Abstract
Targeted therapy with epidermal growth factor receptor (EGFR)-tyrosine kinase inhibitors (TKIs) is a standard modality of the 1st-line treatments for patients with advanced EGFR-mutated non-small cell lung cancer (NSCLC), and substantially improves their prognosis. However, EGFR T790M mutation is the primary mechanism of 1st- and 2nd-generation EGFR-TKI resistance. Osimertinib is a representative of the 3rd-generation EGFR-TKIs that target T790M mutation, and has satisfactory efficacy in the treatment of T790M-positive NSCLC with disease progression following use of 1st- or 2nd-generation EGFR-TKIs. Other 3rd-generation EGFR-TKIs, such as abivertinib, rociletinib, nazartinib, olmutinib and alflutinib, are also at various stages of development. However, the occurrence of acquired resistance is inevitable, and the mechanisms of 3rd-generation EGFR-TKI resistance are complex and incompletely understood. Genomic studies in tissue and liquid biopsies of resistant patients reveal multiple candidate pathways. The present review summarizes the recent findings in mechanisms of resistance to 3rd-generation EGFR-TKIs in advanced NSCLC, and provides possible strategies to overcome this resistance. The mechanisms of acquired resistance mainly include an altered EGFR signaling pathway (EGFR tertiary mutations and amplification), activation of aberrant bypassing pathways (hepatocyte growth factor receptor amplification, human epidermal growth factor receptor 2 amplification and aberrant insulin-like growth factor 1 receptor activation), downstream pathway activation (RAS/RAF/MEK/ERK and PI3K/AKT/mTOR) and histological/phenotypic transformations (SCLC transformation and epithelial-mesenchymal transition). The combination of targeted therapies is a promising strategy to treat osimertinib-resistant patients, and multiple clinical studies on novel combined therapies are ongoing.
Keywords: epidermal growth factor receptor-tyrosine kinase inhibitor, osimertinib, non-small cell lung cancer, resistance mechanism, targeted therapy
1. Introduction
Lung cancer is the leading cause of cancer-related death throughout the whole world. More than half (57%) of all patients with lung cancer have metastasis at the time of diagnosis, and the 5-year survival rate is only 5% (1). Non-small cell lung cancer (NSCLC) constitutes 85% of all lung cancer cases (2). Epidermal growth factor receptor (EGFR) is one of the most common driver genes in NSCLC. Female patients have higher mutation rates than male patients. Adenocarcinomas and non-smoking are also associated with EGFR mutations (3). The EGFR belongs to the HER/ErbB family; it locates on the cell surface, and binds to its ligands, such as EGF and transforming growth factor. Upon ligand binding, EGFR undergoes dimer formation and auto-phosphorylation at the tyrosine residues, and activates downstream signaling through MAPK and PI3K pathways, leading to cancer cell proliferation, differentiation and migration (4). Approximately 20% of NSCLC patients bear activating mutations in EGFR. The deletions in exon 19 (Ex19del) and the L858R point mutation within exon 21 constitute ~90% of EGFR activating mutations (5). The activating mutations of EGFR are the targets of EGFR tyrosine kinase inhibitors (EGFR-TKIs). Current treatment guidelines recommend EGFR-TKIs as the 1st-line treatment for patients with advanced NSCLC with EGFR activating mutations.
The 1st-generation EGFR-TKIs, including gefitinib, erlotinib and icotinib, are reversible inhibitors that can inhibit the EGFR tyrosine kinase domain in an ATP-competitive and -reversible manner (Fig. 1) (6). The Iressa Pan-Asia study is a phase III and open-label study comparing gefitinib with carboplatin-paclitaxel as the 1st-line treatment for patients with advanced NSCLC in East Asia. The results showed that in the patients with EGFR-activating mutations, the gefitinib group experienced longer progression-free survival (PFS) than the carboplatin/paclitaxel group, while in patients without EGFR mutations, there was no PFS benefit in the gefitinib group (7). In 2015, gefitinib was approved by the U.S. Food and Drug Administration (FDA) for the 1st-line treatment of advanced NSCLC with EGFR-activating mutations (8). The European Tarceva versus Chemotherapy study is a multicenter, open-label and randomized phase III trial, which aimed to compare erlotinib with platinum-based chemotherapy as the 1st-line treatment for European patients with advanced NSCLC. In this study, erlotinib significantly improved PFS time compared with platinum-based chemotherapy in the patients with EGFR-activating mutations (9.7 vs. 5.2 months; HR, 0.37; 95% CI, 0.25-0.54; P<0.0001) (9). Icotinib is independently developed in China, and the CONVINCE study was designed to compare the efficacy and safety of 1st-line icotinib versus cisplatin/pemetrexed plus pemetrexed maintenance in patients with advanced lung adenocarcinoma. The results have demonstrated superior efficacy in that the PFS time of the icotinib group was significantly longer than that of the pemetrexed plus cisplatin chemotherapy group following the 1st-line treatment of EGFR-mutated NSCLC (11.2 vs. 7.9 months; HR, 0.61; 95% CI, 0.43-0.87; P=0.006) (10).
Figure 1.
Chemical structures of different generations of EGFR-TKIs. Chemical structures were sourced from https://pubchem.ncbi.nlm.nih.gov/. EGFR-TKI, epidermal growth factor receptor-tyrosine kinase inhibitor.
The 2nd-generation EGFR-TKIs, such as afatinib and dacomitinib, were originally designed to overcome the resistance to the 1st-generation EGFR-TKIs (Fig. 1). Afatinib is an irreversible dual specificity EGFR/human epidermal growth factor receptor 2 (HER2) inhibitor that is designed to covalently bind to EGFR and HER2, while dacomitinib is an irreversible pan-HER inhibitor. The broad spectrum of activity enables them to improve inhibition of EGFR-dependent tumor growth compared with the 1st-generation EGFR-TKIs. In the LUX-Lung7 and ARCHER studies, afatinib and dacomitinib significantly prolonged PFS and overall survival (OS) times compared with gefitinib in the 1st-line treatment of patients with NSCLC and EGFR activating mutations (11,12). However, afatinib and dacomitinib exhibited low maximum tolerated doses in the patients. Compared to gefitinib, they were associated with a higher incidence of adverse events, including increased skin and gastrointestinal toxicity. The 2nd-generation EGFR-TKIs therefore have limited roles in patients with developed resistance to the 1st-generation EGFR-TKIs (13-15). Most patients inevitably develop acquired resistance through various mechanisms after 9-13 months of treatment with the 1st- and 2nd-generation EGFR-TKIs. The T790M mutation in EGFR exon 20 is the predominant cause, occurring in 50-60% of patients (15-17).
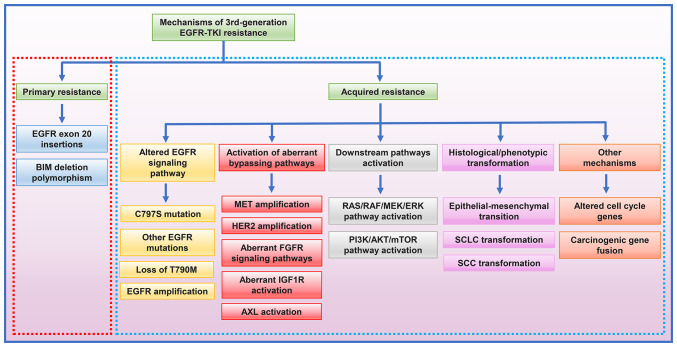
The so-called 'gatekeeper' T790M mutation increases the competition between ATP and the reversible EGFR-TKIs by exerting effects on both steric hindrance and increased ATP affinity to mutant EGFR receptor, thereby decreasing the efficacy of 1st- and 2nd-generation EGFR-TKIs (15). The 3rd-generation EGFR-TKIs, represented by osimertinib, have satisfactory efficacy in overcoming acquired resistance to the 1st- and 2nd-generation EGFR-TKIs mediated by T790M mutation (Fig. 1). Osimertinib selectively targets the EGFR T790M mutation, forming covalent bonds with the C797 residue in the ATP-binding site of mutant EGFR. Since their binding is irreversible, this agent overcomes the enhanced ATP affinity conferred by the T790M mutation; it exhibits ~200 times greater potency against L858R/T790M mutant EGFR than wild-type EGFR (18). The 3rd-generation EGFR-TKIs have promising clinical activity and tolerability. For patients who progressed on the 1st-generation EGFR-TKI treatment and have a T790M mutation, osimertinib has significantly improved their PFS and OS times compared with chemotherapy (17). Furthermore, osimertinib has shown better efficacy than gefitinib or erlotinib in untreated NSCLC with EGFR mutations (19). Patients receiving osimertinib have a lower incidence of serious adverse events than those treated with the 1st- or 2nd-generation EGFR-TKIs (12,19). However, patients with EGFR-activating mutations administered osimertinib as the 1st-line treatment have also been found to inevitably develop resistance after 18.9 months (19). Previous studies on the mechanisms of 3rd-generation EGFR-TKI resistance have revealed both primary and acquired resistance (Fig. 2). For patients with NSCLC progression after the 3rd-generation EGFR-TKI treatments, the use of next-generation sequencing (NGS) of plasma or tissue samples to clarify the resistance mechanism is important to guide further treatment. The molecular targets and resistance mechanisms of different generations of EGFR-TKIs are shown in Table I. Comprehensive and in-depth studies on the resistance to the 3rd-generation EGFR-TKIs are urgently required to provide better therapeutic strategies.
Figure 2.
Mechanisms of primary and acquired 3rd-generation EGFR-TKI resistance in advanced EGFR-mutated non-SCLC. BIM, B-cell lymphoma-2 (BCL-2)-like 11; MET, hepatocyte growth factor receptor; HER2, human epidermal growth factor receptor 2; FGFR, fibroblast growth factor receptor; IGF1R, insulin-like growth factors 1 receptor; AXL, anexelekto; SCLC, small cell lung cancer; SCC, squamous cell carcinoma; EGFR-TKI, epidermal growth factor receptor-tyrosine kinase inhibitor.
Table I.
Different generations of EGFR-TKIs.
| A, 1st-generation EGFR-TKIs
| |||||
|---|---|---|---|---|---|
| Drug name | Molecular target | Target binding mode | Resistance mechanisms | Status | Adverse events |
| Gefitinib (ZD1839) | EGFR Ex19del, L858R | Reversible; competitive | T790M, EGFR amp, HER2 amp, MET amp | Approved by the FDA in 2005 | Skin rash/acne, abnormal LFT, anorexia (7) |
| Erlotinib (OSI-774) | EGFR Ex19del, L858R | Reversible; competitive | T790M, EGFR amp, HER2 amp, MET amp | Approved by the FDA in 2003 | Skin rash, abnormal LFT, diarrhea (9) |
| Icotinib (BPI-2009H) | EGFR Ex19del, L858R | Reversible; competitive | T790M, EGFR amp, HER2 amp, MET amp | Approved by the SFDA in 2011 | Skin rash, cough, diarrhea, abnormal LFT (10) |
|
| |||||
| B, 2nd-generation EGFR-TKIs
| |||||
| Drug name | Molecular target | Target binding mode | Resistance mechanisms | Status | Adverse events |
| Afatinib (BIBW 2992) | EGFR Ex19del, L858R, HER2, HER4, uncommon mutations | Irreversible; covalent | T790M, EGFR amp, HER2 amp, MET amp | Approved by the FDA in 2013 | Diarrhea, paronychia, skin rash (11) |
| Dacomitinib (PF-00299804) | EGFR Ex19del, L858R, HER2, HER4 | Irreversible; covalent | T790M, EGFR amp, HER2 amp, MET amp | Approved by the FDA in 2018 | Diarrhea, skin rash/acne, paronychia (12) |
|
| |||||
| C, 3rd-generation EGFR-TKIs
| |||||
| Drug name | Molecular target | Target binding mode | Resistance mechanisms | Status | Adverse events |
| Osimertinib (AZD9291) | EGFR Ex19del, L858R, T790M | Irreversible; covalent | C797S, MET amp, EGFR amp, HER2 amp/mut, PIK3CA amp/mut, SCLC transformation | Approved by the FDA in 2015 | Diarrhea, skin rash, dry skin, paronychia (17-19) |
| Rociletinib (AVL301/CO1686) | EGFR Ex19del, L858R, T790M | Irreversible; covalent | C797S, EGFR amp, MET amp, HER2 amp, KRAS mut | After phase I/II trial, rejected by the FDA | Hyperglycemia, QTc prolong, cataracts (20,26) |
| Abivertinib (AC0010) | EGFR Ex19del, L858R, T790M | Irreversible; covalent | C797S, EGFR amp, MET amp, HER2 amp, KRAS mut | Phase II | Diarrhea, skin rash, abnormal LFT (22,27) |
| Nazartinib (EGF816) | EGFR Ex19del, L858R, T790M | Irreversible; covalent | C797S, MET amp | Phase III | Rash, diarrhea, pruritus (23,28) |
| Olmutinib (HM61713) | EGFR Ex19del, L858R, T790M | Irreversible; covalent | C797S | Approved by the FDA in 2015 | Diarrhea, skin exfoliation, nausea (25,29) |
| Alflutinib (AST2818) | EGFR G719X, Ex19del, L858R, L861Q, T790M | Irreversible; covalent | N/A | Approved in China in 2021 | Diarrhea, skin rash, abnormal LFT (30) |
| Almonertinib (HS-10296) | EGFR G719X, Ex19del, L858R, L861Q, T790M | Irreversible; covalent | N/A | Approved in China in 2020 | Creatine phosphokinase elevation, skin rash, abnormal LFT, leukopenia (31) |
| Lazertinib (YH25448/GNS-1480) | EGFR Ex19del, L858R, T790M | Irreversible; covalent | C797S, EGFR amp, PIK3CA, MET amp, HER2 amp | Approved in South Korea in 2021 | Skin rash, pruritus, paresthesia (21,32) |
| Naquotinib (ASP8273) | EGFR Ex19del, L858R, T790M | Irreversible; covalent | N/A | Phase III discontinued | Diarrhea, hyponatremia, abnormal LFT, nausea (24,33) |
|
| |||||
| D, 4th-generation EGFR-TKIs
| |||||
| Drug name | Molecular target | Target binding mode | Resistance mechanisms | Status | Adverse events |
| EAI045 | EGFR L858R, C797S, T790M | Non-ATP- competitive; reversible | N/A | Preclinical (91) | N/A |
| BLU-945 | EGFR Ex19del, L858R, C797S, T790M | N/A | N/A | Preclinical (93) | N/A |
| TQB3804 | EGFR Ex19del, L858R, C797S, T790M | N/A | N/A | Phase I (94) | N/A |
|
| |||||
| E, Multi-target TKIs
| |||||
| Drug name | Molecular target | Target binding mode | Resistance mechanisms | Status | Adverse events |
| Brigatinib (AP26113) | EGFR Ex19del, L858R, C797S, T790M | ATP- competitive; reversible | N/A | Approved by the FDA in 2016 | Nausea, diarrhea, headache, creatine phosphokinase elevation (92,95) |
amp, amplification; Ex19del, exon 19 deletion; FDA, Food and Drug Administration; SFDA, Chinese State Food and Drug Administration; LFT, liver function test; mut, mutation; EGFR-TKI, epidermal growth factor receptor tyrosine kinase inhibitor; QTc, corrected QT interval; SCLC, small cell lung cancer; MET, hepatocyte growth factor receptor; HER2, human epidermal growth factor receptor 2.
The present review summarizes the emerging evidence on mechanisms of 3rd-generation EGFR-TKI resistance in advanced NSCLC, and outlines the latest clinical strategies used to overcome this problem.
2. Third-generation EGFR-TKIs
In addition to osimertinib, several other 3rd-generation EGFR-TKIs targeting the T790M mutation (Fig. 1), such as rociletinib (CO1686), lazertinib (YH25448), abivertinib (AC0010), nazartinib (EGF816), naquotinib (ASP8273) and olmutinib (HM61713), are at various stages of development (Table I) (17,20-33). Osimertinib, rociletinib and olmutinib are currently approved for clinical use by the U.S. FDA. Osimertinib has dominated the global landscape of treatment of EGFR-positive NSCLC, since it is the current standard of care for the 1st-line treatment of advanced EGFR-mutated NSCLC and for EGFR T790M-positive NSCLC after 1st- or 2nd-generation EGFR-TKIs (17,19).
Osimertinib
In 2015, the FDA approved osimertinib for the treatment of patients with metastatic EGFR-mutant NSCLC who have acquired the EGFR T790M resistance mutation. The drug was then approved as the 1st-line therapy for advanced EGFR-mutated NSCLC in 2018 (34). Osimertinib is an oral, irreversible EGFR-TKI that is selective for both EGFR and T790M mutations and has activity in the central nervous system (CNS). AURA3 is a randomized, open-label, phase 3 trial that has been conducted to show the superiority of osimertinib over platinum therapy plus pemetrexed in patients with T790M-positive advanced NSCLC after 1st-generation EGFR-TKI therapy. The results showed that the median PFS time was significantly longer with osimertinib than that with platinum therapy plus pemetrexed (10.1 vs. 4.4 months; HR, 0.30; 95% CI, 0.23-0.41; P<0.001), which establish a standard protocol for the treatment of EGFR T790M-positive patients after failure of 1st-generation EGFR-TKI therapy, as well as targeted therapy for brain metastasis (17). The double-blind, phase 3 FLAURA trial compared the efficacy and safety of osimertinib with the standard EGFR-TKIs (gefitinib or erlotinib) in patients with untreated EGFR mutation-positive advanced NSCLC. It demonstrated that the median PFS time of untreated patients with EGFR mutation was significantly longer following osimertinib administration compared with that following gefitinib or erlotinib administration (18.9 vs. 10.2 months; HR, 0.80; 95% CI, 0.37-0.57; P<0.001) (19), as was the median OS time (38.6 vs. 31.8 months; HR, 0.46; 95% CI, 0.64-1.00; P=0.046) (35). In patients with measurable brain metastases, the objective response rate (ORR) was 91% with osimertinib versus 68% with the 1st-generation EGFR-TKIs (odds ratio, 4.6; 95% CI, 0.9-34.9; P=0.066) (36). Several studies have investigated the safety and efficacy of EGFR-TKIs in combination with other targeted therapies (37-39). The phase Ib TATTON study assessed the safety and tolerability of osimertinib in combination with selumetinib (MEK inhibitor), savolitinib [hepatocyte growth factor receptor (MET) inhibitor] or durvalumab, a human anti-programmed cell death-ligand-1 (PD-L1) antibody. The ORR in the selumetinib, savolitinib and durvalumab arms was 42, 44 and 43%, respectively (39).
Overall, osimertinib is a promising 3rd-generation EGFR-TKI in current lung cancer treatment regimens, and osimertinib-based combination therapy deserves broad investigations to improve outcomes in patients with advanced EGFR-mutated NSCLC.
Rociletinib
Rociletinib (CO1686) is a small-molecule, orally administered and irreversibly selective EGFR-TKI that inhibits EGFR mutations, including Ex19del, L858R and T790M, but not exon 20 insertion (e20ins) (26). The TIGER-X trial (phase I/II study) and the TIGER-2 trial (phase II, open-label, multi-center study) evaluated the safety and efficacy of rociletinib in previously treated patients with EGFR-mutated NSCLC. A total of 130 patients were enrolled in the TIGER-X study. Of the 46 evaluable T790M patients, the ORR was 59% (95% CI, 45-73), and the estimated median PFS time was 13.1 months (95% CI, 5.4-13.1) (26). However, results of the TIGER-X and TIGER-2 trials using a pooled cohort of patients found that the rate of confirmed response was 28-34% for rociletinib, which significantly differed from the result of the TIGER-X trial. After independent analysis, the maturation confirmation response rate was updated to 45% and the median PFS time to 6.1 months (20). Sequist et al (40) reported that 9 patients with progression on rociletinib were subsequently treated with osimertinib. Among them, 2 patients exhibited a partial response, 3 presented with stable disease and 4 developed progressive disease. The 3 patients with brain metastasis after rociletinib treatment also showed improvement in CNS disease response to osimertinib. The development of rociletinib was terminated on May 6, 2016, due to a lower efficacy than osimertinib and incidences of adverse events such as high-grade hyperglycemia and corrected QT interval prolongation (41).
Abivertinib
Abivertinib (AC0010) is one of the 3rd-generation EGFR-TKIs developed in China; it is a pyrrolopyrimidine compound, which is highly selective, irreversible and potent (42). A phase I study of abivertinib presented significant clinical benefits and a good safety profile in patients with NSCLC and acquired resistance to the 1st-generation EGFR-TKIs. The ORR in patients with T790M mutation was 42%, and the median PFS time estimated by Kaplan-Meier ranged between 14.0 and 35.6 weeks in those patients receiving a daily dose of 350-600 mg (42). A phase I, open, multicenter study explored the efficacy of abivertinib in patients with NSCLC and CNS metastasis. The median intracranial PFS time was 142 days (95% CI, 31.1-252.9) in 7 patients with brain metastasis, suggesting that abivertinib had good control of asymptomatic brain metastasis (43). A recent study reported that 9 patients received osimertinib treatment after progression on abiverinib, with a median total treatment duration of 15.9 months (95% CI, 12.5-19.3) (27). These results have demonstrated that osimertinib might be an option for subsequent therapy in patients with disease progression after abivertinib treatment. Further studies on abivertinib are required to investigate its interaction with osimertinib.
Other 3rd-generation EGFR-TKIs
Several other 3rd-generation EGFR-TKIs are also under development. Nazartinib (EGF816) is a covalent and irreversible EGFR-TKI. Preliminary results from an open-label, multicenter, phase I/II study of 180 patients at 9 academic medical centers in Europe, Asia and North America demonstrated that nazartinib had an excellent safety profile, with low dermal toxicity and a primary adverse event of maculopapular rash (40%) (28). Olmutinib (HM61713), approved by the FDA in December 2015 for the treatment of NSCLC, has potent inhibitory activity against L858R/T790M mutant NSCLC cells (44). In a recently published single-arm, open-label, phase I/II trial, EGFR T790M-positive patients receiving olmutinib (800 mg/day) had an ORR of 55% (38/69 evaluable patients; 95% CI, 42.6-67.1) and an estimated median PFS time of 6.9 months (95% CI, 5.6-9.7). The common adverse events included diarrhea (59%), pruritus (42%), rash (41%) and nausea (40%) (29). On September 30, 2016, two cases of toxic epidermal necrolysis/Stevens-Johnson syndrome, one of which was fatal, were reported in a phase II trial of olmutinib in South Korea. On April 13, 2018, the development of olmutinib was terminated (41). Alflutinib (AST2818) is a newly developed 3rd-generation EGFR-TKI; its ORR was 77% (89 out of 116 patients), and 9% of patients (11 out of 130 patients) experienced grade 3 or higher drug-related adverse events (30). Almonertinib, a 3rd-generation EGFR-TKI developed in China, has also shown good efficacy in the treatment of T790M-positive NSCLC after EGFR-TKI resistance. In a phase II trial, it exhibited a median PFS time of 12.3 months, an ORR of 69% and acceptable toxicity. On March 19, 2020, almonertinib was approved by the Center for Drug Evaluation of the China National Medical Products Administration (31). Phase I/II clinical studies on lazertinib showed that lazertinib had a favorable safety profile and exhibited promising antitumor activity in patients with EGFR T790M-positive NSCLC, with a median PFS time of 11.0 months and an ORR of 58%. Lazertinib was approved for EGFR T790M-positive NSCLC in South Korea in 2021 (21,32). Naquotinib has anti-tumor activity in preclinical models of EGFR-mutant NSCLC that targets mutant EGFR, including EGFR T790M. In phase I trials, it had an ORR of 31% against T790M-positive NSCLC. However, the phase III clinical trial of naquotinib was terminated due to toxicity and limited predicted efficacy (24,33). Further investigations on 3rd-generation EGFR-TKIs are still ongoing.
3. Primary resistance mechanisms
Similar to chemotherapy-associated resistance, resistance to EGFR-TKIs includes both primary (intrinsic) and acquired (secondary) types. The primary type is generally defined as resistance that is present prior to the EGFR-TKI treatment. There are few studies related to primary resistance, and the mechanisms are unclear. Possible causes include EGFR e20ins and B-cell lymphoma-2 (BCL-2)-like 11 (BIM) deletion polymorphism.
EGFR e20ins
EGFR e20ins occur in ~3% of lung adenocarcinoma patients and 9% of EGFR-mutated tumors. EGFR e20ins block EGFR-TKI binding to EGFR target sites, leading to primary resistance (45). For patients with EGFR e20ins, the overall effectiveness of 1st-line treatment with 1st- and 2nd-generation EGFR-TKIs is 3-8%, with a median PFS time of only 2 months. An in vitro study showed that NSCLC cells stably expressing EGFR e20ins were resistant to the 3rd-generation EGFR-TKIs, suggesting that EGFR e20ins might be primary mechanisms of 3rd-generation EGFR-TKI resistance (46). Qin et al (47) demonstrated that p.A767_V769dup (25%) and p.S768_D770dup (18%) were the 2 most common EGFR e20ins, accounting for 44% of cases and serving as primary targets for future drug development. In addition, different EGFR e20ins might be associated with different outcomes in patients with advanced NSCLC. p.A763_Y764insFQEA conferred better PFS times than the other e20ins when patients were treated with various EGFR-TKIs, while p.S768_D770dup showed a limited response and p.D770delinsGY patients experienced a short PFS time. Therefore, it is important to select the appropriate EGFR-TKIs according to the specific EGFR e20in type (47). van Veggel et al (48) reported that osimertinib (80 or 160 mg/day) treatment had an ORR of 5% and a median PFS time of 3.6 months (95% CI, 2.6-4.5) in 21 patients with advanced NSCLC and EGFR e20ins (48). Phase II clinical studies on osimertinib for EGFR e20ins in NSCLC are currently underway (NCT03414814 and NCT03191149).
BIM deletion polymorphism
BIM is a member of the BCL-2 family and a pro-apoptotic molecule. The BIM deletion polymorphism is present in ~21% of East Asian individuals, and is absent in African and European populations (49,50). Tanimoto et al (51) demonstrated that EGFR-mutant NSCLC cells with BIM deletion polymorphism were resistant to 3rd-generation EGFR-TKIs. Li et al (49) first reported that a patient with NSCLC plus the EGFR L858R/T790M mutation and the BIM deletion polymorphism exhibited a poor clinical outcome after treatment with osimertinib (49). In addition, another study indicated that the median PFS time of patients with BIM deletion polymorphism was significantly shorter than that of wild-type BIM patients (227 vs. 533 days; P<0.001). Multivariate Cox regression analysis showed that the BIM deletion polymorphism was an independent indicator of shorter PFS time (52). However, Liu et al (53) concluded that it was the concomitant genetic alterations, rather than BIM deletion polymorphism, that influenced the EGFR-TKI response. Cox multivariate regression analysis showed that TP53 mutations, NTRK1 mutations and amplifications, RB1 mutations and PIK3CA mutations were associated with poorer PFS. In addition, TP53 mutations, KEAP1 mutations and deletions were shown to have an impact on OS in patients treated with EGFR-TKI. By contrast, neither analysis showed an effect of BIM deletion polymorphisms on PFS and OS (53). The roles of BIM deletion polymorphism in the prediction of EGFR-TKI responses are still controversial. Further studies are required to explore the exact functions of BIM deletion polymorphism in the primary resistance of 3rd-generation EGFR-TKIs.
4. Acquired resistance mechanisms
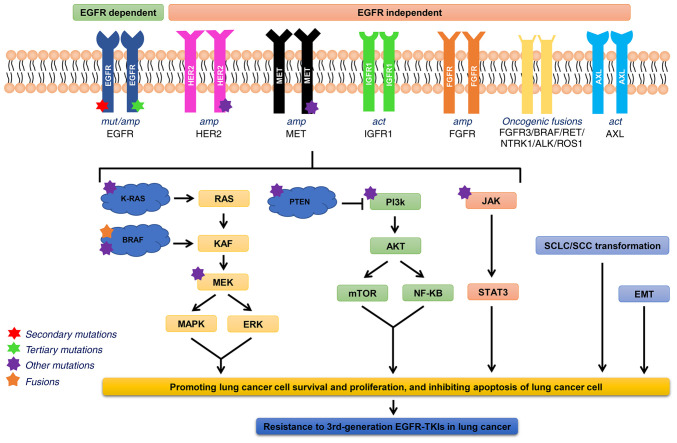
Acquired resistance refers to the evading effects of EGFR-TKIs in tumor cells via alteration of their metabolic pathways after exposure to the drugs. With the application of NGS-based genomic profiling, the mechanisms of 3rd-generation EGFR-TKI resistance have been extensively studied. The acquired resistance of osimertinib as the 2nd- and 1st-line therapy (54-60), as well as rociletinib and abivertinib (42,61-63), is summarized in Fig. 3. The mechanisms of acquired resistance to 3rd-generation EGFR-TKIs include an altered EGFR signaling pathway, aberrant activation of bypass and downstream signaling pathways, and histological transformation. The molecular mechanisms are depicted in Fig. 4.
Figure 3.
Mechanisms of acquired 3rd-generation EGFR-TKI resistance in advanced EGFR-mutated non-SCLC. (A) Osimertinib as the 2nd-line treatment. (B) Osimertinib as the 1st-line treatments. (C) Abivertinib. (D) Rociletinib. These pie-charts summarize data from studies that enrolled >15 patients. Different resistant mechanisms might co-exist in the same patient. mut, mutation; amp, amplification; PIK3CA, phosphatidylinositol-3-kinase catalytic α; PTEN, phosphatase and tensin homolog; del, deletion; EGFR-TKI, epidermal growth factor receptor-tyrosine kinase inhibitor; MET, hepatocyte growth factor receptor; HER2, human epidermal growth factor receptor 2; FGFR, fibroblast growth factor receptor; IGF1R, insulin-like growth factors 1 receptor; HGF, hepatocyte growth factor; AXL, anexelekto; SCLC, small cell lung cancer; SCC, squamous cell carcinoma; EGFR-TKI, epidermal growth factor receptor-tyrosine kinase inhibitor; ROS1, c-ros oncogene 1, receptor tyrosine kinase.
Figure 4.
Molecular mechanisms of acquired 3rd-generation EGFR-TKI resistance. act, activation; NTRK1, neurotrophic receptor tyrosine kinase 1; ALK, anaplastic lymphocyte kinase; ROS1, c-ros oncogene 1, receptor tyrosine kinase; STAT3, signal transducer and activator of transcription 3; EMT, epithelial-mesenchymal transition; SCLC, small cell lung cancer; SCC, squamous cell carcinoma; MET, hepatocyte growth factor receptor; HER2, human epidermal growth factor receptor 2; FGFR, fibroblast growth factor receptor; IGF1R, insulin-like growth factors 1 receptor; AXL, anexelekto.
Altered EGFR signaling pathway
EGFR tertiary mutations
EGFR tertiary mutations are common resistance mechanisms against 3rd-generation EGFR-TKIs in lung cancer, and multiple EGFR tertiary mutations have been observed. The C797S mutation at EGFR exon 20 is the most common tertiary mutation mediating 3rd-generation EGFR-TKI resistance. C797S is a missense mutation in the tyrosine kinase region of EGFR, which prevents the 3rd-generation EGFR-TKIs forming covalent bonds in the ATP-binding domain of EGFR kinase, resulting in EGFR-TKI dysfunction. The C797S mutation has been found to affect the sensitivity of NSCLC to 3rd-generation EGFR-TKIs in both in vitro and in vivo models (54,56,59). Ercan et al (64) evaluated the sensitivity of EGFR inhibitors in models harboring drug-resistant EGFR mutations. It was found that NSCLC cell lines harboring C797S mutation were resistant to irreversible EGFR-TKIs such as osimertinib, rociletinib and olmutinib (64). NGS analysis of plasma samples from 73 patients with progression after 2nd-line treatment of osimertinib in the AURA3 study showed that 10 patients (14%) had the C797S mutation, and all retained the T790M mutation (54). Le et al (56) found 8 (19%) C797S mutations in 42 patients coinciding with the T790M mutation. In the FLAURA study, the frequency of the C797S mutation was 7% (6/91) (59). In addition to that in osimertinib, the C797S mutation was reported to mediate resistance to other 3rd-generation TKIs, such as olmutinib, larzertinib, rociletinib and abivertinib (61-63,65,66). Zhang et al (61) first indicated that EGFR tertiary mutations mediated abivertinib resistance. The EGFR C797S mutation was detected in 3 patients (10%), all occurred in cis with the EGFR T790M mutation. By contrast, the EGFR C797S mutation is an infrequent mechanism of rociletinib resistance, only observed in 2.3-4.5% of patients (62,63).
Other rare EGFR tertiary mutations associated with drug resistance observed in the AURA3 and FLAURA studies are L792X (3%), G796S (1%), L718Q (1-2%) and S768I (1%), which have the potential to sterically interfere with the osimertinib-EGFR interaction (59). Osimertinib treatment may be ineffective against G724S mutation (67,68). Structural analysis revealed that the G724S mutation might induce a glycine-rich loop conformation, which is incompatible with the binding of 3rd-generation EGFR-TKIs (68). Further studies indicated that the L718Q mutation showed cross-resistance to all the 3rd-generation EGFR-TKIs (69). In addition, the L718V mutation was observed in abivertinib-resistant patients, while the E709K, L692V and L798I mutations were detected in rociletinib-resistant patients (61,62).
Loss of T790M
Patients enrolled in the AURA3 study all developed the T790M mutation after the 1st-line treatment with 1st- or 2nd-generation EGFR-TKIs, and 49% (36/73) of them had loss of T790M after osimertinib resistance (54). The frequency of T790M mutation loss in osimertinib-resistant patients in the studies by Le et al (56) and Oxnard et al (57) was 53% (21/40) and 68% (28/41), respectively. Oxnard et al (57) conducted tumor biopsies for genomic analysis in 41 patients, and suggested that the loss of T790M was associated with a small decrease in EGFR-activating mutations after 1-3 weeks of treatment. Another recent study demonstrated a markedly shorter median PFS time in the T790M-loss group than that in the T790M group (5.93 vs. 11.87 months, P=0.0004). Loss of T790M mutation was correlated with earlier disease progression and worse survival in the osimertinib-resistant patients (70). In addition, the loss of T790M was present in 50% (6/12) of patients resistant to rociletinib (71). In T790M cases, the resistant mechanism is mostly related to C797S mutation or bypass pathway activation, while patients without T790M often show EGFR-independent mechanisms (56,71).
EGFR amplification
Le et al (56) demonstrated that EGFR amplification occurred in 8 (19%) of 42 patients who developed resistance to osimertinib (56). One patient with progression after osimertinib treatment developed amplified copies of EGFR Ex19del, with amplification levels parallel to clinical and radiological progression (72). EGFR amplification is more commonly observed in patients treated with rociletinib. Piotrowska et al (71) reported that 25% (3/12) of patients resistant to rociletinib showed EGFR amplification. This was comparable to 29% (17/58) in the study by Helman et al (63). In addition, EGFR amplification in 7 (23%) patients was considered as putative mechanisms of abivertinib resistance. Among them, 4 patients had EGFR amplification after abivertinib treatment, and the other 3 had EGFR amplification prior to treatment but had elevated EGFR copy numbers at progressive disease (61).
Activation of aberrant bypassing pathways
MET amplification
Amplification of c-Met plays an important role in 1st- and 3rd-generation EGFR-TKI resistance; it functions as a transmembrane receptor tyrosine kinase. When hepatocyte growth factor ligands bind to c-Met, c-Met is activated, inducing homodimerization and phosphorylation of intracellular tyrosine residues, which activates the downstream RAS/ERK/MAPK and PI3K-AKT signaling pathways (73,74). These pathways can drive cell survival, proliferation, motility, migration, invasion, angiogenesis and epithelial-mesenchymal transition (EMT), providing a bypass pathway in the presence of an EGFR inhibitor (73,74). The AURA3 study showed that 19% (14/73) of patients developed MET gene amplification after the 2nd-line treatment with osimertinib, which is the second most common cause of resistance following the C797S mutation (54). Moreover, the incidence of MET amplification was 14% (6/42) in the study by Le et al (56). In the FLAURA study, 15% (14/91) of patients resistant to osimertinib developed MET amplification, exceeding the 7% (6/91) of the C797S mutation (59). MET amplification might be the most common cause of resistance when osimertinib is used as 1st-line treatment. It is also one of the most common resistance mechanisms to rociletinib, occurring in 8-26% of rociletinib-resistant patients (62,63). In the abivertinib-resistant cohort, MET amplification was detected in 3 (10%) patients (61).
HER2 amplification
HER2 belongs to the EGFR family. HER2 amplification has a sustained effect on the PI3K/AKT and MAPK signaling pathways downstream of EGFR, thereby promoting osimertinib resistance in lung cancer cells (54,56,59). In the AURA3 study, HER2 amplification occurred in 6% of patients resistant to 2nd-line treatment with osimertinib (4/73) (54), and in 10% (4/42) of patients in the study by Le et al (56). In the FLAURA study, for the patients resistant to 1st-line treatment with osimertinib, the incidence of HER2 amplification was 2% (2/91) (59). Moreover, HER2 e20ins and E1247K were also identified in osimertinib-resistant patients (60). A recent in vitro study found that HER2 D16 can form a homodimer and activate downstream signaling pathways in NSCLC cells, resulting in acquired resistance to osimertinib. The combination of osimertinib and afatinib could synergistically inhibit H1975-HER2 D16 cells (75). In addition, the HER2 amplification also occurred in rociletinib-resistant patients with a frequency of 9% (62).
Aberrant fibroblast growth factor receptor (FGFR) signaling pathway
FGFR signaling pathways regulate cell proliferation, migration, cycle progression, metabolism and survival (76). Kim et al (77) found that FGFR1 amplification and FGF2 expression were upregulated in patients resistant to osimertinib, suggesting that the FGF2-FGFR1 autocrine loop might be associated with osimertinib resistance (77). The plasma-based circulating tumor DNA (ctDNA) assay revealed that the FGFR3-TACC3 fusion mutation was present in patients with the T790M mutation, and disease progression occurred upon osimertinib and naquotinib treatment (78). These results suggested that abnormalities of the FGFR signaling pathway might contribute to 3rd-generation EGFR-TKI resistance.
Aberrant insulin-like growth factor 1 receptor (IGF1R) activation
IGF1R is present in numerous cell types in various tissues, such as muscle, cartilage, bone and brain; it is a tyrosine kinases receptor that is involved in the pathogenesis and progression of various malignancies, including lung cancer (79). Abnormalities of IGF1R confer resistance to 3rd-generation EGFR-TKIs by activating the EGFR bypass signaling pathway (79). Manabe et al (80) demonstrated for the first time that IGF2 autocrine-mediated activation of the IGF1R pathway in lung cancer cells was involved in osimertinib resistance. Immunohistochemistry confirmed the increased IGF2 expression in lung cancer patients with acquired osimertinib resistance (80). Phosphate-receptor tyrosine kinase array analysis of osimertinib-resistant cells showed that IGF1R was activated in PC9/T790M/AZDR and H1975/AZDR cells. Inhibition of IGF1R activation by small interfering RNA or linstinib (IGF1R inhibitor) significantly restored osimertinib sensitivity, suggesting that aberrant IGF1R activation might be one of the causes of 3rd-generation EGFR-TKI resistance (79).
Anexelekto (AXL) activation
AXL is a member of the receptor tyrosine kinases, which are involved in the regulation of cell survival, proliferation, migration and metabolism. A study by Taniguchi et al (81) demonstrated that the overexpression of AXL was associated with a poor response to osimertinib. This study showed that osimertinib stimulated AXL by inhibiting the feedback inhibition of SPRY4. Activated AXL interacted with EGFR and HER3 to maintain cell survival and induced osimertinib resistance, which could be delayed by AXL inhibitors (81). Another study demonstrated that AXL was upregulated in osimertinib-resistant lung adenocarcinoma cells. Osimertinib combined with cabozantinib (AXL inhibitor) inhibited the growth of these cells both in vitro and in vivo (82). In addition, AXL amplification was also detected in abivertinib-resistant patients (61). Therefore, AXL activation and AXL amplification might also contribute to the primary and acquired resistance to 3rd-generation EGFR-TKIs.
Downstream pathways activation
RAS/RAF/MEK/ERK pathway activation
Acquired resistance to the 3rd-generation EGFR-TKIs is associated with the activation of the RAS-MAPK pathway. BRAF is a serine/threonine protein kinase, which regulates cell survival, proliferation, differentiation and apoptosis, and plays a crucial role in RAS/RAF/MEK/ERK pathways. Nakatani et al (83) observed KRAS overexpression in osimertinib-resistant cells. One case (1%) in the AURA3 study had a KRAS (G12D) mutation after resistance and 2 cases (3%) had BRAF V600E mutations (54). KRAS Q61K mutation, BRAF mutations and ESYT2-BRAF gene fusion have also been detected in patients with acquired osimertinib resistance (57). KRAS mutations in the FLAURA study included A146T (1%), G12C (1%) and G12D (1%), and the incidence of the BRAF V600E mutation was 3% (3/91) (59).
PI3K/AKT/mTOR pathway activation
Phosphatidylinositol-3- kinase catalytic α (PIK3CA) amplification or mutations promote tumor infiltration and activate the PI3K/AKT/mTOR pathway. In the AURA3 study, PIK3CA amplification and E545K mutation were detected in patients with osimertinib resistance (54). Oxnard et al (57) reported a 10% (4/41) incidence of PIK3CA amplification in osimertinib-resistant patients (57). PIK3CA mutations in the FLAURA study included E453K (1%), E545K (4%) and H1047R (1%) (59). These results suggested that PI3K/AKT/mTOR pathway activation might be involved in the 3rd-generation EGFR-TKI resistance.
Histological/phenotypic transformation
Marcoux et al (84) reported that 19 NSCLC patients developed SCLC transformation after treatment with the 3rd-generation EGFR-TKIs. The median time of transformation was 17.8 months (95% CI, 14.3-26.2) and the median survival time after SCLC transformation was 10.9 months (95% CI, 8.0-13.7). The incidence of squamous cell carcinoma (SCC) transformation after the 1st- and 2nd-line osimertinib treatment was 15 and 14%, respectively (85). SCLC and SCC transformation have not yet been identified in vitro, suggesting that the tumor microenvironment might play potential roles in these processes. The transdifferentiation of epithelial cells into mesenchymal cells, a process known as EMT, involves the loss of epithelial cell markers, such as E-cadherin, and the acquisition of mesenchymal markers, such as vimentin, N-cadherin, fibronectin, β-catenin and zeb-1, which confers on cancer cells increased tumor-initiating and metastatic potential (86). Nukaga et al (87) demonstrated that osimertinib- and rociletinib-resistant H1975 cells exhibited a shuttle cell-like morphology and acquired a more mesenchymal phenotype in vitro. NSCLC cells from patients with acquired osimertinib resistance were reported to exhibit EMT features, including decreased epithelial junction proteins and increased mesenchymal markers, confirming that EMT was closely associated with 3rd-generation EGFR-TKI resistance (88). Therefore, in addition to liquid biopsy to clarify the relevant mutant genes, it is also important to clarify the presence of histological/phenotypic transformation by tissue biopsy in osimertinib-resistant patients.
Altered cell cycle genes
The AURA3 and FLAURA studies demonstrated that the frequency of cell cycle gene alterations was 11 and 12%, respectively, in patients with disease progression after osimertinib treatment as the 1st- or 2nd-line therapy (54,59). Both CCNE1 and CDK6 amplification occurred at a frequency of 7% (5/73) as the most common cell cycle gene alterations in the AURA3 study (54). The most common cell cycle gene alteration in the FLAURA study was CDK6 amplification (3%) (59). In the study by Le et al (56), CCND1, CCNE1 and CDKN2A deletions were also found. Alterations in cell cycle genes may be involved in the development of osimertinib resistance.
Carcinogenic gene fusion
RET-ERC1 and NTRK1-TPM3 gene fusions were found in osimertinib-resistant patients in the AURA3 study (54). ALK gene fusion was detected in the FLAURA study (59). Schrock et al (89) identified BRAF, ALK, RET and FGFR3 gene fusions in 31 patients with osimertinib resistance. One of the patients with the T790M mutation had disease progression after osimertinib treatment. Analysis of tumor and blood-based ctDNA samples by NGS revealed a PLEKHA7-ALK gene fusion. Combined treatment of osimertinib and the ALK inhibitor alectinib alleviated the disease. A blood-based ctDNA test 4 months later revealed the disappearance of the PLEKHA7-ALK gene fusion (89). Batra et al (90) reported the case of a patient who developed echinoderm microtubule-associated protein like 4-ALK fusion after osimertinib resistance, and revealed that further treatment with the combination of osimertinib and alectinib was effective, suggesting that the oncogenic gene fusion might contribute to EGFR-TKI resistance (90).
5. Strategies to overcome resistance
Scientists are exploring approaches to overcome the different mechanisms of 3rd-generation EGFR-TKI resistance. Combination of targeted therapies is a promising strategy. For the reversion of 3rd-generation EGFR-TKI resistance, possible strategies to overcome different mechanisms are shown in Fig. 5. Some representative 4th-generation EGFR-TKIs are summarized in Table I (91-95). Case reports and ongoing clinical trials exploring the emerging combination are shown in Table II (84,89,96-104).
Figure 5.
Possible overcome strategies to the 3rd-generation EGFR-TKI resistance in advanced EGFR-mutated NSCLC. For patients who developed resistance to the 3rd-generation EGFR-TKI after 1st- or 2nd-line treatment with osimertinib, according to the status of the C797S mutation, different treatment regimens can be adopted. If the C797S and T790M mutations are in the cis structure, a combination of cetuximab with brigatinib or EAI045 is effective, while C797S and T790M mutations in trans are sensitive to a combination of 1st- and 3rd-generation TKIs. For NSCLC with C797S mutation but without T790M mutation, 1st- or 2nd-generation EGFR-TKIs are promising agents. For other heterogeneous resistance mechanisms, combinations of 3rd-generation EGFR-TKIs and other targeted agents, such as MEK inhibitors, MET inhibitors and HER2 inhibitors, are effective treatment options. gen, generation; act, activation; EGFR-TKI, epidermal growth factor receptor tyrosine kinase inhibitor; MET, hepatocyte growth factor receptor; HER2, human epidermal growth factor receptor 2; AXL, anexelekto.
Table II.
Combined therapies to overcome 3rd-generation EGFR-tyrosine kinase inhibitor resistance.
| Targeted resistance | Target | Combined strategies | Primary outcomes | Clinical trial identifier | (Refs.) |
|---|---|---|---|---|---|
| EGFR-dependent resistance | |||||
| EGFR C797S, in cis | C797S | Brigatinib + cetuximab | mPFS time of 14 months and an ORR of 60% | None | (96) |
| Osimertinib + bevacizumab + brigatinib | PR after 1 month of treatment and had fewer EGFR mutations | None | (97) | ||
| EGFR C797S, in trans | C797S | Osimertinib + erlotinib | PR after 1 month of treatment, PD after 3 months | None | (98) |
| Osimertinib + geftinib | Clinical improvement within 3 days, PD after 1 month | None | Recruiting | ||
| EGFR-independent resistance | |||||
| MET amplification | c-Met | Osimertinib + crizotinib | PR after 1 month of treatment | None | (99) |
| Osimertinib + savolitinib | Safety (DLTs and incidence of AEs) and efficacy (ORR, PFS, DCR and OS) | NCT02143466 | (100) | ||
| Nazartinib + INC280 | Safety (DLTs and MTD) and efficacy (ORR and PFS) | NCT02335944 | None | ||
| HER2 amplification | HER2 | Osimertinib + trastuzumab | Safety (intensity and incidence of AEs) and efficacy (ORR, PFS, DCR and OS) | NCT03784599 | None |
| Osimertinib + necitumumab + trastuzumab | Safety (intensity and incidence of AEs) and efficacy (ORR, PFS and OS) | NCT04285671 | None | ||
| AXL amplification | AXL | Osimertinib + DS-1205c | Safety (DLTs and incidence of AEs) and efficacy (ORR, PFS, DCR and OS) | NCT03255083 | None |
| MEK | Osimertinib + selumetinib | Safety (DLTs and incidence of AEs) and efficacy (ORR, PFS, DCR and OS) | NCT02143466 | None | |
| RAS/RAF/MEK/ERK pathway aberrant activation | |||||
| BRAF mutation | BRAF | Dabrafenib + trametinib | ORR of 63% and a DCR of 79% | None | (101) |
| BRAF | Dabrafenib + trametinib | Safety (intensity and incidence of AEs) and efficacy (ORR, PFS, DCR and OS) | NCT04452877/N CT04507919 | None | |
| mTOR | Osimertinib + sapanisertib | Safety (DLTs and incidence of AEs) and efficacy (ORR, PFS, DCR and OS) | NCT02503722 | None | |
| JAK/STAT3 | JAK | Osimertinib + itacitinib | Safety (intensity and incidence of AEs) and efficacy (ORR, PFS and OS) | NCT02917993 | None |
| Osimertinib + AZD4205 | Safety (incidence of AEs) and efficacy (ORR) | NCT03450330 | None | ||
| Histological transformation | |||||
| SCLC transformation | None | Etoposide + cisplatin | Responded well to chemotherapy | None | (102) |
| Etoposide + cisplatin | Clinical response rate of 54% and an estimated mPFS time of 3.4 months | None | (84) | ||
| Others | |||||
| CCDC6-RET fusion | RET | Osimertinib + BLU-667 | PR after 2 months of treatment with grade 1 toxicities | None | (103) |
| PLEKHA7-ALK fusion | ALK | Osimertinib + alectinib | PR and a duration of response of 6 months | None | (89) |
| CDK4, CDK6 amplification | CDK4/6 | Osimertinib + G1T38 | Safety (DLTs and incidence of AEs) and efficacy (PFS and OS) | NCT03455829 | None |
| Unknown | VEGFR | Osimertinib + apatinib | Optimal dosage, safety (intensity and incidence of AEs) and efficacy (PFS and OS) | NCT03050411 | None |
| Osimertinib+ ramucirumab | Safety (intensity and incidence of AEs) and efficacy (CR, PR, SD, PFS and OS) | NCT02411448 | None | ||
| VEGF | Osimertinib + bevacizumab | Safety (MTD and intensity and incidence of AEs) and efficacy (ORR, PFS and OS) | NCT02803203/N CT02971501 | None | |
| BCL-2 | Osimertinib + navitoclax | Safety (intensity and incidence of AEs) and efficacy (ORR) | NCT02520778 | None | |
| BIM | Osimertinib + aspirin | The mPFS time was 15.3 and 9.3 months for the combination therapy and osimertinib groups, respectively. | None | (104) |
mPFS, median progression free survival; ORR, objective response rate; PR, partial response; PD, progressive disease; DLTs, dose limiting toxicities; AEs, adverse events; DCR, disease control rate; OS, overall survival; VEGFR, vascular endothelial growth factor receptor; CR, complete response; SD, stable disease; MTD, maximum tolerated dose; BIM, B-cell lymphoma-2 (BCL-2)-like 11; MET, hepatocyte growth factor receptor; HER2, human epidermal growth factor receptor 2; AXL, anexelekto; EGFR, epidermal growth factor receptor.
Targeting EGFR tertiary mutations
Fourth-generation EGFR-TKIs
If the C797S and T790M mutations are cis-structured (located in the same allele; 85%), patients are resistant to 1st-, 2nd- and 3rd-generation EGFR-TKIs (105). The 4th-generation EGFR-TKI, EAI045, is the first selective, non-ATP-competitive allosteric TKI targeting the L858R, T790M and C797S mutations (Fig. 1). However, EGFR dimerization affects the efficacy of allosteric EGFR inhibitors. EAI045 is ineffective when used alone. Cetuximab is a chimeric monoclonal antibody that inhibits EGFR dimerization (91). EAI045 in combination with cetuximab was demonstrated to significantly inhibit the proliferation of lung cancer cells with EGFR L858R/T790M/C797S mutations both in vitro and in vivo. However, it was ineffective against drug resistance caused by EGFR Ex19del/C797S/T790M mutation (91). Brigatinib, an ALK and EGFR inhibitor, was effective against both EGFR Ex19del/C797S/T790M and L858R/C797S/T790M mutant cells in vitro and in vivo (96). Uchibori et al (92) demonstrated that brigatinib fits into the ATP-binding pocket of triple-mutant EGFR. The structure-activity relationship analysis revealed that the chloro, phosphine oxide group and the methoxy group of brigatinib worked as key components to inhibit the triple-mutant EGFR. The efficacy of brigatinib was significantly enhanced when used in combination with anti-EGFR antibody due to the reduction of surface and total EGFR expression (92). A recent study reported the cases of 15 osimertinib-resistant patients with EGFR T790M-cis-C797S mutation, 5 of whom were treated with a combination of brigatinib and cetuximab, and the remainder of whom received cisplatin-based doublet chemotherapy. The median PFS time was 14 and 3 months, respectively, with an ORR of 60 and 10%, respectively (96). Blu-945 is a 4th-generation EGFR-TKI designed to target EGFR T790M/C797S and T790M-resistant mutations. A recent study reported that BLU-945 alone or in combination with osimertinib significantly inhibited osimertinib-resistant lung cancer cell growth both in vitro and in vivo (93). TQB3804 is a 4th-generation EGFR-TKI developed in China (Fig. 1). A preclinical study showed that TQB3804 exhibits a prominent inhibitory effect on the Ex19del/T790M/C797S and L858R/T790M/C797S triple mutations (94). A phase I clinical trial of TQB3804 is currently recruiting (NCT04128085). More clinical trials are required to validate the efficacy of the novel EGFR-TKIs in patients with advanced NSCLC.
Combination of the 1st- and 3rd-generation EGFR-TKIs
When EGFR C797S and T790M mutations are in the trans structure (on separate alleles; 10%), the tumor is resistant to 3rd-generation TKIs, but sensitive to combined therapy with 1st- and 3rd-generation TKIs (105). Erlotinib combined with osimertinib was effective for a patient developing T790M and C797S trans mutations after osimertinib treatment. However, resistance reappeared 3 months later and liquid biopsies showed T790M and C797S mutations in cis (106). An ongoing phase I/II study investigated the safety and efficacy of osimertinib plus gefitinib as the 1st-line treatment for EGFR-mutated NSCLC. The adverse events were tolerable, and the ORR was 85.2% (95% CI, 67.5-94.1), consistent with the 1st-line ORR to osimertinib. The median PFS time was not yet reached (107).
1st- or 2nd-generation EGFR-TKIs
In vitro studies have shown that NSCLC cells with EGFR Ex19del/C797S mutation and without EGFR T790M mutation are resistant to 3rd-generation EGFR-TKIs, but remain sensitive to 1st- or 2nd-generation EGFR-TKIs (105). One patient with EGFR Ex19del/C797S mutation had a partial tumor remission after gefitinib treatment (108). Rangachari et al (109) demonstrated that osimertinib-resistant NSCLC with EGFR C797S mutation responded transiently to the 1st-generation EGFR-TKIs, but acquired EGFR T790M/C797S mutation eventually (109). Yang et al (110) reported that afatinib was effective against EGFR L858R/L718Q mutation with T790M loss after osimertinib resistance, indicating that 2nd-generation EGFR-TKIs might be promising agents for selected osimertinib-resistant patients.
Targeting EGFR-independent resistance
Bypassing pathway inhibitors
Against drug resistance due to MET or HER2 amplification, use of c-Met or HER2 inhibitors alone or in combination with the 3rd-generation EGFR-TKIs is a potential therapeutic strategy. Romaniello et al (111) demonstrated that the combination of cetuximab, trastuzumab and osimertinib inhibited ERK activation and reduced the abundance of c-Met, AXL and HER3 in vitro and in vivo, suppressing tumor growth and preventing recurrence (111). An ongoing phase I study demonstrated that patritumab deruxtecan (a HER3-directed antibody drug conjugate) had good efficacy in patients with various types of osimertinib resistance, including EGFR C797S, MET amplification and HER2 mutations (112). The combination of crizotinib and osimertinib substantially reduced tumor size after a 1-month treatment in a patient with MET amplification (99). In a multicenter, open-label, phase Ib TATTON study, osimertinib in combination with the c-Met inhibitor savolitinib was used to treat patients with advanced NSCLC harboring EGFR mutations and MET amplification after the 3rd-generation EGFR-TKI treatment, and encouraging results were obtained. In 69 patients with disease progression on the 3rd-generation EGFR-TKI treatments, the ORR was 30% (20-43) and the median PFS time was 5.4 months (95% CI, 4.1-8.0) (100). In addition, amivantamab (EGFR-MET bispecific antibody) with lazertinib has demonstrated synergistic inhibition of tumor growth in preclinical studies. Ongoing studies are exploring the safety and preliminary efficacy of this combined therapy in patients with disease progression upon osimertinib treatment (113).
Currently, the studies on AXL inhibitors are mainly at the preclinical stages, and there are few clinical trials. Okura et al (114) demonstrated that the novel AXL inhibitor ONO-7475 increased the sensitivity of AXL-overexpressing NSCLC cells to osimertinib and suppressed the emergence of osimertinib-resistant cells in vitro (114). Kim et al (115) found that promoting AXL degradation in combination with osimertinib delayed or overcame osimertinib resistance in EGFR-mutant patient-derived xenograft models (115). In addition, the newly developed drug CB469 inhibited both AXL and c-Met activation and phosphorylation, thereby overcoming acquired resistance to EGFR-TKIs mediated by AXL and c-Met activation both in vitro and in vivo (116).
Downstream signaling pathway inhibitors
For drug resistance caused by RAS/RAF/MEK/ERK signaling pathway activation, 3rd-generation EGFR-TKIs combined with MEK inhibitors are potential therapeutic strategies. Preclinical studies demonstrated that the combination of osimertinib and selumetinib overcame the resistance caused by KRAS amplification and overexpression (83). In another in vitro study, MEK inhibitors restored the sensitivity of EGFR ex19del/T790M/C797S-mutated PC9 cells to osimertinib by modulating MEK/ERK-dependent degradation of BIM and MCL-1 (117). Osimertinib and MEK/ERK inhibitors synergistically promoted apoptosis and inhibited survival in osimertinib-resistant cells, and this combination could delay the emergence of osimertinib resistance both in vitro and in vivo (118). In 2017, the FDA approved the combination of dabrafenib (BRAF V600E inhibitor) plus trametinib (MEK inhibitor) for the treatment of patients with BRAF V600E mutations, with an ORR of 63 and 61%, respectively, for the 1st- and 2nd-line treatments (101). Xie et al (119) demonstrated that osimertinib combined with vemurafenib was effective to overcome BRAF V600E-mediated osimertinib resistance (119). More clinical trials are required to validate the roles of RAS/RAF/MEK/ERK signaling pathway inhibitors in osimertinib-resistant patients.
Targeting EMT- and BIM-mediated resistance
In a recent study, the 3rd-generation EGFR inhibitor WZ-4002 and the FGFR inhibitor dovitinib synergistically inhibited EMT-mediated resistant cell survival and prevented the generation of resistant clones. It was shown that FGFR signaling is critical for the emergence of mesenchymal-like drug tolerant clones. Dual EGFR plus FGFR inhibition might be a promising strategy to avoid resistance (120). Yochum et al (121) reported that the transcription factor TWIST1 inhibitor harmine and the Bcl-2/BclXL inhibitor ABT737 were able to target the EMT transcription factor TWIST1 in EGFR-mutant NSCLC. The study demonstrated that downregulated TWIST1 and upregulated BIM could overcome EMT- and BIM-mediated drug resistance (121). Another recent study demonstrated that combined treatment with osimertinib and aspirin promoted BIM-dependent apoptosis in osimertinib-resistant lung cancer cells. A retrospective analysis of 45 patients with NSCLC also confirmed that the median PFS time of the osimertinib plus aspirin group was significantly longer than that of the osimertinib alone group (104).
Targeting EGFR 20 exon insertion mutations
A recent study included 693 patients with EGFR e20ins and uncommon mutations (G719X, L861Q and S768). Afatinib was demonstrated to have broad activity against uncommon EGFR mutations and several e20ins, with an ORR of 60.0 and 24.3%, respectively (122). In vitro studies showed that cetuximab combined with afatinib was specifically sensitive to EGFR e20ins tumors, and this was also confirmed in clinical studies (123-125). Fang et al (123) reported that afatinib and cetuximab achieved long-term disease control in a patient with NSCLC and a rare EGFR e20ins. Poziotinib is a potent inhibitor of EGFR and HER2 e20ins mutations; it can effectively inhibit EGFR e20ins cells in vitro with 100-fold greater potency than osimertinib. In a phase II clinical study, the ORR of poziotinib in patients with NSCLC and EGFR e20ins was 64%, and the median OS was not achieved (46). In addition, the promising clinical activity of amivantamab and mobocertinib (oral EGFR/HER2 inhibitor) in ongoing phase I/II studies also provides hope for the treatment of patients with EGFR e20ins in future (126,127).
Other strategies
Vascular endothelial growth factor receptor (VEGFR) inhibitors
VEGFR inhibitors have synergistic antitumor effects with EGFR inhibitors. Phase I/II clinical trials of osimertinib in combination with ramucirumab (128) or bevacizumab (129) in patients with advanced NSCLC and EGFR mutations are ongoing. In addition, an open-label, phase II, multicenter, single-arm trial is exploring the efficacy of afatinib plus bevacizumab in patients with EGFR-mutant NSCLC and osimertinib resistance (130).
Immunotherapy
A retrospective analysis showed that anti-programmed cell death-1 (PD-1) monotherapy was poorly effective in EGFR-mutated patients, even for patients with PD-L1 expression ≥50% (131). In phase Ib TATTON study, osimertinib in combination with durvalumab had an ORR of 67% in patients with EGFR T790M mutation. However, the incidence of interstitial lung disease was 38% (132). The osimertinib plus durvalumab combination arm of the TATTON study was suspended due to severe adverse events. Immunotherapy combined with chemotherapy may provide new hope for patients resistant to the 3rd-generation EGFR-TKIs. A recent study reported that combined therapy of toripalimab (anti-PD-1 anti-body) and pemetrexed/carboplatin achieved a partial response for >8 months in a osimertinib-resistant patient. A phase II clinical trial of toripalimab combined with chemotherapy in patients without EGFR T790M mutation achieved an ORR of 50% and a median PFS time of 7.0 months (133). Clinical trials of nivolumab (anti-PD-1 antibody) in combination with chemotherapy or ipilimumab (anti-cytotoxic T lymphocyte-associated antigen-4 antibody) (NCT02864251) and pembrolizumab (anti-PD-1 antibody) in combination with chemotherapy (NCT03515837) in patients who were resistant to the 1st- or 2nd-line treatment of osimertinib are currently underway.
Chemotherapy
For patients who developed SCLC transformation, chemotherapy after osimertinib resistance is an option. Marcoux et al (84) demonstrated that patients with SCLC transformation had higher response rates to etoposide, cisplatin and paclitaxel. For patients with unknown resistant mechanisms, chemotherapy is still an option. If the patient is asymptomatic or has symptomatic local progression, osimertinib can be combined with local treatment according to National Comprehensive Cancer Network (NCCN) guidelines (134). Furthermore, carboplatin/paclitaxel/bevacizumab/atezolizumab (anti-PD-L1 antibody) are also options for patients who experience systemic progression after osimertinib treatment (135). Whether chemotherapy can delay resistance to the 3rd-generation EGFR-TKIs remains unknown. A study on osimertinib with or without chemotherapy as the 1st-line treatment in patients with EGFR-mutated NSCLC is currently recruiting (NCT04035486).
6. Conclusions and perspectives
The resistance mechanism of 3rd-generation EGFR-TKIs for advanced NSCLC is very complex. The mechanism of 3rd-generation EGFR-TKI resistance in EGFR-mutated tumors is different between patients, and heterogeneous among different tumor sites. Therefore, NGS of blood-based ctDNA or tissue samples to clarify the resistance mechanism is of importance to guide the next treatment, which will be conducive to the clinical research of novel combined therapy to overcome drug resistance. According to the NCCN guidelines (version 4. 2021) (136), radical local therapy combined with osimertinib continuation should be considered for patients who progressed on osimertinib, except those who developed extensive progression after resistance, for whom platinum-based two-drug chemotherapy with or without bevacizumab is recommended. To improve survival in patients with EGFR mutations, a range of new therapies targeting different resistance mechanisms are being developed. 4th-generation EGFR-TKIs have emerged, such as EAI045, TQB3804 and BLU-945, and the multi-target TKI brigatinib, showing high potential and being worthy of attention with regard to targeting EGFR T790M/C797S mutation. EAI045 is a representative of allosteric EGFR-mutant selective compounds. A number of other allosteric EGFR inhibitors, including JBJ-04-125-02 and DDC-01-163, are currently in development. Studies have suggested that EGFR dimerization affects the efficacy of allosteric EGFR inhibitors, so it is more effective when allosteric EGFR inhibitors are combined with osimertinib or EGFR-targeted monoclonal antibodies (cetuximab), than when used as single agents. Furthermore, the allogeneic EGFR inhibitors have potential to improve the clinical efficacy of osimertinib and delay the emergence of mutant EGFR-mediated resistance in NSCLC (91,137,138). Receptor structure-based drug design and library screening provides novel allogeneic EGFR inhibitors. Combined therapy of allogeneic EGFR inhibitors with conventional EGFR-TKIs, EGFR-targeted monoclonal antibodies, chemotherapy or immunotherapy offers an effective treatment option for patients with 3rd-generation EGFR-TKI-resistant NSCLC. More preclinical and clinical studies are required to validate the efficacy of combined therapies. The combinations of EGFR-TKIs with agents targeting aberrant EGFR bypassing pathways, as well as downstream pathways, are also promising to overcome EGFR-TKI resistance. Mutations in some EGFR loci are insensitive to conventional EGFR-TKIs, and the emergence of new agents such as mobocertinib, amivantamab and poziotinib have shown promising efficacy against EGFR e20ins. Recent studies have shown that chemotherapy plus EGFR-TKIs or bevacizumab is superior to chemotherapy alone in SCLC-transformed EGFR-mutant lung adenocarcinoma. In addition, the rapid development of immunotherapy has brought new hope for EGFR-TKI resistant patients. The combination of immune checkpoint inhibitors, anti-angiogenic agents and chemotherapy may be a promising research direction in future. Although the combination of immunotherapy and EGFR-TKIs has achieved satisfactory efficacy, serious adverse events have hindered further promotion of clinical trials. The manner in which the occurrence of adverse reactions can be reduced in the process of combination therapy is an important concern. Most combined therapies are still under development, several of which have shown satisfactory results at the preclinical stage, but failed in clinical trials. Therefore, more studies should be conducted in the future to improve our understanding of the complex signaling pathways involved in the resistance to 3rd-generation EGFR-TKIs and to find the best combination treatments modality to overcome the 3rd-generation EGFR-TKI resistance under the guidance of NGS.
Acknowledgments
The authors acknowledge that the 2D structures of different EGFR-TKIs were sourced from https://pubchem.ncbi.nlm.nih.gov/(Gifitinib: CID 123631; https://pubchem.ncbi.nlm.nih.gov/compound/123631#section=2D-Structure; Erlotinib: CID 176870; https://pubchem.ncbi.nlm.nih.gov/compound/176870#section=2D-Structure; Icotinib: CID 22024915; https://pubchem.ncbi.nlm.nih.gov/compound/22024915#section=2D-Structure; Afatinib: CID 10184653; https://pubchem.ncbi.nlm.nih.gov/compound/10184653#section=2D-Structure; Dacomitinib: CID 11511120; https://pubchem.ncbi.nlm.nih.gov/compound/11511120#section=2D-Structure; Osimertinib: CID 71496458; https://pubchem.ncbi.nlm.nih.gov/compound/71496458#section=2D-Structure; Rociletinib, CID 57335384; https://pubchem.ncbi.nlm.nih.gov/compound/57335384#section=2D-Structure; Abivertinib: CID 72734520; https://pubchem.ncbi.nlm.nih.gov/compound/72734520#section=2D-Structure; Nazartinib: CID 72703790; https://pubchem.ncbi.nlm.nih.gov/compound/72703790#section=2D-Structure; Olmutinib: CID 54758501; https://pubchem.ncbi.nlm.nih.gov/compound/54758501#section=2D-Structure; Alflutinib: CID 118861389; https://pubchem.ncbi.nlm.nih.gov/compound/118861389#section=2D-Structure; Almonertinib: CID 121280087; https://pubchem.ncbi.nlm.nih.gov/compound/121280087#section=2D-Structure; Lazertinib: CID 121269225; https://pubchem.ncbi.nlm.nih.gov/compound/121269225#section=2D-Structure; Lazertinib: CID 121269225; https://pubchem.ncbi.nlm.nih.gov/compound/121269225#section=2D-Structure; Naquotinib: CID 71667668; https://pubchem.ncbi.nlm.nih.gov/compound/71667668#section=2D-Structure; EAI045: CID 121231412; https://pubchem.ncbi.nlm.nih.gov/compound/121231412#section=2D-Structure; TQB3804: CID 138911391; https://pubchem.ncbi.nlm.nih.gov/compound/138911391#section=2D-Structure; Brigatinib: CID 68165256; https://pubchem.ncbi.nlm.nih.gov/compound/68165256#section=2D-Structure).
Abbreviations
- AXL
anexelekto
- BIM
B-cell lymphoma-2-like 11
- CNS
central nervous system
- ctDNA
circulating tumor DNA
- del
deletion
- EGFR e20ins
EGFR exon 20 insertions
- EGFR-TKI
epidermal growth factor receptor-tyrosine kinase inhibitor
- EMT
epithelial-mesenchymal transition
- Ex19del
exon 19 deletion
- FDA
Food and Drug Administration
- FGF2
fibroblast growth factor 2
- FGFR
fibroblast growth factor receptor
- HER2
human epidermal growth factor receptor 2
- IGF1R
insulin-like growth factor 1 receptor
- c-Met
hepatocyte growth factor receptor
- NGS
next-generation sequencing
- NSCLC
non-small cell lung cancer
- ORR
objective response rate
- OS
overall survival
- PD-1
programmed cell death-1
- PD-L1
programmed cell death-ligand-1
- PFS
progression free survival
- PIK3CA
phosphatidylinositol-3-kinase catalytic α
- SCC
squamous cell carcinoma
- VEGFR
vascular endothelial growth factor receptor
Funding Statement
This study was supported by the National Natural Science Foundation of China (grant nos. 81773236, 81800429 and 81972852), the Key Research & Development Project of Hubei Province (grant no. 2020BCA069), the Nature Science Foundation of Hubei Province (grant no. 2020CFB612), the Health Commission of Hubei Province Medical Leading Talent Project, Young and Middle-Aged Medical Backbone Talents of Wuhan (grant no. WHQG201902), the Application Foundation Frontier Project of Wuhan (grant no. 2020020601012221), the Zhongnan Hospital of Wuhan University Science, Technology and Innovation Seed Fund (grant nos. znpy2019001 and znpy 2019048) and the Translational Medicine and Interdisciplinary Research Joint Fund of Zhongnan Hospital of Wuhan University (grant nos. ZNJC201922 and ZNJC202007).
Availability of data and materials
Not applicable.
Authors' contributions
JH, YG and CX were responsible for the study conception and design. Administrative support was provided by YG and CX. JH and ZH contributed to the provision of study materials or patients. JH, ZH and LH collected and assembled the data, and JH, ZH and LH were responsible for data analysis and interpretation. All authors helped to write the manuscript and have read and approved the final manuscript. Data authentication is not applicable.
Ethics approval and consent to participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
References
- 1.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2020. CA Cancer J Clin. 2020;70:7–30. doi: 10.3322/caac.21590. [DOI] [PubMed] [Google Scholar]
- 2.Testa U, Castelli G, Pelosi E. Lung cancers: Molecular characterization, clonal heterogeneity and evolution, and cancer stem cells. Cancers (Basel) 2018;10:248. doi: 10.3390/cancers10080248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rosell R, Moran T, Queralt C, Porta R, Cardenal F, Camps C, Majem M, Lopez-Vivanco G, Isla D, Provencio M, et al. Screening for epidermal growth factor receptor mutations in lung cancer. New Engl J Med. 2009;361:958–967. doi: 10.1056/NEJMoa0904554. [DOI] [PubMed] [Google Scholar]
- 4.da Cunha Santos G, Shepherd FA, Tsao MS. EGFR mutations and lung cancer. Annu Rev Pathol. 2011;6:49–69. doi: 10.1146/annurev-pathol-011110-130206. [DOI] [PubMed] [Google Scholar]
- 5.Roskoski R., Jr Small molecule inhibitors targeting the EGFR/ErbB family of protein-tyrosine kinases in human cancers. Pharmacol Res. 2019;139:395–411. doi: 10.1016/j.phrs.2018.11.014. [DOI] [PubMed] [Google Scholar]
- 6.Huang L, Fu L. Mechanisms of resistance to EGFR tyrosine kinase inhibitors. Acta Pharm Sin B. 2015;5:390–401. doi: 10.1016/j.apsb.2015.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mok TS, Wu YL, Thongprasert S, Yang CH, Chu DT, Saijo N, Sunpaweravong P, Han B, Margono B, Ichinose Y, et al. Gefitinib or carboplatin-paclitaxel in pulmonary adenocarcinoma. N Engl J Med. 2009;361:947–957. doi: 10.1056/NEJMoa0810699. [DOI] [PubMed] [Google Scholar]
- 8.Kazandjian D, Blumenthal GM, Yuan W, He K, Keegan P, Pazdur R. FDA approval of gefitinib for the treatment of patients with metastatic EGFR mutation-positive non-small cell lung cancer. Clin Cancer Res. 2016;22:1307–1312. doi: 10.1158/1078-0432.CCR-15-2266. [DOI] [PubMed] [Google Scholar]
- 9.Rosell R, Carcereny E, Gervais R, Vergnenegre A, Massuti B, Felip E, Palmero R, Garcia-Gomez R, Pallares C, Sanchez JM, et al. Erlotinib versus standard chemotherapy as first-line treatment for European patients with advanced EGFR mutation-positive non-small-cell lung cancer (EURTAC): A multicentre, open-label, randomised phase 3 trial. Lancet Oncol. 2012;13:239–246. doi: 10.1016/S1470-2045(11)70393-X. [DOI] [PubMed] [Google Scholar]
- 10.Shi YK, Wang L, Han BH, Li W, Yu P, Liu YP, Ding CM, Song X, Ma ZY, Ren XL, et al. First-line icotinib versus cisplatin/pemetrexed plus pemetrexed maintenance therapy for patients with advanced EGFR mutation-positive lung adenocarcinoma (CONVINCE): A phase 3, open-label, randomized study. Ann Oncol. 2017;28:2443–2450. doi: 10.1093/annonc/mdx359. [DOI] [PubMed] [Google Scholar]
- 11.Park K, Tan EH, O'Byrne K, Zhang L, Boyer M, Mok T, Hirsh V, Yang JC, Lee KH, Lu S, et al. Afatinib versus gefitinib as first-line treatment of patients with EGFR mutation-positive non-small-cell lung cancer (LUX-Lung 7): A phase 2B, open-label, randomised controlled trial. Lancet Oncol. 2016;17:577–589. doi: 10.1016/S1470-2045(16)30033-X. [DOI] [PubMed] [Google Scholar]
- 12.Wu YL, Cheng Y, Zhou X, Lee KH, Nakagawa K, Niho S, Tsuji F, Linke R, Rosell R, Corral J, et al. Dacomitinib versus gefitinib as first-line treatment for patients with EGFR-mutation-positive non-small-cell lung cancer (ARCHER 1050): A randomised, open-label, phase 3 trial. Lancet Oncol. 2017;18:1454–1466. doi: 10.1016/S1470-2045(17)30608-3. [DOI] [PubMed] [Google Scholar]
- 13.Miller VA, Hirsh V, Cadranel J, Chen YM, Park K, Kim SW, Zhou C, Su WC, Wang M, Sun Y, et al. Afatinib versus placebo for patients with advanced, metastatic non-small-cell lung cancer after failure of erlotinib, gefitinib, or both, and one or two lines of chemotherapy (LUX-Lung 1): A phase 2b/3 randomised trial. Lancet Oncol. 2012;13:528–538. doi: 10.1016/S1470-2045(12)70087-6. [DOI] [PubMed] [Google Scholar]
- 14.Ellis PM, Shepherd FA, Millward M, Perrone F, Seymour L, Liu G, Sun S, Cho BC, Morabito A, Leighl NB, et al. Dacomitinib compared with placebo in pretreated patients with advanced or metastatic non-small-cell lung cancer (NCIC CTG BR.26): A double-blind, randomised, phase 3 trial. Lancet Oncol. 2014;15:1379–1388. doi: 10.1016/S1470-2045(14)70472-3. [DOI] [PubMed] [Google Scholar]
- 15.Westover D, Zugazagoitia J, Cho BC, Lovly CM, Paz-Ares L. Mechanisms of acquired resistance to first- and second-generation EGFR tyrosine kinase inhibitors. Ann Oncol. 2018;29(Suppl-1):i10–i19. doi: 10.1093/annonc/mdx703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Passaro A, Guerini-Rocco E, Pochesci A, Vacirca D, Spitaleri G, Catania CM, Rappa A, Barberis M, de Marinis F. Targeting EGFR T790M mutation in NSCLC: From biology to evaluation and treatment. Pharmacol Res. 2017;117:406–415. doi: 10.1016/j.phrs.2017.01.003. [DOI] [PubMed] [Google Scholar]
- 17.Mok TS, Wu YL, Ahn MJ, Garassino MC, Kim HR, Ramalingam SS, Shepherd FA, He Y, Akamatsu H, Theelen WS, et al. Osimertinib or platinum-pemetrexed in EGFR T790M-positive lung cancer. New Engl J Med. 2017;376:629–640. doi: 10.1056/NEJMoa1612674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cross DA, Ashton SE, Ghiorghiu S, Eberlein C, Nebhan CA, Spitzler PJ, Orme JP, Finlay MR, Ward RA, Mellor MJ, et al. AZD9291, an irreversible EGFR TKI, overcomes T790M-mediated resistance to EGFR inhibitors in lung cancer. Cancer Discov. 2014;4:1046–1061. doi: 10.1158/2159-8290.CD-14-0337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Soria JC, Ohe Y, Vansteenkiste J, Reungwetwattana T, Chewaskulyong B, Lee KH, Dechaphunkul A, Imamura F, Nogami N, Kurata T, et al. Osimertinib in untreated EGFR-mutated advanced non-small-cell lung cancer. New Engl J Med. 2018;378:113–125. doi: 10.1056/NEJMoa1713137. [DOI] [PubMed] [Google Scholar]
- 20.Sequist LV, Soria JC, Camidge DR. Update to rociletinib data with the RECIST confirmed response rate. New Engl J Med. 2016;374:2296–2297. doi: 10.1056/NEJMc1602688. [DOI] [PubMed] [Google Scholar]
- 21.Yun J, Hong MH, Kim SY, Park CW, Kim S, Yun MR, Kang HN, Pyo KH, Lee SS, Koh JS, et al. YH25448, an irreversible EGFR-TKI with potent intracranial activity in EGFR mutant non-small cell lung cancer. Clin Cancer Res. 2019;25:2575–2587. doi: 10.1158/1078-0432.CCR-18-2906. [DOI] [PubMed] [Google Scholar]
- 22.Xu X, Mao L, Xu W, Tang W, Zhang X, Xi B, Xu R, Fang X, Liu J, Fang C, et al. AC0010, an irreversible EGFR inhibitor selectively targeting mutated EGFR and overcoming T790M-induced resistance in animal models and lung cancer patients. Mol Cancer Ther. 2016;15:2586–2597. doi: 10.1158/1535-7163.MCT-16-0281. [DOI] [PubMed] [Google Scholar]
- 23.Jia Y, Juarez J, Li J, Manuia M, Niederst MJ, Tompkins C, Timple N, Vaillancourt MT, Pferdekamper AC, Lockerman EL, et al. EGF816 exerts anticancer effects in non-small cell lung cancer by irreversibly and selectively targeting primary and acquired activating mutations in the EGF receptor. Cancer Res. 2016;76:1591–1602. doi: 10.1158/0008-5472.CAN-15-2581. [DOI] [PubMed] [Google Scholar]
- 24.Yu HA, Spira A, Horn L, Weiss J, West H, Giaccone G, Evans T, Kelly RJ, Desai B, Krivoshik A, et al. A phase I, dose escalation study of oral ASP8273 in patients with non-small cell lung cancers with epidermal growth factor receptor mutations. Clin Cancer Res. 2017;23:7467–7473. doi: 10.1158/1078-0432.CCR-17-1447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Park K, Lee JS, Lee KH, Kim JH, Min YJ, Cho JY, Han JY, Kim BS, Kim JS, Lee DH, et al. Updated safety and efficacy results from phase I/II study of HM61713 in patients (pts) with EGFR mutation positive non-small cell lung cancer (NSCLC) who failed previous EGFR-tyrosine kinase inhibitor (TKI) J Clin Oncol. 2015;33(Suppl 15):S8084. doi: 10.1200/jco.2015.33.15_suppl.8084. [DOI] [Google Scholar]
- 26.Sequist LV, Rolfe L, Allen AR. Rociletinib in EGFR-mutated non-small-cell lung cancer. New Engl J Med. 2015;373:578–579. doi: 10.1056/NEJMc1506831. [DOI] [PubMed] [Google Scholar]
- 27.Wang H, Pan R, Zhang X, Si X, Wang M, Zhang L. Abivertinib in patients with T790M-positive advanced NSCLC and its subsequent treatment with osimertinib. Thorac Cancer. 2020;11:594–602. doi: 10.1111/1759-7714.13302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tan DS, Leighl NB, Riely GJ, Yang JC, Sequist LV, Wolf J, Seto T, Felip E, Aix SP, Jonnaert M, et al. Safety and efficacy of nazartinib (EGF816) in adults with EGFR-mutant non-small-cell lung carcinoma: A multicentre, open-label, phase 1 study. Lancet Resp Med. 2020;8:561–572. doi: 10.1016/S2213-2600(19)30267-X. [DOI] [PubMed] [Google Scholar]
- 29.Kim DW, Lee DH, Han JY, Lee J, Cho BC, Kang JH, Lee KH, Cho EK, Kim JS, Min YJ, et al. Safety, tolerability, and anti-tumor activity of olmutinib in non-small cell lung cancer with T790M mutation: A single arm, open label, phase 1/2 trial. Lung Cancer. 2019;135:66–72. doi: 10.1016/j.lungcan.2019.07.007. [DOI] [PubMed] [Google Scholar]
- 30.Shi Y, Zhang S, Hu X, Feng J, Ma Z, Zhou J, Yang N, Wu L, Liao W, Zhong D, et al. Safety, clinical activity, and pharmacokinetics of alflutinib (AST2818) in patients with advanced NSCLC With EGFR T790M mutation. J Thorac Oncol. 2020;15:1015–1026. doi: 10.1016/j.jtho.2020.01.010. [DOI] [PubMed] [Google Scholar]
- 31.Yang JC, Camidge DR, Yang CT, Zhou J, Guo R, Chiu CH, Chang GC, Shiah HS, Chen Y, Wang CC, et al. Safety, efficacy, and pharmacokinetics of almonertinib (HS-10296) in pretreated patients with EGFR-mutated advanced NSCLC: A multicenter, open-label, phase 1 Trial. J Thorac Oncol. 2020;15:1907–1918. doi: 10.1016/j.jtho.2020.09.001. [DOI] [PubMed] [Google Scholar]
- 32.Ahn MJ, Han JY, Lee KH, Kim SW, Kim DW, Lee YG, Cho EK, Kim JH, Lee GW, Lee JS, et al. Lazertinib in patients with EGFR mutation-positive advanced non-small-cell lung cancer: Results from the dose escalation and dose expansion parts of a first-in-human, open-label, multicentre, phase 1-2 study. Lancet Oncol. 2019;20:1681–1690. doi: 10.1016/S1470-2045(19)30504-2. [DOI] [PubMed] [Google Scholar]
- 33.Kelly RJ, Shepherd FA, Krivoshik A, Jie F, Horn L. A phase III, randomized, open-label study of ASP8273 versus erlotinib or gefitinib in patients with advanced stage IIIB/IV non-small-cell lung cancer. Ann Oncol. 2019;30:1127–1133. doi: 10.1093/annonc/mdz128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mezquita L, Varga A, Planchard D. Safety of osimertinib in EGFR-mutated non-small cell lung cancer. Expert Opin Drug Saf. 2018;17:1239–1248. doi: 10.1080/14740338.2018.1549222. [DOI] [PubMed] [Google Scholar]
- 35.Ramalingam SS, Vansteenkiste J, Planchard D, Cho BC, Gray JE, Ohe Y, Zhou C, Reungwetwattana T, Cheng Y, Chewaskulyong B, et al. Overall Survival with Osimertinib in Untreated, -Mutated Advanced NSCLC. New Engl J Med. 2020;382:41–50. doi: 10.1056/NEJMoa1913662. [DOI] [PubMed] [Google Scholar]
- 36.Reungwetwattana T, Nakagawa K, Cho BC, Cobo M, Cho EK, Bertolini A, Bohnet S, Zhou C, Lee KH, Nogami N, et al. CNS response to osimertinib versus standard epidermal growth factor receptor tyrosine kinase inhibitors in patients with untreated EGFR-mutated advanced non-small-cell lung cancer. J Clin Oncol. 2018 Aug 28; doi: 10.1200/JCO.2018.78.3118. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 37.Saito H, Fukuhara T, Furuya N, Watanabe K, Sugawara S, Iwasawa S, Tsunezuka Y, Yamaguchi O, Okada M, Yoshimori K, et al. Erlotinib plus bevacizumab versus erlotinib alone in patients with EGFR-positive advanced non-squamous non-small-cell lung cancer (NEJ026): Interim analysis of an open-label, randomised, multicentre, phase 3 trial. Lancet Oncol. 2019;20:625–635. doi: 10.1016/S1470-2045(19)30035-X. [DOI] [PubMed] [Google Scholar]
- 38.Goldberg SB, Redman MW, Lilenbaum R, Politi K, Stinchcombe TE, Horn L, Chen EH, Mashru SH, Gettinger SN, Melnick MA, et al. Randomized trial of afatinib plus cetuximab versus afatinib alone for first-line treatment of -mutant non-small-cell lung cancer: Final results from SWOG S1403. J Clin Oncol. 2020;38:4076–4085. doi: 10.1200/JCO.20.01149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Oxnard GR, Yang JC, Yu H, Kim SW, Saka H, Horn L, Goto K, Ohe Y, Mann H, Thress KS, et al. TATTON: A multi-arm, phase Ib trial of osimertinib combined with selumetinib, savolitinib, or durvalumab in EGFR-mutant lung cancer. Ann Oncol. 2020;31:507–516. doi: 10.1016/j.annonc.2020.01.013. [DOI] [PubMed] [Google Scholar]
- 40.Sequist LV, Piotrowska Z, Niederst MJ, Heist RS, Digumarthy S, Shaw AT, Engelman JA. Osimertinib responses after disease progression in patients who had been receiving rociletinib. JAMA Oncol. 2016;2:541–543. doi: 10.1001/jamaoncol.2015.5009. [DOI] [PubMed] [Google Scholar]
- 41.Nagasaka M, Zhu VW, Lim SM, Greco M, Wu F, Ou SI. Beyond osimertinib: The development of third-generation EGFR tyrosine kinase inhibitors for advanced EGFR+ NSCLC. J Thorac Oncol. 2021;16:740–763. doi: 10.1016/j.jtho.2020.11.028. [DOI] [PubMed] [Google Scholar]
- 42.Ma Y, Zheng X, Zhao H, Fang W, Zhang Y, Ge J, Wang L, Wang W, Jiang J, Chuai S, et al. First-in-human phase I study of AC0010, a mutant-selective EGFR inhibitor in non-small cell lung cancer: Safety, efficacy, and potential mechanism of resistance. J Thorac Oncol. 2018;13:968–977. doi: 10.1016/j.jtho.2018.03.025. [DOI] [PubMed] [Google Scholar]
- 43.Wang H, Zhang L, Hu P, Zheng X, Si X, Zhang X, Wang M. Penetration of the blood-brain barrier by avitinib and its control of intra/extra-cranial disease in non-small cell lung cancer harboring the T790M mutation. Lung Cancer. 2018;122:1–6. doi: 10.1016/j.lungcan.2018.05.010. [DOI] [PubMed] [Google Scholar]
- 44.Kim ES. Olmutinib: First global approval. Drugs. 2016;76:1153–1157. doi: 10.1007/s40265-016-0606-z. [DOI] [PubMed] [Google Scholar]
- 45.Arcila ME, Nafa K, Chaft JE, Rekhtman N, Lau C, Reva BA, Zakowski MF, Kris MG, Ladanyi M. EGFR exon 20 insertion mutations in lung adenocarcinomas: Prevalence, molecular heterogeneity, and clinicopathologic characteristics. Mol Cancer Ther. 2013;12:220–229. doi: 10.1158/1535-7163.MCT-12-0620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Robichaux JP, Elamin YY, Tan Z, Carter BW, Zhang S, Liu S, Li S, Chen T, Poteete A, Estrada-Bernal A, et al. Mechanisms and clinical activity of an EGFR and HER2 exon 20-selective kinase inhibitor in non-small cell lung cancer. Nat Med. 2018;24:638–646. doi: 10.1038/s41591-018-0007-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Qin Y, Jian H, Tong X, Wu X, Wang F, Shao YW, Zhao X. Variability of EGFR exon 20 insertions in 24 468 Chinese lung cancer patients and their divergent responses to EGFR inhibitors. Mol Oncol. 2020;14:1695–1704. doi: 10.1002/1878-0261.12710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.van Veggel B, Madeira R, Santos JFV, Hashemi SMS, Paats MS, Monkhorst K, Heideman DAM, Groves M, Radonic T, Smit EF, et al. Osimertinib treatment for patients with EGFR exon 20 mutation positive non-small cell lung cancer. Lung Cancer. 2020;141:9–13. doi: 10.1016/j.lungcan.2019.12.013. [DOI] [PubMed] [Google Scholar]
- 49.Li X, Wang S, Li B, Wang Z, Shang S, Shao Y, Sun X, Wang L. BIM deletion polymorphism confers resistance to osimertinib in EGFR T790M lung cancer: A case report and literature review. Target Oncol. 2018;13:517–523. doi: 10.1007/s11523-018-0573-2. [DOI] [PubMed] [Google Scholar]
- 50.Zhao M, Zhang Y, Cai W, Li J, Zhou F, Cheng N, Ren R, Zhao C, Li X, Ren S, et al. The Bim deletion polymorphism clinical profile and its relation with tyrosine kinase inhibitor resistance in Chinese patients with non-small cell lung cancer. Cancer. 2014;120:2299–2307. doi: 10.1002/cncr.28725. [DOI] [PubMed] [Google Scholar]
- 51.Tanimoto A, Takeuchi S, Arai S, Fukuda K, Yamada T, Roca X, Ong ST, Yano S. Histone deacetylase 3 inhibition overcomes BIM deletion polymorphism-mediated osimertinib resistance in EGFR-mutant lung cancer. Clin Cancer Res. 2017;23:3139–3149. doi: 10.1158/1078-0432.CCR-16-2271. [DOI] [PubMed] [Google Scholar]
- 52.Isobe K, Hata Y, Tochigi N, Kaburaki K, Kobayashi H, Makino T, Otsuka H, Sato F, Ishida F, Kikuchi N, et al. Clinical significance of BIM deletion polymorphism in non-small-cell lung cancer with epidermal growth factor receptor mutation. J Thorac Oncol. 2014;9:483–487. doi: 10.1097/JTO.0000000000000125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Liu SY, Zhou JY, Li WF, Sun H, Zhang YC, Yan HH, Chen ZH, Chen CX, Ye JY, Yang JJ, et al. Concomitant genetic alterations having greater impact on the clinical benefit of EGFR-TKIs in EGFR-mutant advanced NSCLC than BIM deletion polymorphism. Clin Transl Med. 2020;10:337–345. doi: 10.1002/ctm2.12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Papadimitrakopoulou VA, Wu YL, Han JY, Ahn MJ, Ramalingam SS, John T, Okamoto I, Yang JCH, Bulusu KC, Laus G, et al. Analysis of resistance mechanisms to osimertinib in patients with EGFR T790M advanced NSCLC from the AURA3 study. Ann Oncol. 2018;29(Suppl 8):VIII741. doi: 10.1093/annonc/mdy424.064. [DOI] [Google Scholar]
- 55.Yang Z, Yang N, Ou Q, Xiang Y, Jiang T, Wu X, Bao H, Tong X, Wang X, Shao YW, et al. Investigating novel resistance mechanisms to third-generation EGFR tyrosine kinase inhibitor osimertinib in non-small cell lung cancer patients. Clin Cancer Res. 2018;24:3097–3107. doi: 10.1158/1078-0432.CCR-17-2310. [DOI] [PubMed] [Google Scholar]
- 56.Le X, Puri S, Negrao MV, Nilsson MB, Robichaux J, Boyle T, Hicks JK, Lovinger KL, Roarty E, Rinsurongkawong W, et al. Landscape of EGFR-dependent and -independent resistance mechanisms to osimertinib and continuation therapy beyond progression in -mutant NSCLC. Clin Cancer Res. 2018;24:6195–6203. doi: 10.1158/1078-0432.CCR-18-1542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Oxnard GR, Hu Y, Mileham KF, Husain H, Costa DB, Tracy P, Feeney N, Sholl LM, Dahlberg SE, Redig AJ, et al. Assessment of resistance mechanisms and clinical implications in patients with EGFR T790M-positive lung cancer and acquired resistance to osimertinib. JAMA Oncol. 2018;4:1527–1534. doi: 10.1001/jamaoncol.2018.2969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Lin CC, Shih JY, Yu CJ, Ho CC, Liao WY, Lee JH, Tsai TH, Su KY, Hsieh MS, Chang YL, et al. Outcomes in patients with non-small-cell lung cancer and acquired Thr790Met mutation treated with osimertinib: A genomic study. Lancet Resp Med. 2018;6:107–116. doi: 10.1016/S2213-2600(17)30480-0. [DOI] [PubMed] [Google Scholar]
- 59.Ramalingam SS, Cheng Y, Zhou C, Ohe Y, Imamura F, Cho BC, Lin MC, Majem M, Shah R, Rukazenkov Y, et al. Mechanisms of acquired resistance to first-line osimertinib: Preliminary data from the phase III FLAURA study. Ann Oncol. 2018;29(Suppl 8):viii740. doi: 10.1093/annonc/mdy424.063. [DOI] [Google Scholar]
- 60.Ramalingam SS, Yang JC, Lee CK, Kurata T, Kim DW, John T, Nogami N, Ohe Y, Mann H, Rukazenkov Y, et al. Osimertinib as first-line treatment of EGFR mutation-positive advanced non-small-cell lung cancer. J Clin Oncol. 2018;36:841–849. doi: 10.1200/JCO.2017.74.7576. [DOI] [PubMed] [Google Scholar]
- 61.Zhang YC, Chen ZH, Zhang XC, Xu CR, Yan HH, Xie Z, Chuai SK, Ye JY, Han-Zhang H, Zhang Z, et al. Analysis of resistance mechanisms to abivertinib, a third-generation EGFR tyrosine kinase inhibitor, in patients with EGFR T790M-positive non-small cell lung cancer from a phase I trial. EBioMedicine. 2019;43:180–187. doi: 10.1016/j.ebiom.2019.04.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Chabon JJ, Simmons AD, Lovejoy AF, Esfahani MS, Newman AM, Haringsma HJ, Kurtz DM, Stehr H, Scherer F, Karlovich CA, et al. Circulating tumour DNA profiling reveals heterogeneity of EGFR inhibitor resistance mechanisms in lung cancer patients. Nat Commun. 2016;7:11815. doi: 10.1038/ncomms11815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Helman E, Nguyen M, Karlovich CA, Despain D, Choquette AK, Spira AI, Yu HA, Camidge DR, Harding TC, Lanman RB, Simmons AD. Cell-Free DNA next-generation sequencing prediction of response and resistance to third-generation EGFR inhibitor. Clin Lung Cancer. 2018;19:530.e7. doi: 10.1016/j.cllc.2018.07.008. [DOI] [PubMed] [Google Scholar]
- 64.Ercan D, Choi HG, Yun CH, Capelletti M, Xie T, Eck MJ, Gray NS, Jänne PA. EGFR mutations and resistance to irreversible pyrimidine-based EGFR inhibitors. Clin Cancer Res. 2015;21:3913–3923. doi: 10.1158/1078-0432.CCR-14-2789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Song HN, Jung KS, Yoo KH, Cho J, Lee JY, Lim SH, Kim HS, Sun JM, Lee SH, Ahn JS, et al. Acquired C797S mutation upon treatment with a T790M-specific third-generation EGFR inhibitor (HM61713) in non-small cell lung cancer. J Thorac Oncol. 2016;11:e45–e47. doi: 10.1016/j.jtho.2015.12.093. [DOI] [PubMed] [Google Scholar]
- 66.Park S, Ku BM, Jung HA, Sun JM, Ahn JS, Lee SH, Park K, Ahn MJ. EGFR C797S as a resistance mechanism of lazertinib in non-small cell lung cancer with EGFR T790M mutation. Cancer Res Treat. 2020;52:1288–1290. doi: 10.4143/crt.2020.278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Oztan A, Fischer S, Schrock AB, Erlich RL, Lovly CM, Stephens PJ, Ross JS, Miller V, Ali SM, Ou SI, Raez LE. Emergence of EGFR G724S mutation in EGFR-mutant lung adenocarcinoma post progression on osimertinib. Lung Cancer. 2017;111:84–87. doi: 10.1016/j.lungcan.2017.07.002. [DOI] [PubMed] [Google Scholar]
- 68.Fassunke J, Muller F, Keul M, Michels S, Dammert MA, Schmitt A, Plenker D, Lategahn J, Heydt C, Bragelmann J, et al. Overcoming EGFRG724S-mediated osimertinib resistance through unique binding characteristics of second-generation EGFR inhibitors. Nat Commun. 2018;9:4655. doi: 10.1038/s41467-018-07078-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Bersanelli M, Minari R, Bordi P, Gnetti L, Bozzetti C, Squadrilli A, Lagrasta CA, Bottarelli L, Osipova G, Capelletto E, et al. L718Q mutation as new mechanism of acquired resistance to AZD9291 in EGFR-Mutated NSCLC. J Thorac Oncol 11: e121-e123, 2016 doi: 10.1016/j.jtho.2016.05.019. [DOI] [PubMed] [Google Scholar]
- 70.Zhao S, Li X, Zhao C, Jiang T, Jia Y, Shi J, He Y, Li J, Zhou F, Gao G, et al. Loss of T790M mutation is associated with early progression to osimertinib in Chinese patients with advanced NSCLC who are harboring EGFR T790M. Lung Cancer. 2019;128:33–39. doi: 10.1016/j.lungcan.2018.12.010. [DOI] [PubMed] [Google Scholar]
- 71.Piotrowska Z, Niederst MJ, Karlovich CA, Wakelee HA, Neal JW, Mino-Kenudson M, Fulton L, Hata AN, Lockerman EL, Kalsy A, et al. Heterogeneity underlies the emergence of EGFRT790 wild-type clones following treatment of T790M-positive cancers with a third-generation EGFR inhibitor. Cancer Discov. 2015;5:713–722. doi: 10.1158/2159-8290.CD-15-0399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Knebel FH, Bettoni F, Shimada AK, Cruz M, Alessi JV, Negrão MV, Reis LFL, Katz A, Camargo AA. Sequential liquid biopsies reveal dynamic alterations of EGFR driver mutations and indicate EGFR amplification as a new mechanism of resistance to osimertinib in NSCLC. Lung Cancer. 2017;108:238–241. doi: 10.1016/j.lungcan.2017.04.004. [DOI] [PubMed] [Google Scholar]
- 73.Wang Q, Yang S, Wang K, Sun SY. MET inhibitors for targeted therapy of EGFR TKI-resistant lung cancer. J Hematol Oncol. 2019;12:63. doi: 10.1186/s13045-019-0759-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Drilon A, Cappuzzo F, Ou SI, Camidge DR. Targeting MET in lung cancer: Will expectations finally Be MET? J Thorac Oncol. 2017;12:15–26. doi: 10.1016/j.jtho.2016.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Hsu CC, Liao BC, Liao WY, Markovets A, Stetson D, Thress K, Yang JC. Exon 16-Skipping HER2 as a Novel Mechanism of Osimertinib Resistance in EGFR L858R/T790M-positive non-small cell lung cancer. J Thorac Oncol. 2020;15:50–61. doi: 10.1016/j.jtho.2019.09.006. [DOI] [PubMed] [Google Scholar]
- 76.Desai A, Adjei AA. FGFR signaling as a target for lung cancer therapy. J Thorac Oncol. 2016;11:9–20. doi: 10.1016/j.jtho.2015.08.003. [DOI] [PubMed] [Google Scholar]
- 77.Kim TM, Song A, Kim DW, Kim S, Ahn YO, Keam B, Jeon YK, Lee SH, Chung DH, Heo DS. Mechanisms of acquired resistance to AZD9291: A mutation-selective, irreversible EGFR inhibitor. J Thorac Oncol. 2015;10:1736–1744. doi: 10.1097/JTO.0000000000000688. [DOI] [PubMed] [Google Scholar]
- 78.Ou SI, Horn L, Cruz M, Vafai D, Lovly CM, Spradlin A, Williamson MJ, Dagogo-Jack I, Johnson A, Miller VA, et al. Emergence of FGFR3-TACC3 fusions as a potential by-pass resistance mechanism to EGFR tyrosine kinase inhibitors in EGFR mutated NSCLC patients. Lung Cancer. 2017;111:61–64. doi: 10.1016/j.lungcan.2017.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Hayakawa D, Takahashi F, Mitsuishi Y, Tajima K, Hidayat M, Winardi W, Ihara H, Kanamori K, Matsumoto N, Asao T, et al. Activation of insulin-like growth factor-1 receptor confers acquired resistance to osimertinib in non-small cell lung cancer with EGFR T790M mutation. Thorac Cancer. 2020;11:140–149. doi: 10.1111/1759-7714.13255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Manabe T, Yasuda H, Terai H, Kagiwada H, Hamamoto J, Ebisudani T, Kobayashi K, Masuzawa K, Ikemura S, Kawada I, et al. IGF2 Autocrine-Mediated IGF1R activation is a clinically relevant mechanism of osimertinib resistance in lung cancer. Mol Cancer Res. 2020;18:549–559. doi: 10.1158/1541-7786.MCR-19-0956. [DOI] [PubMed] [Google Scholar]
- 81.Taniguchi H, Yamada T, Wang R, Tanimura K, Adachi Y, Nishiyama A, Tanimoto A, Takeuchi S, Araujo LH, Boroni M, et al. AXL confers intrinsic resistance to osimertinib and advances the emergence of tolerant cells. Nat Commun. 2019;10:259. doi: 10.1038/s41467-018-08074-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Namba K, Shien K, Takahashi Y, Torigoe H, Sato H, Yoshioka T, Takeda T, Kurihara E, Ogoshi Y, Yamamoto H, et al. Activation of AXL as a preclinical acquired resistance mechanism against osimertinib treatment in EGFR-mutant non-small cell lung cancer cells. Mol Cancer Res. 2019;17:499–507. doi: 10.1158/1541-7786.MCR-18-0628. [DOI] [PubMed] [Google Scholar]
- 83.Nakatani K, Yamaoka T, Ohba M, Fujita KI, Arata S, Kusumoto S, Taki-Takemoto I, Kamei D, Iwai S, Tsurutani J, Ohmori T. KRAS and EGFR amplifications mediate resistance to rociletinib and osimertinib in acquired afatinib-resistant NSCLC harboring exon 19 deletion/T790M in. Mol Cancer Ther. 2019;18:112–126. doi: 10.1158/1535-7163.MCT-18-0591. [DOI] [PubMed] [Google Scholar]
- 84.Marcoux N, Gettinger SN, O'Kane G, Arbour KC, Neal JW, Husain H, Evans TL, Brahmer JR, Muzikansky A, Bonomi PD, et al. EGFR-mutant adenocarcinomas that transform to small-cell lung cancer and other neuroendocrine carcinomas: Clinical outcomes. J Clin Oncol. 2019;37:278–285. doi: 10.1200/JCO.18.01585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Schoenfeld AJ, Chan JM, Kubota D, Sato H, Rizvi H, Daneshbod Y, Chang JC, Paik PK, Offin M, Arcila ME, et al. Tumor analyses reveal squamous transformation and off-target alterations as early resistance mechanisms to first-line osimertinib in EGFR-mutant lung cancer. Clin Cancer Res. 2020;26:2654–2663. doi: 10.1158/1078-0432.CCR-19-3563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Dongre A, Weinberg RA. New insights into the mechanisms of epithelial-mesenchymal transition and implications for cancer. Nat Rev Mol Cell Biol. 2019;20:69–84. doi: 10.1038/s41580-018-0080-4. [DOI] [PubMed] [Google Scholar]
- 87.Nukaga S, Yasuda H, Tsuchihara K, Hamamoto J, Masuzawa K, Kawada I, Naoki K, Matsumoto S, Mimaki S, Ikemura S, et al. Amplification of EGFR wild-type alleles in non-small cell lung cancer cells confers acquired resistance to mutation-selective EGFR tyrosine kinase inhibitors. Cancer Res. 2017;77:2078–2089. doi: 10.1158/0008-5472.CAN-16-2359. [DOI] [PubMed] [Google Scholar]
- 88.Weng CH, Chen LY, Lin YC, Shih JY, Lin YC, Tseng RY, Chiu AC, Yeh YH, Liu C, Lin YT, et al. Epithelial-mesenchymal transition (EMT) beyond EGFR mutations per se is a common mechanism for acquired resistance to EGFR TKI. Oncogene. 2019;38:455–468. doi: 10.1038/s41388-018-0454-2. [DOI] [PubMed] [Google Scholar]
- 89.Schrock AB, Zhu VW, Hsieh WS, Madison R, Creelan B, Silberberg J, Costin D, Bharne A, Bonta I, Bosemani T, et al. Receptor tyrosine kinase fusions and BRAF kinase fusions are rare but actionable resistance mechanisms to EGFR tyrosine kinase inhibitors. J Thorac Oncol. 2018;13:1312–1323. doi: 10.1016/j.jtho.2018.05.027. [DOI] [PubMed] [Google Scholar]
- 90.Batra U, Sharma M, Amrith BP, Mehta A, Jain P. EML4-ALK fusion as a resistance mechanism to osimertinib and its successful management with osimertinib and alectinib: Case report and review of the literature. Clin Lung Cancer. 2020;21:e597–e600. doi: 10.1016/j.cllc.2020.05.016. [DOI] [PubMed] [Google Scholar]
- 91.Jia Y, Yun CH, Park E, Ercan D, Manuia M, Juarez J, Xu C, Rhee K, Chen T, Zhang H, et al. Overcoming EGFR(T790M) and EGFR(C797S) resistance with mutant-selective allosteric inhibitors. Nature. 2016;534:129–132. doi: 10.1038/nature17960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Uchibori K, Inase N, Araki M, Kamada M, Sato S, Okuno Y, Fujita N, Katayama R. Brigatinib combined with anti-EGFR antibody overcomes osimertinib resistance in EGFR-mutated non-small-cell lung cancer. Nat Commun. 2017;8:14768. doi: 10.1038/ncomms14768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Schalm SS, Dineen T, Lim SM, Park CW, Hsieh J, Woessner R, Zhang Z, Wilson K, Eno M, Wilson D, et al. 1296P BLU-945, a highly potent and selective 4th generation EGFR TKI for the treatment of EGFR T790M/C797S resistant NSCLC. Ann Oncol. 2020;31(Suppl 6):S1386–S1406. [Google Scholar]
- 94.Liu X, Zhang X, Yang L, Tian X, Dong T, Ding CZ, Hu L, Wu L, Zhao L, Mao J, et al. Abstract 1320: Preclinical evaluation of TQB3804, a potent EGFR C797S inhibitor. 2019;79(Suppl 13):S1320. [Google Scholar]
- 95.Kim DW, Tiseo M, Ahn MJ, Reckamp KL, Hansen KH, Kim SW, Huber RM, West HL, Groen HJ, Hochmair MJ, et al. Brigatinib in patients with crizotinib-refractory anaplastic lymphoma kinase-positive non-small-cell lung cancer: A randomized, multicenter phase II trial. J Clin Oncol. 2017;35:2490–2498. doi: 10.1200/JCO.2016.71.5904. [DOI] [PubMed] [Google Scholar]
- 96.Wang Y, Yang N, Zhang Y, Li L, Han R, Zhu M, Feng M, Chen H, Lizaso A, Qin T, et al. Effective treatment of lung adenocarcinoma harboring EGFR-activating mutation, T790M, and cis-C797S triple mutations by brigatinib and cetuximab combination therapy. J Thorac Oncol. 2020;15:1369–1375. doi: 10.1016/j.jtho.2020.04.014. [DOI] [PubMed] [Google Scholar]
- 97.Zhao J, Zou M, Lv J, Han Y, Wang G, Wang G. Effective treatment of pulmonary adenocarcinoma harboring triple EGFR mutations of L858R, T790M, and -C797S by osimertinib, bevacizumab, and brigatinib combination therapy: A case report. Onco Targets Ther. 2018;11:5545–5550. doi: 10.2147/OTT.S170358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Arulananda S, Do H, Musafer A, Mitchell P, Dobrovic A, John T. Combination osimertinib and gefitinib in C797S and T790M EGFR-mutated non-small cell lung cancer. J Thorac Oncol. 2017;12:1728–1732. doi: 10.1016/j.jtho.2017.08.006. [DOI] [PubMed] [Google Scholar]
- 99.Giroux-Leprieur E, Dumenil C, Chinet T. Combination of crizotinib and osimertinib or erlotinib might overcome MET-mediated resistance to EGFR Tyrosine kinase inhibitor in EGFR-mutated adenocarcinoma. J Thorac Oncol. 2018;13:e232–e234. doi: 10.1016/j.jtho.2018.07.012. [DOI] [PubMed] [Google Scholar]
- 100.Sequist LV, Han JY, Ahn MJ, Cho BC, Yu H, Kim SW, Yang JC, Lee JS, Su WC, Kowalski D, et al. Osimertinib plus savolitinib in patients with EGFR mutation-positive, MET-amplified, non-small-cell lung cancer after progression on EGFR tyrosine kinase inhibitors: Interim results from a multicentre, open-label, phase 1b study. Lancet Oncol. 2020;21:373–386. doi: 10.1016/S1470-2045(19)30785-5. [DOI] [PubMed] [Google Scholar]
- 101.Odogwu L, Mathieu L, Blumenthal G, Larkins E, Goldberg KB, Griffin N, Bijwaard K, Lee EY, Philip R, Jiang X, et al. FDA approval summary: Dabrafenib and trametinib for the treatment of metastatic non-small cell lung cancers harboring BRAF V600E mutations. Oncologist. 2018;23:740–745. doi: 10.1634/theoncologist.2017-0642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Oh DK, Ji WJ, Kim WS, Choi CM, Yoon SK, Rho JK, Lee JC. Efficacy, safety, and resistance profile of osimertinib in T790M mutation-positive non-small cell lung cancer in real-world practice. PLoS One. 2019;14:e0210225. doi: 10.1371/journal.pone.0210225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Piotrowska Z, Isozaki H, Lennerz JK, Gainor JF, Lennes IT, Zhu VW, Marcoux N, Banwait MK, Digumarthy SR, Su W, et al. Landscape of acquired resistance to osimertinib in EGFR-Mutant NSCLC and clinical validation of combined EGFR and RET inhibition with osimertinib and BLU-667 for acquired fusion. Cancer Discov. 2018;8:1529–1539. doi: 10.1158/2159-8290.CD-18-1022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Han R, Hao S, Lu C, Zhang C, Lin C, Li L, Wang Y, Hu C, He Y. Aspirin sensitizes osimertinib-resistant NSCLC cells in vitro and in vivo via Bim-dependent apoptosis induction. Mol Oncol. 2020;14:1152–1169. doi: 10.1002/1878-0261.12682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Niederst MJ, Hu H, Mulvey HE, Lockerman EL, Garcia AR, Piotrowska Z, Sequist LV, Engelman JA. The allelic context of the C797S mutation acquired upon treatment with third-generation EGFR inhibitors impacts sensitivity to subsequent treatment strategies. Clin Cancer Res. 2015;21:3924–3933. doi: 10.1158/1078-0432.CCR-15-0560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Wang Z, Yang JJ, Huang J, Ye JY, Zhang XC, Tu HY, Han-Zhang H, Wu YL. Lung Adenocarcinoma Harboring EGFR T790M and in trans C797S responds to combination therapy of first- and third-generation EGFR TKIs and shifts allelic configuration at resistance. J Thorac Oncol. 2017;12:1723–1727. doi: 10.1016/j.jtho.2017.06.017. [DOI] [PubMed] [Google Scholar]
- 107.Rotow JK, Costa DB, Paweletz CP, Awad MM, Marcoux P, Rangachari D, Barbie DA, Sands J, Cheng ML, Johnson BE, et al. Concurrent osimertinib plus gefitinib for first-line treatment of EGFR-mutated non-small cell lung cancer (NSCLC) J Clin Oncol. 2020;38:9507. doi: 10.1200/JCO.2020.38.15_suppl.9507. [DOI] [Google Scholar]
- 108.Chic N, Mayo-de-Las-Casas C, Reguart N. Successful treatment with gefitinib in advanced non-small cell lung cancer after acquired resistance to osimertinib. J Thorac Oncol. 2017;12:e78–e80. doi: 10.1016/j.jtho.2017.02.014. [DOI] [PubMed] [Google Scholar]
- 109.Rangachari D, To C, Shpilsky JE, VanderLaan PA, Kobayashi SS, Mushajiang M, Lau CJ, Paweletz CP, Oxnard GR, Jänne PA, Costa DB. EGFR-mutated lung cancers resistant to osimertinib through EGFR C797S respond to first-generation reversible EGFR inhibitors but eventually acquire EGFR T790M/C797S in preclinical models and clinical samples. J Thorac Oncol. 2019;14:1995–2002. doi: 10.1016/j.jtho.2019.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Yang X, Huang C, Chen R, Zhao J. Resolving resistance to osimertinib therapy with afatinib in an NSCLC patient with EGFR L718Q mutation. Clin Lung Cancer. 2020;21:e258–e260. doi: 10.1016/j.cllc.2019.12.002. [DOI] [PubMed] [Google Scholar]
- 111.Romaniello D, Mazzeo L, Mancini M, Marrocco I, Noronha A, Kreitman M, Srivastava S, Ghosh S, Lindzen M, Salame TM, et al. A combination of approved antibodies overcomes resistance of lung cancer to osimertinib by blocking bypass pathways. Clin Cancer Res. 2018;24:5610–5621. doi: 10.1158/1078-0432.CCR-18-0450. [DOI] [PubMed] [Google Scholar]
- 112.Yu HA, Baik CS, Gold K, Hayashi H, Johnson M, Koczywas M, Murakami H, Nishio M, Steuer C, Su WC, et al. LBA62 Efficacy and safety of patritumab deruxtecan (U3-1402), a novel HER3 directed antibody drug conjugate, in patients (pts) with EGFR-mutated (EGFRm) NSCLC. Ann Oncol. 2020;31:S1189–S1190. doi: 10.1016/j.annonc.2020.08.2295. [DOI] [Google Scholar]
- 113.Cho BC, Lee KH, Cho EK, Kim DW, Lee JS, Han JY, Kim SW, Spira A, Haura EB, Sabari JK, et al. 1258O Amivantamab (JNJ-61186372), an EGFR-MET bispecific antibody, in combination with lazertinib, a 3rd-generation tyrosine kinase inhibitor (TKI), in advanced EGFR NSCLC. Ann Oncol. 2020;31(Suppl 4):S813. doi: 10.1016/j.annonc.2020.08.1572. [DOI] [Google Scholar]
- 114.Okura N, Nishioka N, Yamada T, Taniguchi H, Tanimura K, Katayama Y, Yoshimura A, Watanabe S, Kikuchi T, Shiotsu S, et al. ONO-7475, a Novel AXL inhibitor, suppresses the adaptive resistance to initial EGFR-TKI treatment in -mutated non-small cell lung cancer. Clin Cancer Res. 2020;26:2244–2256. doi: 10.1158/1078-0432.CCR-19-2321. [DOI] [PubMed] [Google Scholar]
- 115.Kim D, Bach DH, Fan YH, Luu TT, Hong JY, Park HJ, Lee SK. AXL degradation in combination with EGFR-TKI can delay and overcome acquired resistance in human non-small cell lung cancer cells. Cell Death Dis. 2019;10:361. doi: 10.1038/s41419-019-1601-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Yang YM, Jang Y, Lee SH, Kang B, Lim SM. AXL/MET dual inhibitor, CB469, has activity in non-small cell lung cancer with acquired resistance to EGFR TKI with AXL or MET activation. Lung Cancer. 2020;46:70–77. doi: 10.1016/j.lungcan.2020.05.031. [DOI] [PubMed] [Google Scholar]
- 117.Shi P, Oh YT, Deng L, Zhang G, Qian G, Zhang S, Ren H, Wu G, Legendre B, Jr, Anderson E, et al. Overcoming acquired resistance to AZD9291, A third-generation EGFR inhibitor, through modulation of MEK/ERK-dependent Bim and Mcl-1 Degradation. Clin Cancer Res. 2017;23:6567–6579. doi: 10.1158/1078-0432.CCR-17-1574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Gu J, Yao W, Shi P, Zhang G, Owonikoko TK, Ramalingam SS, Sun SY. MEK or ERK inhibition effectively abrogates emergence of acquired osimertinib resistance in the treatment of epidermal growth factor receptor-mutant lung cancers. Cancer. 2020;126:3788–3799. doi: 10.1002/cncr.32996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Xie Z, Gu Y, Xie X, Lin X, Ouyang M, Qin Y, Zhang J, Lizaso A, Chen S, Zhou C. Lung adenocarcinoma harboring concomitant EGFR mutations and BRAF V600E responds to a combination of osimertinib and vemurafenib to overcome osimertinib resistance. Clin Lung Cancer. 2021;22:e390–e394. doi: 10.1016/j.cllc.2020.06.008. [DOI] [PubMed] [Google Scholar]
- 120.Raoof S, Mulford IJ, Frisco-Cabanos H, Nangia V, Timonina D, Labrot E, Hafeez N, Bilton SJ, Drier Y, Ji F, et al. Targeting FGFR overcomes EMT-mediated resistance in EGFR mutant non-small cell lung cancer. Oncogene. 2019;38:6399–6413. doi: 10.1038/s41388-019-0887-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Yochum ZA, Cades J, Wang H, Chatterjee S, Simons BW, O'Brien JP, Khetarpal SK, Lemtiri-Chlieh G, Myers KV, Huang EH, et al. Targeting the EMT transcription factor TWIST1 overcomes resistance to EGFR inhibitors in EGFR-mutant non-small-cell lung cancer. Oncogene. 2019;38:656–670. doi: 10.1038/s41388-018-0482-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Yang JC, Schuler M, Popat S, Miura S, Heeke S, Park K, Märten A, Kim ES. Afatinib for the treatment of NSCLC harboring uncommon EGFR mutations: A database of 693 cases. J Thorac Oncol. 2020;15:803–815. doi: 10.1016/j.jtho.2019.12.126. [DOI] [PubMed] [Google Scholar]
- 123.Fang W, Huang Y, Gan J, Shao YW, Zhang L. Durable response of low-dose afatinib plus cetuximab in an adenocarcinoma patient with a novel EGFR exon 20 insertion mutation. J Thorac Oncol. 2019;14:e220–e221. doi: 10.1016/j.jtho.2019.05.023. [DOI] [PubMed] [Google Scholar]
- 124.Hasegawa H, Yasuda H, Hamamoto J, Masuzawa K, Tani T, Nukaga S, Hirano T, Kobayashi K, Manabe T, Terai H, et al. Efficacy of afatinib or osimertinib plus cetuximab combination therapy for non-small-cell lung cancer with EGFR exon 20 insertion mutations. Lung Cancer. 2019;127:146–152. doi: 10.1016/j.lungcan.2018.11.039. [DOI] [PubMed] [Google Scholar]
- 125.van Veggel B, de Langen AJ, Hashemi SMS, Monkhorst K, Heideman DAM, Thunnissen E, Smit EF. Afatinib and cetuximab in four patients with EGFR exon 20 insertion-positive advanced NSCLC. J Thorac Oncol. 2018;13:1222–1226. doi: 10.1016/j.jtho.2018.04.012. [DOI] [PubMed] [Google Scholar]
- 126.Yun J, Lee SH, Kim SY, Jeong SY, Kim JH, Pyo KH, Park CW, Heo SG, Yun MR, Lim S, et al. Antitumor activity of amivantamab (JNJ-61186372), an EGFR-MET bispecific antibody, in diverse models of exon 20 insertion-driven NSCLC. Cancer Discov. 2020;10:1194–1209. doi: 10.1158/2159-8290.CD-20-0116. [DOI] [PubMed] [Google Scholar]
- 127.Riely GJ, Neal JW, Camidge DR, Spira A, Piotrowska Z, Horn L, Costa DB, Tsao A, Patel J, Gadgeel S, et al. 1261MO Updated results from a phase I/II study of mobocertinib (TAK-788) in NSCLC with EGFR exon 20 insertions (exon20ins) Ann Oncol. 2020;31:S815–S816. doi: 10.1016/j.annonc.2020.08.1575. [DOI] [Google Scholar]
- 128.Nakahara Y, Kato T, Isomura R, Seki N, Furuya N, Naoki K, Yamanaka T, Okamoto H. A multicenter, open label, randomized phase II study of osimertinib plus ramucirumab versus osimertinib alone as initial chemotherapy for EGFR mutation-positive non-squamous non-small cell lung cancer: TORG1833. J Clin Oncol. 2019;37:TPS9120. doi: 10.1200/JCO.2019.37.15_suppl.TPS9120. [DOI] [Google Scholar]
- 129.Yu HA, Schoenfeld AJ, Makhnin A, Kim R, Rizvi H, Tsui D, Falcon C, Houck-Loomis B, Meng F, Yang JL, et al. Effect of osimertinib and bevacizumab on progression-free survival for patients with metastatic EGFR-mutant lung cancers: A Phase 1/2 single-group open-label trial. JAMA Oncol. 2020;6:1048–1054. doi: 10.1001/jamaoncol.2020.1260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Kobayashi N, Hashimoto H, Kamimaki C, Nagasawa R, Tanaka K, Kubo S, Katakura S, Chen H, Hirama N, Ushio R, et al. Afatinib + bevacizumab combination therapy in EGFR-mutant NSCLC patients with osimertinib resistance: Protocol of an open-label, phase II, multicenter, single-arm trial. Thorac Cancer. 2020;11:2125–2129. doi: 10.1111/1759-7714.13503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Lisberg A, Cummings A, Goldman JW, Bornazyan K, Reese N, Wang T, Coluzzi P, Ledezma B, Mendenhall M, Hunt J, et al. A phase II study of pembrolizumab in EGFR-Mutant, PD-L1+, tyrosine kinase inhibitor naïve patients with advanced NSCLC. J Thorac Oncol. 2018;13:1138–1145. doi: 10.1016/j.jtho.2018.03.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Ahn MJ, Yang J, Yu H, Saka H, Ramalingam S, Goto K, Kim SW, Yang L, Walding A, Oxnard GR. 136O: Osimertinib combined with durvalumab in EGFR-mutant non-small cell lung cancer: Results from the TATTON phase Ib trial. J Thorac Oncol. 2016;11:S115. doi: 10.1016/S1556-0864(16)30246-5. [DOI] [Google Scholar]
- 133.Zhou J, Zhou F, Xie H, Wu Y, Zhao J, Su C. An advanced non-small cell lung cancer patient with epidermal growth factor receptor sensitizing mutation responded to toripalimab in combination with chemotherapy after resistance to osimertinib: A case report. Transl Lung Cancer Res. 2020;9:354–359. doi: 10.21037/tlcr.2020.02.09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Zhang YC, Zhou Q, Wu YL. Clinical management of third-generation EGFR inhibitor-resistant patients with advanced non-small cell lung cancer: Current status and future perspectives. Cancer Lett. 2019;459:240–247. doi: 10.1016/j.canlet.2019.05.044. [DOI] [PubMed] [Google Scholar]
- 135.Wu YL, Planchard D, Lu S, Sun H, Yamamoto N, Kim DW, Tan DSW, Yang JC, Azrif M, Mitsudomi T, et al. Pan-Asian adapted clinical practice guidelines for the management of patients with metastatic non-small-cell lung cancer: A CSCO-ESMO initiative endorsed by JSMO, KSMO, MOS, SSO and TOS. Ann Oncol. 2019;30:171–210. doi: 10.1093/annonc/mdy554. [DOI] [PubMed] [Google Scholar]
- 136.Non-Small Cell Lung Cancer Version 4. NCCN Clinical Practice Guidelines in Oncology. 2021. https://www.nccn.org. Accessed at 25 Jun 2021.
- 137.Jang J, To C, De Clercq DJH, Park E, Ponthier CM, Shin BH, Mushajiang M, Nowak RP, Fischer ES, Eck MJ, et al. Mutant-selective allosteric EGFR degraders are effective against a broad range of drug-resistant mutations. Angew Chem Int Ed Engl. 2020;59:14481–14489. doi: 10.1002/anie.202003500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.To C, Jang J, Chen T, Park E, Mushajiang M, De Clercq DJH, Xu M, Wang S, Cameron MD, Heppner DE, et al. Single and dual targeting of mutant EGFR with an allosteric inhibitor. Cancer Discov. 2019;9:926–943. doi: 10.1158/2159-8290.CD-18-0903. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.