Abstract
Background
Shift work is associated with increased cardiometabolic disease risk. This observation may be partly explained by cardiometabolic risk factors having a role in the selection of individuals into or out of shift work. We performed Mendelian randomization (MR) analyses in the UK Biobank (UKB) to test this hypothesis.
Methods
We used genetic risk scores (GRS) to proxy nine cardiometabolic risk factors and diseases (including educational attainment, body mass index (BMI), smoking, and alcohol consumption), and tested associations of each GRS with self-reported frequency of current shift work among employed UKB participants of European ancestry (n = 190 573). We used summary-level MR sensitivity analyses to assess robustness of the identified effects, and we tested whether effects were mediated through sleep timing preference.
Results
Genetically instrumented liability to lower educational attainment (odds ratio (OR) per 3.6 fewer years in educational attainment = 2.40, 95% confidence interval (CI) = 2.22–2.59, P = 4.84 × 10–20) and higher body mass index (OR per 4.7 kg/m2 higher BMI = 1.30, 95% CI = 1.14–1.47, P = 5.85 × 10–5) increased odds of reporting participation in frequent shift work. Results were unchanged in sensitivity analyses allowing for different assumptions regarding horizontal pleiotropy. No selection effects were evident for the remaining exposures, nor for any exposures on selection out of shift work. Sleep timing preference did not mediate the effects of BMI and educational attainment on selection into shift work.
Conclusions
Liability to lower educational attainment and higher BMI may influence selection into shift work. This phenomenon may bias epidemiological studies of shift work that are performed in the UKB.
Keywords: Body mass index, cardiometabolic disease, confounding, educational attainment, Mendelian randomization, obesity, shift work, UK Biobank
Key Messages
Although it has been hypothesized that cardiometabolic risk factors and diseases influence selection into shift work, little evidence for such an effect is currently available.
Using Mendelian randomization, we assessed whether cardiometabolic risk factors and diseases influenced selection into or out of shift work in UK Biobank participants.
Our findings were consistent with a causal effect of both higher body mass index (BMI) and liability to lower educational attainment on selection into shift work, with a stronger magnitude of effect for shift work that is more frequent and that includes more night shifts.
Using multivariable Mendelian randomization, we found independent effects of higher BMI and liability to lower educational attainment on selection into shift work. The effect of sleep timing preference on shift work selection was consistent with the null and therefore did not mediate these effects.
Selection effects through educational attainment and BMI may bias the epidemiological relationships of shift work with cardiometabolic disease, particularly for analyses performed using UK Biobank data. Social mechanisms underlying these effects may warrant further investigation.
Introduction
Shift work is an increasingly common health exposure and is a risk factor for cardiometabolic diseases1, including type 2 diabetes (T2D)2 and coronary artery disease (CAD)3. The baseline characteristics of shift workers in observational studies often differ systematically from non-shift workers, including: higher rates of cigarette smoking2,4,5, lower rates of alcohol consumption2,4, lower educational attainment6, and higher body mass index (BMI)6–8. Such baseline differences may reflect either a selection effect9,10 of these factors on shift work, a causal effect of shift work on these factors, or confounding by shared influences. To date, few studies have examined evidence for a selection effect in shift workers11,12.
Clarifying which factors influence participation in shift work is of public health interest. First, a selection effect of cardiometabolic risk factors on shift work may confound epidemiological associations between shift work and adverse health outcomes. Understanding these biases is critical, as epidemiological associations of shift work with disease have potential to inform public policy13. Estimating the magnitude of this selection effect could facilitate development of methodology to better account for confounding. Observational data have several limitations for identifying selection factors, including timing of exposure measurement, unmeasured confounding and causal effects of shift work on cardiometabolic risk factors and disease. Mendelian randomization (MR) is an analytical method that can overcome these limitations by using genetic variants as proxies for epidemiological exposures to estimate causal effects14. This approach is well suited for identifying selection effects on shift work because genetic variants are precisely measured and are less prone to bias through confounding or reverse causality15,16. Previous MR analyses supported a causal effect of BMI on lower income and socioeconomic status17,18, but MR has not been applied to characterize the determinants of selection into shift work. We used MR to assess for causal effects of cardiometabolic risk factors and diseases on selection into shift work in the UK Biobank (UKB)19.
Methods
The UK Biobank study was approved by the National Health Service National Research Ethics Service (ref. 11/NW/0382). All participants provided written informed consent, and data used in this study were de-identified.
Population
The UKB is a population-based cohort study that enrolled over 500 000 volunteers aged 40–69 over 2006–10, participation rate: 5.5%19. Data collection included questionnaire and nurse interview information, anthropometric and physiological measurements and genomic data as previously described19,20.
Exposures
The exposures were single genetic variants or weighted genetic risk scores (GRS) derived from genome-wide association studies (GWAS) for cardiometabolic risk factors and diseases: alcohol consumption, smoking heaviness, body mass index (BMI), waist-to-hip ratio adjusted for BMI, type 2 diabetes (T2D), coronary artery disease (CAD), low-density lipoprotein (LDL) cholesterol and educational attainment (Table 1)21–30. We prioritized risk factors with publicly available GWAS summary statistics from datasets that were not overlapping with UKB, and with putative causal effects on cardiometabolic outcomes, as supported by previous MR studies21,26,31–34. Although T2D and CAD are typically analysed as outcomes, they were treated as exposures in this analysis to determine whether liability to these diseases may influence shift work participation through a ‘healthy worker effect’ (e.g. angina reducing tolerability of night shift work). Supplementary Figure S1, available as Supplementary data at IJE online, shows a causal diagram by which these exposures may influence selection into shift work.
Table 1.
Genetic data sources used to construct genetic risk scores
| Exposure | Author/consortium name (Pubmed ID) | n SNPs | Phenotype units in GWAS |
|---|---|---|---|
| Alcohol consumption—primary analysis |
Holmes (25011450) |
1 | 17% less weekly intake of alcohol |
| Alcohol consumption—secondary analysis |
GSCAN (30643251) |
89 | Log-transformed drinks per week |
| Smoking heaviness—primary analysis |
TAG consortium (20418890) |
1 | 1 cigarette per effect allele |
| Smoking heaviness—secondary analysis |
GSCAN (30643251) |
45 | Binned cigarettes/day |
| Body mass index (BMI) |
GIANT (25673413) |
91 | 1 SD unit increase in BMI |
| Waist-hip-ratio adjusted for BMI (WHRadjBMI) |
GIANT (25673412) |
47 | 1 SD unit increase in WHRadjBMI |
| Type 2 diabetes (T2D) |
DIAGRAM (28566273) |
103 | Log-odds of T2D |
| Coronary artery disease (CAD) |
CARDIoGRAMplusC4D (26343387) |
57 | Log-odds of CAD |
| Low-density lipoprotein cholesterol (LDL-c) |
GLGC (24097068) |
190 | 1 SD increase in LDL-c |
| Educational attainment |
SSGAC (27225129) |
68 | 1 SD increase in years of schooling completed |
| Sleep timing preference |
Jones, 23andme estimates (30696823) |
331 | Log-odds of morning sleep timing preference |
GWAS, genome-wide association study; SD, standard deviation; SNP, single nucleotide polymorphism.
Alcohol consumption was proxied through a single nucleotide polymorphism (SNP) in the alcohol dehydrogenase 1B (ADH1B) gene robustly associated with lower alcohol consumption21. Consistent with previous analyses21, we coded this missense variant (rs1229984) under a dominant model of inheritance (cases combining homozygotes and heterozygotes), with the effect allele oriented to reduced alcohol consumption. A missense variant (rs16969968) in the cholinergic receptor nicotinic alpha 5 subunit (CHRNA5) gene associated at genome-wide significance with one additional cigarette smoked per day22, was used as the genetic instrument for smoking heaviness. We first performed this analysis limiting the sample to current smokers. For all other exposures, we used SNP associations from GWAS meta-analyses that did not include UKB data, to generate multi-SNP GRS23,24,27,29. Genetic proxies for each of the exposures were further clumped to a between-SNP r2 <0.10.
We used risk profiling in PLINK35 v1.9 to construct beta-weighted GRS using individual-level data in the UKB for participants of White British ancestry. External GRS weights were obtained from the respective GWAS. We regressed each GRS on the respective exposure to determine the strength of each genetic instrument, considering an F statistic >10 to indicate minimal weak instrument bias36.
Outcomes
We derived the primary study outcomes using baseline shift work characteristics from employed UKB participants. Participants were asked to indicate shift work participation by answering the following question: ‘Does your work involve shift work?’ defined as ‘a work schedule that falls outside the normal daytime working hours of 9am-5pm. This may involve working afternoons, evenings or nights or rotating through these kinds of shifts’. Response options included: ‘never/rarely,’ ‘sometimes,’ ‘usually,’ ‘always,’ ‘prefer not to answer’ and ‘do not know’. ‘Never/rarely’ served as the reference group, ‘sometimes’ was an intermediate group and ‘usually’ and ‘always’ were collapsed into ‘frequent shift work’, totalling three groups in this analysis. ‘Prefer not to answer’ and ‘do not know’ were coded as missing. Participants indicating that they currently worked shifts were further queried, ‘Does your work involve night shifts?’ defined as ‘a work schedule that involves working through the normal sleeping hours, for instance working through the hours from 12am to 6am’. Response options were identical to those for the primary shift work question and were coded similarly. In the night shift work analyses, we compared employed individuals not participating in shift work (reference group) with individuals working ‘shift work, but no night shift work’, ‘some night shift work’, and ‘frequent night shift work’, resulting in four groups in this analysis.
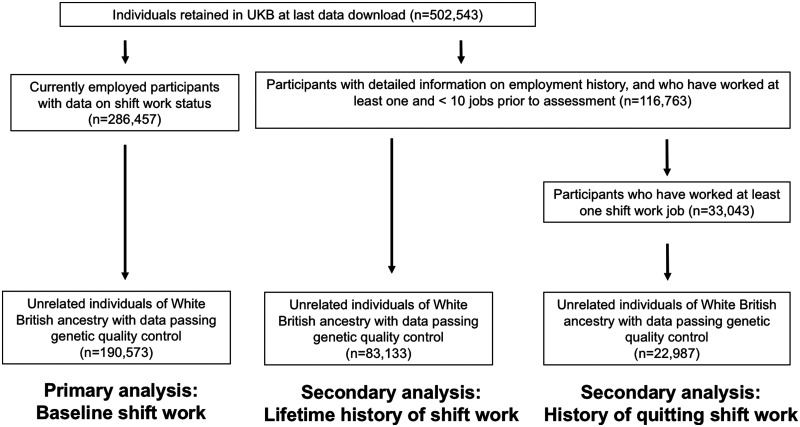
UKB participants with e-mail addresses ( n ∼330 000) were invited to complete an online follow-up questionnaire on lifetime employment information. This questionnaire queried information on each job worked over the participant’s lifetime (n = 118 699). For each job, participants were asked ‘Did you ever work shifts (day and/or night shifts) for this job?’ To determine whether associations generalized beyond current shift work participation, we derived a secondary outcome of lifetime history of shift work, regardless of current employment status. To identify exposures influencing selection out of shift work, we also used these data to derive an outcome of history of quitting shift work. Cases were defined as participants who reported previously working a shift work job and subsequently working a non-shiftwork job (Figure 1).
Figure 1.
Study design
Individual-level analyses
We first tested the association of each GRS37 with the shift work outcomes, using multinomial or binomial logistic regression adjusted for age, sex and the top ten principal components (PCs) of ancestry. We transformed the effects of binary exposures (GRS for CAD and T2DM) to reflect a doubling in the odds of the exposure on the odds of the outcome38.
We assessed the robustness of results through a series of sensitivity analyses. Using a previously described GxE interaction test, we first tested the effect of the CHRNA5 smoking heaviness proxy on participation in frequent shift work in ever smokers, and then tested for heterogeneity in this estimate relative to never smokers39. Such an approach may be more powerful (n = 80 847) than testing for an effect in the smaller sample of current smokers (n = 19 675), and permits detection of horizontal pleiotropy through the interaction test. As defined above, we examined the outcomes of lifetime history of shift work participation and history of leaving shift work. To assess potential bias by population stratification40, we controlled for: (i) 40 PCs of ancestry; and (ii) the 22 UKB assessment centres. We additionally adjusted for the Townsend Deprivation Index to assess whether the effects of the exposures on selection into shift work were independent of socioeconomic status. Previous work suggested sex differences in the effects of BMI on socioeconomic outcomes17, so we tested for GRS interactions with sex using a model with an interaction term. We used UKB job codes to stratify jobs as ‘skilled’ and ‘unskilled’, using the classification laid out in Howe et al.18, and tested for interactions of job skill with the GRS using a model with an interaction term. Finally, we expanded the alcohol consumption and smoking heaviness instruments to include SNPs identified in a recent large-scale meta-analysis41. We used GRS weights from summary statistics excluding UKB41. Although these instruments explain more variance in the respective exposures relative to the ADH1B and CHRNA5 instruments, analyses using these expanded instruments may be at greater risk for bias by pleiotropy due to the inclusion of variants with uncharacterized biological function42. As such, these instruments were only used in sensitivity analyses.
Summary-level MR analyses and sensitivity analyses
Results from Mendelian randomization analyses may be biased by horizontal pleiotropy, whereby the variants influence the outcome through pathways that are independent of the exposure. We therefore performed summary-level MR analyses to assess sensitivity of results to this bias. We first calculated Cochran’s Q as a global test for heterogeneity, which may indicate the presence of pleiotropy. We then estimated MR effects using inverse-variance weighted (IVW) random-effects regression, which is unbiased in the setting of balanced pleiotropy43. We used the following sensitivity analyses that are robust to various forms of unbalanced horizontal pleiotropy: MR Egger44, weighted median45 and MR-PRESSO46 (Supplementary Methods, available as Supplementary data at IJE online). We used the estimate of the MR Egger model intercept to further assess for balanced horizontal pleiotropy, although this method is typically underpowered47. For effects identified in univariable MR, we undertook summary-level multivariable MR (MVMR)48 simultaneously controlling for each exposure49. We confirmed instrument strength using the Qstrength adapted for MVMR48. The Qstrength divided by the number of variants is analogous to the F statistic, with values >10 indicating minimal weak instrument bias. Previous MR analyses identified a nominal effect of greater BMI on earlier sleep timing preference, so we tested for a causal effect of earlier sleep timing preference on selection into shift work using GWAS estimates that did not overlap with UKB50. We hypothesized that earlier sleep timing preference would be negatively associated with current shift work, reflecting a negative selection effect. We planned to perform mediation analyses only if the first-stage association of sleep timing preference with shift work demonstrated evidence of effect.
Interpretation of results
We used a two-sided alpha threshold of 3.13x10-3 to account for eight exposures and two outcomes in our primary analysis. This adjustment was not made to dichotomize results on the basis of statistical significance, but rather to serve as a means of grading the strength of evidence51.
Software
These analyses were performed using R version 3.5.0, including the TwoSampleMR49 and MVMR48 packages.
Results
Individual level analyses
The median age of employed participants in the UKB was 53 years [interquartile range (IQR) 47–59] and 49% were male. Shift workers were more likely to be male and report lower indicators of socioeconomic and health status (Table 2). Each GRS was strongly associated with the respective exposure (Supplementary Table S1, available as Supplementary data at IJE online).
Table 2.
Sample characteristics by current shift work status (n = 190 569). Summary statistics are shown as mean (standard deviation) or count (percentage)
| Exposure or covariate | Employed, not shift worker (n = 159 900) | Some shift work (n = 13 411) | Frequent shift work (n = 17 258) |
|---|---|---|---|
| Age (years) | 53.13 (7.08) | 52.34 (7.00) | 51.94 (6.87) |
| Male sex (%) | 75794 (47.4) | 7477 (55.8) | 9692 (56.2) |
| Household income < 18,000 (%) | 12898 (8.9) | 1496 (12.4) | 2295 (14.7) |
| Household income > 100,000 (%) | 12056 (8.3) | 641 (5.3) | 268 (1.7) |
| University education (%) | 63824 (39.9) | 3749 (28.0) | 2433 (14.1) |
| Educational attainment (years) | 16.01 (4.70) | 15.40 (4.82) | 14.55 (4.78) |
| Townsend deprivation index | −1.72 (2.79) | −1.10 (3.06) | −0.83 (3.12) |
| Lives with spouse or partner (%) | 121599 (83.3) | 9486 (79.6) | 11844 (77.9) |
| Current smoker (%) | 15002 (9.4) | 1928 (14.4) | 2745 (16.0) |
| Drinks per week (standardized alcohol units) | 15.78 (16.02) | 16.49 (17.32) | 14.61 (16.61) |
| Body mass index (kg/m2) | 27.08 (4.62) | 27.94 (4.89) | 28.14 (4.91) |
| Waist-hip-ratio | 0.86 (0.09) | 0.88 (0.09) | 0.89 (0.09) |
| LDL cholesterol (mmol/L) | 3.67 (0.82) | 3.70 (0.85) | 3.68 (0.83) |
| Coronary artery disease (%) | 2130 (1.3) | 227 (1.7) | 289 (1.7) |
| Type 2 diabetes (%) | 3757 (2.3) | 379 (2.8) | 529 (3.1) |
| Definitely a ‘morning’ person (%)a | 36,572 (25.5) | 3,230 (26.9) | 3,823 (25.1) |
| Excellent self-rated health (%)b | 31588 (19.8) | 2019 (15.1) | 2356 (13.7) |
| Poor self-rated health (%)b | 3084 (1.9) | 336 (2.5) | 544 (3.2) |
Scale for sleep timing preference includes: “Definitely a ‘morning’ person”, ‘”More a ‘morning’ than ‘evening’ person”, “More an ‘evening’ than a ‘morning’ person”, and “Definitely an ‘evening’ person”.
Scale for self-rated health includes: poor, fair, good, and excellent.
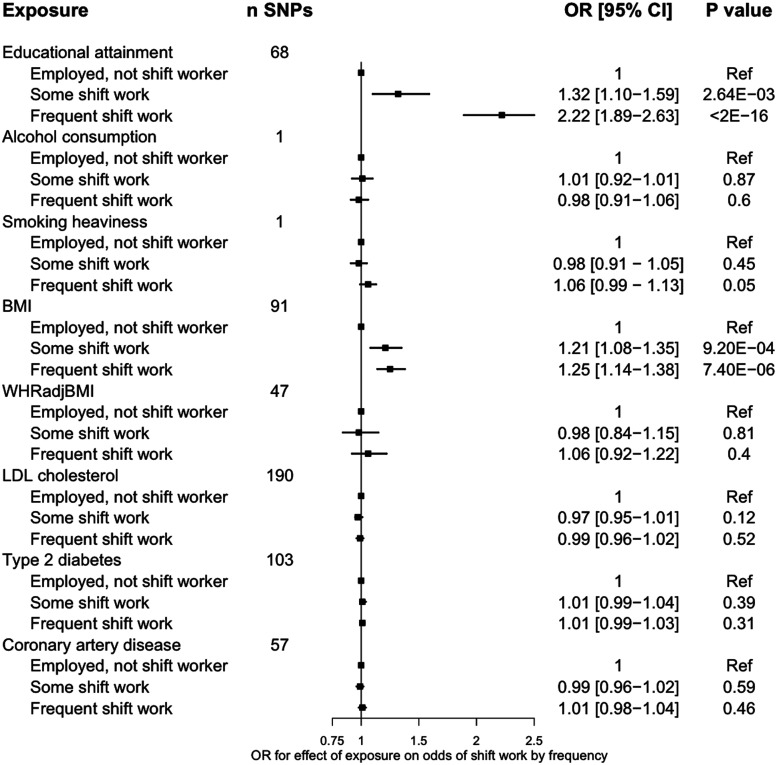
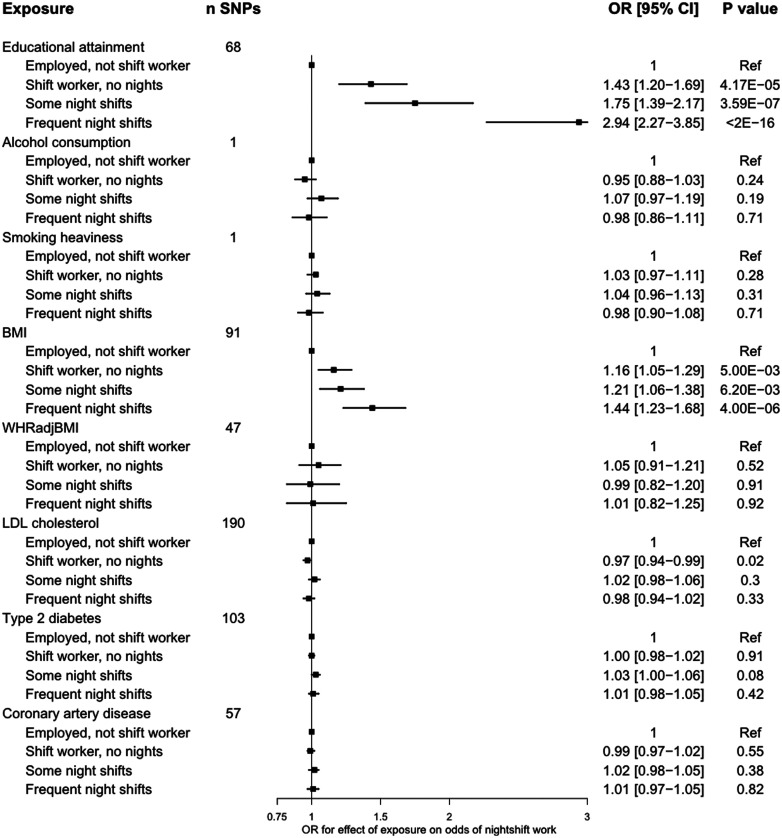
A genetically proxied liability to lower educational attainment [a 1-standard deviation (SD) increase, or ∼3.6 years] increased odds of working some shift work [odds ratio (OR) = 1.32, 95% confidence interval (CI) = 1.10–1.59, P = 2.64 × 10–3], frequent shift work (OR = 2.22, 95% CI = 1.89–2.63, P < 2 × 10–16) and frequent night shift work (OR = 2.94, 95% CI = 2.27–3.85, P < 2 × 10–16; Figures 2–3). Genetically proxied higher BMI increased odds of working some shift work (OR = 1.21, 95% CI = 1.08–1.35, P = 9.20 × 10–4), frequent shift work (OR = 1.25, 95% CI = 1.14–1.38, P = 7.40 × 10–6) and frequent night shift work (OR = 1.44, 95% CI = 1.23–1.68, P = 4.00 × 10–6; Figures 2–3).
Figure 2.
Associations of genetic proxies for cardiometabolic traits with current shift work patterns1. The plotted estimates correspond to a unit change in the GRS, or to an additional effect allele for the single-SNP instruments. All effects are oriented to an increase in the exposure, except for educational attainment which is oriented to lower levels of educational attainment. 1 ncontrols = 159 903, nsome shift work = 13 411, nfrequent shift work = 17 259. BMI, body mass index; CAD, coronary artery disease; CI, confidence interval; GRS, genetic risk score; SNP, single nucleotide polymorphism; DM, diabetes mellitus; OR, odds ratio; WHRadjBMI, waist-to-hip ratio adjusted for BMI
Figure 3.
Associations of genetic proxies for cardiometabolic traits with current night-shift work patterns1. The plotted estimates correspond to a unit change in the GRS, or to an additional effect allele for the single-SNP instruments. All effects are oriented to an increase in the exposure, except for educational attainment which is oriented to lower levels of educational attainment. ncontrols = 159 903, nshifts but no nights = 15 288, nsome nights = 8726, nfrequent nights=6629. BMI, body mass index; CAD, coronary artery disease; CI, confidence interval; GRS, genetic risk score; SNP, single nucleotide polymorphism; DM, diabetes mellitus; OR, odds ratio; WHRadjBMI, waist-to-hip ratio adjusted for BMI
Genetically proxied liability to lower educational attainment (OR = 1.40, 95% CI = 1.20–1.64, P = 2.68 × 10–5) and higher BMI (OR = 1.17, 95% CI = 1.06–1.28, P = 1.68 × 10–3) increased the odds of working at least one shift work job across the life course (Supplementary Table S2, available as Supplementary data at IJE online). No other exposures associated with shift work participation, and no exposures associated with history of leaving shift work (Figures 2–3; Supplementary Table S3, available as Supplementary data at IJE online).
Secondary and sensitivity analyses in individual level analyses
When performing analyses in ever smokers, the effect of genetically proxied smoking heaviness on participation in some shift work (OR = 0.98, 95% CI = 0.95–1.02, P = 0.36) and frequent shift work (OR = 1.02, 95% CI 0.99–1.06, P = 0.17) remained consistent with the null, and did not interact with smoking status (Psome = 0.20, Pfrequent = 0.55). The estimates for the effect of BMI and educational attainment on shift work did not vary by sex or job skill classification (Pinteraction >0.05). Additional control for population stratification through statistical adjustment for 40 principal components, UKB assessment centere and the Townsend Deprivation Index did not influence the estimates (Supplementary Table S4, available as Supplementary data at IJE online). Results were similar when using an expanded GRS for alcohol consumption (OR = 1.07, 95% CI = 0.88–1.32, P = 0.50) and smoking heaviness (OR = 1.03, 95% CI = 1.00–1.05, P = 0.02) on the outcome of frequent shift work. There was no effect of genetically proxied early sleep timing preference on frequent shift work (OR - 0.99, 95% CI = 0.96–1.04, P = 0.79) or frequent night shift work (OR = 0.96, 95% CI 0.90–1.02, P = 0.16).
Summary-level MR analyses
We next performed summary-level MR analyses to estimate causal effects robust to certain forms of horizontal pleiotropy. There was overall minimal evidence of statistical heterogeneity between the causal effects estimated by each SNP in the genetic instruments beyond that expected by chance, with no outlier SNPs identified by MR-PRESSO (Table 3). Estimates from random-effects inverse-variance weighted analyses further supported effects of higher BMI (OR = 1.30, 95% CI = 1.14–1.47, P = 5.58 × 10–5) and liability to lower educational attainment (OR = 2.40, 95% CI = 2.22–2.59, P = 4.84 × 10–20) on selection into shift work (Table 3). Similar estimates were obtained in the MR Egger and weighted median analyses (Table 3), and the Egger intercept test for unbalanced pleiotropy was consistent with the null for analyses of BMI (intercept = 0.00, P = 0.37) and educational attainment (intercept = 0.02, P = 0.07). We next turned to multivariable MR to assess the independence of the effects of BMI and educational attainment on shift work. The genetic instruments for educational attainment (Qstrength = 2,407, Fadjusted = 15.4) and BMI (Qstrength = 4,393, Fadjusted = 28) were conditionally strong in multivariable MR. The effects of BMI (OR = 1.18, 95% CI = 1.08–1.31, P = 1.94 × 10–3) and of educational attainment (OR = 2.56, 95% CI = 2.12–3.13, P = 1.48 × 10–23) on self-reported ‘frequent’ shift work were independent in MVMR.
Table 3.
Summary-level Mendelian randomization analyses for the effects of liability to lower educational attainment and higher body mass index on shift work status (17 259 cases of frequent shift work/159 903 controls)
| MR technique |
||||||||
|---|---|---|---|---|---|---|---|---|
| Exposure | Inverse-variance weighted |
MR Egger |
Weighted median |
Heterogeneity statistic |
||||
| OR [95% CI] | P-value | OR [95% CI] | P-value | OR [95% CI] | P-value | Q | P-value | |
|
Education |
2.40 [2.22-2.59] |
4.84x10-20 |
5.26 [2.22-12.5] |
3.30x10-4 |
2.22 [1.72-2.94] |
1.01x10-9 | 76 | 0.13 |
| BMI |
1.30 [1.14-1.47] |
5.58x10-5 |
1.51 [1.05-2.16] |
0.03 |
1.34 [1.13-1.59] |
8.68x10-4 | 126 | 0.01 |
BMI, body mass index; OR, odds ratio; CI, confidence interval; MR, Mendelian randomization.
Discussion
Mendelian randomization analyses revealed strong evidence for effects of liability to lower educational attainment and higher BMI on selection into shift work, with particularly pronounced effects for night shift work. These effects were not mediated by sleep timing preference. There was minimal evidence for selection through the other tested exposures.
Our findings provide evidence for a causal effect of liability to lower educational attainment and higher BMI, but not of other cardiometabolic risk factors, on selection into shift work within the UKB population. The effect estimates were larger when shift work involved night shifts, and with increasing frequency of shift work, supporting a dose-dependent effect. Selection into shift work by cardiometabolic risk factors (including alcohol consumption, BMI, smoking, lipids and glucose) has been evaluated in previous observational studies (n ∼2800)11,12. These studies identified an association of smoking with future participation in shift work. This contrasts with our results, where we did not identify strong evidence for an effect of smoking heaviness on shift work selection, potentially due to smoking heaviness reflecting a different dimension of smoking behaviour. Given the inverse effect of educational attainment on shift work, and the known effect of educational attainment on reduced smoking33, it is also possible that educational attainment confounded the previously observed relationship of smoking with shift work. Previous work has not evaluated the role of educational attainment in shift work selection, although shift workers typically report lower levels of educational attainment relative to non-shift workers6,52. Our findings suggest that these baseline imbalances may be driven by a causal effect of educational attainment on selection into shift work. We identified an effect of higher BMI on shift work selection, which is consistent with the growing evidence for a causal effect of higher BMI on lower socioeconomic status17,18. This finding does, however, contrast the null associations reported in previous observational analyses of shift work selection11,12. There are several potential explanations for these differences. First, the observational estimates11,12 had wide confidence intervals, and could not exclude moderate-strength relationships between BMI and selection into shift work. Second, the influence of BMI on selection into shift work may accrue over time, such that the effects are only observed later in life. Such a phenomenon would affect the present analysis, given the use of an older sample in contrast to the younger samples recruited in earlier studies of selection into shift work. Finally, our findings may reflect selection effects unique to analyses of the UKB shift work population.
There are several potential pathways by which educational attainment and BMI may influence selection into shift work. Lower educational attainment is related to lower-skilled employment53, which is generally more typical of shift work54. It is less clear what mediates the effect of BMI on selection into shift work. One potential social mechanism driving this effect is weight stigma, which is a highly prevalent55 and well-characterized driver of employment inequities56. Although women experience a greater burden of this discrimination than men57, we observed no effect modification by sex, suggesting that men and women experience similar selection pressures into shift work on the basis of differences in BMI and educational attainment. Although we hypothesized that differences in sleep timing preference may be a mediating mechanism50, we did not observe an effect of earlier sleep timing preference on night shift odds. This is surprising, given that night shift workers who sleep earlier may experience the greatest burden of circadian misalignment58,59, and would therefore be more likely to leave night shift work. A possible interpretation of this finding is that selection effects of other social factors, such as socioeconomic status or educational attainment, preclude leaving a job on the basis of tolerability. We did not identify factors influencing selection out of shift work, but the sample size for this analysis was an order of magnitude smaller than the sample size in the primary analysis, and we may have been underpowered to detect modest effects.
The present results have several implications for the investigation of health effects of shift work. First, the associations of shift work with cardiometabolic outcomes in the UKB may be partly confounded by BMI and educational attainment. This does not invalidate the contribution of circadian misalignment to the excess disease risk conferred by shift work, particularly because these two variables are typically included for adjustment in multivariable models. Cross-sectional studies of shift work that investigate obesity as an outcome may be at high risk for bias, as any estimated associations will reflect a combination of forward and reverse causal effects60. Second, analyses restricted to shift workers may be affected by collider bias61, which can induce false-positive associations. Finally, our findings demonstrate that MR can be a useful tool in occupational epidemiology to explore potential selection effects and biases.
Our study has some limitations. Given that UKB is a relatively healthy population62, our results may not generalize to sicker populations or to other cohorts63 with different occupation structures. Moreover, the identified effects may be driven by selection and collider bias, given a 5% response rate for recruitment into the UKB cohort62. As such, the present results may be most conservatively interpreted as reflecting biases inherent to analyses of shift work in the UKB cohort. The mechanisms mediating our results remain unknown and should be investigated in future studies. Despite consistent results in sensitivity analyses, the MR estimates may be biased by horizontal pleiotropy. Additional sources of bias include confounding by cryptic population stratification of the variants used as instruments, as well as assortative mating on the traits used as exposures64. Furthermore, we cannot exclude an influence of liability to lower educational attainment, rather than variation in educational attainment itself, on our effect estimates65. Our findings should therefore be triangulated with results from within-family studies and analyses leveraging other forms of natural experiments, such as compulsory educational reform66,67. Future analyses may investigate other cardiometabolic or non-cardiometabolic traits of interest. Finally, future studies may explore the use of genetics to investigate whether shift work causally influences cardiometabolic disease risk.
In conclusion, higher BMI and liability to lower educational attainment influenced selection into shift work and night shift work in the UKB, but no other cardiometabolic risk factors were associated with selection into or out of shift work. Consequent bias attributable to these effects should be considered in the design and interpretation of epidemiological studies investigating the health effects of shift work in the UKB.
Supplementary data
Supplementary data are available at IJE online.
Funding
This work was supported by NIH grants R01DK105072 (R.S., H.S.D., C.V.), R21OH011052 (E.S.S., C.V.), R01DK107859 (H.S.D., R.S.), and R01DK102696 (R.S.). This work was also supported by grants from the MGH Research Scholar Fund (R.S.), Diabetes UK (17/0005700; M.K.R.), University of Manchester Research Infrastructure Fund (M.K.R.), and the Instrumentarium Science Foundation, Yrjö Jahnsson Foundation and Academy of Finland (#269517; H.O.). R.C.R. and G.D.S. are members of the MRC Integrative Epidemiology Unit at the University of Bristol funded by the Medical Research Council (MC_UU_00011/1). R.C.R. is a de Pass Vice Chancellor’s Research Fellow at the University of Bristol.
Data availability
All individual-level data from UKB used to support this manuscript may be accessed by applying to the UKB Central Access Committee [http://www.ukbiobank.ac.uk/register-apply/].
Supplementary Material
Acknowledgements
This research has been conducted using the UKB Resource (application number 6818). We thank the participants and researchers from the UKB who contributed or collected data. I.D. is the guarantor.
Author contributions
R.S. obtained data for the study. R.S. and C.V. obtained funding for the study. I.D. and C.V. designed the study with input from all co-authors. I.D. performed the statistical analyses. I.D. and C.V. drafted the manuscript. All authors actively participated in interpretation of results and critical revision of the manuscript, and provided approval of the final version of the manuscript.
Conflicts of interest
C.V., during the conduct of the study, was a scientific advisory board member of Circadian Light Therapy Inc., and served as a paid consultant to the US Department of Energy outside the submitted work. M.K.R. reports receiving research funding from Novo Nordisk, consultancy fees from Novo Nordisk and Roche Diabetes Care, and modest owning of shares in GlaxoSmithKline.
References
- 1.Kantermann T, Juda M, Vetter C, Roenneberg T.. Shift-work research: Where do we stand, where should we go? Sleep Biol Rhythms 2010;8:95–105. [Google Scholar]
- 2.Vetter C, Dashti HS, Lane JM. et al. Night shift work, genetic risk, and type 2 diabetes in the UK biobank. Diabetes Care 2018;41:762–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Vetter C, Devore EE, Wegrzyn LR. et al. Association between rotating night shift work and risk of coronary heart disease among women. JAMA 2016;315:1726–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tamimi RM, Schernhammer ES, Eliassen AH. et al. Rotating night-shift work and the risk of breast cancer in the nurses’ health studies. Am J Epidemiol 2017;186:532–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Amelsvoort LGPM, van, Jansen NWH, Kant I.. Smoking among shift workers: more than a confounding factor. Chronobiol Int 2006;23:1105–13. [DOI] [PubMed] [Google Scholar]
- 6.Amelsvoort Lgpm van, Schouten EG, Kok FJ.. Impact of one year of shift work on cardiovascular disease risk factors. J Occup Environ Med 2004;46:699–706. [DOI] [PubMed] [Google Scholar]
- 7.Ramin C, Devore EE, Wang W, Pierre-Paul J, Wegrzyn LR, Schernhammer ES.. Night shift work at specific age ranges and chronic disease risk factors. Occup Environ Med 2015;72:100–07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fujishiro K, Lividoti Hibert E, Schernhammer E, Rich-Edwards JW.. Shift work, job strain and changes in the body mass index among women: a prospective study. Occup Environ Med 2017;74:410–16. [DOI] [PubMed] [Google Scholar]
- 9.Puttonen S, Viitasalo K, Härmä M.. The relationship between current and former shift work and the metabolic syndrome. Scand J Work Environ Health 2012;38:343–48. [DOI] [PubMed] [Google Scholar]
- 10.Ritonja J, Aronson KJ, Matthews RW, Boivin DB, Kantermann T.. Working Time Society consensus statements: Individual differences in shift work tolerance and recommendations for research and practice. Ind Health 2019;57:201–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yong M, Germann C, Lang S, Oberlinner C.. Primary selection into shift work and change of cardiovascular risk profile. Scand J Work Environ Health 2015;41:259–67. [DOI] [PubMed] [Google Scholar]
- 12.Nabe-Nielsen K, Garde AH, Tüchsen F, Hogh A, Diderichsen F.. Cardiovascular risk factors and primary selection into shift work. Scand J Work Environ Health 2008;34:206–12. [DOI] [PubMed] [Google Scholar]
- 13.Wise J. Danish night shift workers with breast cancer awarded compensation. BMJ 2009;338:b1152. [DOI] [PubMed] [Google Scholar]
- 14.Davey Smith G, Ebrahim S. ‘ Mendelian randomization’: can genetic epidemiology contribute to understanding environmental determinants of disease? Int J Epidemiol 2003;32:1–22. [DOI] [PubMed] [Google Scholar]
- 15.Davey Smith G, Lawlor DA, Harbord R, Timpson N, Day I, Ebrahim S.. Clustered environments and randomized genes: a fundamental distinction between conventional and genetic epidemiology. PLoS Med 2007;4:e352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Davies NM, Holmes MV, Davey Smith. G. Reading Mendelian randomisation studies: a guide, glossary, and checklist for clinicians. BMJ 2018;362:k601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tyrrell J, Jones SE, Beaumont R. et al. Height, body mass index, and socioeconomic status: mendelian randomisation study in UK Biobank. BMJ 2016;352:i582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Howe LD, Kanayalal R, Harrison S. et al. Effects of body mass index on relationship status, social contact and socio-economic position: Mendelian randomization and within-sibling study in UK Biobank. Int J Epidemiol 2019;49:1173–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bycroft C, Freeman C, Petkova D. et al. The UK Biobank resource with deep phenotyping and genomic data. Nature 2018;562:203–09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dashti HS, Jones SE, Wood AR. et al. Genome-wide association study identifies genetic loci for self-reported habitual sleep duration supported by accelerometer-derived estimates. Nat Commun 2019;10:1100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Holmes MV, Dale CE, Zuccolo L. et al. ; on behalf of the InterAct Consortium. Association between alcohol and cardiovascular disease: Mendelian randomisation analysis based on individual participant data. BMJ 2014;349:g4164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.The Tobacco and Genetics Consortium. Genome-wide meta-analyses identify multiple loci associated with smoking behavior. Nat Genet 2010;42:441–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Locke AE, Kahali B, Berndt SI. et al. The LifeLines Cohort Study. Genetic studies of body mass index yield new insights for obesity biology. Nature 2015;518:197–206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Shungin D, Winkler TW, Croteau-Chonka DC. et al. ; the ADIPOGen Consortium. New genetic loci link adipose and insulin biology to body fat distribution. Nature 2015;518:187–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Okbay A, Beauchamp JP, Fontana MA. et al. Genome-wide association study identifies 74 loci associated with educational attainment. Nature 2016;533:539–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tillmann T, Vaucher J, Okbay A. et al. Education and coronary heart disease: mendelian randomisation study. BMJ 2017;358:j3542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Scott RA, Scott LJ, Mägi R. et al. An expanded genome-wide association study of type 2 diabetes in Europeans. Diabetes 2017;66:2888–902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Natarajan P, Young R, Stitziel NO. et al. Polygenic risk score identifies subgroup with higher burden of atherosclerosis and greater relative benefit from statin therapy in the primary prevention setting. Circulation 2017;135:2091–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nikpay M, Goel A, Won H-H. et al. A comprehensive 1000 Genomes–based genome-wide association meta-analysis of coronary artery disease. Nat Genet 2015;47:1121–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Willer CJ, Schmidt EM, Sengupta S. et al. Discovery and refinement of loci associated with lipid levels. Nat Genet 2013;45:1274–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lyall DM, Celis-Morales C, Ward J. et al. Association of body mass index with cardiometabolic disease in the UK biobank. JAMA Cardiol 2017;2:882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Emdin CA, Khera AV, Natarajan P. et al. Genetic association of waist-to-hip ratio with cardiometabolic traits, type 2 diabetes, and coronary heart disease. JAMA 2017;317:626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Carter AR, Gill D, Davies NM. et al. Understanding the consequences of education inequality on cardiovascular disease: mendelian randomisation study. BMJ 2019;365:1185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Frayling TM, Stoneman CE.. Mendelian randomisation in type 2 diabetes and coronary artery disease. Curr Opin Genet Dev 2018;50:111–20. [DOI] [PubMed] [Google Scholar]
- 35.Purcell S, Neale B, Todd-Brown K. et al. PLINK: a tool set for whole-genome association and population-based linkage analyses. Am J Hum Genet 2007;81:559–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Pierce BL, Ahsan H, VanderWeele TJ.. Power and instrument strength requirements for Mendelian randomization studies using multiple genetic variants. Int J Epidemiol 2011;40:740–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Burgess S, Thompson SG.. Use of allele scores as instrumental variables for Mendelian randomization. Int J Epidemiol 2013;42:1134–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Burgess S, Labrecque JA.. Mendelian randomization with a binary exposure variable: interpretation and presentation of causal estimates. Eur J Epidemiol 2018;33:947–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Millard LAC, Munafò MR, Tilling K, Wootton RE, Davey Smith G.. MR-pheWAS with stratification and interaction: Searching for the causal effects of smoking heaviness identified an effect on facial aging. PLOS Genet 2019;15:e1008353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Haworth S, Mitchell R, Corbin L. et al. Apparent latent structure within the UK Biobank sample has implications for epidemiological analysis. Nat Commun 2019;10:333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Liu M, Jiang Y, Wedow R. et al. ; 23andMe Research Team. Association studies of up to 1.2 million individuals yield new insights into the genetic etiology of tobacco and alcohol use. Nat Genet 2019;51:237–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Burgess S, Davey Smith G.. How humans can contribute to Mendelian randomization analyses. Int J Epidemiol 2019;48:661–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bowden J, Greco MF, Del Minelli C. et al. A framework for the investigation of pleiotropy in two-sample summary data Mendelian randomization. Stat Med 2017;36:1783–802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bowden J, Davey Smith G, Burgess S.. Mendelian randomization with invalid instruments: Effect estimation and bias detection through Egger regression. Int J Epidemiol 2015;44:512–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Bowden J, Davey Smith G, Haycock PC, Burgess S.. Consistent estimation in mendelian randomization with some invalid instruments using a weighted median estimator. Genet Epidemiol 2016;40:304–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Verbanck M, Chen C-Y, Neale B, Do R.. Detection of widespread horizontal pleiotropy in causal relationships inferred from Mendelian randomization between complex traits and diseases. Nat Genet 2018;50:693–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Qi G, Chatterjee NA.. A comprehensive evaluation of methods for Mendelian randomization using realistic simulations and an analysis of 38 biomarkers for risk of type 2 diabetes. Int J Epidemiol 2021;50:1335–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sanderson E, Davey Smith G, Windmeijer F, Bowden J.. An examination of multivariable Mendelian randomization in the single-sample and two-sample summary data settings. Int J Epidemiol 2019;48:713–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hemani G, Zheng J, Elsworth B. et al. The MR-Base platform supports systematic causal inference across the human phenome. Elife 2018;7:e34408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Jones SE, Lane JM, Wood AR. et al. Genome-wide association analyses of chronotype in 697,828 individuals provides insights into circadian rhythms. Nat Commun 2019;10:343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Sterne JAC. Sifting the evidence - what’s wrong with significance tests? Another comment on the role of statistical methods. BMJ 2001;322:226–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Papantoniou K, Devore EE, Massa J. et al. Rotating night shift work and colorectal cancer risk in the nurses’ health studies. Int J Cancer 2018;143:2709–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Dieckhoff M. Skills and occupational attainment: a comparative study of Germany, Denmark and the UK. Work Employ Soc 2008;22:89–108. [Google Scholar]
- 54.Jirjahn U. On the determinants of shift work and overtime work: evidence from German establishment data. Br J Ind Relations 2008;46:133–68. [Google Scholar]
- 55.Andreyeva T, Puhl RM, Brownell KD.. Changes in perceived weight discrimination among Americans, 1995-1996 through 2004-2006. Obesity 2008;16:1129–34. [DOI] [PubMed] [Google Scholar]
- 56.Puhl RM, Heuer CA.. The stigma of obesity: a review and update. Obesity 2009;17:941–64. [DOI] [PubMed] [Google Scholar]
- 57.Roehling MV, Roehling PV, Pichler S.. The relationship between body weight and perceived weight-related employment discrimination: The role of sex and race. J Vocat Behav 2007;71:300–18. [Google Scholar]
- 58.Vetter C, Fischer D, Matera JL, Roenneberg T.. Aligning work and circadian time in shift workers improves sleep and reduces circadian disruption. Curr Biol 2015;25:907–11. [DOI] [PubMed] [Google Scholar]
- 59.Juda M, Vetter C, Roenneberg T.. Chronotype modulates sleep duration, sleep quality, and social jet lag in shift-workers. J Biol Rhythms 2013;28:141–51. [DOI] [PubMed] [Google Scholar]
- 60.Sun M, Feng W, Wang F. et al. Meta-analysis on shift work and risks of specific obesity types. Obes Rev 2018;19:28–40. [DOI] [PubMed] [Google Scholar]
- 61.Munafò MR, Tilling K, Taylor AE, Evans DM, Davey Smith G.. Collider scope: when selection bias can substantially influence observed associations. Int J Epidemiol 2018;47:226–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Fry A, Littlejohns TJ, Sudlow C. et al. Comparison of sociodemographic and health-related characteristics of UK biobank participants with those of the general population. Am J Epidemiol 2017;186:1026–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Wang X-S, Travis RC, Reeves G. et al. Characteristics of the Million Women Study participants who have and have not worked at night. Scand J Work Environ Health 2012;38:590–99. [DOI] [PubMed] [Google Scholar]
- 64.Howe LJ, Lawson DJ, Davies NM. et al. Genetic evidence for assortative mating on alcohol consumption in the UK Biobank. Nat Commun 2019;10:5039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Howe LJ, Tudball M, Smith G, Davies NM.. Interpreting Mendelian randomization estimates of the effects of categorical exposures such as disease status and educational. medRxiv 2020. doi: 10.1101/2020.12.14.20248168. [DOI] [PMC free article] [PubMed]
- 66.Lawlor DA, Tilling K, Davey Smith. G. Triangulation in aetiological epidemiology. Int J Epidemiol 2017;45:1866–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Barcellos SH, Carvalho LS, Turley P.. Education can reduce health differences related to genetic risk of obesity. Proc Natl Acad Sci U S A 2018;115:E9765–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All individual-level data from UKB used to support this manuscript may be accessed by applying to the UKB Central Access Committee [http://www.ukbiobank.ac.uk/register-apply/].