Abstract
Background and purpose:
Stachys pilifera is used in traditional medicine due to its antioxidant, anti-inflammatory, and antimicrobial effects. The goal of this study was to examine the renoprotective activity of the hydroalcoholic extract of aerial parts of S. pilifera on paracetamol (PCM)-induced nephrotoxicity.
Experimental approach:
The Wistar female rats were randomly divided into four groups including control, PCM, S. pilifera hydroalcoholic extract (SPE), and PCM + SPE. The animals received SPE (500 mg/kg) for one week and PCM (3 g/kg) on the 6th day orally. Kidney function tests and oxidant/antioxidant markers were determined in serum and tissue homogenate, respectively. Protein and mRNA levels of TNF-α, as well as hematoxylin and eosin staining, were assessed in the kidney tissue.
Findings/Results:
Treatment with SPE in the PCM group significantly decreased blood urea nitrogen and creatinine against the merely PCM rats (P < 0.05). The amount of nitric oxide metabolite and superoxide dismutase activity in the group receiving SPE showed a significant increase compared to PCM rats (P < 0.05). A significant difference in TNF-α levels between the groups was not observed. Histological changes were improved in the rats treated with SPE.
Conclusion and implications:
Totally, our findings showed that SPE can inhibit PCM nephrotoxicity by enhancing kidney function markers, antioxidant status, and histological changes. Though, more researches are required to estimate the possible mechanism of SPE.
Keywords: Antioxidant, Nephrotoxicity, Paracetamol, Stachys pilifera
INTRODUCTION
The kidneys are dynamic organs that are exposed to toxic damage due to their unique biochemical and physiological properties (1). Paracetamol (PCM, N-acetyl-para-aminophenol) or acetaminophen is a drug used as a painkiller and to control fever. It is safe in common doses, but overdose can induce hepatotoxicity and lead to nephrotoxicity if it progresses (2). However, acute kidney damage can also occur in the absence of liver damage and lead to death in humans and animals (3). Renal impairment is seen in 1 to 2% of patients receiving high-dose of PCM (4). The liver, kidneys, and intestines are the most important organs involved in PCM metabolism (5). Following a therapeutic dose, PCM is inactivated mainly by conjugation with glucuronide and sulfate, and a small amount of it is oxidized to the N-acetyl-p-benzoquinone-imine (NAPQI) metabolite. This reactive metabolite is detoxified through glutathione (GSH), but in case of an overdose of PCM, the amount of NAPQI increases and causes depletion of GSH stores, oxidative stress, and eventually hepatorenal injury (6).
In addition, PCM is converted to the nephrotoxic metabolite para-aminophenol by deacetylation in the kidneys, which causes necrosis of the renal cortex (4). Because oxidative stress is involved in the progress of kidney-liver damage caused by PCM, the use of natural compounds with antioxidant properties has been considered (5). Today, the renal protection effects of medicinal plants on PCM-induced nephrotoxicity have been proven (3).
The genus Stachys contains 300 species, which is one of the largest members of the Lamiaceae family and is distributed in tropical and subtropical regions (7). Thirty-four species of this genus are known in different regions of Iran, one of which is Stachys pilifera. Benth (8). In traditional medicine, several species of Stachys have been used as antinephritis, antidiarrhea, wound disinfectant, and anti-inflammation (9). In addition, the antibacterial, antioxidant, and cytotoxic properties of some species of Stachys have been proven (7). Flavonoids, iridoids, diterpenoids, and phenolic acids are secondary metabolites of various species of this genus (10). Also in our previous study, the antioxidant and hepatoprotective activity of the hydroalcoholic extract of this plant were observed in PCM-induced liver damage (11). The purpose of the current study was to evaluate the protective impact of Stachys pilifera hydroalcoholic extract (SPE) on PCM-induced nephrotoxicity in female rats.
MATERIAL AND METHODS
Preparation of the S. pilifera extract
S. pilifera plant was gathered in Yasuj, Iran, and identified by Dr. Jafari, from the Department of Botany, Yasuj University. A proof specimen (Voucher No. 1897) was deposited in the herbarium of the Department of Botany, Yasuj University, Kohgilouyeh, and Boyerahmad province, Yasuj, Iran. The dried aerial parts of S. pilifera were kept for 48 h at 25 °C in 70% ethanol. Then, the extract was filtered and the remnant was re-extracted with fresh ethanol (50%) for 24 h. The extract solution was concerted under condensed pressure at approximately 50 °C. The extraction yield of solid SPE was 20%.
Animals
This study used Wistar female rats that were approximately 200-250 g (adults, 8-week old). The rats were acquired from Shahrekord University of Medical Sciences, Shahrekord, Iran. The animals were maintained in a room under a controlled temperature (24 ± 2 °C) and were maintained in a 12/12-h light/dark cycle with free access to a normal diet and water to fed and drink ad libitum. All procedures used in the current study were carried out based on the “Principles of Laboratory Animal Care” (NIH Publication No. 86-23) and approved by the Ethics Committee of Yasuj University of Medical Sciences, Yasuj, Iran (Ethical code: IR.YUMS.REC.1398.012). All efforts were made to reduce animals from suffering and minimize the number of animals used in the study.
Experimental protocol
Twenty-four rats were randomly divided into four groups as follows: group I (the control): orally distilled water (vehicle) for one week; group II (PCM group): orally distilled water for one week and on the 6th day PCM (3 g/kg) (12,13); group III (SPE): orally ethanolic SPE (500 mg/kg) (8)for one week; and group IV (PCM + SPE): orally SPE (500 mg/kg) for one week and PCM (3 g/kg) on the 6th day. PCM was provided from Sigma Chemical Co (St Louis, MO, USA).
Specimen collection and biochemical assays
Twenty-four h after the administration of PCM, blood specimens were gathered from the cardiac puncture under ethyl ether anesthesia. They were centrifuged at 3500 g for 10 min and the serum was obtained for the assessment of biochemical parameters. After killing the rats, both kidneys were removed, washed entirely with ice-cold saline. One of them was kept in 10% formalin solution for histopathological analysis and the other was homogenized (10%, w/v) in phosphate-buffered saline (PBS; 10 mmol/l, pH 7.4) for biochemical analyses. Kidney homogenates were centrifuged at 10000 g for 5 min at 4 °C to determine nitric oxide (NO) metabolite, ferric reducing antioxidant power (FRAP), total thiols (T-SH), tumor necrosis factor alpha (TNF-α), and antioxidant enzymes including GSH peroxidase (GPX), catalase (CAT), and superoxide dismutase (SOD).
Serum levels of urea and creatinine were evaluated using commercial kits (Pars Azmoon, Iran). The T-SH content was determined using the spectrophotometric method (14). Benzie and Strain procedure was applied to measure FRAP (14). Tissue NO metabolite (nitrite) was measured as indices of NO production according to the Griess reaction (15). Renal tissue homogenate was examined for the protein level of TNF-α according to the manufacturer’s guidelines (ELISA kit, Kermania pars Gene, Kerman, Iran). For the determination of antioxidant enzymes activity (SOD, CAT, and GPX) in tissue homogenate, a routine kit (Zell Bio GmbH, Ulm, Germany) was used based on the manufacturer’s procedure.
To explore mRNA TNF-α, the total RNA was extracted from kidney homogenate (RNX Plus kit, Sinaclon, Tehran, Iran) in line with the manufacturer’s guidelines. First-strand complementary DNA (cDNA) was synthesized (cDNA Synthesis kit, Sinaclon, Tehran, Iran), and real-time polymerase chain reaction (RT-PCR) was done (Rotor Gene 3000 instrument, Bio-Rad, USA). The specific primer sequences were as follows: TNF-α and GAPDH (Table 1). PCR program was including the denaturation stage (95 °C for 15 S), annealing stage (62 °C for 30 S), and elongation stage (72 °C for 30 S) in 40 cycles. The relative mRNA expression was calculated by the 2-ΔΔCt formula.
Table 1.
The sequences of the primers for TNF-α and GAPDH.
| Forward (5’ to 3’) | Reverse (5’ to 3’) | |
|---|---|---|
| TNF-α | TGAGCACAGAAAGCATGATC | CATCTGCTGGTACCACCAGTT |
| GAPDH | AGTTCAACGGCACAGTCAAGG | AGACTCCACGACATACTCAGC |
Histological assessment
For light microscopic evaluation, kidney tissue portions were sectioned, fixed (in 10% formalin), dehydrated, and embedded. These samples were sliced into 5-mm thick segments, stained with hematoxylin & eosin, and observed under a light microscope by a pathologist who was blinded to the groups.
Statistical analysis
SPSS version 18 software was used to analyze the data. ANOVA followed by Tukey’s tests were utilized to detect differences between groups. The values were reported as mean ± SEM. P values < 0.05 were considered as a statistically noteworthy variation.
RESULTS
Biochemical markers
The results of this study demonstrated that serum urea and creatinine levels in the PCM group were considerably increased in comparison to the healthy rats. Treatment of PCM group with 500 mg/kg of SPE markedly decreased urea and creatinine levels compared to the merely PCM rats (Fig. 1A and B).
Fig. 1.
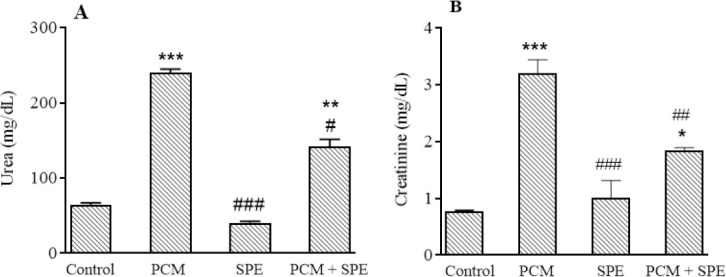
Impact of SPE (500 mg/kg) on (A) serum urea and creatinine (B) amounts in nephrotoxicity caused by PCM. Data are expressed as mean ± SEM. *P ≤ 0.05, **P ≤ 0.01, and ***P ≤ 0.001 indicate significant differences compared to the control group; #P ≤ 0.05, ##P ≤ 0.01, and ###P ≤ 0.001 against the PCM group. PCM, Paracetamol, SPE, Stachys pilifera ethanolic extract.
TNF-α level
The renal tissue levels and mRNA expression of TNF-α slightly increased in the merely PCM rats against the control group but were not significant. Administration of SPE had no significant change on protein and mRNA levels of TNF-α in comparison to the PCM-treated animals (Fig. 2).
Fig. 2.
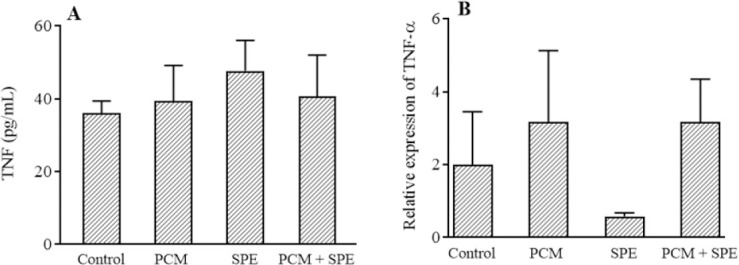
The impact of SPE (500 mg/kg) on (A) the protein and (B) mRNA expression of TNF-α in nephrotoxicity caused by PCM. Data are expressed as mean ± SEM. PCM, Paracetamol; SPE, Stachys pilifera ethanolic extract; TNF, tumor necrosis factor alpha.
Oxidative stress parameters
As shown in Fig. 3, PCM-induced rats demonstrated a remarkable increment in FRAP level and a marked reduction in T-SH level in comparison to the control rats. In the PCM + SPE rats, FRAP and NO metabolite amounts were significantly increased, although, administration of SPE insignificantly increased T-SH content in contrast to the PCM group.
Fig. 3.
The effect of SPE (500 mg/kg) on oxidative stress parameters; (A) FRAP, (B) T-SH, (C) NO metabolite in nephrotoxicity caused by PCM. Data are expressed as mean ± SEM. *P ≤ 0.05 indicates significant differences compared to the control group; #P ≤ 0.05 against the PCM group. PCM, Paracetamol, SPE, Stachys pilifera ethanolic extract; FRAP, ferric reducing antioxidant power; T-SH, total thiols; NO, nitric oxide.
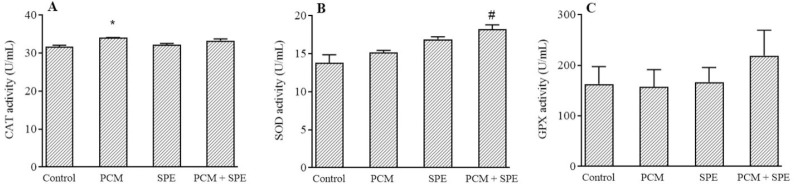
Antioxidant enzymes evaluation
PCM significantly increased CAT activity in comparison to the control rats. Moreover, SOD activity was significantly augmented in the PCM + SPE group in contrast to PCM rats. However, the use of SPE had no considerable effect on the CAT and GPX enzymes activity. (Fig. 4).
Fig. 4.
The impact of SPE (500 mg/kg) on the activity of the antioxidant enzymes of (A) CAT, (B) SOD, and (C) GPX in nephrotoxicity caused by PCM. Data are expressed as mean ± SEM. *P ≤ 0.05 indicates significant differences compared to the control group; #P ≤ 0.05 against the PCM group. PCM, Paracetamol, SPE, Stachys pilifera ethanolic extract; CAT, catalase; SOD, superoxide dismutase; GPX, glutathione peroxidase.
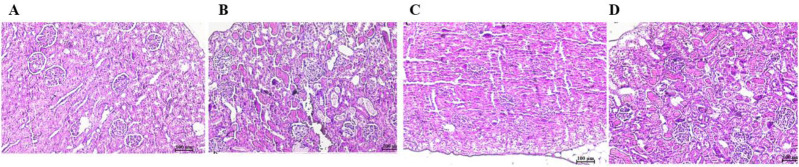
Histological observation of kidney
Histological examination of renal tissue demonstrated that PCM causes cell necrosis and inflammation in renal glomeruli. White blood cells granulation was shown in the PCM rats. However, it was observed that the SPE could decrease these injuries (Fig. 5).
Fig. 5.
Histological examination of kidney tissues. (A) Control group; (B) paracetamol; (C); Stachys pilifera ethanolic extract; and (D) Stachys pilifera ethanolic extract + paracetamol.
DISCUSSION
PCM is one of the most effective analgesics and antipyretics belonging to the class of para-aminophenol and nonsteroidal anti-inflammatory drugs (16). Although safe in therapeutic doses, overdose is the leading cause of liver damage among all drug toxicities (17). PCM-induced renal toxicity is mainly manifested after liver injury, but renal impairment in the absence of liver damage can also occur (1). Studies have shown that PCM-induced renal toxicity leads to acute kidney injury and death in experimental animals (18). Previous studies have demonstrated the renal-protection effects of medicinal plants on PCM-induced nephrotoxicity. In most of these studies, a dose of PCM in the range of 400 to 2000 mg/kg was used orally or intraperitoneally (3,19). Since plants belonging to the genus Stachys contain compounds such as flavonoids and phenolic acids, they can have an antioxidant role (9,10). Phenolic groups react with reactive oxygen species (ROS) as hydrogen donors and neutralize them (20). Therefore, this study was performed to evaluate the impact of SPE on renal injury caused by PCM in rats.
Damage caused by drugs such as PCM in the kidney is characterized by tubular necrosis followed by crystalline nephropathy, uremia, glomerular hemodynamics, and inflammation (21). Increased serum levels of urea and creatinine are important diagnostic indicators for renal dysfunction because, in kidney damage, the rate of production of these substances increases relative to the rate of their clearance (22). The findings of the current study indicated that following the PCM administration (3 g/kg), serum urea and creatinine amounts increased significantly in comparison to the control group, which could indicate kidney tissue damage. These results were consistent with the findings of our previous study (13). Similar results were obtained in the previous studies by ingestion of 2 g/kg PCM (23). We observed that urea and creatinine levels were remarkably reduced in the group receiving SPE at a dose of 500 mg/kg. In accordance with the finding of the present study, Sadeghi et al. showed that ethanolic extract of SPE at a dose of 500 mg/kg reduced urea and creatinine in cisplatin-induced renal damage (8). Therefore, it can be said that SPE is effective in preventing the progression of PCM-induced kidney damage.
As mentioned, the oxidative stress following the use of PCM can lead to severe kidney damage (3). Oxidative stress manifests itself as an imbalance between the production of oxidants, including ROS, and antioxidant compounds, leading to oxidative damage to proteins, lipids, nucleic acids, and ultimately destruction of tissue integrity (24). In our body, the antioxidant defense system traps ROS to inhibit oxidative stress (25). Among various antioxidant markers, SOD and CAT act as the chief enzymes in the elimination of ROS (25). In the present study, according to our previous investigation, consumption of PCM increased CAT enzyme activity (13). Although this change may be unexpected based on other studies (17,18), one study suggested that an increase in CAT could be due to the overproduction of free radicals (26). Thus, an augmentation in CAT in the present study is probably a compensatory response to an increase in free radical production after PCM. The use of SPE had no effect on CAT activity, although it significantly increased SOD activity. SOD is the main enzyme in reducing free radicals, including superoxide anions (11). Intracellular oxidative stress begins with the formation of superoxide, which is converted to oxygen and hydrogen peroxide by cytosolic or mitochondrial SOD (27). Semnani et al. showed that some Stachys species have antioxidant activity due to the reduction of superoxide anions (28). In a study performed by Sadeghi et al. it was observed that SPE with its scavenging properties has an antioxidant role against cisplatin-induced nephrotoxicity (8). Therefore, in the present study, increasing SOD activity in the SPE group may play a protective role against PCM-induced renal impairment through free radical scavenging activity and removal of superoxide anions.
T-SH groups of proteins are sensitive oxidative markers that are involved in the antioxidant defense system. T-SH contains protein thiol groups and GSH (29). Consistent with our previous study (13), in this work, it was observed that in rats treated with PCM, T-SH levels were significantly reduced, which could be due to GSH depletion by NAPQI. Similar to our previous study (8,11)T-SH level enlarged in the SPE group in contrast to the PCM group. The FRAP method is a sensitive test for measuring the antioxidant power of biological fluids (30). In this study, it was observed that FRAP levels increased in both PCM and SPE-treated groups. Nitric oxide as an endogenous vasodilator is involved in the physiology and normal blood flow of the kidney and has been shown to reduce kidney damage in renal disease (31). In the present study, the amount of NO metabolite was slightly decreased in the PCM rats in contrast to the control group. Nagappan et al. observed that NO was reduced in indomethacin-induced renal injury via inhibiting NO synthase enzyme activity (32). Meng et al. showed that a decrease in NO in rats with urinary tract obstruction promotes the development of fibrosis in the renal tubules. However, they observed that consumption of Astragalus membranaceus and Angelica sinensis was able to increase NO levels by scavenging ROS and keeping NO (33). Therefore, in this study, it was suggested that the increase in NO metabolite following the injection of SPE is due to the trapping of free radicals by this plant.
Inflammation has been shown to be involved in the pathogenesis of PCM-induced renal damage (34). The nuclear factor kappa-B (NF-κB) is a transcription factor that regulates immune responses and inflammatory diseases in many tissues (35). The NF-κB pathway, which is involved in the activation of proinflammatory cytokines such as interleukine-6, TNF-α, and interleukine-1β, is activated following oxidative stress and stimulates transcription of these cytokines (6). In this work, TNF-α levels were measured to evaluate the inflammatory process in the kidney. It was observed that PCM consumption insignificantly increased TNF-α. In agreement with this study, Ozatik et al observed an increase in TNF-α in PCM-induced nephrotoxicity (4). We observed that SP extract had no significant effect on TNF-α levels.
CONCLUSION
Briefly, in this study, it was observed that the toxic dose of PCM can induce kidney damage. It has been speculated that the SPE may inhibit the development of nephrotoxicity due to its antioxidant properties as well as the improvement of biochemical and histological parameters. Nevertheless, more studies are needed in the future to show the exact mechanism of the impact of SPE on renal injury caused by PCM.
Conflict of interest statement
All authors declared no conflict of interest in this study.
Authors’ contribution
M.R. Rabani and H. Sadeghi designed the study, N. Azarmehr, Z. Moslemi, and H. Amini-Khoei performed the study, as well as A.H. Doustimotlagh analyzed the data and wrote the manuscript.
Acknowledgements
This study was financially supported by the Vice-Chancellor for Research of Yasuj University of Medical Sciences, Yasuj, I.R. Iran through Grant No. 960393).
REFERENCES
- 1.Dokumacioglu E, Iskender H, Aktaş MS, Hanedan B, Dokumacioglu A, Mazlum Şen T, et al. The effect of sulforaphane on the levels of serum cystatin-c in acetaminophen-induced nephrotoxicity in rats. Dicle Med J. 2016;43(3):383–389. DOI: 10.5798/diclemedj.0921.2016.03.0701. [Google Scholar]
- 2.Li C, Liu J, Saavedra JE, Keefer LK, Waalkes MP. The nitric oxide donor, V-PYRRO/NO, protects against acetaminophen-induced nephrotoxicity in mice. Toxicology. 2003;189(3):173–180. doi: 10.1016/s0300-483x(03)00129-x. DOI: 10.1016/s0300-483x(03)00129-x. [DOI] [PubMed] [Google Scholar]
- 3.Chinnappan SM, George A, Thaggikuppe P, Choudhary Y, Choudhary VK, Ramani Y, et al. Nephroprotective effect of herbal extract Eurycoma longifolia on paracetamol-induced nephrotoxicity in rats. Evid Based Complement Alternat Med 2019. 2019:1–6. doi: 10.1155/2019/4916519. 4916519. DOI: 10.1155/2019/4916519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ozatik FY, Teksen Y, Kadioglu E, Ozatik O, Bayat Z. Effects of hydrogen sulfide on acetaminophen-induced acute renal toxicity in rats. Int Urol Nephrol. 2019;51(4):745–754. doi: 10.1007/s11255-018-2053-0. DOI: 10.1007/s11255-018-2053-0. [DOI] [PubMed] [Google Scholar]
- 5.Ozioko OM, Ozioko US, Mba CE, Atuadu V, Egwuatu IA, Okoro A. Curative effect of aqueous leaf extract of Solanum macrocarpon on acetaminophen induced nephrotoxicity on adult wistar rats. World J Pharm Res. 2020;9(6):158–172. DOI: 10.20959/wjpr20206-17650. [Google Scholar]
- 6.Eraky SM, El-Magd NFA. Omega-3 fatty acids protect against acetaminophen-induced hepatic and renal toxicity in rats through HO-1-Nrf2-BACH1 pathway. Arch. Biochem Biophys. 2020;687:108387. doi: 10.1016/j.abb.2020.108387. DOI: 10.1016/j.abb.2020.108387. [DOI] [PubMed] [Google Scholar]
- 7.Kokhdan EP, Sadeghi H, Ghafoori H, Sadeghi H, Danaei N, Javadian H, et al. Cytotoxic effect of methanolic extract, alkaloid and terpenoid fractions of Stachys pilifera against HT-29 cell line. Res Pharm Sci. 2018;13(5):404–412. doi: 10.4103/1735-5362.236833. DOI: 10.4103/1735-5362.236833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sadeghi H, Mansourian M, Panahi kokhdan E, Salehpour Z, Sadati I, Abbaszadeh-Goudarzi K, et al. Antioxidant and protective effect of Stachys pilifera Benth against nephrotoxicity induced by cisplatin in rats. J Food Biochem. 2020;44(5):e13190. doi: 10.1111/jfbc.13190. DOI: 10.1111/jfbc.13190. [DOI] [PubMed] [Google Scholar]
- 9.Panahi Kokhdan E, Ahmadi K, Sadeghi H, Sadeghi H, Dadgary F, Danaei N, et al. Hepatoprotective effect of Stachys pilifera ethanol extract in carbon tetrachloride-induce hepatotoxicity in rats. Pharm Biol. 2017;55(1):1389–1393. doi: 10.1080/13880209.2017.1302484. DOI: 10.1080/13880209.2017.1302484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Garjani A, Maleki N, Nazemieh H. Effects of hydroalcoholic extract from aerial parts of the sterile stems of Stachys inflata on myocardial infarct size in rats. Iran J Pharm Sci. 2004;3(3):165–170. DOI: 10.22037/IJPR.2010.595. [Google Scholar]
- 11.Mansourian M, Mirzaei A, Azarmehr N, Vakilpour H, Kokhdan EP, Doustimotlagh AH. Hepatoprotective and antioxidant activity of hydroalcoholic extract of Stachys pilifera. Benth on acetaminophen-induced liver toxicity in male rats. Heliyon. 2019;5(12):1–5. doi: 10.1016/j.heliyon.2019.e03029. e03029. DOI: 10.1016/j.heliyon.2019.e03029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cristani M, Speciale A, Mancari F, Arcoraci T, Ferrari D, Fratantonio D, et al. Protective activity of an anthocyanin-rich extract from bilberries and blackcurrants on acute acetaminophen-induced hepatotoxicity in rats. Nat Prod Res. 2016;30(24):2845–2849. doi: 10.1080/14786419.2016.1160235. DOI: 10.1080/14786419.2016.1160235. [DOI] [PubMed] [Google Scholar]
- 13.Ansari S, Azarmehr N, Barmoudeh Z, Moslemi Z, Ghahremani H, Mirzaei A, et al. Evaluation of the protective potential of hydroalcoholic extract of Thymus daenensis on acetaminophen-induced nephrotoxicity in rats. Heliyon. 2020;6(5):e03898. doi: 10.1016/j.heliyon.2020.e03898. DOI: 10.1016/j.heliyon.2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sadeghi A, Bastin AR, Ghahremani H, Doustimotlagh AH. The effects of rosmarinic acid on oxidative stress parameters and inflammatory cytokines in lipopolysaccharide-induced peripheral blood mononuclear cells. Mol Biol Rep. 2020;47(5):3557–3566. doi: 10.1007/s11033-020-05447-x. DOI: 10.1007/s11033-020-05447-x. [DOI] [PubMed] [Google Scholar]
- 15.Doustimotlagh AH, Dehpour AR, Etemad-Moghadam S, Alaeddini M, Ostadhadi S, Golestani A. A study on OPG/RANK/RANKL axis in osteoporotic bile duct-ligated rats and the involvement of nitrergic and opioidergic systems. Res Pharm Sci. 2018;13(3):239–249. doi: 10.4103/1735-5362.228954. DOI: 10.4103/1735-5362.228954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Palani S, Kumar R, Kumar B. Effect of the ethanolic extract of Indigofera barberi (L.) in acute acetaminophen induced nephrotoxic rats. New Biotechnol. 2009;(25):S14. DOI: 10.1016/j.nbt.2009.06.989. [Google Scholar]
- 17.Ko JW, Shin JY, Kim JW, Park SH, Shin NR, Lee IC, et al. Protective effects of diallyl disulfide against acetaminophen-induced nephrotoxicity: a possible role of CYP2E1 and NF-κB. Food Chem Toxicol. 2017;102:156–165. doi: 10.1016/j.fct.2017.02.021. DOI: 10.1016/j.fct.2017.02.021. [DOI] [PubMed] [Google Scholar]
- 18.Karaali HF, Fahmi RR, Borjac JM. Effect of Ocimum basilicum leaves extract on acetaminophen-induced nephrotoxicity in BALB/c mice. J Complement Integr Med. 2018;16(2) doi: 10.1515/jcim-2018-0111. /j/jcim.2019.16.issue-2/jcim-2018-0111/jcim-2018-0111.xml. DOI: 10.1515/jcim-2018-0111. [DOI] [PubMed] [Google Scholar]
- 19.Ezeonwu V, Dahiru D. Protective effect of bi-herbal formulation of Ocimum gratissimum and Gongronema latifolium aqueous leaf extracts on acetaminophen-induced hepato-nephrotoxicity in rats. Am J Biochem. 2013;3(1):18–23. DOI: 10.5923/j.ajb.20130301.03. [Google Scholar]
- 20.Kukic J, Petrovic S, Niketic M. Antioxidant activity of four endemic Stachys taxa. Biol Pharm Bull. 2006;29(4):725–729. doi: 10.1248/bpb.29.725. DOI: 10.1248/bpb.29.725. [DOI] [PubMed] [Google Scholar]
- 21.Hussain Z, Khan JA, Arshad A, Asif P, Rashid H, Arshad MI. Protective effects of Cinnamomum zeylanicum L. (Darchini) in acetaminophen-induced oxidative stress, hepatotoxicity and nephrotoxicity in mouse model. Biomed Pharmacother. 2019;109:2285–2292. doi: 10.1016/j.biopha.2018.11.123. DOI: 10.1016/j.biopha.2018.11.123. [DOI] [PubMed] [Google Scholar]
- 22.Reshi MS, Yadav D, Uthra C, Shrivastava S, Shukla S. Acetaminophen-induced renal toxicity: preventive effect of silver nanoparticles. Toxicol Res. 2020;9(4):406–412. doi: 10.1093/toxres/tfaa040. DOI: 10.1093/toxres/tfaa040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Haidara MA, Al-Ani B, Eid RA, Mohammed ME, Al-Hashem F, Dallak M. Acetaminophen induces alterations to the renal tubular ultrastructure in a rat model of acute nephrotoxicity protected by resveratrol and quercetin. Int J Morphol. 2020;38(3):585–591. DOI.10.4067/S0717-95022020000300585. [Google Scholar]
- 24.Klimiuk A, Zalewska A, Sawicki R, Knapp M, Maciejczyk M. Salivary oxidative stress increases with the progression of chronic heart failure. J Clin Med. 2020;9(3):769–788. doi: 10.3390/jcm9030769. DOI: 10.3390/jcm9030769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ghosh J, Das J, Manna P, Sil PC. Acetaminophen induced renal injury via oxidative stress and TNF-α production: therapeutic potential of arjunolic acid. Toxicology. 2010;268(1-2):8–18. doi: 10.1016/j.tox.2009.11.011. DOI: 10.1016/j.tox.2009.11.011. [DOI] [PubMed] [Google Scholar]
- 26.Mansour MA, Nagi MN, El-Khatib AS, Al-Bekairi AM. Effects of thymoquinone on antioxidant enzyme activities, lipid peroxidation and DT-diaphorase in different tissues of mice: a possible mechanism of action. Cell Biochem Funct. 2002;20(2):143–151. doi: 10.1002/cbf.968. DOI: 10.1002/cbf.968. [DOI] [PubMed] [Google Scholar]
- 27.Jaeschke H, Ramachandran A. The role of oxidant stress in acetaminophen-induced liver injury. Curr Opin Toxicol. 2020;20-21:9–14. doi: 10.1016/j.cotox.2020.03.003. DOI: 10.1016/j.cotox.2020.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Morteza-Semnani K, Saeedi M, Shahani S. Antioxidant activity of the methanolic extracts of some species of Phlomis and Stachys on sunflower oil. Afr J Biotechnol. 2006;5(24):2428–2432. [Google Scholar]
- 29.Azarmehr N, Afshar P, Moradi M, Sadeghi H, Sadeghi H, Alipoor B, et al. Hepatoprotective and antioxidant activity of watercress extract on acetaminophen-induced hepatotoxicity in rats. Heliyon. 2019;5(7):1–5. doi: 10.1016/j.heliyon.2019.e02072. e02072. DOI: 10.1016/j.heliyon.2019.e02072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gohari A, Hajimehdipoor H, Saeidnia S, Ajani Y, Hadjiakhoondi A. Antioxidant activity of some medicinal species using FRAP assay. J Medicinal Plants. 2011;10(37):54–60. [Google Scholar]
- 31.Huang A, Palmer LS, Hom D, Valderrama E, Trachtman H. The role of nitric oxide in obstructive nephropathy. J Urol. 2000;163(4):1276–1281. [PubMed] [Google Scholar]
- 32.Nagappan AS, Varghese J, Pranesh GT, Jeyaseelan V, Jacob M. Indomethacin inhibits activation of endothelial nitric oxide synthase in the rat kidney: possible role of this effect in the pathogenesis of indomethacin-induced renal damage. Chem Biol Interact. 2014;221:77–87. doi: 10.1016/j.cbi.2014.07.014. DOI: 10.1016/j.cbi.2014.07.014. [DOI] [PubMed] [Google Scholar]
- 33.Meng L, Qu L, Tang J, Cai SQ, Wang H, Li X. A combination of Chinese herbs, Astragalus membranaceus var. mongholicus and Angelica sinensis, enhanced nitric oxide production in obstructed rat kidney. Vascul Pharmacol. 2007;47(2-3):174–183. doi: 10.1016/j.vph.2007.06.002. DOI: 10.1016/j.vph.2007.06.002. [DOI] [PubMed] [Google Scholar]
- 34.Hua H, Ge X, Wu M, Zhu C, Chen L, Yang G, et al. Rotenone protects against acetaminophen-induced kidney injury by attenuating oxidative stress and inflammation. Kidney Blood Press Res. 2018;43(4):1297–1309. doi: 10.1159/000492589. DOI: 10.1159/000492589. [DOI] [PubMed] [Google Scholar]
- 35.Temel Y, Kucukler S, Yildirim S, Caglayan C, Kandemir FM. Protective effect of chrysin on cyclophosphamide-induced hepatotoxicity and nephrotoxicity via the inhibition of oxidative stress, inflammation, and apoptosis. Naunyn Schmiedebergs Arch Pharmacol. 2020;393(3):325–337. doi: 10.1007/s00210-019-01741-z. DOI: 10.1007/s00210-019-01741-z. [DOI] [PubMed] [Google Scholar]