Abstract
Background:
We conducted a two-part study to evaluate the incorporation of veliparib, a PARP inhibitor, into chemoradiotherapy (CRT) for stage III non-small cell lung cancer.
Patients and Methods:
In the phase I part, patients were treated successively at 3 dose levels of veliparib (40, 80, and 120 mg) twice daily during CRT. In the phase II part, patients were randomized to receive veliparib or placebo during thoracic radiotherapy with concurrent weekly carboplatin and paclitaxel, followed by two cycles of consolidation carboplatin and paclitaxel with velirapib or placebo. The study was prematurely discontinued due to the emergence of adjuvant immunotherapy as standard of care.
Results:
Of 21 patients enrolled in phase I, 2 patients developed dose-limiting toxicities (DLTs): one grade 3 esophagitis with dysphagia (at 40 mg) and one grade 3 esophagitis with dehydration (at 80 mg). No DLTs were seen at veliparib dose of 120 mg twice daily which was selected for the phase II part that enrolled 31 eligible patients. Progression-free survival (PFS) was not different between the two arms (p = 0.20). For the veliparib and placebo arms, response rates were 56% and 69%, PFS at 1 year 47% and 46%, and overall survival at 1 year 89% and 54%, respectively.
Conclusions:
Veliparib with CRT was feasible and well tolerated. Efficacy could not accurately be determined due to early study closure. Nonetheless, there is enthusiasm for the evaluation of PARP inhibitors in lung cancer as predictive biomarkers are being developed and combinations with immunotherapy are attractive.
MICRO-ABSTRACT
We evaluated the incorporation of veliparib, a PARP inhibitor, into chemoradiotherapy with carboplatin and paclitaxel for stage III non-small cell lung cancer. In the phase I part that enrolled 21 patients we selected veliparib dose of 120 mg twice daily as the recommended phase II dose. The phase II part that enrolled 31 eligible patients. Progression-free survival (PFS) was not different between the two arms (p = 0.20). Veliparib with chemoradiotherapy was feasible and well tolerated. Efficacy could not accurately be determined due to early study closure.
INTRODUCTION
Lung cancer is the leading cause of cancer-related deaths in the United States among both sexes, while worldwide it is the most common cause of cancer deaths among men and the second leading cause of cancer death in women [1]. Approximately 20% of patients with non–small-cell lung cancer (NSCLC) have stage III disease at presentation [1]. Combined modality treatment with radiation therapy (RT) and concurrent chemotherapy using either etoposide and cisplatin or carboplatin and paclitaxel is the standard of care for the treatment of stage III, unresectable non-small cell lung cancer (NSCLC) [2–4]. Unfortunately only 20% of patients achieve long-term survival [5]. Studies that attempted to escalate radiotherapy dose failed to demonstrate any benefit [6]. Recent advances are limited to the addition of an immune checkpoint inhibitor, durvalumab, as consolidation therapy after chemoradiotherapy (CRT). This approach has demonstrated an improvement in both progression-free survival (PFS) and overall survival (OS) over CRT alone in a phase III trial (“PACIFIC” trial) [7]. Further advances in treatment outcomes continue to be an area of clinical need in this patient population.
Veliparib (ABT-888) is an oral poly (ADP-ribose) polymerase (PARP) inhibitor that may potentiate the effect of DNA-damaging agents, including radiation and chemotherapy [8].
Gain of DNA repair capacity of the tumor represents a common mechanism used by cancer cells to survive DNA-damaging therapy. PARP-1 is a nuclear enzyme that is activated by DNA damage and plays a critical role in base excision repair. Inhibition of PARP represents an attractive approach for the treatment of cancer. A single-dose pharmacokinetic and pharmacodynamic endpoint study (single doses of veliparib at 10, 25, or 50 mg) was conducted in 13 patients with advanced cancer [9]. Target inhibition of poly (ADP-ribose) levels was reported in tumor biopsies and peripheral blood mononuclear cells at the 25-mg and 50-mg dose levels. An initial phase I trial of carboplatin, paclitaxel plus veliparib evaluated the recommended phase II doses and drug pharmacokinetics [10], whereas a subsequent phase II trial with this regimen using a veliparib dose of 120 mg twice daily showed promising results in advanced NSCLC, especially in squamous cell carcinoma [11]. Synergy has been noted with combined veliparib and radiation in lung cancer models [12]. Based on this data we conducted a phase I dose-finding trial followed by a randomized phase II study to evaluate the safety, tolerability, and preliminary efficacy of veliparib added to the backbone of standard CRT with carboplatin and paclitaxel in the curative setting treatment of stage III NSCLC (SWOG trial S1206; ClinicalTrials.gov Identifier: NCT01386385).
PATIENTS AND METHODS
Patient selection
Eligibility included adult patients with previously untreated, unresectable stage IIIA/IIIB (AJCC 7th edition), NSCLC (adenocarcinoma, large cell carcinoma, squamous cell carcinoma, or mixed). All patients underwent brain imaging with computed tomography (CT) or magnetic resonance imaging (MRI) scan. Documentation of measurable disease was required within 28 days of registration. All patients had measurable or evaluable disease as per RECIST 1.1 [13], ECOG performance status 0–1, and adequate hematologic, renal, hepatic, and pulmonary function as evidenced by all of the following: absolute neutrophil count (ANC) ≥ 1,500/μl; platelets ≥ 100,000/μl; hemoglobin ≥ 9.0 g/dl; total bilirubin within institutional upper limit of normal (IULN) and aspartate aminotransferase or alanine aminotransferase ≤ 2.5 × IULN; serum creatinine ≤ the IULN or creatinine clearance ≥ 60 cc/min using the Cockroft-Gault formula (for patients with creatinine levels above institutional normal); forced expiratory volume in one second (FEV1) ≥ 1.2 liters/second and/or ≥ 50% predicted. Patients must have been able to swallow whole capsules and had no more than 10% weight loss in the prior 6 months. No other prior malignancy was allowed except for the following: adequately treated basal cell or squamous cell skin cancer, in situ cervical cancer, adequately treated stage I or II cancer in complete remission, or any other cancer from which the patient was disease free for a minimum of five years: Patients were also excluded if they had grade ≥ 1 symptomatic neuropathy-sensory (National Cancer Institute Common Terminology Criteria Version 4.0), history of seizures, any known immune deficiencies, uncontrolled intercurrent illness including, active infections, symptomatic congestive heart failure, unstable angina pectoris, cardiac arrhythmia, or psychiatric illness/social situations that would limit compliance with study requirements.
The institutional review board (IRB) of each of the participating centers approved the conduct of this trial led by/ SWOG in accordance with the Declaration of Helsinki and Good Clinical Practice. Written informed consent was obtained from all patients prior to inclusion onto the study according to institutional guidelines.
Treatment plan
Patients received paclitaxel 45 mg/m2 intravenously over 1 hour followed by carboplatin (AUC 2) intravenously over 30 minutes once weekly during RT, which was given to a total dose of 60 Gy in 30 fractions (Monday-Friday) over 6 weeks utilizing standard fractionation (one fraction per day). Either 3D conformal treatment planning or intensity-modulated RT (IMRT) was utilized. Proton therapy was not allowed on this study. The primary tumor and clinically positive lymph nodes seen either on the planning CT (> 1 cm short axis diameter) or pretreatment PET scan (SUV > 3) constituted the gross tumor tumor volume (GTV). The clinical target volume (CTV) was defined to be the GTV plus a 0.5 cm to 1 cm margin as appropriate to account for microscopic tumor extension. Elective treatment of the mediastinum and supraclavicular fossae was not done. Any mediastinal node detected by CT scan >1.0 cm (short axis) or with SUV > 3 on pre-treatment PET scan was included as GTV, with the appropriate margin to create the CTV. The planning target volume (PTV) was the CTV plus a margin to ensure that the prescribed dose was actually delivered to the GTV. The PTV was within a range of 0.5 to 1.5 cm, depending on variations in treatment delivery, including variations in set-up between treatments, patient motion during treatment, movement of the tissues that contain the GTV, and size variations in the tissue containing the GTV. The prescribed dose to the PTV was 60 Gy. The spinal cord dose limitation was the highest priority dose constraint. Total “direct” plus “scatter” dose to the spinal cord was not to exceed 50.5 Gy. The dose-volume constraint to the lungs was the second highest priority. The volume of both lungs that receive more than 20 Gy (the V20) should not exceed 37% of the total lung volume. Alternatively, the mean lung dose should optimally be ≤ 20 Gy. The mean dose to the esophagus was optimally kept below 34 Gy. The V60 (% volume of esophagus exceeding 60 Gy) was calculated for each patient. For the heart, the following limits were recommended: 60 Gy to < 1/3, 45 Gy to < 2/3, and 40 Gy to < 100% of the heart. Patients registered to this study underwent radiation therapy review by the Quality Assurance Review Center (QARC). Materials were required to be submitted within 3 days after initiation of treatment.
Veliparib (NSC 737664/ IND 77840) and matching placebo were provided free of charge by Abbott Laboratories and distributed by the National Cancer Institute (NCI). In the phase I part of the study, veliparib (supplied as 20 mg capsules) was administered at escalating doses (40 mg, 80 mg, 120 mg) twice a day starting the first day of RT, continuously during RT and for one additional day after RT completion. In the phase II part, veliparib at 120 mg twice daily or placebo was administered during RT and for one additional day after RT completion. Capsules were taken without regard to meals. Missed doses were not made up. Four to 6 weeks after completing CRT, patients without disease progression on restaging scans, who met protocol specified criteria, received 2 cycles of carboplatin (AUC 6), paclitaxel (200 mg/m2) every 21 days and veliparib 80 mg (or placebo) twice daily on days 1–7 of each cycle. No dose reductions of carboplatin and paclitaxel were allowed during CRT, but weekly doses were skipped for protocol specified hematologic (e.g. grade 3–4 neutropenia or grade 2–4 thrombocytopenia) and non-hematologic toxicities on the day of due treatment. Patients who skipped chemotherapy due to toxicity also held veliparib until treatment resumed per protocol specified criteria. RT was temporarily held for in-field non-hematologic toxicity (grade 4 or persistent grade 3 esophagitis, grade 3 pneumonitis, grade 4 radiation induced dermatitis) or permanently discontinued for grade 4 pneumonitis.
Dose delays and/or one dose reduction of carboplatin, paclitaxel and veliparib were allowed during consolidation therapy for hematologic and non-hematologic grade 3–4 toxicities. Grade 3 or greater neuropathy required discontinuation of paclitaxel and temporary hold of carboplatin and veliparib. The protocol was amended in November of 2016 to allow for prophylactic growth factor support during consolidation. During the phase I portion of the trial adverse events (AEs) were monitored by the protocol management team and the disease committee chair through weekly toxicity reports and biweekly conference calls in which decisions about dose escalation were made based on DLT data defined below. Per SWOG policy the conduct of the randomized phase II portion of the study was supervised by an independent Data and Safety
Monitoring Committee.
Tumor assessments were performed at 4 weeks (+/− 3 days) after completion of CRT and after completion of consolidation therapy. Subsequently, repeat imaging with CT scans of chest and abdomen were performed every 4 months for up to 2 years after initial registration, then every 6 months for up to 3 years after initial registration.
Statistical methods
The phase I study evaluated 3 dose levels of veliparib in following a 3+3 design. The planned dose escalation highest dose cohort was 120 mg twice daily. No intra-patient dose escalation was allowed. DLT (dose-limiting toxicity) was evaluated during RT and the 2 weeks following completion of RT. Treatment-related AEs were captured for the duration of study treatment and during patients’ follow up in the study. DLT was defined as any of the following events attributable to the study regimen: radiation esophagitis or dermatitis grade 4 or grade 3 (lasting > 7 consecutive days), grade 4 neutropenia lasting for > 7 days or neutropenic fever (defined as ANC < 500 and a temperature of 38.5° C or above), grade 4 thrombocytopenia, grade 4 nausea/vomiting despite appropriate antiemetic therapy and delays in radiotherapy, chemotherapy or veliparib due to toxicity > 3 weeks. Non-hematological DLTs also included any grade 3 or higher toxicities with the following exceptions: anorexia, fatigue, infection without neutropenia, grade 3 AST/ALT elevations ≤ 7 days, grade 3 or 4 lymphopenia, grade 3 or 4 electrolyte abnormalities corrected to grade 2 or less in < 48 hours, grade 3 dehydration lasting < 7 days and infusion reactions. Patients with ≥ grade 3 infusion reactions were removed from study and replaced and were not considered evaluable for DLT. Patients were evaluable for DLT if they received at least 66% of the planned doses of veliparib at the assigned dose level, at least one dose each of carboplatin and paclitaxel, and initiated radiation, or if they experienced a DLT. Patients who were not evaluable for DLT were replaced.
The primary endpoint for the phase II part of the study was PFS. Patients were randomized equally between the two arms (veliparib vs. placebo) with randomization stratified by stage (IIIA vs. IIIB) and histology (squamous vs. non-squamous). The analysis followed a modified intention-to-treat principle, evaluating all randomized and eligible patients. The study was designed with 85% power to detect a hazard ratio of 0.57 (akin to a 75% improvement in median PFS) using a 1-sided 10% level log-rank test which would require the observation of 68 progression events. Assuming exponentially distributed PFS, a median of 11 months in the placebo arm, 120 eligible patients accrued over 15 months with a minimum of 12 months of follow-up from the completion of accrual, were needed based on the design assumptions. Assuming a 10% ineligibility rate, the projected accrual was 132 patients. Secondary endpoints included OS, response rate and toxicities. Response rate was evaluated by RECIST1.1 [13]. Disease control was defined as the sum of complete response, partial response and stable disease. OS and PFS were estimated using the method of Kaplan-Meier. Confidence intervals for the median OS survival and PFS were calculated according to the method of Brookmeyer and Crowley [14]. Time-to-event outcomes were compared between the arms using a 1-sided stratified log-rank test, and binary endpoints were compared using stratified Cochran–Mantel–Haenszel test at the 10% level. Hazard ratios (HR) and corresponding confidence intervals were estimated using a stratified Cox proportional-hazard model. Toxicities were graded according to the version 4.0 of the NCI Common Terminology Criteria for Adverse Events.
RESULTS
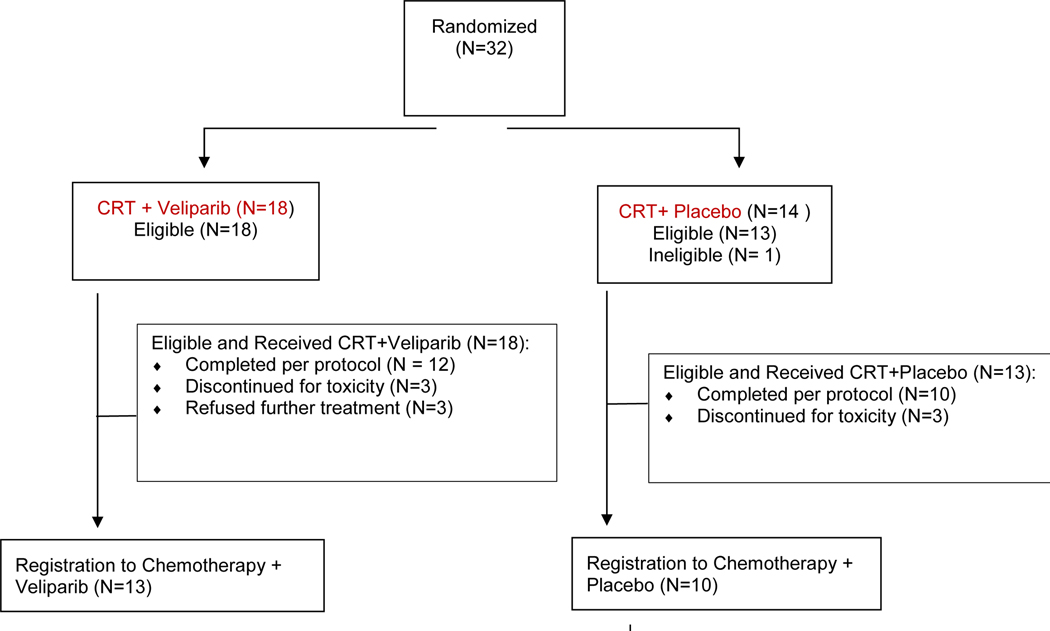
Between January 2013 and December 2015, the phase I portion of the study accrued 21 patients from 6 SWOG institutions. The phase II portion accrued 32 patients between July 2016 and October 2017 and was open to accrual to all Clinical Trials Support Unit (CTSU) network members. All 21 patients in the phase I portion met the eligibility criteria; 1 patient on the phase II portion was not eligible due to inadequate renal function. Of the 31 eligible patients on the phase II, 18 were randomized to the velparib-containing arm and 13 to the placebo-containing arm (Figure 1). The median follow-up time among alive patients in phase I is 40.6 months and 26.9 months for phase II patients.
Figure 1.
CONSORT flow diagram
Table 1 displays patient demographics and disease characteristics of both the phase I and phase II components of the study. Patients enrolled in the phase II part had more advanced disease than patients enrolled in phase I (61% had stage IIIB in phase II vs. 24% in phase I).
Table 1:
Patient characteristics
| Phase I (n = 21) | Phase II (n=31) |
|||
|---|---|---|---|---|
| All Phase II Patients (N = 31) | CRT+ Veliparib (N=18) | CRT + Placebo (N=13) | ||
|
| ||||
| Age, median (range) | 70 (53 – 81) | 64.7(47.0–78.9) | 65.0(56.6–75.6) | |
|
| ||||
| Sex | ||||
| Male | 14 (67%) | 14(45%) | 7(39%) | 7(54%) |
| Female | 7 (33%) | 17(55%) | 11(61%) | 6(46%) |
|
| ||||
| Zubrod PS | ||||
| 0 | 11 (52%) | 10(32%) | 7(39%) | 3(23%) |
| 1 | 10 (48%) | 21(68%) | 11(61%) | 10(77%) |
|
| ||||
| Race | ||||
| White | 18 (86%) | 25(81%) | 13(72%) | 12(92%) |
| African American | 1 (5%) | 3(10%) | 2(11%) | 1(8%) |
| Asian | 1 (5%) | 2(6%) | 2(11%) | 0(0%) |
| Unknown | 1 (5%) | 1(3%) | 1(6%) | 0(0%) |
|
| ||||
| Stage | ||||
| IIIA | 16 (76%) | 12(39%) | 7(39%) | 5(38%) |
| IIIB | 5 (24%) | 19(61%) | 11(61%) | 8(62%) |
|
| ||||
| Histology | ||||
| Squamous cell | 8 (38%) | 15(48%) | 10(56%) | 5(38%) |
| Adenocarcinoma | 12 (57%) | 16(52%) | 8(44%) | 8(62%) |
| Other | 1(4.8%) | 0 | 0 | 0 |
|
| ||||
| Baseline LDH | ||||
| Normal | 15 (71%) | 25(81%) | 16(89%) | 9(69%) |
| Elevated | 3 (14%) | 5(16%) | 2(11%) | 3(23%) |
| Not reported | 3 (14%) | 1(3%) | 0(0%) | 1(8%) |
|
| ||||
| Smoking status | ||||
| Current smoker | 4 (19%) | 14(45%) | 8(44%) | 6(46%) |
| Former smoker Never | 13 (62%) | 16(52%) | 9(50%) | 7(54%) |
| smoker | 4 (19%) | 1(3%) | 1(6%) | 0(0%) |
|
| ||||
| Weight loss past 6 months | ||||
| < 5% | 15 (71%) | 24(71%) | 14(78%) | 8(62%) |
| 5–10% | 6 (29%) | 9(29%) | 4(22%) | 5(38%) |
PS, performance status; LDH, lactate dehydrogenase; CRT, concurrent chemoradiotherapy
The phase II study was prematurely discontinued because of the results of the PACIFIC trial [7] that led to a change in the standard of care in stage III NSCLC with adoption of adjuvant durvalumab after CRT as standard practice in the United States.
Phase I Dose-Finding Results
The phase I portion of the study enrolled 21 patients; 8 to the 40 mg cohort, 7 to the 80 mg cohort, 6 to the 120 mg cohort. Of the 21 patients enrolled, 17 were evaluable for DLT (6 patients each within the 40 and 80 mg cohorts and 5 patients within the 120 mg cohort). One DLT was reported within both the 40 mg and 80 mg cohorts (grade 3 dysphagia, esophagitis lasting > 7 days, and esophageal pain on the 40 mg cohort and grade 3 dehydration and esophagitis lasting > 7 days on the 80 mg cohort), and no DLTs were reported in the 120 mg cohort. The patient who experienced DLT in the 80 mg cohort had an actual weight ≥ 140% over ideal weight and was erroneously treated with a carboplatin dose calculated on the basis of his actual weight versus ideal weight (as specified in the protocol for these patients) which resulted in an increased dosing of 20–24% during CRT over per protocol-planned. However, this patient completed all protocol treatment, including consolidation chemotherapy without experiencing any additional serious adverse events. All of the 4 patients not evaluable for DLTs received less than 66% of the planned doses of veliparib for the following reasons: 2 received less than 66% of the planned doses for reasons unrelated to study drug (associated co-morbidities and/or non-compliance), 1 declined treatment after developing atrial fibrillation during the first month, and 1 discontinued treatment after a grade 3 drug-infusion reaction related to paclitaxel. Per design, the 120 mg twice daily was chosen as the veliparib dose for the phase II part of the study.
Treatment Delivery
Sixteen of the 21 patients enrolled on the phase I portion of the study completed CRT and veliparib as planned and 14 completed consolidation therapy as planned (i.e. as specified in protocol). In the phase II part, of the 18 patients who were randomized to the veliparib arm, 12 completed CRT, 3 were removed due to adverse events and 3 refused further treatment for reasons other than adverse events. All 12 patients who completed CRT, and 1 who did not receive all doses of CRT due to adverse events, registered to receive consolidation therapy. Of the 13 patients registered to receive consolidation, one had progressive disease discovered the day after registration (a major protocol deviation) and did not receive any protocol treatment. Of the remaining 12 patients, 8 completed consolidation treatment as planned, 2 discontinued treatment due to adverse events, and 2 refused further treatment. Of the 13 patients randomized to the placebo arm, 10 completed CRT and 3 were removed from treatment due to adverse events. All 10 patients who completed CRT received consolidation, and 8 completed consolidation treatment as planned. One was removed from treatment due to adverse events, and 1 was removed due to progressive disease.
During CRT, patients received veliparib for an average of 35 days compared to 40 days of placebo. During consolidation, more patients in the veliparib arm experienced dose delays (17%), reductions (17%) or discontinuation (17%) compared to the placebo arm (10% dose and reductions and 10% discontinuation); patients received an average of 9.7 days of veliparib compared to 13.2 days of placebo.
Adverse Events due to Chemoradiotherapy
Treatment-related adverse events (AE) due to CRT, from treatment start to end of CRT and the period before starting consolidation chemotherapy (i.e. a period of approximately 10–12 weeks) are summarized in Table 2.
Table 2:
Treatment-related grade 3–5 adverse events due to chemoradiotherapy
| Phase I (n=21) | Phase II CRT+ Veliparib (n=17) | Phase II CRT + Placebo (n=13) | |||||||
|---|---|---|---|---|---|---|---|---|---|
| ADVERSE EVENTS | 3 | 4 | 5 | 3 | 4 | 5 | 3 | 4 | 5 |
| Anemia | 1 (5%) | 1 (6%) | |||||||
| Anorexia | 1 (6%) | 1 (8%) | |||||||
| Dehydration | 1 (5%) | 1 (8%) | |||||||
| Dysphagia | 1 (5%) | ||||||||
| Esophageal pain | 1 (5%) | 1 (6%) | |||||||
| Esophageal perforation | 1 (5%) | ||||||||
| Esophagitis | 4 (19%) | 1 (6%) | 1 (8%) | ||||||
| Fatigue | 2 (10%) | 1 (6%) | 1 (8%) | ||||||
| Febrile neutropenia | 1 (5%) | ||||||||
| Hyperglycemia | 1 (6%) | ||||||||
| Hypocalcemia | 1 (8%) | ||||||||
| Hypoglycemia | 1 (8%) | ||||||||
| Hypokalemia | 1 (5%) | 1 (8%) | |||||||
| Hypomagnesemia | 1 (8%) | ||||||||
| Hyponatremia | 1 (5%) | 3 (23%) | |||||||
| Hypotension | 1 (6%) | ||||||||
| Infusion related reaction | 1 (5%) | ||||||||
| Lung infection | 1 (5%) | 1 (8%) | |||||||
| Lymphocyte count decreased | 9 (43%) | 3 (14%) | 1 (6%) | 2 (12%) | 2 (15%) | 2 (15%) | |||
| Mucositis oral | 1 (6%) | ||||||||
| Neutrophil count decreased | 7 (33%) | 1 (5%) | 3 (18%) | 1 (8%) | |||||
| Platelet count decreased | 1 (5%) | 1 (6%) | |||||||
| Pneumonitis | 1 (8%) | ||||||||
| Vomiting | 1 (8%) | ||||||||
| Weight loss | 1 (6%) | ||||||||
| White blood cell decreased | 5 (24%) | 2 (10%) | 2 (12%) | 2 (15%) | |||||
| Any esophagitis, dysphagia, esophageal pain, or/and perforation | 3 (14%) | 0 | 1(5%) | 2 (12%) | 0 | 0 | 1(8%) | 0 | 0 |
| MAX. GRADE ANY ADVERSE EVENT | 12 (57%) | 4 (19%) | 1 (5%) | 6 (35%) | 2 (12%) | 0 | 6 (46%) | 3 (23%) | 0 |
In the phase I portion, there was 1 treatment-related death due to esophageal perforation that occurred 8 months after completing CRT. This patient had developed a mid-esophageal stricture after CRT requiring endoscopic dilations. Four patients (19%) experienced grade 4 adverse events, which included lymphopenia (3) and neutropenia (1), noting that patients could have multiple events. In addition, 12 (57%) patients experienced grade 3 adverse events, which were mostly hematologic and had no relationship with dose levels.
In the phase II portion, there were no treatment-related deaths. Of 17 evaluable patients on concurrent CRT plus veliparib, 6 patients (35%) had treatment-related grade 3 adverse events, and 2 (12%) had treatment-related grade 4 lymphopenia. Of the 13 patients evaluable for adverse events on concurrent CRT plus placebo arm, 3 (23%) experienced treatment-related grade 4 adverse events;1 hypoglycemia, 2 lymphopenia, and 6 (46%) treatment-related grade 3 adverse events. No late grade 3–5 AEs were reported.
Adverse Events after Consolidation Therapy
Treatment-related adverse events related to consolidation therapy are reported in Supplemental Table 1. Fourteen patients in the phase I portion and 22 patients in the phase II portion (12 patients on the veliparib arm and 10 on the placebo arm) received consolidation therapy. In the phase I portion, there was 1 treatment-related death due to neutropenic sepsis that occurred during the second cycle of consolidation therapy.An additional 3 patients (21%) experienced grade 4 neutropenia during cycle 1.
In the phase II part, on the veliparib arm, 3 patients (25%) experienced treatment-related grade 4 adverse events: 2 neutropenia and 1 lymphopenia, and 5 patients (42%) had treatment-related grade 3 adverse events. One additional patient on the veliparib arm developed grade 3 esophageal stenosis and grade 4 esophageal fistula 15 months after completing consolidation chemotherapy (this event that occurred during long-term follow up is not included in the Supplemental Table 1).
On the placebo arm, there was 1 treatment-related death due to grade 5 pneumonitis that occurred 6 weeks after completing CRT. This patient was noted to have grade 4 lymphopenia as well. In addition, 1 patient on the placebo arm developed grade 4 hyperglycemia, and 1 patient developed grade 3 adverse events. No other patient developed a grade 3 or higher late toxicity after completing protocol treatment. Of note is that in the placebo arm, 3 patients (23%) had grade 1 esophagitis and 3 patients (23%) had grade 2 esophagitis, whereas in the veliparib arm, 1 patient (6%) had grade 1 esophagitis and 5 patients (29%) had grade 2 esophagitis. In terms of less than grade 3 pneumonitis, only a single patient on placebo had grade 1 pneumonitis.No other patient had grade 2 or less pneumonitis.
Efficacy Outcomes
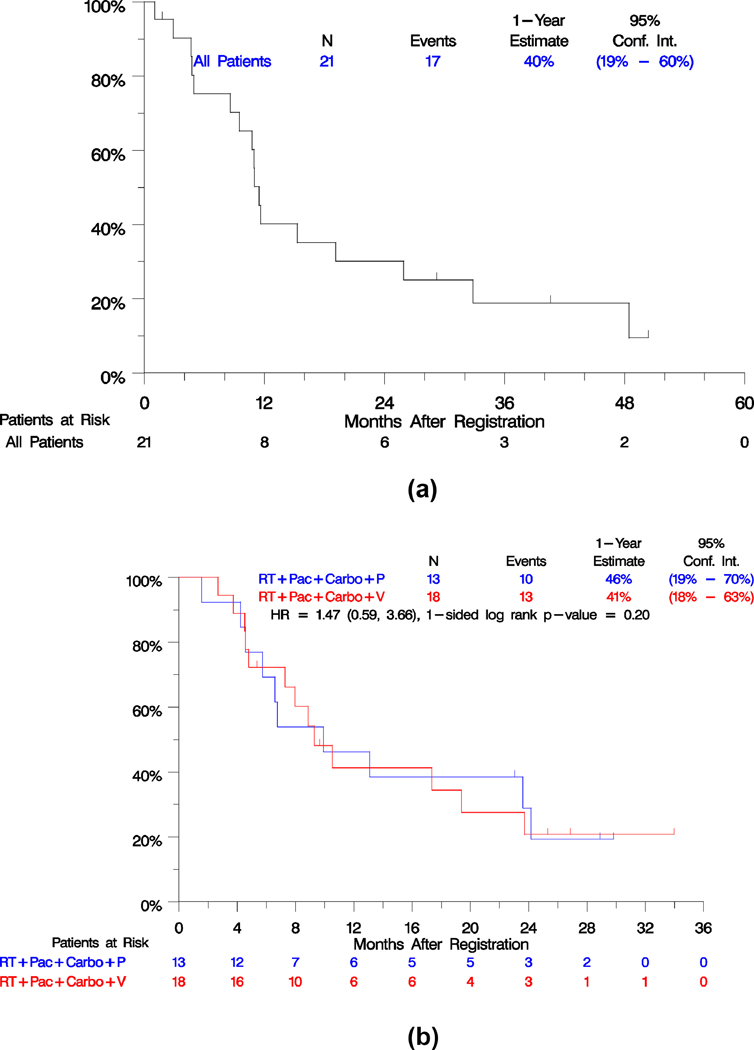
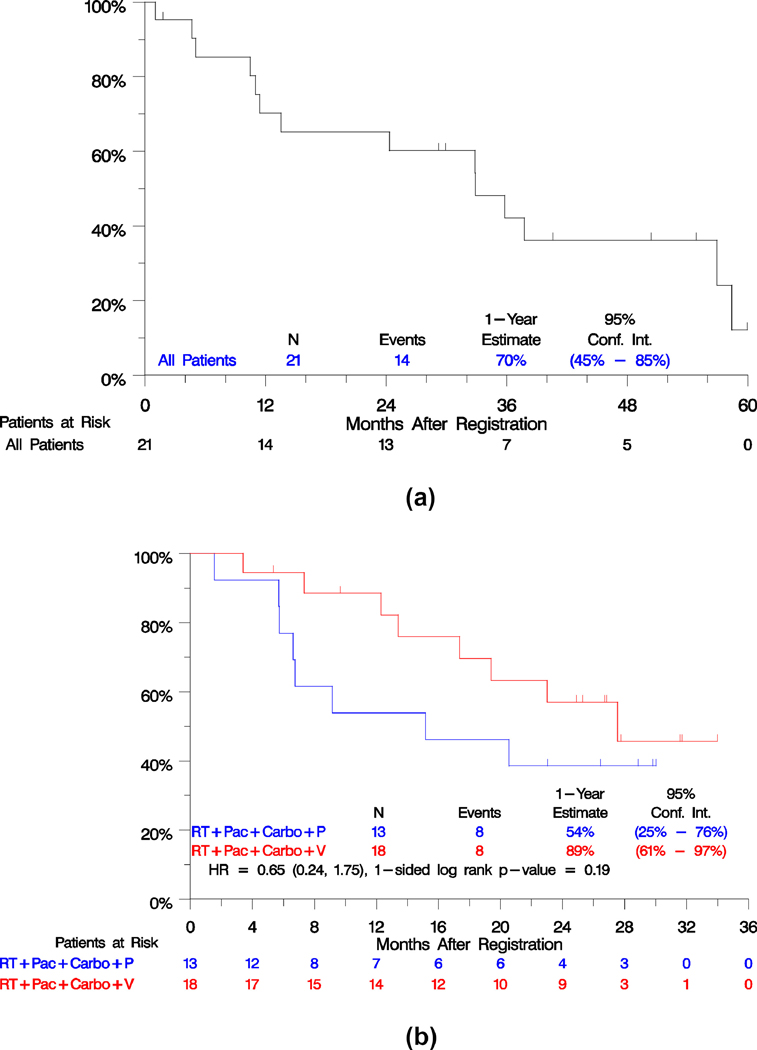
Efficacy outcomes are summarized in Table 3. PFS and OS survival curves of the phase I and phase II parts of study are shown in Figures 2 and 3. The phase II part did not meet the primary objective to evaluate whether there was a difference in PFS between the arms. With progression events reported for 23 of 31 patients and over 2 years from study closure, the estimated hazard ratio of veliparib vs. placebo was 1.47 (95% confidence intervals, 0.59– 3.66), 1-sided p=0.20. Median PFS with veliparib was 9.3 months versus 9.9 months with placebo (Figure 2A). Veliparib relative to placebo was also not associated with improved response (56% on veliparib and 69% on placebo, p=0.69) or disease control (83% on veliparib and 85% on placebo, p= 0.56). Nevertheless, we acknowledge the difficulties in assessing response using CT scans in a previously irradiated field in this setting. However, the OS rate at 1 year was numerically superior in the veliparib arm. The estimated percentage alive at 1 year was 89% with veliparib and 54% with placebo (Figure 3A). The estimated relative risk of death between the arms was a 35% reduction, (HR = 0.65; 95% CI, 0.24–1.75), although this difference was not statistically significant (p = 0.19). In both arms combined, there were 20 patients with progression (11 in the veliparib arm and 9 in the placebo arm), of whom 8 (40%) progressed in local sites, 9 (45%) progressed in regional sites, 7 (35%) progressed in distant sites (4/11 in the veliparib arm and 3/9 in the placebo arm), 2 (10%) died due to disease, and 1 (5%) had missing information. A patient might have multiple progression sites recorded.
Table 3:
Efficacy outcomes
| Phase I CRT+ Veliparib (n = 21) | Phase II | |||
|---|---|---|---|---|
| CRT+ Veliparib (n = 18) | CRT + Placebo (n = 13) | p-value | ||
| Outcomes from Initial Registration (Start of CRT) | ||||
| Objective Response | n = 18 86% (64%, 97%) | n = 10 56% (31%, 78%) | n = 9 69% (38%, 91%) | 0.69 |
| Disease Control (objective response + stable disease) | n = 18 86% (64%, 97%) | n = 15 83% (59%, 96%) | n = 11 85% (55%, 98%) | 0.56 |
| PFS | ||||
| HR* (95% CI) | N/A | 1.47 (0.59, 3.66) | 0.20 | |
| Median in months (95%CI) | 11.5 (9.5, 19.2) | 9.3 (7.3, 17.4) | 9.9 (5.7, 23.6) | |
| 1-year estimate (95% CI) | 40% (19%, 60%) | 41% (18%, 63%) | 46% (19%, 70%) | |
| Overall Survival | ||||
| HR* (95% CI) | N/A | 0.65 (0.24, 1.75) | 0.19 | |
| Median in months (95% CI) | 32.9 (13.8, 37.8) | 27.6 (17.4, 27.6) | 15.2 (6.6, 20.6) | |
| 1-year estimate | 70% (45%, 85%) | 89% (61%, 97%) | 54% (25%, 76%) | |
| Outcomes from start of Consolidation | N = 14 | N = 13 | N = 10 | |
| PFS | ||||
| HR* (95% CI) | N/A | 1.65 (0.54,5.01) | 0.19 | |
| 1-year estimate (95% CI) | 50% (23%, 72%) | 43% (16%, 68%) | 40% (12%, 67%) | |
| Overall Survival | ||||
| HR* (95% CI) | N/A | 0.71 (0.23, 2.20) | 0.28 | |
| 1-year estimate | 79% (47%, 93%) | 76% (42%, 91%) | 50% (18%, 76%) | |
95% confidence intervals in parenthesis
HR, hazard ratio, stratified by stage and histologic subtype
CRT, concurrent chemoradiotherapy; PR, partial response; CR, complete response; SD, stable disease; DCR, disease control rate; PFS, progression-free survival; OS, overall survival; NR, not reached; M, months
Figure 2.
Kaplan-Meier estimates of progression-free survival, phase I part (2A), phase II part (2B).
Figure 3.
Kaplan-Meier estimates of overall survival, phase I part (3A), phase II part (3B).
PFS and OS measured from registration to the consolidation step, were not different between the arms (p= 0.19 for PFS and p=0.28 for OS). PFS at 1-year from consolidation, was 43% with veliparib and 40% with placebo. OS at 1-year from consolidation was 76% with veliparib and 50% with placebo.
The outcomes for patients enrolled to the phase I portion are also displayed in Table 3. The median PFS was 11.5 months (95% CI: 9.5, 19.2), median OS of 32.9 months (95% CI: 13.6, 37.8), with 70% alive at one year (Figures 2B and 3B).Of 14 patients with progression, 4 (28%) progressed in local sites, 3 (21%) in regional sites, 5 (35%) in distant sites, 1 (7%) died without documentation of progression and 1 (7%) had missing data.
DISCUSSION
Our study achieved the first goal of demonstrating veliparib was safe and tolerable when added to standard weekly carboplatin/paclitaxel and chest radiotherapy followed by consolidation full dose chemotherapy. We determined veliparib 120 mg twice daily as the recommended phase II dose for the randomized component of our study. The randomized study substantiated the safety of veliparib with no increased toxicity observed over placebo. Due to the premature closure of the phase II part of the study prompted by a change in standard of care in the treatment of stage III NSCLC with the adoption of adjuvant immunotherapy with durvalumab, veliparib efficacy could not be fully evaluated. The disease control rate and the PFS outcomes were similar in both arms, but there was an intriguing numerical advantage in OS in the veliparib treated patients. It is unclear whether this is suggestive of potential benefits of veliparib, pure chance or related to imbalances favoring the veliparib group.
Our results are similar to those from a phase I trial conducted by another cooperative group, the Alliance for Clinical Trials in Oncology, that mirrored our study design (NCT02412371). Veliparib in doses ranging from 60 mg twice daily to 240 mg twice daily was given concurrently with weekly paclitaxel, carboplatin and thoracic radiotherapy followed by consolidation paclitaxel plus carboplatin and 120 mg or 240 twice daily of veliparib in patients with stage III NSCLC [15]. In that trial, veliparib 240 mg twice daily during CRT and veliparib 120 mg with consolidation chemotherapy were declared the recommended phase II doses. However, grade 3 treatment related adverse events were increased in this study occurring in 58% of the 13 patients treated at their recommended phase II dose versus 35% in patients who received 120 mg twice daily of veliparib in the randomized arm of our study. This is not surprising given the double dose of veliparib administered. A preliminary efficacy analysis of the 48 patients enrolled into the six treatment cohorts, of whom the majority were treated at 200 mg twice daily or above, revealed a median PFS of 19.1 months with a median OS of 32.6 months. Although the sample size was small, these results suggest that a higher dose of veliparib compared to the one utilized in our study may be needed to achieve a clinical benefit. However, veliparib 240 mg twice daily was associated with increased toxicity. Since our trial was initiated there are now several PARP inhibitors in clinical trials. A notable difference between veliparib and the others is its lower PARP trapping capability [16]. However, it is unclear if this mechanism is important in lung cancer. A phase I trial of daily cisplatin with olaparib plus thoracic radiotherapy (NCT01562210) may provide insight into deciphering relevant toxicity and efficacy differences between PARP inhibitors [17]. There many challenges in the clinical development of drug-RT combinations and novel trial designs may be needed, for example, employing a biomarker-driven patient selection [18, 19].
Although immunotherapy with durvalumab has become standard adjuvant therapy after CRT for unresectable stage III NSCLC there is still a need to develop better CRT and adjuvant treatment regimens. Even though PARP inhibitors have not yet demonstrated therapeutic success in lung cancer, enthusiasm remains high for their continued evaluation. Predictive biomarkers that could identify patients most likely to benefit from PARP inhibitors are emerging such as LOH, DNA repair scores and SLFN11. In vitro sensitivity to platinum plus veliparib was associated with DNA-PKcs protein expression and a 5-gene expression signature [20]. SLFN11 expression is among the top biomarker candidates. An evaluation of the NCI 60 cell line panel revealed an association between SLFN11 expression and sensitivity to DNA damaging repair agents [21] and PARP inhibitors in preclinical and clinical evaluations across several tumor types [22]. Although most of the data with SLFN11 as a predictor of PARP responsiveness is in SCLC, its evaluation in NSCLC is under investigation. In our study, blood and tissue samples were collected for exploratory biomarker analysis but were not performed due to the limited sample size.
The combination of PARP inhibitors and immune checkpoint inhibitors (ICI) is a rational and highly attractive approach due to the well-known interplay between the DNA repair pathway and immune activation [22]. In mouse models, PARP inhibitors have demonstrated upregulation of tumor PD-L1 and greater tumor killing than either agent alone [23].
In lung cancer there is an ongoing phase II trial of niraparib plus ICI in ICI naïve metastatic NSCLC patients (NCT03308942). In previously treated patients with non-squamous NSCLC, avelumab plus talazoparib is being evaluated in patients whose tumor express an STK11 mutation, a negative predictor of ICI responsiveness (NCT04173507). In SCLC, atezolizumab plus talazoparib will be studied as maintenance therapy in patients with SLFN11 positive extensive stage SCLC after chemotherapy plus atezolizumab (NCT04334941). If these studies show promising efficacy with acceptable toxicity, one could envision a trial in stage III NSCLC evaluating the combination of immunotherapy plus a PARP inhibitor.
In conclusion, the combination of veliparib and CRT in stage III NSCLC is feasible and well tolerated. No new safety signals were observed. Treatment efficacy could not be fully evaluated due to early study closure. Despite the limitations of our study, PARP inhibitors should continue to be studied in NSCLC. The identification of promising predictive biomarkers and the rational combination of PARP inhibitors with ICIs is expected to define the future role of PARP inhibition in lung cancer treatment.
Supplementary Material
CLINICAL PRACTICE POINTS.
Chemoradiotherapy is a standard treatment option for stage III non-small cell lung cancer. Efficacy outcomes remain suboptimal and new agents that can augment chemoradiotherapy are warranted.
Our phase I/II trial evaluated the addition of a PARP inhibitor to a backbone of chest radiotherapy, carboplatin, paclitaxel followed by consolidation carboplatin and paclitaxel.
Veliparib with chemoradiotherapy was feasible and well tolerated. However, efficacy could not accurately be determined in the phase II randomized part due to early study closure because of the emergence of adjuvant immunotherapy as standard of care in stage III non-small cell lung cancer.
Acknowledgments
Support: Research reported in this publication was supported by the National Cancer Institute of the National Institutes of Health under Award Numbers CA180888, CA180819, CA180826, CA180846, CA189830, CA189957, CA189858, CA180858, CA189861, CA46282, CA13612 and CA46368. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Torre LA, Siegel RL, Jemal A. Lung Cancer Statistics. Adv Exp Med Biol 2016; 893: 1–19. [DOI] [PubMed] [Google Scholar]
- 2.Auperin A, Le Pechoux C, Rolland E et al. Meta-analysis of concomitant versus sequential radiochemotherapy in locally advanced non-small-cell lung cancer. J Clin Oncol 2010; 28: 2181–2190. [DOI] [PubMed] [Google Scholar]
- 3.Santana-Davila R, Devisetty K, Szabo A et al. Cisplatin and etoposide versus carboplatin and paclitaxel with concurrent radiotherapy for stage III non-small-cell lung cancer: an analysis of Veterans Health Administration data. J Clin Oncol 2015; 33: 567–574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Steuer CE, Behera M, Ernani V et al. Comparison of Concurrent Use of Thoracic Radiation With Either Carboplatin-Paclitaxel or Cisplatin-Etoposide for Patients With Stage III Non-Small-Cell Lung Cancer: A Systematic Review. JAMA Oncol 2016. [DOI] [PubMed] [Google Scholar]
- 5.Salama JK, Vokes EE. New radiotherapy and chemoradiotherapy approaches for non-small-cell lung cancer. J Clin Oncol 2013; 31: 1029–1038. [DOI] [PubMed] [Google Scholar]
- 6.Bradley JD, Paulus R, Komaki R et al. Standard-dose versus high-dose conformal radiotherapy with concurrent and consolidation carboplatin plus paclitaxel with or without cetuximab for patients with stage IIIA or IIIB non-small-cell lung cancer (RTOG 0617): a randomised, two-by-two factorial phase 3 study. Lancet Oncol 2015; 16: 187–199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Antonia SJ, Villegas A, Daniel D et al. Durvalumab after Chemoradiotherapy in Stage III Non-Small-Cell Lung Cancer. N Engl J Med 2017; 377: 1919–1929. [DOI] [PubMed] [Google Scholar]
- 8.Barazzuol L, Jena R, Burnet NG et al. Evaluation of poly (ADP-ribose) polymerase inhibitor ABT-888 combined with radiotherapy and temozolomide in glioblastoma. Radiat Oncol 2013; 8: 65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kummar S, Kinders R, Gutierrez ME et al. Phase 0 clinical trial of the poly (ADP-ribose) polymerase inhibitor ABT-888 in patients with advanced malignancies. J Clin Oncol 2009; 27: 2705–2711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Appleman LJ, Beumer JH, Jiang Y et al. Phase 1 study of veliparib (ABT-888), a poly (ADP-ribose) polymerase inhibitor, with carboplatin and paclitaxel in advanced solid malignancies. Cancer Chemother Pharmacol 2019; 84: 1289–1301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ramalingam SS, Blais N, Mazieres J et al. Randomized, Placebo-Controlled, Phase 2 Study of Veliparib in Combination with Carboplatin and Paclitaxel for Advanced/Metastatic Non-Small Cell Lung Cancer. Clin Cancer Res 2017;23: 1937–44 [DOI] [PubMed] [Google Scholar]
- 12.Albert JM, Cao C, Kim KW et al. Inhibition of poly(ADP-ribose) polymerase enhances cell death and improves tumor growth delay in irradiated lung cancer models. Clin Cancer Res 2007; 13: 3033–3042. [DOI] [PubMed] [Google Scholar]
- 13.Eisenhauer EA, Therasse P, Bogaerts J et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer 2009; 45: 228–247. [DOI] [PubMed] [Google Scholar]
- 14.Mantel N. Evaluation of survival data and two new rank order statistics arising in its consideration. Cancer Chemother Rep 1966; 50: 163–170. [PubMed] [Google Scholar]
- 15.Kozono DE, Stinchcombe T, Salama JK et al. Veliparib (Vel) in combination with chemoradiotherapy (CRT) of carboplatin/paclitaxel (C/P) plus radiation in patients (pts) with stage III non-small cell lung cancer (NSCLC) (M14–360/AFT-07). ASCO 2019; A8510. [Google Scholar]
- 16.Hopkins TA, Shi Y, Rodriguez LE et al. Mechanistic Dissection of PARP1 Trapping and the Impact on In Vivo Tolerability and Efficacy of PARP Inhibitors. Mol Cancer Res 2015; 13: 1465–1477. [DOI] [PubMed] [Google Scholar]
- 17.de Haan R, van Werkhoven E, van den Heuvel MM et al. Study protocols of three parallel phase 1 trials combining radical radiotherapy with the PARP inhibitor olaparib. BMC Cancer 2019; 19: 901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ahmad SS, Crittenden MR, Tran PT et al. Clinical Development of Novel Drug-Radiotherapy Combinations. Clin Cancer Res 2019; 25: 1455–1461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sharma RA, Plummer R, Stock JK et al. Clinical development of new drug-radiotherapy combinations. Nat Rev Clin Oncol 2016; 13: 627–642. [DOI] [PubMed] [Google Scholar]
- 20.Owonikoko TK, Zhang G, Deng X et al. Poly (ADP) ribose polymerase enzyme inhibitor, veliparib, potentiates chemotherapy and radiation in vitro and in vivo in small cell lung cancer. Cancer Med 2014; 3: 1579–1594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Murai J, Feng Y, Yu GK et al. Resistance to PARP inhibitors by SLFN11 inactivation can be overcome by ATR inhibition. Oncotarget 2016; 7: 76534–76550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pilie PG, Gay CM, Byers LA et al. PARP Inhibitors: Extending Benefit Beyond BRCA-Mutant Cancers. Clin Cancer Res 2019; 25: 3759–3771. [DOI] [PubMed] [Google Scholar]
- 23.Jiao S, Xia W, Yamaguchi H et al. PARP Inhibitor Upregulates PD-L1 Expression and Enhances Cancer-Associated Immunosuppression. Clin Cancer Res 2017; 23: 3711–3720. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.