Abstract
Cancer is a disease advanced via surplus angiogenesis. The development of new anti-angiogenic therapeutic agents with more efficacy and fewer side effects is still quite necessary. Conventional therapies saving the life of many cancer patients but due to drug resistance and lack of specificity utilizing these methods is faced with limits. Recently, new therapeutic agents have been developed and used to treat cancers such as scaffold proteins, monoclonal antibodies, tyrosine kinase inhibitors, and peptides. In antiangiogenic drug development, anti-angiogenic peptides design is a significant aim. Peptides have developed as substantial therapeutics that are being carefully investigated in angiogenesis-dependent diseases because of their high penetrating rate into the cancer cells, high specificity, and low toxicity. In this review, we focus on anti-angiogenic peptides in the field of cancer therapy that are designed, screened, or derived from nanobodies, mimotopes, phage displays, and natural resources.
Keywords: Angiogenesis, Cancer, Nanobodies, Natural resource, Peptide, Phage display
1. INTRODUCTION
Angiogenesis refers to the process of the formation of new blood vessels from existing blood vessels, which occurs in pathological conditions like cancers (1). This process is needed for supplying oxygen and nutrients during cancer growth and progression (2). Angiogenesis is caused by an imbalance between several proangiogenic and antiangiogenic endogenous factors that result in the progression of the disease. Important factors involved in the process include vascular endothelial growth factor (VEGF), fibroblast growth factor (FGF), platelet-derived growth factor (PDGF), and angiopoietins that act through interaction with the cell-extracellular matrix (Fig. 1) (3). Interaction between cell-extracellular matrix and endothelial cells is an important step in the progression of cellular processes including migration, proliferation, differentiation, and apoptosis in several cancers. Many antiangiogenic drugs are widely available, however, many of them exhibit high long-term toxicity and drug resistance (4). Recently, various anti-angiogenic therapies have been designed and shown to efficiently block the tumor angiogenesis process. Many anti-angiogenic proteins are large and have complex structures that hardly penetrate the tissues. On the other hand, the production process of them in large volumes for maintaining the essential drug dose is very expensive (5). Among anti-angiogenic proteins, peptides have received special attention because of their specific advantages.
Fig. 1.
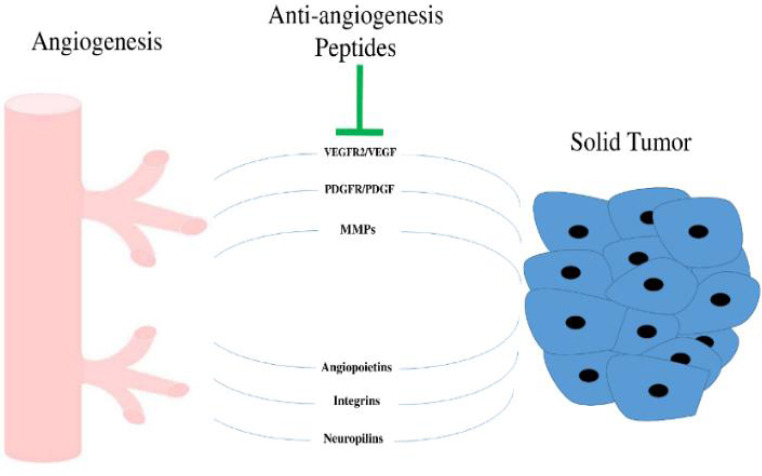
Important enzymes and factors involved in the angiogenesis process.
Peptides are polymeric structures linked together by an amide bond. As a result of their low toxicity and high specificity, several numbers of peptides have been considered as candidate drugs and various drugs have been approved (6). Also, they could rapidly penetrate into tumor tissues because of their small size. Therefore, for solving the mentioned barriers, the development of small peptide fragments with similar functionality to antiangiogenic proteins has been considered. In recent years, it is demonstrated that anticancer and anti-angiogenic peptides have a substantial role in cancer treatment and peptides have been recognized as future therapeutics (7). Peptides are composed of usually less than 50 amino acids in length and are often stabilized by disulfide bonds. They can be designed by methods that bind to a specific protein with high specificity and induce interaction with the desired protein (8). Peptides are easily synthesized and less immunogenic than antibodies (9,10). Therapeutic peptides are novel and promising tools for the development of anti-angiogenic drugs (11).
The focus of this review is on anti-angiogenic peptides with application in cancer therapy that not exceeding 50 residues in length and are rationally designed or derived from nanobodies, mimotopes, phage display, and natural resources.
2. THERAPEUTIC PEPTIDES DERIVED FROM NANOBODIES AND MIMOTOPES
Treatment of cancer via monoclonal antibody has been recognized as one of the most effective therapeutic schemes for both solid tumors and hematologic malignancies in the last 20 years (12). For example, Oliner et al. generated peptide-Fc fusion proteins and antibodies via phage display peptide and Fab libraries which panned versus human Ang2. Anti-angiopoietin-2 therapy via this method hampered angiogenesis and tumor growth in the human epidermoid carcinoma cell line (A-431) (13). Although monoclonal antibodies (mAbs) are commonly used in cancer therapies, they have several disadvantages that restrict their applications. The production is often costly in eukaryotic expression systems and a high therapeutic dose is needed for the treatment of solid tumors (14). To overcome these problems, mimotopes derived from phage display could be a suitable substitute. The mimotopes or peptidomimetics are small peptides that are identified via the immune system, and they have some ‘key’ structural features that mimic the spatial structure of the presented epitope, and not necessarily its sequence (15). Mimotopes mimic proteins, lipids, or carbohydrates behavior and are produced through different methods. They have introduced a new approach to treat diseases like cancers (7,16). Mimotopes in comparison to mAbs have special advantages. For example, high-affinity mimotopes are easily obtained by phage display and biopanning techniques. Their safety and efficacy are usually higher than mAbs because of the epitopic features. In addition, they are more cost-effective than mAbs because they linger the immune responses and require only a low dose to achieve the therapeutic goal. Also, the administration of mimotopes in comparison to mAbs has special advantages for the induction of specific endogenous immune responses (17,18,19).
Computational approaches are also used for designing mimotopes. However, the designed mimotopes should be more evaluated in vitro and in vivo to ensure safety and efficacy (20). It must be mentioned that in recent years, monoclonal antibodies have been gradually substituted with nanobodies. Nanobodies are derived from heavy chain antibodies (HCAbs) of a camel by genetic engineering (Table 1) (21).
Table 1.
Anticancer peptides from nanobodies and mimotopes resources and their respective oncolytic properties against tumors.
| Source | Peptide | Cancer type | Anticancer activity | Reference |
|---|---|---|---|---|
| VEGF mimotope | Human umbilical vein endothelial cells | Inhibition VEGF signaling | (15) | |
| CEA mimotope | Human colorectal adenocarcinoma | Effectively inhibit the growth of CEA-positive tumors. | (22) | |
| CDR3 domain of HER3 nanobody | Breast cancer | Targeting HER-3 | (23) | |
| EGFR mimotope | Unilateral ureteral obstruction | Inhibition of EGFR signaling | (24) | |
| Nanobodies and mimotopes | EGFR mimotope | Lewis lung carcinoma xenograft model | Inhibition of EGFR signaling | (25) |
| keyhole limpet hemocyanin-12P | Human umbilical vein endothelial cells | Block VEGF binding with VEFGR | (27) | |
| peptides 12/16 | MyLa | Induce cytolytic responses of the cytotoxic T lymphocytes | (28) | |
| Glypican 3 peptide | Ovarian clear cell carcinoma | Induce cytolytic responses of the cytotoxic T lymphocytes | (29) |
VEGF, Vascular endothelial growth factor; CDR3, complementarity determining region 3; HER-3, receptor tyrosineprotein kinase erbB-3; EGFR, epidermal growth factor receptor; VEGFR, vascular endothelial growth factor receptor.
In approximately 50% of all human and veterinarian tumors, a glycoprotein named carcinoembryonic antigen (CEA) is overexpressed. Bramswig et al. used the monoclonal anti-CEA antibody Col-1 and the biopanning method to design mimotopes of the Col-1 epitope. This mimotope which exhibited a specific and significant inhibition of tumor growth was in a murine tumor transplant model (22).
Recently, Karami et al. developed a nanobody-derived mimotope against VEGF which effectively inhibited cell proliferation and angiogenesis (tube formation) in the HUVEC cell line (15). The human epidermal growth factor receptor 3 (HER3) is an important therapeutic target in cancer therapy. In 2017, Pourhashem et al. considered the complementarity-determining region 3 (CDR3) domain of HER3 nanobody as a mimicking peptide using bioinformatics studies. The resulted CDR3 fragment of the HER3 nanobody has a similar binding affinity to the HER3 receptor when compared to the full HER3 nanobody (23). In another study, Yang et al. evaluated the effect of epidermal growth factor receptor (EGFR) mimotope (WHTEILKSYPHEGGGSGGGS) as an EGFR inhibitor. The designed EGFR mimotope showed a decrease in renal fibrosis in a unilateral ureteral obstruction animal model through inhibition of EGFR signaling and apoptosis-induction in macrophages (24). In another study by Javanmardi et al. triple tandem repeat of EGFR mimotope displaying particles (3M) and single EGFR mimotope displaying phage particle prepared by utilizing m13-PVIII phage display system. With treatment of C57BL/6 mice Lewis lung carcinoma xenograft model via 3M phage vaccine, 1M phage vaccine, and control agents, the anti-angiogenesis properties of phage vaccine were established (25). Although EGFR inhibitors and mAbs against EGFR are commercially available, they are expensive and require sequential injections which are oftentimes neglected by the patients (26). Therefore, producing a mimotope that can mimic the EGFR inhibitors’ behavior is useful. The design of peptide vaccines is another interesting field in cancer therapy. In 2013, Li et al. utilized a phage display technique to design mimotopes that complemented the Avastin antibody. The chemically synthesized DHTLYTPYHTHP, named 12P, was conjugated to keyhole limpet hemocyanin (KLH) via glutaraldehyde to produce the vaccine (KLH-12P). Their results showed that the peptide can prompt specific antibody production against VEGF and angiogenesis. The sera obtained from KLH-12P-immunized mice captured VEGF and blocked its binding to VEGF receptor (VEGFR), and therefore, successfully inhibited the proliferation and migration of human umbilical vein endothelial cells (HUVECs) (27). Linnemann et al. produced mimotopes of a tumor-associated T cell epitope via combinatorial peptide library screening. These mimotopes, nonapeptides 12 (PVKTKDIKL), and 16 (PVKTYDIKL) were generated according to the HLA restriction of the original cytotoxic T lymphocytes (CTL) clone for presentation by HLA-B8. The peptides induced IFN-γ secretion by the MyLa CTL and evoked tumor-specific T cell responses in lymphoma patients and, therefore, are suitable for therapeutic vaccination in cancers (28). Suzuki et al. purposed a peptide vaccine derived from a carcinoembryonic antigen, glypican-3 (GPC3), which is approved for the treatment of ovarian clear cell carcinoma. Thirty-two cancerous patients received an intradermal injection of GPC3298-306 (EYILSLEEL) and GPC3144-152 peptides (FVGEFFTDV) emulsified with incomplete Freund’s adjuvant near the bilateral axillary lymph nodes. GPC3 peptide vaccines exhibited strong antitumor effects and prolong the patients’ survival in certain populations with refractory ovarian clear cell carcinoma (29).
3. PEPTIDES FROM NATURAL RESOURCES
There are numerous drugs with anti-angiogenic and antiproliferative effects that are either natural products or natural product derivatives (30). Nature provides a wide range of peptides expressed in various organisms. Natural selection has led to the selection and optimization of these peptides for binding to specific molecules with high affinity (31).
Studies have shown that nearly 7000 peptides/peptidomimetics with different therapeutic properties have been discovered from nature (32,33). In recent years, in the field of biotechnology, peptides derived from the venom of various organisms have been extensively used for therapeutic purposes. For example, peptides isolated from the venom of a snake, bee (34), and scorpion can act as potent anticancer agents in malignant tumors or peptides from bacteria, animals, and plants also can play an anti-angiogenic role (35). On the other hand, recent advances in chromatographic or spectrometric techniques such as high-performance liquid chromatography (HPLC) and mass spectrometry have enabled us to identify and isolate the biologically active peptides and proteins from any natural resources (36). In this section, we explain peptides derived from natural resources that showed anti-angiogenic activity in cancers (Table 2).
Table 2.
Anticancer peptides from natural resources and their respective oncolytic properties against tumors.
| Source | Peptide | Source | Cancer type | Anticancer activity | Reference |
|---|---|---|---|---|---|
| ICD-85 | Venoms of an Iranian brown snake (Agkistrodon halys) and a yellow scorpion (Hemiscorpius lepturus) | HeLa cancer cell | Caspase 8 activation and apoptosis | (44) | |
| Venoms | Melittin | Bee venom | Breast, lung, and leukemia | Membrane disruption | (41) |
| Scorpion venom | |||||
| Gonearrestide | Androctonus mauritanicus Androctonus australis | Leukemia and colon cancers | Inhibiting cyclin-dependent kinases | (43) | |
| P8 Protein | Lactobacillus rhamnosus | Colorectal, cervical, lung and stomach cancers | Inhibition of the p53-p21-cyclin B1/Cdk1 signal pathway | (45) | |
| Bacteria | Apratoxin A | Lyngbya majuscula | HeLa cervical carcinoma and HT29 colon adenocarcinoma | Inducing G1-phase cell cycle arrest | (47) |
| Ieodoglucomides | Bacillus licheniformis | lung cancer and stomach cancer cell lines | - | (48) | |
| Fungi | Beauvericin | Fusarium sp. | Human epidermoid carcinoma | Release of cytochrome C, caspase-9/3 activation | (49) |
| Sponges | Jaspamide | Jaspis and Hemiastrella | Leukemia | Caspase-3 activation and reducing the expression of Bcl-2 | (51) |
| Kahalalides | Elysia refescens | Non-small cell lung cancer and androgen-independent prostate cancer | Induces disturbances in lysosomal function, anti-tubulin | (53) | |
| Animals | Aurein 1.2 | Litoria aurea | Human glioblastoma cells | Disruption of the mitochondrial membrane and/or plasma membrane | (54) |
| Deoxybourvardin | Rubia yannanesis | Human breast cancer cells | Inducing mitochondria- mediated apoptosis | (55) | |
| Plants | Lunasin | Soy and wheat | Cancerous mammalian cells which were induced by chemical carcinogens and viral oncogenes | Bind to chromatin, anti-transformation (foci formation) | (56) |
CDK1, Cyclin-dependent kinase 1; Bcl-2, B-cell lymphoma 2.
3.1. Therapeutic peptides derived from venoms
Most snake venoms contain contortrostatin that exhibits antitumor activity against various cancer cells. This peptide exerts its deleterious effect on the cancer cells by binding to carcinoma epithelial receptors and apoptosis induction in the cancer cells (36,37). Studies also showed bee venom consists of different anticancer peptides such as melittin, adolapin, and apamin (38,39). Melittin, a small linear basic 26 amino acid peptide with a molecular weight of 2847.5 Da, has a powerful haemolytic activity (40). In vitro and in vivo studies revealed the beneficial effects of melittin in the treatment of breast and lung cancer, as well as leukemia (41). Scorpion venoms also contain anticancer peptides. For example, a peptide extracted from a black Indian scorpion showed anticancer activity against human leukemia cancer cells (42). In 2018, a new peptide derived from scorpion venom was discovered using the next-generation sequencing technique and mass spectrometry (MS/MS) proteome platform. This peptide which was named gonearrestide exhibited strong anticancer activity against colon cancer cells and solid tumors. It inhibits the growth of cancer cells and solid tumors through inhibiting cyclin-dependent kinases (43). Some peptides are also concurrently isolated from the venom of snakes and scorpions. For instance, ICD-85 peptide is extracted from both Agkistrodon Halys (an Iranian brown snake) and Hemiscorpius lepturus (a yellow scorpion). According to in vitro toxicity tests, isolated ICD-85 showed a slight effect on natural killer cells, while had significant dose-dependent toxicity on Hela tumor cells (44).
3.2. Therapeutic peptides derived from bacteria, fungi, and sponges
Peptides from bacteria are popularly used in medicine for therapeutic purposes. In 2019, a protein (P8) was extracted from lactic acid bacteria and showed significant cytotoxicity against colorectal cancer (45). Cyanobacteria are one of the bacterial resources that contain proteins with anti-cancer activity (46). Apratoxin A is a peptide extracted from lyngbya that inhibits the cell cycle in cervical cancer (47). Ieodoglucomides are another peptide derived from bacteria. It is a glycoprotein extracted from marine-derived bacterium Bacillus licheniformis, and has been reported it shows antitumor activity against lung and stomach cancer cell lines with GI50 values of 25.18 and 17.78 μg/mL, respectively (48). Fungi are another source of peptides that can be used in medicine. Beauvericin (CAS 26048-05-5, MW 783.957 Da), derived from mangrove endophytic fungus Fusarium sp, is a cyclic hexadepsipeptide that comprises three N-methyl-phenylalanyl and three D-hydroxy-isovaleryl residues in the sequence. Beauvericin induces apoptosis in human epidermoid carcinoma cells via different mechanisms including a decrease in reactive oxygen species, loss of mitochondrial membrane potential, the release of cytochrome C, caspase 9/3 activation, and poly (ADP-ribose) polymerase cleavage (49). In different studies, various peptides and oligopeptides with anticancer effects were extracted from sponges. Jaspamide is a cyclic depsipeptide derived from Jasipsis and Hemiastrella. It owns a 15-carbon macrocyclic ring that comprises three amino acid residues. Jaspamide induces apoptosis in the human leukemic cell line by activating caspase-3 and reducing the expression of the B-cell lymphoma 2 (Bcl-2) gene at the transcriptional level (50,51).
3.3. Therapeutic peptides derived from animals and plants
Mollusca is the second largest phylum of invertebrates that has an important role in medicine. Some mollusca-extracted peptides exhibit antitumor activity. For example, kahalalid, a dehydroaminobutyric acid-containing peptide isolated from Elysia refescens, showed promising results in disturbances of lysosomal function and suppressed the expression of particular genes that are involved in DNA replication and cell proliferation. It successfully prevented tumor spreading and growth in androgen-independent prostate and non-small cell lung cancers (52,53). Aurein 1.2 (GLFDIIKKIAESF) is a peptide derived from Litoria aurea that showed antitumor effects against human glioblastoma cell lines via disruption of the mitochondrial and/or plasma membranes. Notably, besides the antitumor activity, this peptide also has potent antimicrobial activity against different microbial pathogens (54).
Recently, it was shown that peptides derived from plants also exhibit antitumor activity. For instance, deoxybouvardin (RA-V), is a plant cyclopeptide extracted from Rubia yunnanensis that its anticancer activity has been identified in human breast cancer cells by inducing mitochondria-mediated apoptosis. RA-V also repressed phosphorylation of pyruvate dehydrogenase kinase 1 and protein kinase B in breast cancer MCF-7 cells (55). Lunasin, a 43-amino acid chemopreventive peptide derived from soy and wheat, significantly prevents the development of cancers induced by viral oncogenes and chemical carcinogens in mammalian cells. Lunasin exerts its anticancer effects via anti-transformation capacity by binding to the chromatin (56).
4. TARGETING AND THERAPEUTIC PEPTIDES DERIVED BY PHAGE LIBRARY SCREENING
Screening of phage display libraries is one of the efficient tools for obtaining targeting and therapeutic peptides against cancer. These screening could be performed against any molecular library involved in remodeling the vasculature of tumor tissues. Many peptides isolated by in vivo panning of combinatorial phage libraries efficiently target endothelial cells (Table 3). For example, peptides isolated via in vivo selection of phage display libraries composed of an αV integrin-binding motif (Arg-Gly-Asp) that coupled with drug and boosted the efficacy of the drug against human cancer cells (57). Some of these peptides concurrently blocked αV integrin from binding to their ligands and were utilized for targeting angiogenic tissues like tumors (58). Matrix metalloproteinase-2 (MMP-2) and MMP-9 are known to play a vital role in tumor invasion, angiogenesis, and metastasis. Koivunen et al. isolated cyclic peptides by phage library screening that are selective potent inhibitors for MMP-2 and MMP-9. One of these peptides, CTTHWGFTLC, showed potent efficacy for blocking blood vessels angiogenesis and migration of human endothelial cells in vivo (59). In another study, Lu et al. isolated MMP-2 specific inhibitors via a phage display technology that suppressed cell invasion of pancreatic cancer in vitro and reduced the growth of tumor blood vessels in nude mice (60). Another isolated peptide, named CRV (CRVYGPYLLC), inhibited MMP-9, efficiently. The CRV-treated mice exhibited inhibition of angiogenesis and less developed tumor vasculature (61).
Table 3.
List of therapeutic peptides derived by phage library screening for cancer treatment.
| Peptide sequence | Target antigen | Mechanism of action | Cancer type | Reference |
|---|---|---|---|---|
| CDCRGDCFC | Integrin | Target tumor blood vessels with doxorubicin | Human breast cancer | (57) |
| CTTHWGFTLC | MMP-2/9 | Inhibit the migration of tumor cells | Ovarian carcinoma, fibrosarcoma | (59) |
| HWWQWPSSLQLRGGGS | MMP-2 | Reduce MMP-2-mediated invasion of tumor cells | Pancreatic cancer | (60) |
| CRVYGPYLLC | MMP-9 | Inhibits proenzyme activation and cell migration | Tongue squamous cell carcinoma, fibrosarcoma | (61) |
| SMSIARL | Prostate-homing phage | Deliver a proapoptotic peptide to tumor tissue | Prostate cancer (TRAMP mice) | (62) |
| CTPSPFSHC | Vasculature of the colorectal cancer tissue | Deliver a pro-apoptotic peptide to tumor tissue | Orthotopic colorectal cancer | (63) |
| CPGPEGAGC | Aminopeptidase-P | Deliver a conjugated drug to tumor tissue | Human breast cancer | (64) |
| Cyclic RGD | MMP-2 | Antiangiogenic activity by inhibiting of MMP2 binding to αVβ3 | Human fibrosarcoma | (66) |
| ATWLPPR | VEGF | Abolish VEGF-induced angiogenesis | Rabbit corneal angiogenesis | (68) |
| WHSDMEWWYLLG | Flt-1 receptor | Reduce Flt-1-induced angiogenesis | Human breast cancer | (69) |
| CNGRCVSGCAGRC | Aminopeptidase-N | Inhibit APN-mediated angiogenesis | Human melanoma cancer | (71) |
| CGNSNPKSC | Endothelial cells of human gastric cancer | Anti-angiogenesis activity | Human gastric cancer | (74) |
| TLTYTWS | Collagen IV | Reduce endothelial differentiation and angiogenesis | Murine Lewis lung carcinoma | (75) |
| CRGDKGPDC | Neuropilin-1 | Target tumor tissue with Abraxane | Human prostate cancer | (76) |
| SVSVGMKPSPRP | Human umbilical vein endothelial cells | Target tumor tissue with doxorubicin | Human oral and lung cancer | (77) |
| CPRECESIC | Aminopeptidase-A | Suppress proliferation of endothelial cells, inhibit angiogenesis | Human breast carcinoma | (78) |
MMP, Matrix metalloproteinase-2; TRAMP, transgenic adenocarcinoma of the mouse prostate; VEGF, vascular endothelial growth factor; Flt-1, vascular endothelial growth factor receptor 1.
In other studies, Arap et al. introduced a novel peptide selected via phage display that homes specifically to human vasculature prostate. They linked this peptide (SMSIARL) to a pro-apoptotic peptide, D(KLAKLAK)2, which is an amphipathic D-amino acid peptide. The chimeric peptide was selectively toxic to angiogenic endothelial cells and disrupted mitochondrial membranes after cellular uptake (62). Also, in another study in 2010, Li et al. separated a vasculature targeting peptide TCP-1 (CTPSPFSHC) using the in vivo phage library selection against an orthotopic colorectal cancer model. This peptide could significantly deliver the apoptosis-inducing peptide D(KLAKLAK)2 to the tumor site and inhibit angiogenesis (63). Essler et al. introduced a cyclic nonapeptide (CPGPEGAGC) that homes to breast cancer tissue with high selectivity.
Aminopeptidase P was the receptor for this breast-homing peptide (64). A construct containing CPGPEGAGC, a cell-penetrating peptide, and an optional linker were coupled together to make an angiogenesis blocker with antitumor activity (65).
Studies revealed that the interaction between upregulated αvβ3 integrin and membrane-bound MMP-2 in proliferating endothelial cells, which commonly occur in the tumors, plays a vital role in angiogenesis. To block such interaction, a phage display libraries screening was conducted by Boger et al. in which in vivo results indicated that the selected peptides demonstrate a potent antitumor and anti-angiogenic activity (66).
As mentioned before, the VEGF mediates angiogenesis with binding to the kinase domain receptors such as VEGFR-2 and kinase insert domain receptor/fetal liver kinase1 (KDR/FLK1). Via a phage library screening, a single-chain antibody was selected against VEGF that selectively homes to tumors tissue after intravenous administration. Therefore, the screened peptide was potentially proposed for targeting tumor vasculature (67). In another study, Binetruy et al. isolated peptides that blocked the binding of VEGF to KDR using screening of a peptide library displayed on filamentous phages. ATWLPPR specifically inhibited human endothelial cell proliferation in vitro and eliminated VEGF-induced angiogenesis in vivo (68). The screening of a filamentous phage M13 library resulted in a 12-mer peptide (WHSDMEWWYLLG), which was named F56. Further experiments on this peptide indicated that F56 inhibits the efficient binding of VEGF to Flt-1 in vitro. Furthermore, F56 displayed an antiangiogenic effect and inhibited blood vessel formation in implanted human breast cancer cells (BICR-H1) in severe combined immunodeficient mice (69). Also, Hamzeh-Mivehroud et al. developed two novel peptides specific for EGFR that were identified using phage display technology which can lead to compounds for designing new pharmaceuticals effective in anti-angiogenesis cancer therapy (70).
Aminopeptidase N (APN), a type II metalloprotease, is often up-regulated in tissues that undergo angiogenesis. Pasqualini et al. screened that peptides containing the NGR motif could specifically bind to APN. A conjugate of doxorubicin to CNGRC peptide was specifically toxic to APN-expressing cells and inhibited angiogenesis (71). Also, NGR peptide (CNGRC) was fused with human tumor necrosis factor-alpha protein (TNF-α) to form a novel recombinant protein that could significantly boost the activity of TNF- α at a very lower dose (0.3 μg) in mice with tumor (72). Further studies revealed that 0.1 ng of NGR-TNF-α could increase synergistically the tumor toxicity of cisplatin, doxorubicin, paclitaxel, gemcitabine, and melphalan in mouse tumor models, without any amplification in the side effects (73). Also, a cyclic 7-mer peptide (CGNSNPKSC), named GX1, binds specifically to the human gastric cancer vasculature. This peptide was isolated using in vivo screening of a phage-displayed peptide library. It was shown that the coupling of this peptide to recombinant mutant human tumor necrosis factor (rmhTNFα) inhibits efficiently angiogenesis in tumor tissue (74).
Proteolytic degradation of collagen IV by MMP-2 plays also a vital role in tumor angiogenesis. In one study, Essler et al. carried out a combined in vitro and in vivo screening via a library of recombinant phage-displayed peptides. They found a peptide sequence (TLTYTWS) that selectively binds to collagen IV modified by MMP-2. This novel tumor-homing peptide significantly blocked angiogenesis in vivo by accumulating in the tumor environment (75).
Neuropilin-1 (NRP-1) is a receptor on the cell surface of endothelial cells involved in the angiogenesis process. A peptide, termed iRGD (CRGDKGPDC), was screened using in vivo phage library versus a human prostate cancer mouse model. This peptide can specifically bind to the NRP-1 receptor. Chemical conjugation of iRGD to Abraxane could enhance the accumulation of the drug in tumor tissues and improved the therapeutic index (76). Via a phage-displayed random peptide library, Lee et al. recognized a targeting peptide, SVSVGMKPSPRP (SP5-52). The peptide could selectively bind to the tumor vessels of several tumor types. In a further study, SP5-52 was coupled to liposomes comprising doxorubicin, and noticeably, the targeted liposomes could reduce tumor blood vessels and angiogenesis in both human oral and lung cancer xenografts in severe combined immunodeficient mice (77).
Aminopeptidase A (APA) is a zinc metallopeptidase cell surface protein that is upregulated in the angiogenic tumor blood vessels. Enzymatic activity of aminopeptidase A plays a critical role in the angiogenesis of tumor tissues. Marchio et al. separated a selective peptide inhibitor of aminopeptidase A (CPRECESIC) from a phage display peptide library. The inhibition of aminopeptidase A activity using this peptide led to a reduction in proliferation and migration of endothelial cells in tumor tissues (78).
5. RATIONALLY DESIGNED ANTI- ANGIOGENIC PEPTIDES
Multiple therapeutic peptides have been designed for targeting cell cycle, signal transduction pathways, proto-oncogenes, cell adhesion molecules (laminin and integrin), and angiogenesis growth factors (VEGF, PDGF) (Table 4). For example, after identifying the vital role of laminin in the angiogenesis process in 1987, Iwamoto et al. designed and evaluated a nonapeptide (peptide 11) and its amide form (peptide 11-amide). In vivo tests revealed that these peptides successfully inhibit angiogenesis in the lung tumor colonization murine model (79). In another study, Sakamoto et al. evaluated the inhibitory effects of a synthetic laminin peptide-NH2 (CDPGYIGSR) on tumor growth and angiogenesis. This peptide significantly inhibited the embryonic angiogenesis of 3LL Lewis lung carcinoma and chick chorioallantoic membrane (80).
Table 4.
List of rationally designed antiangiogenic peptides for cancer treatment.
| Peptide name | Sequence | Target molecule | Effects of treatment | Cancer type | Reference |
|---|---|---|---|---|---|
| Peptide 11 | CDPGYIGSR | Laminin | Inhibit of endothelial cell migration and angiogenesis | Lewis lung carcinoma | (80) |
| Cilengitide (EMD 121974) | RGD -DPhe-(NMeVal) | ανβ3/ανβ5 integrin | Antiangiogenic activity | Ocular melanoma, adenoid cystic cancer | (81) |
| IM862 | L-glutamine L-tryptophan | VEGF | Inhibition of β-FGF and VEGF-induced angiogenesis | AIDS-Kaposi’s sarcoma | (82) |
| JV-1-38 | PhAc-Tyr1, D-Arg2, Phe(4-Cl)6, Har9, Tyr(Me)10, Abu15, Nle27, D-Arg28, Har29 | Growth hormone-releasing hormone | Anti-proliferative effects | Human androgen-sensitive prostate cancer | (83) |
| TM peptide | ILITIIAMSALGVLLGAVCG VVLYRKR | pTM-NRP1 | Anti-angiogenic effect | Human breast cancer | (84) |
| ATN-161 | Ac-PHSCNNH2 | Integrin α5β1 | Reduction of phosphorylated MAPK expression | Prostate, colon and renal cancers | (85) |
| IS4 | Ac-VMDGYPMP | MMP-14 | Inhibition of MMP-14-enhanced cell migration | Human fibrosarcoma | (88) |
| Peptide [SRSRY] | SRSRY | uPAR | Inhibition of extra-cellular matrix invasion | Chondrosarcoma and osteosarcoma | (89) |
| A6 | Ac-KPSSPPEE | CD44 | Inhibition of cell-cell interactions mediated migration | Breast, lung, glioma, ovarian, and prostate cancer | (90) |
| CTCE-9908 | KGVSLSYRKKGVSLSYR | CXCR4 | Inhibition of the rate of metastases | Human breast adenocarcinoma | (94) |
| Peptide 47-70 | NGRKICLDLQAPLYKKIIKK LLES | Platelet factor-4 | Inhibition of cell proliferation | Colon carcinoma | (97) |
| CXCL4L1 | CXCL4 variant with three- amino acid replacements (P58L, K66E, and L67H) | CXCL4 | Inhibition of angiogenesis | B16 melanoma | (98) |
| Met-CCL5 | CCL5 from residues 24-91 with an N-terminus methionine | CCR1/CCR5 | Reduction leukocyte infiltration and tumor volume | Human breast cancer | (99) |
| RC-3095 | D-Tpi6, Leu13 psi[CH2NH]-Leu14 | Gastrin-releasing peptide | Reduction of the tumor size | Estrogen- independent human breast carcinoma | (102) |
VEGF, Vascular endothelial growth factor; AIDS, acquired immunodeficiency syndrome; β-FGF, Basic fibroblast growth factor; TM-NRP1, transmembrane domain of neuropilin 1; MAPK, mitogen-activated protein kinase; MMP- 14, matrix metalloproteinase-14; uPAR, urokinase-type plasminogen activator receptor; CD44, cluster of differentiation 44; CXCR4, C-X-C chemokine receptor type 4; CXCL4, C-X-C chemokine ligand 4; CCL5, C-C motif chemokine ligand 5; CCR, C-C chemokine receptor type.
Cilengitide (EMD 121974) is a cyclized pentapeptide that selectively inhibits ανβ3 and ανβ5 integrins. These proteins are involved in the attachment and migration of endothelial cells.
The efficacy and safety of this peptide were evaluated by Hariharan and his colleague on 20 patients who received 50 doses of the drug. The results revealed that cilengitide is well-tolerated as an antiangiogenic agent (81). A synthetic dipeptide IM862 (l-glutamine l-tryptophan) with antiangiogenic properties is well established in vitro and in vivo with no known toxic metabolites. Albeit it was demonstrated to be ineffectual in acquired immunodeficiency syndrome (AIDS) Kaposi’s sarcoma in phase III clinical trial, there are still some promising results for other forms of cancers (82).
Several studies elucidate growth hormone-releasing hormone (GHRH) antagonists can attenuate the VEGF expression, which is considered to be one of the main factors in angiogenesis. GHRH antagonist peptide was synthesized in the Letsch laboratory by a solid phase method. In an MDA-PCa-2b model of human androgen-sensitive prostate cancer, tumor inhibition was observed by injection of JV-1-38. This inhibition has occurred via suppression of VEGF synthesis at both transcriptional and translational levels (83).
It was shown that the binding of NRP-1 to VEGF leads to tumor progression and pathological angiogenesis. TM peptide (ILITIIAMSALGVLLGAVCGVVLYRKR), which targets the transmembrane domain (TMD) of neuropilin-1 (pTM-NRP1), blocked glioma growth in vivo and inhibited angiogenesis and primary tumor growth in breast cancer models (84).
A five-amino acid peptide, Ac-PHSCNNH2 (ATN-161), which was manufactured via a solution-phase method, demonstrated abilities to inhibit angiogenesis. It also blocked metastasis with prolonged survival in multiple animals tumor models, such as prostate, colon, and breast cancers, either when given as a single compound or when accompanied with radiotherapy and chemotherapy (85). A very well safety profile was obtained for utilizing ATN-161 in the phase I clinical trial (86). But phase II for renal cancer and phase I/II for recurrent glioma in the United States were terminated in 2016 due to the low activity of this peptide (87).
Recently, new investigations indicated that MMPs might have non-proteolytic activity via the c-terminal hemopexin domain that is essential for MMP-mediated tumor tissue angiogenesis. For example, it was shown that the c-terminus hemopexin domain of matrix MMP-14, which is responsible for the homodimerization of MMP-14 and cross-talking between MMP-14 and cluster of differentiation 44 (CD44), is essential for both invasion and angiogenesis. Zarrabi et al. introduced synthetic peptides that mimic the critical outermost strand motifs inside the c-terminal hemopexin domain of MMP-14 and found that the peptides interrupt MMP-14-induced cancer cell angiogenesis and metastasis in vivo (88).
The urokinase-type plasminogen activator receptor (uPAR) is generally known as the principal regulator of cell migration and residues 88 to 92 (SRSRY) are the minimal sequence needed to promote angiogenesis and cell motility via interacting with the formyl peptide receptor type 1. The synthetic linear SRSRY peptide hampers the angiogenic and chemotactic activity of uPAR in vitro and in vivo. Moreover, the cyclized SRSRY peptide prevents neovascularization, suppresses trans-endothelial migration of monocytes, intravasation of chondrosarcoma, and osteosarcoma cells (89). An 8-mer capped peptide (A6), which is taken from the non-receptor binding region of uPA, prevented the interaction between uPA and uPAR in a non-competitive manner. Additionally, anti-invasive and anti-angiogenic aspects of A6 peptide were illustrated in preclinical studies via modulation of CD44 receptor function (90). Regarding hopeful outcomes in phase I clinical trials (91), a phase II assessment of A6 peptide is presently ongoing to check the side effects and the efficacy in patients with persistent or recurrent ovarian epithelial cancer, fallopian tube cancer, and primary peritoneal cancer (92) (see at ClinicalTrials.gov).
Chemokines are essential paracrine and autocrine players in tumor expansion. So, blocking of chemokine signaling pathways can be considered as a hopeful strategy to treat cancers (93). For instance, it was revealed the overexpression of C-X-C chemokine receptor type 4 (CXCR4) in cancer cells contributes to angiogenesis and metastasis. Since CXCR4 is a valuable target in tumor tissues, various antagonists are already in clinical trial studies (93). Another example is a 17-amino acid peptide (CTCE-9908) which is a CXCR4 antagonist. Surprisingly, it exhibited promising results in the reduction of the adhesion and growth of tumor cells via inhibition of angiogenesis in several in vivo models (94). Currently, the compound is in phase I/II clinical trials in patients with advanced metastatic cancer (95). Several peptides were also designed based on chemokines that exhibit angiostatic behavior. For example, platelet factor-4 (CXCL4/PF-4), which is released from alpha-granules of activated platelets, is the first described angiostatic chemokine (96). A short peptide (NGRKICLDLQAPLYKKIIKKLLES) derived from PF-4 is a well-established angiogenesis inhibitor (97). In another example, a CXCL4 variant, termed CXCL4L1, was designed via three-amino acid replacements (P58L, K66E, and L67H). This peptide showed antitumor and anti-angiogenic effects in a B16 melanoma in vivo model (98). In another study, Robinson et al. designed a peptide comprising 69 amino acids of C-C motif chemokine ligand 5 (CCL5) from residues 24-91 with an N-terminus methionine. This peptide which is called Met-CCL5 inhibited significantly angiogenesis in a breast cancer model via antagonistic effects on C-C CCR1 and CCR5 proteins (99).
The gastrin-releasing peptide (GRP) and other mammalian bombesin (BB)-like peptides are also considered as the main autocrine growth factors involved in the expansion of multiple human cancers. Irregular expression of GRP/BB-like peptides and their receptors (GRP/BB-R) have been described in many cancers such as prostate, breast, colon, pancreas, lung, and brain cancers (100). Several GRP/BB-R antagonists including RC-3950, RC-3940-II, and RC-3095 were designed and synthesized (101). These synthetic peptides displayed remarkable anti-angiogenic properties in numerous in vivo tumor models and represented hopeful outcomes in preclinical studies of breast and prostate cancers (102).
6. CONCLUSION
As cancer therapies, peptides are appropriate constituents due to their advantages in production, for instance, possibilities for automated chemical synthesis and possibilities of several kinds of modifications. In near future, peptides can create a huge impact in the area of cancer treatment and anti-angiogenesis drugs. Aside from the fact that small peptide inhibitors have numerous benefits over larger anti-angiogenic drugs, they may also be used to help create improved, more active anti-angiogenic peptides or even small molecule mimetics. However, due to and lack of oral bioavailability, enzymatic degradation, and renal clearance the most peptides suffer from short serum half-life, which has hindered their shift from an investigation into viable clinical drugs. The appearance of novel technologies in recent decades such as macromolecule conjugation, pegylation, non-natural amino acids, peptidomimetics, and polymer-supported formulation has enabled a number of recent clinical entries and commercialization of peptide-based therapeutics. Recent advancements in cost-effective solid-phase synthesis and novel drug delivery technologies have also surely helped new peptide therapeutics discovery.
To attaining synergistic results, conjugations of anti-angiogenesis via traditional chemotherapy are presently using in clinical trials. For instance, in newly diagnosed glioblastoma patients cilengitide-temozolomide conjugation was utilized in a phase I/IIa which demonstrated better overall survival rates. Recent work by numerous researchers in the area of peptidomimetics, phage display, and natural peptides has also helped significantly in the development and design of approaches for improving the serum half-life of anti-angiogenesis peptides.
New methods and principles in drug design in phage display and peptidomimetics are now contributed to the improved pharmacokinetic profile of anti-angiogenesis peptides. Continued investigation in the structural chemistry of proteins and peptides, joined with the expansion of novel templates and scaffolds which can predictably mimic the stereo-structural properties of peptides continue to offer the approaches for effective de novo peptidomimetic design (103). Anyway, rapid systemic clearance due to renal filtration and excretion always happens to proteins smaller than 40 kDa. Although, the anti-angiogenic peptides being discussed here would fall in this classification but rapid renal clearance can be overwhelmed via conjugating the peptides with water-soluble polymers resulting in molecules larger than 40 kDa (104).
Thus, further possibilities for modification of anti-angiogenesis peptides introduce a promising way to make better and more active drugs for cancer treatment.
Conflict of interest statement
The authors declared no conflict of interest in this study.
Authors’ contribution
All authors were equally responsible for the article and approved the final draft of the manuscript.
7. REFERENCES
- 1.Ramadhani AH, Ahkam AH, Suharto AR, Jatmiko YD, Tsuboi H, Rifa’i M. Suppression of hypoxia and inflammatory pathways by Phyllanthus niruri extract inhibits angiogenesis in DMBA-induced breast cancer mice. Res Pharm Sci. 2021;16(2):217–226. doi: 10.4103/1735-5362.310528. DOI: 10.4103/1735-5362.310528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Figg WD, Folkman J. Angiogenesis: An Integrative Approach from Science to Medicine. 1st ed. Springer US. :1–14. DOI: 10.1007/978-0-387-71518-6. [Google Scholar]
- 3.Carmeliet P. Angiogenesis in life, disease and medicine. Nature. 2005;438(7070):932–936. doi: 10.1038/nature04478. DOI: 10.1038/nature04478. [DOI] [PubMed] [Google Scholar]
- 4.Vicari D, Foy KC, Liotta EM, Kaumaya PTP. Engineered conformation-dependent VEGF peptide mimics are effective in inhibiting VEGF signaling pathways. J Biol Chem. 2011;286(15):13612–13625. doi: 10.1074/jbc.M110.216812. DOI: 10.1074/jbc.M110.216812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bhutia SK, Maiti TK. Targeting tumors with peptides from natural sources. Trends Biotechnol. 2008;26(4):210–217. doi: 10.1016/j.tibtech.2008.01.002. DOI: 10.1016/j.tibtech.2008.01.002. [DOI] [PubMed] [Google Scholar]
- 6.Saladin PM, Zhang BD, Reichert JM. Current trends in the clinical development of peptide therapeutics. IDrugs. 2009;12(12):779–784. PMID: 19943221. [PubMed] [Google Scholar]
- 7.Pourjafar M, Samadi P, Khoshinani HM, Saidijam M. Are mimotope vaccines a good alternative to monoclonal antibodies? Immunotherapy. 2019;11(9):795–800. doi: 10.2217/imt-2018-0213. DOI: 10.2217/imt-2018-0213. [DOI] [PubMed] [Google Scholar]
- 8.Marqus S, Pirogova E, Piva TJ. Evaluation of the use of therapeutic peptides for cancer treatment. J Biomed Sci. 2017;24(1):21–35. doi: 10.1186/s12929-017-0328-x. DOI: 10.1186/s12929-017-0328-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Boohaker RJ, Lee MW, Vishnubhotla P, Perez JM, Khaled AR. The use of therapeutic peptides to target and to kill cancer cells. Curr Med Chem. 2012;19(22):3794–3804. doi: 10.2174/092986712801661004. DOI: 10.2174/092986712801661004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.McGregor DP. Discovering and improving novel peptide therapeutics. Curr Opin Pharmacol. 2008;8(5):616–619. doi: 10.1016/j.coph.2008.06.002. DOI: 10.1016/j.coph.2008.06.002. [DOI] [PubMed] [Google Scholar]
- 11.Blanco-Míguez A, Gutiérrez-Jácome A, Pérez-Pérez M, Pérez-Rodríguez G, Catalán-García S, Fdez-Riverola F, et al. From amino acid sequence to bioactivity: the biomedical potential of antitumor peptides. Protein Sci. 2016;25(6):1084–1095. doi: 10.1002/pro.2927. DOI: 10.1002/pro.2927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Scott AM, Allison JP, Wolchok JD. Monoclonal antibodies in cancer therapy. Cancer Immun. 2012;12:14–21. PMID: 22896759. [PMC free article] [PubMed] [Google Scholar]
- 13.Oliner J, Min H, Leal JA, Yu D, Rao S, You E, et al. Suppression of angiogenesis and tumor growth by selective inhibition of angiopoietin-2. Cancer Cell. 2004;6(5):507–516. doi: 10.1016/j.ccr.2004.09.030. DOI: 10.1016/j.ccr.2004.09.030. [DOI] [PubMed] [Google Scholar]
- 14.Kimiz-Gebologlu I, Gulce-Iz S, Biray-Avci C. Monoclonal antibodies in cancer immunotherapy. Mol Biol Rep. 2018;45:2935–2940. doi: 10.1007/s11033-018-4427-x. DOI: 10.1007/s11033-018-4427-x. [DOI] [PubMed] [Google Scholar]
- 15.Karami E, Sabatier JM, Behdani M, Irani S, Kazemi-Lomedasht F. A nanobody-derived mimotope against VEGF inhibits cancer angiogenesis. J Enzyme Inhib Med Chem. 2020;35(1):1233–1239. doi: 10.1080/14756366.2020.1758690. DOI: 10.1080/14756366.2020.1758690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Knittelfelder R, Riemer AB, Jensen-Jarolim E. Mimotope vaccination-from allergy to cancer. Expert Opin Biol Ther. 2009;9(4):493–506. doi: 10.1517/14712590902870386. DOI: 10.1517/14712590902870386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Witsch EJ, Mahlknecht G, Wakim J, Sertchook R, Bublil E, Yarden Y, et al. Generation and characterization of peptide mimotopes specific for anti ErbB-2 monoclonal antibodies. Int Immunol. 2011;23(6):391–403. doi: 10.1093/intimm/dxr028. DOI: 10.1093/intimm/dxr028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Leung NYH, Wai CYY, Ho MHK, Liu R, Lam KS, Wang JJ, et al. Screening and identification of mimotopes of the major shrimp allergen tropomyosin using one-bead-one-compound peptide libraries. Cell Mol Immunol. 2017;14(3):308–318. doi: 10.1038/cmi.2015.83. DOI: 10.1038/cmi.2015.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Aghebati-Maleki L, Bakhshinejad B, Baradaran B, Motallebnezhad M, Aghebati-Maleki A, Nickho H, et al. Phage display as a promising approach for vaccine development. J Biomed Sci. 2016;23:66–83. doi: 10.1186/s12929-016-0285-9. DOI: 10.1186/s12929-016-0285-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Soria-Guerra RE, Nieto-Gomez R, Govea-Alonso DO, Rosales-Mendoza S. An overview of bioinformatics tools for epitope prediction: implications on vaccine development. J Biomed Inform. 2015;53:405–414. doi: 10.1016/j.jbi.2014.11.003. DOI: 10.1016/j.jbi.2014.11.003. [DOI] [PubMed] [Google Scholar]
- 21.Muyldermans S, Baral TN, Retamozzo VC, De Baetselier P, De Genst E, Kinne J, et al. Camelid immunoglobulins and nanobody technology. Vet Immunol Immunopathol. 2009;128(1-3):178–183. doi: 10.1016/j.vetimm.2008.10.299. DOI: 10.1016/j.vetimm.2008.10.299. [DOI] [PubMed] [Google Scholar]
- 22.Brämswig KH, Knittelfelder R, Gruber S, Untersmayr E, Riemer AB, Szalai K, et al. Immunization with mimotopes prevents growth of carcinoembryonic antigen-positive tumors in BALB/c mice. Clin Cancer Res. 2007;13(21):6501–6508. doi: 10.1158/1078-0432.CCR-07-0692. DOI: 10.1158/1078-0432.CCR-07-0692. [DOI] [PubMed] [Google Scholar]
- 23.Pourhashem Z, Mehrpouya M, Yardehnavi N, Eslamparast A, Kazemi-Lomedasht F. An in-silico approach to find a peptidomimetic targeting extracellular domain of HER3 from a HER3 Nanobody. Comput Biol Chem. 2017;68:39–42. doi: 10.1016/j.compbiolchem.2017.02.001. DOI: 10.1016/j.compbiolchem.2017.02.001. [DOI] [PubMed] [Google Scholar]
- 24.Yang L, Yuan H, Yu Y, Yu N, Ling L, Niu J, et al. Epidermal growth factor receptor mimotope alleviates renal fibrosis in murine unilateral ureteral obstruction model. Clin Immunol. 2019;205:57–64. doi: 10.1016/j.clim.2019.05.014. DOI: 10.1016/j.clim.2019.05.014. [DOI] [PubMed] [Google Scholar]
- 25.Javanmardi M, Rasaee M, Modjtahedi H, Asadi GM, Maghami MG. Triple tandem mimotope peptide of epidermal growth factor receptor displaying on the surface of M13 phage induces anti-tumor response in mice tumor model. Iran J Biotech. 2014;12(3):e1017,1–9. DOI: 10.15171/ijb.1017. [Google Scholar]
- 26.Imai K, Takaoka A. Comparing antibody and small-molecule therapies for cancer. Nat Rev Cancer. 2006;6(9):714–727. doi: 10.1038/nrc1913. DOI: 10.1038/nrc1913. [DOI] [PubMed] [Google Scholar]
- 27.Li W, Ran Y, Li M, Zhang K, Qin X, Xue X, et al. Mimotope vaccination for epitope-specific induction of anti-VEGF antibodies. BMC Biotechnol. 2013;13:77–86. doi: 10.1186/1472-6750-13-77. DOI: 10.1186/1472-6750-13-77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Linnemann T, Tumenjargal S, Gellrich S, Wiesmüller K, Kaltoft K, Sterry W, et al. Mimotopes for tumor-specific T lymphocytes in human cancer determined with combinatorial peptide libraries. Eur J Immunol. 2001;31(1):156–165. doi: 10.1002/1521-4141(200101)31:1<156::aid-immu156>3.0.co;2-p. DOI: 10.1002/1521-4141(200101)31:1<156::aid-immu156>3.0.co;2-p. [DOI] [PubMed] [Google Scholar]
- 29.Suzuki S, Sakata J, Utsumi F, Sekiya R, Kajiyama H, Shibata K, et al. Efficacy of glypican-3-derived peptide vaccine therapy on the survival of patients with refractory ovarian clear cell carcinoma. Oncoimmunology. 2016;5(11):e1238542,1–8. doi: 10.1080/2162402X.2016.1238542. DOI: 10.1080/2162402X.2016.1238542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Dana N, Javanmard SH, Rafiee L. Antiangiogenic and antiproliferative effects of black pomegranate peel extract on melanoma cell line. Res Pharm Sci. 2015;10(2):117–124. PMID: 26487888. [PMC free article] [PubMed] [Google Scholar]
- 31.Sable R, Parajuli P, Jois S. Peptides, peptidomimetics, and polypeptides from marine sources: a wealth of natural sources for pharmaceutical applications. Mar Drugs. 2017;15(4):124–160. doi: 10.3390/md15040124. DOI: 10.3390/md15040124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Fosgerau K, Hoffmann T. Peptide therapeutics: current status and future directions. Drug Discov Today. 2015;20(1):122–128. doi: 10.1016/j.drudis.2014.10.003. DOI: 10.1016/j.drudis.2014.10.003. [DOI] [PubMed] [Google Scholar]
- 33.Padhi A, Sengupta M, Sengupta S, Roehm KH, Sonawane A. Antimicrobial peptides and proteins in mycobacterial therapy: current status and future prospects. Tuberculosis. 2014;94(4):363–373. doi: 10.1016/j.tube.2014.03.011. DOI: 10.1016/j.tube.2014.03.011. [DOI] [PubMed] [Google Scholar]
- 34.Eteraf-Oskouei T, Shafiee-Khamneh A, Heshmati-Afshar F, Delazar A. Anti-inflammatory and anti-angiogenesis effect of bee pollen methanolic extract using air pouch model of inflammation. Res Pharm Sci. 2020;15(1):66–75. doi: 10.4103/1735-5362.278716. DOI: 10.4103/1735-5362.278716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Pennington MW, Czerwinski A, Norton RS. Peptide therapeutics from venom: current status and potential. Bioorg Med Chem. 2018;26(10):2738–2758. doi: 10.1016/j.bmc.2017.09.029. DOI: 10.1016/j.bmc.2017.09.029. [DOI] [PubMed] [Google Scholar]
- 36.Waheed H, Moin SF, Choudhary MI. Snake venom: from deadly toxins to life-saving therapeutics. Curr Med Chem. 2017;24(17):1874–1891. doi: 10.2174/0929867324666170605091546. DOI: 10.2174/0929867324666170605091546. [DOI] [PubMed] [Google Scholar]
- 37.Siddiqua A, Khattak K, Nwaz S. Venom proteins; prospects for anticancer therapy. Pak J Biochem Mol Biol. 2019;52(2):15–26. [Google Scholar]
- 38.Światły-Błaszkiewicz A, Mrówczyńska L, Matuszewska E, Lubawy J, Urbański A, Kokot ZJ, et al. The effect of bee venom peptides melittin, tertiapin, and apamin on the human erythrocytes ghosts: a preliminary study. Metabolites. 2020;10(5):191–203. doi: 10.3390/metabo10050191. DOI: 10.3390/metabo10050191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Koburova KL, Michailova SG, Shkenderov SV. Further investigation on the antiinflammatory properties of adolapin-bee venom polypeptide. Acta Physiol Pharmacol Bulg. 1985;11(2):50–55. PMID: 2996298. [PubMed] [Google Scholar]
- 40.Chen J, Guan SM, Sun W, Fu H. Melittin, the major pain-producing substance of bee venom. Neurosci Bull. 2016;32(3):265–272. doi: 10.1007/s12264-016-0024-y. DOI: 10.1007/s12264-016-0024-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Rady I, Siddiqui IA, Rady M, Mukhtar H. Melittin, a major peptide component of bee venom, and its conjugates in cancer therapy. Cancer Lett. 2017;402:16–31. doi: 10.1016/j.canlet.2017.05.010. DOI: 10.1016/j.canlet.2017.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ma R, Mahadevappa R, Kwok HF. Venom-based peptide therapy: insights into anti-cancer mechanism. Oncotarget. 2017;8(59):100908–100930. doi: 10.18632/oncotarget.21740. DOI: 10.18632/oncotarget.21740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Li B, Lyu P, Xi X, Ge L, Mahadevappa R, Shaw C, et al. Triggering of cancer cell cycle arrest by a novel scorpion venom-derived peptide-Gonearrestide. J Cell Mol Med. 2018;22(9):4460–4473. doi: 10.1111/jcmm.13745. DOI: 10.1111/jcmm.13745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sarzaeem A, Zare MA, Moradhaseli S, Morovvati H, Lotfi M. Cytotoxic effect of ICD-85 (venom-derived peptides) on HeLa cancer cell line and normal LK cells using MTT assay. Arch Iran Med. 2012;15(11):696–701. PMID: 23102247. [PubMed] [Google Scholar]
- 45.Chung MJ. Protein p8 derived from lactic acid bacteria and its use as anti-cancer agent. Google Patents, 2019. Publication No. 20190201481. https://patents.justia.com/patent/20190201481.
- 46.Vijayakumar S, Menakha M. Pharmaceutical applications of cyanobacteria-a review. J Acute Med. 2015;5(1):15–23. DOI: 10.1016/j.jacme.2015.02.004. [Google Scholar]
- 47.Kang HK, Choi MC, Seo CH, Park Y. Therapeutic properties and biological benefits of marine-derived anticancer peptides. Int J Mol Sci. 2018;19(3):919–958. doi: 10.3390/ijms19030919. DOI: 10.3390/ijms19030919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Tareq FS, Kim JH, Lee MA, Lee HS, Lee YJ, Lee JS, et al. Ieodoglucomides A and B from a marine-derived bacterium Bacillus licheniformis. Org Lett. 2012;14(6):1464–1467. doi: 10.1021/ol300202z. DOI: 10.1021/ol300202z. [DOI] [PubMed] [Google Scholar]
- 49.Tao Y, Lin Y, She Z, Lin M, Chen P, Zhang J. Anticancer activity and mechanism investigation of beauvericin isolated from secondary metabolites of the mangrove endophytic fungi. Anticancer Agents Med Chem. 2015;15(2):258–266. doi: 10.2174/1871520614666140825112255. DOI: 10.2174/1871520614666140825112255. [DOI] [PubMed] [Google Scholar]
- 50.Braekman JC, Daloze D, Moussiaux B, Riccio R. Jaspamide from the marine sponge jaspis johnstoni. J Nat Prod. 1987;50(5):994–995. DOI: 10.1021/np50053a048. [Google Scholar]
- 51.Odaka C, Sanders ML, Crews P. Jasplakinolide induces apoptosis in various transformed cell lines by a caspase-3-like protease-dependent pathway. Clin Diagn Lab Immunol. 2000;7(6):947–952. doi: 10.1128/cdli.7.6.947-952.2000. DOI: 10.1128/cdli.7.6.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Garcia-Rocha M, Bonay P, Avila J. The antitumoral compound Kahalalide F acts on cell lysosomes. Cancer Lett. 1996;99(1):43–50. doi: 10.1016/0304-3835(95)04036-6. DOI: 10.1016/0304-3835(95)04036-6. [DOI] [PubMed] [Google Scholar]
- 53.Suárez Y, González L, Cuadrado A, Berciano M, Lafarga M, Muñoz A. Kahalalide F, a new marine-derived compound, induces oncosis in human prostate and breast cancer cells. Mol Cancer Ther. 2003;2(9):863–872. PMID: 14555705. [PubMed] [Google Scholar]
- 54.Rozek T, Wegener KL, Bowie JH, Olver IN, Carver JA, Wallace JC, et al. The antibiotic and anticancer active aurein peptides from the Australian bell frogs Litoria aurea and Litoria raniformis: the solution structure of aurein 1.2. Eur J Biochem. 2000;267(17):5330–5341. doi: 10.1046/j.1432-1327.2000.01536.x. DOI: 10.1046/j.1432-1327.2000.01536.x. [DOI] [PubMed] [Google Scholar]
- 55.Fang XY, Chen W, Fan JT, Song R, Wang L, Gu YH, et al. Plant cyclopeptide RA-V kills human breast cancer cells by inducing mitochondria-mediated apoptosis through blocking PDK1-AKT interaction. Toxicol Appl Pharmacol. 2013;267(1):95–103. doi: 10.1016/j.taap.2012.12.010. DOI: 10.1016/j.taap.2012.12.010. [DOI] [PubMed] [Google Scholar]
- 56.Hernandez-Ledesma B, Hsieh CC, Ben O. Lunasin, a novel seed peptide for cancer prevention. Peptides. 2009;30(2):426–430. doi: 10.1016/j.peptides.2008.11.002. DOI: 10.1016/j.peptides.2008.11.002. [DOI] [PubMed] [Google Scholar]
- 57.Arap W, Pasqualini R, Ruoslahti E. Cancer treatment by targeted drug delivery to tumor vasculature in a mouse model. Science. 1998;279(5349):377–380. doi: 10.1126/science.279.5349.377. DOI: 10.1126/science.279.5349.377. [DOI] [PubMed] [Google Scholar]
- 58.Pasqualini R, Ruoslahti E. Organ targeting in vivo using phage display peptide libraries. Nature. 1996;380(6572):364–366. doi: 10.1038/380364a0. DOI: 10.1038/380364a0. [DOI] [PubMed] [Google Scholar]
- 59.Koivunen E, Arap W, Valtanen H, Rainisalo A, Medina OP, Heikkilä P, et al. Tumor targeting with a selective gelatinase inhibitor. Nat Biotechnol. 1999;17(8):768–774. doi: 10.1038/11703. DOI: 10.1038/11703. [DOI] [PubMed] [Google Scholar]
- 60.Lu G, Zheng M, Zhu Y, Sha M, Wu Y, Han X. Selection of peptide inhibitor to matrix metalloproteinase-2 using phage display and its effects on pancreatic cancer cell lines PANC-1 and CFPAC-1. Int J Biol Sci. 2012;8(5):650–662. doi: 10.7150/ijbs.3897. DOI: 10.7150/ijbs.3897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Björklund M, Heikkilä P, Koivunen E. Peptide inhibition of catalytic and noncatalytic activities of matrix metalloproteinase-9 blocks tumor cell migration and invasion. J Biol Chem. 2004;279(28):29589–29597. doi: 10.1074/jbc.M401601200. DOI: 10.1074/jbc.M401601200. [DOI] [PubMed] [Google Scholar]
- 62.Arap W, Haedicke W, Bernasconi M, Kain R, Rajotte D, Krajewski S, et al. Targeting the prostate for destruction through a vascular address. Proc Natl Acad Sci U S A. 2002;99(3):1527–1531. doi: 10.1073/pnas.241655998. DOI: 10.1073/pnas.241655998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Li ZJ, Wu WKK, Ng SSM, Yu L, Li HT, Wong CCM, et al. A novel peptide specifically targeting the vasculature of orthotopic colorectal cancer for imaging detection and drug delivery. J Control release. 2010;148(3):292–302. doi: 10.1016/j.jconrel.2010.09.015. DOI: 10.1016/j.jconrel.2010.09.015. [DOI] [PubMed] [Google Scholar]
- 64.Essler M, Ruoslahti E. Molecular specialization of breast vasculature: a breast-homing phage-displayed peptide binds to aminopeptidase P in breast vasculature. Proc Natl Acad Sci U S A. 2002;99(4):2252–2257. doi: 10.1073/pnas.251687998. DOI: 10.1073/pnas.251687998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Langel Ü, Ruoslahti E, Myrberg H, Mäe M, Zhang L. Chimeric constructs between cancer-homing peptides and cell-penetrating peptides coupled to anticancer drugs and/or diagnostic agent/agents. Google Patents, 2010. Publication No. WO2007108749A8. https://patents.google.com/patent/WO2007108749A8.
- 66.Boger DL, Goldberg J, Silletti S, Kessler T, Cheresh DA. Identification of a novel class of small-molecule antiangiogenic agents through the screening of combinatorial libraries which function by inhibiting the binding and localization of proteinase MMP2 to integrin αVβ3. J Am Chem Soc. 2001;123(7):1280–1288. doi: 10.1021/ja003579+. DOI: 10.1021/ja003579+ [DOI] [PubMed] [Google Scholar]
- 67.Takahashi S. Vascular endothelial growth factor (VEGF), VEGF receptors and their inhibitors for antiangiogenic tumor therapy. Biol Pharm Bull. 2011;34(12):1785–1788. doi: 10.1248/bpb.34.1785. DOI: 10.1248/bpb.34.1785. [DOI] [PubMed] [Google Scholar]
- 68.Binétruy-Tournaire R, Demangel C, Malavaud B, Vassy R, Rouyre S, Kraemer M, et al. Identification of a peptide blocking vascular endothelial growth factor (VEGF)-mediated angiogenesis. EMBO J. 2000;19(7):1525–1533. doi: 10.1093/emboj/19.7.1525. DOI: 10.1093/emboj/19.7.1525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.An P, Lei H, Zhang J, Song S, He L, Jin G, et al. Suppression of tumor growth and metastasis by a VEGFR-1 antagonizing peptide identified from a phage display library. Int J Cancer. 2004;111(2):165–173. doi: 10.1002/ijc.20214. DOI: 10.1002/ijc.20214. [DOI] [PubMed] [Google Scholar]
- 70.Hamzeh-Mivehroud M, Dastmalchi S. Identification of new peptide ligands for epidermal growth factor receptor using phage display technology. Res Pharm Sci. 2012;7(5):S452. doi: 10.1111/j.1747-0285.2011.01282.x. [DOI] [PubMed] [Google Scholar]
- 71.Pasqualini R, Koivunen E, Kain R, Lahdenranta J, Sakamoto M, Stryhn A, et al. Aminopeptidase N is a receptor for tumor-homing peptides and a target for inhibiting angiogenesis. Cancer Res. 2000;60(3):722–727. PMID: 10676659. [PMC free article] [PubMed] [Google Scholar]
- 72.Curnis F, Sacchi A, Borgna L, Magni F, Gasparri A, Corti A. Enhancement of tumor necrosis factor a antitumor immunotherapeutic properties by targeted delivery to aminopeptidase N (CD13) Nat Biotechnol. 2000;18(11):1185–1190. doi: 10.1038/81183. DOI: 10.1038/81183. [DOI] [PubMed] [Google Scholar]
- 73.Sacchi A, Gasparri A, Gallo-Stampino C, Toma S, Curnis F, Corti A. Synergistic antitumor activity of cisplatin, paclitaxel, and gemcitabine with tumor vasculature-targeted tumor necrosis factor-a. Clin Cancer Res. 2006;12(1):175–182. doi: 10.1158/1078-0432.CCR-05-1147. DOI: 10.1158/1078-0432.CCR-05-1147. [DOI] [PubMed] [Google Scholar]
- 74.Hui X, Han Y, Liang S, Liu Z, Liu J, Hong L, et al. Specific targeting of the vasculature of gastric cancer by a new tumor-homing peptide CGNSNPKSC. J Control Release. 2008;131(2):86–93. doi: 10.1016/j.jconrel.2008.07.024. DOI: 10.1016/j.jconrel.2008.07.024. [DOI] [PubMed] [Google Scholar]
- 75.Mueller J, Gaertner FC, Blechert B, Janssen KP, Essler M. Targeting of tumor blood vessels: a phage-displayed tumor-homing peptide specifically binds to matrix metalloproteinase-2-processed collagen IV and blocks angiogenesis in vivo. Mol Cancer Res. 2009;7(7):1078–1085. doi: 10.1158/1541-7786.MCR-08-0538. DOI: 10.1158/1541-7786.MCR-08-0538. [DOI] [PubMed] [Google Scholar]
- 76.Sugahara KN, Teesalu T, Karmali PP, Kotamraju VR, Agemy L, Girard OM, et al. Tissue-penetrating delivery of compounds and nanoparticles into tumors. Cancer Cell. 2009;16(6):510–520. doi: 10.1016/j.ccr.2009.10.013. DOI: 10.1016/j.ccr.2009.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Lee TY, Lin CT, Kuo SY, Chang DK, Wu HC. Peptide-mediated targeting to tumor blood vessels of lung cancer for drug delivery. Cancer Res. 2007;67(22):10958–10965. doi: 10.1158/0008-5472.CAN-07-2233. DOI: 10.1158/0008-5472. [DOI] [PubMed] [Google Scholar]
- 78.Marchiò S, Lahdenranta J, Schlingemann RO, Valdembri D, Wesseling P, Arap MA, et al. Aminopeptidase A is a functional target in angiogenic blood vessels. Cancer Cell. 2004;5(2):151–162. doi: 10.1016/s1535-6108(04)00025-x. DOI: 10.1016/s1535-6108(04)00025-x. [DOI] [PubMed] [Google Scholar]
- 79.Iwamoto Y, Robey FA, Graf J, Sasaki M, Kleinman HK, Yamada Y, et al. YIGSR, a synthetic laminin pentapeptide, inhibits experimental metastasis formation. Science. 1987;238(4830):1132–1134. doi: 10.1126/science.2961059. DOI: 10.1126/science.2961059. [DOI] [PubMed] [Google Scholar]
- 80.Sakamoto N, Iwahana M, Tanaka NG, Osada Y. Inhibition of angiogenesis and tumor growth by a synthetic laminin peptide, CDPGYIGSR-NH2. Cancer Res. 1991;51(3):903–906. PMID: 1703042. [PubMed] [Google Scholar]
- 81.Hariharan S, Gustafson D, Holden S, McConkey D, Davis D, Morrow M, et al. Assessment of the biological and pharmacological effects of the ανβ3 and ανβ5 integrin receptor antagonist, cilengitide (EMD 121974), in patients with advanced solid tumors. Ann Oncol. 2007;18(8):1400–1407. doi: 10.1093/annonc/mdm140. DOI: 10.1093/annonc/mdm140. [DOI] [PubMed] [Google Scholar]
- 82.Noy A, Scadden DT, Lee J, Dezube BJ, Aboulafia D, Tulpule A, et al. Angiogenesis inhibitor IM862 is ineffective against AIDS-Kaposi’s sarcoma in a phase III trial, but demonstrates sustained, potent effect of highly active antiretroviral therapy: from the AIDS malignancy consortium and IM862 study team. J Clin Oncol. 2005;23(5):990–998. doi: 10.1200/JCO.2005.11.043. DOI: 10.1200/JCO.2005.11.043. [DOI] [PubMed] [Google Scholar]
- 83.Letsch M, Schally AV, Busto R, Bajo AM, Varga JL. Growth hormone-releasing hormone (GHRH) antagonists inhibit the proliferation of androgen-dependent and-independent prostate cancers. Proc Natl Acad Sci U S A. 2003;100(3):1250–1255. doi: 10.1073/pnas.0337496100. DOI: 10.1073/pnas.0337496100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Nasarre C, Roth M, Jacob L, Roth L, Koncina E, Thien A, et al. Peptide-based interference of the transmembrane domain of neuropilin-1 inhibits glioma growth in vivo. Oncogene. 2010;29(16):2381–2392. doi: 10.1038/onc.2010.9. DOI: 10.1038/onc.2010.9. [DOI] [PubMed] [Google Scholar]
- 85.Khalili P, Arakelian A, Chen G, Plunkett ML, Beck I, Parry GC, et al. A non-RGD-based integrin binding peptide (ATN-161) blocks breast cancer growth and metastasis in vivo. Mol Cancer Ther. 2006;5(9):2271–2280. doi: 10.1158/1535-7163.MCT-06-0100. DOI: 10.1158/1535-7163.MCT-06-0100. [DOI] [PubMed] [Google Scholar]
- 86.Cianfrocca ME, Kimmel KA, Gallo J, Cardoso T, Brown MM, Hudes G, et al. Phase 1 trial of the antiangiogenic peptide ATN-161 (Ac-PHSCN-NH(2)), a beta integrin antagonist, in patients with solid tumours. Br J Cancer. 2006;94(11):1621–1626. doi: 10.1038/sj.bjc.6603171. DOI: 10.1038/sj.bjc.6603171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Ellert-Miklaszewska A, Poleszak K, Kaminska B. Short peptides interfering with signaling pathways as new therapeutic tools for cancer treatment. Future Med Chem. 2017;9(2):199–221. doi: 10.4155/fmc-2016-0189. DOI: 10.4155/fmc-2016-0189. [DOI] [PubMed] [Google Scholar]
- 88.Zarrabi K, Dufour A, Li J, Kuscu C, Pulkoski-Gross A, Zhi J, et al. Inhibition of matrix metalloproteinase 14 (MMP-14)-mediated cancer cell migration. J Biol Chem. 2011;286(38):33167–33177. doi: 10.1074/jbc.M111.256644. DOI: 10.1074/jbc.M111.256644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Ingangi V, Bifulco K, Yousif AM, Ragone C, Motti ML, Rea D, et al. The urokinase receptor-derived cyclic peptide [SRSRY] suppresses neovascularization and intravasation of osteosarcoma and chondrosarcoma cells. Oncotarget. 2016;7(34):54474–54487. doi: 10.18632/oncotarget.9976. DOI: 10.18632/oncotarget.9976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Finlayson M. Modulation of CD44 activity by A6-peptide. Front Immunol. 2015;6:135–142. doi: 10.3389/fimmu.2015.00135. DOI: 10.3389/fimmu.2015.00135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Berkenblit A, Matulonis UA, Kroener JF, Dezube BJ, Lam GN, Cuasay LC, et al. Å6, a urokinase plasminogen activator (uPA)-derived peptide in patients with advanced gynecologic cancer: a phase I trial. Gynecol Oncol. 2005;99(1):50–57. doi: 10.1016/j.ygyno.2005.05.023. DOI: 10.1016/j.ygyno.2005.05.023. [DOI] [PubMed] [Google Scholar]
- 92.Gold MA, Brady WE, Lankes HA, Rose PG, Kelley JL, De Geest K, et al. A phase II study of a urokinase-derived peptide (A6) in the treatment of persistent or recurrent epithelial ovarian, fallopian tube, or primary peritoneal carcinoma: a Gynecologic Oncology Group study. Gynecol Oncol. 2012;125(3):635–639. doi: 10.1016/j.ygyno.2012.03.023. DOI: 10.1016/j.ygyno.2012.03.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Vicari AP, Caux C. Chemokines in cancer. Cytokine Growth Factor Rev. 2002;13(2):143–154. doi: 10.1016/s1359-6101(01)00033-8. DOI: 10.1016/s1359-6101(01)00033-8. [DOI] [PubMed] [Google Scholar]
- 94.Huang EH, Singh B, Cristofanilli M, Gelovani J, Wei C, Vincent L, et al. A CXCR4 antagonist CTCE-9908 inhibits primary tumor growth and metastasis of breast cancer. J Surg Res. 2009;155(2):231–236. doi: 10.1016/j.jss.2008.06.044. DOI: 10.1016/j.jss.2008.06.044. [DOI] [PubMed] [Google Scholar]
- 95.Hotte S, Hirte H, Iacobucci A, Wong D, Cantin L, Korz W, et al. Phase I/II study of CTCE-9908, a novel anticancer agent that inhibits CXCR4, in patients with advanced solid cancers. Mol Cancer Ther. 2007;6(11):A153. [Google Scholar]
- 96.Goodarzi A, Yari F, Mohammadipour M, Deyhim MR, Timori Naghadeh H. Capability of platelet factor 4 to induce apoptosis in the cancerous cell lines in vitro. Int J Med Lab. 2018;5(3):195–207. [Google Scholar]
- 97.Valdivia-Silva J, Medina-Tamayo J, Garcia-Zepeda EA. Chemokine-derived peptides: novel antimicrobial and antineoplasic agents. Int J Mol Sci. 2015;16(6):12958–12985. doi: 10.3390/ijms160612958. DOI: 10.3390/ijms160612958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Vandercappellen J, Liekens S, Bronckaers A, Noppen S, Ronsse I, Dillen C, et al. The COOH-terminal peptide of platelet factor-4 variant (CXCL4L1/PF-4var47-70) strongly inhibits angiogenesis and suppresses B16 melanoma growth in vivo. Mol Cancer Res. 2010;8(3):322–334. doi: 10.1158/1541-7786.MCR-09-0176. DOI: 10.1158/1541-7786.MCR-09-0176. [DOI] [PubMed] [Google Scholar]
- 99.Robinson SC, Scott KA, Wilson JL, Thompson RG, Proudfoot AEI, Balkwill FR. A chemokine receptor antagonist inhibits experimental breast tumor growth. Cancer Res. 2003;63(23):8360–8365. PMID: 14678997. [PubMed] [Google Scholar]
- 100.Moreno P, Ramos-Álvarez I, Moody TW, Jensen RT. Bombesin related peptides/receptors and their promising therapeutic roles in cancer imaging, targeting and treatment. Expert Opin Ther Targets. 2016;20(9):1055–1073. doi: 10.1517/14728222.2016.1164694. DOI: 10.1517/14728222.2016.1164694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Kahán Z, Sun B, Schally AV, Arencibia JM, Cai R, Groot K, et al. Inhibition of growth of MDA-MB-468 estrogen-independent human breast carcinoma by bombesin/gastrin-releasing peptide antagonists RC-3095 and RC-3940-II. Cancer. 2000;88(6):1384–1392. doi: 10.1002/(sici)1097-0142(20000315)88:6<1384::aid-cncr16>3.0.co;2-q. DOI: 10.1002/(sici)1097-0142(20000315)88: 6<1384::aid-cncr16>3.0.co;2-q. [DOI] [PubMed] [Google Scholar]
- 102.de Oliveira MS, Cechim G, Braganhol E, Santos DG, Meurer L, de Castro CG, et al. Anti-proliferative effect of the gastrin-release peptide receptor antagonist RC-3095 plus temozolomide in experimental glioblastoma models. J Neurooncol. 2009;93(2):191–201. doi: 10.1007/s11060-008-9775-2. DOI: 10.1007/s11060-008-9775-2. [DOI] [PubMed] [Google Scholar]
- 103.Hruby VJ. Prospects for peptidomimetic drug design. Drug Discov Today. 1997;2(5):165–167. DOI: 10.1016/S1359-6446(96)20009-1. [Google Scholar]
- 104.Rosca EV, Koskimaki JE, Rivera CG, Pandey NB, Tamiz AP, Popel AS. Anti-angiogenic peptides for cancer therapeutics. Curr Pharm Biotechnol. 2011;12(8):1101–1116. doi: 10.2174/138920111796117300. DOI: 10.2174/138920111796117300. [DOI] [PMC free article] [PubMed] [Google Scholar]


