Abstract
Exposure of animals/biological samples to human-made electromagnetic fields (EMFs), especially in the extremely low frequency (ELF) band, and the microwave/radio frequency (RF) band which is always combined with ELF, may lead to DNA damage. DNA damage is connected with cell death, infertility and other pathologies, including cancer. ELF exposure from high-voltage power lines and complex RF exposure from wireless communication antennas/devices are linked to increased cancer risk. Almost all human-made RF EMFs include ELF components in the form of modulation, pulsing and random variability. Thus, in addition to polarization and coherence, the existence of ELFs is a common feature of almost all human-made EMFs. The present study reviews the DNA damage and related effects induced by human-made EMFs. The ion forced-oscillation mechanism for irregular gating of voltage-gated ion channels on cell membranes by polarized/coherent EMFs is extensively described. Dysfunction of ion channels disrupts intracellular ionic concentrations, which determine the cell's electrochemical balance and homeostasis. The present study shows how this can result in DNA damage through reactive oxygen species/free radical overproduction. Thus, a complete picture is provided of how human-made EMF exposure may indeed lead to DNA damage and related pathologies, including cancer. Moreover, it is suggested that the non-thermal biological effects attributed to RF EMFs are actually due to their ELF components.
Keywords: EMF, ion forced-oscillation, VGICs, free radicals, OS, ROS, DNA damage, cancer
1. Introduction
Experimental and epidemiological findings connecting exposure of living organisms to ELF and complex RF human-made EMFs with genetic damage, infertility and cancer
There is a plethora of experimental findings connecting the in vivo or in vitro exposure of experimental animals or cells to extremely low frequency (ELF) (3-3000 Hz) or radio-frequency (RF)/microwave (300 kHz-300 GHz) electromagnetic fields (EMFs), with genetic damage/alterations (DNA damage, chromosome damage and mutations, among others), cell death and related effects (1-4). Most findings concern exposure to wireless communication (WC) EMFs [from mobile phones/antennas, cordless domestic phones (DECT: digitally enhanced cordless telecommunications), internet (Wi-Fi: wireless fidelity) or 'Bluetooth' wireless connections, among others], which necessarily combine RF/microwave carrier frequencies with ELF pulsing and modulation, and ultra low frequency (ULF) (0-3 Hz) random variability of the signal. Today, almost all technical RF EMFs (not only of WC, but also from radars, radio and television antennas, among others) contain ELF/ULF components in the form of on/off pulsations, modulation, and signal variability. These are usually called simply 'RF', but actually they are a combination of RF and ELF/ULF (4).
The number of experimental-laboratory studies showing genetic damage and related effects induced by human-made ELF or RF (combined with ELF) EMFs on a variety of organisms/cell types under different experimental conditions has rapidly increased, especially in recent years (5-55).
Several of the aforementioned findings involve DNA damage and consequent cell death in reproductive cells of different animals, resulting in decreased reproduction. In particular, the effects of pulsing WC EMFs on the DNA of reproductive cells, as reported by different studies on a variety of animals (25,30,31,36,40,41,46), display a marked similarity and explain other findings that connect WC EMF exposure with insect, bird and mammalian (including human) infertility (56-64), or declines in bird and insect populations (especially bees) during the past 15 years (65-69). A significant decrease in reproduction (decrease in egg laying or embryonic death) after exposure to mobile telephony (MT) radiation was identically observed in fruit flies (30,40,57,58), chicken eggs (61), birds (65-67), and bees (63). Similar effects are reported for amphibians (70,71), rats (31,62), and human sperm (decreased number and motility of spermatozoa) (59,60). These markedly similar findings in different organisms by different research groups can be explained by the observed cell death in reproductive cells after DNA damage, as seen in fruit fly ovarian cells (30,40,41,46), human sperm cells (36), mouse and rat sperm cells (25,31). Decreased reproduction after DNA damage and cell death in reproductive cells or embryonic death induced by purely ELF EMF-exposure is also reported (4,9,14,22,47).
At the same time, epidemiological/statistical studies increasingly link man-made EMF exposure with health problems, genetic damage and cancer in human populations. More specifically, ELF EMFs from power lines and high-voltage transformers (mainly 50-60 Hz plus additional frequencies due to harmonics, noise and discharges, among others) are linked with childhood leukemia (72-82) for magnetic field intensities down to 2 mG (0.2 µT) (76,82), or distances from power lines up to 600 m (81), and electric field intensities down to 10 V/m (78). RF exposure from various antennas always containing ELF components, especially MT antennas, is linked to various forms of cancer. Hallberg and Johansson (83) found a connection between skin cancer (melanoma) incidence in humans and residential exposure to radio broadcasting antennas, while two recent studies found significantly increased genetic damage in the peripheral blood lymphocytes of people residing in the vicinity of MT base antennas (84,85). During the past 15 years, epidemiological studies have found an increasing association between mobile or cordless phone use and brain tumors in humans (86-98). Moreover, during the past 20 years, statistical studies have found associations between exposure to MT base station antennas and devices, and reported symptoms of un-wellness referred to as 'microwave syndrome' or 'electro-hypersensitivity' (EHS). The symptoms include headaches, fatigue, sleep disorders, etc. (99-107). A high percentage (~80%) of EHS self-reporting patients were recently found with increased oxidative stress (OS) [intracellular increase in free radicals/reactive oxygen species (ROS)] in their peripheral blood (108).
A review of studies involving exposure to complex RF EMFs with ELF pulsation/modulation revealed that 93% of them reported induction of OS/ROS overproduction in biological systems (109).
Induction of cancer in experimental animals by long-term MT exposure, including ELF pulsations, has also been reported (110,111). A recent study of the USA National Toxicology Program (NTP) found that rats exposed for 2 years, 9 h per day, in the near-field of simulated 2nd generation (2G) or 3rd generation (3G) MT emissions, developed brain cancer (glioma) and heart cancer (malignant schwannoma), with both lower and higher radiation levels than the officially accepted limits (112). Moreover the study found significantly increased DNA damage (strand breaks) in the brains of exposed animals (113), confirming that DNA damage is closely related to carcinogenesis. An Italian life-span exposure study of rats in a simulated 2G MT far-field also found induction of heart schwannomas and brain glial tumors, confirming the results of the NTP study (114).
These findings on animal carcinogenicity along with the epidemiological cancer findings on humans, the DNA damage and OS findings, and the adverse effects on reproduction due to DNA damage in the gametes or embryonic death, point towards the same direction, i.e., that human-made EMF exposure causes OS and DNA damage that may lead to cancer, reproductive declines and related diseases. It is important to note that the exposure levels in the vast majority of all the aforementioned studies (1-114) were significantly below the officially accepted exposure limits for ELF and RF EMFs, which have been set to prevent discharges on humans in the case of ELF and heating of living tissues in the case of RF (115,116).
At the same time, several other studies have reported no effects of ELF or RF EMFs in all the aforementioned end-points (1-4,47,57,115-124), especially studies that employed simulated MT/WC exposure from generators with invariable parameters (intensity, frequency and pulsations, among others) and no modulation or random variability. By contrast, more than 95% of the studies that employed real-life MT/WC exposure from commercially available devices (mobile/cordless phones and Wi-Fi, among others) with high signal variability found effects (4,121,122). Regardless of real-life or simulated exposure, the majority of experimental studies (more than 70%) both in the RF (combined with ELF) and purely ELF bands do find effects (4,109,123,124). In a recent review of 138 RF studies with frequencies >6 GHz evaluating potential effects of the under deployment 5th generation (5G) MT/WC system, it was not specifically examined whether there were ELF components in the exposure and what type, or whether there was any similarity between the signals produced by generators in the studies, and those of the 5G, apart from the carrier frequency. While most of the reviewed studies reported effects, they were criticised in this review for not being 'independently replicated' and for employing 'low quality methods of exposure assessment and control' (125). Thus, despite the incomplete review methodology, the authors of the review attempted to downgrade any reported effects.
Under the increasing weight of scientific evidence, the International Agency for Research on Cancer (IARC) has for a long time now classified both ELF and RF EMFs as possibly carcinogenic to humans (group 2B) (117-119). Based on additional scientific evidence after the 2011 IARC classification for RF EMFs, several studies have suggested that RF/WC EMFs should be re-evaluated and classified as probably carcinogenic (group 2A) or carcinogenic (group 1) to humans (92,97,126,127). As already emphasized, in the vast majority of studies characterized as 'RF', the ELF/ULF components were present.
While the reported effects in the vast majority of the above studies (1-124) induced by ELF or complex RF (containing ELF) EMFs were not accompanied by any significant heating of the exposed living tissues, it is well established that purely RF/microwave EMFs cause heating of exposed materials (e.g. microwave ovens). The heating becomes significant for high power/intensity (≥0.1 mW/cm2) and high frequency (at GHz range) microwaves (128). In addition, purely RF EMFs, which are of very limited technological use, are scarcely reported to induce non-thermal effects, and it is questionable in such cases, whether the presence of any ELFs was carefully excluded (129).
DNA damage and related pathologies
It is well documented that DNA damage is connected with cell senescence (cell aging and loss of replicative capacity), cell death, neurodegenerative diseases and aging of an organism, and is the main cause of carcinogenesis induced by environmental stressors (3,130-138). DNA damaging events take place at any time in the cells of any living organism due to a variety of events (such as exposure to ultraviolet radiation, natural radioactivity or cytotoxic chemicals), but efficient DNA repair mechanisms have evolved to provide protection. Damage in the DNA is any modification in a nucleotide base, deoxyribose, a break in a covalent bond between deoxyribose and nucleotide base, or a break in a phosphodiester bond in one or both strands (3,130-139).
Replication of damaged (or inaccurately repaired) DNA that may occur before repair or blocking can lead to gene mutations, which will then give rise to altered proteins. Mutations in oncogenes, tumor-suppressor genes, DNA repair genes or genes that control the cell cycle can generate a clonal cell population with a distinct ability to proliferate. DNA methylation that may prohibit the expression of DNA repair genes and synthesis of related proteins can result in inaccurate ('error-prone') DNA repair. Many such events, which may accumulate over a long period of time in cases of chronic exposure to carcinogens, can lead to genomic instability and cancer (133,134,136,139).
When the genomic DNA of a cell is damaged by an external stressor and the damage is either not reparable or inaccurately repaired, the following outcomes are possible: i) The cell dies (necrosis) or is led to suicide (induced apoptosis). In the case of cell types with the ability to proliferate, the organism compensates for their loss by creating new cells, practically with no adverse consequences apart from energy consumption, which may lead to accelerated aging when such events occur at a high rate. In the case of cell types that do not have ability to proliferate, such as neural cells or chondrocytes, the loss of a significant number of cells will probably result in the inability of certain tissues/organs to operate normally. In the case of neural cells, this may lead to neurodegenerative diseases such as Alzheimer and Parkinson, and autoimmune disorders, among others. ii) The cell does not die but survives with modified DNA. In the case of somatic cells that proliferate, the modified genome will reproduce itself. Even though the organism may recognize such mutant cells as foreign and try to isolate them and remove them, they strive to survive and may start proliferating uncontrollably, initiating cancer. In the case of reproductive cells (oocytes and spermatocytes), this may lead to mutated new organisms that may be problematic in many ways or cancer-prone. In both cases (somatic or reproductive cells) cell senescence is an alternative pathway for eliminating surviving genetically defective cells. Thus, cells with irreparably damaged genomic DNA will result in cell senescence, cell death, cancer or mutated offspring, depending on cell type and specific biological/environmental conditions (3,4,122,130-132,135-137).
The duration of cancer development (latency period) after irreparable DNA damage may be a number of years, depending on the organism and the type of cancer. The latency period for gliomas (a type of brain cancer) is usually >20 years in humans (140). This probably explains why only during the past ~15 years epidemiological studies have started showing an association between mobile phone use and cancer (86), whereas cancer from power lines, which are several decades older than MT/WC, has been indicated long before (72).
Purpose of the present study
As aforementioned, a growing number of experimental and epidemiological/statistical findings connect man-made EMF exposure with genetic damage and cancer, and this involves the breakage of chemical/electronic bonds in molecules/atoms, in other words ionization. The human-made EMFs with frequencies up to the lower limit of infrared (0-3×1011 Hz) discussed in the present study cannot directly cause ionization, except for very strong field intensities (≥106 V/m) (141,142). Such field intensities rarely exist environmentally, apart from atmospheric discharges (lightning) or in very close proximity to high-voltage power lines and transformers. The question therefore is how human-made EMFs at environmental intensities are capable of damaging DNA and other biological molecules. Obviously they have the ability of breaking chemical bonds indirectly through the action of some primary biophysical mechanism(s) and subsequent initiation of intracellular biochemical processes.
Visible and infrared natural light cannot break chemical bonds, even though they expose us at higher frequencies and radiation intensities than human-made EMFs in daily life (143). There must be a unique property of the human-made EMFs that makes them capable of inducing adverse biological/health effects and ionization, in contrast to natural infrared and visible light. This unique property is that human-made EMFs/radiation are totally polarized and coherent, meaning that they possess net electric and magnetic fields, apart from radiation intensity, which exert forces on any electrically charged (or polar) particle/molecule such as mobile/dissolved ions and charged macromolecules in any biological system (143).
The purpose of the present study is to suggest a realistic primary biophysical mechanism for polarized and coherent EMFs at environmentally relevant intensities, to impair cellular function and initiate plausible intracellular biochemical processes resulting in genetic damage and carcinogenesis, as reported in the aforementioned studies.
2. Biophysical action of polarized/coherent EMFs resulting in voltage-gated ion channel (VGIC) dysfunction and disruption of cell electrochemical balance
It has been shown that polarized/coherent EMFs, even at very low field intensities in the ULF and ELF bands, can cause irregular gating of electro-sensitive ion channels or VGICs on the cell membranes through the 'ion forced-oscillation mechanism' (143-146), with consequent disruption of the cell's electrochemical balance (the electrical and osmotic equilibrium maintained by specific concentrations of all dissolved/mobile ions across all cell membranes according to the Nernst equation) (144,147,148). Since, as explained, ELF/ULF components exist also in the complex WC/RF EMFs, this mechanism, which will be thoroughly reviewed next, accounts for the biological effects of the vast majority of human-made (polarized and coherent) EMFs.
The mechanism is based on molecular/physical data, and the forces on mobile ions, in the vicinity of the voltage-sensors of VGICs, exerted by an applied polarized oscillating EMF. The oscillating field will force mobile ions to oscillate on parallel planes and in phase with the field. This coordinated motion of electrically charged particles exerts electric forces on the voltage-sensors, similar to the forces exerted on them by changes in the transmembrane electric field known to physiologically gate these channels, and thus the channels are gated irregularly by the applied EMF. The forces are proportional to the amplitude of the forced-oscillation, and thus, the amplitude is a direct measure of the bioactivity of the applied EMF. It has been shown that the amplitude (bioactivity) is proportional to EMF intensity, inversely proportional to EMF frequency and doubles for pulsed EMFs. The validity of the proposed mechanism has been verified by numerical testing, while other previously suggested mechanisms have failed to pass the same test (149,150). Repeated irregular gating of electro-sensitive ion channels disrupts cellular electrochemical balance and homeostasis (147,148), leading to overproduction of ROS/free radicals as described next.
It is known from a plethora of experimental data that the most bioactive EMFs are the lower frequency ones (ELF/ULF). In numerous cases of induced biological effects by complex RF EMFs modulated by ELFs, it has been found that the modulation (ELF) and not the carrier (RF) is responsible for the recorded effects. In addition, it has been repeatedly found that pulsing RF EMFs with ELF pulse-repetition rates are more active biologically than continuous (non-pulsed) fields of identical other parameters (1-5,44,45,47,151-159). These findings are in direct agreement with the described mechanism.
Biological molecules of critical importance such as ions, water molecules, proteins, nucleic acids and lipids, among others, are either polar or carry a net electric charge (147,148). The net electric field from an infinite number of individual electric pulses of random polarization and/or random phase (as e.g. photons of natural light) tends to zero at any moment (and similarly the net magnetic field).
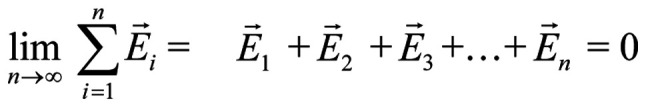 |
Thus, non-polarised/incoherent EMFs (as e.g. light and cosmic microwaves) at any radiation intensity cannot cause any parallel/coherent oscillation of charged/polar molecules (143). On the contrary, polarized and coherent (human-made) oscillating EMFs force all charged/polar molecules in biological tissue to oscillate on planes parallel to their polarization and in phase with them. This is crucially important for understanding the mechanism described. The forced-oscillation will be most intense on the mobile ions, the smallest charged particles dissolved in large concentrations in the cytosolic and extracellular aqueous solutions in all living cells/tissues controlling practically all cellular/biological functions (147,148).
Even though all molecules move randomly with much greater velocities/displacements due to thermal energy, this has no biological effect other than increasing tissue temperature. By contrast, a polarized and coherent oscillation of much lower energy than average thermal molecular energy can initiate biological effects (143-145).
The majority of cation channels (Ca2+, K+, Na+ and H+, among others) on the membranes of all animal cells are voltage-gated (147,148). These ion channels convert between open and closed states when the electrostatic force on their voltage sensors, due to transmembrane voltage changes, exceeds some critical value. The voltage sensors are four symmetrically arranged, transmembrane, positively charged α-helices, each one named S4. The S4 helices occupy the 4 th position in a group of 6 parallel α-helices (S1-S6). The channel consists of four identical such groups in symmetrical positions around the pore of the channel. The S5-S6 helices of the four groups form the pore walls (147,148). More specifically, the sensors are positive Lys and Arg amino acids in the S4 helices. Changes in the transmembrane voltage of the order of ~30 mV are normally required to gate electrosensitive channels (change their status from opened to closed and vice-versa) (160,161). Among the S1-S4 α-helices, the S4 helices are the closest to the pore-forming S5-S6 helices, being <1 nm in distance from the pore (162,163). Several ions may interact simultaneously at any instant with an S4 sensor from a distance of the order of 1 nm, as, except for the ion(s) that may be passing through the pore any moment or are just outside the gate ready to pass, a few more ions are bound close to the pore at specific ion-binding sites (e.g. three in potassium channels) (164,165). Proton voltage-gated channels studied more recently also contain S4 transmembrane helices with charged Arg residues as voltage-sensors, similar to the metallic cation channels (166,167).
Let us consider four identical mobile ions at distances of the order of 1 nm from the channel-sensors (S4) and an externally applied oscillating EMF. The average electric (and magnetic) force on each ion due to any non-polarized EMF is zero (Eq. 1). By contrast, the force due to a polarized field with an electrical component E, is F=Ezqe, (with zqe the electric charge of the ion).
In the most usual and simplest case of a sinusoidal alternating electric field, E=Eo sinωt, the motion (forcedoscillation) equation of a mobile ion is as follows (143-146):
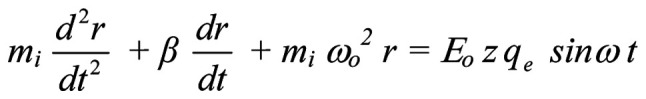 |
where mi is the mass of the ion, r is the displacement of the ion due to the forced-oscillation, z is the valence of the ion (z=1 for K+, Na+ or z=2 for Ca2+ ions), qe =1.6×10−19C is the elementary charge, β is the damping coefficient (being within channels
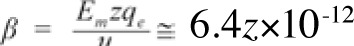 kg/s, with Em (~107 V/m) the transmembrane electric field, and uo=0.25 m/s the velocity of the ion through an open channel calculated from patch-clamp measurements of channel ion-currents). ωo=2πνo (νo the ion's oscillation self-frequency accepted to be equal to the recorded spontaneous intracellular ionic oscillation frequencies on the order of 0.1 Hz), ω=2πν (ν the frequency of the applied field) and Eo is the intensity amplitude of the applied oscillating field. Detailed calculations of the parameters are provided in Panagopoulos et al 2000 (144).
kg/s, with Em (~107 V/m) the transmembrane electric field, and uo=0.25 m/s the velocity of the ion through an open channel calculated from patch-clamp measurements of channel ion-currents). ωo=2πνo (νo the ion's oscillation self-frequency accepted to be equal to the recorded spontaneous intracellular ionic oscillation frequencies on the order of 0.1 Hz), ω=2πν (ν the frequency of the applied field) and Eo is the intensity amplitude of the applied oscillating field. Detailed calculations of the parameters are provided in Panagopoulos et al 2000 (144).
The right part of Eq. 2 is the force on the ion due to the applied E-field. The first term of the left part
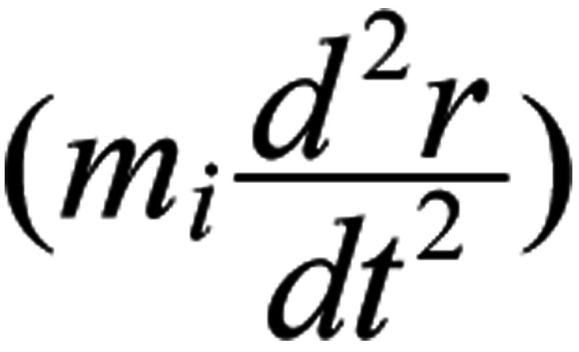 is the resultant force on the ion, the second term
is the resultant force on the ion, the second term
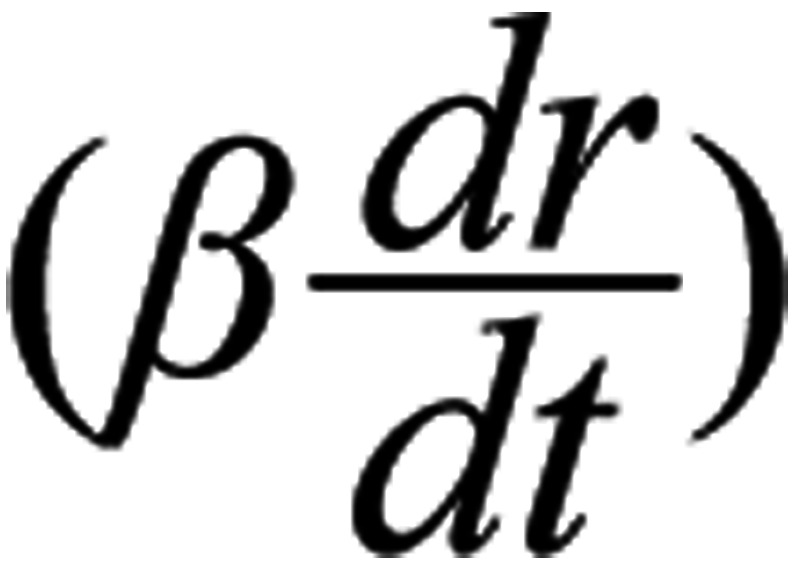 is a damping force and the third term (mi ωo2
r) a restoration force exerted by the medium (144,145). While an oscillating ion close to the S4 sensors exerts gating forces on them, it receives zero opposite force, as the S4 charges are paired with opposite charges from adjacent helices of the channel (148). Eq. 2 is a second-order linear differential equation with constant coefficients, which is solvable once we know the values of the different parameters.
is a damping force and the third term (mi ωo2
r) a restoration force exerted by the medium (144,145). While an oscillating ion close to the S4 sensors exerts gating forces on them, it receives zero opposite force, as the S4 charges are paired with opposite charges from adjacent helices of the channel (148). Eq. 2 is a second-order linear differential equation with constant coefficients, which is solvable once we know the values of the different parameters.
The general solution of Equation 2 (144) is:
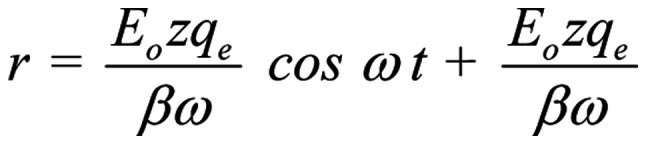 |
The constant term
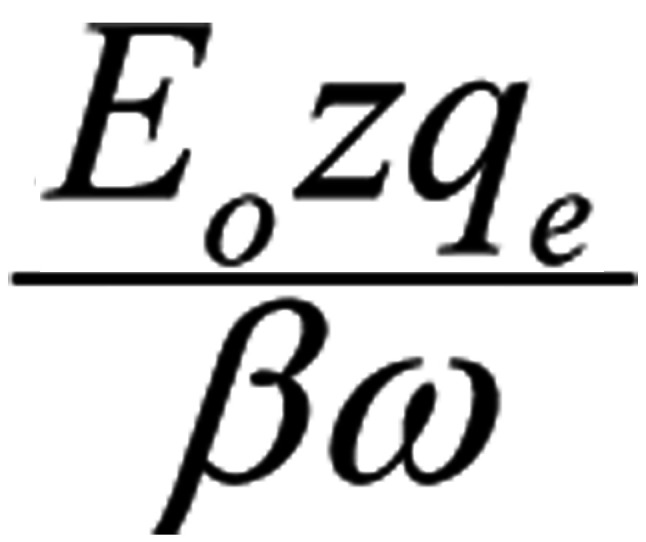 in the solution represents a constant displacement of the ion and has no effect on the oscillating term
in the solution represents a constant displacement of the ion and has no effect on the oscillating term
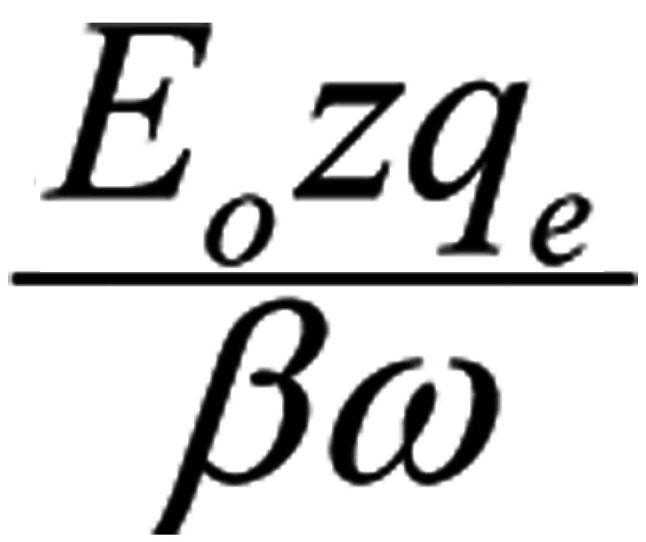 . This constant displacement represents a jump of the whole oscillation at a distance equal to the amplitude, in other words it doubles the amplitude
. This constant displacement represents a jump of the whole oscillation at a distance equal to the amplitude, in other words it doubles the amplitude
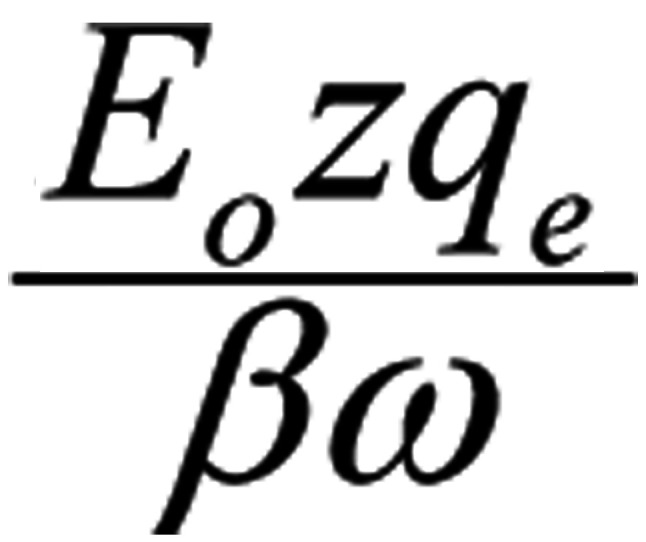 of the oscillation at the moment when the field is applied or interrupted. For pulsed fields (such as the vast majority of human-made complex RF/microwave EMFs, especially those employed in modern WC), this interruption/repetition occurs constantly with every repeated pulse. Therefore, pulsed fields are predicted to be twice as bioactive as continuous/non-pulsed fields of the same other parameters, and this explains a plethora of experimental findings showing increased bioactivity of pulsed compared with non-pulsed RF EMFs, which were previously unexplained (44,45,154, 155,157-159).
of the oscillation at the moment when the field is applied or interrupted. For pulsed fields (such as the vast majority of human-made complex RF/microwave EMFs, especially those employed in modern WC), this interruption/repetition occurs constantly with every repeated pulse. Therefore, pulsed fields are predicted to be twice as bioactive as continuous/non-pulsed fields of the same other parameters, and this explains a plethora of experimental findings showing increased bioactivity of pulsed compared with non-pulsed RF EMFs, which were previously unexplained (44,45,154, 155,157-159).
Ignoring the constant term in Eq. 3, the amplitude of the forced-oscillation is:
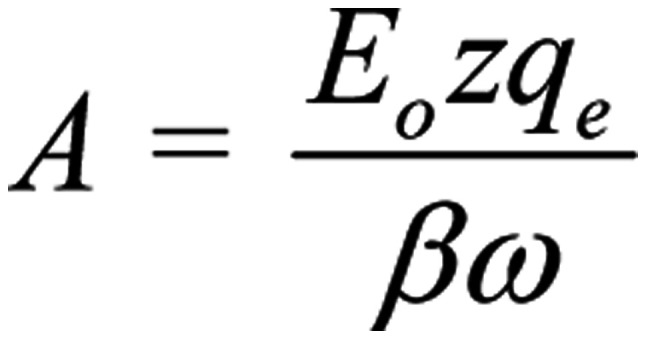 |
An oscillating ion of charge zqe (whose motion is described by Eq. 3) close to the S4 helices of a voltage-gated channel exerts a force F on the effective charge q of each S4, as described by Coulomb's law:
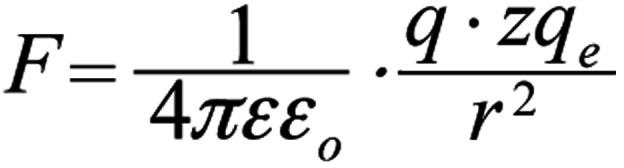 , (r here is the distance of the oscillating ion from the S4). The ion displaced by dr during its oscillation, induces an additional force dF on each S4 sensor:
, (r here is the distance of the oscillating ion from the S4). The ion displaced by dr during its oscillation, induces an additional force dF on each S4 sensor:
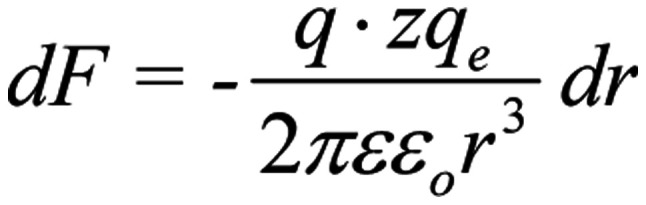 |
While in the case of a random/chaotic movement of the ion due to e.g. thermal motion
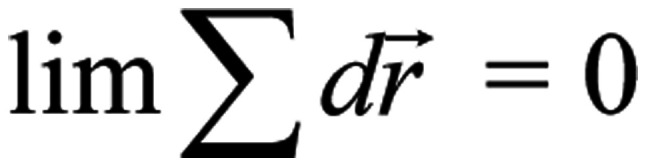 , and
, and
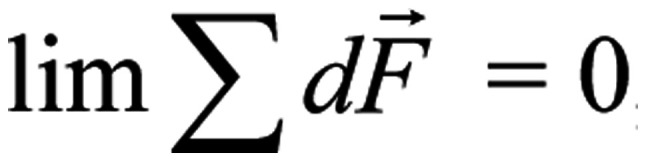 , in the case of a coordinated polarized and coherent forcedoscillation, the sum force on each S4 from all four ions, is:
, in the case of a coordinated polarized and coherent forcedoscillation, the sum force on each S4 from all four ions, is:
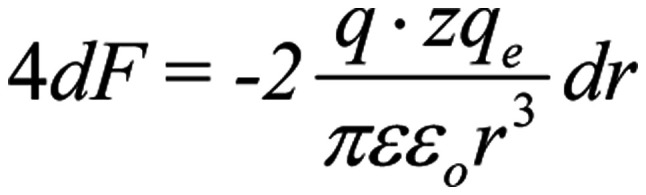 |
The effective charge of each S4 domain is found to be: q=1.7qe (161). The force on this charge exerted by a change of 30 mV in the transmembrane voltage required normally to gate the channel, is calculated to be (144): dF=8.16×10−13 N.
The displacement of one single-valence ion within the channel corresponding to this minimum force, according to Eq. 5 (for z=1, ε ≅ 4, and r ~1 nm), is: dr=4×10−12 m.
The dielectric constant within proteins is significantly lower than in the aqueous solutions (4/80), and ion concentration in cells is of the order of 1 ion per nm3 (144,147,148).
For 4 single-valence ions oscillating on parallel planes and in phase with an applied polarized (and coherent) oscillating field, the minimum displacement is (according to Eq. 6) reduced to: dr=10−12 m. The corresponding necessary displacement for ions outside the channel would be about 20-fold higher due to the higher dielectric constant of the aqueous solutions.
Thus, a crucial finding has been reached: Any external polarized and coherent oscillating EMF (like all technical/human-made EMFs) able to force mobile ions to oscillate with amplitude
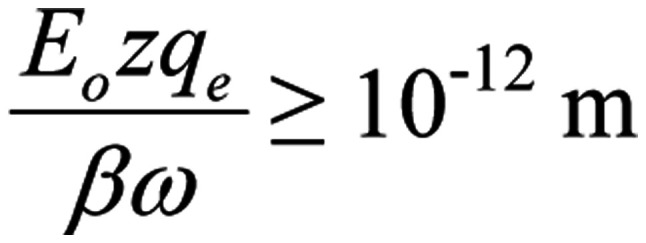 |
is able to irregularly gate VGICs on cell membranes.
For z=1 (e.g. K+ ions), and replacing qe, β by their values in Condition 7, we get:
| (8) |
(ν in Hz, Eo in V/m)
For double-valence cations (z=2) (e.g. Ca2+) the condition becomes:
| (9) |
(ν in Hz, Eo in V/m)
For pulsed fields (such as all MT/WC fields) the right part of Condition 9 is further divided by 2, becoming:
| (10) |
(ν in Hz, Eo in V/m)
It is clear that the amplitude of the forced-oscillation given by Eq. 4 is the critical parameter to determine the ability of a polarized/coherent EMF to induce biological/health effects. We shall name it 'Bioactivity of the EMF' or 'EMF-Bioactivity'. Thus:
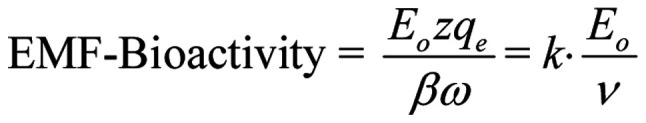 |
where
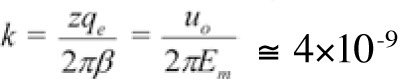 is a constant quantity (depending upon the membrane electric field Em and the velocity of the ion through an open channel uo), Eo is the intensity amplitude and ν is the frequency of the applied electric field. We shall name k the 'bioactivity constant'.
is a constant quantity (depending upon the membrane electric field Em and the velocity of the ion through an open channel uo), Eo is the intensity amplitude and ν is the frequency of the applied electric field. We shall name k the 'bioactivity constant'.
Thus, a most reasonable and elegant result is reached, that the bioactivity of a polarized oscillating EMF is proportional to its maximum intensity (Eo) and inversely proportional to its frequency (ν), meaning that lower frequency fields are predicted to be more bioactive than higher frequency ones of the same intensity and waveform. Although this result was obtained considering the most usual/simple case of harmonically oscillating polarized EMFs, it is evident that non-harmonically oscillating polarized fields can also be approximately described in terms of their bioactivity by Eq. 11.
For pulsed EMFs with harmonically oscillating carriers, the amplitude doubles and so does the bioactivity:
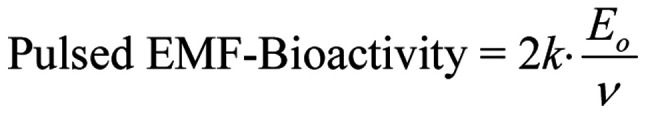 |
The same mechanism explains the biological action of polarized oscillating magnetic fields as well, if we replace in Eq. 2 the electric force FE=Ezqe, by a magnetic force:
| (13) |
exerted on an ion with charge zqe, moving with velocity u, vertically to the direction of a magnetic field of intensity B (in which case the magnetic force is maximum). In the simplest (and most usual) case of an alternating magnetic field B=Bosinωt with intensity amplitude Bo and based on the same reasoning as aforementioned, corresponding bioactivity conditions are obtained for an oscillating magnetic field.
For one single-valence ion moving through an open channel vertically to the direction of the applied magnetic field with u=uo=0.25 m/s (the velocity calculated for ions moving through an open channel) (144) and for the case of a continuous oscillating magnetic field, the corresponding bioactivity condition is:
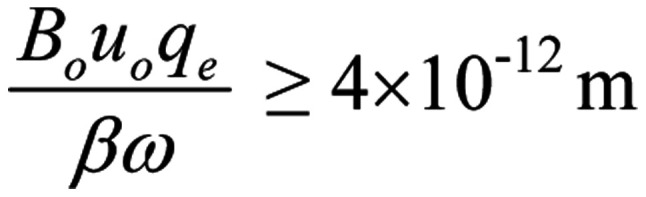 |
from which is obtained:
| (15) |
(ν in Hz, Bo in T), or
| (16) |
(ν in Hz, Bo in ¼T), or
For double-valence ions the right part of Condition 16 is divided by 2:
| (17) |
(ν in Hz, Bo in µT)
For double-valence ions and pulsing magnetic field the right part of Condition 17 is further divided by 2, and the bioactivity condition becomes:
| (18) |
(ν in Hz, Bo in µT)
It should be noted that apart from the drift velocity of the ion through the channel (uo=0.25 m/s) that is accepted as initial velocity, the ion will acquire an additional velocity dr/dt due to the forced-oscillation. From Eq. 3, the following is obtained:
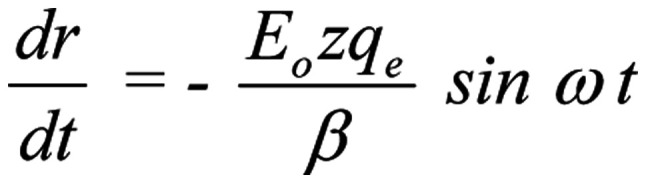 |
(or respectively:
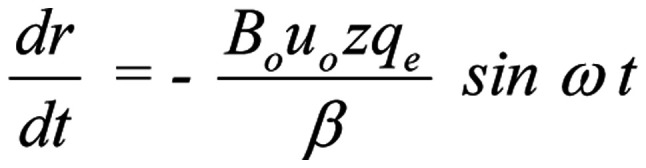 for a sinusoidal magnetic field)
for a sinusoidal magnetic field)
The corresponding magnetic force due to this additional velocity, Bzqe(dr/dt), is negligible (more than 108 times smaller) compared with the damping force β(dr/dt), and thus, it is not taken into account in Eq. 2.
The maximum
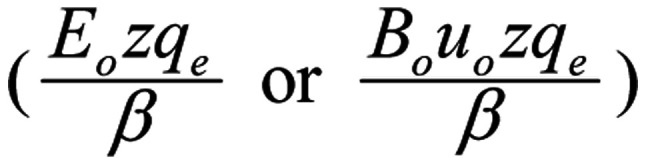 of this additional velocity is independent of the frequency of the field (ω), and is much smaller for usual field intensities than the ion velocity through an open channel (uo=0.25 m/s), which in turn is more than 103 times smaller than its corresponding average thermal velocity ukT (168). Thus, the described ion forced-oscillation does not add to tissue temperature and this mechanism is 'non-thermal', in contrast to the known heating ability of the high intensity microwaves (128). The non-thermal nature of human-made EMF-bioeffects, including those of low power modulated/pulsing RF/microwaves, in contrast to high power microwaves, has also been discussed in previous studies (169,170).
of this additional velocity is independent of the frequency of the field (ω), and is much smaller for usual field intensities than the ion velocity through an open channel (uo=0.25 m/s), which in turn is more than 103 times smaller than its corresponding average thermal velocity ukT (168). Thus, the described ion forced-oscillation does not add to tissue temperature and this mechanism is 'non-thermal', in contrast to the known heating ability of the high intensity microwaves (128). The non-thermal nature of human-made EMF-bioeffects, including those of low power modulated/pulsing RF/microwaves, in contrast to high power microwaves, has also been discussed in previous studies (169,170).
This theory allows certain predictions for the bioactivity of some human-made EMFs widely present in the modern environment: For the sinusoidal alternating (continuous) 50-Hz E and B fields of high-voltage power lines with intensities of the order of E ~10 kV/m and B ~0.1-1 G (or ~10-100 µT) at close distances (10-20 m) from such lines the conditions 9 and 17 for double valence cations (e.g. Ca2+) give: Eo≥6×10−3 V/m or Eo≥6 mV/m (which is satisfied by more than 106 times), and Bo ≥105 µT, which is not satisfied, showing that the recorded effects from high-voltage power lines are due to the electric rather than the magnetic component of the resultant EMF, in contrast to what is usually considered. Thus, the electric component of power line EMFs is certainly capable of inducing biological effects in living organisms according to the mechanism presented, even for intensities down to 1-10 V/m, which exist in most homes and work places.
For the pulsing ELF E and B fields of MT/WC EMFs with a pulsing repetition frequency of ~100 Hz (3G/4G MT, DECT), E ~10 V/m and B ~1 mG (or ~0.1 µT) (30,40,54,55), the bioactivity conditions 10 and 18 respectively give: Eo≥6×10−3 V/m or Eo≥6 mV/m, which is satisfied by more than 103 times, and Bo ≥105µT, which is not satisfied for direct action, but it may be satisfied by the magnetically induced electric field, which is significant in this case due to the short rise/fall times of the pulses (143). Similar results are obtained for the 217-Hz pulsing E/B fields of 2G MT (30,40).
For Wi-Fi and Bluetooth wireless connections with a pulsing frequency of ~10 Hz, E ~1 V/m and B ~0.1 mG (or ~0.01 µT) (171), the bioactivity conditions 10 and 18 respectively give: Eo ≥0.6×10−3 V/m or Eo≥0.6 mV/m, which is satisfied by more than 103 times, and Bo≥104 µT, which is not satisfied for direct action.
The aforementioned numerical examples show that it is the electric field that seems to be the bioactive component of an EMF and not the magnetic field, in contrast to what has been considered before by health agencies (117). The magnetically induced electric field can also be bioactive in the case of ELF pulses of WC signals with short rise/fall times (143).
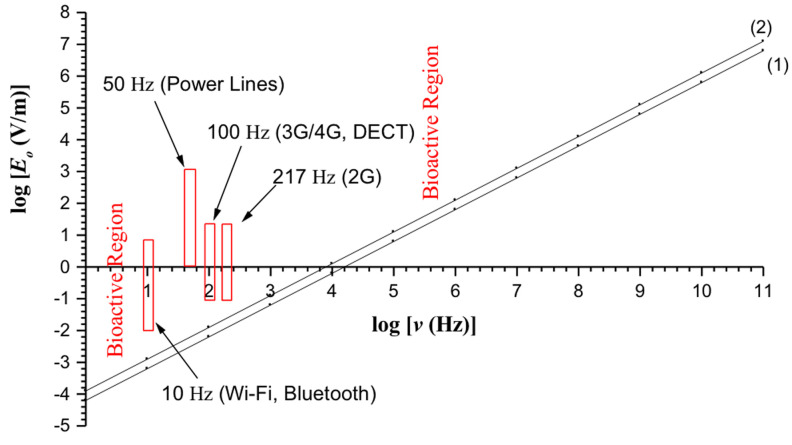
The bioactivity conditions 9 and 10 for continuous and pulsed electric fields respectively are depicted in Fig. 1. The region above line 1 (including the line) represents the bioactive combinations of intensity amplitude (Eo) and frequency (ν) for pulsed fields, and above line 2 (including the line) for continuous fields. The ELF electric field of power lines, 2G/3G/4G MT, DECT, WiFi and 'Bluetooth', lie within the bioactive region predicted by the presented theory.
Figure 1.
E-field bioactivity diagram showing the bioactive combinations of electric field intensity and frequency capable of inducing biological/health effects according to the ion forced-oscillation mechanism for dysfunction of voltage-gated ion channels in cells. The ELF electric fields of power lines, 2G/3G/4G MT, DECT, Wi-Fi and Bluetooth, are within the bioactive region (above lines 1 and 2). Line 1 refers to pulsed fields, such as the ELF pulsations of WC EMFs (Condition 10), while line 2 refers to continuous (non-pulsed) fields such as those from power lines (Condition 9).
3. Biochemical processes activated by irregular gating of VGICs, leading to DNA damage
Irregular gating of ion channels and ROS
Irregular gating of VGICs by oscillating polarized and coherent ELF EMFs as described [and originally in (143-146)] has been verified experimentally for calcium (Ca2+), potassium (K+) and sodium (Na+) VGICs (172-174). This can alter intracellular ionic concentrations, disrupting the electrochemical balance of the cell and leading to DNA damage by OS/ROS overproduction (175-179).
Most ROS are free radicals. Free radicals are highly unstable molecules containing an unpaired electron, which is denoted by a dot (•), and have a tremendous tendency to chemically react with surrounding molecules and/or with each other in order to couple the unpaired electron and become stable. This is the reason why they have extremely short lifetimes. Most ROS react rapidly with surrounding biomolecules inducing chemical alterations (180). Overproduction of ROS in living cells due to EMF exposure has been reliably documented, with two important ROS found after EMF exposure being superoxide anion (O2•−) and nitric oxide (NO•) (109). These may result in hydroxyl radical (OH•) and peroxynitrite (ONOO−) correspondingly, both of which ROS are very reactive with biological molecules and specifically DNA, as discussed next. ONOO− may interact directly with DNA, as, similarly with NO•, it can be diffused everywhere in the cell (181). Superoxide anion radical (O2•−) is catalyzed by superoxide dismutase enzymes in the cytosol or the mitochondria and is converted to hydrogen peroxide (H2O2) (109,182):
| (20) |
H2O2 is a critical molecule in oxidative damage since it can move to any intracellular site (including the nucleus), where it can be converted to the most potent OH•, which can damage any biological molecule, including DNA (183-187).
DNA damage by ROS leading to mutations and disease has been well studied (188,189). Pall (190), in a review of EMF-bioeffects studies with calcium channel blockers, noted a connection between voltage-gated calcium channels (VGCCs) and NO•/ONOO− overproduction. This verified earlier observations of EMF-induced effects on intracellular calcium concentrations, and the unique role of VGCCs (1,151-153,191,192).
It is known that the intracellular redox status can activate Ca2+, Na+ and K+ channels in order to reinstate homeostasis (178), and inversely, activation of these channels determines the redox status and the electrochemical balance of the cell (179). Multiple studies have found connections between the impaired function of calcium, potassium, sodium and chloride channels with the induction of OS and related pathologies (175-177). These studies provide additional evidence for the validity of the presented biophysical mechanism (143-146).
Calcium signaling and mitochondrial ROS production
Alteration of intracellular ionic concentrations will affect key cellular signaling pathways, including the Ca2+ signaling system, which regulates a variety of cellular functions including cell proliferation, differentiation, the ROS regulatory system and apoptosis (192-196). Impaired function of VGCCs in the plasma or in the mitochondrial membranes leading to critical changes in cytosolic or mitochondrial concentrations of Ca2+ ions, such as those following EMF exposure, is connected with pathogenesis and cytotoxicity (195,196).
Voltage-gated anion channels in the outer membrane of the mitochondria regulate Ca2+ entry into the inter-membrane space and in the matrix, which is crucial for mitochondrial ROS production. Increased level of Ca2+ stimulates O2•− production by the electron transport chain in the mitochondria and/or activation of nitric oxide synthase (NOS), to generate more NO•. NO• inhibits complex IV of the electron transport chain, triggering production of even more ROS (109,193). ROS overproduction in the mitochondria can damage DNA both in the mitochondria and the nucleus, and initiate a signaling cascade leading to apoptosis, as found in human spermatozoa after MT EMF exposure (36). Moreover, increased concentrations of NO• in living cells due to activation of NOS at different locations of the cell may lead to formation of ONOO− (181,182).
Regulation of apoptosis is crucial for anticancer control (197). However, excessive apoptosis, induced by increased ROS levels, is connected with inflammatory diseases and cancer (198). When overproduction of ROS in a cell overloads the capacity of the antioxidant system of the cell, the cell/organism is under OS. This condition may lead to significant DNA damage with consequent genomic instability and carcinogenesis (182,183,194-198).
K+ channels have also been shown to be involved in the activation of apoptosis (194), and voltage-gated Ca2+ and K+ channels have been shown to be connected with cell proliferation and carcinogenesis (199). Thus, cytosolic concentrations of Ca2+ and K+ ions play major roles in cellular function and metabolism. In addition, voltage-gated calcium and potassium channels play important roles in iron entry into the cells. Iron catalyzes the production of OH• via the Fenton reaction and thus, impaired function of these channels can promote cellular toxicity (200-202).
NADPH oxidase and ROS production
Apart from the effect of EMFs on metallic cation voltage-gated channels (such as Ca2+, Na+ and K+), proton (H+) voltage-gated channels will be affected as well, as they operate in a very similar way (166,167). This in turn would affect the function of NADPH oxidase, a plasma membrane enzyme found in abundance in all cells, which normally generates ROS for the elimination of invading microorganisms (203,204). The activity of NADPH oxidase is strongly associated with H+ channels and it may even act directly as a H+ voltage-gated channel due to its gp91phox transmembrane subunit (205,206). NADPH oxidase generates an electron flux for the reduction of extracellular O2 to O2•− (203,207).
NADPH oxidase is activated by cytosolic Ca2+ and possesses a Ca2+-binding site in addition to its H+ voltage-gated channel (gp91phox transmembrane region) (204). Thus, perturbation of intracellular concentrations of either H+ or Ca2+, after irregular gating of their voltage-gated channels, will affect the function of NADPH oxidase and trigger irregular ROS production.
NADPH oxidase has been reasonably suggested as a primary target of EMF exposure in living cells. In 2007, Friedman et al (208) found rapid ROS production in cultured cells after a few min of exposure to RF EMF emitted by a generator.
Na+/K+-ATPase and ROS production
Impaired function of Na+, K+, Mg2+ and Ca2+ voltage-gated channels may also affect the function of the Na+/K+ pump (ATPase) and Ca2+ pumps in the plasma membranes of all cells. The ion pumps (active ion transporters) across all cell membranes in coordination with the ion channels (passive ion transporters) determine the membrane voltage, the volume of the cell and the electrochemical balance (147,148). A positive-feedback amplification loop between Na+/K+-ATPase signaling and ROS production by the mitochondria was experimentally demonstrated in primary cultures of cardiac myocytes (209). Na+/K+-ATPase became a target for ROS-initiated signaling, and in turn, stimulation of Na+/K+-ATPase signaling function led to increased ROS production. This model can definitely be associated with dysfunction in living cells under EMF-exposure.
Therefore, it is clearly indicated that irregular gating of VGICs on plasma and intracellular membranes due to EMF-exposure will most likely trigger ROS overproduction and consequent cellular damage. Although plenty of data connecting ion channel dysfunction and the induction of cell death or cancer have been available for a long time (194,199), the connection between the dysfunction of VGICs and ROS overproduction (175-179,190-192) leading to DNA damage has not perhaps gained the attention it deserves.
Apart from action via ROS/free radicals, DNA damage may be brought about by irregular activation of DNases after alteration of intracellular ionic concentrations. Of the two forms of endonucleases implicated in the initiation of apoptosis, one of them is Ca2+-dependent (DNase I). An increased level of intracellular Ca2+ in some cases is associated with increased apoptosis, possibly due to the activation of DNase I (210). Thus, the possible activation of DNase I by increased levels of intracellular Ca2+ may be an alternative way for DNA damage and related pathologies.
ROS and DNA damage
OH• is considered the most potent oxidant of DNA. The main mechanism for OH• production involves the iron-catalyzed conversion of H2O2 via the Fenton reaction (211): Fe2+ is oxidized by H2O2 to Fe3+, producing an OH• radical and a hydroxide ion (OH−) (Eq. 21). Fe3+ is then reduced back to Fe2+ by another molecule of H2O2, producing a hydroperoxyl radical and a proton (Eq. 22).
| (21) |
| (22) |
The net effect is the conversion of two hydrogen peroxide molecules to produce two different oxygen-radical species, with water (H+ + OH−) as a byproduct.
| (23) |
The OH• radical reacts with any biological molecule in its immediate environment, including DNA. For example, it can break macromolecules (R-R or R-H) or abstract atoms from them (such as the various hydrogen atoms of the deoxyribose) by breakage of covalent bonds. This results in chemical alterations of the macromolecules and production of new free radicals (R• or RO•):
| (24) |
| (25) |
| (26) |
The new free radicals will further react with other molecules resulting in additional chemical alterations. Corresponding evidence for DNA damage by ONOO− is available as well (181).
In conclusion, there is a clear sequence of events starting from the irregular gating of VGICs by EMFs up to DNA damage and related pathologies, including carcinogenesis.
4. Discussion
The present study reviewed experimental and epidemiological findings connecting exposure to purely ELF, and RF (containing ELF) human-made EMFs, with DNA damage and related pathologies, including cancer. It is documented that both such types of human-made EMF-exposure can induce OS (3,34,36-39,43,45,109), DNA damage (1-55,84,85) and infertility (56-71). It is also documented that the same types of EMF-exposure are linked with increased cancer risk both in humans and experimental animals (72-83,86-98,110-114).
We attempted to provide a complete, plausible explanation of these DNA damage-related findings on a biophysical and biochemical basis. According to the ion forced-oscillation mechanism for dysfunction of VGICs (143-146), human-made (polarized and coherent) ELF/ULF EMFs or the ELF/ULF modulation/pulsing/variability components of modern RF/WC EMFs can alter intracellular ionic concentrations by irregular gating of VGICs on cell membranes. This leads to immediate OS by ROS (over)production in the cytosol and/or the mitochondria, which can damage DNA when cells are unable to reinstate electrochemical balance (normal intracellular ionic concentrations). Consequently, DNA damage can lead to reproductive disabilities, neurodegenerative diseases, aging, genetic alterations and cancer.
According to the presented biophysical mechanism, the bioactivity of a polarized/coherent EMF is proportional to its intensity, inversely proportional to its frequency and doubles for pulsed fields, meaning that the ELF/ULF EMFs and even more the pulsing RF EMFs with ELF pulsations such as all WC EMFs, are predicted to be the most bioactive. This explains the recorded effects of purely ELF EMFs (1-5,9,13-18,22,47, 50,72-82,117,212) and those of modulated/pulsing/variable RF EMFs (1,3,4,6-8,19-21,23-46,48,49,51-55,57-71,84-107, 109-114,118,121-126). As emphasized, all types of RF exposure from all types of antennas and WC devices (WC EMFs) necessarily combine RF carrier signals with ELF/ULF components in the form of pulsing, modulation and random variability. The RF carrier signal alone does not contain information. The information is always contained in the ELF signals that modulate the RF (4). Significant experimental evidence shows that the bioactive parameters in a complex signal are its ELF components, and that non-modulated and non-pulsed RF signals alone do not usually induce biological effects (4,44,45,151-159), apart from heating when they possess high enough frequency and intensity (128,168-170). Therefore, the present study suggests that the vast majority of non-thermal effects attributed till now to various types of RF EMF-exposure, are actually due to their ELF/ULF components.
The presented biophysical mechanism and the provided numerical examples show that it is the direct ELF electric fields (and the magnetically induced electric fields in the case of sudden pulses), not the magnetic, that are the bioactive components, in contrast to what has been considered before by health agencies (117), and in agreement with previous experimental findings (191). Although electric fields are less penetrating in living tissue than magnetic fields, penetration depends upon the inverse square root of frequency, and thus ELF electric fields are significantly penetrating. Penetration depends also upon the inverse square root of the medium conductivity (213). Even though seawater is much more conductive than living tissue, ELF electromagnetic waves (thus both the electric and the magnetic parts of the waves) are penetrating several meters into seawater, accommodating communications with submarines (214). Moreover, it is known that isolated tissues respond to externally applied pulsed or sinusoidal ELF electric fields at very low thresholds (~10−3 V/m) similar to those predicted by this theory (143,215-217). This evidence shows that ELF electric fields penetrate enough to induce effects into living tissue, even at very low field intensities. Finally, skin cells, nerve terminals, eyes and organs close to the surface, such as the brain and heart, are directly exposed to externally applied EMFs. For all these reasons, no distinction is made between externally applied ELF electric fields and internally induced ones.
The ion forced-oscillation mechanism/theory was described in the present study by realistic equations based on the forces exerted on mobile ions in the vicinity of the voltage-sensors of VGICs on cell membranes by externally applied human-made (polarized) EMFs. The solution of the basic Eq. 2 resulted in bioactivity conditions connecting the intensity of an applied polarized EMF with its frequency. The bioactivity conditions 8-10, and 16-18, provided the bioactive intensity-frequency combinations for continuous and pulsed electric and magnetic fields. The final numbers explain almost all the experimental and epidemiological findings connecting biological/health effects with human-made EMF-exposure.
Although the mechanism was first published in 2000 (144) based on the available data on the structure and function of the VGICs, newer details on the roles of S1-S6 helices, channel structure, relaxation, hysteresis and gating, have not refuted but verified and extended that knowledge (162,163,165,218-221).
What is more difficult to explain is the existence of non-linear phenomena such as the increased bioactivity 'windows' reported occasionally in the EMF-bioeffects literature, where certain effects are intensified within certain values of an EMF-exposure parameter (intensity in most cases, or frequency) (1,40,151-153,222). The existence of 'windows' shows that the response of living cells/organisms to EMFs is not generally proportional to the aforementioned EMF-parameters. Non-linear responses of living cells have not been explored in depth and it will take a number of years until they are. A possible explanation of observed intensity 'windows' according to the described mechanism has been suggested as being due to an existing upper limit in the membrane gating voltage change (222). Indeed, such an upper limit seems to exist. The VGICs respond to membrane voltage changes from ~30 mV (minimum) to ~100 mV (maximum) where the conductivity of the channel saturates (218,221). Apart from this possible explanation, no other explanation for the observed 'window' effects has been provided so far.
An effect not included in the bioactivity Eqs. 11 and 12 is the increased bioactivity of highly and unpredictably varying exposure such as those from WC devices (including mobile phones and Wi-Fi) and corresponding antennas (4,121,122). The described mechanism results in accurate predictions when the applied EMFs have constant parameters (intensity and frequency, among others). When the parameters are highly and unpredictably variable, the mechanism, and any possible mechanism, can only estimate effects according to the average and maximum exposure values of the varying EMFs. Finally, the bioactivity equations include field (and tissue) parameters and not exposure variables such as exposure duration or intermittence, which are also very important (16,17,19,41,55,122). One way to include such parameters is to multiply the right parts of Eqs. 11 and 12 by certain coefficient(s), which would be estimated experimentally. This could be a subject for future development of the theory.
This theory has successfully explained for the first time the sensing of upcoming earthquakes by animals, and the sensing of upcoming thunderstorms by sensitive individuals through the action of the partially polarized natural EMFs associated with these phenomena (146,223).
Any 'mechanism' in science (particularly in physics) must be based on simple and reasonable postulates, and must necessarily be expressed quantitatively (by solvable equations and numbers). The values of the different parameters in the equations must be based on physical/molecular data. Qualitative descriptions alone or incomplete quantitative descriptions based on incomplete or unsolvable equations do not constitute a 'mechanism'. The presented biophysical mechanism (143-146) is the only one that fulfills the afore-mentioned criteria in the case of EMF-induced bioeffects. Previous important attempts on mechanisms focusing on ions moving inside membrane channels or other proteins (224-227) were not successful, mainly for the following reasons: i) They had not taken into account damping and restoration forces (224,226), or did not calculate them (225,227). The difficulty was not related with considering such forces, as this is standard in oscillation mechanics, but with calculating their parameters such as β and ωo, or the maximum velocity of the ion (uo) within a channel. ii) They did not consider coordinated motion of several ions oscillating in parallel and in phase due to polarization and coherence, exerting additive forces on channel sensors, which prevail against the greater but chaotic forces due to the random thermal motion of the ions. iii) They focused on magnetic fields and magnetically induced electric ones, and ignored externally applied electric fields, which eventually seem to be more bioactive (191). iv) They did not result in numbers for field intensity versus frequency necessary to affect cells, although some experimental reports have indicated bioactive frequencies close to those predicted by Liboff's ion cyclotron resonance (ICR) model (224,228), possibly indicating some additional/secondary resonance mechanism involving ICR phenomenon (169). v) Apart from the study by Balcavage et al (226), there was no focus on the gating of VGICs, which is by far a more probable event to initiate biological effects, but simply on the motion of ions within channels/proteins.
Several other suggestions on possible mechanisms also face problems on fundamental issues (229-231). What is termed by Pall 'VGCC activation mechanism' and presented as his own discovery is none other than the mechanism presented here. A commentary paper/letter to the editor was published on this major ethical issue (129). An extended review of suggested mechanisms has been written by Creasey and Goldberg (169).
It has been claimed that the ELF components of complex RF-ELF EMFs of WC need to be 'demodulated' in order to be sensed by living organisms (232). 'Demodulated' or not, the fact is that the ELF components of modulated/pulsed WC signals can be directly sensed by both ELF meters/spectrum analyzers and living organisms (40,55).
Although there have been successive publications of this mechanism since 2000 (144), the subject is of great importance and in each consecutive publication additional important aspects are elucidated and/or refined. In our previous study in 2002 (145), the mechanism was extended to include oscillating magnetic fields and the thermal noise problem was discussed in more depth, while in 2015 (143) the mechanism was applied to reveal the importance of polarization/coherence in the bioactivity of man-made EMFs. In 2017 (223) and 2020 (146), it was applied to explain the sensing of upcoming thunderstorms and earthquakes, respectively, by sensitive humans/animals. In the present study, several aspects are further refined, including: i) The distance of S4 sensors from the channel pore; ii) more details on damping coefficient β and bioactivity constant k (Eq. 11); iii) further explanation of the role of the constant term in the solution (Eq. 3); iv) the similarity of proton voltage-gated channels with the other VGICs; v) numerical examples demonstrating the ability of the pulsing ELF electric and magnetic fields of 2G/3G/4G MT, DECT, Wi-Fi, Bluetooth, and the power line ELF fields to induce biological/health effects; vi) the velocity of oscillating ions; vii) bioactivity diagram extended to intensities down to 10−5 V/m; and viii) discussion on other suggested mechanisms.
Moreover, the present study documented how the impaired function of VGICs on the membranes of living cells triggers (over)production of free radicals/ROS, such as the most potent OH• produced by H2O2 via the Fenton reaction, and ONOO− produced by NO•. These are considered the main damaging species for DNA and other critical biological molecules. It is estimated that approximately two-thirds of the DNA damage caused by ionizing radiation is due to OH• (233,234). Although OH• can only diffuse at distances comparable to the length of a macromolecule, H2O2 can move to any intracellular site. Thus, even though the most potent OH• due to its high reactivity has an extremely short lifetime (of the order of 10−9-10−4 s depending on the presence of other molecules) it can be formed by H2O2 at any location within the cell (including the nucleus) and act instantly upon DNA or other macromolecules (233,234). As for NO•/ONOO−, they can be diffused anywhere in the cell and thus directly affect any molecule, including DNA (181). Even though the present study identified specific pathways of ROS over-production or the release of DNases connected with disrupted ionic concentrations in EMF-exposed cells, the exact molecular mechanisms need to be further explored and elucidated.
Finally, the present study discussed how unrepaired/misrepaired DNA lesions/damage such as strand breaks, covalent bond breakage or nucleotide base damages, lead to cell senescence, cell death or mutations, and related pathologies, including cancer. Even though effective mechanisms have evolved in all animals/cells for repairing DNA damage induced by environmental stressors, it is very different when the damaging events are isolated or random (e.g. radioactive particles or γ-photons of cosmic/natural radioactivity, or sporadic x-ray diagnostic exposure), compared with persisting/repeated exposure to cytotoxic agents, even when these agents are relatively weaker. Exposure to human-made EMFs and especially to the most detrimental ones from WC antennas/devices and high-voltage transmission lines (4) has become a new reality in modern life. Billions of people are exposed to such EMFs on a daily basis. Although they are less cytotoxic than radioactivity or certain cytotoxic chemicals, they represent the most persistent daily cytotoxic stressors against which any repair mechanisms cannot be efficient enough. By contrast, previously existing cytotoxic agents expose us randomly as isolated events. When an organism is constantly under OS due to a totally new cytotoxic agent such as human-made EMFs, no protective mechanism, evolved in the billions of years of biological evolution to protect from natural (non-polarized) EMFs/radiation or isolated hazardous events, can be effective enough.
The repair capability of cells in response to DNA damage is crucial for the final outcome. The threshold of damage above which it becomes irreparable depends on cell type and the health and status of the organism. An organism with poor health and/or under stress and inflammation due to OS is expected to have decreased repair capability and increased cancer risk. Epigenetic effects such as altered gene expression may also lead to cellular dysfunction and carcinogenesis (133,235,236).
Both DNA damage and alterations in protein synthesis, especially increased levels of stress proteins, are reported to be induced similarly by both ELF and pulsing RF EMFs (237,238). However, the effects of pulsing RF were attributed to the carrier frequency, and it was not considered that perhaps in both cases (ELF and pulsing RF) the ELF components might be responsible for the effects, as suggested now by the present study.
To the best of our knowledge, the present study provides for the first time a complete and precise biophysical/biochemical picture to explain the great number of experimental and epidemiological findings connecting human-made EMF exposure with DNA damage and related pathologies such as cancer, infertility and neurodegenerative diseases.
The long-existing experimental and epidemiological findings connecting exposure to human-made EMFs and DNA damage, infertility and cancer, are now explained by the presented complete mechanism. The present study should provide a basis for further research and encourage health authorities to take measures for the protection of life on Earth against unrestricted use of human-made EMFs.
Acknowledgments
Not applicable.
Abbreviations
- DECT
digitally enhanced cordless telecommunications
- ELF
extremely low frequency
- EMF
electromagnetic field
- MT
mobile telephony
- OS
oxidative stress
- RF
radio frequency
- ROS
reactive oxygen species
- ULF
ultra low frequency
- VGICs
voltage-gated ion channels
- VGCCs
voltage-gated calcium channels
- WC
wireless communications
- Wi-Fi
wireless fidelity
- 2G/3G/4G/5G
second/third/fourth/fifth-generation of mobile telephony
Funding Statement
The study is supported by the Special Account for Research Grants of the National and Kapodistrian University of Athens (grant number 16599).
Availability of data and materials
Not applicable.
Authors' contributions
DJP designed the study and wrote the main manuscript. AK verified all equations and calculations. IY coauthored section 3 on biochemical processes. GPC reviewed and evaluated all data. All authors have read and approved the manuscript. Data authentication is not applicable.
Ethics approval and consent to participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
References
- 1.Goodman EM, Greenebaum B, Marron MT. Effects of electromagnetic fields on molecules and cells. Int Rev Cytol. 1995;158:279–338. doi: 10.1016/S0074-7696(08)62489-4. [DOI] [PubMed] [Google Scholar]
- 2.Santini MT, Ferrante A, Rainaldi G, Indovina P, Indovina PL. Extremely low frequency (ELF) magnetic fields and apoptosis: A review. Int J Radiat Biol. 2005;81:1–11. doi: 10.1080/09553000400029502. [DOI] [PubMed] [Google Scholar]
- 3.Phillips JL, Singh NP, Lai H. Electromagnetic fields and DNA damage. Pathophysiology. 2009;16:79–88. doi: 10.1016/j.pathophys.2008.11.005. [DOI] [PubMed] [Google Scholar]
- 4.Panagopoulos DJ. Comparing DNA damage induced by mobile telephony and other types of man-made electromagnetic fields. Mutat Res Rev Mutat Res. 2019;781:53–62. doi: 10.1016/j.mrrev.2019.03.003. [DOI] [PubMed] [Google Scholar]
- 5.Delgado JMR. Biological effects of extremely low frequency electromagnetic fields. J Bioelectricity. 1985;4:75–92. doi: 10.3109/15368378509040362. [DOI] [Google Scholar]
- 6.Garaj-Vrhovac V, Horvat D, Koren Z. The effect of microwave radiation on the cell genome. Mutat Res. 1990;243:87–93. doi: 10.1016/0165-7992(90)90028-I. [DOI] [PubMed] [Google Scholar]
- 7.Garaj-Vrhovac V, Horvat D, Koren Z. The relationship between colony-forming ability, chromosome aberrations and incidence of micronuclei in V79 Chinese hamster cells exposed to microwave radiation. Mutat Res. 1991;263:143–149. doi: 10.1016/0165-7992(91)90054-8. [DOI] [PubMed] [Google Scholar]
- 8.Garaj-Vrhovac V, Fucić A, Horvat D. The correlation between the frequency of micronuclei and specific chromosome aberrations in human lymphocytes exposed to microwave radiation in vitro. Mutat Res. 1992;281:181–186. doi: 10.1016/0165-7992(92)90006-4. [DOI] [PubMed] [Google Scholar]
- 9.Ma TH, Chu KC. Effect of the extremely low frequency (ELF) electromagnetic field (EMF) on developing embryos of the fruit fly (Drosophila melanogaster L.) Mutat Res. 1993;303:35–39. doi: 10.1016/0165-7992(93)90006-H. [DOI] [PubMed] [Google Scholar]
- 10.Sarkar S, Ali S, Behari J. Effect of low power microwave on the mouse genome: A direct DNA analysis. Mutat Res. 1994;320:141–147. doi: 10.1016/0165-1218(94)90066-3. [DOI] [PubMed] [Google Scholar]
- 11.Lai H, Singh NP. Acute low-intensity microwave exposure increases DNA single-strand breaks in rat brain cells. Bioelectromagnetics. 1995;16:207–210. doi: 10.1002/bem.2250160309. [DOI] [PubMed] [Google Scholar]
- 12.Lai H, Singh NP. Single- and double-strand DNA breaks in rat brain cells after acute exposure to radiofrequency electro-magnetic radiation. Int J Radiat Biol. 1996;69:513–521. doi: 10.1080/095530096145814. [DOI] [PubMed] [Google Scholar]
- 13.Lai H, Singh NP. Acute exposure to a 60 Hz magnetic field increases DNA strand breaks in rat brain cells. Bioelectromagnetics. 1997;18:156–165. doi: 10.1002/(SICI)1521-186X(1997)18:2<156::AID-BEM8>3.0.CO;2-1. [DOI] [PubMed] [Google Scholar]
- 14.Svedenstål BM, Johanson KJ, Mild KH. DNA damage induced in brain cells of CBA mice exposed to magnetic fields. In Vivo. 1999;13:551–552. [PubMed] [Google Scholar]
- 15.Koana T, Okada MO, Takashima Y, Ikehata M, Miyakoshi J. Involvement of eddy currents in the mutagenicity of ELF magnetic fields. Mutat Res. 2001;476:55–62. doi: 10.1016/S0027-5107(01)00082-3. [DOI] [PubMed] [Google Scholar]
- 16.Ivancsits S, Diem E, Pilger A, Rüdiger HW, Jahn O. Induction of DNA strand breaks by intermittent exposure to extremely-low-frequency electromagnetic fields in human diploid fibroblasts. Mutat Res. 2002;519:1–13. doi: 10.1016/S1383-5718(02)00109-2. [DOI] [PubMed] [Google Scholar]
- 17.Ivancsits S, Diem E, Jahn O, Rüdiger HW. Intermittent extremely low frequency electromagnetic fields cause DNA damage in a dose-dependent way. Int Arch Occup Environ Health. 2003;76:431–436. doi: 10.1007/s00420-003-0446-5. [DOI] [PubMed] [Google Scholar]
- 18.Winker R, Ivancsits S, Pilger A, Adlkofer F, Rüdiger HW. Chromosomal damage in human diploid fibroblasts by intermittent exposure to extremely low-frequency electromagnetic fields. Mutat Res. 2005;585:43–49. doi: 10.1016/j.mrgentox.2005.04.013. [DOI] [PubMed] [Google Scholar]
- 19.Diem E, Schwarz C, Adlkofer F, Jahn O, Rüdiger H. Non-thermal DNA breakage by mobile-phone radiation (1800 MHz) in human fibroblasts and in transformed GFSH-R17 rat granulosa cells in vitro. Mutat Res. 2005;583:178–183. doi: 10.1016/j.mrgentox.2005.03.006. [DOI] [PubMed] [Google Scholar]
- 20.Mausset-Bonnefont AL, Hirbec H, Bonnefont X, Privat A, Vignon J, de Sèze R. Acute exposure to GSM 900-MHz electromagnetic fields induces glial reactivity and biochemical modifications in the rat brain. Neurobiol Dis. 2004;17:445–454. doi: 10.1016/j.nbd.2004.07.004. [DOI] [PubMed] [Google Scholar]
- 21.Ji S, Oh E, Sul D, Choi JW, Park H, Lee E. DNA damage of lymphocytes in volunteers after 4 h use of mobile phone. J Prev Med Public Health. 2004;37:373–380. [PubMed] [Google Scholar]
- 22.Hong R, Zhang Y, Liu Y, Weng EQ. Effects of extremely low frequency electromagnetic fields on DNA of testicular cells and sperm chromatin structure in mice. Zhonghua Lao Dong Wei Sheng Zhi Ye Bing Za Zhi. 2005;23:414–417. In Chinese. [PubMed] [Google Scholar]
- 23.Belyaev IY, Hillert L, Protopopova M, Tamm C, Malmgren LO, Persson BR, Selivanova G, Harms-Ringdahl M. 915 MHz microwaves and 50 Hz magnetic field affect chromatin conformation and 53BP1 foci in human lymphocytes from hypersensitive and healthy persons. Bioelectromagnetics. 2005;26:173–184. doi: 10.1002/bem.20103. [DOI] [PubMed] [Google Scholar]
- 24.Markovà E, Hillert L, Malmgren L, Persson BR, Belyaev IY. Microwaves from GSM mobile telephones affect 53BP1 and gamma-H2AX foci in human lymphocytes from hypersensitive and healthy persons. Environ Health Perspect. 2005;113:1172–1177. doi: 10.1289/ehp.7561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Aitken RJ, Bennetts LE, Sawyer D, Wiklendt AM, King BV. Impact of radio frequency electromagnetic radiation on DNA integrity in the male germline. Int J Androl. 2005;28:171–179. doi: 10.1111/j.1365-2605.2005.00531.x. [DOI] [PubMed] [Google Scholar]
- 26.Nikolova T, Czyz J, Rolletschek A, Blyszczuk P, Fuchs J, Jovtchev G, Schuderer J, Kuster N, Wobus AM. Electromagnetic fields affect transcript levels of apoptosis-related genes in embryonic stem cell-derived neural progenitor cells. FASEB J. 2005;19:1686–1688. doi: 10.1096/fj.04-3549fje. [DOI] [PubMed] [Google Scholar]
- 27.Zhang DY, Xu ZP, Chiang H, Lu DQ, Zeng QL. Effects of GSM 1800 MHz radiofrequency electromagnetic fields on DNA damage in Chinese hamster lung cells. Zhonghua Yu Fang Yi Xue Za Zhi. 2006;40:149–152. In Chinese. [PubMed] [Google Scholar]
- 28.Lixia S, Yao K, Kaijun W, Deqiang L, Huajun H, Xiangwei G, Baohong W, Wei Z, Jianling L, Wei W. Effects of 1.8 GHz radiofrequency field on DNA damage and expression of heat shock protein 70 in human lens epithelial cells. Mutat Res. 2006;602:135–142. doi: 10.1016/j.mrfmmm.2006.08.010. [DOI] [PubMed] [Google Scholar]
- 29.Ferreira AR, Knakievicz T, Pasquali MA, Gelain DP, Dal-Pizzol F, Fernández CE, de Salles AA, Ferreira HB, Moreira JC. Ultra high frequency-electromagnetic field irradiation during pregnancy leads to an increase in erythrocytes micronuclei incidence in rat offspring. Life Sci. 2006;80:43–50. doi: 10.1016/j.lfs.2006.08.018. [DOI] [PubMed] [Google Scholar]
- 30.Panagopoulos DJ, Chavdoula ED, Nezis IP, Margaritis LH. Cell death induced by GSM 900-MHz and DCS 1800-MHz mobile telephony radiation. Mutat Res. 2007;626:69–78. doi: 10.1016/j.mrgentox.2006.08.008. [DOI] [PubMed] [Google Scholar]
- 31.Yan JG, Agresti M, Bruce T, Yan YH, Granlund A, Matloub HS. Effects of cellular phone emissions on sperm motility in rats. Fertil Steril. 2007;88:957–964. doi: 10.1016/j.fertnstert.2006.12.022. [DOI] [PubMed] [Google Scholar]
- 32.Yao K, Wu W, Wang K, Ni S, Ye P, Yu Y, Ye J, Sun L. Electromagnetic noise inhibits radiofrequency radiation-induced DNA damage and reactive oxygen species increase in human lens epithelial cells. Mol Vis. 2008;14:964–969. [PMC free article] [PubMed] [Google Scholar]
- 33.Yadav AS, Sharma MK. Increased frequency of micronucleated exfoliated cells among humans exposed in vivo to mobile telephone radiations. Mutat Res. 2008;650:175–180. doi: 10.1016/j.mrgentox.2007.11.005. [DOI] [PubMed] [Google Scholar]
- 34.Sokolovic D, Djindjic B, Nikolic J, Bjelakovic G, Pavlovic D, Kocic G, Krstic D, Cvetkovic T, Pavlovic V. Melatonin reduces oxidative stress induced by chronic exposure of microwave radiation from mobile phones in rat brain. J Radiat Res. 2008;49:579–586. doi: 10.1269/jrr.07077. [DOI] [PubMed] [Google Scholar]
- 35.Lee KS, Choi JS, Hong SY, Son TH, Yu K. Mobile phone electromagnetic radiation activates MAPK signaling and regulates viability in Drosophila. Bioelectromagnetics. 2008;29:371–379. doi: 10.1002/bem.20395. [DOI] [PubMed] [Google Scholar]
- 36.De Iuliis GN, Newey RJ, King BV, Aitken RJ. Mobile phone radiation induces reactive oxygen species production and DNA damage in human spermatozoa in vitro. PLoS One. 2009;4:e6446. doi: 10.1371/journal.pone.0006446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Agarwal A, Desai NR, Makker K, Varghese A, Mouradi R, Sabanegh E, Sharma R. Effects of radiofrequency electromagnetic waves (RF-EMW) from cellular phones on human ejaculated semen: An in vitro pilot study. Fertil Steril. 2009;92:1318–1325. doi: 10.1016/j.fertnstert.2008.08.022. [DOI] [PubMed] [Google Scholar]
- 38.Mailankot M, Kunnath AP, Jayalekshmi H, Koduru B, Valsalan R. Radio frequency electromagnetic radiation (RF-EMR) from GSM (0.9/1.8 GHz) mobile phones induces oxidative stress and reduces sperm motility in rats. Clinics (Sao Paulo) 2009;64:561–565. doi: 10.1590/S1807-59322009000600011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Luukkonen J, Hakulinen P, Mäki-Paakkanen J, Juutilainen J, Naarala J. Enhancement of chemically induced reactive oxygen species production and DNA damage in human SH-SY5Y neuroblastoma cells by 872 MHz radiofrequency radiation. Mutat Res. 2009;662:54–58. doi: 10.1016/j.mrfmmm.2008.12.005. [DOI] [PubMed] [Google Scholar]
- 40.Panagopoulos DJ, Chavdoula ED, Margaritis LH. Bioeffects of mobile telephony radiation in relation to its intensity or distance from the antenna. Int J Radiat Biol. 2010;86:345–357. doi: 10.3109/09553000903567961. [DOI] [PubMed] [Google Scholar]
- 41.Chavdoula ED, Panagopoulos DJ, Margaritis LH. Comparison of biological effects between continuous and intermittent exposure to GSM-900-MHz mobile phone radiation. Detection of apoptotic cell death features Mutat Res. 2010;700:51–61. doi: 10.1016/j.mrgentox.2010.05.008. [DOI] [PubMed] [Google Scholar]
- 42.Guler G, Tomruk A, Ozgur E, Seyhan N. The effect of radio-frequency radiation on DNA and lipid damage in non-pregnant and pregnant rabbits and their newborns. Gen Physiol Biophys. 2010;29:59–66. doi: 10.4149/gpb_2010_01_59. [DOI] [PubMed] [Google Scholar]
- 43.Tomruk A, Guler G, Dincel AS. The influence of 1800 MHz GSM-like signals on hepatic oxidative DNA and lipid damage in nonpregnant, pregnant, and newly born rabbits. Cell Biochem Biophys. 2010;56:39–47. doi: 10.1007/s12013-009-9068-1. [DOI] [PubMed] [Google Scholar]
- 44.Franzellitti S, Valbonesi P, Ciancaglini N, Biondi C, Contin A, Bersani F, Fabbri E. Transient DNA damage induced by high-frequency electromagnetic fields (GSM 1.8 GHz) in the human trophoblast HTR-8/SVneo cell line evaluated with the alkaline comet assay. Mutat Res. 2010;683:35–42. doi: 10.1016/j.mrfmmm.2009.10.004. [DOI] [PubMed] [Google Scholar]
- 45.Campisi A, Gulino M, Acquaviva R, Bellia P, Raciti G, Grasso R, Musumeci F, Vanella A, Triglia A. Reactive oxygen species levels and DNA fragmentation on astrocytes in primary culture after acute exposure to low intensity microwave electromagnetic field. Neurosci Lett. 2010;473:52–55. doi: 10.1016/j.neulet.2010.02.018. [DOI] [PubMed] [Google Scholar]
- 46.Panagopoulos DJ. Effect of microwave exposure on the ovarian development of Drosophila melanogaster. Cell Biochem Biophys. 2012;63:121–132. doi: 10.1007/s12013-012-9347-0. [DOI] [PubMed] [Google Scholar]
- 47.Panagopoulos DJ, Karabarbounis A, Lioliousis C. ELF alternating magnetic field decreases reproduction by DNA damage induction. Cell Biochem Biophys. 2013;67:703–716. doi: 10.1007/s12013-013-9560-5. [DOI] [PubMed] [Google Scholar]
- 48.Liu C, Gao P, Xu SC, Wang Y, Chen CH, He MD, Yu ZP, Zhang L, Zhou Z. Mobile phone radiation induces mode-dependent DNA damage in a mouse spermatocyte-derived cell line: A protective role of melatonin. Int J Radiat Biol. 2013;89:993–1001. doi: 10.3109/09553002.2013.811309. [DOI] [PubMed] [Google Scholar]
- 49.Pesnya DS, Romanovsky AV. Comparison of cytotoxic and genotoxic effects of plutonium-239 alpha particles and mobile phone GSM 900 radiation in the Allium cepa test. Mutat Res. 2013;750:27–33. doi: 10.1016/j.mrgentox.2012.08.010. [DOI] [PubMed] [Google Scholar]
- 50.Mihai CT, Rotinberg P, Brinza F, Vochita G. Extremely low-frequency electromagnetic fields cause DNA strand breaks in normal cells. J Environ Health Sci Eng. 2014;12:15. doi: 10.1186/2052-336X-12-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Daroit NB, Visioli F, Magnusson AS, Vieira GR, Rados PV. Cell phone radiation effects on cytogenetic abnormalities of oral mucosal cells. Braz Oral Res. 2015;29:1–8. doi: 10.1590/1807-3107BOR-2015.vol29.0114. [DOI] [PubMed] [Google Scholar]
- 52.Banerjee S, Singh NN, Sreedhar G, Mukherjee S. Analysis of the genotoxic effects of mobile phone radiation using buccal micronucleus assay: A comparative evaluation. J Clin Diagn Res. 2016;10:ZC82–ZC85. doi: 10.7860/JCDR/2016/17592.7505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.D'Silva MH, Swer RT, Anbalagan J, Rajesh B. Effect of radiofrequency radiation emitted from 2G and 3G cell phone on developing liver of chick embryo-a comparative study. J Clin Diagn Res. 2017;11:AC05–AC09. doi: 10.7860/JCDR/2017/26360.10275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Panagopoulos DJ. Chromosome damage in human cells induced by UMTS mobile telephony radiation. Gen Physiol Biophys. 2019;38:445–454. doi: 10.4149/gpb_2019032. [DOI] [PubMed] [Google Scholar]
- 55.Panagopoulos DJ. Comparing chromosome damage induced by mobile telephony radiation and a high caffeine dose: Effect of combination and exposure duration. Gen Physiol Biophys. 2020;39:531–544. doi: 10.4149/gpb_2020036. [DOI] [PubMed] [Google Scholar]
- 56.Magras IN, Xenos TD. RF radiation-induced changes in the prenatal development of mice. Bioelectromagnetics. 1997;18:455–461. doi: 10.1002/(SICI)1521-186X(1997)18:6<455::AID-BEM8>3.0.CO;2-1. [DOI] [PubMed] [Google Scholar]
- 57.Panagopoulos DJ, Karabarbounis A, Margaritis LH. Effect of GSM 900-MHz mobile phone radiation on the reproductive capacity of Drosophila melanogaster. Electromagn Biol Med. 2004;23:29–43. doi: 10.1081/JBC-120039350. [DOI] [Google Scholar]
- 58.Panagopoulos DJ, Chavdoula ED, Karabarbounis A, Margaritis LH. Comparison of bioactivity between GSM 900 MHz and DCS 1800 MHz mobile telephony radiation. Electromagn Biol Med. 2007;26:33–44. doi: 10.1080/15368370701205644. [DOI] [PubMed] [Google Scholar]
- 59.Wdowiak A, Wdowiak L, Wiktor H. Evaluation of the effect of using mobile phones on male fertility. Ann Agric Environ Med. 2007;14:169–172. [PubMed] [Google Scholar]
- 60.Agarwal A, Deepinder F, Sharma RK, Ranga G, Li J. Effect of cell phone usage on semen analysis in men attending infertility clinic: An observational study. Fertil Steril. 2008;89:124–128. doi: 10.1016/j.fertnstert.2007.01.166. [DOI] [PubMed] [Google Scholar]
- 61.Batellier F, Couty I, Picard D, Brillard JP. Effects of exposing chicken eggs to a cell phone in 'call' position over the entire incubation period. Theriogenology. 2008;69:737–745. doi: 10.1016/j.theriogenology.2007.12.006. [DOI] [PubMed] [Google Scholar]
- 62.Gul A, Celebi H, Uğraş S. The effects of microwave emitted by cellular phones on ovarian follicles in rats. Arch Gynecol Obstet. 2009;280:729–733. doi: 10.1007/s00404-009-0972-9. [DOI] [PubMed] [Google Scholar]
- 63.Sharma VP, Kumar NR. Changes in honey bee behaviour and biology under the influence of cell phone radiations. Curr Sci. 2010;98:1376–1378. [Google Scholar]
- 64.La Vignera S, Condorelli RA, Vicardi E, D'Agata R, Calogero AE. Effects of the exposure to mobile phones on male reproduction: A review of the literature. J Androl. 2012;33:350–356. doi: 10.2164/jandrol.111.014373. [DOI] [PubMed] [Google Scholar]
- 65.Balmori A. Possible effects of electromagnetic fields from phone masts on a population of white stork (Ciconia ciconia) Electromagn Biol Med. 2005;24:109–119. doi: 10.1080/15368370500205472. [DOI] [Google Scholar]
- 66.Balmori A, Hallberg O. The urban decline of the house sparrow (Passer domesticus): A possible link with electromagnetic radiation. Electromagn Biol Med. 2007;26:141–151. doi: 10.1080/15368370701410558. [DOI] [PubMed] [Google Scholar]
- 67.Everaert J, Bauwens D. A possible effect of electromagnetic radiation from mobile phone base stations on the number of breeding house sparrows (Passer domesticus) Electromagn Biol Med. 2007;26:63–72. doi: 10.1080/15368370701205693. [DOI] [PubMed] [Google Scholar]
- 68.Bacandritsos N, Granato A, Budge G, Papanastasiou I, Roinioti E, Caldon M, Falcaro C, Gallina A, Mutinelli F. Sudden deaths and colony population decline in Greek honey bee colonies. J Invertebr Pathol. 2010;105:335–340. doi: 10.1016/j.jip.2010.08.004. [DOI] [PubMed] [Google Scholar]
- 69.Cucurachi S, Tamis WL, Vijver MG, Peijnenburg WJ, Bolte JF, de Snoo GR. A review of the ecological effects of radio-frequency electromagnetic fields (RF-EMF) Environ Int. 2013;51:116–140. doi: 10.1016/j.envint.2012.10.009. [DOI] [PubMed] [Google Scholar]
- 70.Balmori A. The incidence of electromagnetic pollution on the amphibian decline: Is this an important piece of the puzzle? Toxicol Environ Chem. 2006;88:287–299. doi: 10.1080/02772240600687200. [DOI] [Google Scholar]
- 71.Balmori A. Mobile phone mast effects on common frog (Rana temporaria) tadpoles: The city turned into a laboratory. Electromagn Biol Med. 2010;29:31–35. doi: 10.3109/15368371003685363. [DOI] [PubMed] [Google Scholar]
- 72.Wertheimer N, Leeper E. Electrical wiring configurations and childhood cancer. Am J Epidemiol. 1979;109:273–284. doi: 10.1093/oxfordjournals.aje.a112681. [DOI] [PubMed] [Google Scholar]
- 73.Savitz DA, Wachtel H, Barnes F, John EM, Tvrdik JG. Case-control study of childhood cancer and exposure to 60-Hz magnetic fields. Am J Epidemiol. 1988;128:21–38. doi: 10.1093/oxfordjournals.aje.a114943. [DOI] [PubMed] [Google Scholar]
- 74.Coleman MP, Bell CM, Taylor HL, Primic-Zakelj M. Leukaemia and residence near electricity transmission equipment: A case-control study. Br J Cancer. 1989;60:793–798. doi: 10.1038/bjc.1989.362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Feychting M, Ahlbom A. Magnetic fields and cancer in children residing near Swedish high-voltage power lines. Am J Epidemiol. 1993;138:467–481. doi: 10.1093/oxfordjournals.aje.a116881. [DOI] [PubMed] [Google Scholar]
- 76.Feychting M, Ahlbom A. Magnetic fields, leukemia, and central nervous system tumors in Swedish adults residing near high-voltage power lines. Epidemiology. 1994;5:501–509. [PubMed] [Google Scholar]
- 77.Feychting M, Ahlbom A. Childhood leukemia and residential exposure to weak extremely low frequency magnetic fields. Environ Health Perspect. 1995;103(Suppl 2):S59–S62. doi: 10.1289/ehp.95103s259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Coghill RW, Steward J, Philips A. Extra low frequency electric and magnetic fields in the bed place of children diagnosed with leukaemia: A case-control study. Eur J Cancer Prev. 1996;5:153–158. doi: 10.1097/00008469-199606000-00002. [DOI] [PubMed] [Google Scholar]
- 79.Ahlbom A, Day N, Feychting M, Roman E, Skinner J, Dockerty J, Linet M, McBride M, Michaelis J, Olsen JH, et al. A pooled analysis of magnetic fields and childhood leukaemia. Br J Cancer. 2000;83:692–698. doi: 10.1054/bjoc.2000.1376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Greenland S, Sheppard AR, Kaune WT, Poole C, Kelsh MA. A pooled analysis of magnetic fields, wire codes, and childhood leukemia. Childhood leukemia-EMF study group Epidemiology. 2000;11:624–634. doi: 10.1097/00001648-200011000-00003. [DOI] [PubMed] [Google Scholar]
- 81.Draper G, Vincent T, Kroll ME, Swanson J. Childhood cancer in relation to distance from high voltage power lines in England and Wales: A case-control study. BMJ. 2005;330:1290. doi: 10.1136/bmj.330.7503.1290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Kheifets L, Ahlbom A, Crespi CM, Draper G, Hagihara J, Lowenthal RM, Mezei G, Oksuzyan S, Schüz J, Swanson J, et al. Pooled analysis of recent studies on magnetic fields and childhood leukaemia. Br J Cancer. 2010;103:1128–1135. doi: 10.1038/sj.bjc.6605838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Hallberg O, Johansson O. Melanoma incidence and frequency modulation (FM) broadcasting. Arch Environ Health. 2002;57:32–40. doi: 10.1080/00039890209602914. [DOI] [PubMed] [Google Scholar]
- 84.Gulati S, Yadav A, Kumar N, Kanupriya, Aggarwal NK, Kumar R, Gupta R. Effect of GSTM1 and GSTT1 polymorphisms on genetic damage in humans populations exposed to radiation from mobile towers. Arch Environ Contam Toxicol. 2016;70:615–625. doi: 10.1007/s00244-015-0195-y. [DOI] [PubMed] [Google Scholar]
- 85.Zothansiama, Zosangzuali M, Lalramdinpuii M, Jagetia GC. Impact of radiofrequency radiation on DNA damage and anti-oxidants in peripheral blood lymphocytes of humans residing in the vicinity of mobile phone base stations. Electromagn Biol Med. 2017;36:295–305. doi: 10.1080/15368378.2017.1350584. [DOI] [PubMed] [Google Scholar]
- 86.Hardell L, Carlberg M, Söderqvist F, Mild KH, Morgan LL. Long-term use of cellular phones and brain tumours: Increased risk associated with use for > or =10 years. Occup Environ Med. 2007;64:626–632. doi: 10.1136/oem.2006.029751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Hardell L, Carlberg M, Hansson Mild K. Epidemiological evidence for an association between use of wireless phones and tumor diseases. Pathophysiology. 2009;16:113–122. doi: 10.1016/j.pathophys.2009.01.003. [DOI] [PubMed] [Google Scholar]
- 88.Khurana VG, Teo C, Kundi M, Hardell L, Carlberg M. Cell phones and brain tumors: A review including the long-term epidemiologic data. Surg Neurol. 2009;72:205–214. doi: 10.1016/j.surneu.2009.01.019. [DOI] [PubMed] [Google Scholar]
- 89.Hardell L, Carlberg M. Mobile phones, cordless phones and the risk for brain tumours. Int J Oncol. 2009;35:5–17. doi: 10.3892/ijo_00000307. [DOI] [PubMed] [Google Scholar]
- 90.Hardell L, Carlberg M, Söderqvist F, Mild KH. Pooled analysis of case-control studies on acoustic neuroma diagnosed 1997-2003 and 2007-2009 and use of mobile and cordless phones. Int J Oncol. 2013;43:1036–1044. doi: 10.3892/ijo.2013.2025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Hardell L, Carlberg M, Söderqvist F, Mild KH. Case-control study of the association between malignant brain tumours diagnosed between 2007 and 2009 and mobile and cordless phone use. Int J Oncol. 2013;43:1833–1845. doi: 10.3892/ijo.2013.2111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Hardell L, Carlberg M, Hansson Mild K. Use of mobile phones and cordless phones is associated with increased risk for glioma and acoustic neuroma. Pathophysiology. 2013;20:85–110. doi: 10.1016/j.pathophys.2012.11.001. [DOI] [PubMed] [Google Scholar]
- 93.Wang Y, Guo X. Meta-analysis of association between mobile phone use and glioma risk. J Cancer Res Ther. 2016;12(Suppl):C298–C300. doi: 10.4103/0973-1482.200759. [DOI] [PubMed] [Google Scholar]
- 94.Carlberg M, Hardell L. Evaluation of mobile phone and cordless phone use and glioma risk using the bradford hill viewpoints from 1965 on association or causation. Biomed Res Int. 2017;2017:9218486. doi: 10.1155/2017/9218486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Hardell L. Effects of mobile phones on children's and adolescents' health: A commentary. Child Dev. 2018;89:137–140. doi: 10.1111/cdev.12831. [DOI] [PubMed] [Google Scholar]
- 96.Momoli F, Siemiatycki J, McBride ML, Parent MÉ, Richardson L, Bedard D, Platt R, Vrijheid M, Cardis E, Krewski D. Probabilistic multiple-bias modelling applied to the Canadian data from the INTERPHONE study of mobile phone use and risk of glioma, meningioma, acoustic neuroma, and parotid gland tumors. Am J Epidemiol. 2017;186:885–893. doi: 10.1093/aje/kwx157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Miller AB, Morgan LL, Udasin I, Davis DL. Cancer epidemiology update following the 2011 IARC evaluation of radiofrequency electromagnetic fields (Monograph 102) Environ Res. 2018;167:673–683. doi: 10.1016/j.envres.2018.06.043. [DOI] [PubMed] [Google Scholar]
- 98.Miller AB, Sears ME, Morgan LL, Davis DL, Hardell L, Oremus M, Soskolne CL. Risks to health and well-being from radio-frequency radiation emitted by cell phones and other wireless devices. Front Public Health. 2019;7:223. doi: 10.3389/fpubh.2019.00223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Santini R, Santini P, Danze JM, Le Ruz P, Seigne M. Study of the health of people living in the vicinity of mobile phone base stations: I. Influences of distance and sex Pathol Biol. 2002;50:369–373. doi: 10.1016/S0369-8114(02)00311-5. [DOI] [PubMed] [Google Scholar]
- 100.Navarro A, Garcia JS, Portoles M, Gómez-Perretta G. The microwave syndrome: A preliminary study in Spain. Electromagn Biol Med. 2003;22:161–169. doi: 10.1081/JBC-120024625. [DOI] [Google Scholar]
- 101.Salama OE, Abou El Naga RM. Cellular phones: Are they detrimental? J Egypt Public Health Assoc. 2004;79:197–223. [PubMed] [Google Scholar]
- 102.Hutter HP, Moshammer H, Wallner P, Kundi M. Subjective symptoms, sleeping problems, and cognitive performance in subjects living near mobile phone base stations. Occup Environ Med. 2006;63:307–313. doi: 10.1136/oem.2005.020784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Abdel-Rassoul G, El-Fateh OA, Salem MA, Michael A, Farahat F, El-Batanouny M, Salem E. Neurobehavioral effects among inhabitants around mobile phone base stations. Neurotoxicology. 2007;28:434–440. doi: 10.1016/j.neuro.2006.07.012. [DOI] [PubMed] [Google Scholar]
- 104.Blettner M, Schlehofer B, Breckenkamp J, Kowall B, Schmiedel S, Reis U, Potthoff P, Schüz J, Berg-Beckhoff G. Mobile phone base stations and adverse health effects: Phase 1 of a population-based, cross-sectional study in Germany. Occup Environ Med. 2009;66:118–123. doi: 10.1136/oem.2007.037721. [DOI] [PubMed] [Google Scholar]
- 105.Viel JF, Clerc S, Barrera C, Rymzhanova R, Moissonnier M, Hours M, Cardis E. Residential exposure to radiofrequency fields from mobile phone base stations, and broadcast transmitters: A population-based survey with personal meter. Occup Environ Med. 2009;66:550–556. doi: 10.1136/oem.2008.044180. [DOI] [PubMed] [Google Scholar]
- 106.Kundi M, Hutter HP. Mobile phone base stations-effects on wellbeing and health. Pathophysiology. 2009;16:123–135. doi: 10.1016/j.pathophys.2009.01.008. [DOI] [PubMed] [Google Scholar]
- 107.Shahbazi-Gahrouei D, Karbalae M, Moradi HA, Baradaran-Ghahfarokhi M. Health effects of living near mobile phone base transceiver station (BTS) antennae: A report from Isfahan, Iran. Electromagn Biol Med. 2014;33:206–210. doi: 10.3109/15368378.2013.801352. [DOI] [PubMed] [Google Scholar]
- 108.Irigaray P, Caccamo D, Belpomme D. Oxidative stress in electrohypersensitivity self-reporting patients: Results of a prospective in vivo investigation with comprehensive molecular analysis. Int J Mol Med. 2018;42:1885–1898. doi: 10.3892/ijmm.2018.3774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Yakymenko I, Tsybulin O, Sidorik E, Henshel D, Kyrylenko O, Kyrylenko S. Oxidative mechanisms of biological activity of low-intensity radiofrequency radiation. Electromagn Biol Med. 2016;35:186–202. doi: 10.3109/15368378.2015.1043557. [DOI] [PubMed] [Google Scholar]
- 110.Tillmann T, Ernst H, Streckert J, Zhou Y, Taugner F, Hansen V, Dasenbrock C. Indication of cocarcinogenic potential of chronic UMTS-modulated radiofrequency exposure in an ethylnitrosourea mouse model. Int J Radiat Biol. 2010;86:529–541. doi: 10.3109/09553001003734501. [DOI] [PubMed] [Google Scholar]
- 111.Lerchl A, Klose M, Grote K, Wilhelm AF, Spathmann O, Fiedler T, Streckert J, Hansen V, Clemens M. Tumor promotion by exposure to radiofrequency electromagnetic fields below exposure limits for humans. Biochem Biophys Res Commun. 2015;459:585–590. doi: 10.1016/j.bbrc.2015.02.151. [DOI] [PubMed] [Google Scholar]
- 112.National Toxicology Program. Toxicology and carcinogenesis studies in Sprague Dawley (Hsd:Sprague Dawley SD) rats exposed to whole-body radio frequency radiation at a frequency (900 MHz) and modulations (GSM and CDMA) used by cell phones. Natl Toxicol Program Tech Rep Ser: NTP-TR-595. 2018 doi: 10.22427/NTP-TR-595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Smith-Roe SL, Wyde ME, Stout MD, Winters JW, Hobbs CA, Shepard KG, Green AS, Kissling GE, Shockley KR, Tice RR, et al. Evaluation of the genotoxicity of cell phone radiofrequency radiation in male and female rats and mice following subchronic exposure. Environ Mol Mutagen. 2020;61:276–290. doi: 10.1002/em.22343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Falcioni L, Bua L, Tibaldi E, Lauriola M, De Angelis L, Gnudi F, Mandrioli D, Manservigi M, Manservisi F, Manzoli I, et al. Report of final results regarding brain and heart tumors in Sprague-Dawley rats exposed from prenatal life until natural death to mobile phone radiofrequency field representative of a 1.8 GHz GSM base station environmental emission. Environ Res. 2018;165:496–503. doi: 10.1016/j.envres.2018.01.037. [DOI] [PubMed] [Google Scholar]
- 115.International Commission on Non-Ionizing Radiation Protection Guidelines for limiting exposure to time-varying electric and magnetic fields (1 Hz to 100 kHz) Health Phys. 2010;99:818–836. doi: 10.1097/HP.0b013e3181f06c86. [DOI] [PubMed] [Google Scholar]
- 116.International Commission on Non-Ionizing Radiation Protection Guidelines for limiting exposure to electromagnetic fields (100 kHz to 300 GHz) Health Phys. 2020;118:483–524. doi: 10.1097/HP.0000000000001210. [DOI] [PubMed] [Google Scholar]
- 117.IARC: Non-ionizing radiation, part 1: Static and extremely low-frequency (ELF) electric and magnetic fields. Vol. 80. World Health Organization. IARC Press; Lyon: 2002. [PMC free article] [PubMed] [Google Scholar]
- 118.IARC: Non-Ionizing Radiation, Part 2: Radiofrequency Electromagnetic Fields. Vol. 102. IARC Press; Lyon: 2013. [PMC free article] [PubMed] [Google Scholar]
- 119.Baan R, Grosse Y, Lauby-Secretan B, El Ghissassi F, Bouvard V, Benbrahim-Tallaa L, Guha N, Islami F, Galichet L. Carcinogenicity of radiofrequency electromagnetic fields. Lancet Oncol. 2011;12:624–626. doi: 10.1016/S1470-2045(11)70147-4. [DOI] [PubMed] [Google Scholar]
- 120.Verschaeve L. Misleading scientific papers on health effects from wireless communication devices. In: Geddes CD, editor. Microwave Effects on DNA and Proteins. Springer; Cham: 2017. pp. 159–233. [DOI] [Google Scholar]
- 121.Panagopoulos DJ, Johansson O, Carlo GL. Real versus simulated mobile phone exposures in experimental studies. Biomed Res Int. 2015;2015:607053. doi: 10.1155/2015/607053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Panagopoulos DJ. Mobile telephony radiation effects on insect ovarian cells. The necessity for real exposures bioactivity assessment The key role of polarization, and the 'ion forced-oscillation mechanism'. In: Geddes CD, editor. Microwave Effects on DNA and Proteins. Springer; Cham: 2017. pp. 1–48. [Google Scholar]
- 123.Manna D, Ghosh R. Effect of radiofrequency radiation in cultured mammalian cells: A review. Electromagn Biol Med. 2016;35:265–301. doi: 10.3109/15368378.2015.1092158. [DOI] [PubMed] [Google Scholar]
- 124.Leach V, Weller S, Redmayne M. A novel database of bio-effects from non-ionizing radiation. Rev Environ Health. 2018;33:273–280. doi: 10.1515/reveh-2018-0017. [DOI] [PubMed] [Google Scholar]
- 125.Karipidis K, Mate R, Urban D, Tinker R, Wood A. 5G mobile networks and health-a state-of-the-science review of the research into low-level RF fields above 6 GHz. J Expo Sci Environ Epidemiol. 2021;31:585–605. doi: 10.1038/s41370-021-00297-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Hardell L, Nyberg R. Appeals that matter or not on a moratorium on the deployment of the fifth generation, 5G, for microwave radiation. Mol Clin Oncol. 2020;12:247–257. doi: 10.3892/mco.2020.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Hardell L, Carlberg M. Health risks from radiofrequency radiation, including 5G, should be assessed by experts with no conflicts of interest. Oncol Lett. 2020;20:15. doi: 10.3892/ol.2020.11876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Metaxas AC. Microwave heating. Power Eng. 1991;5:237–247. doi: 10.1049/pe:19910047. [DOI] [Google Scholar]
- 129.Panagopoulos DJ. Comments on Pall's 'Millimeter (MM) wave and microwave frequency radiation produce deeply penetrating effects: The biology and the physics'. Rev Environ Health. Jul 12, 2021. [DOI] [PubMed]
- 130.Ames BN. Endogenous DNA damage as related to cancer and aging. Mutat Res. 1989;214:41–46. doi: 10.1016/0027-5107(89)90196-6. [DOI] [PubMed] [Google Scholar]
- 131.Lieber MR. Pathological and physiological double-strand breaks: Roles in cancer, aging, and the immune system. Am J Pathol. 1998;153:1323–1332. doi: 10.1016/S0002-9440(10)65716-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Helleday T, Loc J, van Gentd DC, Engelward BP. DNA double-strand break repair: From mechanistic understanding to cancer treatment. DNA Repair (Amst) 2007;6:923–935. doi: 10.1016/j.dnarep.2007.02.006. [DOI] [PubMed] [Google Scholar]
- 133.Lahtz C, Pfeifer GP. Epigenetic changes of DNA repair genes in cancer. J Mol Cell Biol. 2011;3:51–58. doi: 10.1093/jmcb/mjq053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Yao Y, Dai W. Genomic instability and cancer. J Carcinog Mutagen. 2014;5:1000165. doi: 10.4172/2157-2518.1000165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Bernstein C, Prasad AR, Nfonsam V, Bernstein H. DNA damage, DNA repair and cancer. In: Clarc C, editor. New Research Directions in DNA Repair. InTech; Rijeka: 2013. pp. 413–465. [Google Scholar]
- 136.Basu AK. DNA damage, mutagenesis and cancer. Int J Mol Sci. 2018;19:970. doi: 10.3390/ijms19040970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.von Zglinicki T, Saretzki G, Ladhoff J, d'Adda di Fagagna F, Jackson SP. Human cell senescence as a DNA damage response. Mech Ageing Dev. 2005;126:111–117. doi: 10.1016/j.mad.2004.09.034. [DOI] [PubMed] [Google Scholar]
- 138.Shah DJ, Sachs RK, Wilson DJ. Radiation-induced cancer: A modern view. Br J Radiol. 2012;85:e1166–e1173. doi: 10.1259/bjr/25026140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Rodgers K, McVey M. Error-prone repair of DNA double-strand breaks. J Cell Physiol. 2016;231:15–24. doi: 10.1002/jcp.25053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Nadler DL, Zurbenko IG. Estimating cancer latency times using a weibull model. Adv Epidemiol. 2014;2014:746769. doi: 10.1155/2014/746769. [DOI] [Google Scholar]
- 141.Francis G. Ionization Phenomena in Gases. Butterworths Scientific Publications; London: 1960. [Google Scholar]
- 142.Gomer R. Field Emission and Field Ionization. Harvard University Press; Cambridge: 1961. [Google Scholar]
- 143.Panagopoulos DJ, Johansson O, Carlo GL. Polarization: A key difference between man-made and natural electromagnetic fields, in regard to biological activity. Sci Rep. 2015;5:14914. doi: 10.1038/srep14914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Panagopoulos DJ, Messini N, Karabarbounis A, Filippetis AL, Margaritis LH. A mechanism for action of oscillating electric fields on cells. Biochem Biophys Res Commun. 2000;272:634–640. doi: 10.1006/bbrc.2000.2746. [DOI] [PubMed] [Google Scholar]
- 145.Panagopoulos DJ, Karabarbounis A, Margaritis LH. Mechanism for action of electromagnetic fields on cells. Biochem Biophys Res Commun. 2002;298:95–102. doi: 10.1016/S0006-291X(02)02393-8. [DOI] [PubMed] [Google Scholar]
- 146.Panagopoulos DJ, Balmori A, Chrousos GP. On the biophysical mechanism of sensing upcoming earthquakes by animals. Sci Total Environ. 2020;717:136989. doi: 10.1016/j.scitotenv.2020.136989. [DOI] [PubMed] [Google Scholar]
- 147.Alberts B, Johnson A, Lewis J, Raff M, Roberts K, Walter P. Molecular Biology of the Cell. Garland Publishing, Inc; New York: 1994. [Google Scholar]
- 148.Stryer L. In: Biochemistry. 4th edition. Freeman WH, editor. Freeman and Company; New York, NY: 1995. [Google Scholar]
- 149.Halgamuge MN, Abeyrathne CD. Behavior of charged particles in a biological cell exposed to AC-DC electromagnetic fields. Environ Eng Sci. 2001;28:1–10. doi: 10.1089/ees.2010.0045. [DOI] [Google Scholar]
- 150.Panagopoulos DJ, Karabarbounis A. Comments on study of charged particle's behavior in a biological cell exposed to AC-DC electromagnetic fields, and on comparison between two models of interaction between electric and magnetic fields and proteins in cell membranes. Environ Eng Sci. 2011;28:749–751. doi: 10.1089/ees.2011.2810.com. [DOI] [Google Scholar]
- 151.Bawin SM, Kaczmarek LK, Adey WR. Effects of modulated VHF fields, on the central nervous system. Ann N Y Acad Sci. 1975;247:74–81. doi: 10.1111/j.1749-6632.1975.tb35984.x. [DOI] [PubMed] [Google Scholar]
- 152.Bawin SM, Adey WR, Sabbot IM. Ionic factors in release of 45Ca2+ from chicken cerebral tissue by electromagnetic fields. Proc Natl Acad Sci USA. 1978;75:6314–6318. doi: 10.1073/pnas.75.12.6314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Blackman CF, Benane SG, Elder JA, House DE, Lampe JA, Faulk JM. Induction of calcium-ion efflux from brain tissue by radiofrequency radiation: Effect of sample number and modulation frequency on the power density window. Bioelectromagnetics. 1980;1:35–43. doi: 10.1002/bem.2250010104. [DOI] [PubMed] [Google Scholar]
- 154.Frei M, Jauchem J, Heinmets F. Physiological effects of 2.8 GHz radio-frequency radiation: A comparison of pulsed and continuous-wave radiation. J Microw Power Electromagn Energy. 1988;23:85–93. doi: 10.1080/08327823.1988.11688043. [DOI] [PubMed] [Google Scholar]
- 155.Bolshakov MA, Alekseev SI. Bursting responses of Lymnea neurons to microwave radiation. Bioelectromagnetics. 1992;13:119–129. doi: 10.1002/bem.2250130206. [DOI] [PubMed] [Google Scholar]
- 156.Penafiel LM, Litovitz T, Krause D, Desta A, Mullins JM. Role of modulation on the effect of microwaves on ornithine decarboxylase activity in L929 cells. Bioelectromagnetics. 1997;18:132–141. doi: 10.1002/(SICI)1521-186X(1997)18:2<132::AID-BEM6>3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]
- 157.Huber R, Treyer V, Borbély AA, Schuderer J, Gottselig JM, Landolt HP, Werth E, Berthold T, Kuster N, Buck A, Achermann P. Electromagnetic fields, such as those from mobile phones, alter regional cerebral blood flow and sleep and waking EEG. J Sleep Res. 2002;11:289–295. doi: 10.1046/j.1365-2869.2002.00314.x. [DOI] [PubMed] [Google Scholar]
- 158.Höytö A, Luukkonen J, Juutilainen J, Naarala J. Proliferation, oxidative stress and cell death in cells exposed to 872 MHz radio-frequency radiation and oxidants. Radiat Res. 2008;170:235–243. doi: 10.1667/RR1322.1. [DOI] [PubMed] [Google Scholar]
- 159.Mohammed HS, Fahmy HM, Radwan NM, Elsayed AA. Non-thermal continuous and modulated electromagnetic radiation fields effects on sleep EEG of rats. J Adv Res. 2013;4:181–187. doi: 10.1016/j.jare.2012.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Noda M, Ikeda T, Kayano T, Suzuki H, Takeshima H, Kurasaki M, Takahashi H, Numa S. Existence of distinct sodium channel messenger RNAs in rat brain. Nature. 1986;320:188–192. doi: 10.1038/320188a0. [DOI] [PubMed] [Google Scholar]
- 161.Liman ER, Hess P, Weaver F, Koren G. Voltage-sensing residues in the S4 region of a mammalian K+ channel. Nature. 1991;353:752–756. doi: 10.1038/353752a0. [DOI] [PubMed] [Google Scholar]
- 162.Tombola F, Pathak MM, Isacoff EY. How does voltage open an ion channel? Annu Rev Cell Dev Biol. 2006;22:23–52. doi: 10.1146/annurev.cellbio.21.020404.145837. [DOI] [PubMed] [Google Scholar]
- 163.Schmidt WF, Thomas CG. More precise model of α-helix and transmembrane α-helical peptide backbone structure. J Biophy Chem. 2012;3:295–303. doi: 10.4236/jbpc.2012.34036. [DOI] [Google Scholar]
- 164.Miller C. An overview of the potassium channel family. Genome Biol. 2000;1:REVIEWS0004. doi: 10.1186/gb-2000-1-4-reviews0004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Zhang XC, Yang H, Liu Z, Sun F. Thermodynamics of voltage-gated ion channels. Biophys Rep. 2018;4:300–319. doi: 10.1007/s41048-018-0074-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.DeCoursey TE. Interactions between NADPH oxidase and voltage-gated proton channels: Why electron transport depends on proton transport. FEBS Lett. 2003;555:57–61. doi: 10.1016/S0014-5793(03)01103-7. [DOI] [PubMed] [Google Scholar]
- 167.Seredenina T, Demaurex N, Krause KH. Voltage-gated proton channels as novel drug targets: From NADPH oxidase regulation to sperm biology. Antioxid Redox Sign. 2015;23:490–513. doi: 10.1089/ars.2013.5806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Panagopoulos DJ, Johansson O, Carlo GL. Evaluation of specific absorption rate as a dosimetric quantity for electromagnetic fields bioeffects. PLoS One. 2013;8:e62663. doi: 10.1371/journal.pone.0062663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Creasey WA, Goldberg RB. A new twist on an old mechanism for EMF bioeffects? EMF Health Rep. 2001;9:1–11. [Google Scholar]
- 170.Belyaev IY. Non-thermal biological effects of microwaves. Microw Rev. 2005;11:13–29. [Google Scholar]
- 171.Zhou R, Xiong Y, Xing G, Sun L, Ma J. ZiFi: Wireless LAN discovery via ZigBee interference signatures. MobiCom'10. September 20-24; Chicago, Illinois, USA. 2010 Proceedings of the 16th Annual International Conference on Mobile Computing and Networking; [DOI] [Google Scholar]
- 172.Piacentini R, Ripoli C, Mezzogori D, Azzena GB, Grassi C. Extremely low-frequency electromagnetic fields promote in vitro neurogenesis via upregulation of Ca(v)1-channel activity. J Cell Physiol. 2008;215:129–139. doi: 10.1002/jcp.21293. [DOI] [PubMed] [Google Scholar]
- 173.Cecchetto C, Maschietto M, Boccaccio P, Vassanelli S. Electromagnetic field affects the voltage-dependent potassium channel Kv1.3. Electromagn Biol Med. 2020;39:316–322. doi: 10.1080/15368378.2020.1799386. [DOI] [PubMed] [Google Scholar]
- 174.Zheng Y, Xia P, Dong L, Tian L, Xiong C. Effects of modulation on sodium and potassium channel currents by extremely low frequency electromagnetic fields stimulation on hippocampal CA1 pyramidal cells. Electromagn Biol Med. 2021;40:274–285. doi: 10.1080/15368378.2021.1885433. [DOI] [PubMed] [Google Scholar]
- 175.Batcioglu K, Uyumlu AB, Satilmis B, Yildirim B, Yucel N, Demirtas H, Onkal R, Guzel RM, Djamgoz MB. Oxidative stress in the in vivo DMBA rat model of breast cancer: Suppression by a voltage-gated sodium channel inhibitor (RS100642) Basic Clin Pharmacol Toxicol. 2012;111:137–141. doi: 10.1111/j.1742-7843.2012.00880.x. [DOI] [PubMed] [Google Scholar]
- 176.Ramírez A, Vázquez-Sánchez AY, Carrión-Robalino N, Camacho J. Ion channels and oxidative stress as a potential link for the diagnosis or treatment of liver diseases. Oxid Med Cell Longev. 2016;2016:3928714. doi: 10.1155/2016/3928714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.O'Hare Doig RL, Chiha W, Giacci MK, Yates NJ, Bartlett CA, Smith NM, Hodgetts SI, Harvey AR, Fitzgerald M. Specific ion channels contribute to key elements of pathology during secondary degeneration following neurotrauma. BMC Neurosci. 2017;18:62. doi: 10.1186/s12868-017-0380-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Akbarali HI. Oxidative stress and ion channels. In: Lahers I, editor. Systems Biology of Free Radicals and Antioxidants. Springer; Berlin, Heidelberg: 2014. pp. 355–373. [DOI] [Google Scholar]
- 179.Kourie JI. Interaction of reactive oxygen species with ion transport mechanisms. Am J Physiol. 1998;275:C1–C24. doi: 10.1152/ajpcell.1998.275.1.C1. [DOI] [PubMed] [Google Scholar]
- 180.Lushchak VI. Free radicals, reactive oxygen species, oxidative stress and its classification. Chem Biol Interact. 2014;224:164–175. doi: 10.1016/j.cbi.2014.10.016. [DOI] [PubMed] [Google Scholar]
- 181.Pacher P, Beckman JS, Liaudet L. Nitric oxide and peroxynitrite in health and disease. Physiol Rev. 2007;87:315–424. doi: 10.1152/physrev.00029.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Valko M, Leibfritz D, Moncol J, Cronin MT, Mazur M, Telser J. Free radicals and antioxidants in normal physiological functions and human disease. Int J Biochem Cell Biol. 2007;39:44–84. doi: 10.1016/j.biocel.2006.07.001. [DOI] [PubMed] [Google Scholar]
- 183.Halliwell B. Biochemistry of oxidative stress. Biochem Soc Trans. 2007;35:1147–1150. doi: 10.1042/BST0351147. [DOI] [PubMed] [Google Scholar]
- 184.Balasubramanian B, Pogozelski WK, Tullius TD. DNA strand breaking by the hydroxyl radical is governed by the accessible surface areas of the hydrogen atoms of the DNA backbone. Proc Natl Acad Sci USA. 1998;95:9738–9743. doi: 10.1073/pnas.95.17.9738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Cadet J, Delatour T, Douki T, Gasparutto D, Pouget JP, Ravanat JL, Sauvaigo S. Hydroxyl radicals and DNA base damage. Mutat Res. 1999;1424:9–21. doi: 10.1016/S0027-5107(99)00004-4. [DOI] [PubMed] [Google Scholar]
- 186.Cadet J, Wagner JR. DNA base damage by reactive oxygen species, oxidizing agents, and UV radiation. Cold Spring Harb Perspect Biol. 2013;5:a012559. doi: 10.1101/cshperspect.a012559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Tsunoda M, Sakaue T, Naito S, Sunami T, Abe N, Ueno Y, Matsuda A, Takénaka A. Insights into the structures of DNA damaged by hydroxyl radical: Crystal structures of DNA duplexes containing 5-formyluracil. J Nucleic Acids. 2010;2010:107289. doi: 10.4061/2010/107289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Cooke MS, Evans MD, Dizdaroglu M, Lunec J. Oxidative DNA damage: Mechanisms, mutation, and disease. FASEB J. 2003;17:1195–1214. doi: 10.1096/fj.02-0752rev. [DOI] [PubMed] [Google Scholar]
- 189.Barzilai A, Yamamoto K. DNA damage responses to oxidative stress. DNA Repair (Amst) 2004;3:1109–1115. doi: 10.1016/j.dnarep.2004.03.002. [DOI] [PubMed] [Google Scholar]
- 190.Pall ML. Electromagnetic fields act via activation of voltage-gated calcium channels to produce beneficial or adverse effects. J Cell Mol Med. 2013;17:958–965. doi: 10.1111/jcmm.12088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Liburdy RP. Calcium signaling in lymphocytes and ELF fields. Evidence for an electric field metric and a site of interaction involving the calcium ion channel. FEBS Lett. 1992;301:53–59. doi: 10.1016/0014-5793(92)80209-Y. [DOI] [PubMed] [Google Scholar]
- 192.Walleczek J. Electromagnetic field effects on cells of the immune system: The role of calcium signaling. FASEB J. 1992;6:3177–3185. doi: 10.1096/fasebj.6.13.1397839. [DOI] [PubMed] [Google Scholar]
- 193.Brookes PS, Yoon Y, Robotham JL, Anders MW, Sheu SS. Calcium, ATP, and ROS: A mitochondrial love-hate triangle. Am J Physiol Cell Physiol. 2004;287:C817–C833. doi: 10.1152/ajpcell.00139.2004. [DOI] [PubMed] [Google Scholar]
- 194.Lang F, Föller M, Lang KS, Lang PA, Ritter M, Gulbins E, Vereninov A, Huber SM. Ion channels in cell proliferation and apoptotic cell death. J Membr Biol. 2005;205:147–157. doi: 10.1007/s00232-005-0780-5. [DOI] [PubMed] [Google Scholar]
- 195.Görlach A, Bertram K, Hudecova S, Krizanova O. Calcium and ROS: A mutual interplay. Redox Biol. 2015;6:260–271. doi: 10.1016/j.redox.2015.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Lombardi AA, Gibb AA, Arif E, Kolmetzky DW, Tomar D, Luongo TS, Jadiya P, Murray EK, Lorkiewicz PK, Hajnóczky G, et al. Mitochondrial calcium exchange links metabolism with the epigenome to control cellular differentiation. Nat Commun. 2019;10:4509. doi: 10.1038/s41467-019-12103-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Lowe SW, Lin AW. Apoptosis in cancer. Carcinogenesis. 2000;21:485–495. doi: 10.1093/carcin/21.3.485. [DOI] [PubMed] [Google Scholar]
- 198.Ikwegbue PC, Masamba P, Oyinloye BE, Kappo AP. Roles of heat shock proteins in apoptosis, oxidative stress, human inflammatory diseases, and cancer. Pharmaceuticals (Basel) 2017;11:2. doi: 10.3390/ph11010002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Becchetti A. Ion channels and transporters in cancer. 1. Ion channels and cell proliferation in cancer. Am J Physiol Cell Physiol. 2011;301:C255–C265. doi: 10.1152/ajpcell.00047.2011. [DOI] [PubMed] [Google Scholar]
- 200.Gaasch JA, Geldenhuys WJ, Lockman PR, Allen DD, Van der Schyf CJ. Voltage-gated calcium channels provide an alternate route for iron uptake in neuronal cell cultures. Neurochem Res. 2007;32:1686–1693. doi: 10.1007/s11064-007-9313-1. [DOI] [PubMed] [Google Scholar]
- 201.Chattipakorn N, Kumfu S, Fucharoen S, Chattipakorn S. Calcium channels and iron uptake into the heart. World J Cardiol. 2011;3:215–218. doi: 10.4330/wjc.v3.i7.215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Salsbury G, Cambridge EL, McIntyre Z, Arends MJ, Karp NA, Isherwood C, Shannon C, Hooks Y, Sanger Mouse Genetics Project. Ramirez-Solis R, et al. Disruption of the potassium channel regulatory subunit KCNE2 causes iron-deficient anemia. Exp Hematol. 2014;42:1053–1058.e1. doi: 10.1016/j.exphem.2014.07.269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Gamaley I, Augsten K, Berg H. Electrostimulation of macrophage NADPH oxidase by modulated high-frequency electromagnetic fields. Bioelectrochem Bioenerg. 1995;38:415–418. doi: 10.1016/0302-4598(95)01836-4. [DOI] [Google Scholar]
- 204.Panday A, Sahoo MK, Osorio D, Batra S. NADPH oxidases: An overview from structure to innate immunity-associated pathologies. Cell Mol Immunol. 2015;12:5–23. doi: 10.1038/cmi.2014.89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Henderson LM. NADPH oxidase subunit gp91phox: A proton pathway. Protoplasma. 2001;217:37–42. doi: 10.1007/BF01289411. [DOI] [PubMed] [Google Scholar]
- 206.Musset B, Cherny VV, Morgan D, DeCoursey TE. The intimate and mysterious relationship between proton channels and NADPH oxidase. FEBS Lett. 2009;583:7–12. doi: 10.1016/j.febslet.2008.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.DeCoursey T, Morgan D, Cherny V. The voltage dependence of NADPH oxidase reveals why phagocytes need proton channels. Nature. 2003;422:531–534. doi: 10.1038/nature01523. [DOI] [PubMed] [Google Scholar]
- 208.Friedman J, Kraus S, Hauptman Y, Schiff Y, Seger R. Mechanism of short-term ERK activation by electromagnetic fields at mobile phone frequencies. Biochem J. 2007;405:559–568. doi: 10.1042/BJ20061653. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 209.Pratt RD, Brickman CR, Cottrill CL, Shapiro JI, Liu J. The Na/K-ATPase signaling: from specific ligands to general reactive oxygen species. Int J Mol Sci. 2018;19:2600. doi: 10.3390/ijms19092600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Nitahara JA, Cheng W, Liu Y, Li B, Leri A, Li P, Mogul D, Gambert SR, Kajstura J, Anversa P. Intracellular calcium, DNase activity and myocyte apoptosis in aging Fischer 344 rats. J Mol Cell Cardiol. 1998;30:519–535. doi: 10.1006/jmcc.1997.0616. [DOI] [PubMed] [Google Scholar]
- 211.Fenton HJH. Oxidation of tartaric acid in presence of iron. J Chem Soc Trans. 1894;65:899–911. doi: 10.1039/CT8946500899. [DOI] [Google Scholar]
- 212.Bawin SM, Adey WR. Sensitivity of calcium binding in cerebral tissue to weak environmental electric fields oscillating at low frequency. Proc Natl Acad Sci USA. 1976;73:1999–2003. doi: 10.1073/pnas.73.6.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Jackson JD. Classical Electrodynamics. 2nd edition. John Wiley & Sons, Inc; New York, NY: 1975. [Google Scholar]
- 214.Barr R, Llanwyn Jones D, Rodger CJ. ELF and VLF radio waves. J Atmos Sol-Terr Phys. 2000;62:1689–1718. doi: 10.1016/S1364-6826(00)00121-8. [DOI] [Google Scholar]
- 215.McLeod KJ, Lee RC, Ehrlich HP. Frequency dependence of electric field modulation of fibroblast protein synthesis. Science. 1987;236:1465–1469. doi: 10.1126/science.3589667. [DOI] [PubMed] [Google Scholar]
- 216.Cleary SF, Liu LM, Graham R, Diegelmann RF. Modulation of tendon fibroplasia by exogenous electric currents. Bioelectromagnetics. 1988;9:183–194. doi: 10.1002/bem.2250090210. [DOI] [PubMed] [Google Scholar]
- 217.Lee RC, Canaday DJ, Doong H. A review of the biophysical basis for the clinical application of electric fields in soft-tissue repair. J Burn Care Rehabil. 1993;14:319–335. doi: 10.1097/00004630-199305000-00003. [DOI] [PubMed] [Google Scholar]
- 218.Sandipan C, Baron C. Basic mechanisms of voltage sensing. In: Zheng J, Trudeau MC, editors. Handbook of Ion Channels. CRC Press; London: 2015. pp. 25–39. [Google Scholar]
- 219.Groome JR, Bayless-Edwards L. Roles for countercharge in the voltage sensor domain of ion channels. Front Pharmacol. 2020;11:160. doi: 10.3389/fphar.2020.00160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Shi YP, Thouta S, Claydon TW. Modulation of hERG K+ channel deactivation by voltage sensor relaxation. Front Pharmacol. 2020;11:139. doi: 10.3389/fphar.2020.00139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Villalba-Galea CA, Chiem AT. Hysteretic behavior in voltage-gated channels. Front Pharmacol. 2020;11:579596. doi: 10.3389/fphar.2020.579596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Panagopoulos DJ, Margaritis LH. The identification of an intensity 'window' on the bioeffects of mobile telephony radiation. Int J Radiat Biol. 2010;86:358–366. doi: 10.3109/09553000903567979. [DOI] [PubMed] [Google Scholar]
- 223.Panagopoulos DJ, Balmori A. On the biophysical mechanism of sensing atmospheric discharges by living organisms. Sci Total Environ. 2017;599-600:2026–2034. doi: 10.1016/j.scitotenv.2017.05.089. [DOI] [PubMed] [Google Scholar]
- 224.Liboff AR. Cyclotron resonance in membrane transport. In: Chiabrera A, Nicolini C, Schwan HP, editors. Interactions between Electromagnetic Fields and Cells. Plenum Press; London: 1985. pp. 281–296. [Google Scholar]
- 225.Bianco B, Chiabrera A, Morro A, Parodi M. Effects of magnetic exposure on ions in electric fields. Ferroelectrics. 1988;86:159–168. doi: 10.1080/00150198808227011. [DOI] [Google Scholar]
- 226.Balcavage WX, Alvager T, Swez J, Goff CW, Fox MT, Abdullyava S, King MW. A mechanism for action of extremely low frequency electromagnetic fields on biological systems. Biochem Biophys Res Commun. 1996;222:374–378. doi: 10.1006/bbrc.1996.0751. [DOI] [PubMed] [Google Scholar]
- 227.Zhadin MN. Combined action of static and alternating magnetic fields on ion motion in a macromolecule: Theoretical aspects. Bioelectromagnetics. 1998;19:279–292. doi: 10.1002/(SICI)1521-186X(1998)19:5<279::AID-BEM2>3.0.CO;2-Z. [DOI] [PubMed] [Google Scholar]
- 228.Liboff AR. Ion cyclotron resonance in biological systems: Experimental evidence. In: Stavroulakis P, editor. Biological Effects of Electromagnetic Fields. Springer; Berlin: 2003. pp. 76–113. [Google Scholar]
- 229.Lednev VV. Possible mechanism for the influence of weak magnetic fields on biological systems. Bioelectromagnetics. 1991;12:71–75. doi: 10.1002/bem.2250120202. [DOI] [PubMed] [Google Scholar]
- 230.Kirschvink JL. Magnetite biomineralization and geomagnetic sensitivity in higher animals: An update and recommendations for future study. Bioelectromagnetics. 1989;10:239–259. doi: 10.1002/bem.2250100304. [DOI] [PubMed] [Google Scholar]
- 231.Brocklehurst B, McLauchlan KA. Free radical mechanism for the effects of environmental electromagnetic fields on biological systems. Int J Radiat Biol. 1996;69:3–24. doi: 10.1080/095530096146147. [DOI] [PubMed] [Google Scholar]
- 232.Sheppard AR, Swicord ML, Balzano Q. Quantitative evaluations of mechanisms of radiofrequency interactions with biological molecules and processes. Health Phys. 2008;93:365–396. doi: 10.1097/01.HP.0000319903.20660.37. [DOI] [PubMed] [Google Scholar]
- 233.Hall EJ, Giaccia AJ. Radiobiology for the Radiologist. 6th edition. Lippincott Williams & Wilkins; Philadelphia, PA: 2006. [Google Scholar]
- 234.Coggle JE. Biological Effects of Radiation. Taylor & Francis; 1983. [Google Scholar]
- 235.Hanahan D, Weinberg RA. The hallmarks of cancer. Cell. 2000;100:57–70. doi: 10.1016/S0092-8674(00)81683-9. [DOI] [PubMed] [Google Scholar]
- 236.Hanahan D, Weinberg RA. Hallmarks of cancer: The next generation. Cell. 2011;144:646–674. doi: 10.1016/j.cell.2011.02.013. [DOI] [PubMed] [Google Scholar]
- 237.Blank M, Goodman R. Electromagnetic fields stress living cells. Pathophysiology. 2009;16:71–78. doi: 10.1016/j.pathophys.2009.01.006. [DOI] [PubMed] [Google Scholar]
- 238.Blank M, Goodman R. DNA is a fractal antenna in electromagnetic fields. Int J Radiat Biol. 2011;87:409–415. doi: 10.3109/09553002.2011.538130. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.