Abstract
Background and purpose:
Clostridium perfringens is an anaerobic, spore-forming, and pathogenic bacterium that causes intestinal diseases in humans and animals. In these cases, therapeutic intervention is challenging; because the disease progresses much rapidly. This bacterium can produce 5 main toxins (alpha, beta, epsilon, iota, and a type of enterotoxin) among which the epsilon toxin (ETX) is used for bioterrorism. This toxin can be prevented by immunization with specific immunogenic vaccines. In the present research, we aimed at developing a recombinant chitosan-based nano-vaccine against ETX of C. perfringens and evaluate its effects on the antibody titration against epsilon toxin in BALB/c mice as the vaccine model.
Experimental approach:
The etx gene from C. perfringens type D was cloned and expressed in E. coli. After analysis by SDS-PAGE and western blotting, the expressed products were purified, and the obtained proteins were used for immunization in mice as a chitosan nanoparticle containing recombinant, purified ETX, and protein.
Findings/Results:
The results of ELISA showed that IgA antibody serum level increased sufficiently using recombinant protein with nanoparticle as an oral and injectable formulation. IgG antibody titers increased significantly after administrating the recombinant proteins with nanoparticles through both oral delivery and intravenous injection.
Conclusion and implication:
In conclusion, the recombinant ETX is suggested as a good candidate for vaccine production against diseases caused by ETX of C. perfringens type D.
Keywords: Chitosan, Clostridium perfringens, Epsilon toxin, Immunization, Nano-vaccine
INTRODUCTION
Clostridium perfringens (C. perfringens) is an anaerobic, spore-forming, gram-positive, and rod-shaped pathogen bacterium (1,2). It is a major cause of systemic and gastrointestinal diseases such as gas gangrene, food poisoning, non-food diarrhea, and enterocolitis in humans and domestic animals (3,4). C. perfringens has also been shown to be the main pathogen causing food poisoning in the United States and Canada (5). About 250,000 people in the US are infected each year through contaminated meat which is the main reservoir for this bacterium. Mortality due to the disease has mainly occurred in older and susceptible people with immune deficiency disorders (6).
C. perfringens is the most important cause of antibiotic-associated diarrhea, especially in the hospital environment. In a study on 2280 fecal samples from (> 50 years old) Iranian diarrhea patients suspected of having antibiotic-associated diarrhea, this pathogen was diagnosed in 13.3% of patients (303 out of 2280) (7). C. perfringens is divided into five categories (A-G) according to the type of toxins it produces: alpha (CPA), beta (CPB), epsilon (ETX), iota (ITX), and C. perfringens-enterotoxin (CPE) (2). Spores and toxins, especially the ETX, have been identified as the bioterrorism agents by the US Government Centers for Disease Control and Prevention (CDC) (5,8). ETX results in diarrhea in lambs and enterotoxemia (pulpy kidney) in goats and sheep but rarely is pathogenic to cattle (8,9). It is the third most pathogenic clostridial toxin after botulinum and tetani toxins (8). This toxin is primarily produced as a 32.98 kDa protoxin (10)that can be converted from relatively inactive to active form with the help of the protease enzymes, such as trypsin and chymotrypsin (9,11912). The active form of epsilon has high toxicity and lethal activity (13). The mechanism of its activity relies on inserting the cell membrane by seven protein monomers (13)and increasing the permeability of the intestinal cells that cause the toxin to enter the bloodstream and spread between organs such as the brain, lungs, and kidneys (14). Briefly, the most important effect of this toxin on the brain is disrupting the movement membrane leading to apoptosis (15).
The biosafety problems can be possibly lessened using Escherichia coli (E. coli) BL21 (DE3) as an expression system to produce a recombinant vaccine against ETX, because of the non-pathogenic nature of this strain (16). According to studies, expression of the complete sequence of ETX and immunization with the recombinant version of this toxin has the ability to stimulate the immune system. In addition, the recombinant ETX (rETX) has been produced in E. coli to formulate a trivalent vaccine (16).
Chitosan-based nano-vaccines have shown the tremendous potential of providing immunity against a variety of diseases in animal models. But most of these vaccines have been shown to be highly functional when administered via the mucosal pathways. On the other hand, intranasal and oral nano-vaccines are preferred, thus, several water-soluble products such as trimethylated chitosan are studied as proper adjuvants (17,18). One of the biggest challenges in oral-routed vaccinology is the process of antigen transmission through the digestive tract. In this pathway, the target antigen is absorbed through the intestine, then transmitted by M cells to the dendritic cells, macrophages, and lymphocytes (19). Various studies have aimed at designing effective vaccines against C. perfringens. Most of these researches have been focused on oral vaccines using pure protein in the immunization process.
The present study, also, aimed at designing an oral and injectable vaccine using the recombinant protein both as pure and combined with nanoparticles for immunization and their results were compared (20,21,22). We developed a chitosan-based recombinant nano-vaccine against the ETX of C. perfringens using bioinformatic tools. For this purpose, the epsilon protoxin gene (etx) was expressed in E. coli as a host and prepared with trimethylated chitosan as an adjuvant for the subsequent oral and parenteral delivery in the BALB/c mice as the vaccine model.
MATERIALS AND METHODS
Kits, enzymes, and reagents
The pET28-a plasmid, DH5a, and BL21 (DE3) strains were prepared from the National Institute of Genetic Engineering and Biotechnology (NIGEB, Iran). The EcoRI and HinDIII restriction enzymes (Thermo, USA) were used for sub-cloning the epsilon toxin gene into the pET28-a vector.
Sequence retrieval and codon optimization
The complete annotated sequence of the ETX of the C. perfringens type D (accession no. Q02307), were obtained from the Entrez protein database available at NCBI (www.ncbi.nlm.nih.gov/) and submitted to the Basic Local Alignment Search Tool (BLAST) search against the non-redundant protein database available at Uni-Prot (www.uniprot.org/). The propeptide (13 aa) and epsilon toxin (283 aa) regions of C. perfringens type D which are responsible for a rapidly fatal enterotoxemia were selected. The codons of the ETX were optimized by the GenScript service (http://www.genscript.com/gene_synthesis.html), based on the codon usage table of E. coli for the back-translation of the sequence. Some parameters that are critical to the efficiency of gene expression including codon adaptation index and GC content adjustment were optimized. The necessary restriction enzyme sites (for EcoRI and HinDIII) were introduced at the ends of the sequence for subsequent cloning purposes. The gene was synthesized by Biomatik Co. (Canada).
Recombinant ETX plasmids construction
E. coli was grown in Luria-Bertani (LB) broth supplemented by kanamycin (Merck, Germany) in a shaking incubator at 37 °C for 24 h was store bacteria. Primary DNA denaturation was performed at 95 °C for 5 min followed by secondary denaturation at 94 °C for 20 s, annealing at 52 °C for 20 s, extension at 72 °C for 72 s, and the final extension at 75 °C for 5 m. The final polymerase chain reaction (PCR) product was analyzed by electrophoresis with 1% agarose gel and ethidium bromide. The construction was transformed into the E. coli BL21 (DE3) through the heat shock method. The recombinant colonies were verified by PCR using universal T7 primers and digestion with restriction endonucleases.
Expression and purification of the recombinant protein
E. coli BL21 (DE3) strain carrying the recombinant plasmid of pET28a-etx was grown in LB agar at 37 °C until OD600 = 0.7 was achieved. The culture was induced by 1 mM isopropyl-β-D-1-thiogalactopyranoside (IPTG, Sigma) and incubated at 37 °C for a further 4 h. Cells were centrifuged (5000 g, 10 m, 25 °C) and were re-suspended in the lysis buffer (100 mM NaH2PO4, 10 mM Tris-Cl, 8 M urea, pH = 7.4). After glass bead treatment (3 times for 3 m), the lysate was centrifuged (15 m, 1000 g, 4 °C) and the supernatant was loaded on a Ni-NTA column (Thermo, USA). The column was equilibrated with lysis buffer, then, the protein solution was applied to it. The impurity was removed by washing buffer (100 mM NaH2PO4, 10 mM Tris-Cl, 8 M urea, pH = 5.9) and the protein was eluted by the same buffer (pH = 4.5). Solubilization and refolding were done by the dialysis method. In this method, the gradient of urea was gradually reduced to remove protein from urea. At first, urea and protein were poured into the dialysis bag and the surrounding area was covered with 6.0 M urea buffer. Then, the urea buffer concentration was gradually reduced from 6.0 M to 1.0 M with intervals of 1 h between each variation. In the final stage, the area around the dialysis bag was covered by phosphate-buffered saline (PBS) and 10% glycerol. Gradual reduction of urea gradient causes less shock and prevents protein deposition. Finally, the concentration of the recombinant protein was estimated using the Bradford assay. The concentrations of insoluble proteins parts was 17 and 11 μg/mL, respectively.
Western blot analysis
The recombinant protein ETX was separated by 12% sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and transferred onto polyvinylidene difluoride (PVDF) membrane (Roche, Germany) with transfer buffer and electricity flow. The membrane blocking was done with skim milk (5%) and tris-buffered saline (TBS) buffer (50 mM Tris-Cl, 150 mM NaCl, pH = 7.5) containing 0.05% Tween® 20 at 37 °C for 2 h. Then, the mouse anti-His horseradish peroxidase (HRP)-conjugated antibody (1:2000) was used for affinity purification of recombinant proteins. Finally, the membrane was soaked in 3,3’-diaminobenzidine (tablet DAB reagent, Sigma) for signal development.
Preparation of chitosan nanoparticles
The acetic acid 2% was initially mixed with 100 mg chitosan and passed through a filter paper 30 min after mixing. Simultaneously with mixing on stirrer, 1 mg/mL of antigen was added dropwise and the pH was adjusted to 4.5 based on the isoelectric point of the ETX. Then, 5 mL of sodium tripolyphosphate was added slowly to the solution. The mixture was stirred for 1 h until the nanoparticles appeared. Finally, 100 μL of glycerol was added and centrifuged for 5 min (13000 rpm). The resulting solution was applied to the Bradford reagent and the optical absorption was measured at 595 nm (23,24). Determination of size and zeta potential of the synthesized nanoparticles was performed using a zeta sizer SZ3000 (Malvern instrument, Worcestershire, UK).
Animal immunization
Twelve female BALB/c mice (20-22 g), 4-6 weeks old (NIGE, Tehran, Iran) were used to determine the antigenicity of the ETX protein. Animal testing was done according to the University’s Internal Ethic Rules and Regulations (Ethical code: IR.BMSU.REC.1396.950). Mice were divided into 4 experimental groups including oral, oral + injection, injection, and control and acclimatized for 1 week. The mice in the oral group were gavaged three times with 200 μg of the rETX-containing nanoparticles with an interval of two weeks between each booster. The oral + injection group was gavaged 3 times with 200 μg of rETX-containing nanoparticles plus one booster (100 μg) via the subcutaneous route. The injection group was inoculated with 15 μg (20,22)of the purified ETX (without nanoparticles) subcutaneously and the complete Freund’s adjuvant (CFA, Sigma, USA) in the first week. The immunization of the injection group continued by two booster doses, every 15 μg of the recombinant antigen with the incomplete Freund’s adjuvant (IFA, Sigma, USA) and finally (2 μg) via intraperitoneal route at the intervals of two weeks. The control group was gavaged with PBS (20 μg). Blood samples were taken from mice eyes with a capillary tube and allowed to clot at 37 °C for 1 h, then, the serum was collected by centrifugation (5 min, 10000 g). The serum samples of each mice group were prepared and pooled for the immunological analyses. Finally, the fecal samples (0.5 g) were mixed with 500 μL PBS and incubated at 37 °C for 1 h, then centrifuged as explained above (25,26,27).
ELISA analysis of the recombinant protein
ELISA was performed using antigen with a serially diluted concentration of recombinant ETX (insoluble proteins) and a constant concentration of antibodies. The purified ETX (500 ng per well) in 100 μL bicarbonate buffer (15 mM Na2CO3 and 35 mM NaHCO3) was used to coat Maxisorb plates (Nunc, Denmark) at 37 °C for 2 h. After washing wells with PBS Tween® 20 (PBST) 3 times, the blocking buffer (skim milk 5% in PBST) was added and incubated for 1 h at 37 °C. Then, the wells were exposed to 100 μL of diluted immunized serum and fecal samples at 37 °C for 1 h. After that, the secondary antibodies including goat anti-IgG HRP-conjugated antibody and rabbit anti-IgA HRP-conjugated antibody (1:5000) were used for 1 h at 37 °C. Mouse anti-IgG antibody has been used in experimental and control samples. The wells were washed again with PBST (3 times), and O-phenyl de amine (ODP) was added as a substrate and remained at room temperature as long as the controls were colored. The process was finally stopped by adding 2.5 M sulfuric acid and the absorbance in each well was measured at 492 nm.
Cytotoxicity assay
To analyze the cell toxicity of ETX, 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay was done. The MDCK cell line was prepared from the pasture institute of Tehran, Iran, and cultured in Dulbecco’s modified eagles medium (DMEM) containing 15% FBS and 1% penicillin/streptomycin (Gibco, USA) at 37 °C and 5% CO2. Briefly, 4 × 103 cells were plated to each well of 96-well plates and incubated at 37 °C and 5% CO2. After 24 h, the cells were treated with 1, 5, 10, 25, 50, 100, 150, 200, 300, and 400 ng/μL TEX and incubated at 37 °C and 5% CO2 for 24 h. Afterward, the culture medium of each well was replaced by 100 μL of fresh medium and 10 μL of MTT reagent (5 mg/mL) (Sigma-Aldrich, USA) was added to each well and the plate was incubated at 37 °C and 5% CO2 for 4 h. After incubation, the content of all wells was emptied, 100 μL of dimethyl sulfoxide (DMSO, Merck, Germany) was added, and the optical density was recorded at 570 nm using an ELISA reader (BioRad, USA). All experiments were done in triplicate and the PBS was tested as the negative control.
Neutralization assay
This test was performed to assess the ETX neutralizing ability of immunized mice sera. Briefly, 4 × 103 MDCK cells were cultured in each well of a 96-well plate and incubated at 37 °C in 5% CO2. A serial dilution of serum collected from the mice immunized by epsilon-loaded chitosan was prepared. The diluted sera were incubated with ETX (300 ng/μL as TC50 for 50% cell toxicity) for 60 min at 37 °C. Then, the MDCK cells were treated with a mixture of sera and ETX for 24 h at 37 °C and 5% CO2. Finally, the percent of cell viability was measured by the MTT method as was described above. The serum obtained from unimmunized mice and PBS were tested as the negative control.
Statistical analysis
The data were presented as mean ± SD. Differences between tested groups were analyzed using one-way ANOVA and Mann-Whitney tests by the SPSS software version 11 (Chicago, USA). P < 0.05 was reported statistically significant.
RESULTS
Amplification and subcloning of etx gene
The etx gene was extracted and amplified by PCR with primer M13. The 906-bp PCR product is shown in Fig. 1. The fragment was sub-cloned in pET28-a plasmid, and then, transferred into E. coli DH5a.
Fig. 1.

(A-C) the genomic DNA extraction, PCR products by primer M13, and the digestion analysis on agarose gel 1%. (A) Lane M: DNA size marker; lane 1: the positive control; and lane 2: etx gene digested by EcoRI and HindIII restriction enzymes. (B) Lane M: DNA size marker and lane 1: etx gene extracted from pBSK. (C) Lane M: DNA size marker and lane 1: digested pET28a by EcoRI and HindIII restriction enzymes. ETX, Epsilon toxin.
Both pET28-a and pBSK plasmids were extracted from E. coli DH5α and digested by restriction enzymes EcoRI and HindIII. Then, they were further analyzed by agarose gel electrophoresis (Figs. 1 and 2). The recombinant pET28a-etx was extracted from E. coli DH5α and transformed to E. coli BL21 (DE3) for the expression of ETX protein.
Fig. 2.
Expression, solubility, and purification of epsilon toxin stained with Coomassie blue on sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) 10%.
Expression and purification of the recombinant proteins
The protein solubility determination process started by centrifugation of the E. coli suspension. Accordingly, 2 mL of the native lysis buffer (chemical method) and 1 mg/μL of lysozyme buffer (enzymatic method) were added respectively to the bacteria suspension and the mixture was left at room temperature for 1 h. For every 3 mL of the sample, 3 g glass bead was added and was vortexed (physical method). The centrifuged sample is the soluble protein. Then, 2 mL denature lysis buffer was added to the sample and after being left for 1 h at 37 °C, the insoluble protein was obtained through centrifugation.
The SDS-PAGE result showed that the recombinant ETX protein has been successfully expressed in E. coli BL21 (DE3) and purified on the nickel-nitrilotriacetic acid (Ni-NTA) column. The purified protein in the soluble and insoluble phases are shown in Fig. 2C and D. The western blotting with the anti-His-tag antibody confirmed obtaining the ETX protein of 36 kDa molecular weight (Fig. 3).
Fig. 3.
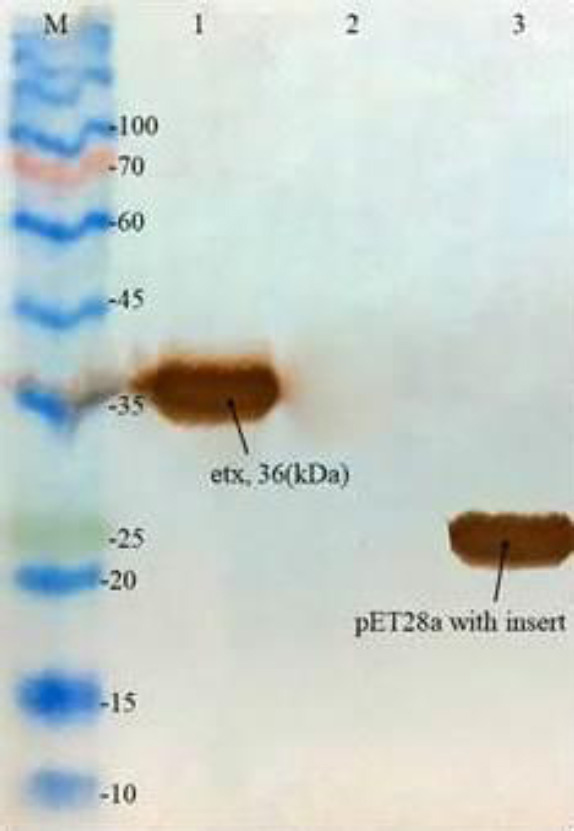
Western blotting of ETX with specific anti-His tag. Lane M: protein size marker; lane 1: purified recombinant protein ETX; lane 2: non-induced transformed BL21(DE3) as the negative control, and lane 3: pET28a with insert as the positive control. ETX, Epsilon toxin.
Epsilon-loaded chitosan nanoparticle properties
Examination and comparison of size and zeta potential of chitosan nanoparticles before and after protein loading using a zeta sizer SZ3000 (Malvern instrument, Worcestershire, UK) were done. The results showed that it increased in size after loading with protein (Fig. 4).
Fig. 4.
Determination of size and zeta potential of nanoparticles (A) before loading of protein, (B) after loading of protein, and (C) zeta potential after loading of protein.
ELISA analysis
The patterns of antibody production followed by the immunization at oral, oral + injection, and injection groups showed the successful increase of antibody titer after each phase of the immunization. The sera and fecal samples were tested with ELISA for detection of the ETX results. IgG serum antibody response with recombinant ETX protein is shown in Fig. 5. In the oral group, antibody increase was observed after the first dose (Fig. 5A), while in oral + injection and injection groups, it happened after the second dose (Fig. 5B and C). Comparison of oral and oral + injected groups showed a further increase in IgG antibodies in the oral group after the first dose (Fig. 5D).
Fig. 5.
IgG serum antibody response of BALB/c mice immunized with recombinant epsilon toxin protein after two doses in (A) oral, (B) oral + injection, (C) injection group, and (D) comparison of oral and oral + injection groups IgG antibody titration. The highest stimulation of antibodies was observed in the oral + injection group. The data represent mean + SD.
The increase of IgA antibodies was observed after the second dose in all three groups (Fig. 6). Among all, the oral group showed better results for IgA stimulation (Fig. 6D). The ELISA results of the injection group, which received only the purified recombinant protein, showed that the antibody level increased even after the first injection (Figs. 5C and 6C). However, IgG stimulation after the first and second injections was different and much greater than that of IgA. Examination of the fecal samples confirmed the results of the serological examination. This means that the IgA antibody increased after the second dose (Fig. 7).
Fig. 6.
Serum IgA response of BALB/c mice immunized with recombinant epsilon toxin protein after two doses in (A) oral, (B) oral+injection, (C) injection group, and (D) comparison of oral, oral + injection, and injection groups. The highest stimulation of antibodies was observed in the oral group. The data represent mean + SD.
Fig. 7.
IgA antibody titration in fecal samples of (A) oral; (B) oral + injection; (C) injection groups, and (D) comparison of oral, oral + injection, and injection fecal groups IgA antibody titration. More stimulation of antibodies was shown in the oral group. The data represent mean + SD.
Cell toxicity
Results from the MTT assay showed that ETX reduced the cell viability percentage at concentrations above 50 ng/μL, significantly (P < 0.05; Fig. 8). The TC50 was approximately 300 ng/μL.
Fig. 8.

Effect of epsilon toxin toxicity on MDCK cells. The data represent mean +SD Phosphate-buffered saline has been used as the negative control group. *P < 0.05 Indicates significant differences compared to the control group.
Neutralization result
As illustrated in Fig. 9, the serum from immunized mice is able to protect the toxicity properties of ETX against MDCK after 1/8 dilution. On the other hand, serum from unimmunized mice showed no protective effect.
Fig. 9.

Protective effect of sera from immunized mice against MDCK cells. The data represent mean +SD Phosphate-buffered saline has been used as the negative control group. *P < 0.05 Indicates significant differences compared to the control group.
DISCUSSION
C. perfringens is one of the most important foodborne bacteria in the world that causes necrosis, emphysematous cholecystitis, and gas gangrene (28). One of the toxins produced by C. perfringens type D is ETX. In the UK and USA, this bacterium is the third most common cause of meat-borne diseases (29). In Iran, this bacterium causes diseases mostly in people aged over 507. Due to its high worldwide prevalence and importance among human and animal health-threatening pathogens, many pieces of research have been carried out aimed at the development of new vaccine candidates. Such a vaccine against C. perfringens can be also important for animal husbandry purposes. Then, extensive studies all over the world have been advocated for developing a new vaccine candidate against the toxins of this bacterium. Additionally, developing novel methods of vaccine production, especially those based on recombinant DNA technologies, has attracted the attention of many researchers (22,30,31).
In the present study, we have shown that the rETX can be produced efficiently in E. coli as the host and induce strong immune in the animal models. Our findings indicated that antibodies specified to the recombinant protoxin of C. perfringens type D could be detected in the sera obtained from the immunized mice. To express and purify the rETX, the pET28a vector was used. Interestingly, we found that the rETX was produced in both soluble and insoluble fractions of the bacterial cell lysate. It was also demonstrated that the degree of protein expression in the soluble form versus insoluble form depends on the nature of the target protein (30). However, the recombinant proteins expressed in the E. coli cytoplasm are mainly insoluble because of the hydrophobic residues which existing in the surface proteins structure such as ETX (32). In similar research, Langroudi et al. reported producing the soluble epsilon-beta fusion protoxin with the biological activity using pET22b as the expression vector (31). Also, Souza and colleagues cloned the etx gene in the expression vector of pET11-a and obtained the purified protein mostly in the soluble phase (22). The rETX from the soluble fraction was purified using diethyl aminoethyl-sepharose anion-exchange chromatography in their study (33).
Since the purpose of this study was to use the ETX for immunization, the presence of the antibody against the toxin in mice sera compared to the control group was evaluated by western blotting to verify the expression of recombinant proteins. The immunoblotting results revealed an appropriate interaction between all immunized mice sera raised against rETX protein.
In the current study, chitosan was used as a nanoparticle adjuvant for the production of the rETX-based vaccine. The mice immunized with the nanoparticle rETX protein showed significant IgA and IgG antibody titers two weeks after the first vaccination through oral (Fig. 5B and C) and injection (Fig. 6B and C) administrations. According to the results of the oral + injection group, the oral pathway showed to be more effective than the injection route for increasing the IgA antibody titer (Fig. 6B). The IgA and IgG antibody titers had an upward trend even after the first booster of the rETX protein (Figs. 5C and 6C). The rETX-administered mice showed their response mostly by increasing the production of IgG before they receive the booster (OD = 0.3 - 3.2). On the other side, the nanoparticle-rETX protein could more increase the production of IgA antibodies (OD = 0.30 - 0.35). Finally, the achieved results showed that the etx gene expressed in E. coli could elicit a raise of antibody in the immunized mice sufficient to be detected by ELISA. Oral administration of the nanoparticle-rETX protein was shown to be the best way for stimulation of IgA antibodies, while the injected rETX protein was more suitable for raising IgG antibodies. In a similar research by Yao et al. ELISA data showed that the intraperitoneal immunization method using the rETX vaccine was more effective than the subcutaneous method during the first administration (23). ELISA results of fecal samples showed that antibody titer increase in oral administration is more than injection (Fig. 7). In 2004, vaccination of sheep against C. perfringens type D was performed using aluminum hydroxide as an adjuvant. The results showed that there was no relationship between the immune response and the vaccination period in 24 sheep that were vaccinated twice at 2 and 4 weeks intervals (24). Also, the oral immunization by LC-pT1NX-ε produced a high level of IgG and specifically IgA antibodies in mice (20). Vaccine injection can activate humoral immunity and increase IgG and IgA antibody titer. Better immunity responses to expressed antigens depend on the appropriate presentation of proteins and their capturing by antigen-presenting cells (34).
CONCLUSION
The current study showed that the rETX can be one of the excellent candidates for producing nano-vaccine against C. perfringens.
Conflict of interest statement
The authors declared no conflicts of interest in this study.
Authors’ contribution
Experimental works were performed by F. Poorhassan and M.J. Motamedi. The concept, and study design, and manuscript preparation were carried out by S.A. Mirhosseini. F. Nemati and P. Saffarian contributed in data analysis and manuscript preparation.
REFERENCES
- 1.Hassan KA, Elbourne LD, Tetu SG, Melville SB, Rood JI, Paulsen IT. Genomic analyses of Clostridium perfringens isolates from five toxinotypes. Res Microbiol. 2015;166(4):255–263. doi: 10.1016/j.resmic.2014.10.003. DOI: 10.1016/j.resmic.2014.10.003. [DOI] [PubMed] [Google Scholar]
- 2.Kiu R, Hall LJ. An update on the human and animal enteric pathogen Clostridium perfringens. Emerg Microbes Infect. 2018;7(1):141–155. doi: 10.1038/s41426-018-0144-8. DOI: 10.1038/s41426-018-0144-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sim K, Shaw AG, Randell P, Cox MJ, McClure ZE, Li MS, et al. Dysbiosis anticipating necrotizing enterocolitis in very premature infants. Clin Infect Dis. 2015;60(3):389–397. doi: 10.1093/cid/ciu822. DOI: 10.1093/cid/ciu822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Heida FH, van Zoonen AG, Hulscher JB, Te Kiefte BJ, Wessels R, Kooi EM, et al. A necrotizing enterocolitis-associated gut microbiota is present in the meconium: results of a prospective study. Clin Infect Dis. 2016;62(7):863–870. doi: 10.1093/cid/ciw016. DOI: 10.1093/cid/ciw016. [DOI] [PubMed] [Google Scholar]
- 5.Titball RW. Clostridium perfringens vaccines. Vaccine. 2009;27(Suppl 4):D44–D477. doi: 10.1016/j.vaccine.2009.07.047. DOI: 10.1016/j.vaccine.2009.07.047. [DOI] [PubMed] [Google Scholar]
- 6.Songer JG. Clostridia as agents of zoonotic disease. Vet Microbiol. 2010;140(3-4):399–404. doi: 10.1016/j.vetmic.2009.07.003. DOI: 10.1016/j.vetmic.2009.07.003. [DOI] [PubMed] [Google Scholar]
- 7.Azimirad M, Gholami F, Yadegar A, Knight DR, Shamloei S, Aghdaei HA, et al. Prevalence and characterization of Clostridium perfringens toxinotypes among patients with antibiotic-associated diarrhea in Iran. Sci Rep. 2019;9(1):7792–7800. doi: 10.1038/s41598-019-44281-5. DOI: 10.1038/s41598-019-44281-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Garcia J, Adams V, Beingesser J, Hughes ML, Poon R, Lyras D, et al. Epsilon toxin is essential for the virulence of Clostridium perfringens type D infection in sheep, goats, and mice. Infect Immun. 2013;81(7):2405–2414. doi: 10.1128/IAI.00238-13. DOI: 10.1128/IAI.00238-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Miyata S, Minami J, Tamai E, Matsushita O, Shimamoto S, Okabe A. Clostridium perfringens ε-toxin forms a heptameric pore within the detergent-insoluble microdomains of Madin-Darby canine kidney cells and rat synaptosomes. J Biol Chem. 2002;277(42):39463–39468. doi: 10.1074/jbc.M206731200. DOI: 10.1074/jbc.M206731200. [DOI] [PubMed] [Google Scholar]
- 10.Minami J, Katayama S, Matsushita O, Matsushita C, Okabe A. Lambda-toxin of Clostridium perfringens activates the precursor of epsilon-toxin by releasing its N-and C-terminal peptides. Microbiol Immunol. 1997;41(7):527–535. doi: 10.1111/j.1348-0421.1997.tb01888.x. DOI: 10.1111/j.1348-0421.1997.tb01888.x. [DOI] [PubMed] [Google Scholar]
- 11.Bokori-Brown M, Savva CG, da Costa SPF, Naylor CE, Basak AK, Titball RW. Molecular basis of toxicity of Clostridium perfringens epsilon toxin. FEBS J. 2011;278(23):4589–4601. doi: 10.1111/j.1742-4658.2011.08140.x. DOI: 10.1111/j.1742-4658.2011.08140.x. [DOI] [PubMed] [Google Scholar]
- 12.Petit L, Gibert M, Gillet D, Laurent-Winter C, Boquet P, Popoff MR. Clostridium perfringens epsilon-toxin acts on MDCK cells by forming a large membrane complex. J Bacteriol. 1997;179(20):6480–6487. doi: 10.1128/jb.179.20.6480-6487.1997. DOI: 10.1128/jb. 179.20.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Casadevall A. Passive antibody administration (immediate immunity) as a specific defense against biological weapons. Emerg Infect Dis. 2002;8(8):833–841. doi: 10.3201/eid0808.010516. DOI: 10.3201/eid0808.010516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.McDonel JL. Clostridium perfringens toxins (type A, B, C, D, E) Pharmacol Ther. 1980;10(3):617–655. doi: 10.1016/0163-7258(80)90031-5. DOI: 10.1016/0163-7258(80)90031-5. [DOI] [PubMed] [Google Scholar]
- 15.Lonchamp E, Dupont JL, Wioland L, Courjaret R, Mbebi-Liegeois C, Jover E, et al. Clostridium perfringens epsilon toxin targets granule cells in the mouse cerebellum and stimulates glutamate release. PloS One. 2010;5(9):e13046,1–15. doi: 10.1371/journal.pone.0013046. DOI: 10.1371/journal.pone.0013046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Moreira GMSG, Salvarani FM, Da Cunha CEP, Mendonça M, Moreira ÂN, Gonçalves LA, et al. Immunogenicity of a trivalent recombinant vaccine against Clostridium perfringens alpha, beta, and epsilon toxins in farm ruminants. Sci Rep. 2016;6:22816–22824. doi: 10.1038/srep22816. DOI: 10.1038/srep22816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Singla A, Chawla M. Chitosan: some pharmaceutical and biological aspects-an update. J Pharm Pharmacol. 2001;53(8):1047–1467. doi: 10.1211/0022357011776441. DOI: 10.1211/0022357011776441. [DOI] [PubMed] [Google Scholar]
- 18.Amidi M, Romeijn SG, Borchard G, Junginger HE, Hennink WE, Jiskoot W. Preparation and characterization of protein-loaded N-trimethyl chitosan nanoparticles as nasal delivery system. J Control Release. 2006;111(1-2):107–116. doi: 10.1016/j.jconrel.2005.11.014. DOI: 10.1016/j.jconrel.2005.11.014. [DOI] [PubMed] [Google Scholar]
- 19.des Rieux A, Fievez V, Garinot M, Schneider YJ, Préat V. Nanoparticles as potential oral delivery systems of proteins and vaccines: a mechanistic approach. J Control Release. 2006;116(1):1–27. doi: 10.1016/j.jconrel.2006.08.013. DOI: 10.1016/j.jconrel.2006.08.013. [DOI] [PubMed] [Google Scholar]
- 20.Alimolaei M, Golchin M, Daneshvar H. Oral immunization of mice against Clostridium perfringens epsilon toxin with a Lactobacillus casei vector vaccine expressing epsilon toxoid. Infect Genet Evol. 2016;40:282–287. doi: 10.1016/j.meegid.2016.03.020. DOI: 10.1016/j.meegid.2016.03.020. [DOI] [PubMed] [Google Scholar]
- 21.Fernandez-Miyakawa ME, Sayeed S, Fisher DJ, Poon R, Adams V, Rood JI, et al. Development and application of an oral challenge mouse model for studying Clostridium perfringens type D infection. Infect Immun. 2007;75(9):4282–4288. doi: 10.1128/IAI.00562-07. DOI: 10.1128/IAI.00562-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Souza A, Reis J, Assis R, Horta C, Siqueira F, Facchin S, et al. Molecular cloning and expression of epsilon toxin from Clostridium perfringens type D and tests of animal immunization. Genet Mol Res. 2010;9(1):266–276. doi: 10.4238/vol9-1gmr711. DOI: 10.4238/vol9-1gmr711. [DOI] [PubMed] [Google Scholar]
- 23.Yao W, Kang J, Kang L, Gao S, Yang H, Ji B, et al. Immunization with a novel Clostridium perfringens epsilon toxin mutant rETX Y196E-C confers strong protection in mice. Sci Rep. 2016;6:24162–24168. doi: 10.1038/srep24162. DOI: 10.1038/srep24162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bernath S, Fabian K, Kádár I, Szita G, Barna T. Optimum time interval between the first vaccination and the booster of sheep for Clostridium perfringens type D. Acta Vet Brno. 2004;73(4):473–475. DOI: 10.2754/avb200473040473. [Google Scholar]
- 25.Mirhosseini SA, Fooladi AAI, Amani J, Sedighian H. Production of recombinant flagellin to develop ELISA-based detection of Salmonella Enteritidis. Braz J Microbiol. 2017;48(4):774–781. doi: 10.1016/j.bjm.2016.04.033. DOI: 10.1016/j.bjm.2016.04.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fattahian Y, Rasooli I, Gargari SLM, Rahbar MR, Astaneh SDA, Amani J. Protection against Acinetobacter baumannii infection via its functional deprivation of biofilm associated protein (Bap) Microb Pathog. 2011;51(6):402–406. doi: 10.1016/j.micpath.2011.09.004. DOI: 10.1016/j.micpath.2011.09.004. [DOI] [PubMed] [Google Scholar]
- 27.Ghasemi A, Mohammad N, Mautner J, Karsabet MT, Amani J, Ardjmand A, et al. Immunization with a recombinant fusion protein protects mice against Helicobacter pylori infection. Vaccine. 2018;36(34):5124–5132. doi: 10.1016/j.vaccine.2018.07.033. DOI: 10.1016/j.vaccine.2018.07.033. [DOI] [PubMed] [Google Scholar]
- 28.Juckett G, Bardwell G, McClane B, Brown S. The microbiology of salt rising bread. W V Med J. 2008;104(4):26–27. PMID: 18646681. [PubMed] [Google Scholar]
- 29.Warrell DA, Benz EJ, Jr, Cox TM, Firth JD. Oxford Textbook of Medicine. 4th ed. Oxford: Oxford University Press; 2003. p. 3. [Google Scholar]
- 30.Hwang PM, Pan JS, Sykes BD. Targeted expression, purification, and cleavage of fusion proteins from inclusion bodies in Escherichia coli. FEBS Lett. 2014;588(2):247–252. doi: 10.1016/j.febslet.2013.09.028. DOI: 10.1016/j.febslet.2013.09.028. [DOI] [PubMed] [Google Scholar]
- 31.Langroudi RP, Shamsara M, Aghaiypour K. Expression of Clostridium perfringens epsilon-beta fusion toxin gene in E. coli and its immunologic studies in mouse. Vaccine. 2013;31(32):3295–3299. doi: 10.1016/j.vaccine.2013.04.061. DOI: 10.1016/j.vaccine.2013.04.061. [DOI] [PubMed] [Google Scholar]
- 32.Sørensen HP, Mortensen KK. Soluble expression of recombinant proteins in the cytoplasm of Escherichia coli. Microb Cell Fact. 2005;4(1):1–7. doi: 10.1186/1475-2859-4-1. DOI: 10.1186/1475-2859-4-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mathur DD, Deshmukh S, Kaushik H, Garg LC. Functional and structural characterization of soluble recombinant epsilon toxin of Clostridium perfringens D, causative agent of enterotoxaemia. Appl Microbiol Biotechnol. 2010;88(4):877–884. doi: 10.1007/s00253-010-2785-y. DOI: 10.1007/s00253-010-2785-y. [DOI] [PubMed] [Google Scholar]
- 34.Kulkarni R, Parreira V, Sharif S, Prescott J. Oral immunization of broiler chickens against necrotic enteritis with an attenuated Salmonella vaccine vector expressing Clostridium perfringens antigens. Vaccine. 2008;26(33):4194–4203. doi: 10.1016/j.vaccine.2008.05.079. DOI: 10.1016/j.vaccine.2008.05.079. [DOI] [PubMed] [Google Scholar]







