Abstract
Off-label use of dalbavancin for deep-seated and endovascular infections has been increasing. We performed a scoping review to evaluate the evidence for use of multiple-dose dalbavancin regimens as the predominant therapy for these indications. Predominant therapy was defined as use of dalbavancin without other concurrent antibiotics for more than half of the total treatment duration. Fifteen publications were identified; 2 were small, open-label randomized controlled trials and the remainder were retrospective observational studies or case reports. A total of 144 cases from these publications met eligibility criteria for inclusion in this review. Types of infections included osteoarticular infections, catheter-related or complicated bloodstream infections, and infective endocarditis. Overall, the evidence for use of multiple-dose regimens of dalbavancin for deep-seated and endovascular infections is limited by a paucity of data from controlled trials, heterogeneity of dosing regimens, and a lack of standardized clinical outcomes.
Keywords: dalbavancin, gram-positive infection, efficacy
Evidence for the use of multiple-dose dalbavancin regimens as the predominant therapy for deep-seated or endovascular infections is limited. Limitations of the literature include a paucity of data from controlled trials and heterogeneity of dosing regimens and outcome reporting.
Dalbavancin (Dalvance) was approved by the United States Food and Drug Administration in 2014 for the treatment of acute bacterial skin and skin structure infections (ABSSSIs) [1]. It is a long-acting injectable lipoglycopeptide designed to inhibit cell wall formation of gram-positive pathogens such as Staphylococcus aureus, Streptococcus pyogenes, Streptococcus agalactiae, Streptococcus anginosus, and Enterococcus species. Due to its long lipophilic side chain, dalbavancin has a half-life of about 346 hours, which allows for once-weekly dosing. For ABSSSIs, the recommended dose is either a 1-time dose of 1500 mg intravenously or 1000 mg intravenously on day 1, followed by 500 mg intravenously on day 8. Pharmacokinetic studies have shown that two 1500-mg doses of dalbavancin given 1 week apart would result in therapeutic concentrations in serum as well as bone and articular tissue for up to 8 weeks [2]. Dalbavancin’s spectrum of activity, long half-life, and tissue penetration make it an appealing option for the treatment of gram-positive infections that typically require prolonged antibiotic courses such as deep-seated infections including osteomyelitis, septic arthritis, and complicated bloodstream infections, and endovascular infections including infective endocarditis and septic deep thrombophlebitis. Oritavancin (Orbactiv), another long-acting lipoglycopeptide, was not be included in this review for the purpose of specificity.
Although the standard of care for the above-mentioned deep-seated or endovascular infections has traditionally been intravenous antibiotic therapy, this treatment approach may pose challenges. Outpatient parenteral antibiotic therapy (OPAT) has been shown to be safe and effective; however, this requires insertion and maintenance of a central venous catheter, can be costly, and may not be a feasible option for patients with substance use disorders or those with housing instability. More recently, randomized controlled trials have shown that oral antibiotic regimens have similar efficacy as intravenous antibiotics for infective endocarditis and bone and joint infections [3, 4]. Of note, these trials include very few patients with methicillin-resistant Staphylococcus aureus infections, which may be associated with worse outcomes compared with methicillin-susceptible S aureus. Unfortunately, patients who are not candidates for OPAT due to substance use or housing instability may also have difficulties adhering to a prolonged oral antibiotic regimen. Such patients are often kept in the hospital to complete an entire intravenous antibiotic course, leading to strain on both patients and hospitals. In such clinical scenarios, a long-acting antibiotic such as dalbavancin that can be dosed once weekly in the outpatient setting offers a potentially attractive alternative to prolonged daily intravenous or oral antibiotic therapy. Furthermore, dalbavancin has an excellent safety profile and may result in fewer treatment-related adverse events than standard of care agents such as vancomycin, daptomycin, linezolid, and cefazolin [5].
In light of the challenges associated with prolonged daily intravenous or oral therapy as well as dalbavancin’s relative tolerability, lack of drug interactions, and convenient administration, off-label use of this agent for invasive gram-positive infections that require prolonged antibiotic therapy has been increasing. In these clinical settings, dalbavancin has often been used as secondary therapy to complete treatment after a patient has medically stabilized and received several weeks or more of a daily antibiotic. This has proven to be an effective approach to decrease hospital length of stay and reduce health care costs [6]. However, whether dalbavancin is safe and effective as the sole therapy or predominant therapy for these complicated infections is less clear. The purpose of this scoping review was to summarize existing evidence and ongoing studies of dalbavancin for deep-seated or endovascular infections caused by gram-positive pathogens and to identify current knowledge gaps.
METHODS
We identified published and ongoing studies involving the use of multiple-dose dalbavancin regimens for deep-seated or endovascular gram-positive infections including osteomyelitis, septic arthritis, epidural abscess, pyomyositis, bacteremia, infective endocarditis, or other endovascular infections. Predominant therapy was defined as use of dalbavancin without other concurrent antibiotics for more than half of the total treatment duration. Since the above indications generally require 4 weeks or more of antibiotic therapy, cases were excluded when >14 days of antibiotics were given prior to initiating dalbavancin or if other antibiotics, including rifamycins, were given during dalbavancin treatment. Infections that require shorter treatment durations or can be treated with a single dose of dalbavancin, such as ABSSSIs or pneumonia, were excluded.
To identify potentially eligible publications and studies in development or underway, we searched the Medline and ClinicalTrials.gov databases using the Medical Subject Headings (MeSH) term “dalbavancin.” This initial search was not restricted based on date of publication, type of study, indication for dalbavancin, or clinical setting. All studies identified through this search strategy were screened for relevance through an assessment of the title and abstract by 2 review authors (M. M. C. and C. R. P.). When necessary, a third author (K. C. S.) adjudicated disagreements. Only publications in English were evaluated for inclusion. Full-text copies of the studies deemed to be of potential relevance were retrieved. These full-text studies were evaluated to assess for eligibility for inclusion in the review. When only a subset of cases from a publication met eligibility criteria, only the eligible cases were extracted.
Publications were eligible for inclusion if they involved patients who (1) had an invasive infection as defined above caused by a gram-positive pathogen, (2) received no more than 14 days of antibiotic therapy prior to the initiation of dalbavancin, (3) received at least 2 doses of dalbavancin, and (4) did not receive concomitant antibiotic therapy during the treatment with dalbavancin. Clinical trials of ABSSSI or cases where only a single dose of dalbavancin was administered were excluded, as the focus of this review was infections that require prolonged antibiotic therapy. Publications or cases where the duration of antibiotics administered prior to dalbavancin was not reported were excluded. In vitro studies, animal studies, pharmacokinetic studies, and studies of chronic suppressive therapy with dalbavancin were also excluded. Data from each included publication were extracted and summarized using a standardized data collection tool. Given heterogeneity in the definitions and reporting of clinical outcomes across studies, we classified cases as clinical success or failure according to the definitions used in individual publications.
RESULTS
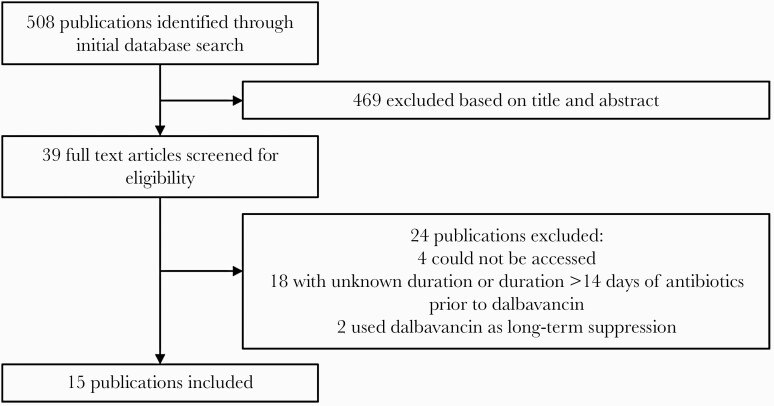
Fifteen publications were identified that reported at least 1 patient meeting the above eligibility criteria as outlined in Figure 1. Two were randomized controlled trials: 1 for osteomyelitis (70 participants) and 1 for catheter-related bloodstream infections (23 participants). The remainder of the publications were retrospective observational studies or case reports. A total of 144 cases met the eligibility criteria for this review; data for these cases are summarized in Table 1. The 2 randomized trials contributed 90 of the 144 (63%) cases, while 54 (38%) were from observational studies or case reports.
Figure 1.
Schematic of publications identified, screened, and included.
Table 1.
Data Summary of Cases Included in the Review
| Study and Study Design | Patient Demographics | Reason for Dalbavancin | Infection | Pathogen | Antibiotics Prior to Dalbavancin | Dalbavancin Dose | Clinical Outcomes |
|---|---|---|---|---|---|---|---|
| Rappo et al, 2019 [7] Randomized, open-label, comparator-controlled trial |
70 patients Age, mean: 49.2 y (range, 26–79) Men: 59 (84.3%) |
Clinical trial | Osteomyelitis; 4 with bacteremia | 67 with gram-positive infection included: 42 (60%) Staphylococcus aureus with 4 MRSA; 14 (20%) CoNS; 8 enterococci with 1 Enterococcus faecium; 3 streptococci; 9 anaerobes; 5 other | Excluded patients who received >24 h of IV antibiotics within 96 h of randomization | 1500 mg on day 1 and day 8 | Clinical cure at day 42 = 65/67 (97%); 2 patients lost to follow-up |
| Raad et al, 2005 [8] Phase 2, open-label, randomized, controlled, multicenter study |
23 patients Age, mean: 54 y (range, 20–78) Men: 13 (57%) |
Clinical trial | Catheter-related bloodstream infections | Gram-positive pathogens; 11 (42.3%) S aureus with 5 MRSA; 13 (50%) CoNS; 2 (7.7%) Enterococcus faecalis | Excluded patients who received gram-positive antibiotics for >24 h within 48 h of study initiation | 1000 mg on day 1, 500 mg on day 8 | Overall success = 21/23 (91.3%) Clinical success = 21/23 (91.3%) Microbiological success = 22/23 (95.6%) |
| Almangour et al, 2020 [9] Retrospective matched cohort |
11 patients Age, mean: 50.8 y Male: 9 (81.8%) |
Clinical trial | Osteomyelitis | S aureus; 6 (54.5%) MRSA | Patients excluded if they received >7 d of empiric or targeted therapy before initiation of dalbavancin | 1500 mg on day 1 and day 8 (n = 5) 1500 mg on day 1, 500 mg ×3 on days 15, 22, 29 (n = 1) 1000 mg, 500 mg ×4 weekly (n = 2) 1000 mg, 500 mg ×5 weekly (n = 1) 1000 mg, 500 mg ×10 weekly (n = 1) 1000 mg, 500 mg ×13 weekly (n = 1) |
Clinical success 90 d after treatment = 11/11 (100%) |
| Ajaka et al, 2020 [10] Retrospective review |
38 M | No active drug use, but patient not appropriate for OPAT | Bacteremia | MRSA | Vancomycin ×2 d | 1500 mg on day 1, 1000 mg on day 8 | Cure at 90 d |
| 50 M | PWID | Disseminated infection | MRSA | Vancomycin ×13 d | 1500 mg weekly ×2 | Failure (readmitted for infection) | |
| 51 M | PWID | Osteomyelitis with bacteremia | MRSA | Vancomycin ×11 d | 1500 mg on day 1, 1000 mg on day 8 | Cure at 90 d | |
| 55 F | No active drug use, but patient not appropriate for OPAT | Catheter-associated bloodstream infection | MRSA | Vancomycin ×8 d | 1500 mg ×2 weekly | Cure at 90 d | |
| Almangour et al, 2019 [11] Retrospective review |
Adult ≥18 | Retrospective review included 32% of PWID. Dalbavancin was used to facilitate early discharge, for patients who did not qualify for OPAT, or those with treatment failure | Osteomyelitis with abscess | MSSA | None | 1500 mg weekly ×2 | Clinical success at 90 d |
| Adult ≥18 | Osteomyelitis | MRSA | None | 1500 mg weekly ×2 | Clinical success at 90 d | ||
| Adult ≥18 | Osteomyelitis | MRSA and group G Streptococcus | Vancomycin and piperacillin-tazobactam ×3 d | 1500 mg weekly ×2 | Clinical success at 90 d | ||
| Adult ≥18 | Osteomyelitis | MRSA | Vancomycin and piperacillin-tazobactam ×2 d | 1500 mg weekly ×2 | Clinical success at 90 d | ||
| Adult ≥18 | Osteomyelitis with abscess | MRSA | Daptomycin ×14 d | 1000 mg, 500 mg weekly ×3 | Treatment failure resulting in bilateral AKA | ||
| Adult ≥18 | Osteomyelitis with myositis | MSSA | Vancomycin ×3 d followed by cefazolin ×3 d | 1000 mg, 500 mg weekly ×4 | Clinical success at 90 d | ||
| Adult ≥18 | Osteomyelitis | MSSA | None | 1000 mg, 500 mg weekly ×5 | Clinical success at 90 d | ||
| Adult ≥18 | Osteomyelitis | MRSA | None | 1000 mg, 500 mg weekly ×13 | Clinical success at 90 d | ||
| Adult ≥18 | Osteomyelitis | MRSA | Linezolid and piperacillin-tazobactam ×7 d | 1500 mg on day 1, 500 mg on days 15 and 22 | Treatment failure resulting in BKA | ||
| Bryson-Cahn et al, 2019 [12] Retrospective review |
Adult ≥18 | PWUD | Osteomyelitis | S aureus | Vancomycin and sulfamethoxazole-trimethoprim ×10 d | 1500 mg, 1000 mg, 500 mg × weekly ×3 | Clinical success at follow-up |
| Adult ≥18 | PWUD | Bacteremia with infected thrombophlebitis | S aureus | Vancomycin ×6 d | 1500 mg, 1000 mg weekly ×2 | Clinical success at follow-up | |
| Adult ≥18 | PWUD | Osteomyelitis | S aureus | Vancomycin ×1 d | 1000 mg, 500 mg weekly ×1 | Clinical success at follow-up | |
| Adult ≥18 | PWUD | Endocarditis | S aureus | Ceftaroline ×4 d | 1000 mg, 500 mg weekly ×1 | Clinical success at follow-up | |
| Adult ≥18 | PWUD | Endocarditis | S aureus | Vancomycin ×8 d | 1000 mg, 500 mg weekly ×1 | Clinical success at follow-up | |
| Adult ≥18 | PWUD | Bacteremia with subdural and epidural abscess | S aureus | Nafcillin ×12 d | 1000 mg, 500 mg weekly ×1 | Treatment failure; development of vertebral osteomyelitis 8 mo later | |
| Adult ≥18 | PWUD | Septic arthritis | S aureus | Vancomycin ×14 d | 1000 mg, 500 mg weekly ×1 | Loss to follow-up | |
| Tobudic et al, 2018 [13] Retrospective review |
Adult | No comment on drug use or homelessness. Dalbavancin was used for patients with poor venous access or as an OPAT regimen | Native valve endocarditis | E faecalis | Ceftriaxone and ampicillin ×2 wk | 1000 mg, 500 mg weekly ×3 | Clinical success at 6 mo |
| Adult | Prosthetic valve endocarditis | Streptococcus equi | Penicillin G ×1 wk | 1500 mg on day 1, 1000 mg on day 14 | Clinical success at 6 mo | ||
| Adult | Native valve endocarditis | Streptococcus mitis | Ceftriaxone and gentamicin ×2 wk | 1500 mg on day 1, 1000 mg on day 14 | Clinical success at 6 mo | ||
| Adult | Prosthetic valve endocarditis | S aureus | Flucloxacillin and rifampicin × 2 wk | 1000 mg, 500 mg weekly ×5 | Clinical success at 6 mo | ||
| Adult | Native valve endocarditis | Staphylococcus hominis | Flucloxacillin and daptomycin ×2 wk | 1000 mg, 500 mg weekly ×5 | Clinical success at 6 mo | ||
| Adult | Prosthetic valve endocarditis | Streptococcus equinus | Penicillin G ×1 wk | 1500 mg, 1000 mg every other week ×2 | Clinical success at 6 mo | ||
| Adult | Suspected prosthetic valve | Streptococcus sanguinis | None | 1500 mg, 1000 mg every other week ×2 | Clinical success at 6 mo | ||
| Adult | Native valve endocarditis | Enterococcus faecalis | Ceftriaxone and ampicillin ×1 wk | 1500 mg, 1000 mg every other week ×2 | Clinical success at 6 mo | ||
| Adult | Native valve endocarditis | Aerococcus urinae | Penicillin G × 2 wk | 1500 mg, 1000 mg every other week ×2 | Clinical success at 6 mo | ||
| Adult | Native valve endocarditis | S aureus | Flucloxacillin and daptomycin ×1 wk | 1500 mg, 1000 mg every other week ×2 | Clinical success at 6 mo | ||
| Adult | Native valve endocarditis | S aureus | Flucloxacillin and fosfomycin ×1 wk | 1500 mg, 1000 mg every other week ×2 | Clinical success at 6 mo | ||
| Adult | Native valve endocarditis | S sanguinis and S hominis | Flucloxacillin and ampicillin ×1 wk | 1500 mg, 1000 mg every other week ×2 | Clinical success at 6 mo | ||
| Durante-Mangoni et al, 2021 [14] Retrospective review |
86 F | Shorten hospital stay | Transcatheter aortic valve endocarditis | E faecium | Daptomycin and teicoplanin ×2 wk | 1000 mg, 500 mg weekly ×3 wk | Clinical cure at 6 wk |
| 74 F | Shorten hospital stay | Pacemaker-associated endocarditis | MSSA | Ceftriaxone ×2 wk | 1000 mg, 500 mg weekly ×5 wk | Clinical cure at 6 wk | |
| 60 M | Shorten hospital stay | Native aortic valve endocarditis | Streptococcus gallolyticus | Amoxicillin/clavulanate and gentamicin ×2 wk | 1500 mg weekly ×2 | Clinical cure at 6 wk | |
| Morrisette et al, 2019 [15] Retrospective cohort |
63 M | Not OPAT candidate | Bacteremia | MRSA | Vancomycin, cefepime/ceftriaxone/ ciprofloxacin ×7 d | 1500 mg on day 1, 500 mg on day 7 | Loss to follow-up |
| 40 M | PWID | Tricuspid valve endocarditis | Culture negative | Vancomycin and cefepime ×5 d | 1000 mg, 500 mg weekly ×2 | Loss to follow-up | |
| Azamgarhi et al, 2019 [16] Case report |
76 F | Intolerances to other agents | Prosthetic joint infection | Methicillin-resistant S epidermidis | 5 d of vancomycin, ceftriaxone and 2 doses of amikacin | 1500 mg 10 d apart ×2 | Clinical cure at 4 mo |
| Cho et al, 2015 [17] Case report |
54 M | Alcohol abuse and history of noncompliance | Bacteremia with septic thrombophlebitis | MSSA | Cefazolin ×6 d | 1000 mg on day 1, 500 mg on day 8 | Clinical success at end of therapy |
| Guzek et al, 2018 [18] Case report |
60 M | No clinical improvement on vancomycin | Deep sternal wound infection | MRSA | Vancomycin ×9 d, rifampicin ×4 d | 1500 mg every other week ×2 | Clinical success at end of therapy |
| Hakim et al, 2020 [19] Case report |
27 F | PWID | Tricuspid valve endocarditis with septic pulmonary emboli | MSSA | Sulfamethoxazole-trimethoprim PO ×2 d | 1500 mg, 500 mg weekly ×5 | Clinical cure at 6 wk |
| Martinez-Sanz et al, 2018 [20] Case report |
46 M | Difficulty maintaining IV access | Bacteremia with septic thrombophlebitis | MSSA | Amoxicillin-clavulanate ×1 d, cloxacillin ×3 d, cefazolin and daptomycin ×3 d | 1500 mg every other week ×3 | Clinical success at 12 wk |
| Ramirez Hidalgo et al, 2018 [21] Case report |
55 F | Avoid line placement | Prosthetic joint infection | Methicillin-resistant S epidermidis | Daptomycin ×10 d | 1000 mg, 500 mg weekly ×3 | Clinical success at 2 mo |
Abbreviations: AKA, above-knee amputation; BKA, below-knee amputation; CoNS, coagulase-negative staphylococci; IV, intravenous; MRSA, methicillin-resistant Staphylococcus aureus; MSSA, methicillin-susceptible Staphylococcus aureus; OPAT, outpatient parenteral antibiotic therapy; PO, per oral; PWID, person who injects drugs; PWUD, person who uses drugs.
The average age of included patients was 51 years; the majority were male. Not all studies disclosed information on substance use disorders or injection drug use, but at least 11 patients were reported to have an active substance use disorder. Outside of the 2 clinical trials, other reasons cited for use of dalbavancin included poor venous access, failure or intolerance of other antibiotics, a desire to reduce hospital length of stay, or unspecified reasons that a patient was not eligible for OPAT.
The indications for dalbavancin and the infecting pathogens among the 144 cases are shown in Table 2. Ninety-four (65%) cases involved bone or joint infections, with the vast majority (96%) being osteomyelitis. Eighty-five (90%) of these patients had documentation of surgery or debridement. Other indications included catheter-related bloodstream infection (n = 24 [17%]), infective endocarditis (n = 19 [13%]), and complicated bacteremia (n = 7 [5%]). Fifty-four patients had positive blood cultures and 50 (93%) cleared blood cultures prior to administration of dalbavancin. A bacterial pathogen was identified in all but 1 case; 16 (11%) of the infections were polymicrobial. Pathogens included S aureus (93 [57%]), coagulase-negative Staphylococcus species (31 [19%]), Enterococcus species (13 [8%]), Streptococcus species (10 [6%]), and other gram-positive organisms (15 [9%]). One-third of S aureus isolates were methicillin-resistant; 2 cases involved Enterococcus faecium.
Table 2.
Pathogens Identified by Indication for Dalbavancin
| Indication for Dalbavancin (No. of Bacterial Isolates) |
Pathogena | ||||
|---|---|---|---|---|---|
| Staphylococcus aureus | Coagulase-Negative Staphylococci | Streptococci | Enterococci | Otherb | |
| Bone or joint infection (n = 109) | 67 (61) | 16 (15) | 4 (4) | 8 (7) | 14 (13) |
| Catheter-related bloodstream infection (n = 27) | 12 (44) | 13 (48) | 0 | 2 (7) | 0 |
| Infective endocarditis (n = 19) | 7 (37) | 2 (11) | 6 (32) | 3 (16) | 1 (5) |
| Complicated bacteremia (n = 7) | 7 (100) | 0 | 0 | 0 | 0 |
| Total (N = 162) | 93 (57) | 31 (19) | 10 (6) | 13 (8) | 15 (9) |
Data are presented as No. (%).
aMore than 1 pathogen was identified in 16 cases; thus, the sum of the number of pathogens (n = 162) exceeds the total number of cases (n = 144) included in the review.
bOther pathogens include Corynebacterium striatum, Aerococcus viridans, Aerococcus urinae, Globicatella sp, Micrococcus luteus, and undefined gram-positive anaerobic organisms.
Ninety-five (66%) patients did not receive any antibiotic therapy prior to initiation of dalbavancin; however, 90 of these patients were from the 2 randomized controlled trials. One retrospective cohort excluded patients who received more than a week of antibiotics prior to dalbavancin but did not report the type or duration of antibiotics prior to dalbavancin [9]. Of the remaining 38 patients who received antibiotics prior to dalbavancin, the mean duration of treatment was 8.6 days (range, 1–14 days). The antibiotics given prior to dalbavancin primarily included β-lactams and vancomycin. Dalbavancin dosing regimens across publications varied. In the majority of publications, including the 2 randomized trials, 2 dalbavancin doses were given 1 week apart; however, the initial dose varied from 1000 to 1500 mg and the second dose varied from 500 to 1500 mg. The median total dose was 3 g but ranged from 1.5 to 7.5 g. The greatest variability in dosing was for osteoarticular infections; 3 patients received >10 doses of dalbavancin. Patients with catheter-related bloodstream infection tended to receive a lower total dalbavancin dose while those with infective endocarditis tended to receive a higher total dose. A summary of dalbavancin dosing by indication is shown in Table 3.
Table 3.
Dalbavancin Dosing by Indication
| Indication | No. of Doses, Median (Range) | Total Dalbavancin Dose, g, Median (Range) |
|---|---|---|
| Bone or joint infection (n = 94) | 2 (2–14) | 3 (1.5–7.5) |
| Catheter-related bloodstream infection (n = 24) | 2 (2–2) | 1.5 (1.5–3) |
| Infective endocarditis (n = 19) | 3 (2–6) | 3.5 (1.5–4) |
| Complicated bacteremia (n = 7) | 2 (2–3) | 2.5 (1.5–4.5) |
Treatment outcomes are outlined in Table 4. Clinical success, as reported in each individual publication, occurred in 133 (92%) patients. There were 6 reported cases of treatment failure. Of those, 4 involved recurrence of infection and 2 required amputation for progression of osteomyelitis. Clinical outcomes could not be assessed in 5 patients who were lost to follow-up. There were few adverse events reported; the most common included nausea, diarrhea, and infusion-related reactions. One case of acute kidney injury was reported. In no cases was therapy with dalbavancin reported to have been discontinued due to an adverse event.
Table 4.
Clinical Outcomes by Indication
| Indication | Clinical Success | Treatment Failure | Loss to Follow-upa |
|---|---|---|---|
| Bone or joint infection (n = 94) | 89 (94.7) | 2 (2.1) | 3 (3.2) |
| Catheter-related bloodstream infection (n = 24) | 22 (91.7) | 2 (8.3) | 0 |
| Infective endocarditis (n = 19) | 18 (95) | 0 | 1 (5) |
| Complicated bacteremia (n = 7) | 4 (57.1) | 2 (28.6) | 1 (14.3) |
Data are presented as No. (%).
aPatient received at least 2 doses of dalbavancin, but did not have any hospital readmissions or ambulatory care visits to assess treatment outcome.
Several ongoing trials were identified that are evaluating dalbavancin for invasive gram-positive infections. In a phase 2b open-label study conducted by the Antibacterial Resistance Leadership Group of the National Institutes of Health (NCT04775953), a total of 200 participants with complicated S aureus bacteremia or right-sided infective endocarditis will be randomized to receive dalbavancin 1500 mg on days 1 and 8 or standard of care antibiotic therapy. Among the exclusion criteria for this trial are left-sided endocarditis and prosthetic valve, cardiac device, or vascular graft infections where the prosthesis is not promptly removed. In an ongoing pilot study (NCT03426761), 50 participants with native or prosthetic joint septic arthritis caused by a gram-positive pathogen will be randomized to dalbavancin 1500 mg every 14 days or standard of care antibiotic therapy. The total duration of therapy is not specified. Last, an open-label, single-arm study (NCT04847921) is evaluating the use of dalbavancin for invasive gram-positive infections in up to 60 participants with a substance use disorder who are not candidates for OPAT. No trials were identified that plan to evaluate dalbavancin for left-sided infective endocarditis.
DISCUSSION
To our knowledge, this is the first scoping review of the evidence for dalbavancin as the predominant therapy for invasive gram-positive infections requiring prolonged antibiotic treatment. There have been a number of studies evaluating the safety and efficacy of dalbavancin for off-label indications such as osteoarticular infection or infective endocarditis. However, most of these studies included patients who received extended courses of standard of care antibiotic therapy prior to initiation of dalbavancin. For example, in 1 retrospective review of dalbavancin for the treatment of osteoarticular infections [22], the median duration of antibiotic therapy prior to dalbavancin treatment was 32 days in the native infection group (n = 19) and 41 days in the implant-related infection group (n = 44). In another retrospective review, among 34 patients with infective endocarditis treated with dalbavancin, the median duration of antibiotics prior to the initiation of dalbavancin was 28 days [23]. Given that national guidelines for osteomyelitis, prosthetic joint infections, and infective endocarditis recommend total treatment durations of 4 – 6 weeks (28–42 days) [24–26], the extended treatment courses prior to dalbavancin in these studies make it difficult, if not impossible, to attribute clinical outcomes to dalbavancin itself. To avoid this problem in the present scoping review, we included only publications or cases in which no more than 14 days of antibiotics were given prior to dalbavancin. In fact, more than two-thirds of patients received no other antibiotics prior to starting dalbavancin, and in those who did receive prior antibiotics, the mean treatment duration was <9 days. This approach served to reduce heterogeneity of cases and allow for a more meaningful assessment of clinical outcomes.
Overall, the findings of this scoping review demonstrate a relative paucity of data and low-quality evidence for dalbavancin for deep-seated or endovascular infections. Of the 2 published randomized controlled trials, both were industry-sponsored and had a small sample size and open-label design limiting interpretation of their results. The randomized trial performed by Rappo and colleagues was designed as a pilot study with patients randomized 7:1 to dalbavancin or standard of care, respectively. A larger randomized trial meant to confirm the findings of the pilot study was initiated, but never completed (NCT03091439). One-third of the cases identified in this review were from retrospective observational studies or case reports. There are currently no prospective data for the use of dalbavancin for right- or left-sided infective endocarditis. The randomized trials underway for complicated S aureus bacteremia (including right-sided endocarditis) and septic arthritis may ultimately shed additional light on the safety and effectiveness of dalbavancin for these conditions; however, these may be years away from completion and the trial of septic arthritis is a small pilot study. As off-label use of dalbavancin is increasing, including for infective endocarditis, these findings highlight an important knowledge gap for the field. It is clear that additional randomized controlled trials will be necessary to develop a complete understanding of the role of dalbavancin for deep-seated or endovascular infections caused by gram-positive pathogens.
We found that dalbavancin dosing across publications was variable. Two doses given 1 week apart was the most commonly used regimen, but with a wide range of total dalbavancin doses. In a smaller number of cases, doses were given at weekly intervals for more prolonged periods. This heterogeneity makes it difficult to draw conclusions about the optimal dosing of dalbavancin for complicated infections; however, 2 doses 1 week apart appears to be an appropriate regimen based on the findings of this review. The definitions of clinical outcomes used in the included publications were also heterogenous, and for that reason, we were only able to report clinical success or failure as they were classified in individual publications. The overall 92% clinical success rate in this review is somewhat higher than might be expected for these types of complicated infections. This indicates the possibility of reporting bias in that cases with positive clinical outcomes may have been more likely to be submitted or accepted for publication. This high success rate also contrasts with findings from a recent meta-analysis evaluating use of dalbavancin or oritavancin for a variety of complicated infections [27]. In this meta-analysis, the median success rate for osteoarticular infections, catheter-related bloodstream infections, and infective endocarditis was 73% (n = 426), 75% (n = 123), and 68% (n = 133), respectively. It should be noted that numerous dosing regimens were used in studies included in this meta-analysis, and many patients received prolonged durations of antibiotic therapy prior to dalbavancin or oritavancin or received concomitant antibiotics during treatment. As such, these results are not directly comparable with this scoping review focused on multiple-dose dalbavancin regimens used as the predominant therapy.
A number of cases included in this review involved the use of dalbavancin as an alternative to prolonged antibiotic therapy in the hospital for patients with a substance use disorder or housing instability that precluded use of OPAT. In these clinical scenarios, the potential for treatment with only 2 doses of dalbavancin 1 week apart is an attractive option since care continuity for prolonged community-based antibiotic therapy within these populations can be challenging due to high loss to follow-up rates [12, 28], the potential for central venous catheter complications or misuse, and other barriers such as lack of housing, transportation, or telephones. Infective endocarditis is particularly common in patients with infections related to injection drug use. In these clinical scenarios, it is important for clinicians to recognize that there are currently no prospective data for the use of dalbavancin for infective endocarditis. Furthermore, it should be noted that even with use of a once-weekly antibiotic like dalbavancin, nonadherence to subsequent doses and loss to follow-up have been commonly reported [12]. Thus, use of dalbavancin in such patients must be coupled with appropriate measures to promote completion of therapy.
This scoping review had several important limitations. First, the relatively small number of cases identified, the observational nature of many of the studies, and the lack of standardized clinical outcomes preclude conclusions regarding the safety and effectiveness of dalbavancin for the infections included in this review. Furthermore, all adverse events may not have been captured and reported, particularly in the observational studies. Second, two-thirds of the S aureus isolates were susceptible to methicillin, and it is unclear if dalbavancin is as efficacious as β-lactams for serious methicillin-susceptible S aureus infections. Third, since one-third of included cases were from retrospective observational studies or case reports, reporting bias may have contributed to a falsely high reported rate of clinical success. Fourth, by limiting the review to cases in which at least 2 doses of dalbavancin were given, cases in which the patient became lost to follow-up after a single dose would have been excluded. This also may have contributed to a falsely high clinical success rate, and a falsely low rate of patients lost to follow-up. Fifth, our literature search was limited to Medline and ClinicalTrials.gov databases; we therefore may have not identified all relevant publications or ongoing clinical trials. Finally, although we attempted to limit the review to use of dalbavancin as the predominant therapy, some patients may still have received a substantial amount of antibiotic therapy (up to 14 days) prior to starting dalbavancin.
In summary, the evidence for use of a multiple-dose dalbavancin regimens as the predominant therapy for deep-seated or endovascular infections is limited by a paucity of data from controlled trials, heterogeneity of dosing regimens, and a lack of standardized clinical outcomes. Although it may be reasonable to consider treatment with multiple doses of dalbavancin for patients in whom the standard of care is not feasible, clinicians should be aware of the limitations of the evidence supporting this treatment approach, particularly for infective endocarditis. Clinical trials underway are likely to address some of the existing knowledge gaps; however, additional trials will be necessary to determine the role of dalbavancin for invasive infections caused by gram-positive pathogens that require prolonged antibiotic therapy.
Notes
Potential conflicts of interest. All authors: No reported conflicts of interest.
All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1.Durata Therapeutics. Dalbavancin [package insert]. Parsippany, NJ: Durata Therapeutics; 2014. [Google Scholar]
- 2. Dunne MW, Puttagunta S, Sprenger CR, et al. Extended-duration dosing and distribution of dalbavancin into bone and articular tissue. Antimicrob Agents Chemother 2015; 59:1849–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Iversen K, Ihlemann N, Gill SU, et al. Partial oral versus intravenous antibiotic treatment of endocarditis. N Engl J Med 2019; 380:415–24. [DOI] [PubMed] [Google Scholar]
- 4. Li HK, Rombach I, Zambellas R, et al. ; OVIVA Trial Collaborators. Oral versus intravenous antibiotics for bone and joint infection. N Engl J Med 2019; 380:425–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Dunne MW, Talbot GH, Boucher HW, et al. Safety of dalbavancin in the treatment of skin and skin structure infections: a pooled analysis of randomized, comparative studies. Drug Saf 2016; 39:147–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Vazquez Deida AA, Shihadeh KC, Preslaski CR, et al. Use of a standardized dalbavancin approach to facilitate earlier hospital discharge for vulnerable patients receiving prolonged inpatient antibiotic therapy. Open Forum Infect Dis 2020; 7:ofaa293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Rappo U, Puttagunta S, Shevchenko V, et al. Dalbavancin for the treatment of osteomyelitis in adult patients: a randomized clinical trial of efficacy and safety. Open Forum Infect Dis 2019; 6:ofy331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Raad I, Darouiche R, Vazquez J, et al. Efficacy and safety of weekly dalbavancin therapy for catheter-related bloodstream infection caused by gram-positive pathogens. Clin Infect Dis 2005; 40:374–80. [DOI] [PubMed] [Google Scholar]
- 9. Almangour TA, Perry GK, Alhifany AA. Dalbavancin versus standard of care for the treatment of osteomyelitis in adults: a retrospective matched cohort study. Saudi Pharm J 2020; 28:460–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Ajaka L, Heil E, Schmalzle S. Dalbavancin in the treatment of bacteremia and endocarditis in people with barriers to standard care. Antibiotics 2020; 9:700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Almangour TA, Perry GK, Terriff CM, et al. Dalbavancin for the management of gram-positive osteomyelitis: effectiveness and potential utility. Diagn Microbiol Infect Dis 2019; 93:213–8. [DOI] [PubMed] [Google Scholar]
- 12. Bryson-Cahn C, Beieler AM, Chan JD, et al. Dalbavancin as secondary therapy for serious Staphylococcus aureus infections in a vulnerable patient population. Open Forum Infect Dis 2019; 6:ofz028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Tobudic S, Forstner C, Burgmann H, et al. Dalbavancin as primary and sequential treatment for gram-positive infective endocarditis: 2-year experience at the general hospital of Vienna. Clin Infect Dis 2018; 67:795–8. [DOI] [PubMed] [Google Scholar]
- 14. Durante-Mangoni E, Boccia F, Ursi MP, et al. Dalbavancin for infective endocarditis: a single centre experience. J Chemotherapy. 2021; 33:256–62. [DOI] [PubMed] [Google Scholar]
- 15. Morrisette T, Miller MA, Montague BT, et al. On- and off-label utilization of dalbavancin and oritavancin for gram-positive infections. J Antimicrob Chemother 2019; 74:2405–16. [DOI] [PubMed] [Google Scholar]
- 16. Azamgarhi T, Donaldson J, Shah A, Warren S. Dalbavancin to treat infected massive endoprostheses: a case report and cost comparison analysis. J Bone Jt Infect 2019; 4:234–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Cho JC, Estrada SJ, Beltran AJ, Revuelta MP. Treatment of methicillin-sensitive Staphylococcus aureus bacteremia secondary to septic phlebitis using dalbavancin. J Clin Pharm Ther 2015; 40:604–6. [DOI] [PubMed] [Google Scholar]
- 18. Guzek A, Suwalski G, Tomaszewski D, Rybicki Z. Dalbavancin treatment in a deep sternal wound MRSA infection after coronary artery bypass surgery: a case report. J Cardiothorac Surg 2018; 13:3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Hakim A, Braun H, Thornton D, Strymish J. Successful treatment of methicillin-sensitive Staphylococcus aureus tricuspid-valve endocarditis with dalbavancin as an outpatient in a person who injects drugs: a case report. Int J Infect Dis 2020; 91:202–5. [DOI] [PubMed] [Google Scholar]
- 20. Martínez-Sanz J, Gijón de la Santa L, Torralba M. Treatment with dalbavancin in a patient with septic thrombophlebitis of the internal jugular vein due to Staphylococcus aureus after insertion of an implantable cardioverter defibrillator. Enferm Infecc Microbiol Clin 2018; 36:389–90. [DOI] [PubMed] [Google Scholar]
- 21. Ramírez Hidalgo M, Jover-Sáenz A, García-González M, Barcenilla-Gaite F. Dalbavancin treatment of prosthetic knee infection due to oxacillin-resistant Staphylococcus epidermidis. Enferm Infecc Microbiol Clin (Engl Ed) 2018; 36:142–3. [DOI] [PubMed] [Google Scholar]
- 22. Morata L, Cobo J, Fernandez-Sampedro M, et al. Safety and efficacy of prolonged use of dalbavancin in bone and joint infections. Antimicrob Agents Chemother 2019; 63:e02280-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Hidalgo-Tenorio C, Vinuesa D, Plata A, et al. DALBACEN cohort: dalbavancin as consolidation therapy in patients with endocarditis and/or bloodstream infection produced by gram-positive cocci. Ann Clin Microbiol Antimicrob 2019; 18:30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lipsky BA, Berendt AR, Cornia PB, et al. Infectious Diseases Society of America clinical practice guideline for the diagnosis and treatment of diabetic foot infections. Clin Infect Dis 2012; 54:e132–73. [DOI] [PubMed] [Google Scholar]
- 25. Osmon DR, Berbari EF, Berendt AR, et al. ; Infectious Diseases Society of America. Diagnosis and management of prosthetic joint infection: clinical practice guidelines by the Infectious Diseases Society of America. Clin Infect Dis 2013; 56:e1–25. [DOI] [PubMed] [Google Scholar]
- 26. Baddour LM, Wilson WR, Bayer AS, et al. Infective endocarditis in adults: diagnosis, antimicrobial therapy, and management of complications: a scientific statement for healthcare professionals from the American Heart Association. Circulation 2015; 132:1435–86. [DOI] [PubMed] [Google Scholar]
- 27. Thomas G, Henao-Martínez AF, Franco-Paredes C, Chastain DB. Treatment of osteoarticular, cardiovascular, intravascular-catheter-related and other complicated infections with dalbavancin and oritavancin: a systematic review. Int J Antimicrob Agents 2020; 56:106069. [DOI] [PubMed] [Google Scholar]
- 28. Bork JT, Heil EL, Berry S, et al. Dalbavancin use in vulnerable patients receiving outpatient parenteral antibiotic therapy for invasive gram-positive infections. Infect Dis Ther 2019; 8:171–84. [DOI] [PMC free article] [PubMed] [Google Scholar]