Abstract
Coronavirus disease 2019 (COVID-19) pandemic has caused an urgent need for investigating potential treatments. Traditional medicine offers many potential remedies that have been historically used and have the advantage of bypassing the cultural obstacles in the practice of medicine. We aimed to investigate the efficacy of Zufa syrup in the treatment of suspected patients with mild to moderate symptoms of COVID-19. This triple-blind randomized controlled trial recruited patients with evidence of COVID-19 on chest computed tomography without an indication of hospital admission from March 2020 until April 2020. Participants were assessed by a physician and completed a pre-specified form to assess the duration and severity of symptoms. Patients were randomized to receive Zufa syrup (a combination of herbal medicines: Nepetabracteata, Ziziphus jujube, Glycyrrhizaglabra, Ficuscarica, Cordia myxa, Papaver somniferum, Fennel, Adiantumcapillusveneris, Viola, Viper‘s-buglosses, Lavender, Iris, and sugar) or identical-looking placebo syrup at a dose of 7.5 mL (one tablespoon) every 4 hours for 10 days. After applying the eligibility criteria, 116 patients (49.1% male) were randomized to trial arms with a mean age of 44.3. During the follow-up, Cough, dyspnea, headache, myalgia, anorexia, anxiety, and insomnia improved gradually in both groups, and showed no difference between Zufa syrup and placebo. Oxygen saturation and pulse rate had stable trends throughout the follow-up and were similar between study arms. No patient required hospital admission or supplemental oxygen therapy during the study period. To conclude, in patients with mild to moderate symptoms of COVID-19, Zufa syrup did not show any difference in symptomatology over a 10 days’ period when compared with placebo. Due to potential effects of medicinal plants in the treatment of respiratory infections, further studies are warranted to clarify their role in COVID-19. The study was approved by the Ethics Committee of the Qom University of Medical Science (Ethics committee reference number IR.MUQ.REC.1398.165) on March 10, 2020 and was registered in Iranian Clinical Trial Center (approval ID: IRCT20200404046934N1) on April 13, 2020.
Keywords: corona, COVID-19, herbal drug, Iran, Nepetabracteata, pandemic, respiratory disease, traditional medicine
INTRODUCTION
Coronavirus disease 2019 (COVID-19) emerged in late 2019,1 and has since become a global threat and provoked a pandemic.2 As of July 16, 2020, more than 13 million individuals have contracted the virus globally and over 574,000 people have died from COVID-19.2,3 Although, vaccination have already started and is on its way to cover major population in some of the western countries, but it is still awaiting sensitivity analyses to evaluate the effectiveness of the vaccine after different intervals following vaccination.3 Besides lethal forms of COVID-19, many patients show mild to moderate symptoms and do not require hospitalization.4 Such patients may require appropriate treatments to alleviate symptoms and stop the progression of disease.
Traditional and alternative medicine approaches have been employed in the care of COVID-19 patients during this pandemic, especially in China.5 The traditional Persian medicine is a widely practiced alternative medicine intertwined with the culture and history of Iran, which has the advantage of bypassing the cultural obstacles in the practice of medicine.6 Several herbs are used in the practice of traditional Persian medicine for alleviation of symptoms associated with common viral respiratory infections. Nepeta species have been found to be effective in chronic productive cough and in patients with chronic bronchitis.7,8 In another study, Viola syrup reduced coughing in children who suffered from asthma.9 A mixture of several traditional herbs was also proven effective to ameliorate the symptoms of common cold in asthmatic children.10 Moreover, studies have observed evidence of antiviral effects in a handful of these traditionally used herbal remedies, which might prove helpful with future investigations.11,12
The Zufa syrup is an herbal mixture of traditional Persian medicine remedies used for the treatment of respiratory illnesses which contains elements from several plants. As the search for effective treatments for COVID-19 continues, we decided to conduct a randomized clinical trial to investigate the effectiveness of Zufa syrup in patients with mild to moderate manifestations of COVID-19.
SUBJECTS AND METHODS
This randomized, placebo-controlled, triple-blind clinical trial was conducted in compliance with the principles of the Declaration of Helsinki and was approved by the Ethics Committee of the Qom University of Medical Science (Ethics committee reference number IR.MUQ.REC.1398.165) on March 10, 2020 and was registered in Iranian Clinical Trial Center (approval ID: IRCT20200404046934N1) on April 13, 2020. All participants were required to provide informed written consent.
Study design and participants
Patients were recruited from Shahid Beheshti Hospital of respiratory disease with mild to moderate symptoms suggestive of COVID-19 without an indication for hospital admission from March 2020 until April 2020. The definition of mild to moderate symptoms was based on physician examination and oxygen saturation, respiratory rate and self-declaration of patients based on a checklist (Additional file 1). All patients who were visited by a physician in one of the assigned hospital outpatient clinics or offices were initially screened for eligibility and a chest computed tomography (CT) was requested. Inclusion in the study required the following criteria: 20–70 years of age, presence of COVID-19 lung involvement on chest CT, and indication of outpatient pharmacotherapy for COVID-19 with moderate symptoms. Key exclusion criteria included a history of heart, lung, kidney, or liver disease, diabetes mellitus, systolic blood pressure higher than 160 mmHg, fever of 39°C or higher, pregnancy, and breastfeeding.
Following the confirmation of eligibility and review of the CT scan, the demographic and baseline clinical data were recorded. A table of relevant symptoms was provided to patients as they were educated about each symptom and instructed to fill the table for each day during follow-up. The patients rated each symptom as mild, moderate, or severe. Following enrollment, patients were randomized 1:1 to receive either the Zufa syrup or placebo syrup. The randomization code was generated by computer in permuted blocks of 4. The patients, the recruiting physicians who provided the syrups, the researchers who conducted the follow-ups and those who analyzed the data were blind to the treatment arms. Each syrup bottle had a unique code on its label and the recruiting physicians were instructed to provide bottles in the chronological order of the label code. The recruiting physicians were not aware of the randomization sequence or the block size. After the completion of data collection, a member of the staff, who was not at any stage involved in the process of patient enrollment and follow-up, used the label codes to assign patients to treatment arms named A and B. Un-blinding was done after the conduction of statistical analysis.
Study medication
Zufa syrup (IRC:2129211562044973) was manufactured by Booalidaroo Pharmaceutical Company (Qom, Iran) and the placebo was prepared at the same appearance too. Zufa syrup is a poly herbal medicine that is a combination of Nepetabracteata, Ziziphus jujube, Glycyrrhizaglabra, Ficuscarica, Cordia myxa, Papaver somniferum, Fennel, Adiantumcapillus-veneris, Viola, Viper‘s-buglosses, Lavender, Iris, and sugar. Patients were instructed to take 7.5 mL of their syrup every 4 hours for 10 days. The remaining treatments except for Zufa syrup were the same between two groups and the patients were on the recommended treatments based Ministry of Health of Iran protocols. Routine treatment is generally considered according to protocol of COVID-19.13,14
Follow-up and endpoints
The time from the onset of COVID-19 symptoms to the presentation to investigating physicians was determined at first visit. Every other day, patients were followed through phone calls which inquired about treatment adherence and changes in symptomatology. Each symptom was assessed by the patient assigning a number describing its severity (1 = mild, 2 = moderate, 3 = severe). Oxygen saturation was recorded with a pulse oximetry (ChoiceMMed, Beijing, China) through home-visits on alternate days. The indications of hospital admission were oxygen saturation < 93%, respiratory rate > 30 breaths/min and acute respiratory disease.
Some symptoms include of fever, shaking, cough, dyspnea, headache, myalgia, fatigue, weakness, anorexia, and insomnia were registered. We have not any side effect in patients, one of them has headache and been excluded.
Statistical analysis
Statistical analysis conducted using SPSS version 20 (IBM, Armonk, NY, USA) and sample size calculation estimated by MedCalc Statistical Software version 15.8 (MedCalc Software bvba, Ostend, Belgium). Categorical variables are shown as number (percentage) and compared by Chi-square test. Continuous variables are shown as mean ± standard deviation (SD) and compared by independent samples t-test. For comparison of the follow-up variables, the symptoms were assessed by severity scores of 1 to 3. In each group, repeated measurements analysis of variance was performed according to measurements on each day of the follow-up. Tukey’s post hoc test was used for further pairwise comparison. The Zufa syrup and placebo arms were compared by independent samples t-tests.
Assuming a treatment effect size with a difference of at least 10% in symptoms between the two arms, with a power of 80% and a type 1 error of 5%, the appropriate sample size was calculated to be 65 participants in each group.
Six of participants were excluded, three of whom were excluded because of interference with other herbal medicine, and two of whom were admitted in hospital later for headache.
RESULTS
Trial population
Figure 1 shows the CONsolidated Standards Of Reporting Trials (CONSORT) trial flowchart of participants through each stage of a randomized trial. From March 2020 until April 2020, were screened among whom 116 patients (49.1% male) with evidence of COVID-19 on chest CT were randomized.
Figure 1.
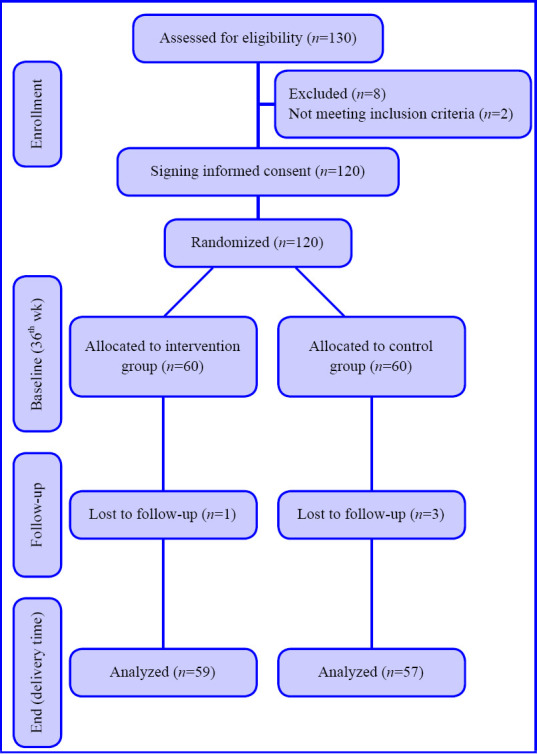
CONsolidated Standards Of Reporting Trials (CONSORT) diagram showing the flow of participants through each stage of a randomized trial.
The age of the population was 44.3 ± 11.5 years. Table 1 summarizes the baseline characteristics of study participants. At the first visit, the time from symptom onset until presentation was documented for each symptom and is presented in Table 2 The mean duration of symptoms was the longest for dry cough (5.4 days), followed by dyspnea (4.3 days), and was the least for productive cough (2.2 days). All patients were assessed through the 10 days’ follow-up period and data gathering was completed without loss to follow-up. No major adverse events were reported by study participants.
Table 1.
Baseline characteristics of COVID-19 participants
| Characteristics | Intervention (n=59) | Control (n=57) | P-value |
|---|---|---|---|
| Age (yr)a | 44.32±12.86 | 44.02 ±11.34 | 0.893 |
| Sexb | 0.136 | ||
| Female | 26 (44) | 33 (58) | |
| Male | 33 (56) | 24 (42) | |
| Weight (kg)a | 77.36±15.66 | 79.72±15.70 | 0.535 |
| Height (cm)a | 169.06±9.7 | 165.27±10.61 | 0.125 |
| Temperature (°C)a | 36.27±0.45 | 33.18±10.49 | 0.063 |
| Body mass index (kg/m2)a | 27.01±4.49 | 29.25±5.28 | 0.063 |
| Marital statusb | 0.189 | ||
| Single | 10 (17) | 5 (9) | |
| Married | 49 (83) | 52 (91) | |
| Occupationb | 0.353 | ||
| Employed | 13 (22) | 9 (16) | |
| Self-employed | 13 (22) | 12 (21) | |
| Worker | 6 (10) | 1 (2) | |
| Housewife | 22 (37) | 29 (51) | |
| Unemployed | 2 (3) | 3 (5) | |
| Retired | 3 (5) | 2 (4) | |
| Educationb | 0.945 | ||
| Under educated | 0 | 1 (2) | |
| High school | 47 (80) | 46 (81) | |
| Academic | 11 (19) | 10(182) | |
| education | |||
| Religious | 1 (2) | 1 (2) | |
| education | |||
| Smoking | 5 (8) | 5 (9) | 0.955 |
| Baseline total symptoms scorea | 21.39 ± 12.30 | 23.26 ± 11.38 | 0.4 |
Note: aData are presented as number (percentage) and were analyzed by Chi-square test. bData are presented as mean ± SD, and were analyzed by independent samples f-test. COVID-19: Coronavirus disease 2019.
Table 2.
Time from COVID-19 symptoms onset to presentation
| Number of days since symptom onset until presentation | Intervention (n=59) | Control (n=57) | P-value |
|---|---|---|---|
| All symptoms | 12.46 ± 11.48 | 13.98 ± 2.07 | 0.485 |
| Dry cough | 5.93 ± 9.06 | 5.05 ± 8.46 | 0.59 |
| Productive cough | 2.19 ± 4.7 | 1.77 ± 4.43 | 0.629 |
| Dyspnea | 4.34 ± 6.67 | 4.68 ± 7.69 | 0.797 |
Note: Data are presented as mean ± SD, and were analyzed by independent samples t-test. COVID-19: Coronavirus disease 2019.
COVID-19 symptomatology
Among the study population during the follow-up, none were admitted to the hospital or require oxygen therapy. Most patients improved during the 10 days’ treatment period. The symptomatology follow-ups assigned scored according to the severity of each symptom, and the data are demonstrated in Tables 3–9. The cough, dyspnea, headache, myalgia, anorexia, anxiety, and insomnia were improved significantly over the 10 days’ period in both the intervention and placebo group compared with those before treatment (P < 0.001). There was no significant difference in the severity of symptoms at each time point between those who the patients the Zufa syrup and placebo (Tables 3–9), with the exception of dyspnea on the 10th day of treatment which was reported to be less severe by the placebo group patients (P = 0.007).
Table 3.
Cough during the study period
| Group | Day 0 | Day 2 | Day 4 | Day 6 | Day 8 | Day 10 | P-value |
|---|---|---|---|---|---|---|---|
| Intervention (n=59) | 1.74±1.00 | 0.93±0.93 | 0.65±0.80 | 0.53±0.77 | 0.41±0.72 | 0.32±0.63 | < 0.001 |
| Control (n=57) | 1.40±1.16 | 0.80±0.95 | 0.61±0.83 | 0.43±0.78 | 0.38±0.70 | 0.29±0.70 | < 0.001 |
|
| |||||||
| P-value | 0.98 | 0.482 | 0.789 | 0.510 | 0.835 | 0.815 | |
Note: Data are presented as mean ± SD, and were analyzed by repeated measurements analysis of variance followed by Tukey’s post hoc test.
Table 9.
Anosmia during the study period
| Group | Day 0 | Day 2 | Day 4 | Day 6 | Day 8 | Day 10 | P-value |
|---|---|---|---|---|---|---|---|
| Intervention (n=59) | 1.03±1.16 | 0.70±0.99 | 0.48±0.80 | 0.41±0.75 | 0.24±0.60 | 0.34±0.78 | < 0.001 |
| Control (n=57) | 0.91±1.18 | 0.66±0.95 | 0.47±0.86 | 0.24±0.66 | 0.15±0.52 | 0.14±0.47 | < 0.001 |
|
| |||||||
| P-value | 0.579 | 0.825 | 0.954 | 0.205 | 0.431 | 0.095 | |
Note: Data are presented as mean ± SD, and were analyzed by repeated measurements analysis of variance followed by Tukey’s post hoc test.
Table 4.
Headache during the study period
| Group | Day 0 | Day 2 | Day 4 | Day 6 | Day 8 | Day 10 | P-value |
|---|---|---|---|---|---|---|---|
| Intervention (n=59) | 1.01±1.22 | 0.55±0.97 | 0.43±0.81 | 0.17±0.50 | 0.10±0.35 | 0.10±0.30 | < 0.001 |
| Control (n=57) | 1.08±1.18 | 0.57±0.99 | 0.22±0.62 | 0.28±0.64 | 0.21±0.58 | 0.19±0.61 | < 0.001 |
|
| |||||||
| P-value | 0.754 | 0.883 | 0.139 | 0.317 | 0.241 | 0.321 | |
Note: Data are presented as mean ± SD, and were analyzed by repeated measurements analysis of variance followed by Tukey’s post hoc test.
Table 5.
Dyspnea during the study period
| Group | Day 0 | Day 2 | Day 4 | Day 6 | Day 8 | Day 10 | P-value |
|---|---|---|---|---|---|---|---|
| Intervention (n=59) | 1.44±1.11 | 0.75±0.86 | 0.53±0.64 | 0.51±0.62 | 0.43±0.59 | 0.48±0.70 | < 0.001 |
| Control (n=57) | 1.36±1.06 | 0.84±0.94 | 0.49±0.68 | 0.38±0.67 | 0.31±0.53 | 0.17±0.46 | < 0.001 |
|
| |||||||
| P-value | 0.695 | 0.621 | 0.730 | 0.282 | 0.280 | 0.007 | |
Note: Data are presented as mean ± SD, and were analyzed by repeated measurements analysis of variance followed by Tukey’s post hoc test.
Table 6.
Myalgia during the study period
| Group | Day 0 | Day 2 | Day 4 | Day 6 | Day 8 | Day 10 | P-value |
|---|---|---|---|---|---|---|---|
| Intervention (n=59) | 1.43±1.25 | 0.27±0.64 | 0.15±0.58 | 0.12±0.46 | 0.12±0.49 | 0.03±0.18 | < 0.001 |
| Control (n=57) | 1.31±1.29 | 0.38±0.77 | 0.29±0.68 | 0.14±0.47 | 0.12±0.42 | 0.07±0.31 | < 0.001 |
|
| |||||||
| P-value | 0.630 | 0.408 | 0.229 | 0.823 | 0.981 | 0.464 | |
Note: Data are presented as mean ± SD, and were analyzed by repeated measurements analysis of variance followed by Tukey’s post hoc test.
Table 7.
Anorexia during the study period
| Group | Day 0 | Day 2 | Day 4 | Day 6 | Day 8 | Day 10 | P-value |
|---|---|---|---|---|---|---|---|
| Intervention (n=59) | 0.87±1.28 | 0.60±0.95 | 0.39±0.85 | 0.37±0.87 | 0.17±0.53 | 0.13±0.47 | < 0.001 |
| Control (n=57) | 1.08±1.28 | 0.59±1.03 | 0.49±0.92 | 0.24±0.66 | 0.15±0.56 | 0.10±0.40 | < 0.001 |
|
| |||||||
| P-value | 0.387 | 0.970 | 0.571 | 0.358 | 0.887 | 0.694 | |
Note: Data are presented as mean ± SD, and were analyzed by repeated measurements analysis of variance followed by Tukey’s post hoc test.
Table 8.
Anxiety during the study period
| Group | Day 0 | Day 2 | Day 4 | Day 6 | Day 8 | Day 10 | P-value |
|---|---|---|---|---|---|---|---|
| Intervention (n=59) | 1.20±1.25 | 0.74±0.96 | 0.60±0.85 | 0.39±0.77 | 0.20±0.52 | 0.22±0.62 | < 0.001 |
| Control (n=57) | 1.35±1.24 | 0.84±0.99 | 0.47±0.84 | 0.36±0.79 | 0.15±0.49 | 0.12±0.46 | <0 .001 |
|
| |||||||
| P-value | 0.538 | 0.583 | 0.416 | 0.847 | 0.606 | 0.326 | |
Note: Data are presented as mean ± SD, and were analyzed by repeated measurements analysis of variance followed by Tukey’s post hoc test.
Vital signs
Oxygen saturation and pulse rate were assessed every other day by researchers through home visits. Notably, no patient had a reduced oxygen saturation requiring hospital admission or supplemental oxygen therapy. The overall trend of both saturation and pulse rate were stable in both groups and did not change over time. Essentially, there was no statistically significant difference in the trends of oxygen saturation and pulse rate between the trial arms.
DISCUSSION
In this study, we aimed to investigate the effect of Iranian Polyherbal Syrup by Nepta bractaeta (Zufa syrup), a combination of several plants that have been historically used in traditional Persian medicine for respiratory infections, on the clinical course of patients with mild to moderate symptoms of COVID-19. As compared with placebo, Zufa syrup had no apparent effect on the symptomatology of COVID-19; however, the majority of patients improved during the careful follow-up by home visits and phone calls.
The COVID-19 pandemic presents unprecedented challenges. As the scientific community continues to search for an effective treatment or vaccine, traditional medicine approaches and herbal medicines could provide helpful interventions in this regard.15 Taking such potential into account, studies of plant-based medicines for COVID-19 are scarce. Clinical trials of potential treatments for this novel virus have only rarely been successful. Traditional medicine provides researchers with many potential remedies that have been historically used with evidence of beneficial effect in similar illnesses.16 Several herbal medicines have been studied as potential treatments for respiratory viral infections and alleviation of respiratory symptoms.11,12,17 The efficacy of the components of Zufa syrup has been investigated for respiratory illnesses. Moreover, another study in Iran assessed the effect of Nepeta bracteata benth on chronic obstructive pulmonary disease.18
Glycyrrhizaglabra or licorice has been historically used for medicinal purposes in China, India, and the Middle East and has been reported to be used for symptoms caused by viral respiratory diseases.16 Glycyrrhizin, a component of Glycyrrhizaglabra has been shown in randomized controlled trials to reduce hepatocellular damage in hepatitis B and C.19 In animal studies, glycyrrhizin had antiviral effects against herpes simplex virus and influenza A.19 Moreover, there is evidence of the antiviral activity of glycyrrhizin against human immunodeficiency virus, severe acute respiratory syndrome-related coronavirus, and respiratory syncytial virus.19,20,21 There have been observations of glycyrrhizin and its metabolically active component, glycyrrhetinic acid, to decrease the viral transmission.20 Moreover, anti-inflammatory effects of glycyrrhizin have been documented and are exerted through the Toll-like receptor 4.20 In 2003, at the time of severe acute respiratory syndrome outbreak, a study from Germany reported that glycyrrhizin was quite potent at inhibiting the replication of severe acute respiratory syndrome coronavirus in vitro, and concluded that glycyrrhizin should be investigated as a potential treatment.22 Pharmacologic pathways that have been proposed for the potential of glycyrrhizin in the treatment of COVID-19 include: down regulating inflammation in the lung and other organs, blockage of the accumulating mechanisms of intracellular reactive oxygen species, decreasing the over-production of airway secretions, decreasing thrombotic activity, inducing interferon activity and reduction of viral entry points.20,23
Nepeta species are a well-known anti-cough remedy in the traditional Percian medicine.7 Their effects on chronic cough, bronchitis, asthma, and chronic obstructive pulmonary disease has been studied.7,8 Such potential could translate into a treatment that safely and effectively alleviates symptoms of COVID-19 and reduces the associated anxiety of contracting the virus.24 Ficuscarica has been used for centuries as a medicinal plant for a wide variety of ailments, including symptoms of respiratory infections.25 Moreover, its extracts, hexanic and hexane-ethyl acetate, have been demonstrated to be potent antivirals which could be used against herpes viruses, echovirus, and adenoviruses.25 Viola species are traditional medicinal herbs used for respiratory illnesses and have been demonstrated to reduce the symptoms of asthma and respiratory inflammation.9,26 A study in mice documented the effects of viola on asthma to be related to a reduction in the activity of T helper type 2 cells.26 The effects of viola on respiratory symptoms and immunity could prove beneficial for COVID-19 patients. Plants have antipyretic, antitussive, anti-inflammatory, antioxidant and antimicrobial properties. It seems that it could be potential candidates for animal studies and clinical trials to prove their specific effectiveness.27 In traditional Persian medicine several herbs have proved their effect in alleviation of symptoms associated with common viral respiratory infections.28,29
A major advantage of our study was the close follow-up and observation of patients. This should be emphasized especially during the course of the pandemic. Keeping contact with patients, developing effective support systems, and close follow-up of outpatients could reduce the symptomatic burden of disease as well as the anxiety and psychologic effects of contracting COVID-19 for patients and their families. Such method could also improve education about methods of social distancing and compliance with respiratory hygiene and ultimately reduce the transmission of the virus in the society. Clinicians should be aware of the benefits of such follow-ups and do their best to change their practice accordingly when feasible.
The results of the current study should be interpreted in light of the study limitations. First, the drug dose was low due to prevention of complication. Second, we did not use the high concentration of extract. Third, a portion of patients have been affected to disease a week since the onset of the disease. Fourth, the outpatient follow-up is prone to differences in reporting of symptoms of patients. Therefore, a study on admitted patients could have a much more reliable assessment of the COVID-19 course. Due to the unique circumstances of the pandemic, repeating CT scans and conducting laboratory tests were not feasible options. The social distancing policies and the safety of research staff and patients mandated limited exposure. Moreover, we acknowledge that performing a polymerase chain reaction test for detection of COVID-19 virus could have led to a more reliable selection of patients with COVID-19, rather than chest CT alone. Another limitation was that follow-up phone calls and visits were performed by a group of researchers, not a single individual. This was inevitable due to the relatively large sample size and repeated follow-ups; however, it might have led to variations in the reporting of variables.
In this study, we indicated the effects of the Zufa syrup, a combination of herbal remedies for respiratory infections, for the treatment of patients with mild to moderate symptoms of COVID-19. Over the 10 days’ follow-up, all symptoms improved gradually, and no patient required hospital admission or supplemental oxygen therapy. The patient-reported severity of cough, headache, myalgia, anorexia, anxiety, and insomnia were not different throughout the follow-up. Dyspnea severity remained the same until the eighth day but was lower on the tenth day follow-up in the placebo arm. Oxygen saturation and pulse rate showed a stable trend throughout the follow-up and was similar between the intervention and control arms.
Additional file
Additional file 1: Checklist of demographic information and follow-up data.
Acknowledgements
We are very thankful for patients who participated in this study. Moreover, we are grateful from Vic chancellor of Qom University of Medical Sciences for supporting this project as well as managers of Shahid-Beheshti and Forghani Hospital, management of Baghiatallah Clinic. Moreover, we thank from Dr Maryam Nasiri, Dr Atiehsadat Danesh, Dr Rahimeh Dastranj, Dr SeyedReza Vakilinia, Dr Zahra Sarbazhoseini, Rumella Heidar, Dr Mojdeh Poorhoseini, Dr Fatemeh Toiserkani, Dr Mina Atharizadeh, Dr Batool Khaiatzadeh, Dr Fatemeh Jahani, Dr Morteza Aghahasani for all cooperation, such as patient follow up and call them, coordination with hospital and clinic, and booalidaroo company.
Checklist of demographic information and follow up
Name of questionnaire
Medical center
Date
Patient information
Age
Name
Gender
Occupation
Education
Address
Telephone
History of smoking
Principle illness; with explanation
Clinical symptoms
Severity:3
Intermediate :2
Low :1
Without symptom :0
| Do you have respiratory disease;diabetes;hypertension;coronary disease;chronic disease | day | severity | symptom | |||||
| 3 | 2 | 1 | 0 | |||||
| fever | 1 | |||||||
| shaking | 2 | |||||||
| Feeling cold | 3 | |||||||
| Dry cough | 4 | |||||||
| Productive cough | 5 | |||||||
| Do you use any special medicines?Give the names. | Sore throat | 6 | ||||||
| Irritation of throat | 7 | |||||||
| Itching throat | 8 | |||||||
| Nose dripping | 9 | |||||||
| Currently prescribed drugs by an emergency medicine specialist: | Dry mouth | 10 | ||||||
| Dyspnea | 11 | |||||||
| Body pain | 12 | |||||||
| Fatigue | 13 | |||||||
| Weakness | 14 | |||||||
| Headache | 15 | |||||||
| Anorexia | 16 | |||||||
| vomiting | 17 | |||||||
| thirsty | 18 | |||||||
| Stomach burnt | 19 | |||||||
| ? Do you use any herbal drugs | Reflux | 20 | ||||||
| diarrhea | 21 | |||||||
| nausea | 22 | |||||||
| Abdominal pain | 23 | |||||||
| Anosmia | 24 | |||||||
| No taste | 25 | |||||||
| insomnia | 26 | |||||||
| anxiety | 27 | |||||||
Follow up : after the day of visit
No symptom:0 mild:1 moderate:2 severe:3
| 2nd day | 4th day | 6th day | 8th day | 10th day | |
|---|---|---|---|---|---|
| O2 saturation | |||||
| Feeling of fever | |||||
| Shaking | |||||
| Feeling cold | |||||
| Dry cough | |||||
| Productive cough | |||||
| Sore throat | |||||
| Dry mouth | |||||
| Dyspnea | |||||
| Body pain | |||||
| Fatigue | |||||
| Weakness | |||||
| headache | |||||
| Nausea | |||||
| Vomiting | |||||
| thirsty | |||||
| Reflux | |||||
| Diarrhea | |||||
| Abdominal pain | |||||
| Anosmia | |||||
| No taste | |||||
| Insomnia | |||||
| Anxiety |
Side effects
1-
2-
3-
4-
Footnotes
Conflicts of interest
The authors declare no actual or potential conflicts of interest regarding this study.
Financial support
This study was funded by Deputy of Research and Technology Qom University of Medical Science.
Institutional review board statement
The study was approved by the Ethics Committee of the Qom University of Medical Science (Ethics committee reference number IR.MUQ.REC.1398.165) on March 10, 2020 and was registered in Iranian Clinical Trial Center (approval ID: IRCT20200404046934N1) on April 13, 2020.
Declaration of patient consent
The authors certify that they have obtained patients consent forms. In the form, patients have given their consent for their images and other clinical information to be reported in the journal. The patients understand that their names and initials not be published and due efforts will be made to conceal their identity.
Reporting statement
This study follows the CONsolidated Standards Of Reporting Trials (CONSORT) statement.
Biostatistics statement
The statistical methods of this study were conducted and reviewed by the epidemiologist of Qom University of Medical Sciences, Iran.
Copyright license agreement
The Copyright License Agreement has been signed by all authors xb efore publication.
Data sharing statement
Datasets analyzed during the current study are available from the corresponding author on reasonable request.
Plagiarism check
Checked twice by iThenticate.
Peer review
Externally peer reviewed.
Funding This study was funded by Deputy of Research and Technology Qom University of Medical Science.
REFERENCES
- 1.Huang C, Wang Y, Li X, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395:497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.World Health Organization. COVID-19 situation reports. [Accessed by July 16, 2020]; https:// www.who.int/emergencies/diseases/novel-coronavirus-2019/situation- reports. [Google Scholar]
- 3.Md Insiat Islam R. Current drugs with potential for treatment of COVID-19: a literature review. J Pharm Pharm Sci. 2020;23:58–64. doi: 10.18433/jpps31002. [DOI] [PubMed] [Google Scholar]
- 4.Baud D, Qi X, Nielsen-Saines K, Musso D, Pomar L, Favre G. Real estimates of mortality following COVID-19 infection. Lancet Infect Dis. 2020;20:773. doi: 10.1016/S1473-3099(20)30195-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yang Y, Islam MS, Wang J, Li Y, Chen X. Traditional Chinese medicine in the treatment of patients infected with 2019-new coronavirus (SARS-CoV-2): a review and perspective. Int J Biol Sci. 2020;16:1708–1717. doi: 10.7150/ijbs.45538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Shamsi-Baghbanan H, Sharifian A, Esmaeili S, Minaei B. Hepatoprotective herbs, avicenna viewpoint. Iran Red Crescent Med J. 2014;16:e12313. doi: 10.5812/ircmj.12313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Quddus A, Siddiqui MMH, Siddiqui MY, Aleem S. Clinical evaluation of the efficacy of Qurs Sartan Kafoori and Sharbat Zoofa Murakkab in chronic bronchitis. J Tradit Knowl. 2009;8:417–420. [Google Scholar]
- 8.Sehar N, Alam MI, Ahmad T, Ahmad M, Goswam A. Clinical study of unani formulation ‘Sharbat Zoofa Murakkab’ in the management of sual ratab (productive cough) Hippocratic J Unani Med. 2015;10:1–8. [Google Scholar]
- 9.Qasemzadeh MJ, Sharifi H, Hamedanian M, et al. The effect of viola odorata flower syrup on the cough of children with asthma: a double-blind, randomized controlled trial. J Evid Based Complementary Altern Med. 2015;20:287–291. doi: 10.1177/2156587215584862. [DOI] [PubMed] [Google Scholar]
- 10.Javid A, Motevalli Haghi N, Emami SA, et al. Short-course administration of a traditional herbal mixture ameliorates asthma symptoms of the common cold in children. Avicenna J Phytomed. 2019;9:126–133. [PMC free article] [PubMed] [Google Scholar]
- 11.Nassiri Asl M, Hosseinzadeh H. Review of antiviral effects of Glycyrrhiza glabra L. and its active component, glycyrrhizin. J Med Plants. 2007;6:1–12. [Google Scholar]
- 12.Ziai SA, Hamkar R, Monavari HR, Norooz-Babaei Z, Adibi L. Antiviral effect assay of twenty five species of various medicinal plants families in Iran. J Med Plants. 2007;6:S60112933. [Google Scholar]
- 13.Rahmanzade R, Rahmanzadeh R, Hashemian SM, Tabarsi P. Iran’s approach to COVID-19: evolving treatment protocols and ongoing clinical trials. Front Public Health. 2020;8:551889. doi: 10.3389/fpubh.2020.551889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.World Health Organization. Clinical management of COVID-19. [Accessed by July 16, 2020]. https://www.who.int/publications/i/item/clinical-management-ofcovid- 19.
- 15.Luo H, Tang QL, Shang YX, et al. Can Chinese medicine be used for prevention of corona virus disease 2019 (COVID-19)? A review of historical classics, research evidence and current prevention programs. Chin J Integr Med. 2020;26:243–250. doi: 10.1007/s11655-020-3192-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ang L, Lee HW, Kim A, Lee JA, Zhang J, Lee MS. Herbal medicine for treatment of children diagnosed with COVID-19: A review of guidelines. Complement Ther Clin Pract. 2020;39:101174. doi: 10.1016/j.ctcp.2020.101174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Karsch-Völk M, Barrett B, Kiefer D, Bauer R, Ardjomand-Woelkart K, Linde K. Echinacea for preventing and treating the common cold. Cochrane Database Syst Rev. 2014;2:CD000530. doi: 10.1002/14651858.CD000530.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Abdolahinia A, Naseri M, Eslaminejad A, Ghaffari F, Velayati A. Effect of Nepeta bracteata benth. on chronic obstructive pulmonary disease: a triple-blinded, randomized clinical trial. Iran Red Crescent Med J. 2018;20:e80112. [Google Scholar]
- 19.Fiore C, Eisenhut M, Krausse R, et al. Antiviral effects of Glycyrrhiza species. Phytother Res. 2008;22:141–148. doi: 10.1002/ptr.2295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Murck H. Symptomatic protective action of glycyrrhizin (Licorice) in COVID-19 infection. Front Immunol? 2020;11:1239. doi: 10.3389/fimmu.2020.01239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Knipping K, Garssen J, van’t Land B. An evaluation of the inhibitory effects against rotavirus infection of edible plant extracts. Virol J. 2012;9:137. doi: 10.1186/1743-422X-9-137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cinatl J, Morgenstern B, Bauer G, Chandra P, Rabenau H, Doerr HW. Glycyrrhizin, an active component of liquorice roots, and replication of SARS-associated coronavirus. Lancet. 2003;361:2045–2046. doi: 10.1016/S0140-6736(03)13615-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Luo P, Liu D, Li J. Pharmacological perspective: glycyrrhizin may be an efficacious therapeutic agent for COVID-19. Int J Antimicrob Agents. 2020;55:105995. doi: 10.1016/j.ijantimicag.2020.105995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Šmejkal K, Rjašková V. Use of plant extracts as an efficient alternative therapy of respiratory tract infections. Ceska Slov Farm. 2016;65:139–160. [PubMed] [Google Scholar]
- 25.Badgujar SB, Patel VV, Bandivdekar AH, Mahajan RT. Traditional uses, phytochemistry and pharmacology of Ficus carica: a review. Pharm Biol. 2014;52:1487–1503. doi: 10.3109/13880209.2014.892515. [DOI] [PubMed] [Google Scholar]
- 26.Harati E, Bahrami M, Razavi A, et al. Effects of viola tricolor flower hydroethanolic extract on lung inflammation in a mouse model of chronic asthma. Iran J Allergy Asthma Immunol. 2018;17:409–417. [PubMed] [Google Scholar]
- 27.Mohammadi Kenari H, Yousefsani BS, Eghbalian F, Ghobadi A, Jamshidi A, Mahroozade S. Herbal recommendations for treatment of COVID-19 symptoms according to Persian medicine. J Med Plants. 2021;20:1–14. [Google Scholar]
- 28.Bahramsoltani R, Rahimi R. An evaluation of traditional persian medicine for the management of SARS-CoV-2. Front Pharmacol. 2020;11:571434. doi: 10.3389/fphar.2020.571434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mentis AA, Dalamaga M, Lu C, Polissiou MG. Saffron for "toning down" COVID-19-related cytokine storm: hype or hope? A minireview of current evidence. Metabol Open. 2021;11:100111. doi: 10.1016/j.metop.2021.100111. [DOI] [PMC free article] [PubMed] [Google Scholar]


