Abstract
Background and purpose:
Acute lymphoblastic leukemia (ALL) is a type of cancer of blood and bone marrow characterized by abnormal proliferation of lymphoid progenitor cells. Galectin-9 is a tandem-repeat type galectin expressed in various tumor cells. It seems that the connection between galectin-9 and T cell immunoglobulin mucin-3 receptor acts as a negative regulator of cancer cells proliferation.
Experimental approach:
In this research, the effects of galectin-9 were investigated using MTS cell proliferation colorimetric, colony-forming, annexin V-FITC/PI, and caspase-3 assays in the Jurkat and KE-37 cell lines of ALL. Furthermore, the western blotting technique was used to evaluate the levels of apoptotic proteins such as Bax and Bcl-2 in these cell lines.
Findings/Results:
Our results indicated that galectin-9 can considerably reduce the cell growth and colony formation ability of both Jurkat and KE-37 cell lines in a concentration-dependent manner. Besides, galectin-9 induced apoptosis in a concentration-dependent manner in ALL cells by a mechanism associated with Bax/Bcl-2 expression and activation of the caspase-3 activation.
Conclusion and implications:
Galectin-9 inhibited the growth and proliferation of cell lines with increased programmed cell death, therefore it can be considered as a potential factor in the progression of ALL therapeutics that needs more research in this context.
Keywords: Acute lymphoblastic leukemia, Apoptosis, Galectin-9, T-cell immunoglobulin, mucin-domain 3
INTRODUCTION
Acute lymphoblastic leukemia (ALL) is known as a cancer of the lymphoid lines of the blood cells which is characterized by the development of large numbers of immature lymphocytes. Although the majority of ALL (80%) occurs in children, it is also the second most common acute leukemia in adults. The prevalence of ALL in the United States has been reported as 1.6 per 100,000 population with the number of 6590 new cases of ALL in 2016 (1,2). Due to the prevalence of ALL and despite significant advances in the treatment of ALL, it seems that a small percentage of the patients will achieve long-term remission. Considering the high prevalence of ALL and the long treatment duration in some cases, thus studies searching for novel efficient therapeutics for ALL could be helpful. In general, the immune system fights cancer cells through a series of molecular cascades. Investigation of the molecular mechanisms involved in ALL progression and pathogenesis makes it possible to identify the specific molecules for targeting and consequently the elimination of the cancer cells.
The galectin family is a group of proteins belonging to lectins that have one or two carbohydrate detection domains with affinity to bind beta-galactoside (3). According to the different roles of galectins in apoptosis, angiogenesis, cell migration, and tumor-immune escape, their expression has been thoroughly investigated in several cancers (4). So far, 15 members belonging to the galectins family have been identified in mammals. Galectin-9 is one of the tandem repeat-type of galectins with two distinct beta-galactoside-binding sites (5). Many studies indicated multifaceted functions for galectin-9 in the prevention of various cancers development. Galectin-9 binds to β-galactoside of T cell immunoglobulin mucin-3 (TIM-3) proteins and modulates multiple biological activities such as cell growth, invasion, adhesion, and cell death of tumor cells (6). The previous study demonstrated that the apoptotic effects of galectin-9 in CD4+ Th1 and Th17 cells (7). Moreover, it has been shown that activation of the DR3 signaling pathway by galectin-9 could stimulate the suppressive activity of regulatory T cells (8). It was shown that in solid tumors, the galectin-9 expression is associated with inhibition of tumor cell adhesion or metastasis. For instance, high levels of galectin-9 prevent lung metastasis of melanoma cells in mice (9). Also, clinical researches have shown a reverse correlation between the expression level of galectin-9 and the potential of metastasis in patients with breast cancer and hepatocellular carcinoma (HCC) (10,11). It has been illustrated that the interaction of TIM-3 and galectin-9 can induce apoptosis in mouse colon tumor cells (12). In another study, the inhibitory and suppressive role of galectin-9 on cancer cell proliferation was demonstrated by exploring its effect on the growth of tumor cells in numerous types of gastrointestinal tumors, such as HCC, gastric cancer, gallbladder carcinoma, and cholangiocarcinoma (13,14,15,16). Another study conducted by Kuroda et al. demonstrated the induction of apoptosis by galectin-9 which occurs with the Noxa-dependent pathway in chronic myelogenous leukemia (17).
In a primary study, we examined the expression of TIM-3 and mucin-domain 3 as one of the molecules present in the ALL pathway. Our results indicated that TIM-3 expression was increased in the peripheral blood and bone marrow of these patients (18). Therefore, in the extension of our previous study here we aimed to investigate the effects of galectin-9 (as a TIM-3 ligand) on the proliferation and apoptosis on ALL cell lines. In this procedure, the most available ALL cell lines (KE-37 and Jurkat) were selected as a model for human acute lymphoblastic T cells.
MATERIALS AND METHODS
Cell lines
National Cell Bank of Iran (Pasteur Institute of Iran, Tehran) was a supplier source of Jurkat and KE-37 ALL cell lines. Both cell lines were cultured using RPMI-1640 medium containing 10% fetal bovine serum (FBS), 100 mIU/mL penicillin, and 100 mg/mL streptomycin (Gibco, Germany). The cells were maintained in a humidified incubator at 37 °C with 5% CO2 (19).
Cell proliferation assay
The influence of galectin-9 (R&D Systems, USA) on the proliferation of Jurkat and KE-37 cell lines was measured using 3-(4,5-dimethylthiazol-2-yl) -5-(3- carboxymethoxy-phenyl)-2- (4-sulfophenyl)- 2H-tetrazolium (MTS) cell viability assay (20). For this experiment, Jurkat (3 × 103 cells/well) and KE-37 (3 × 103 cells/well) cell lines were plated in 96-well plates and incubated with galectin-9 at 0.1-100 nM. After 48 h of growth, 20 μL of the MTS substrate (Promega, Tokyo, Japan) was added to each well and then were incubated for an additional 3 h. Then, the absorbance was read at 490 nm using a microplate reading spectrophotometer (BioTek, USA). According to the documents obtained from the MTS assay, the IC50 of galectin-9 for Jurkat and KE-37 cell lines was calculated. The IC50 values were determined for 0.1-100 nM concentration of galectin-9 using the GraphPad Prism software.
Colony-forming assay
The clonogenic assay was accomplished using the soft agar colony formation method for both Jurkat and KE-37 cell lines. The base layer of a 6-well plate was covered with 1 mL of 0.8% agar (Fermentas, USA) in the culture medium and allowed to harden at room temperature. The upper layer in each well comprised 500 μL suspensions of Jurkat or KE-37 cells (1 × 103 cells/well) in a mixture of 0.4% agar that was treated with different galectin-9 (1-100 nM) concentrations. After incubation for 2 weeks at 37 °C in culture media, the crystal violet color was used for plate staining, and colonies were observed with an inverted light microscope (Leica, Germany) (21).
Detection of galectin-9 apoptosis induction in Jurkat and KE-37 cell lines
The flow cytometric technique was used to elucidate whether the galectin-9 shows apoptotic activity in Jurkat and KE-37 cell lines. Jurkat (3 × 105 cells/well) and KE-37 (3 × 105 cells/well) were incubated in a 6-well plate with different concentrations of galectin-9 (1-100 nM) for 48 h. Then, the washed cells were re-suspended in a binding buffer. Subsequently, the cells were exposed to annexin-V fluorescein isothiocyanate (FITC) and propidium iodide (PI) dyes (Biovision, USA) for 15 min Afterward, apoptosis was measured using flow cytometry (FAC Calibur, Bioscience, USA) (22). The untreated Jurkat and KE-37 cells were used as the negative control.
Evaluation of apoptotic proteins
Western blotting was performed to measure Bcl-2 and Bax proteins levels (23). Jurkat and KE-37 cells were incubated with different concentrations of galectin-9 (1-100 nM) for 48 h. After twice washing with ice-cold phosphate-buffered saline (PBS, pH 7.4), the cells were lysed in lysis buffer containing aprotinin (1 mg/mL), pepstatin (1 mg/mL), phenylmethylsulfonyl fluoride (PMSF) (0.05 mmol/mL), sodium orthovanadate (1 mg/mL), NaF (500 mmol/mL), and ethylenediaminetetraacetic acid (EDTA) (500 mmol/mL). The protein samples with the same volumes were loaded to sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and transferred to polyvinylidene difluoride (PVDF) membranes. By embedding the membranes in 5% skim milk for 2 h at room temperature, the membranes were blocked, subsequently incubated overnight at 4 °C with primary anti-Bcl-2, anti-Bax, and anti-glyceraldehyde 3-phosphate dehydrogenase (GAPDH, as a control, housekeeping gene; Santa Cruz Biotechnology, USA), and then were rinsed three times by PBS-Tween® 20 (PBST). Next, the membranes were shaken for 1 h at room temperature with the goat anti-mouse IgG-HRP secondary antibody (Santa Cruz Biotechnology, USA). After rinsing with PBST, the enhanced chemiluminescence detection substance (Bio-rad, USA) was used to identify target proteins.
Caspase-3 activity analysis
Enzymatic activity of caspase-3 was assessed with an R&D kit (Minneapolis, USA) according to the producer’s protocol. Concisely, cells were treated with different concentrations of galectin-9 (0.1-100 nM) for 24 h. Then, cold lysis buffer was added to cells and then cells were kept on ice for 10 min. The supernatants were collected and added to the caspase-3 substrate. The absorbance of p-nitroaniline was determined after 1 h using a microplate reader (BioTek, USA) at 405 nm wavelength (24).
Statistical analysis
Data in the current study are shown as mean ± SEM. The data have been analyzed by a parametric test of variance between groups (ANOVA) followed by Dunnett’s post hoc test. All of the experiments were conducted in three replicates. Statistical analyses were done by software package SPSS version 21 (Statistical Package ver. 18.0; SPSS Inc., Chicago, IL, USA). The difference at P < 0.05 was considered statistically significant. The IC50 values were calculated using the GraphPad Prism software.
RESULTS
Galectin-9 inhibited cell growth of ALL cells
The influence of galectin-9 on viability and proliferation of the Jurkat and KE-37 cell lines was analyzed after 48 h by MTS assay. Our findings showed that treatment with galectin-9 prevented the proliferation of both Jurkat and KE-37 cells in a concentration-dependent manner (P < 0.05). In fact, we observed a noticeable relationship between galectin-9 concentration and cell mortality level (Fig. 1).
Fig. 1.
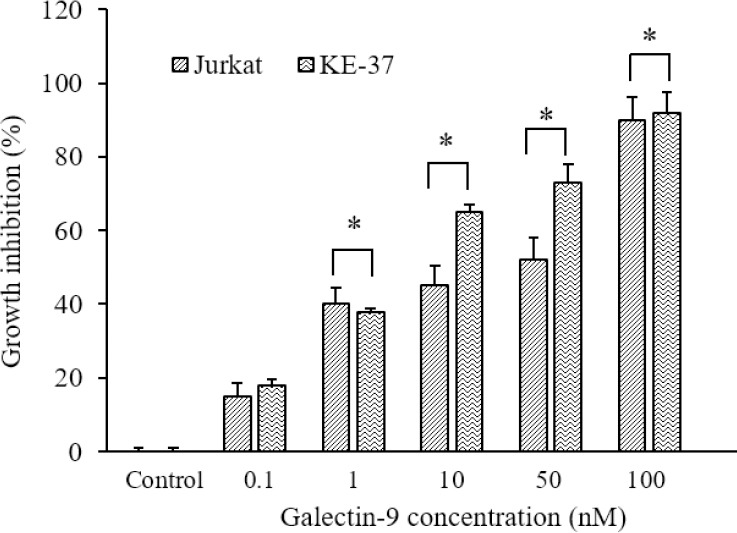
Detection of anti-proliferative effect of galectin-9 on Jurkat and KE-37 ALL cell lines using the MTS assay. Results are reported as mean ± SD of triplicate tests *P < 0.05 Indicates significant differences between defined groups.
The inhibition of cell proliferation was obvious in both cell lines (P < 0.05) when the concentration of galectin-9 is more than 1 nM. On the other hand, the growth inhibition was less sensitive in 0.1 nM of galectin-9. The values of IC50 for two Jurkat and KE-37 cell lines were obtained 3.4 nM and 2.4 nM, respectively. By comparing the percentage of cell growth inhibition at equivalent concentrations of galectin-9 in the two cell lines, it can be concluded that the KE-37 cell line was more susceptible to the galectin-9 toxicity rather than Jurkat cells.
Galectin-9 inhibited clonogenic growth of Jurkat and KE-37 cell lines
The clonogenic inhibitory effect of galectin-9 in Jurkat and KE-37 cell lines was investigated by a soft agar colony formation assay. We found in our research that the colony formation ability of the untreated Jurkat and KE-37 cells was higher than galectin-9-treated Jurkat and KE-37 cells. Actually, we observed that colony formation was significantly suppressed by galectin-9 in a concentration-dependent manner in both Junket (Fig. 2A) and KE-37 (Fig. 2B) cell lines. Similar to the cell viability assay, the colony-forming assay also showed a concentration-dependent manner in reduction of colony survival and percentage of the colony formation efficiency of both cell lines when treated with various galectin-9 concentrations (1, 10, and 100 nM; Fig. 2C).
Fig. 2.
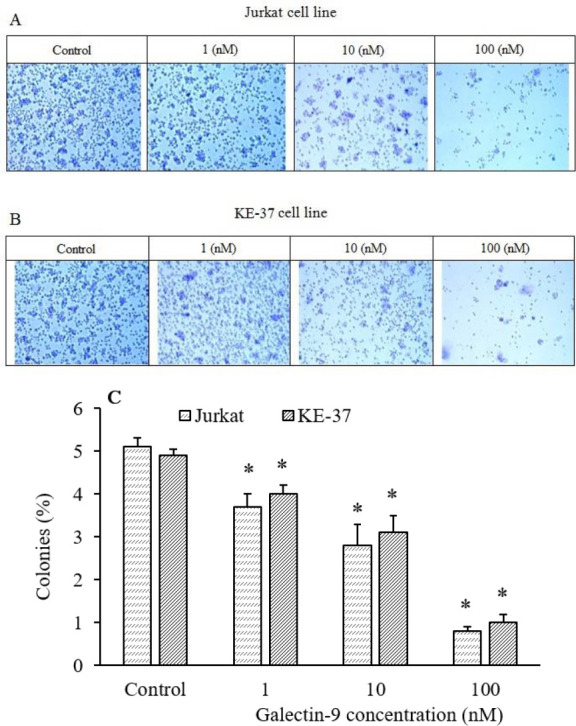
Colony formation assay for (A) Jurkat and (B) KE-37 cell lines in different concentrations of galectin-9 (1-100 nM) compared with the control (non-treated). (C) The histogram demonstrates the percentage of colony-forming efficiency in Jurkat and KE-37 cell lines. Results are reported as mean ± SD of triplicate tests. *P < 0.05 Indicate significant differences compared to the corresponding control group.
Galectin-9 induced cell apoptosis
The flow cytometric analysis was carried out to measure apoptotic cells, which were stained with annexin V/PI. The Jurkat and KE-37 cells were treated with different concentrations of galectin-9 (1-100 nM), and after incubation for 48 h, the apoptotic response was evaluated using an annexin V/PI staining kit. The percentage of the apoptotic, necrotic, and viable Jurkat and KE-37 cells were detected. Our results demonstrated that galectin-9 inhibited cell growth and induced apoptosis indirect relationship with galectin-9 concentration (Fig. 3). Furthermore, the Jurkat and KE-37 cells were significantly induced to undergo apoptosis when exposed to more than 1 nM of galectin-9.
Fig. 3.
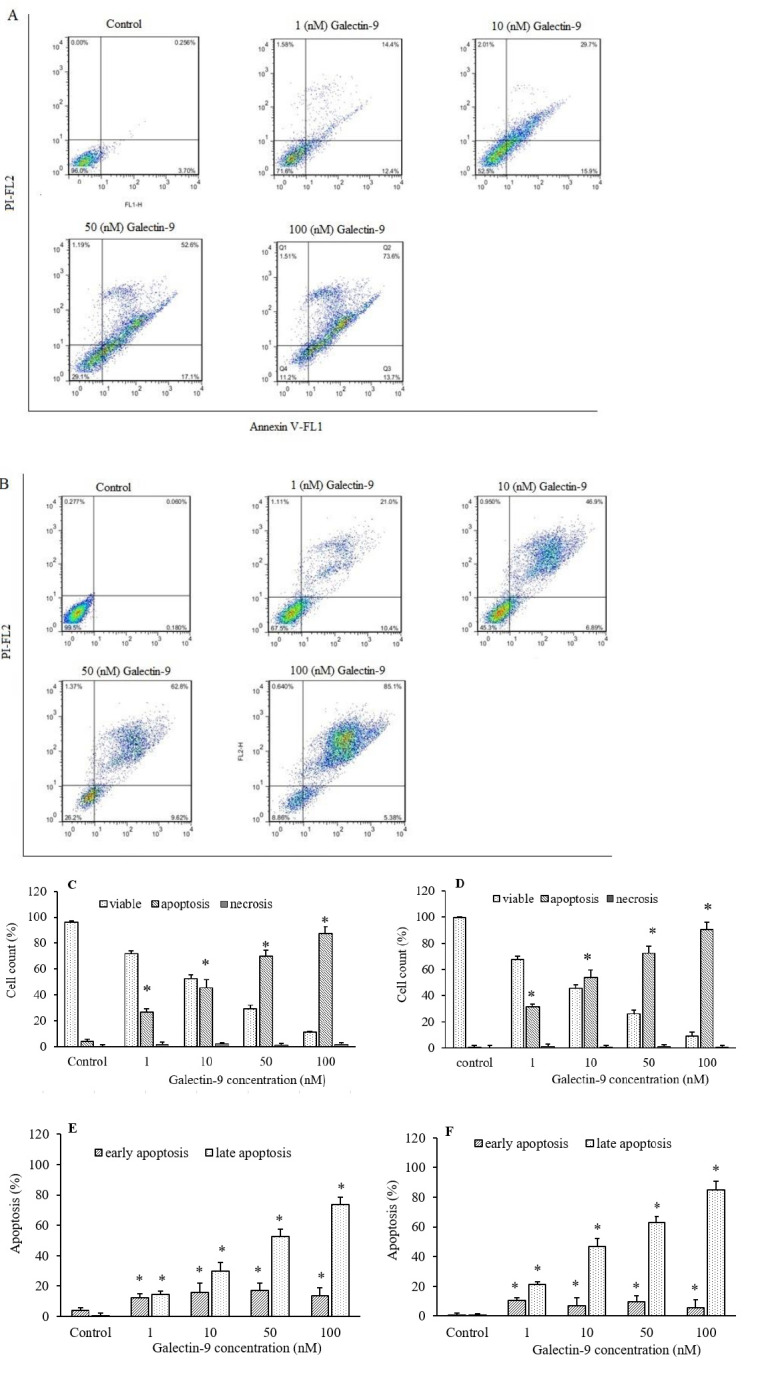
Apoptosis analysis by flow cytometry plots using annexin V-FITC/PI staining for different concentrations of galectin-9 (1-100 nM) in (A) Jurkat and (B) KE-37 cell lines. Apoptosis induction chart of (C) Jurkat and (D) KE-37 cell lines after 48-h treatment with different concentrations of galectin-9 using annexin V/PI flowcytometric assay. Annexin V-FITC and PI were read at FL1 and FL2 channels, respectively. The percentage (%) of early and late apoptotic cells in (E) Jurkat and (F) KE-37. Results are reported as mean ± SD of triplicate tests. *P < 0.05 Indicate significant differences compared to the corresponding control group. FITC, fluorescein isothiocyanate; PI, propidium iodide.
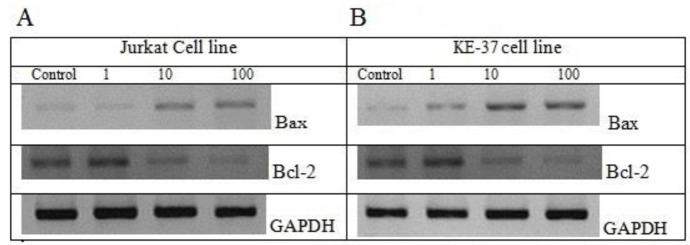
Galectin-9 regulated the expression of apoptotic proteins
For more confirmation, the effect of galectin-9 on the level of the pro-apoptotic protein (Bax) and anti-apoptotic protein (Bcl-2) was investigated using western blot to approve that galectin-9 induces apoptosis in ALL cell lines. The results demonstrated an increased level of the Bax protein and decreased level of the Bcl-2 protein in a concentration-dependent manner of galectin-9 in Jurkat (Fig. 4A) and KE-37 (Fig. 4B) cell lines.
Fig. 4.
The expression levels of Bax and Bcl-2 proteins were determined by western blot in (A) Jurkat and (B) KE-37 cell lines in three different concentrations of galectin-9 (1-100 nM). The ratio of Bax/Bcl-2 has been increased in a concentration-dependent manner of galectin-9.
Galectin-9 increased caspase-3 activity
To investigate the effects of galectin-9 on caspase activity, we measured the activity of caspase-3 after treatment by galectin-9 in Jurkat and KE-37 cell lines. These data showed a remarkable increase in caspase-3 activity directly correlated to galectin-9 concentration (P < 0.05, Fig. 5).
Fig. 5.
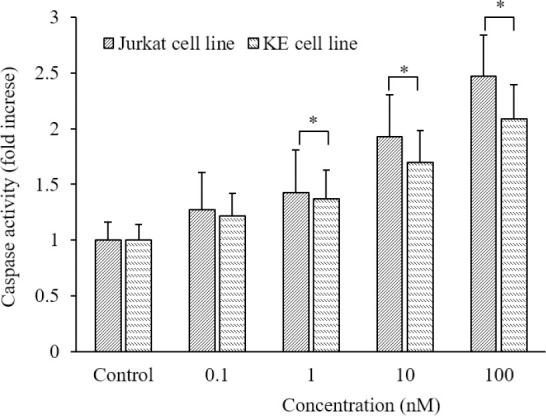
The effects of galectin-9 on caspase-3 activity in Jurkat and KE-37 cells. The activity of caspase-3 enhanced in a concentration-dependent manner after treatment with galectin-9. Results are reported as mean ± SD of triplicate tests. *P < 0.05 Indicates significant differences between defined groups.
DISCUSSION
In the present study, we examined the effect of galectin-9 as a TIM-3 ligand on Jurkat and KE-37 cell lines of ALL. It is well-known that the TIM-3/galectin-9 pathway in some tumor cells can disable the cytotoxic lymphoid cells (25). As mentioned, galectin-9 has been noted because of multiple biological functions in tumor cells. Indeed, some studies confirmed that galectin-9 can bind to the TIM-3 leading to activation of cell death in T lymphocytes, hence it can act as a down-regulator of Th1 lymphocyte (26). It has been shown that exhaustion and apoptosis of specific cytotoxic T lymphocytes in chronic viral infection and cancer to be caused by TIM-3 and galectin-9 interaction (27,28). Interestingly, Folgiero et al. lately reported that TIM-3/galectin-9 interaction in the bone marrow of acute myeloid leukemia patients induces indoleamine 2, 3-dioxygenase 1. This enzyme was applied by cancer cells to hamper immune response in the tumor microenvironment (29).
In another study, Wang et al. evaluated galectin-9 expression in gastric cancer using the tissue microarray immunohistochemistry method. They showed that about 86.2% of gastric cancer tissue was galectin-9 positive. Also, they found that increased galectin-9 expression level was significantly correlated with longer survival in gastric cancer patients (30).
To answer this question that galectin-9 has any effect on cell growth and apoptosis, two ALL cell lines, KE-37 and Jurkat, were treated with galectin-9. Accordingly, our results indicated that the highest inhibition of cell growth was observed at 100 nM galectin-9 (P < 0.001) in Jurkat (87%) and KE-37 (92%) cell lines. Although, in lower concentrations of galectin-9 (1, 10, and 50 nM) the growth inhibition was significantly detected. To further investigate the effect of galectin-9 on cell proliferation inhibition, we used a colony-forming assay. It was observed a distinguished suppressor effect in the colony-forming ability of Jurkat and KE-37 cell lines by increasing the concentration of galectin-9. In addition, further analysis showed that this growth inhibition was related to apoptosis induction in response to galectin-9 exposure in the studied ALL cell lines. Interestingly, at the highest concentration of galectin-9 (100 nM) 87 and 90.5% apoptosis was observed in Jurkat and KE-37 cell lines, respectively (P < 0.001).
In another study, it was clearly stated that the interaction of galectin-9 and TIM-3 could prevent cell proliferation and consequently induced apoptosis in normal immune lymphocytes besides tumor cells. In fact, blocking the TIM-3/galectin-9 signaling pathway increased the functionality of tumor-infiltrating TIM-3+ cells and cell proliferation. Indeed TIM-3/galectin-9 interaction was involved in cells senescence in hepatitis B virus-associated HCC (31).
The effect of galectin-9 on cell proliferation was evaluated in three metastatic liver cell lines derived from pancreatic carcinoma (KMP2, KMP7, and KMP8 cells) in a study by Tadokoro et al. in 2017. They showed that galectin-9 is able to suppress cell proliferation in the three metastatic liver cancer cell lines in a dose-dependent manner (0, 0.1, or 0.3 μM). Furthermore, they determined by flow cytometry analysis that galectin-9 induced early apoptosis of KMP8 cells. Incremental modulatory effects of galectin-9 on apoptosis-associated proteins such as cleaved caspase-3, cytochrome c, Smac/Diablo, and HtrA2 were detected in KMP8 cancer cells by western blotting test, thus they concluded that galectin-9 is involved in the intrinsic pathway of apoptosis (32). Akashi et al. reported that galectin-9 had a dose-dependent inhibitory effect on cell proliferation of four esophageal adenocarcinomas cell lines (OE19, OE33, SK-GT4, and OACM5). Also using the results of annexin V-FITC/PI stained galectin-9 treated SK-GT4 cells measured by flow cytometry, they inferred that the antitumor activity of galectin-9 was associated with the induction of apoptosis in these cells (33).
Consistent with our findings, the induction of apoptosis by galectin-9 has been shown in the MOLT-4 cell line in a dose-dependent manner (0.03-1 (34). Indeed, the maximum apoptosis (80%) was observed at 1 μM galectin-9 after 48 h incubation. As well, they found that galectin-9 can induce apoptosis in other cell lines such as B cells (BALL-1), myelocytes (HL-60), and monocytes (THP-1), except for T cells (35). Our obtained data from the investigation of galectin-9 on Jurkat and KE-37 recommends for analysis of the galectin-9 inhibitory effects on fresh primary ALL malignant cells. TIM-3/galecting-9 interaction has been studied in different cell lines. In this regard, Kobayashi et al. showed that galectin-9 in a dose-related manner inhibited cell growth of AMO-1myeloma cell line through apoptosis mechanism (0.05-1 in 48 h (28). In another research by Silva et al., it was discovered that the expression of galectin-9 and TIM-3, as well as TIM-3-galectin-9 complex, significantly increased in acute myeloid leukemia patients (36). The result of the study conducted by Kuroda et al. displayed a growth inhibitory effect of galectin-9 on chronic myelogenous leukemia cell lines for 48 h in a concentration-dependent manner (100-500 nM) with the MTT assay (37). Another research indicated that galectin-9 is involved in both processes of cell aggregation and apoptosis (93%) in the MM-RU melanoma cell line. In contrast, they found that the MM-RU cells fail to form cell colonies in the absence of galectin-9. (38). However, Chen et al. expressed that galectin-9 infected HCC cell lines presented fewer metastatic colonies than control cells, which was described as an antitumor effect of galectin-9 (39).
The ability of galectin-9 to induce apoptosis was investigated by apoptotic protein expression. The Bcl-2 family of proteins has critical regulatory functions in the apoptosis pathway. Bid, Bax, and Bad are members of this family which contributed to pro-apoptotic function while others including Bcl-2 and Bcl-XL can have an anti-apoptotic role (40). We showed that galectin-9 increased apoptosis via elevation of the ratio of Bax/Bcl-2 proteins. In addition, caspases are a family of proteases that plays a crucial role in apoptosis. These proteases activate and affect the executioner factors of apoptosis (35). Our data showed a high level of caspase-3 activity in the treated Jurkat and KE-37 cell lines comparing to the control groups.
According to the results, it was accepted that induction of apoptosis by galectin-9 was dependent on the caspase-3 pathway. In research by Kashio et al. on MOLT-4 (T cells), it was proposed that apoptosis induced via galectin-9 almost entirely can be repressed by the caspase inhibitor. Overall, they figured out that galectin-9 possibly through activation of caspase-1 can induce programmed cell death, and other caspases including caspases-8, 9, and 10 do not have a role in galectin-9-mediated apoptosis (34). Fujita et al. revealed that caspase-4 and caspase-9 levels increased by galectin-9 treatment in the HCC cell line, but galectin-9 treatment could not alter caspase-8. Thus, they suggested that galectin-9-induced apoptosis by mitochondrial pathway (13). It was reported galectin-9, through the mitochondrial pathway, induces apoptosis on the ovarian cancer cell line (OVCAR-3), in addition to increasing the activity of caspases 3 and 6 in these cells (24).
CONCLUSION
We demonstrated that galectin-9 inhibits ALL cell lines (Jurkat and KE-37) by suppression of cell growth and induction of apoptosis in a mechanism dependent on higher caspase-3 activity and Bax/Bcl2 protein ratio, although other mechanisms are involved in this pathway which needs more research. Furthermore, our data showed that galectin-9 induced apoptosis with a slight expression of Bcl-2 and overexpression of Bax proteins. Moreover, the results indicated that apoptosis has occurred when caspase-3 activity was stimulated by galectin-9. In general, according to the inhibitory role of galectin-9 in the growth of cancer cells it might be used as a potential therapeutic target or as an adjunct to chemotherapy in ALL, which needs to be explored more in the future.
Conflict of interest statement
The authors declared no conflict of interest in this study.
Authors’ contribution
M. Aghaei contributed to the conception and design of the work, analysis, and interpretation of data, drafting the work, M. Shabani contributed in experimental studies, manuscript preparation, editing, and review, N. Zargar Balajam contributed in the literature search, experimental studies, acquisition and analysis of the data, manuscript preparation and review. The final version of the paper was approved by all authors.
Acknowledgments
The current study conducted by Narges Zargar Balajam was partially financially supported by the Vice-Chancellor of research of Isfahan University of Medical Sciences, Isfahan, the I.R. Iran through rant No. 393484.
REFERENCES
- 1.Paul S, Kantarjian H, Jabbour EJ. Adult acute lymphoblastic leukemia. Mayo Clin Proc. 2016;91(11):1645–1666. doi: 10.1016/j.mayocp.2016.09.010. DOI: 10.1016/j.mayocp.2016.09.010. [DOI] [PubMed] [Google Scholar]
- 2.Howlader N, Noone AM, Krapcho M, Miller D, Bishop K, Altekruse SF, et al. SEER cancer statistics review, 1975-2013. National Cancer Institute. Vol. 8. Bethesda, MD: NCI; 2016. p. 19. Available from: https://seer.cancer.gov/archive/csr/1975_2013/ [Google Scholar]
- 3.Klibi J, Niki T, Riedel A, Pioche-Durieu C, Souquere S, Rubinstein E, et al. Blood diffusion and Th1-suppressive effects of galectin-9-containing exosomes released by Epstein-Barr virus-infected nasopharyngeal carcinoma cells. Blood. 2009;113(9):1957–1966. doi: 10.1182/blood-2008-02-142596. DOI: 10.1182/blood-2008-02-142596. [DOI] [PubMed] [Google Scholar]
- 4.Thijssen VL, Heusschen R, Caers J, Griffioen AW. Galectin expression in cancer diagnosis and prognosis: a systematic review. Biochim Biophys Acta. 2015;1855(2):235–247. doi: 10.1016/j.bbcan.2015.03.003. DOI: 10.1016/j.bbcan.2015.03.003. [DOI] [PubMed] [Google Scholar]
- 5.Chou FC, Chen HY, Kuo CC, Sytwu HK. Role of galectins in tumors and in clinical immunotherapy. Int J Mol Sci. 2018;19(2):430–441. doi: 10.3390/ijms19020430. DOI: 10.3390/ijms19020430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Elahi S, Niki T, Hirashima M, Horton H. Galectin-9 binding to Tim-3 renders activated human CD4+ T cells less susceptible to HIV-1 infection. Blood. 2012;119(18):4192–4204. doi: 10.1182/blood-2011-11-389585. DOI: 10.1182/blood-2011-11-389585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Oomizu S, Arikawa T, Niki T, Kadowaki T, Ueno M, Nishi N, et al. Galectin-9 suppresses Th17 cell development in an IL-2-dependent but Tim-3-independent manner. Clin Immunol. 2012;143(1):51–58. doi: 10.1016/j.clim.2012.01.004. DOI: 10.1016/j.clim.2012.01.004. [DOI] [PubMed] [Google Scholar]
- 8.Madireddi S, Eun SY, Mehta AK, Birta A, Zajonc DM, Niki T, et al. Regulatory T cell-mediated suppression of inflammation induced by DR3 signaling is dependent on galectin-9. J Immunol. 2017;199(8):2721–2728. doi: 10.4049/jimmunol.1700575. DOI: 10.4049/jimmunol.1700575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nobumoto A, Nagahara K, Oomizu S, Katoh S, Nishi N, Takeshita K, et al. Galectin-9 suppresses tumor metastasis by blocking adhesion to endothelium and extracellular matrices. Glycobiology. 2008;18(9):735–744. doi: 10.1093/glycob/cwn062. DOI: 10.1093/glycob/cwn062. [DOI] [PubMed] [Google Scholar]
- 10.Irie A, Yamauchi A, Kontani K, Kihara M, Liu D, Shirato Y, et al. Galectin-9 as a prognostic factor with antimetastatic potential in breast cancer. Clin Cancer Res. 2005;11(8):2962–2968. doi: 10.1158/1078-0432.CCR-04-0861. DOI: 10.1158/1078-0432.CCR-04-0861. [DOI] [PubMed] [Google Scholar]
- 11.Zhang ZY, Dong JH, Chen YW, Wang XQ, Li CH, Wang J, et al. Galectin-9 acts as a prognostic factor with antimetastatic potential in hepatocellular carcinoma. Asian Pac J Cancer Prev. 2012;13(6):2503–2509. doi: 10.7314/apjcp.2012.13.6.2503. DOI: 10.7314/apjcp.2012.13.6.2503. [DOI] [PubMed] [Google Scholar]
- 12.Kang CW, Dutta A, Chang LY, Mahalingam J, Lin YC, Chiang JM, et al. Apoptosis of tumor infiltrating effector TIM-3+ CD8+ T cells in colon cancer. Sci Rep. 2015;5:15659,1–12. doi: 10.1038/srep15659. DOI: 10.1038/srep15659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fujita K, Iwama H, Sakamoto T, Okura R, Kobayashi K, Takano J, et al. Galectin-9 suppresses the growth of hepatocellular carcinoma via apoptosis in vitro and in vivo. Int J Oncol. 2015;46(6):2419–2430. doi: 10.3892/ijo.2015.2941. DOI: 10.3892/ijo.2015.2941. [DOI] [PubMed] [Google Scholar]
- 14.Kobayashi K, Morishita A, Iwama H, Fujita K, Okura R, Fujihara S, et al. Galectin-9 suppresses cholangiocarcinoma cell proliferation by inducing apoptosis but not cell cycle arrest. Oncol Rep. 2015;34(4):1761–1770. doi: 10.3892/or.2015.4197. DOI: 10.3892/or.2015.4197. [DOI] [PubMed] [Google Scholar]
- 15.Tadokoro T, Morishita A, Fujihara S, Iwama H, Niki T, Fujita K, et al. Galectin-9: an anticancer molecule for gallbladder carcinoma. Int J Oncol. 2016;48(3):1165–1174. doi: 10.3892/ijo.2016.3347. DOI: 10.3892/ijo.2016.3347. [DOI] [PubMed] [Google Scholar]
- 16.Takano J, Morishita A, Fujihara S, Iwama H, Kokado F, Fujikawa K, et al. Galectin-9 suppresses the proliferation of gastric cancer cells in vitro. Oncol Rep. 2016;35(2):851–860. doi: 10.3892/or.2015.4452. DOI: 10.3892/or.2015.4452. [DOI] [PubMed] [Google Scholar]
- 17.Kuroda J, Yamamoto M, Nagoshi H, Kobayashi T, Sasaki N, Shimura Y, et al. Targeting activating transcription factor 3 by galectin-9 induces apoptosis and overcomes various types of treatment resistance in chronic myelogenous leukemia. Mol Cancer Res. 2010;8(7):994–1001. doi: 10.1158/1541-7786.MCR-10-0040. DOI: 10.1158/1541-7786.MCR-10-0040. [DOI] [PubMed] [Google Scholar]
- 18.Balajam NZ, Shabani M, Aghaei M, Haghighi M, Kompani F. Study of T-cell immunoglobulin and mucin domain-3 expression profile in peripheral blood and bone marrow of human acute lymphoblastic leukemia patients. J Res Med Sci. 2020;25:69–74. doi: 10.4103/jrms.JRMS_759_19. DOI: 10.4103/jrms.JRMS_759_19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Shahali A, Ghanadian M, Jafari SM, Aghaei M. Mitochondrial and caspase pathways are involved in the induction of apoptosis by nardosinen in MCF-7 breast cancer cell line. Res Pharm Sci. 2018;13(1):12–21. doi: 10.4103/1735-5362.220963. DOI: 10.4103/1735-5362.220963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jafari SM, Joshaghani HR, Panjehpour M, Aghaei M, Zargar Balajam N. Apoptosis and cell cycle regulatory effects of adenosine by modulation of GLI-1 and ERK 1/2 pathways in CD 44+ and CD 24-breast cancer stem cells. Cell Prolif. 2017;50(4):e12345,1–12. doi: 10.1111/cpr.12345. DOI: 10.1111/cpr.12345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jafari SM, Panjehpour M, Aghaei M, Joshaghani HR, Enderami SE. A3 Adenosine receptor agonist inhibited survival of breast cancer stem cells via GLI-1 and ERK1/2 pathway. J Cell Biochem. 2017;118(9):2909–2920. doi: 10.1002/jcb.25945. DOI: 10.1002/jcb.25945. [DOI] [PubMed] [Google Scholar]
- 22.Aghaei M, Panjehpour M, Karami-Tehrani F, Salami S. Molecular mechanisms of A3 adenosine receptor-induced G1 cell cycle arrest and apoptosis in androgen-dependent and independent prostate cancer cell lines: involvement of intrinsic pathway. J Cancer Res Clin Oncol. 2011;137(10):1511–1523. doi: 10.1007/s00432-011-1031-z. DOI: 10.1007/s00432-011-1031-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Azemikhah M, Ashtiani HA, Aghaei M, Rastegar H. Evaluation of discoidin domain receptor-2 (DDR2) expression level in normal, benign, and malignant human prostate tissues. Res Pharm Sci. 2015;10:356–363. PMID: 26600862. [PMC free article] [PubMed] [Google Scholar]
- 24.Jafari SM, Nazri A, Shabani M, Balajam NZ, Aghaei M. Galectin-9 induces apoptosis in OVCAR-3 ovarian cancer cell through mitochondrial pathway. Res Pharm Sci. 2018;13(6):557–565. doi: 10.4103/1735-5362.245967. DOI: 10.4103/1735-5362.245967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sakhnevych SS, Yasinska IM, Bratt AM, Benlaouer O, Gonçalves Silva I, Hussain R, et al. Cortisol facilitates the immune escape of human acute myeloid leukemia cells by inducing latrophilin 1 expression. Cell Mol Immunol. 2018;15:994–997. doi: 10.1038/s41423-018-0053-8. DOI: 10.1038/s41423-018-0053-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zhu C, Anderson AC, Kuchroo VK. TIM-3 and its regulatory role in immune responses. Curr Top Microbiol Immunol. 2011;350:1–15. doi: 10.1007/82_2010_84. DOI: 10.1007/82_2010_84. [DOI] [PubMed] [Google Scholar]
- 27.Baghdadi M, Jinushi M. The impact of the TIM gene family on tumor immunity and immunosuppression. Cell Mol Immunol. 2014;11(1):41–48. doi: 10.1038/cmi.2013.57. DOI: 10.1038/cmi.2013.57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mattei F, Schiavoni G. TIM-3 as a molecular switch for tumor escape from innate immunity. Front Immunol. 2013;3:418–420. doi: 10.3389/fimmu.2012.00418. DOI: 10.3389/fimmu.2012.00418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Folgiero V, Cifaldi L, Li Pira G, Maria Goffredo B, Vinti L, Locatelli F. TIM-3/Gal-9 interaction induces IFNgamma-dependent IDO1 expression in acute myeloid leukemia blast cells. J Hematol Oncol. 2015;8:36–41. doi: 10.1186/s13045-015-0134-4. DOI: 10.1186/s13045-015-0134-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wang Z, Zhu J, Gu H, Yuan Y, Zhang B, Zhu D, et al. The clinical significance of abnormal Tim-3 expression on NK cells from patients with gastric cancer. Immunol Invest. 2015;44(6):578–589. doi: 10.3109/08820139.2015.1052145. DOI: 10.3109/08820139.2015.1052145. [DOI] [PubMed] [Google Scholar]
- 31.Li H, Wu K, Tao K, Chen L, Zheng Q, Lu X, et al. Tim-3/galectin-9 signaling pathway mediates T-cell dysfunction and predicts poor prognosis in patients with hepatitis B virus-associated hepatocellular carcinoma. Hepatology. 2012;56(4):1342–1351. doi: 10.1002/hep.25777. DOI: 10.1002/hep.25777. [DOI] [PubMed] [Google Scholar]
- 32.Tadokoro T, Fujihara S, Chiyo T, Oura K, Samukawa E, Yamana Y, et al. Induction of apoptosis by galectin-9 in liver metastatic cancer cells: in vitro study. Int J Oncol. 2017;51(2):607–614. doi: 10.3892/ijo.2017.4053. DOI: 10.3892/ijo.2017.4053. [DOI] [PubMed] [Google Scholar]
- 33.Akashi E, Fujihara S, Morishita A, Tadokoro T, Chiyo T, Fujikawa K, et al. Effects of galectin-9 on apoptosis, cell cycle and autophagy in human esophageal adenocarcinoma cells. Oncol Rep. 2017;38(1):506–514. doi: 10.3892/or.2017.5689. DOI: 10.3892/or.2017.5689. [DOI] [PubMed] [Google Scholar]
- 34.Roth CG, Garner K, Eyck ST, Boyiadzis M, Kane LP, Craig FE. TIM3 expression by leukemic and non-leukemic myeloblasts. Cytometry B Clin Cytom. 2013;84(3):167–172. doi: 10.1002/cyto.b.21080. DOI: 10.1002/cyto.b.21080. [DOI] [PubMed] [Google Scholar]
- 35.Kashio Y, Nakamura K, Abedin MJ, Seki M, Nishi N, Yoshida N, et al. Galectin-9 induces apoptosis through the calcium-calpain-caspase-1 pathway. J Immunol. 2003;170(7):3631–3636. doi: 10.4049/jimmunol.170.7.3631. DOI: 10.4049/jimmunol.170.7.3631. [DOI] [PubMed] [Google Scholar]
- 36.Gonçalves Silva I, Yasinska IM, Sakhnevych SS, Fiedler W, Wellbrock J, Bardelli M, et al. The Tim-3-galectin-9 secretory pathway is involved in the immune escape of human acute myeloid leukemia cells. EBioMedicine. 2017;22:44–57. doi: 10.1016/j.ebiom.2017.07.018. DOI: 10.1016/j.ebiom.2017.07.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kuroda J, Yamamoto M, Nagoshi H, Kobayashi T, Sasaki N, Shimura Y, et al. Targeting activating transcription factor 3 by galectin-9 induces apoptosis and overcomes various types of treatment resistance in chronic myelogenous leukemia. Mol Cancer Res. 2010;8(7):994–1001. doi: 10.1158/1541-7786.MCR-10-0040. DOI: 10.1158/1541-7786. [DOI] [PubMed] [Google Scholar]
- 38.Kageshita T, Kashio Y, Yamauchi A, Seki M, Abedin MJ, Nishi N, et al. Possible role of galectin-9 in cell aggregation and apoptosis of human melanoma cell lines and its clinical significance. Int J Cancer. 2002;99(6):809–816. doi: 10.1002/ijc.10436. DOI: 10.1002/ijc.10436. [DOI] [PubMed] [Google Scholar]
- 39.Chen S, Pu J, Bai J, Yin Y, Wu K, Wang J, et al. EZH2 promotes hepatocellular carcinoma progression through modulating miR-22/galectin-9 axis. J Exp Clin Cancer Res. 2018;37(1):3–14. doi: 10.1186/s13046-017-0670-6. DOI: 10.1186/s13046-017-0670-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Yip KW, Reed JC. Bcl-2 family proteins and cancer. Oncogene. 2008;27(50):6398–6406. doi: 10.1038/onc.2008.307. DOI: 10.1038/onc.2008.307. [DOI] [PubMed] [Google Scholar]