Abstract
Cytokine storm in coronavirus disease 2019 (COVID-19) patients leads to acute lung injury, acute respiratory distress syndrome, multiorgan dysfunction, shock, and thrombosis thus contributing to significant morbidity and mortality. Several agents like steroids, ascorbic acid, vitamins (C, D, E), glutathione, N-acetylcysteine have been used and several studies are underway to identify its efficacy in addressing undesirable effects due to COVID-19 illness. Among several experimental modalities based on expert opinion and anecdotal data, melatonin is one molecule that appears promising. Owing to its anti-inflammatory, anti-oxidant, and immunomodulatory properties, melatonin can be an important agent used as a component of multimodal analgesia in COVID-19 patients, suspected patients, and patients with exposure to positive patients undergoing emergency or urgent surgeries. Further research is required to know the optimal time of initiation, dose, and duration of melatonin as an adjunct.
Keywords: anesthesia, antioxidants, COVID-19, cytokine, inflammation, melatonin, multimodal analgesia, perioperative, surgery
THE SPECTRUM OF COVID-19 INFECTION
In severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infections, there are marked inflammatory and immune responses leading to cytokine storm. This is because of the imbalance between the pro-inflammatory and anti-inflammatory mediators in the lung parenchyma. This eventually leads to apoptosis of epithelial cells and endothelial cells; subsequently, vascular leakage, abnormal T cell, and macrophages responses ensue and induce acute lung injury (ALI), acute respiratory distress syndrome (ARDS), multi-organ dysfunction, or even death.1,2 The components of a cytokine storm are interferons, interleukins, chemokines, colony-stimulating factors, and tumor necrosis factor-α.3 SARS-CoV-2 infection intrudes the host cells via S-spike protein by binding to angiotensin-converting enzyme 2 for internalization which is aided by transmembrane serine protease 2 protease. Once internalized, the viruses attack pulmonary parenchyma, myocytes, and endothelial cells of vasculature which leads to a cascade of inflammatory changes like tissue edema, degeneration, and necrosis. Proinflammatory cytokines like interleukin-6, interleukin-10 and tumor necrosis factor α, granulocyte colony-stimulating factor, monocyte chemoattractant protein 1, macrophage inflammatory protein 1α, and increased expression of programmed cell death 1, T-cell immunoglobulin, and mucin domain 3 are responsible for the insult. The systemic inflammation owing to this leads to ARDS, lowering of immunity, myocardial cell damage, and gastrointestinal system insults like diarrhea, vomiting, and abdominal pain.4,5 Several modalities have been used by clinicians to address the cytokine storm to reduce the damage caused by them. The most commonly used modalities are corticosteroids, immunoglobulins, interleukin antagonists, interferon-α, blood purification therapy like convalescent plasma.6 One such drug under investigation with a lot of promise appears to be melatonin (Figure 1). We searched PubMed, Google Scholar database, and clinicaltrials.gov using keywords: COVID-19; cytokine; melatonin; anesthesia; inflammation; antioxidants; surgery to investigate proven efficacy of melatonin in the perioperative period as an antioxidant, immunomodulator, adjunct to multimodal analgesia, and anti-COVID-19 properties.
Figure 1.
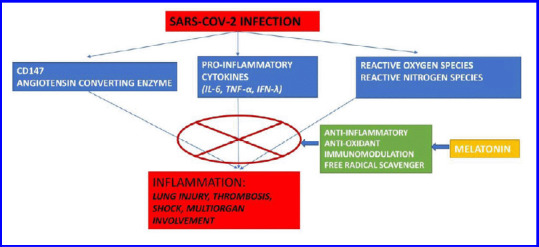
Various beneficial properties of melatonin when used in COVID-19 patients.
Note: COVID-19: Coronavirus disease 2019; IFN-λ: interferon-λ; IL-6: interleukin-6; SARS-CoV2: severe acute respiratory syndrome coronavirus 2; TNF-α: tumor necrosis factor-α.
CD147 AND ITS IMPLICATION IN COVID-19
CD147 is a type I transmembrane protein that is implicated with unfavorable outcomes during viral infections including SARS-CoV-2. This is mediated through pro-inflammatory cytokines like interleukin-6, interferon-λ, tumor necrosis factor-α, and monocyte chemo-attractant protein-1. CD147 has also been found responsible for conditions like multiple sclerosis, myocardial infarction, and cancer proliferation.7,8 In SARS-CoV-2 infections, CD147, along with angiotensin converting enzyme, has been implicated in activation of inflammation. COVID-19 infection has an initial pulmonary phase in which there is viral replication and inflammation. The inflammasome is an important element in the cytokine storm and that reactive oxygen species (ROS) is a potent ligand and direct mediator for triggering NOD-like receptors P3 inflammasome.9 With the activation of ROS and inflammasome along with inflammatory cytokines, there are systemic inflammatory effects like thrombosis, lung injury, and myocardial injury.10 Mitochondria of the cell are the main target of ROS produced due to inflammation. The balance between oxidase and antioxidase determines the generation and elimination of ROS. Increased production of free radical, enhanced activity of mitochondrial inducible nitric oxide synthase, enhanced nitric oxide production, decreased respiratory complex activity, malfunctioning of the electron transport system, and opening of mitochondrial permeability transition pores lead to impaired mitochondrial function.11
MELATONIN – CLINICAL PHARMACOLOGY
Melatonin (5 methoxy-N acetyltryptamine) is a hormone secreted by the pineal gland. Supplemental melatonin has been used by clinicians to manage sleep disorders, delirium, and as an anti-inflammatory agent in diseases like atherosclerosis, respiratory diseases, and viral infections. The anti-oxidative effect of melatonin along with its anti-inflammatory actions like up-regulating anti-oxidative enzymes (e.g., superoxide dismutase), down-regulating pro-oxidative enzymes (e.g., nitric oxide synthase) is well known.12,13,14,15 Melatonin also interacts directly with free radicals, functioning as a free radical scavenger. Another favorable effect of melatonin is its immunomodulatory effects.16 This is supposed to be due to its anti-inflammatory effects in certain conditions and also antioxidative effects.
Melatonin exerts anti-inflammatory effects through various pathways. Sirtuin-1 mediates anti-inflammatory actions of melatonin by inhibiting high mobility group boxechromosomal protein 1, and down-regulating the polarization of macro-phages towards the pro-inflammatory type.17 Nuclear factor kappa-B (NF-κB) has pro-inflammatory and pro-oxidative activities in ALI. The anti-inflammatory effect of melatonin suppresses NF-κB activation by down-regulating NF-κB activation in T cells and lung tissue. Experimental research suggests close interaction of sirtuin-1, NF-κB, and nuclear factor erythroid 2-related factor 2 suggests their participation in the coronavirus-induced ALI/ARDS.18 Melatonin directly scavenges oxygen free radicals, upregulates the expression of antioxidase, and downregulates the expression of oxidase. Therefore, melatonin is also referred to as a mitochondriatargeted antioxidant.19
Melatonin does not have any antiviral effects. However, the anti-inflammatory and anti-oxidant properties of melatonin have been found beneficial in a series of patients with viral lung infections. In a dose ranging from 3 to 10 mg per day, oral melatonin has been found safe in critically ill patients.20 Melatonin deficiency in older adult patients is considered as a factor responsible for adverse outcomes as the deficiency leads to immunosuppression.
MELATONIN AS ADJUVANT IN COVID-19 TREATMENT
Reducing inflammation and enhancing immunity is important in COVID-19 patients to prevent mortality and morbidity. The use of antiviral drugs in early stages when viral replication and infectivity is high appears to be beneficial. Once that stage is crossed, i.e., from the second-week cytokine storm sets in which is responsible for ALI/ARDS, multiorgan dysfunction, and other adverse events like thrombosis.
The safety of melatonin as an adjunct to multimodal analgesia is well established. Melatonin has antinociceptive properties and has been shown efficacious in managing acute postoperative pain and chronic neuropathic pain as a component of multimodal analgesia.21,22,23 Due to its anti-inflammatory, antioxidant, and antinociceptive effects, anesthesiologists can consider starting oral melatonin to COVID-19 positive patients who are coming for emergency or urgent surgeries. When administered orally, the Tmax of oral melatonin is about 50 minutes in healthy volunteers. Ramlall et al.24 retrospectively reviewed data from 791 intubated patients with COVID-19. Authors concluded that patients who were treated with melatonin after intubation had a statistically significant positive outcome (demographics and comorbidities adjusted hazard ratio: 0.131, 95% confidence interval: 0.076 to 0.223). However, the authors did not mention the dose and duration of melatonin therapy in these patients. There are several ongoing studies which are currently ongoing which is investigating the dose, efficacy, safety, and duration of melatonin use in critically ill COVID-19 patients.25,26,27 A study has suggested a dose ranging from 5–8 mg/kg per day in COVID-19 patients for its anti-inflammatory and immunomodulatory effects.28 In critically ill patients, bioavailability will be affected by gut malabsorption and factors affecting the first-pass metabolism. Based on a systematic review done by Harpsøe et al.29 the authors concluded that the bioavailability of oral melatonin is 15%. Results of ongoing studies could guide clinicians regarding the safest possible dose and acceptable duration of melatonin use in critically ill COVID-19 patients. Lower segment caesarean sections posed a unique challenge during the COVID-19 pandemic both while dealing with positive patients and also while dealing with reverse transcriptase polymerase chain reaction negative or suspected patients. Reports which are available from several centers have mentioned that there was an increased rate of caesarean section during the pandemic.30 The safety and efficacy of preoperative melatonin was explored earlier by Khezri et al.31 in patients undergoing caesarean section and it was found that the time to rescue analgesia was prolonged though not significant statistically. In this pandemic, researchers can utilize the opportunity to determine the optimal dose of melatonin as premedication before caesarean section and also the anti-inflammatory, anti-oxidant properties of melatonin in COVID-19 parturients undergoing surgery.
CONCLUSION
Melatonin is a safe drug that could be used in therapeutic doses at any stage when reverse transcriptase polymerase chain reaction is positive for COVID-19 as there are no recommendations right now for the optimal time of starting melatonin. Anesthesiologists could consider using melatonin as a part of multimodal analgesia when dealing with COVID-19 patients, suspected patients, or patients who have history of exposure as it could provide additional benefit. Further, ongoing studies could reveal the optimal time of starting, safest possible dose, and duration of melatonin therapy to provide anti-inflammatory and anti-oxidative benefits in COVID-19 patients.
Footnotes
Conflicts of interest
None.
Financial support
Nil.
Copyright license agreement
The Copyright License Agreement has been signed by the author before publication.
Plagiarism check
Checked twice by iThenticate.
Peer review
Externally peer reviewed.
REFERENCES
- 1.García LF. Immune response, inflammation, and the clinical spectrum of COVID-19. Front Immunol. 2020;11:1441. doi: 10.3389/fimmu.2020.01441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tay MZ, Poh CM, Rénia L, MacAry PA, Ng LFP. The trinity of COVID-19: immunity, inflammation and intervention. Nat Rev Immunol. 2020;20:363–374. doi: 10.1038/s41577-020-0311-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Coperchini F, Chiovato L, Croce L, Magri F, Rotondi M. The cytokine storm in COVID-19: an overview of the involvement of the chemokine/chemokine-receptor system. Cytokine Growth Factor Rev. 2020;53:25–32. doi: 10.1016/j.cytogfr.2020.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Azer SA. COVID-19: pathophysiology, diagnosis, complications and investigational therapeutics. New Microbes New Infect. 2020;37:100738. doi: 10.1016/j.nmni.2020.100738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bhavana V, Thakor P, Singh SB, Mehra NK. COVID-19: Pathophysiology, treatment options, nanotechnology approaches, and research agenda to combating the SARS-CoV2 pandemic. Life Sci. 2020;261:118336. doi: 10.1016/j.lfs.2020.118336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Malik S, Gupta A, Zhong X, Rasmussen TP, Manautou JE, Bahal R. Emerging therapeutic modalities against COVID-19. Pharmaceuticals (Basel) 2020;13:188. doi: 10.3390/ph13080188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zhu X, Song Z, Zhang S, Nanda A, Li G. CD147: a novel modulator of inflammatory and immune disorders. Curr Med Chem. 2014;21:2138–2145. doi: 10.2174/0929867321666131227163352. [DOI] [PubMed] [Google Scholar]
- 8.Ulrich H, Pillat MM. CD147 as a target for COVID-19 treatment: suggested effects of azithromycin and stem cell engagement. Stem Cell Rev Rep. 2020;16:434–440. doi: 10.1007/s12015-020-09976-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mehta P, McAuley DF, Brown M, et al. COVID-19: consider cytokine storm syndromes and immunosuppression. Lancet. 2020;395:1033–1034. doi: 10.1016/S0140-6736(20)30628-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cecchini R, Cecchini AL. SARS-CoV-2 infection pathogenesis is related to oxidative stress as a response to aggression. Med Hypotheses. 2020;143:110102. doi: 10.1016/j.mehy.2020.110102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ganie SA, Dar TA, Bhat AH, et al. Melatonin: a potential anti-oxidant therapeutic agent for mitochondrial dysfunctions and related disorders. Rejuvenation Res. 2016;19:21–40. doi: 10.1089/rej.2015.1704. [DOI] [PubMed] [Google Scholar]
- 12.Zhang HM, Zhang Y. Melatonin: a well-documented antioxidant with conditional pro-oxidant actions. J Pineal Res. 2014;57:131–146. doi: 10.1111/jpi.12162. [DOI] [PubMed] [Google Scholar]
- 13.Karaaslan C, Suzen S. Antioxidant properties of melatonin and its potential action in diseases. Curr Top Med Chem. 2015;15:894–903. doi: 10.2174/1568026615666150220120946. [DOI] [PubMed] [Google Scholar]
- 14.Reiter RJ, Mayo JC, Tan DX, Sainz RM, Alatorre-Jimenez M, Qin L. Melatonin as an antioxidant: under promises but over delivers. J Pineal Res. 2016;61:253–278. doi: 10.1111/jpi.12360. [DOI] [PubMed] [Google Scholar]
- 15.Reiter RJ, Rosales-Corral S, Tan DX, Jou MJ, Galano A, Xu B. Melatonin as a mitochondria-targeted antioxidant: one of evolution’s best ideas. Cell Mol Life Sci. 2017;74:3863–3881. doi: 10.1007/s00018-017-2609-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Carrillo-Vico A, Lardone PJ, Alvarez-Sánchez N, Rodríguez-Rodríguez A, Guerrero JM. Melatonin: buffering the immune system. Int J Mol Sci. 2013;14:8638–8683. doi: 10.3390/ijms14048638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hardeland R. Aging, melatonin, and the pro- and anti-inflammatory networks. Int J Mol Sci. 2019;20:1223. doi: 10.3390/ijms20051223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cristofanon S, Uguccioni F, Cerella C, et al. Intracellular prooxidant activity of melatonin induces a survival pathway involving NF-kappaB activation. Ann N Y Acad Sci. 2009;1171:472–478. doi: 10.1111/j.1749-6632.2009.04896.x. [DOI] [PubMed] [Google Scholar]
- 19.Mistraletti G, Sabbatini G, Taverna M, et al. Pharmacokinetics of orally administered melatonin in critically ill patients. J Pineal Res. 2010;48:142–147. doi: 10.1111/j.1600-079X.2009.00737.x. [DOI] [PubMed] [Google Scholar]
- 20.Qiu X, Wang X, Qiu J, et al. Melatonin rescued reactive oxygen species-impaired osteogenesis of human bone marrow mesenchymal stem cells in the presence of tumor necrosis factor-alpha. Stem Cells Int. 2019;2019:6403967. doi: 10.1155/2019/6403967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhu C, Xu Y, Duan Y, et al. Exogenous melatonin in the treatment of pain: a systematic review and meta-analysis. Oncotarget. 2017;8:100582–100592. doi: 10.18632/oncotarget.21504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Srinivasan V, Zakaria R, Jeet Singh H, Acuna-Castroviejo D. Melatonin and its agonists in pain modulation and its clinical application. Arch Ital Biol. 2012;150:274–289. doi: 10.4449/aib.v150i4.1391. [DOI] [PubMed] [Google Scholar]
- 23.Srinivasan V, Pandi-Perumal SR, Spence DW, et al. Potential use of melatonergic drugs in analgesia: mechanisms of action. Brain Res Bull. 2010;81:362–371. doi: 10.1016/j.brainresbull.2009.12.001. [DOI] [PubMed] [Google Scholar]
- 24.Ramlall V, Zucker J, Tatonetti N. Melatonin is significantly associated with survival of intubated COVID-19 patients. medRxiv. 2020;doi:10. doi: 10.1101/2020.10.15.20213546. [Google Scholar]
- 25.Acuña-Castroviejo D, Escames G, Figueira JC, de la Oliva P, Borobia AM, Acuña-Fernández C. Clinical trial to test the efficacy of melatonin in COVID-19. J Pineal Res. 2020;69:e12683. doi: 10.1111/jpi.12683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rodríguez-Rubio M, Figueira JC, Acuña-Castroviejo D, Borobia AM, Escames G, de la Oliva P. A phase II, single-center, double-blind, randomized placebo-controlled trial to explore the efficacy and safety of intravenous melatonin in patients with COVID-19 admitted to the intensive care unit (MelCOVID study): a structured summary of a study protocol for a randomized controlled trial. Trials. 2020;21:699. doi: 10.1186/s13063-020-04632-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Emadi A, Chua JV, Talwani R, Bentzen SM, Baddley J. Safety and Efficacy of Imatinib for Hospitalized Adults with COVID-19: A structured summary of a study protocol for a randomised controlled trial. Trials. 2020;21:897. doi: 10.1186/s13063-020-04819-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kleszczyński K, Slominski AT, Steinbrink K, Reiter RJ. Clinical trials for use of melatonin to fight against COVID-19 are urgently needed. Nutrients. 2020;12:2561. doi: 10.3390/nu12092561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Harpsøe NG, Andersen LP, Gögenur I, Rosenberg J. Clinical pharmacokinetics of melatonin: a systematic review. Eur J Clin Pharmacol. 2015;71:901–909. doi: 10.1007/s00228-015-1873-4. [DOI] [PubMed] [Google Scholar]
- 30.Debrabandere ML, Farabaugh DC, Giordano C. A review on mode of delivery during COVID-19 between December 2019 and April 2020. Am J Perinatol. 2021;38:332–341. doi: 10.1055/s-0040-1721658. [DOI] [PubMed] [Google Scholar]
- 31.Beigom Khezri M, Delkhosh Reihany M, Oveisy S, Mohammadi N. Evaluation of the analgesic efficacy of melatonin in patients undergoing cesarean section under spinal anesthesia: a prospective randomized double-blind study. Iran J Pharm Res. 2016;15:963–971. [PMC free article] [PubMed] [Google Scholar]


