and therapeutic techniques
4.1. VUS microcirculation detection
VUS is a new imaging examination technique, also known as microcirculation angiography; on the basis of conventional ultrasonography, it increases the difference between tissue echo and surrounding echo by intravenous injection of ultrasound contrast agent and uses the difference in perfusion time between different tissues to improve the imaging resolution, sensitivity and specificity. The entire process from perfusion to withdrawal in organs or tissues can be visualized dynamically and in real time; It can provide the basis for differential diagnosis depending on the perfusion characteristics of different lesions. This technique can be used to quantitatively analyze microcirculatory perfusion with few interfering factors and high reliability; it has great potential value for the detection of microcirculatory perfusion in diabetic foot. VUS can identify changes in time to peak and area under the curve, so as to obtain more accurate information regarding microcirculatory perfusion.
Specific examination steps: 1. The patient is asked to rest in the supine position for 30 min at conventional room temperature, keeping the toes warm; 2. A bolus intravenous injection of SonVue or alternative microbubble contrast agent 4.8 ml is administered, followed by ultrasound observation of microcirculatory perfusion in each section of the hallux. This detection should be performed before surgery; 1 day, 1 week, 1 month, and 3 months after surgery; and the changes in hallux microcirculation should be dynamically observed. (One typical case can be seen in the Fig. 1).
Fig. 1.
Patient, male, 76 years old, diabetic foot, first toe palmar peripheral microcirculation contrast-enhanced ultrasound acoustic curve A. Before treatment; B. after treatment (below-knee balloon dilatation + CD133 + cell reinfusion).
4.2. Laser Doppler imaging technology
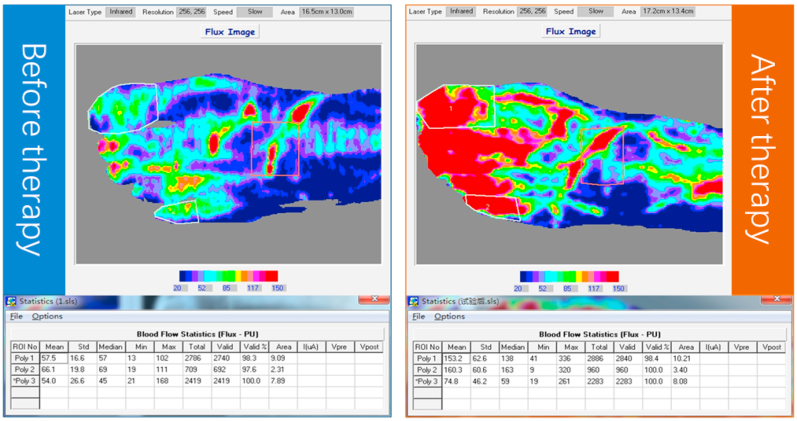
Laser Doppler imaging uses a low-energy laser beam to scan the surface of the skin or other tissues. The blood flowing in the microvessels causes a frequency shift of the scattered laser. This frequency shift is processed by photoelectric detection and plotted as a color-coded blood flow diagram, as shown in Fig. 2. Laser Doppler imaging is a non-contact test that quantitatively analyzes the differences in blood flow in a certain tissue area or uses a repeated scanning mode to continuously evaluate the progressive changes in blood flow over time, thereby evaluating the microcirculatory changes in the diabetic foot and perform quantitative data analysis.
Fig. 2.
A 68-year-old man with diabetic foot underwent laser Doppler imaging A. Before intervention; B. after intervention.
4.3. Cell therapy
Mesenchymal stem cells (MSCs) can effectively promote neovascularization and wound healing and are considered a new method for the treatment of diabetic foot, particularly for patients with CLI. Animal experiments have yielded positive results, and there have been some international clinical trials of cell therapy. Almost all of these studies have shown that cell therapy is significantly effective in objective and subjective outcome measures. Theoretically, cell transplantation can not only achieve the formation of a new vascular circulatory network at sites where blood supply cannot be reconstructed, but also promote subcutaneous tissue repair at sites of protracted ulcers.
Cell therapy is still in the research stage, and rigorous large-sample clinical controlled studies are needed before it can be widely used in clinical practice. At present, most studies use autologous cells; however, allogeneic cell therapy and in vitro cell expansion technology will have more practical clinical value after resolution of safety and ethical issues arising due to obstacles in autologous cell function.26, 27, 28, 29
The research group of Chinese Diabetic Foot Cell and Interventional Therapy Technology Alliance (hereinafter referred to as “Alliance”) conducted the first clinical trial of autologous CD133+ endothelial precursor cells in the treatment of diabetic foot in December 2012. By April 2014, a total of 30 patients had undergone autologous CD133+ endothelial precursor cell transfusion therapy, which met the requirement of the number of cases for phase II clinical studies.
At present, the research group has summarized and analyzed the 6-month follow-up data of 12 patients who completed endovascular intervention + cell therapy (treatment group) and 15 patients who only received endovascular intervention in the control group30: In the 12 patients treated with autologous CD133+ cells, the number of cells sorted from 100 ml arterial blood were 10.44 ± 3.78 × 107 (range of 5.20–15.20 × 107), the cell activity was 96.98 ± 1.16% (range of 95.00–98.70%), and there were no adverse reactions during cell transfusion (itching, palpitation, wheezing, decreased blood pressure, etc.). There were no significant differences in the Rutherford classification (Table 3), ABI index, and TcPO2 (Table 4, in the supplementary appendix) during the 1-month follow-up period after treatment in the control group (P > 0.05); however, cell therapy had obvious advantages regarding long-term efficacy. The Rutherford classification (Table 1), ABI index, and TcPO2 (Tables 4 and 5, in the supplementary appendix) during the 6-month follow-up period were superior in the treatment group compared to those in the control group (P < 0.05), indicating that the action of the MSCs and their secreted cytokines could continuously induce angiogenesis and improve microcirculation at the terminal vessels. The ulcer remission rate in the treatment group was 100% (2/2), while that in the control group was 40% (2/5); the amputation rate was 0 in the treatment group, compared to 20% (3/15) in the control group.
Table 3.
Vascular anatomy + function test + effective microcirculation classification.
| Evaluation item | Standard I | Standard II | Standard III |
|---|---|---|---|
| Vascular Dissection A | Normal 0 | Single Lesion 1 | More than 3 |
| Vascular dissection B | Normal 0 | Mild stenosis 1 | Severe or occluded 3 |
| Function test | Normal 0 | Mild Abnormal 1 | Severely abnormal 3 |
| Microcirculation detection | Normal 0 | Mild Abnormal 1 | Severely abnormal 3 |
Note: Vascular anatomy A refers to the number of involved vessels; vascular anatomy B refers to the degree of vascular stenosis and occlusion; functional tests: patient walking distance, ABI, TBI, peripheral nerve evaluation, each evaluation indicator refers to the existing clinical standard classification; microcirculation tests: ultrasonic microbubbles, laser Doppler scanning, i-flow software determination, each evaluation indicator refers to the existing clinical standard classification.
At present, endothelial progenitor cells (EPCs) and MSCs are used in cell therapy for diabetic foot carried out internationally.31,32 EPCs are precursor cells for vascular endothelial cells. Ulcers are locally treated by mobilizing EPCs within isolated peripheral blood. At present, EPCs are believed to promote blood perfusion, growth of new blood vessels, and repair of vascular injury; thereby achieving the goals for treatment of diabetic foot. As EPCs need to be mobilized and collected from the patient themselves, the proliferation and differentiation ability of the patients' stem cells and other factors affect the efficacy of EPC cell therapy. MSCs are also known as intercellular stem cells, and can differentiate into various mesodermal tissues, which are easier to obtain than EPCs. At present, this is considered to be related to the directional differentiation of MSCs into endothelial cells, the secretion of a variety of vascular growth factors, and the regulation of immune responses.
5. Patient management and follow-up
5.1. Patient education and medication
The occurrence of diabetic foot indicates the cut-off point for neuropathy and vascular diseases. Although active treatment may temporarily control the disease or achieve a “curative” effect, its pathological basis persists. The patient's lifestyle and level of foot care are important factors affecting long-term outcomes. Patient awareness and education is important to prevent the recurrence of diabetic foot symptoms. In addition, it is necessary to regularly inspect the blood glucose, blood pressure, and blood lipid levels of patients (see 3.1 and 3.2 for details), and a corresponding specialist should be responsible for timely adjustment of the drug treatment regimen.
5.2. Periodic inspection
The purpose of regular examination is the timely detection and management of the signs of disease recurrence. The periodic examination (Table 5, in the supplementary appendix) includes the evaluation of peripheral neuropathy, hemodynamics, tissue perfusion levels (see section 2.3 for details), and occurrence of new ulcers. The progression of the patient's condition and the necessity for recanalization is judged on the basis of the results of the physical examination.
5.3. Follow-up schedule and outcome assessment
The development of a detailed and comprehensive follow-up plan can aid in close monitoring of the patient's blood glucose levels, vascular lesions, and other changes. It also facilitates comprehensive recovery of the patient.33 The follow-up period spans 6 months, and the outcome assessment is based on a 7-point score.
6. Nursing care of diabetic foot
6.1. Pre-operative care
6.1.1. Psychological nursing
It mainly includes three aspects: 1. effectively communicating with the patients, helping them understand the course of diabetes and necessity of blood glucose control, etc., and providing satisfactory explanations; 2. briefly introducing the principle and procedure of surgery to the patients, and obtaining their understanding and trust; 3. comforting the patients, eliminating their stress, administering drugs as per the doctor's advice, and providing soothing music and an appropriate rest environment.
6.1.2. Preoperative assessment
Patients were questioned about the duration of diabetes and diabetic foot, blood glucose control, and the presence or absence of other diseases or habits such as smoking and alcohol consumption. Furthermore, they were enquired about the presence of pain, numbness, dysesthesia, loss of sensation in the lower extremities, intermittent claudication, or difficulty in squatting and standing.
Foot ulcer assessment: according to Wagner classification (Table 2).
Limb pain score: Grade 0, no pain; Grade 1, occasional pain; Grade 2, pain is often present but tolerable, and can be relieved with or without occasional general analgesics; Grade 3, pain is often relieved with general analgesics; Grade 4, pain is difficult to relieve with general analgesics and affects sleep.34
Peripheral pressure plate test (microcirculation test): After compression for 30–60 s, observe the time taken for the local skin of the compressed site to recover the same color as the surrounding tissues through the pressure plate on the sole of foot is observed to determine the microcirculation perfusion status of the local tissues. Patients with diabetic foot experience different abnormalities due to ischemia and microcirculatory damage.35
6.1.3. Limb nursing
It mainly includes two aspects: 1. monitoring and recording the temperature and color of the skin of the limb and dorsalis pedis pulse; 2. preventing cold, cleaning the ischemic limb and keeping it warm, and selecting appropriate shoes and socks to avoid extrusion.
6.1.4. Ulcer care
0.5% metronidazole 100 ml + gentamicin 16 U + insulin 4 U/insulin 1 U + anisodamine + fibrate liquid wet compress, twice a day, for 7 consecutive days is recommended for management of ulcers; 0.5% iodophor wet compress is recommended daily for dry gangrene. There is a close relationship between debridement and ulcer healing, and the number of debridements positively correlates with the healing rate of ulcers. Negative pressure therapy facilitates wound healing. Long-term repeated compression causes ulcers, and decompression facilitates ulcer healing.
6.1.5. Blood glucose and skin temperature monitoring
Fasting blood glucose is examined 30 min before meals, three times daily. Skin temperature monitoring is performed using the Tongji five-point method before treatment, during surgery, and during post-operative follow-up. The Tongji five-point method is used to detect the skin temperature at each point of the frontal head, inferior border of the patella (reticular artery of the knee joint), external ankle joint (peroneal artery), central dorsalis pedis (dorsalis pedis artery), and central plantar region (spreading artery). The frontal temperature is used as the baseline. The clinical efficacy is evaluated before and after treatment by comparing the skin temperature measured at the other four points (lower limb artery corresponding to angiosome in parentheses) (Fig. 3, Fig. 4, Fig. 5, Fig. 6). Comparing the skin temperature of both lower limbs is a direct, simple approach for preliminary, quick, and convenient evaluation of the lower limbs of patients.
Fig. 3.
Monitoring of skin temperature changes at the lower edge of the patella by the five-point method for diabetic foot.
Fig. 4.
Changes of ankle joint skin temperature outside the five-point method for diabetic foot.
Fig. 5.
Changes of central skin temperature on dorsum of foot with five-point method for diabetic foot.
Fig. 6.
Changes of central plantar skin temperature in diabetic foot with five-point method.
6.1.6. Nerve sensation nursing
This involves encouraging the patients to express the true degree of numbness and pain, and providing a good environment, music therapy, distraction, etc. to reduce patients' subjective feelings of pain and discomfort. Analgesic drugs are administered as prescribed, if necessary. It also involves timely observation and recording of the improvement of pain in patients, and close monitoring of the possibility of side effects of the drugs.
6.1.7. Local electrical stimulation: A new approach to promote ulcer healing
Electrical stimulation can promote wound healing, reduce painful neuropathy, and improve pressure balance and hemoperfusion of the foot. The types of electrical stimulation include transdermal electrical stimulation, high pulse electrical stimulation, and pulsed electromagnetic waves. There is abundant evidence that electrical stimulation promotes the healing of ulcers; however, they all relate to small sample sizes and short-term observations, and further studies of long-term treatments and stimulation doses are still needed.
6.2. Intraoperative care
Assist the patient in assuming the supine position with the operated lower limb abducted to facilitate femoral artery puncture. Maintain a proper, comfortable position for the patient and keep them warm. Strictly implement aseptic operating procedures. Monitor the patient's consciousness, vital signs and other changes in conditions and record them in a timely manner. Pay attention to control of blood pressure and heart rate. Nitroglycerin is prepared in the operating room, and antihypertensive drugs are administered in a timely manner according to the doctor's advice if high blood pressure is detected. The thrombolytic drugs include urokinase 500,000 U and alteplase 50 U. Cooperate with the physician to perform heparinization at the end of radiography and the formal initiation of treatment. Strictly follow the doctor's advice for measurement, and accurately record the dosage and time of heparinization. The venous access is opened and the drug is applied promptly as prescribed.
6.2.1. Anesthesia care
Cooperate with the anesthesiologist or doctor to help the patient assume the appropriate position and prepare the anesthetic products. Closely monitor the patient's vital signs, timely inform the doctor if any abnormalities are found, and actively cooperate in the treatment or rescue.
6.2.2. Post anesthesia care
Assist the unconscious patient under general anesthesia to take the pillow supine position with the head deviated to one side to avoid aspiration pneumonia or asphyxia; patients under arachnoid anesthesia and spinal anesthesia are kept in the pillow supine position for 6–8 h to prevent headache and those under epidural anesthesia are kept supine for 4–6 h without pillow removal. If the patient experiences agitation during emergence from anesthesia, appropriate restraint and bed railing protection should be provided, when necessary, to prevent fall or injury.
6.3. Post-operative care
6.3.1. Puncture site care
Hemostasis is performed by sandbag compression for 6 h, and the patients are instructed to rest in bed and immobilize the operated limb for 24 h. Wagner grade, cold sensation, numbness, limb pain, pain-free walking distance, ABI (ankle brachial index), and peripheral pressor test (microcirculation test) are reassessed thereafter and compared to the corresponding values before surgery.
6.3.2. Diet care, wound care, and skin color record
Diet care is based on encouraging the consumption of a diabetic diet high in protein and vitamins and regular monitoring of blood glucose. Wound care primarily involves timely dressing change and debridement. In addition, it is necessary to observe the skin color, and record and measure the epidermal temperature of the lower limb (the position is consistent with that determined by the Tongji five-point method).
6.3.3. Functional exercise of the lower limbs
Patients are advised to exercise moderately to improve blood circulation to the limbs. The amount of exercise should be gradual, and it is appropriate to exercise 3 or 4 times a day at intervals of more than 30 min in order to avoid pain after exercise. In addition, the patient can perform Berg-Allen exercise gymnastics as follows: lie flat on the back; elevate the feet 45°–60°, holding this position for 1–3 min; flex the metatarsals on the dorsum of the foot and swing from side to side, extend and then close the feet upwards until the skin of the feet becomes pink; this action lasts for 2–3 min; lie flat, keep warm, rest for 5 min, and repeat the exercise 10 times.
6.4. Health education and telemonitoring
6.4.1. Dietary guidance
Inform patients that the diabetic diet should be regular and quantitative; total daily calories should be controlled; staple food should not be excessive; and the diet should be light, with low fat, less salt, less sugar, a small number of meals, and balanced nutrition.
6.4.2. Medication guidance
Inform the patient to regularly monitor their blood glucose levels, strictly follow the doctor's advice, not alter the dose by themselves, take the prescribed anticoagulant antiplatelet drugs, pay attention to the occurrence of any bleeding points in the skin and mucosa, and seek medical attention if gingival bleeding or hematuria are experienced.
6.4.3. Psychological guidance
Dissuade patients from anxiety, tension, fear, and other adverse emotions to prevent blood glucose fluctuations, and advise them to actively participate in activities (such as walking or Tai Chi) and communicate with friends and family to reduce psychological pressure.
6.4.4. Life guidance
It is recommended that patients pay attention to personal hygiene, change undergarments frequently, live a regular life, and quit smoking and alcohol consumption.
6.4.5. Foot care
Advise patients to wash their feet daily with warm water, wear cotton socks, comfortable shoes, and keep their nails short. Exercise should be based on the amount of blood glucose. It should not be performed under fasting condition; the patient should exercise more if consuming candy, snacks etc.
6.4.6. Rehabilitation instruction
Advise patients to regularly monitor their blood glucose level; use drugs correctly according to the doctor's advice; exercise regularly, step by step; and adhere to it for a long time. Patients with foot ulcers should change the dressings regularly, observe foot skin color and temperature, and seek medical attention if abnormalities are found. The patients should also be asked to perform walking training, that is, walking for 30 min twice daily, and those who could not tolerate this walking duration should be asked to walk for as long as bearable.
6.4.7. Pressure relief insoles and shoes
Recurrence of foot ulcers is an important issue in DFU treatment. Emphasis is placed on prevention of recurrence in healed foot ulcers. It is prevented by reducing the plantar shear force with special shoes and insoles. Reducing foot pressure and adhering to wearing stress-relief footwear are the keys to preventing recurrence. Features of diabetic foot shoes are as follows: they have a cover for the toe tip; the heel to toe tip is flat but protruding in the middle; the cushioning property in the middle is similar to running shoes; the toe tip is additionally widened; they have a 5 mm thick sole (deeper shoe); the material is stretchable and ventilated; the shoe is worn by tying shoelaces and Velcro to facilitate wearing and removal.
6.4.8. Remote home self-monitoring
Monitoring foot movements and correcting pressure abnormalities are effective measures to prevent neuropathic foot ulcers. High-pressure areas are prone to inflammation, and skin temperature increases in the presence of inflammation. A difference of ˃ 2.2 °C in the temperature of both feet indicates the need for immobilization and decompression. Remote monitoring or household self-monitoring of the skin temperature can effectively reduce the occurrence of foot ulcers.36
7. Multidisciplinary team diagnosis and treatment (MDT) and management
7.1. Diabetic foot MDT
At present, there is a lack of overall treatment plans for the diagnosis and treatment of diabetic foot, and a unified multidisciplinary approach is helpful to reduce the number of amputations. Due to long-term continuous hyperglycemic injury, patients with diabetic foot also suffer from multiple organ damage in addition to the diabetic foot itself. Therefore, it is particularly necessary to establish multidisciplinary cooperation for such patients. MDT mainly involves the departments of interventional vascular surgery, endocrinology, neurology, cardiology, nephrology, infectious diseases, orthopedics, outpatient and emergency, nursing, and medical imaging departments. At the same time, the participation of basic researchers including those engaged in metabolism, immunity and stem cell therapy is also required.37 Fig. 7 presents the recommended operating mode.
Fig. 7.
MDT operation mode in diabetes group.
7.2. MDT administration
It mainly includes six aspects: 1. management at the hospital level, taking the lead from the Medical Department (or other similar functional departments), establishing a special management institution with the cooperation of the Department of Science and Education and other departments, under the charge of a specially-assigned person; 2. establishing a full-time MDT secretariat to assist in the daily management of patient with diabetic foot; 3. establishing a diabetic foot management follow-up system and preparing a series of manuals; 4. holding relevant quality control meetings and business studies every month to solve the problems and difficulties encountered in clinical practice; 5. standardizing the daily patient consultation system of each department and having the assigned personnel perform the consultation; and 6. establishing all-patient databases and data platforms and implementing the discussion system for major and difficult surgeries.38,39
8. Internet and large database of diabetic foot
8.1. Background
China has an aging society, and such aged individuals require proper health care. It has been pointed out in the Several Opinions of the State Council on Promoting the Development of the Health Service Industry that, by 2020, China will establish a health service industry system covering the entire life cycle, with a rich connotation and reasonable structure, and the total scale of the health service industry will reach more than 8 trillion yuan. According to the Evaluation Report on the Development Level of Informatization in China in 2013 issued by the China Institute of Electronic Information Industry Development, the development level of informatization in Shanghai ranks first in China. Large cities such as Shanghai have preliminarily met the conditions of being considered “Intelligent cities” and providing “smart medical care.”
It has been stated in the Several Opinions of the State Council on Promoting Information Consumption and Expanding Domestic Demand that, by 2015, the scale of information consumption in China will exceed 3.2 trillion yuan. Modern medical and health services relying on informatization means are important breakthroughs.
A comprehensive management and service platform for diabetic vascular disease was officially proposed in this context. Diabetic angiopathy has a typically high incidence in the elderly, which seriously affects their quality of life. Relying on the comprehensive management and service platform of diabetic vascular disease, the service needs of patients can be effectively linked to hierarchical medical resources, scientific research resources, and medical technology resources. The new service model formed is a typical health service and information consumption service, which is the specific embodiment of the “smart city."
8.2. Task analysis
8.2.1. Enable lifelong monitoring of diabetic patients
From the perspective of residents, patients with diabetic angiopathy not only have a need for treatment, but also have other needs before and after seeking medical attention. We hope to provide appropriate medical resources (mainly hospitals and physicians), treatment, and good rehabilitation services after seeking medical treatment. Therefore, a carrier is needed to open up the medical and health services to patients at all stages of life.
8.2.2. Promote the standardization of clinical medical treatment
From the perspective of hospitals, information exchange of patients with intractable diseases such as diabetic vascular diseases in various regions can be realized and targeted referral can be carried out through industrial alliance. Moreover, subsequent follow-up management can be carried out at the alliance hospitals where the patients are located after treatment. Hospitals of all alliances adopt standardized diagnosis and treatment norms to carry out clinical treatment and collaborative services, which can improve the quality of medical care, improve service efficiency, and allow patients to enjoy better medical services.
8.2.3. Provide basis for national strategy formulation and study implementation
From the perspective of scientific research, physicians in hospitals within the industrial alliance can track the entire course of disease screening, case referral, diagnosis, treatment, and postoperative rehabilitation of patients. A large amount of standardized sample data of scientific research cases accumulated by the alliance platform can be used to carry out case analysis and multi-sample population analysis. With the help of this platform, clinical research exchange and cooperation can be achieved.40 The large number of standardized samples accumulated by the alliance platform will provide scientific research support for new diagnostic and therapeutic technologies, so that the scientific research level of diagnosis and treatment of diabetic vascular disease in China reaches international standards and obtains a voice of authority in this regard.
8.3. Large database on the internet
8.3.1. Platform architecture, features, and effects
The comprehensive management and service platform for diabetic vascular disease (hereinafter referred to as “diabetes specialist platform”), hosted on the internet, can be interconnected with the hospitals of each alliance to obtain case information (including medical imaging), follow-up information, and nursing information of patients from the hospitals of each alliance. This platform can manage and maintain the data integrated from each hospital. Through this platform, case data and group data can be analyzed to provide a reference basis for clinical treatment, rehabilitation and medical scientific research based on large scale sample data of management. Large databases of diabetes specialist cases will become the core competitiveness of this platform. Bidirectional referral and teleconsultation can be achieved between alliance hospitals using a diabetes specialist platform for communication and collaboration.
8.3.2. Features
The large database of diabetes specialty pioneered in China realizes the integration of specialist disease diagnosis, treatment, and management, which aids cross-regional coordination and referral of specialist cases. In addition, it also realizes the integration of specialist medical treatment, education and scientific research.
9. Participating units and individuals
The units and individuals participating in the preparation of this guide include: Zhuhai People's Hospital (Lu Ligong, Li Yong), the First Affiliated Hospital of China University of Science and Technology (Lv Weifu, Ludong), the Second People's Hospital of Hefei (Yin Shiwu, Long Haideng), the First Hospital of Handan (Xin Xuanli, Xia Huawen), Peking University Third Hospital (Li Xuan), China-Japan Friendship Hospital (Liu Peng), Peking University People's Hospital (Zhang Xiaoming), Beijing Chao-Yang Hospital (Zhai Renyou), Shanghai Huangpu District Central Hospital (Cai Xingjuan), Nanjing Drum Tower Hospital (Li Xiaoqiang, Hu Nan), the first hospital of Hebei Medical University (Zhou Huimin), Guiqian International General Hospital (You Jian), Shanghai Putuo District Central Hospital (Li Mengfan), Fujian Medical University Union Medical College Hospital (Yang Weizhu), the Third People's Hospital of Yunnan (Wang Yongping), the First Affiliated Hospital of Lanzhou University (Wang Wenhui), Gansu Provincial People's Hospital of Traditional Chinese Medicine (Che Ming), the Oriental Hospital of Tongji University (Feng Bo), Hunan Provincial People's Hospital (Xiang Hua), Shanghai Xingyuan Technology Co., Ltd. (Lu Xuewei, Huang Weiguo), Wenzhou People's Hospital (Yu Xixiang), 960 Hospital of Chinese People's Liberation Army (Sun Gang), the First Affiliated Hospital of Xinjiang Medical University (Ren Weixin), the First Affiliated Hospital of Jinan University (Wang Xiaobai, Zhang Yan), the Affiliated Hospital of Xuzhou Medical University (Xu Hao, Zu Maoheng), the Second Affiliated Hospital of Nanchang University (Hu Ling, Zhou Weimin), Lishui Central Hospital (Ji Jiansong), Nanfang Hospital of Southern Medical University (Li Yanhao, He Xiaofeng), the Fifth Affiliated Hospital of Zhengzhou University (Wang Bing), Nanjing First Affiliated Hospital of Nanjing Medical University (Gu Jianping, Lou Wensheng), the First Affiliated Hospital of Zhengzhou University (Han Xinwei), Shandong Institute of Medical Imaging (Tang Jun), the Second Xiangya Hospital of Central South University (Li Gang), Shanxi Provincial People's Hospital (Liu Yu’e), the First Affiliated Hospital of Shanxi Medical University (Feng Duiping), the Fifth Affiliated Hospital of Sun Yat-sen University (Shan Hong), the First Affiliated Hospital of Hunan Medical University (Li Qing), the People's Hospital of Guangxi Autonomous Region (Yu Lei), the Third Affiliated Hospital of Yongzhou (Luo jiangtao), National Center for Genetic Testing Technology (Qiu Geng), Henan Provincial People's Hospital (Zhai Shuiting), the Shanghai tenth hospital of Tongji University, (Lian Weishuai, Cheng Jie, Wu Yongfa, Yuan Yifeng, Ni Yebin, Kang Li, Han Jianhong, Zhang Xiaojun, Li Xue). Academic secretaries: Xie Xiaoyun, Li Xue.
Ethical approval
The study was approved by the ethics committee of Shanghai Tenth People's Hospital. All clinical practices and observations were conducted in accordance with the Declaration of Helsinki. Informed consent was obtained from each patient before the study was conducted.
Patient consent
Written informed consent was obtained from patients for publication of these case reports and any accompanying images.
Declaration of competing interest
We declare that we have no financial and personal relationships with other people or organizations that can inappropriately influence our work, there is no professional or other personal interest of any nature or kind in any product, service and/or company that could be construed as influencing the position presented in, or the review of, the manuscript entitled.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.jimed.2021.07.003.
Appendix A. Supplementary data
The following is the supplementary data to this article:
References
- 1.Hingorani A., LaMuraglia G.M., Henke P. The management of diabetic foot: a clinical practice guideline by the society for vascular surgery in collaboration with the American podiatric medical association and the society for vascular medicine. J Vasc Surg. 2016;63:3S–21S. doi: 10.1016/j.jvs.2015.10.003. [DOI] [PubMed] [Google Scholar]
- 2.Tateishi-Yuyama E., Matsubara H., Murohara T. Therapeutic Angiogenesis using Cell Transplantation (TACT) Study Investigators. Therapeutic angiogenesis for patients with limb ischaemia by autologous transplantation of bone-marrow cells: a pilot study and a randomised controlled trial. Lancet. 2002;360:427–435. doi: 10.1016/S0140-6736(02)09670-8. [DOI] [PubMed] [Google Scholar]
- 3.Catterall W.A., Pedersen P.L. Adenosine triphosphatase from rat liver mitochondria. II. Interaction with adenosine diphosphate. J Biol Chem. 1972;247:7969–7976. [PubMed] [Google Scholar]
- 4.Kalish J., Hamdan A. Management of diabetic foot problems. J Vasc Surg. 2010;51:476–486. doi: 10.1016/j.jvs.2009.08.043. [DOI] [PubMed] [Google Scholar]
- 5.Selvin E., Marinopoulos S., Berkenblit G. Meta-analysis: glycosylated hemoglobin and cardiovascular disease in diabetes mellitus. Ann Intern Med. 2004;141:421–431. doi: 10.7326/0003-4819-141-6-200409210-00007. [DOI] [PubMed] [Google Scholar]
- 6.Gershater M.A., Löndahl M., Nyberg P. Complexity of factors related to outcome of neuropathic and neuroischaemic/ischaemic diabetic foot ulcers: a cohort study. Diabetologia. 2009;52:398–407. doi: 10.1007/s00125-008-1226-2. [DOI] [PubMed] [Google Scholar]
- 7.Apelqvist J. The foot in perspective. Diabetes Metab Res Rev. 2008;24:S110–S115. doi: 10.1002/dmrr.834. [DOI] [PubMed] [Google Scholar]
- 8.Apelqvist J., Bakker K., van Houtum W.H. International consensus and practical guidelines on the management and the prevention of the diabetic foot. International Working Group on the Diabetic Foot. Diabetes Metab Res Rev. 2000;16:S84–S92. doi: 10.1002/1520-7560(200009/10)16:1+<::aid-dmrr113>3.0.co;2-s. [DOI] [PubMed] [Google Scholar]
- 9.Jeffcoate W.J., Chipchase S.Y., Ince P. Assessing the outcome of the management of diabetic foot ulcers using ulcer-related and person-related measures. Diabetes Care. 2006;29:1784–1787. doi: 10.2337/dc06-0306. [DOI] [PubMed] [Google Scholar]
- 10.Clairotte C., Retout S., Potier L. Automated ankle-brachial pressure index measurement by clinical staff for peripheral arterial disease diagnosis in nondiabetic and diabetic patients. Diabetes Care. 2009;32:1231–1236. doi: 10.2337/dc08-2230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Norgren L., Hiatt W.R., Dormandy J.A. Inter-society consensus for the management of peripheral arterial disease (TASC II) Eur J Vasc Endovasc Surg. 2007;33:S1–S75. doi: 10.1016/j.ejvs.2006.09.024. [DOI] [PubMed] [Google Scholar]
- 12.European Society of Hypertension-European Society of Cardiology Guidelines Committee European Society of Hypertension-European Society of Cardiology guidelines for the management of arterial hypertension. J Hypertens. 2003;21:1011–1153. doi: 10.1097/00004872-200306000-00001. 2003. [DOI] [PubMed] [Google Scholar]
- 13.American Diabetes Association Standards of medical care in diabetes--2006. Diabetes Care. 2006;29:S4–S42. Erratum in: Diabetes Care. 2006;29:1192. [PubMed] [Google Scholar]
- 14.Lepäntalo M., Apelqvist J., Setacci C. Chapter V: diabetic foot. Eur J Vasc Endovasc Surg. 2011;42:S60–S74. doi: 10.1016/S1078-5884(11)60012-9. [DOI] [PubMed] [Google Scholar]
- 15.Lipsky B.A., Senneville É., Abbas Z.G. Guidelines on the diagnosis and treatment of foot infection in persons with diabetes (IWGDF 2019 update) Diabetes Metab Res Rev. 2020;36 doi: 10.1002/dmrr.3280. [DOI] [PubMed] [Google Scholar]
- 16.Elgzyri T., Larsson J., Thörne J. Outcome of ischemic foot ulcer in diabetic patients who had no invasive vascular intervention. Eur J Vasc Endovasc Surg. 2013;46:110–117. doi: 10.1016/j.ejvs.2013.04.013. [DOI] [PubMed] [Google Scholar]
- 17.Wang A., Lv G., Cheng X. Cuidelines on multidisciplinary approac hes for the prevention and management of diabetic foot disease (2020 edition) Chin J Burns. 2020;36:986. doi: 10.1093/burnst/tkaa017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Adam D.J., Beard J.D., Cleveland T. Bypass versus angioplasty in severe ischaemia of the leg (BASIL): multicentre, randomised controlled trial. Lancet. 2005;366:1925–1934. doi: 10.1016/S0140-6736(05)67704-5. [DOI] [PubMed] [Google Scholar]
- 19.Elraiyah T., Tsapas A., Prutsky G. A systematic review and meta-analysis of adjunctive therapies in diabetic foot ulcers. J Vasc Surg. 2016;63:46S–58S. doi: 10.1016/j.jvs.2015.10.007. e1-2. [DOI] [PubMed] [Google Scholar]
- 20.Meloni M., Izzo V., Vainieri E. Management of negative pressure wound therapy in the treatment of diabetic foot ulcers. World J Orthoped. 2015;6:387–393. doi: 10.5312/wjo.v6.i4.387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Shi R., Jin Y., Cao C. Localization of human adipose-derived stem cells and their effect in repair of diabetic foot ulcers in rats. Stem Cell Res Ther. 2016;7:155. doi: 10.1186/s13287-016-0412-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chen Y.G., Cao Y.M., Chen Q. Perioperative management of amputation for the serious gangrene of diabetic foot in senile patients. China J Orthop Traumatol. 2006;19:333–334. [Google Scholar]
- 23.Guan X.H. Progress of new techniques for treating diabetic foot. Medical Journal of Air Force. 2011;27:96–98. 108. [Google Scholar]
- 24.Wang T., Zhao J. Determination of amputation plane for diabetic foot gangrene. Chin J Curr Adv Gen Surg. 2014;17:76–80. [Google Scholar]
- 25.Gu Y.Q. Determination of amputation level in ischaemic lower limbs. ANZ J Surg. 2004;74:31–33. doi: 10.1046/j.1445-1433.2003.02787.x. [DOI] [PubMed] [Google Scholar]
- 26.Gu Y.Q., Zhang J., Wang Z.K. Surgical treatment of 78 cases with diabetic lower limb ischaemia. Chinese Journal of Diabetes Mellitus. 2004;12:328–331. [Google Scholar]
- 27.Lu C., Zhang X., Zhang D. Short time tripterine treatment enhances endothelial progenitor cell function via heat shock protein 32. J Cell Physiol. 2015;230:1139–1147. doi: 10.1002/jcp.24849. [DOI] [PubMed] [Google Scholar]
- 28.Lu C., Yu X., Zuo K. Tripterine treatment improves endothelial progenitor cell function via integrin-linked kinase. Cell Physiol Biochem. 2015;37:1089–1103. doi: 10.1159/000430234. [DOI] [PubMed] [Google Scholar]
- 29.Zuo K., Li M., Zhang X. MiR-21 suppresses endothelial progenitor cell proliferation by activating the TGFbeta signaling pathway via downregulation of WWP1. Int J Clin Exp Pathol. 2015;8:414–422. [PMC free article] [PubMed] [Google Scholar]
- 30.Zhang X., Lian W., Lou W. Transcatheter arterial infusion of autologous CD133(+) cells for diabetic peripheral artery disease. Stem Cell Int. 2016;2016:6925357. doi: 10.1155/2016/6925357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zhao W.N., Xu S.Q., Liang J.F. Endothelial progenitor cells from human fetal aorta cure diabetic foot in a rat model. Metabolism. 2016;65:1755–1767. doi: 10.1016/j.metabol.2016.09.007. [DOI] [PubMed] [Google Scholar]
- 32.Wu Q., Chen B., Liang Z. Mesenchymal stem cells as a prospective therapy for the diabetic foot. Stem Cell Int. 2016;2016:4612167. doi: 10.1155/2016/4612167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hinchliffe R.J., Forsythe R.O., Apelqvist J. Guidelines on diagnosis, prognosis, and management of peripheral artery disease in patients with foot ulcers and diabetes (IWGDF 2019 update) Diabetes Metab Res Rev. 2020;36 doi: 10.1002/dmrr.3276. [DOI] [PubMed] [Google Scholar]
- 34.Hinchliffe R.J., Brownrigg J.R., Apelqvist J. IWGDF guidance on the diagnosis, prognosis and management of peripheral artery disease in patients with foot ulcers in diabetes. Diabetes Metab Res Rev. 2016;32(Suppl 1):37–44. doi: 10.1002/dmrr.2698. [DOI] [PubMed] [Google Scholar]
- 35.Asadi M.R., Torkaman G., Hedayati M. Angiogenic effects of low-intensity cathodal direct current on ischemic diabetic foot ulcers: a randomized controlled trial. Diabetes Res Clin Pract. 2017;127:147–155. doi: 10.1016/j.diabres.2017.03.012. [DOI] [PubMed] [Google Scholar]
- 36.Chatwin K.E., Abbott C.A., Reddy P.N. A foreign body through the shoe of a person with diabetic peripheral neuropathy alters contralateral biomechanics: captured through innovative plantar pressure technology. Int J Low Extrem Wounds. 2018;17:125–129. doi: 10.1177/1534734618784080. [DOI] [PubMed] [Google Scholar]
- 37.Crisologo P.A., Lavery L.A. Remote home monitoring to identify and prevent diabetic foot ulceration. Ann Transl Med. 2017;5:430. doi: 10.21037/atm.2017.08.40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Yi F., Hou W., Zhou C. Radiofrequency ablation versus antiarrhythmic drug therapy for atrial fibrillation: meta-analysis of safety and efficacy. J Cardiovasc Pharmacol. 2019;73:241–247. doi: 10.1097/FJC.0000000000000654. [DOI] [PubMed] [Google Scholar]
- 39.Wang W., Liu D., Li D. Nanofibrous vascular scaffold prepared from miscible polymer blend with heparin/stromal cell-derived factor-1 alpha for enhancing anticoagulation and endothelialization. Colloids Surf B Biointerfaces. 2019;181:963–972. doi: 10.1016/j.colsurfb.2019.06.065. [DOI] [PubMed] [Google Scholar]
- 40.Shi Z., Zhang Y., Shen X. Fabrication of g-C3N4/BiOBr heterojunctions on carbon fibers as weaveable photocatalyst for degrading tetracycline hydrochloride under visible light. Chem Eng J. 2020:386. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.