Abstract
Background and purpose:
Nonalcoholic steatohepatitis (NASH) is considered a common and serious liver disease, which develops into cirrhosis, fibrosis, and even hepatocellular carcinoma. Oxidative stress is identified as an important factor in the induction and promotion of NASH. Allantoin is a natural and safe compound and has notable effects on lipid metabolism, inflammation, and oxidative stress. Therefore, this study was aimed to assess the role of allantoin on the oxidative stress and SIRT1/Nrf2 pathway in a mouse model of NASH.
Experimental approach:
C57/BL6 male mice received saline and allantoin (saline as the control and allantoin as the positive control groups). NASH was induced by a methionine-choline deficient diet (MCD). In the NASH-allantoin (NASH-Alla) group, allantoin was injected for 4 weeks in the mice feeding on an MCD diet. Afterward, histopathological, serum, oxidative stress, and western blot evaluations were performed.
Findings/Results:
We found NASH provided hepatic lipid accumulation and inflammation. Superoxide dismutase (SOD) and glutathione (GSH) levels decreased, lipid peroxidation increased, and the expression of SIRT1 and Nrf2 downregulated. However, allantoin-treatment decreased serum cholesterol, ALT, and AST. Liver steatosis and inflammation were improved. Protein expression of SIRT1 and Nrf2 were upregulated and SOD, CAT, and GSH levels increased and lipid peroxidation decreased.
Conclusion and implications:
It seems that the antioxidant effects of allantoin might have resulted from the activation of SIRT1/Nrf2 pathway and increase of cellular antioxidant power.
Keywords: Allantoin, NASH, Nrf2, Oxidative stress, SIRT1
INTRODUCTION
Non-alcoholic fatty liver disease (NAFLD) is considered as the extra accumulation of triglycerides in the hepatocytes (steatosis) which can develop into non-alcoholic steatohepatitis (NASH), cirrhosis, and probably hepatocellular carcinoma (1). NASH is identified as hepatic steatosis, inflammation, and with or without fibrosis. The prevalence of NAFLD and NASH are widely growing and becoming the first cause of liver transplantation worldwide (2). NAFLD and NASH are involved in the pathology of some diseases like type 2 diabetes, cardiovascular disease, chronic kidney disease, and even Alzheimer’s disease (3). Although the exact pathophysiological mechanism of NASH has not been understood, the two-hit hypothesis has well been accepted (4). On this basis, hepatic lipid accumulation is provided in three ways; decreased lipolysis, de novo lipogenesis, and increased dietary lipid, which all enhances liver fatty acids and produce excess triglycerides accumulation and lead to hepatic steatosis (5). Increased hepatotoxic levels of triglyceride cause overgeneration of reactive oxygen species (ROS), depletion of antioxidant agents, the release of cytokines/chemokines, and production of inflammation; which finally lead to NASH.
Indeed, it has been shown that high concentrations of ROS are associated with the intensity of NASH disease (6). Oxidative stress is considered an imbalance between antioxidant agents and reactive oxygen species (ROS) production (7). ROS overgeneration activates a cascade of inflammatory, apoptotic, and fibrotic agents, which lead to vast liver damages (8). Therefore, oxidative stress is not only a primary factor for NASH induction, but also progresses liver injuries. Among these, silent information regulator-two 1 (SIRT1) and nuclear factor erythroid 2-related factor 2 (Nrf2) are two important factors in ROS suppression (9). SIRT1 is the nicotine adenine dinucleotide (NAD)-dependent deacetylase, which controls many cellular processes including oxidative stress, lipid metabolism, inflammation, and apoptosis (9). Liver-specific SIRT1 knockout mice with a standard diet presented increased systemic glucose levels, hepatic free fatty acid, and cholesterol content (10). It also raises Nrf2 expression in the liver cells and thereby enhances antioxidant power through increasing superoxide dismutase (SOD), and catalase (CAT) enzymes, and glutathione (GSH) content in the hepatocytes (11). According to the previous documents, SIRT1 and Nrf2 play a critical role in the NASH improvement (10,11). It has been reported that folic acid upregulates SIRT1 expression, then restores peroxisome proliferator-activated receptor alpha (PPARa) and attenuates NASH (12). Resveratrol is a natural SIRT1 activator that also ameliorated mice with NASH through deacetylation of Nrf2 (13).
Allantoin is one of the essential compounds in many herbs such as yam, Nelumbo nucifera rhizome, sugar beet, and leguminous, and also it is a natural and non-toxic agent (14,15). The tissue regeneration and wound healing effects of allantoin are previously well studied (16). It is also considered as an agonist of imidazoline receptor I. It is believed that some of its metabolic effects are mediated through this receptor (17). Allantoin has decreased oxidative stress in rat-induced-gastritis (18). Ma et al. showed that allantoin increased the expression of peroxisome proliferator-activated receptor gamma coactivator 1-alpha (PGC-1α), Sirt-1, and Nrf-1 in skeletal muscle cells (19). Previous studies have also indicated positive effects of allantoin on metabolic functions and streptozotocin-induced β-cells (20). We recently showed that allantoin improved NASH via downregulation of lipid metabolism-related genes such as SREBP1c, PPARα, and apolipoprotein B and attenuation of inflammation and apoptosis in methionine-choline deficient diet (MCD)-induced animals (21). Nevertheless, other mechanisms of allantoin function remained unclear. Therefore, this study was designed to assess the effects of allantoin on oxidative stress and the possible involvement of SIRT1/Nrf2 pathway in a mouse model of NASH.
MATERIALS AND METHODS
Reagents
Allantoin (Aigma-Aldrich, USA), SOD (ZB-SOD-48A, Zellbio, Germany), CAT (ZB-CAT-96A, Zellbio, Germany), GSH (ZB-GSH-96A, Zellbio, Germany), malondialdehyde (MDA; ZB-MDA-96A, Zellbio, Germany), Nrf2 (ab137550, Abcam, USA), SIRT1 (ab110304, Abcam, USA), and β-actin (sc-130657, Sunta Cruz, USA) were purchased from the mentioned companies.
Experimental procedures
Inbred C57/BL6 male mice (25-27 g) were housed in a temperature-controlled room with a 12/12-h light/dark cycle and free access to food and tap water. The animal care and experimental procedure were approved in accordance with the Guidelines for Animal Care and Use at Qom University of Medical Sciences (Ethics No. IR.MUQ.REC.1399.149). Animals were randomly divided into 4 equal groups, 6 each, including the control group, the animals that accessed the standard diet for 8 weeks and received saline daily (ip) from 5th week for 4 weeks; allantoin group, the animals accessed to the standard diet for 8 weeks and received allantoin (5 mg/kg, ip) from 5th week for 4 weeks on a daily basis (22); NASH group, the animals received methionine-choline deficient (MCD) diet for 8 weeks to induce NASH (21); and finally NASH-allantoin group (NASH-Alla), received MCD diet for 8 weeks to induce NASH and received allantoin (5 mg/kg, ip) from 5th week for 4 weeks daily (22). After 8 weeks, the animals were anesthetized by sodium pentobarbital (23). A midline incision was done on the abdomen. After abdomen exposure, the liver was immediately removed and washed by saline. A part of the liver was dissected and kept at -70 °C and another part was placed in the formaldehyde solution. Blood samples were directly collected from the heart.
In this experiment, the MCD diet was used to induce NASH. The most widely used diet for NASH induction is MCD in mice. This diet is enriched with high sucrose (> 40%) and moderate fat (10-20%), which provide insulin resistance. This method causes weight loss and severe steatosis in the liver after 8 weeks due to the reduced lipid export from the liver and lipid accumulation (24).
Histopathological study
The median lobe of the liver was dissected and fixed in 10% buffered-formaldehyde solution. After about 24 h, the tissues were dehydrated by placing them in different concentrations of ethanol solutions, then washed with xylene and embedded in paraffin. They were sectioned with a microtome and stained with hematoxylin and eosin (H&E) stains. A specialist pathologist blinded to the experiment, determined percentages of hepatic steatosis, hepatocytes ballooning, and lobular inflammation by Olympus light microscope (CX23 LED Microscope, Japan). The liver tissue photos were evaluated by Image J software (National Institutes of Health, Image J 1.49f, USA). Histological evaluations were done on the six animals in each group. For steatosis scoring: < 5% (0), 5%-33% (1), 33%-66% (2) and > 66% (3); for ballooning: none (0) and prominent ballooning (1), and lobar inflammation based on overall assessment of all inflammatory foci: no foci (0), < 2 foci per field (1) and > 2 foci per field (2) in magnification of 200×, based on Kleiner et al. scoring system were done (25).
Serum lipids and enzymes measurement
The blood samples were centrifuged (3500 rpm for 20 min) and serum was obtained. Then, total cholesterol, triglyceride (TG), aspartate aminotransferase (AST), and alanine aminotransferase (ALT) levels were measured by enzymatic colorimetric kits, according to the manufacturer’s instructions (Pars Azmoon, Iran).
Oxidative stress indices determination
Briefly, 100 mg of the liver tissue was weighed, 1 mL phosphate-buffered saline was added and centrifuged at 3000-4000 rpm for 20 min. Supernatants were then collected, allocated, and kept at -70 °C for lipid peroxidation by MDA, GSH, CAT, and SOD measurements, according to the manufacturer’s instructions. Antioxidant kits measured quantities assays samples based on colorimetric methods that should be read by ELISA reader (CAT: 405 nm, GSH: 412 nm, MDA: 535 nm, and SOD: 420 nm). According to the kit instruction, MDA-thiobarbituric acid adduct formed by the reaction of MDA and thiobarbituric acid under high temperature. MDA is measured in acidic media and heat (90-100 °C) at 535 nm.
Western blot analysis
SIRT1 and Nrf2 expressions in the liver tissue were measured by western immunoblotting. Briefly, the protein concentration was measured by the Bradford assay kit (Sigma Aldrich, USA). The proteins were separated and transferred to polyvinylidene fluoride (PVDF) membranes. Subsequently, the blocking of the membranes was performed in a 5% skim milk buffer and then probed with primary antibodies against Nrf2 (ab137550), SIRT1(ab110304), and β-actin (sc-130657) overnight. After washing, the HRP-conjugated secondary antibody (1:7000, Cell Signaling) was added to the membranes. After incubation, the membranes were bathed in the wash buffer and washed. Then, the membranes were incubated with the enhanced chemiluminescence (ECL, Amersham) reagents in a darkroom. This was followed by exposing the membrane to an X-ray film and visualization of the chemiluminescence of the binding through a visualizing machine. The intensity of the bands was determined using Image J software (IJ 1.46r version, NIH, USA) and normalized to the bands of the β-actin as an internal control.
Statistical analysis
Data are reported as mean ± SEM. The normality of these was checked by the Kolmogorov-Smirnov test. Statistical analysis was done by one-way analysis of variances (ANOVA) and Tukey’s post hoc test and chi-square test for histopathological results, using SPSS for Windows version 25 (IBM SPSS version 25; USA). A P < 0.05 was considered to be statistically significant.
RESULTS
Allantoin improved ALT, AST, TG, and cholesterol serum in the NASH-induced mice
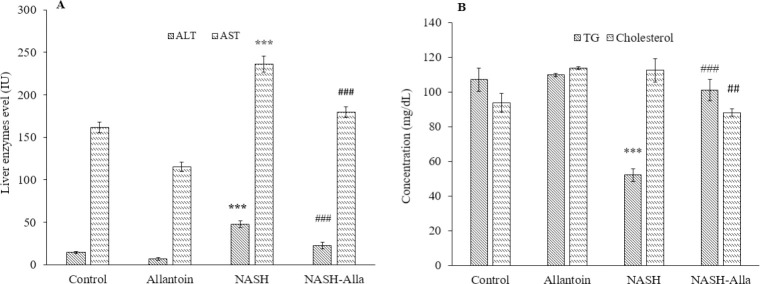
As noted in Fig. 1A, blood sample evaluations showed that ALT levels significantly increased in the NASH group compared with the control (P < 0.001). While administration of allantoin provided a marked decrease in ALT serum level in the NASH-Alla group compared with the NASH group (P < 0.001). Similarly, AST levels significantly increased in the NASH group compared with the control (P < 0.001) and decreased in the NASH-Alla group compared with the NASH group (P < 0.001). Serum levels of TG also significantly decreased in the NASH group compared with the control (P < 0.001), as predicted from the MCD diet method, while in the NASH-Alla group, TG serum reached the control group (P < 0.001). The results showed that allantoin administration after 4 weeks, significantly decreased cholesterol levels in the NASH-Alla group compared with the NASH group (P < 0.01, Fig. 1B).
Fig. 1.
(A) ALT and AST and (B) TG and cholesterol levels of the blood samples between the groups. Data are expressed as mean ± SEM, n = 6. ***P ≤ 0.001 Indicates significant differences compared with the control group, ##P ≤ 0.01 and ###P ≤ 0.001 versus the NASH group. ALT, Alanine aminotransferase; AST, aspartate aminotransferase; NASH, nonalcoholic steatohepatitis; Alla, allantoin; TG, triglyceride.
Allantoin alleviated liver tissue injuries in the NASH induced mice
Our findings stated that in the MCD induced mice, a significant excessive lipid droplets accumulation (empty spaces), cellular ballooning (cell congestion), and lobar inflammation were observed compared with the control group. Whereas, steatosis, edema, and inflammation significantly decreased in the NASH-Alla group compared with the NASH group (Fig. 2 and Table 1).
Fig. 2.
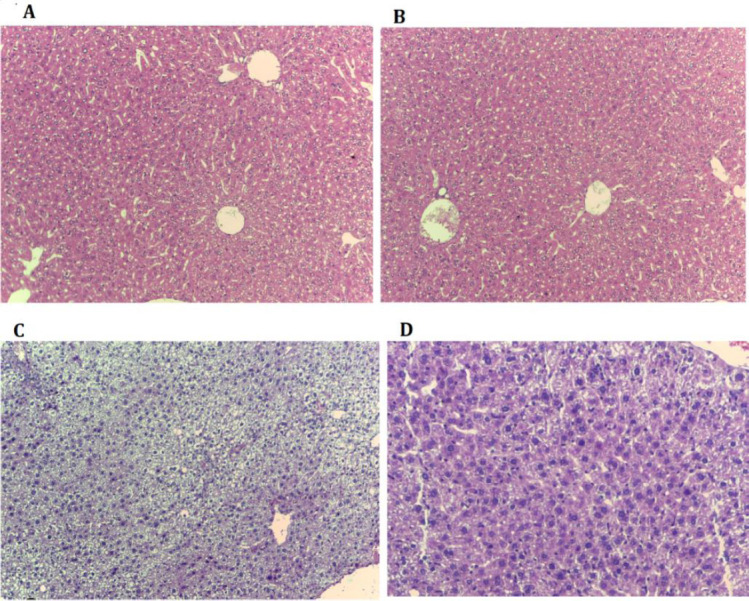
Histological findings of liver tissues after H&E staining (magnification ×200) in different experimental groups. (A) Control group: normal liver histology; (B) allantoin group: normal liver histology; (C) nonalcoholic steatohepatitis group: showing steatosis and ballooning degradation (empty spaces in the cell indicate fat accumulation and enlargement of cells); (D) nonalcoholic steatohepatitis-allantoin group: showing lower steatosis and lobular inflammation (lower empty spaces)
Table 1.
Histopathological findings in experimental groups.
| Control | Allantoin | NASH | NASH + Alla | |
|---|---|---|---|---|
| Steatosis (%) | ||||
| Grade 0 | 100 (6) | 100 (6) | 83.3 (5)b | |
| Grade 1 | 16.6 (1) | |||
| Grade 2 | ||||
| Grade 3 | 100 (6)a | |||
| Ballooning (%) | ||||
| Grade 0 | 100 (6) | 100 (6) | 50 (3) | |
| Grade 1 | 100 (6)a | 50 (3) | ||
| Lobar inflammation (%) | ||||
| Grade 0 | 100 (6) | 100 (6) | 83.3 (5) b | |
| Grade 1 | ||||
| Grade 2 | 100 (6)a | 16.6 (1) |
aP ≤ 0.05 indicates the significant differences compared with the control group and bP ≤ 0.05 versus the NASH group. NASH, Nonalcoholic steatohepatitis; Alla, allantoin.
Allantoin increased liver SIRT1 and Nrf2 expressions in the NASH induced mice
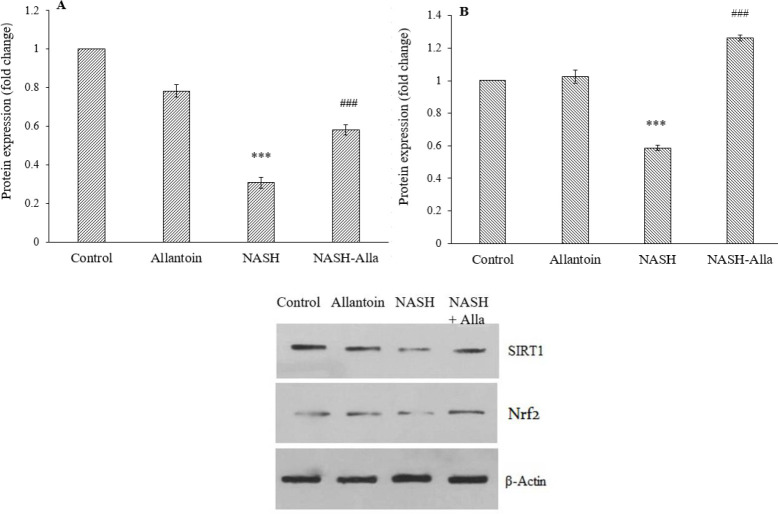
Western blot results showed a significant decrease in protein expression of SIRT1 in the NASH group compared with the control group (P < 0.001). However, allantoin administration markedly increased SIRT1expression compared with the NASH group (P < 0.001, Fig. 3A). Nrf2 protein expression also significantly decreased in the animals with NASH compared with the control group (P < 0.001), while in the NASH-Alla group, it significantly increased compared with the NASH group (P < 0.001, Fig. 3B).
Fig. 3.
(A) Protein expression of SIRT1 and (B) Nrf2 between the groups, (C) the result of western blotting. Data are expressed as mean ± SEM, n = 6. ***P ≤ 0.001 Indicates significant differences compared with the control group, ##P ≤ 0.01 and ###P ≤ 0.001 versus the NASH group. NASH, Nonalcoholic steatohepatitis; Alla, allantoin.
Allantoin enhanced hepatocyte antioxidant defense in the NASH induced mice
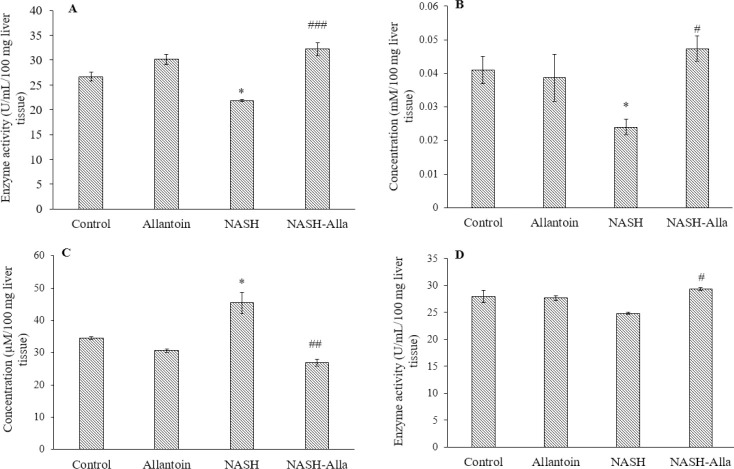
As presented in Fig. 4A, our study displayed that SOD enzyme level significantly decreased in the NASH group compared with the control group (P < 0.05), while in the NASH-Alla group a remarkable increase was observed compared with the NASH group (P < 0.001). In addition, GSH tissue level significantly decreased in the NASH group compared with the control group (P < 0.05) and administration of allantoin provided a significant increase in the GSH tissue levels (P < 0.05) Fig. 4B. Contrary, MDA tissue level, a lipid oxidation index, significantly increased in the NASH group compared with the control group (P < 0.05), whereas in the NASH-Alla group noticeably decreased compared with the NASH group (P < 0.01, Fig. 4C). Moreover, CAT enzyme concentration significantly increased in the NASH-Alla group compared with the NASH group (P < 0.05, Fig. 4D).
Fig. 4.
(A-D) Superoxide dismutase, glutathione, malondialdehyde, and catalase concentrations of the liver tissue between the groups. Data are expressed as mean ± SEM, n = 6. *P ≤ 0.05 Indicates significant differences compared with the control group, ##P ≤ 0.05, ##P ≤ 0.01, and ###P ≤ 0.001 versus the NASH group. NASH, Nonalcoholic steatohepatitis; Alla, allantoin.
DISCUSSION
The major results of this study showed that allantoin decreased serum cholesterol, AST, and ALT in the NASH-induced mice. Allantoin could also increase SIRT1 and Nrf2 proteins expression, enhance SOD, CAT, and GSH levels, decrease lipid peroxidation and ultimately improve NASH. To the best of our knowledge, this is the first report in which the effect of allantoin on the SIRT1/Nrf2 pathway and oxidative stress has been studied in the NASH animal model.
Allantoin is a natural compound, which found in many plants, and its wound healing, anti-inflammatory, antidiabetic, and antihyperlipidemic effects have been demonstrated (19,20,21,22,26).
As shown in the previous study, allantoin lowered steatosis and inflammation in both the liver tissue and blood samples (21). These findings are considered as the first and most important signs for the NAFL and NASH disease, which must be improved. Yang et al. have found undeniable effects of allantoin on the lipid profile decrement in the cell line and animals (22). Similarly, it has been reported that allantoin attenuated obesity and hyperlipidemia via imidazoline receptor activation in mice (17). We had previously demonstrated that allantoin decreased SREBP1c and fatty acid synthase expressions, increased PPARa as a lipolytic transcription factor, declined tumor necrosis factor alpha level, and thereby improved hepatic steatosis, and inflammation in the NASH-induced mice (21). These evidences are compatible with these data and confirm the positive effects of allantoin on MCD-induced NASH in mice. However, involved pathways need to be elucidated.
It is well known that oxidative stress is a major factor in NASH pathophysiology. Indeed, over-production of ROS provides vast damage to the cellular functions and progresses NASH disease (8). Therefore, suppression of oxidative stress is a beneficial way of the NASH improvement. SIRT1 is a histones deacetylase and its undeniable effects on the suppression of ROS, modulation of the metabolic pathway, inflammation, and apoptosis are well demonstrated in some tissues (11,12,27). According to available documents, SIRT1 inhibition is associated with alcoholic and non-alcoholic fatty liver (28). SIRT1 activates many transcriptional factors and cellular enzymes and thereby ameliorates oxidative stress, inflammation, and apoptosis (29). One of these transcriptional factors is Nrf2. Previous studies have indicated regulatory effects of SIRT1 on Nrf2, so that overexpression of SIRT1 as an upstream factor, activates Nrf2 and increases Nrf2/ARE binding (30,31). Then, it increases the expression of target genes such as GSH-peroxidases, SOD, CAT, and GSH ultimately elevates antioxidant resistance in the cells (32). On the other hand, MDA is considered a by-product of membrane lipid peroxidation and oxidative stress index. Zhong et al. have reported that baicalin attenuated NASH through upregulation of SOD and GSH and decrease of MDA level (32). In another study, silicon-enriched restructured pork improved oxidative stress and attenuated the NASH-induced mice (33).
In this experiment, our findings showed that allantoin could increase protein expression of SIRT1 and Nrf2 in the livers with NASH. It also elevated antioxidant resistance by the increase of SOD, CAT, and GSH. Contrarily, allantoin decreased lipid peroxidation. Ma and his colleagues reported that allantoin upregulated SIRT1 and Nrf1 in the liver and pancreas of diabetic mice and increased SOD and CAT (34). Moreover, it has been shown allantoin increases SOD and CAT levels in the rats with gastric ulcers (18). We also previously indicated allantoin increased non-protein sulfhydryl levels in the gastric tissue (35). Altogether, herein it seems that allantoin could elevate expression of SIRT1 and Nrf2 and thereby increase SOD, CAT, and GSH levels, decrease MDA and finally attenuate oxidative stress in the liver of mice with NASH.
CONCLUSION
This study, for the first time, demonstrated that treatment with allantoin could improve NASH disease through activation of SIRT1/Nrf2 pathway, increase of antioxidant enzymes and GSH, and decrease lipid peroxidation. Therefore, allantoin could be considered an antioxidant agent and an attractive candidate for NASH treatment. Future studies can help confirm this hypothesis.
Conflict of interest statement
The authors declared no conflict of interest in this study.
Authors’ contribution
A. Moslehi designed the study and wrote the manuscript; Z. Hamidi-zad and H. Rastegarpanah performed the experiments. The manuscript has been read and approved by all the authors.
Acknowledgments
This work was financially supported by Qom University of Medical Sciences, Qom. I.R. Iran through the Grant No. 991230.;.
REFERENCES
- 1.Gottlieb A, Mosthael W, Sowa JP, Canbay A. Nonalcoholic-fatty-liver-disease and nonalcoholic steatohepatitis: successful development of pharmacological treatment will depend on translational research. Digestion. 2019;100(2):79–85. doi: 10.1159/000493259. DOI: 10.1159/000493259. [DOI] [PubMed] [Google Scholar]
- 2.Wree A, Broderick L, Canbay A, Hoffman HM, Feldstein AE. From NAFLD to NASH to cirrhosis-new insights into disease mechanisms. Nat Rev Gastroenterol Hepatol. 2013;10(11):627–636. doi: 10.1038/nrgastro.2013.149. DOI: 10.1038/nrgastro.2013.149. [DOI] [PubMed] [Google Scholar]
- 3.El-Lakkany NM, Seif El-Din SH, Sabra AA, Hammam OA, Ebeid FA. Co-administration of metformin and N-acetylcysteine with dietary control improves the biochemical and histological manifestations in rats with non-alcoholic fatty liver. Res Pharm Sci. 2016;11(5):374–382. doi: 10.4103/1735-5362.192487. DOI: 10.4103/1735-5362.192487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Liu W, Baker RD, Bhatia T, Zhu L, Baker SS. Pathogenesis of nonalcoholic steatohepatitis. Cell Mol Liff Sci. 2016;73(10):1969–1987. doi: 10.1007/s00018-016-2161-x. DOI: 10.1007/s00018-016-2161-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nili-Ahmadabadi A, Akbari Z, Ahmadimoghaddam D, Larki-Harchegani A. The role of ghrelin and tumor necrosis factor alpha in diazinon-induced dyslipidemia: insights into energy balance regulation. Pestic Biochem Physiol. 2019;157:138–142. doi: 10.1016/j.pestbp.2019.03.013. DOI: 10.1016/j.pestbp.2019.03.013. [DOI] [PubMed] [Google Scholar]
- 6.Ibrahim SH, Hirsova P, Gores GJ. Non-alcoholic steatohepatitis pathogenesis: sublethal hepatocyte injury as a driver of liver inflammation. Gut. 2018;67(5):963–972. doi: 10.1136/gutjnl-2017-315691. DOI: 10.1136/gutjnl-2017-315691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nili-Ahmadabadi A, Alibolandi P, Ranjbar A, Mousavi L, Nili-Ahmadabadi H, Larki-Harchegani A, et al. Thymoquinone attenuates hepatotoxicity and oxidative damage caused by diazinon: an in vivo study. Res Pharm Sci. 2018;13(6):500–508. doi: 10.4103/1735-5362.245962. DOI: 10.4103/1735-5362.245962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Takaki A, Kawai D, Yamamoto K. Molecular mechanisms and new treatment strategies for non-alcoholic steatohepatitis (NASH) Int J Mol Sci. 2014;15(5):7352–7379. doi: 10.3390/ijms15057352. DOI: 10.3390/ijms15057352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Singh V, Ubaid S. Role of silent information regulator 1 (SIRT1) in regulating oxidative stress and inflammation. Inflammation. 2020;43(5):1589–1598. doi: 10.1007/s10753-020-01242-9. DOI: 10.1007/s10753-020-01242-9. [DOI] [PubMed] [Google Scholar]
- 10.Rodgers JT, Puigserver P. Fasting-dependent glucose and lipid metabolic response through hepatic sirtuin 1. Proc Natl Acad Sci U S A. 2007;104(31):12861–12866. doi: 10.1073/pnas.0702509104. DOI: 10.1073/pnas.0702509104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Samimi F, Baazm M, Eftekhar E, Rajabi S, Goodarzi MT, Jalali Mashayekhi F. Possible antioxidant mechanism of coenzyme Q10 in diabetes: impact on Sirt1/Nrf2 signaling pathways. Res Pharm Sci. 2019;14(6):524–533. doi: 10.4103/1735-5362.272561. DOI: 10.4103/1735-5362.272561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Xin FZ, Zhao ZH, Zhang RN, Pan Q, Gong ZZ, Sun C, et al. Folic acid attenuates high-fat diet-induced steatohepatitis via deacetylase SIRT1-dependent restoration of PPARa. World J Gastroenterol. 2020;26(18):2203–2220. doi: 10.3748/wjg.v26.i18.2203. DOI: 10.3748/wjg.v26.i18.2203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jin SH, Yang JH, Shin BY, Seo K, Shin SM, Cho IJ, et al. Resveratrol inhibits LXRa-dependent hepatic lipogenesis through novel antioxidant Sestrin2 gene induction. Toxicol Appl Pharmacol. 2013;271(1):95–105. doi: 10.1016/j.taap.2013.04.023. DOI: 10.1016/j.taap.2013.04.023. [DOI] [PubMed] [Google Scholar]
- 14.Chen MF, Yang TT, Yeh LR, Chung HH, Wen YJ, Lee WJ, et al. Activation of imidazoline I-2B receptors by allantoin to increase glucose uptake into C2C12 cells. Horm Metab Res. 2012;44(4):268–272. doi: 10.1055/s-0032-1301898. DOI: 10.1055/s-0032-1301898. [DOI] [PubMed] [Google Scholar]
- 15.Chen MF, Tsai JT, Chen LJ, Wu TP, Yang JJ, Yin LT, et al. Antihypertensive action of allantoin in animals. Biomed Res Int 2014. 2014:1–6. doi: 10.1155/2014/690135. 690135. DOI: 10.1155/2014/690135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Araújo LU, Grabe-Guimarães A, Mosqueira VC, Carneiro CM, Silva-Barcellos NM. Profile of wound healing process induced by allantoin. Acta Cir Bras. 2010;25(5):460–466. doi: 10.1590/s0102-86502010000500014. DOI: 10.1590/s0102-86502010000500014. [DOI] [PubMed] [Google Scholar]
- 17.Lin KC, Yeh LR, Chen LJ, Wen YJ, Cheng KC, Cheng JT. Plasma glucose-lowering action of allantoin is induced by activation of imidazoline I-2 receptors in streptozotocin-induced diabetic rats. Horm Metab Res. 2012;44(1):41–46. doi: 10.1055/s-0031-1295439. DOI: 10.1055/s-0031-1295439. [DOI] [PubMed] [Google Scholar]
- 18.da Silva DM, Martins JLR, de Oliveira DR, Florentino IF, da Silva DPB, Dos Santos FCA. Effect of allantoin on experimentally induced gastric ulcers: pathways of gastroprotection. Eur J Pharmacol. 2018;821:68–78. doi: 10.1016/j.ejphar.2017.12.052. DOI: 10.1016/j.ejphar.2017.12.052. [DOI] [PubMed] [Google Scholar]
- 19.Ma J, Kang SY, Meng X, Kang AN, Park JH, Park YK, et al. Effects of rhizome extract of Dioscorea batatas and its active compound, allantoin, on the regulation of myoblast differentiation and mitochondrial biogenesis in C2C12 myotubes. Molecules. 2018;23(8):2023–2037. doi: 10.3390/molecules23082023. DOI: 10.3390/molecules23082023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Amitani M, Cheng KC, Asakawa A, Amitani H, Kairupan TS, Sameshima N, et al. Allantoin ameliorates chemically-induced pancreatic β-cell damage through activation of the imidazoline I3 receptors. PeerJ. 2015;3:1–15. doi: 10.7717/peerj.1105. e1105. DOI: 10.7717/peerj.1105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Komeili Movahhed T, Moslehi A, Golchoob M, Ababzadeh S. Allantoin improves methionine-choline deficient diet-induced nonalcoholic steatohepatitis in mice through involvement in endoplasmic reticulum stress and hepatocytes apoptosis-related genes expressions. Iran J Basic Med Sci. 2019;22(7):736–744. doi: 10.22038/ijbms.2019.33553.8012. DOI: 10.22038/ijbms.2019.33553.8012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yang TT, Chiu NH, Chung HH, Hsu CT, Lee WJ, Cheng JT. Stimulatory effect of allantoin on imidazoline I1 receptors in animal and cell line. Horm Metab Res. 2012;44(12):879–884. doi: 10.1055/s-0032-1312624. DOI: 10.1055/s-0032-1312624. [DOI] [PubMed] [Google Scholar]
- 23.Nikoukar LR, Nabavizadeh F, Mohamadi SM, Moslehi A, Hassanzadeh G, Nahrevanian H, et al. Protective effect of ghrelin in a rat model of celiac disease. Acta Physiol Hung. 2014;101(4):438–447. doi: 10.1556/APhysiol.101.2014.4.5. DOI: 10.1556/APhysiol.101.2014.4.5. [DOI] [PubMed] [Google Scholar]
- 24.Machado MV, Michelotti GA, Xie G, Almeida Pereira T, Boursier J, Bohnic B, et al. Mouse models of diet-induced nonalcoholic steatohepatitis reproduce the heterogeneity of the human disease. PLoS One. 2015;10(5):e0127991,1–16. doi: 10.1371/journal.pone.0127991. DOI: 10.1371/journal.pone.0127991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kleiner DE, Brunt EM, Van Natta M, Behling C, Contos MJ, Cummings OW, et al. Design and validation of a histological scoring system for nonalcoholic fatty liver disease. Hepatology. 2005;41(6):1313–1321. doi: 10.1002/hep.20701. DOI: 10.1002/hep.20701. [DOI] [PubMed] [Google Scholar]
- 26.Lee MY, Lee NH, Jung D, Lee JA, Seo CS, Lee H, et al. Protective effects of allantoin against ovalbumin (OVA)-induced lung inflammation in a murine model of asthma. Int Immunopharmacol. 2010;10(4):474–480. doi: 10.1016/j.intimp.2010.01.008. DOI: 10.1016/j.intimp.2010.01.008. [DOI] [PubMed] [Google Scholar]
- 27.Yang R, Song C, Chen J, Zhou L, Jiang X, Cao X, et al. Limonin ameliorates acetaminophen-induced hepatotoxicity by activating Nrf2 antioxidative pathway and inhibiting NF-κB inflammatory response via upregulating Sirt1. Phytomedicine. 2020;69:153211. doi: 10.1016/j.phymed.2020.153211. DOI: 10.1016/j.phymed.2020. [DOI] [PubMed] [Google Scholar]
- 28.Min HK, Kapoor A, Fuchs M, Mirshahi F, Zhou H, Maher J, et al. Increased hepatic synthesis and dysregulation of cholesterol metabolism is associated with the severity of nonalcoholic fatty liver disease. Cell Metab. 2012;15(5):665–674. doi: 10.1016/j.cmet.2012.04.004. DOI: 10.1016/j.cmet.2012.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yan T, Huang J, Nisar MF, Wan C, Huang W. The beneficial roles of SIRT1 in drug-induced liver injury. Oxid Med Cell Longev 2019. 2019:1–14. doi: 10.1155/2019/8506195. 8506195. DOI: 10.1155/2019/8506195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tang W, Jiang YF, Ponnusamy M, Diallo M. Role of Nrf2 in chronic liver disease. World J Gastroenterol. 2014;20(36):13079–13087. doi: 10.3748/wjg.v20.i36.13079. DOI: 10.3748/wjg.v20.i36.13079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Huang K, Huang J, Xie X, Wang S, Chen C, Shen X, et al. Sirt1 resists advanced glycation end products-induced expressions of fibronectin and TGF-β1 by activating the Nrf2/ARE pathway in glomerular mesangial cells. Free Radic Biol Med. 2013;65:528–540. doi: 10.1016/j.freeradbiomed.2013.07.029. DOI: 10.1016/j.freeradbiomed.2013.07.029. [DOI] [PubMed] [Google Scholar]
- 32.Zhong X, Liu H. Baicalin attenuates diet induced nonalcoholic steatohepatitis by inhibiting inflammation and oxidative stress via suppressing JNK signaling pathways. Biomed Pharmacother. 2018;98:111–117. doi: 10.1016/j.biopha.2017.12.026. DOI: 10.1016/j.biopha.2017.12.026. [DOI] [PubMed] [Google Scholar]
- 33.Santos-López JA, Garcimartín A, Merino P, López-Oliva ME, Bastida S, Benedí J, et al. Effects of silicon vs. hydroxytyrosol-enriched restructured pork on liver oxidation status of aged rats fed highsaturated/high-cholesterol diets. PLoS One. 2016;11(1):1–16. doi: 10.1371/journal.pone.0147469. e0147469. DOI: 10.1371/journal.pone.0147469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ma J, Meng X, Liu Y, Yin C, Zhang T, Wang P, et al. Effects of a rhizome aqueous extract of Dioscorea batatas and its bioactive compound, allantoin in high fat diet and streptozotocin-induced diabetic mice and the regulation of liver, pancreas and skeletal muscle dysfunction. J Ethnopharmacol. 2020;259:1–10. doi: 10.1016/j.jep.2020.112926. 112926. DOI: 10.1016/j.jep.2020.112926. [DOI] [PubMed] [Google Scholar]
- 35.Eslami-Farsani M, Moslehi A, Hatami-Shahmir A. Allantoin improves histopathological evaluations in a rat model of gastritis. Physiol Int. 2018;105(4):325–334. doi: 10.1556/2060.105.2018.4.30. DOI: 10.1556/2060.105.2018.4.30. [DOI] [PubMed] [Google Scholar]