Abstract
Introduction
The best management strategy for patients with atrial fibrillation (AF) with heart failure (HF) and preserved left ventricular ejection fraction (HFpEF) is unknown.
Methods and Results
This cohort study was conducted in Olmsted County, Minnesota, with resources of the Rochester Epidemiology Project.Patients with incident AF occurring between 2000 and 2014 with prior or concurrent HF were included.Patients with LVEF ≥ 50% were designated as HFpEF and those with LVEF < 50% were designated as HFrEF.Rhythm control in the first year after AF diagnosis was defined as prescriptions for an antiarrhythmic drug, catheter ablation, or maze procedure.The primary end point was all-cause mortality. The secondary end points were cardiovascular death, cardiovascular hospitalization and stroke or transient ischemic attack. Of 859 patients (age,77.2±12.1 years; 49.2%, female), 447 had HFpEF-AF and 412 had HFrEF-AF. There was no difference in all-cause mortality (10-year mortality, 83% vs 79%; P=.54) or secondary endpoints between the HFpEF-AF and HFrEF-AF, respectively. Compared with the rate control strategy, rhythm control in HFpEF-AF patients (n=40, 15.9%) offered no survival benefits (adjusted HR, 0.70; 95% CI, 0.42–1.16; P=.16), whereas rhythm control in HFrEF-AF patients (n=52, 22.5%) decrease cardiovascular mortality (HR, 0.38; 95% CI, 0.17–0.86; P=.02).
Conclusions
Patients with HFpEF-AF and HFrEF-AF had similar poor prognoses. Rhythm control strategy was seldom adopted in community care in patients with HF and AF. A rhythm control strategy may provide survival benefit for patients with HFrEF-AF and the benefit of rhythm control in patients with HFpEF-AF warrants further study.
Keywords: atrial fibrillation, heart failure with reduced ejection fraction, heart failure with preserved ejection fraction, rate control, rhythm control
Introduction
The aging of the population and improved survival among patients with cardiovascular disease have contributed to an increase in the prevalence of atrial fibrillation (AF) and heart failure (HF),1 which often coexist. In the Framingham Heart Study, AF occurred in more than half the patients with HF, and HF occurred in more than one-third of patients with AF.2 When AF and HF occur in combination, the clinical outcomes are particularly poor.3–8 In addition, outcomes for patients with AF may differ between those with HF and preserved ejection fraction (HFpEF) and those with HF and reduced ejection fraction (HFrEF). In the Framingham Heart Study, AF was associated with a greater risk of death among patients with HFrEF,2 whereas in the Candesartan in Heart Failure Assessment of Reduction in Mortality and Morbidity (CHARM) study, AF-associated death was higher among patients with HFpEF.7 New-onset AF was associated with a 2.3-fold increased risk of cardiovascular events in patients with HFpEF.9 Previous studies have been focused on the association of AF and HF subtypes largely in hospital-based cohorts.10 The objective of our study was to investigate the prognosis for patients with incident AF after a diagnosis of HFpEF or HFrEF in a community-based cohort and the long-term outcomes of treatment of AF according to HF type.
Methods
Study Population
The study was conducted in Olmsted County, Minnesota, using the resources of the Rochester Epidemiology Project (REP), a records-linkage system allowing virtually complete capture of health care data for county residents. This records-linkage system encompasses more than 6 million person-years of follow-up for more than 500,000 unique patients since 1966.11 Demographic and ethnic characteristics of Olmsted County are representative of the state of Minnesota and the US Midwest, and age- and sex-specific mortality rates are similar to the national data, supporting the generalizability of the REP data.12 The present study was approved by the Mayo Clinic and Olmsted Medical Center institutional review boards.
Incident AF and HF cohort
Adults (18 years or older) with newly diagnosed AF and prevalent or concurrent HF were included in the study. AF or atrial flutter occurring from 2000 through 2014was identified with International Classification of Diseases, Ninth Revision, Clinical Modification (ICD-9-CM) codes 427.31 and 427.32 from all providers in the REP and from Mayo Clinic electrocardiograms.13 Diagnostic codes and electrocardiograms from both inpatient and outpatient encounters were captured, and all records were manually reviewed by trained nurse abstractors to validate the events using criteria as we described previously.13 The date of AF diagnosis was defined as the index date.
HF was identified before or concurrent with the diagnosis of AF with the use of ICD-9-CM code 428 assigned during an inpatient or outpatient encounter with any provider in the REP. Baseline was defined as the closest measurement occurring within 1 year after the diagnosis of AF. Patients with left ventricular ejection fraction (LVEF) of at least 50% were designated as having HFpEF, and those with LVEF less than 50% were designated as having HFrEF. Patients without a baseline LVEF were excluded (n=161; Figure 1).
Figure 1. Flow Chart of Patient Selection.
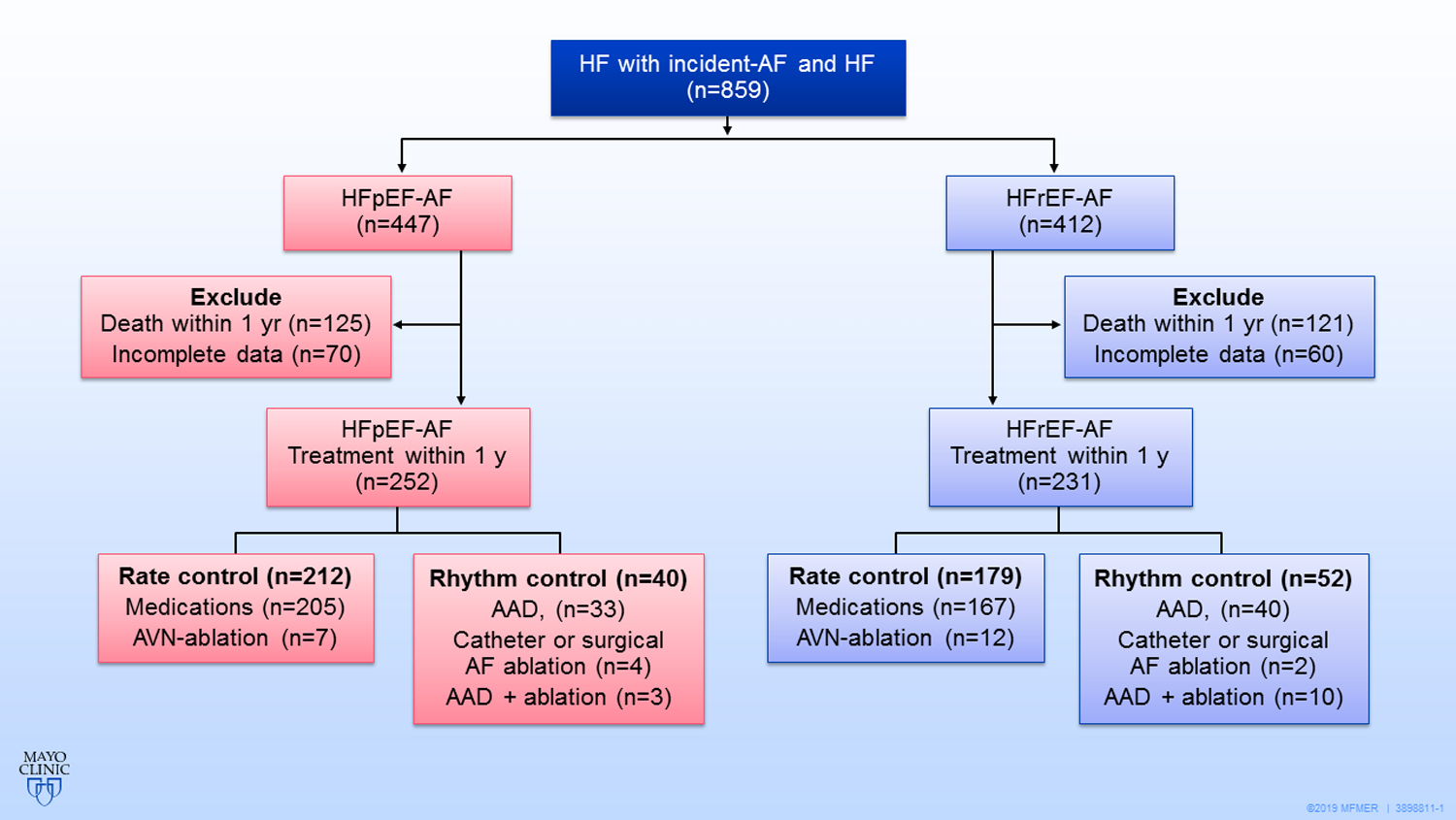
There was no significant difference in all-cause mortality,cardiovascular death, cardiovascular hospitalization and stroke or transient ischemic attack between the HFpEF-AF and HFrEF-AF. Compared with the rate control strategy, rhythm control in HFpEF-AF patients offered no survival benefits,whereas rhythm control decrease cardiovascular mortality in HFrEF-AF patients.
AAD=antiarrhythmic drug;AF=atrial fibrillation;AVN= atrioventricular node;HF=heart failure;
HFpEF-AF= atrial fibrillation and heart failure with preserved left ventricular ejection fraction;
HFrEF-AF= atrial fibrillation and heart failure with reduced left ventricular ejection fraction.
Definitions of Rhythm Control and Rate Control
AF treatment strategies were determined according to the discretion of each provider. The rhythm control group consisted of patients who were prescribed any antiarrhythmic drug (AAD) for at least 30 days or who underwent catheter ablation or the surgical maze procedure for AF within the first year after AF diagnosis.14 The AADs (and their classes) were amiodarone (III), sotalol (III), propafenone (Ic), flecainide (Ic), quinidine (Ia), dofetilide (III), and procainamide (Ia). The rate control group consisted of patients who underwent atrioventricular node (AVN) ablation or who were prescribed a rate control medication for at least 30 days within the first year after AF diagnosis but did not receive any AAD or undergo a rhythm control procedure. The rate control drugs were β-blockers; calcium channel blockers, including diltiazem and verapamil; and digitalis. Thus, patients who received both rhythm control and rate control treatment within the first year after diagnosis were classified in the rhythm control group, and patients who did not receive either rate control or rhythm control were categorized in the rate control group.
Ascertainment of Covariates and Study Outcomes
Comorbidities were identified from inpatient and outpatient ICD-9-CM diagnostic codes, which were defined previously.13 At least 2 codes within the 5 years before the index date were required for confirmation of the diagnosis. Data on medication use were obtained from an REP ambulatory record within 1 month before the index date.
Outcomes were ascertained through December 31, 2017. The primary end point of this study was all-cause mortality, which was determined from death certificates from the state of Minnesota, inpatient and outpatient medical records, and obituaries in local newspapers. The secondary end points included cardiovascular death, cardiovascular hospitalization, and ischemic stroke or transient ischemic attack (TIA). End point events were identified from diagnostic codes as follows: The underlying cause of death on the death certificate and the principal discharge diagnosis from hospitalizations were used to define cardiovascular deaths and cardiovascular hospitalizations with the use of ICD-9-CM codes 390 through 459 and International Classification of Diseases, Tenth Revision, Clinical Modification (ICD-10-CM) codes I00-I99. Ischemic stroke and TIA were identified from ICD-9-CM codes 433 through 436 and ICD-10-CM codes I63 and G45 assigned as the principal discharge diagnosis after hospitalization. Occurrence of a stroke or TIA after diagnosis of AF was identified for the entire cohort, including patients who had a history of a prior stroke.
Statistical Analysis
Continuous variables were summarized as mean (SD). Groups were compared with use of 2-sample t tests. The χ2 test was used to compare categorical variables between groups. The Kaplan-Meier method was used to estimate each of the outcome variable, the overall estimate of AAD use, and the cumulative probability of catheter ablation for AF or AVN ablation. Survival curves were compared between the groups with the log-rank test. The analysis comparing HFpEF and HFrEF outcomes was completed with the date of incident AF as the index date (i.e., the starting point for the study). The association of AF ablation and AVN ablation at any time during follow-up and the association with each end point was completed with time-dependent variables for catheter ablation and AVN ablation in the Cox proportion hazards model for each primary and secondary outcome. Follow-up for this analysis began on the date of AF diagnosis.
These patients were all included in this study as newly diagnosed cases of AF. Given they were newly diagnosed with AF, the patients needed some time to establish an effective treatment strategy. A 1 year window was used to establish the strategy in order to put them into treatment groups for the analysis. The treatment strategy in the first year after AF diagnosis was categorized as rhythm control or rate control. The Kaplan-Meier method was used to compare the 2 strategies within the HFpEF and HFrEF groups. For this analysis, follow-up began 1 year after AF diagnosis to allow for the initiation of therapy, and patients were excluded from this analysis if they died within 1 year of the AF diagnosis (n=246).
A propensity score for the comparison between rhythm control and rate control was constructed and included several covariates (age, sex, time interval from HF to AF, body mass index, hypertension, chronic obstructive pulmonary disease, prior myocardial infarction, and prior stroke). This propensity score was estimated with logistic regression models. Only the variables with significant difference in a univariate regression analysis were chosen for propensity score. Cox proportional hazards models were used to compare the rhythm control group with the rate control group after adjustment for the propensity score. A 2-sided P value less than .05 was considered statistically significant. All statistical analyses were performed with SAS, version 9.4 software (SAS Institute Inc).
Results
Baseline Characteristics of Population
The study included 859 patients who had incident AF (mean [SD] age, 77.2 [12.1] years; 49.2% were female); 447 patients had HFpEF-AF and 412 had HFrEF-AF (Figure 1). Compared with patients who had HFrEF-AF, a higher percentage of patients with HFpEF-AF were female (60.2% vs 37.4%, P<.001), were older (mean, 79.2 [11.1] years vs 74.9 [12.8] years; P<.001), and were more likely to have hypertension, prior stroke, and kidney disease as indicated by a higher CHA2DS2-VASc score (Table 1). The median time from HF diagnosis to development of AF was trending shorter for the HFpEF-AF group compared with the HFrEF-AF group (12 vs 27 days, P=.06).
Table 1.
Baseline Characteristics of Patients With HFpEF-AF or HFrEF-AF
| Characteristic | HFpEF-AF (n=447) | HFrEF-AF (n=412) | P Value |
|---|---|---|---|
| Age, mean (SD), y | 79.2 (11.1) | 74.9 (12.8) | <.001 |
| Female, n (%) | 269 (60.2) | 154 (37.4) | <.001 |
| BMI, mean (SD),kg/m2 | 31.0 (8.0) | 30.7(7.9) | .58 |
| CHA2DS2-VASc score, mean (SD) | 5.2 (1.6) | 4.6(1.8) | <.001 |
| White, n (%) | 426 (95.3) | 398 (96.6) | .34 |
| Smoking , n (%) | 237 (53.0) | 253 (61.4) | .03 |
| Hypertension, n (%) | 382 (85.5) | 310 (75.2) | <.001 |
| Prior MI , n (%) | 83 (18.6) | 134 (32.5) | <.001 |
| Diabetes, n (%) | 168 (37.6) | 154 (37.4) | .95 |
| Prior COPD, n (%) | 106 (23.7) | 105 (25.5) | .55 |
| Peripheral vascular disease, n (%) | 53 (11.9) | 63 (15.3) | .14 |
| Dementia, n (%) | 29 (6.5) | 25 (6.1) | .80 |
| Malignancy, n (%) | 87 (19.5) | 85 (20.6) | .67 |
| Metastatic solid tumor, n (%) | 18 (4.0) | 13 (3.2) | .49 |
| Chronic kidney disease, n (%) | 96 (21.5) | 64 (15.5) | .03 |
| Rheumatologic disease, n (%) | 32 (7.2) | 19 (4.6) | .11 |
| Prior stroke or TIA, n (%) | 105 (23.5) | 64 (15.5) | .003 |
| Time from HF to AF, median, d | 12 | 27 | .06* |
| LVEF, mean (SD),% | 61.2 (6.7) | 32.4 (10.2) | … |
| β-Blocker, n (%) | 297 (66.4) | 283 (68.7) | .48 |
| Digoxin, n (%) | 45 (10.1) | 102 (24.8) | <.001 |
| CCB, n (%) | 64 (14.3) | 43 (10.4) | .09 |
| Statin, n (%) | 160 (35.8) | 203 (49.3) | <.001 |
| Furosemide, n (%) | 215 (48.1) | 248 (60.2) | <.001 |
| Aldosterone, n (%) | 19 (4.3) | 36 (8.7) | .007 |
| ACE-I or ARB, n (%) | 223 (49.9) | 262 (63.6) | <.001 |
| Warfarin or NOAC, n (%) | 178 (39.8) | 186 (45.1) | .11 |
ACE-I=angiotensin-converting enzyme inhibitor; ARB=angiotensin receptor blocker; BMI= body mass index (calculated as weight in kilograms divided by height in meters squared); CCB=cium channel blocker; COPD=chronic obstructive pulmonary disease; HF=heart failure; HFpEF-AF=atrial fibrillation and heart failure with preserved ejection fraction; HFrEF-A=atrial fibrillation and heart failure with reduced ejection fraction; LVEF=left ventricular ejection fraction; MI=myocardial infarction; NOAC=novel oral anticoagulant; TIA=transient ischemic attack.
Wilcoxon rank sum test.
Outcomes in HFpEF-AF and HFrEF-AF
During a mean follow-up of 4.1 years, 354 of 447 patients with HFpEF-AF died and 312 of 412 patients with HFrEF-AF died. The 10-year probability of death was 83% in the HFpEF-AF group and 79% in the HFrEF-AF group (P=.54; Figure 2A). Rates for cardiovascular death, cardiovascular hospitalization, and stroke or TIA were not significantly different between the HFpEF-AF and HFrEF-AF groups (Figure 2B–D).
Figure 2. Kaplan-Meier Survival Curves for Primary and Secondary End Points.
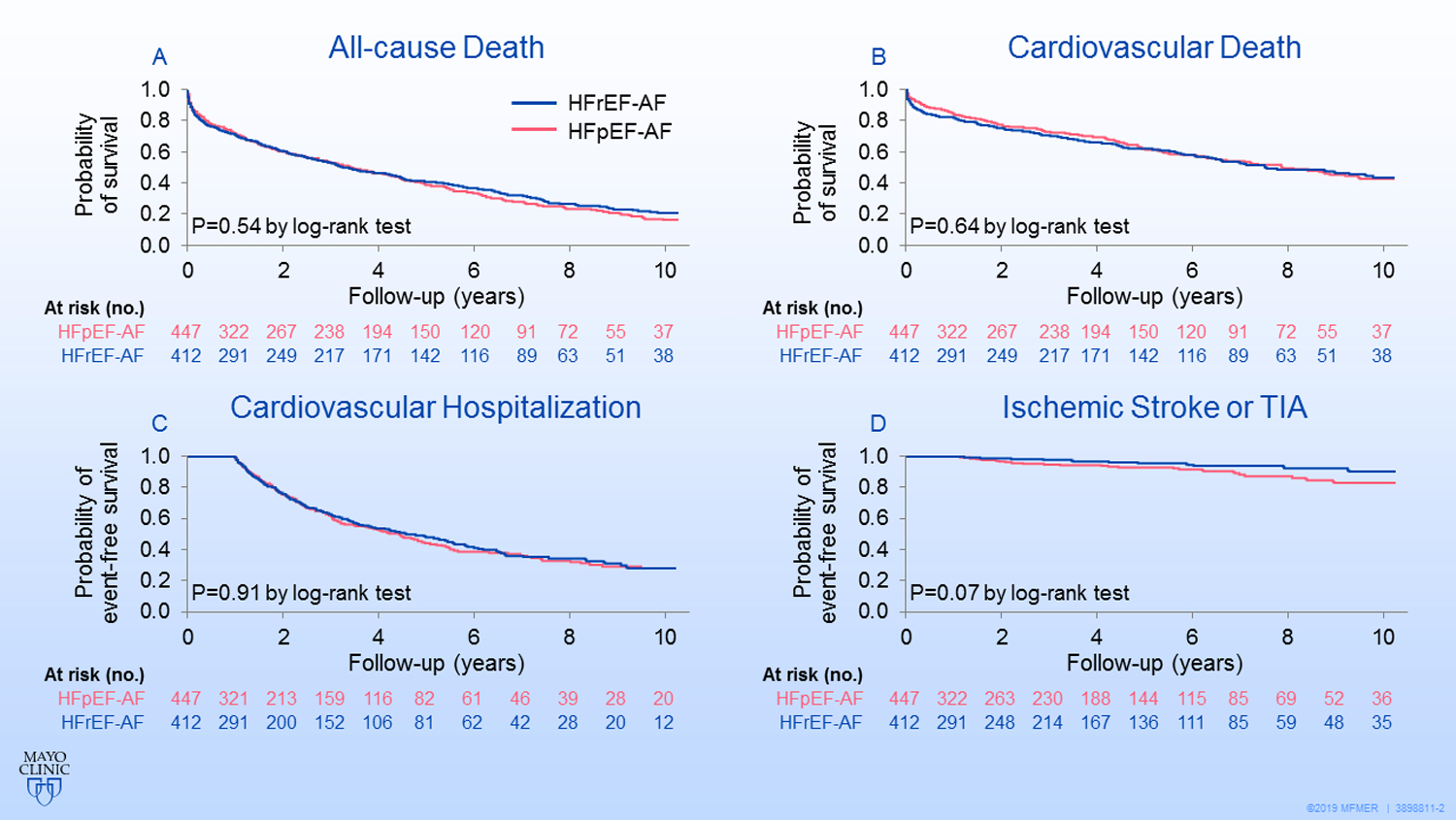
Primary and secondary end points for patients with atrial fibrillation and heart failure with preserved ejection fraction (HFpEF-AF) or atrial fibrillation and heart failure with reduced ejection fraction (HFrEF-AF) were not significantly different (by log-rank test) between the 2 groups.
A, All-cause mortality. B, Cardiovascular mortality. C, Cardiovascular hospitalization. D, Ischemic stroke or transient ischemic attack.
For the cohort, the 10-year probability of undergoing catheter ablation for AF was 3.3% in the HFpEF-AF group and 6.1% in the HFrEF-AF group (P=.05); the 10-year probability of receiving AVN ablation was 4.3% in the HFpEF-AF group and 8.6% in the HFrEF-AF group (P=.05).
Rhythm Control Compared with Rate Control for Patients With HFpEF-AF
Of the 252 patients in the HFpEF-AF group, 40 (15.9%) were treated with rhythm control (33 received AADs alone, 4 underwent AF ablation or the maze procedure, 3 had both AAD and AF ablation) and 212 patients were treated with rate control (205 received medication; 7 underwent AVN ablation). Compared with patients in the rate control group, patients in the rhythm control group were younger and a larger percentage received anticoagulation agents (Table 2). The other variables were not significantly different between the 2 groups. During follow-up (mean, 4.1 [3.9] years) the rhythm control group had a lower all-cause mortality than the rate control group (hazard ratio [HR], 0.51; 95% CI, 0.31–0.85; P=.01) (Figure 3A). However, the difference was no longer significant after adjusting for the propensity score (adjusted HR, 0.70; 95% CI, 0.42–1.16; P=.16) (eTable). There was no significant difference in cardiovascular death, cardiovascular hospital ization, and stroke or TIA between the rhythm control group and the rate control group.
Table 2.
Baseline Characteristics of Patients in Rhythm Control and Rate Control Groups
| Characteristic | HFpEF-AF Rate Control (n=212) | HFpEF-AF Rhythm Control (n=40) | P Value | HFrEF-AF Rate Control (n=179) | HFrEF-AF Rhythm Control (n=52) | P Value |
|---|---|---|---|---|---|---|
| Age, mean (SD), y | 79.1 (11.2) | 73.6 (10.6) | .01 | 74.4 (12.4) | 66.8 (12.3) | .002 |
| Female, n (%) | 122 (57.5) | 22 (55.0) | .77 | 60 (33.5) | 17 (32.7) | .91 |
| BMI, mean (SD), kg/m2 | 31.3 (7.9) | 32.4 (8.1) | .80 | 31.3 (7.5) | 33.3 (8.2) | .09 |
| CHA2DS2-VASc score,b mean (SD) | 5.2 (1.7) | 4.7 (2.0) | .19 | 4.6 (1.6) | 3.5 (1.6) | <.001 |
| White, n (%) | 204 (96.2) | 39 (97.5) | .69 | 173 (96.6) | 50 (96.2) | .86 |
| Smoking, n (%) | 94 (44.3) | 20 (50.0) | .79 | 94 (52.5) | 24 (46.2) | .60 |
| Hypertension, n (%) | 181 (85.4) | 30 (75.0) | .10 | 135 (75.4) | 33 (63.5) | .09 |
| Prior MI, n (%) | 35 (16.5) | 6 (15.0) | .81 | 58 (32.4) | 8 (15.4) | .02 |
| Diabetes, n (%) | 78 (36.8) | 12 (30.0) | .41 | 67 (37.4) | 17 (32.7) | .53 |
| Prior COPD, n (%) | 40 (18.9) | 8 (20.0) | .87 | 54 (30.2) | 2 (3.8) | <.001 |
| Peripheral vascular disease, n (%) | 29 (13.7) | 7 (17.5) | .53 | 20 (11.2) | 2 (3.8) | .11 |
| Dementia, n (%) | 12 (5.7) | 1 (2.5) | .36 | 4 (2.2) | 1 (1.9) | .89 |
| Malignancy, n (%) | 37 (17.5) | 3 (7.5) | .11 | 28 (15.6) | 12 (23.1) | .21 |
| Metastatic solid tumor, n (%) | 9 (4.2) | 0 (0.0) | .18 | 7 (3.9) | 0 (0.0) | .15 |
| Chronic kidney disease, n (%) | 48 (22.6) | 8 (20.0) | .71 | 20 (11.2) | 3 (5.8) | .25 |
| Rheumatologic disease, n (%) | 15 (7.1) | 1 (2.5) | .28 | 7 (3.9) | 1 (1.9) | .49 |
| Prior stroke or TIA, n (%) | 48 (22.6) | 1 (2.5) | .51 | 28 (15.6) | 2 (3.8) | .03 |
| Chronic liver disease, n (%) | 2 (0.9) | 0 (0.0) | .54 | 1 (0.6) | 1 (1.9) | .35 |
| β-Blocker, n (%) | 152 (71.7) | 32 (80.0) | .28 | 152 (84.9) | 48 (92.3) | .17 |
| Digoxin, n (%) | 26 (12.3) | 8 (20.0) | .19 | 71 (39.7) | 15 (28.8) | .16 |
| CCB, n (%) | 42 (19.8) | 12 (30.0) | .15 | 22 (12.3) | 6 (11.5) | .88 |
| Statin, n (%) | 92 (43.4) | 24 (60.0) | .05 | 117 (65.4) | 30 (57.7) | .31 |
| Furosemide, n (%) | 128 (60.4) | 23 (57.5) | .73 | 142 (79.3) | 40 (76.9) | .71 |
| Aldosterone, n (%) | 16 (7.5) | 1 (2.5) | .24 | 26 (14.5) | 9 (17.3) | .62 |
| ACE-I or ARB, n (%) | 111 (52.4) | 27 (67.5) | .08 | 138 (77.1) | 44 (84.6) | .24 |
| Warfarin or NOAC, n (%) | 128 (60.4) | 34 (85.0) | .003 | 113 (63.1) | 48 (92.3) | <.001 |
ACE-I=angiotensin converting enzyme inhibitor; ARB=angiotensin receptor blockers; BMI=body mass index; CCB=calcium channel blocker; COPD=chronic obstructive pulmonary disease; HFpEF-AF=atrial fibrillation and heart failure with preserved ejection fraction; HFrEF-AF=atrial fibrillation and heart failure with reduced ejection fraction; MI=myocardial infarction; NOAC, novel oral anticoagulant; TIA, transient ischemic attack.
3. Kaplan-Meier Survival Curves for Survival.
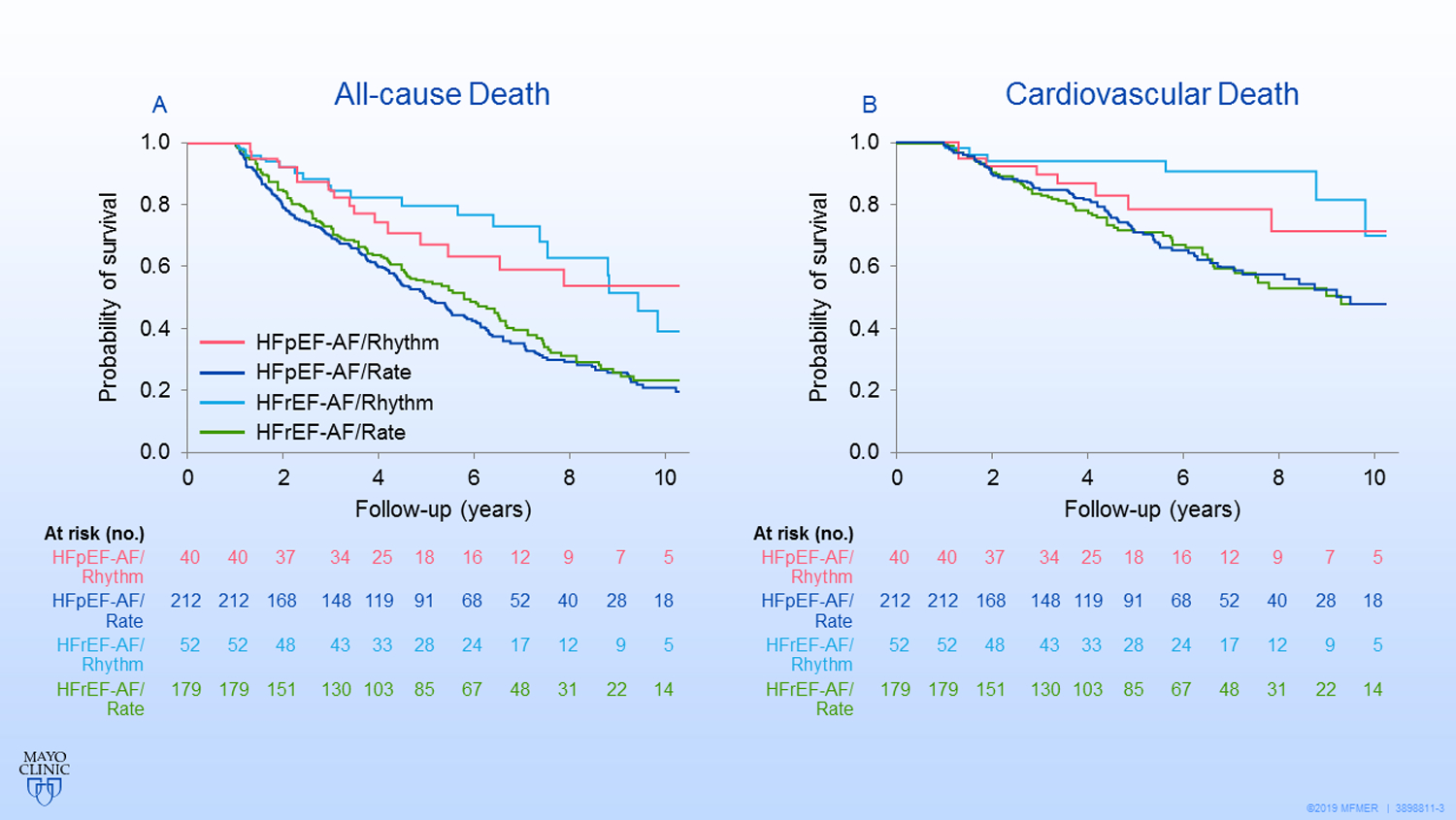
All-cause mortality (A) and cardiovascular mortality (B) compared with the rate control strategy for patients with atrial fibrillation and heart failure with preserved ejection fraction (HFpEF-AF) or atrial fibrillation and heart failure with reduced ejection fraction (HFrEF-AF).
Rhythm Control Compared With Rate Control for Patients With HFrEF-AF
Of the 231 patients in the HFrEF-AF group, 52 (22.5%) patients were in the rhythm control group (40 received AADs alone, 2 underwent AF ablation or the maze procedure, 10 had both AAD and AF ablation) and 179 were in the rate control group (167 received medication; 12 underwent AVN ablation). Patients with HFrEF-AF were more likely to receive rhythm control than patients with HFpEF-AF (22.5% vs 15.9%, P=.06). Patients who were treated with rhythm control were younger; had a lower CHA2DS2-VASc score; were less likely to have had prior myocardial infarction, stroke/TIA, or chronic obstructive pulmonary disease; and were more often were prescribed an anticoagulation agent than were patients in the rate control group (Table 2).
The rhythm control group had lower all-cause mortality than the rate control group (HR, 0.42; 95% CI, 0.26–0.69; P<.001) (Figure 3A) and a trend of lower all-cause mortality after adjusting for the propensity score (adjusted HR, 0.63; 95% CI, 0.38–1.05; P=.08) (eTable). The rhythm control patients had lower cardiovascular mortality than the rate control patients (unadjusted HR, 0.28; 95% CI, 0.13–0.62; P=.002 and adjusted HR, 0.38; 95% CI, 0.17–0.86; P=.02) (Figure 3B).There was no significant difference in cardiovascular hospitalization and stroke or TIA events between the rhythm control group and rate control group.
Discussion
In this community cohort study, we found the following: 1) the patients with HFpEF had a similar prognosis as those with HFrEF after the onset of AF; 2) the vast majority of patients were treated with rate control and very few underwent catheter or surgical pulmonary vein isolation for rhythm control in both the HFpEF group and the HFrEF group; 3) the HFrEF-AF patients who received rhythm control treatment had lower cardiovascular mortality than the rate control group.
Incident AF and Outcomes in HFpEF and HFrEF
Previous studies have shown that patients with HFrEF-AF were associated with a higher all-cause mortality compared with those with HFpEF-AF.2,15 In our study, HFpEF-AF patients and HFrEF-AF patients had similar outcomes for primary and secondary end points, including mortality and cardiovascular events. This finding is consistent with other study results showing that HFpEF-AF carries a comparably poor prognosis as HFrEF-AF despite the preserved LVEF.16 Compared with HFrEF patients, our HFpEF-AF patients in Olmsted county were older, more likely to be female, and more likely to have comorbidities (hypertension, prior stroke, or chronic kidney disease), which may contribute to an unfavorable prognosis as in the HFrEF-AF population.
Early Treatment Strategies for Patients with HFpEF-AF or HFrEF-AF
In the community, less than 20% of patients with HFpEF and approximately 25% of patients with HFrEF who had newly diagnosed AF underwent a rhythm control strategy for maintaining sinus rhythm. Further, no more than 5% patients underwent pulmonary vein isolation with catheter or surgical ablation in the HFpEF and HFrEF groups. In contrast, the vast majority of patients and physicians preferred rate control management with medications or no treatment within 1 year of the AF diagnosis. This finding reflected the real-world practice in the community. Most AF patients may be asymptomatic, and rate control is favorable as first-line therapy for the elderly population.17 Coexisting morbidities further prevent providers and patients from choosing AADs (with potential adverse effects) or AF ablations (with potential procedural complications). In the present study, ablative approaches, including pulmonary vein isolation and AVN ablation, were considered slightly more often for HFrEF patients than for HFpEF patients. More conservative medical therapy was favored by both providers and patients in an older HFpEF population.
Outcomes of Rhythm Control Compared with Rate Control Strategy
Several randomized trials comparing different management strategies on cardiovascular outcomes in AF patients have generated diverse results.18–20 The Catheter Ablation vs Antiarrhythmic Drug Therapy for Atrial Fibrillation (CABANA) trial showed no significant difference in primary composite end points between catheter ablation and antiarrhythmic drug therapy.20However, catheter ablation reduced the rehospitalization rate and AF recurrence compared with medical therapy. In our observational cohort, less than a quarter of patients received rhythm control, including very few who were treated with catheter- or surgical-based AF ablation within 1 year of AF diagnosis. Fukin et al demonstrated that in HFpEF-AF patients, catheter ablation was associated with a reduced HF rehospitalizations compared with medical therapy.21 In our study, although rhythm control appeared to provide a survival benefit, further adjustment by propensity matching did not detect any difference in cardiovascular outcomes in the HFpEF-AF group. A recent study suggested that rhythm control for AF provided no survival advantages over rate control, although more than 80% of patients received AF ablation in the rhythm control group.22 In the HFrEF-AF group, however, all-cause mortality rate was lower (but not significantly different), and the cardiovascular mortality rate was significantly lower for patients treated with rhythm control compared to rate control. This result is in agreement with the finding of “Catheter Ablation for Atrial Fibrillation with Heart Failure”, a randomized study in which AF ablation (compared with medical therapy) improved survival in an HFrEF population.23 Of note, the “Catheter Ablation for Atrial Fibrillation with Heart Failure” study enrolled patients with a EF ≤ 35%, while in our study the HFrEF group included patients with LVEF <50%. The difference in LVEF cut-off may explain some of the discrepancies in conclusions from HF and AF studies.
Limitations
Limitations must be considered to interpret the findings. First, as in any observational cohort study, the effect of unmeasured confounders may limit the findings. The study population was older with a high prevalence of comorbidities (such as diabetes 37%, malignancy 20%), a high number of participants died within 1 year after AF diagnosis and thus were not included in the primary analyses of rhythm control versus rate control strategy. The use of ICD codes alone may capture some degree of patients without HFpEF, or fail to capture some patients with HFpEF, a limitation of comprehensively identifying participants with this syndrome. This study did not investigate the association of rhythm control on quality of life in AF-HFpEF and AF-HFrEF patients, which may be a reason for patients and clinicians to opt for such therapy. The small number of patients who were treated with ablative therapies limits the power to assessment these outcomes. The types of AF and the percentage of patients in whom sinus rhythm was restored during follow-up were not available. Finally, the study population was quite elderly, which limits the study findings to be extrapolated to other populations.
Conclusions
AF patients with HFpEF and HFrEF had similar long-term outcomes, including death, cardiovascular death, cardiovascular hospitalizations, and stroke/TIA. The most common initial therapeutic approach was rate control strategy in the community. Rhythm control strategy may provide better outcomes and thus have a role in patients with HFrE-AF; the benefit of rhythm control in patients with HFpEF-AF warrants further study. Our study may highlight a potential need for major shift in community care in patients with HF and AF.
Supplementary Material
Funding:
This work was made possible using the resources of the Rochester Epidemiology Project, which is supported by the National Institute on Aging of the National Institutes of Health under Award Number R01 AG034676
Footnotes
Conflicts of interest: None
Reference
- 1.Benjamin EJ, Muntner P, Alonso A, et al. Heart Disease and Stroke Statistics-2019 Update: A Report From the American Heart Association. Circulation. 2019;139(10):e56–e528. [DOI] [PubMed] [Google Scholar]
- 2.Santhanakrishnan R, Wang N, Larson MG, et al. Atrial Fibrillation Begets Heart Failure and Vice Versa: Temporal Associations and Differences in Preserved Versus Reduced Ejection Fraction. Circulation. 2016;133(5):484–492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chamberlain AM, Gersh BJ, Alonso A, et al. No decline in the risk of heart failure after incident atrial fibrillation: A community study assessing trends overall and by ejection fraction. Heart Rhythm. 2017;14(6):791–798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zakeri R, Chamberlain AM, Roger VL, Redfield MM. Temporal relationship and prognostic significance of atrial fibrillation in heart failure patients with preserved ejection fraction: a community-based study. Circulation. 2013;128(10):1085–1093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wang TJ, Massaro JM, Levy D, et al. A risk score for predicting stroke or death in individuals with new-onset atrial fibrillation in the community: the Framingham Heart Study. JAMA. 2003;290(8):1049–1056. [DOI] [PubMed] [Google Scholar]
- 6.Dries DL, Exner DV, Gersh BJ, Domanski MJ, Waclawiw MA, Stevenson LW. Atrial fibrillation is associated with an increased risk for mortality and heart failure progression in patients with asymptomatic and symptomatic left ventricular systolic dysfunction: a retrospective analysis of the SOLVD trials. Studies of Left Ventricular Dysfunction. J Am Coll Cardiol. 1998;32(3):695–703. [DOI] [PubMed] [Google Scholar]
- 7.Olsson LG, Swedberg K, Ducharme A, et al. Atrial fibrillation and risk of clinical events in chronic heart failure with and without left ventricular systolic dysfunction: results from the Candesartan in Heart failure-Assessment of Reduction in Mortality and morbidity (CHARM) program. J Am Coll Cardiol. 2006;47(10):1997–2004. [DOI] [PubMed] [Google Scholar]
- 8.Middlekauff HR, Stevenson WG, Stevenson LW. Prognostic significance of atrial fibrillation in advanced heart failure. A study of 390 patients. Circulation. 1991;84(1):40–48. [DOI] [PubMed] [Google Scholar]
- 9.Cikes M, Claggett B, Shah AM, et al. Atrial Fibrillation in Heart Failure With Preserved Ejection Fraction: The TOPCAT Trial. JACC Heart Fail. 2018;6(8):689–697. [DOI] [PubMed] [Google Scholar]
- 10.Cheng M, Lu X, Huang J, Zhang J, Zhang S, Gu D. The prognostic significance of atrial fibrillation in heart failure with a preserved and reduced left ventricular function: insights from a meta-analysis. Eur J Heart Fail. 2014;16(12):1317–1322. [DOI] [PubMed] [Google Scholar]
- 11.Rocca WA, Yawn BP, St Sauver JL, Grossardt BR, Melton LJ 3rd, . History of the Rochester Epidemiology Project: half a century of medical records linkage in a US population. Mayo Clin Proc. 2012;87(12):1202–1213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.St Sauver JL, Grossardt BR, Leibson CL, Yawn BP, Melton LJ 3rd, Rocca WA. Generalizability of epidemiological findings and public health decisions: an illustration from the Rochester Epidemiology Project. Mayo Clin Proc. 2012;87(2):151–160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chamberlain AM, Gersh BJ, Alonso A, et al. Decade-long trends in atrial fibrillation incidence and survival: a community study. Am J Med. 2015;128(3):260–267 e261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Weng CJ, Li CH, Liao YC, et al. Rhythm control better prevents stroke and mortality than rate control strategies in patients with atrial fibrillation - A nationwide cohort study. Int J Cardiol. 2018;270:154–159. [DOI] [PubMed] [Google Scholar]
- 15.Mentias A, Briasoulis A, Shantha G, Alvarez P, Vaughan-Sarrazin M. Impact of Heart Failure Type on Thromboembolic and Bleeding Risk in Patients With Atrial Fibrillation on Oral Anticoagulation. Am J Cardiol. 2019;123(10):1649–1653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sartipy U, Dahlstrom U, Fu M, Lund LH. Atrial Fibrillation in Heart Failure With Preserved, Mid-Range, and Reduced Ejection Fraction. JACC Heart Fail. 2017;5(8):565–574. [DOI] [PubMed] [Google Scholar]
- 17.Kirchhof P, Benussi S, Kotecha D, et al. 2016 ESC Guidelines for the management of atrial fibrillation developed in collaboration with EACTS. Eur Heart J. 2016;37(38):2893–2962. [DOI] [PubMed] [Google Scholar]
- 18.Roy D, Talajic M, Nattel S, et al. Rhythm control versus rate control for atrial fibrillation and heart failure. N Engl J Med. 2008;358(25):2667–2677. [DOI] [PubMed] [Google Scholar]
- 19.Talajic M, Khairy P, Levesque S, et al. Maintenance of sinus rhythm and survival in patients with heart failure and atrial fibrillation. J Am Coll Cardiol. 2010;55(17):1796–1802. [DOI] [PubMed] [Google Scholar]
- 20.Packer DL, Mark DB, Robb RA, et al. Effect of Catheter Ablation vs Antiarrhythmic Drug Therapy on Mortality, Stroke, Bleeding, and Cardiac Arrest Among Patients With Atrial Fibrillation: The CABANA Randomized Clinical Trial. JAMA. 2019;321(13):1261–1274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fukui A, Tanino T, Yamaguchi T, et al. Catheter ablation of atrial fibrillation reduces heart failure rehospitalization in patients with heart failure with preserved ejection fraction. J Cardiovasc Electrophysiol. 2020;31(3):682–688. [DOI] [PubMed] [Google Scholar]
- 22.Machino-Ohtsuka T, Seo Y, Ishizu T, et al. Relationships between maintenance of sinus rhythm and clinical outcomes in patients with heart failure with preserved ejection fraction and atrial fibrillation. J Cardiol. 2019;74(3):235–244. [DOI] [PubMed] [Google Scholar]
- 23.Marrouche NF, Kheirkhahan M, Brachmann J. Catheter Ablation for Atrial Fibrillation with Heart Failure. N Engl J Med. 2018;379(5):492. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


