Abstract
A recent article by Lilascharoen et al. identified two distinct pathways in the globus pallidus (GPe) that are associated with discrete behaviors. Dysfunctions in these pathways were shown to underlie Parkinsonian motor and cognitive deficits in mice, and selective manipulation of these circuits rescued locomotor deficits and improved behavioral flexibility.
The basal ganglia constitute a network of subcortical brain regions that are crucial for motor control as well as for several non-motor functions including decision making, motivation, and associative learning [1,2]. Basal ganglia dysfunction is involved in the pathogenesis of Parkinson's disease (PD), whose cardinal motor symptoms include slow or reduced movements (bradykinesia), rest tremor, and rigidity. Although classified as a movement disorder, accumulating evidence demonstrates that PD is a complex disease that also involves several non-motor symptoms, including cognitive impairment, dementia, and depression [3,4], which frequently begin years before the onset of motor deficits [3,4].
The external segment of the GPe has long been depicted as a relay nucleus in the motor-suppressing 'indirect' pathway of the basal ganglia, where it propagates information from the striatum to output nuclei, predominantly the substantia nigra pars reticulata (SNr) in rodents [5,6]. Although the contribution of the GPe to motor dysfunction in PD is well known, its potential contributions to non-motor symptoms have been underexplored, despite evidence for limbic and associative territories in the GPe where perturbations affect behavioral patterns rather than motor execution [7].
In a recent study, Lilascharoen et al. [8] developed a viral vector approach to establish the function and connectivity of distinct microcircuits in the GPe that regulate locomotion and cognitive flexibility, respectively. Conventional anatomical tracing studies have shown that a majority of parvalbumin-expressing (PV) neurons in the GPe project to the SNr – part of the canonical 'indirect' motor pathway of the basal ganglia (PVGPe-SNr neurons). However, some PV-GPe neurons instead extend axons to the parafascicular nucleus (PVGPe-PF neurons), a brain area that has been implicated in reversal learning [9,10]. The authors found that PVGPe-SNr neurons were distinct from PVGPe-PF neurons. To this end, the authors injected a retrograde virus into the SNr to transfect GPe neurons projecting to the SNr with a Cre-inducable Flp recombinase. When these injections were paired with injections of Flp-dependent, double-floxed, inverted open reading frame (fDIO)-GFP into the GPe of PV-cre transgenic mice, they resulted in GFP expression selectively in PVGPe-SNr neurons, and no fluorescent axons were observed in the PF. Conversely, when retrograde viruses were injected into the PF, fDIO-GFP injections in the GPe resulted in labeling of PVGPe-PF neurons, and no fluorescent axons were observed in the SNr.
In further viral tracing experiments, PVGPe-SNr and PVGPe-PF populations were also found to differ in their inputs. PVGPe-SNr neurons received inputs from canonical indirect pathway structures, including the subthalamic nucleus (STN) and dopamine D2 receptor-expressing spiny projection neurons (D2-SPNs) in the dorsolateral striatum. By contrast, PVGPe-PF neurons received more inputs from the cortex and midbrain, as well as from D1-SPNs in dorsomedial striatum. Taken together, these experiments established that PVGPe-SNr and PVGPe-PF neurons are distinct GPe subpopulations that participate in largely nonoverlapping neural pathways (Figure 1).
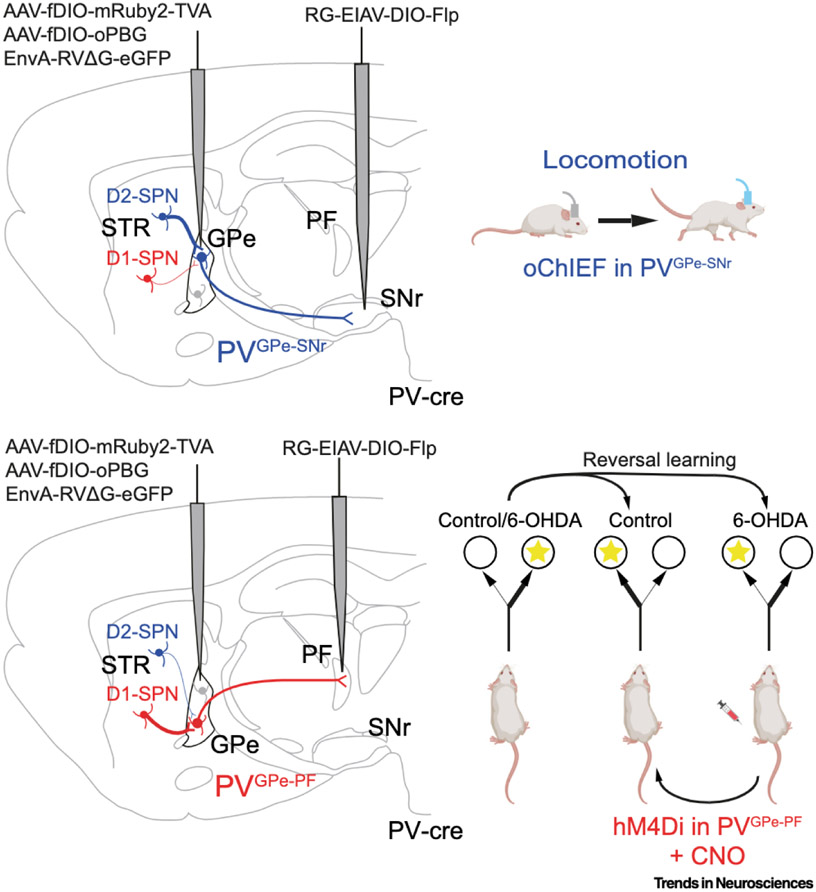
Figure 1. Schematic representation illustrating distinct GPe pathways associated with motor and cognitive functions.
(Upper panel) Viral strategy used by Lilascharoen et al. [8] to map the input and output organization of GPe neurons projecting to the SNr (PVGPe-SNr) in PV–cre mice. PVGPe-SNr neurons receive more inputs from striatal D2-SPNs and fewer inputs from striatal D1-SPNs (left). Optogenetic activation of PVGPe-SNr axon terminals with optical fibers implanted in the SNr increases locomotor activity and was sufficient to restore locomotion in dopamine-depleted mice, with locomotion persisting beyond the photostimulation period. (Lower panel) Viral strategy to map the input and output organization of GPe neurons projecting to the PF (PVGPe-PF) in PV-cre mice. PVGPe-PF neurons preferentially receive projections from striatal D1-SPNs and have fewer projections from striatal D2-SPNs (left). Dopamine depletion (6-OHDA lesion) impairs cognitive flexibility in a context-based food-foraging task. Chemogenetic inhibition of PVGPe-PF neurons significantly improves the reversal-learning performance in the dopamine-depleted mice. Mouse icons adapted from 'Icon Pack: Mice', by BioRender.com (2021); retrieved from https://app.biorender.com/biorender-templates. Abbreviations: AAV, adeno-associated virus; CNO, clozapine-N-oxide; D1/D2, dopamine D1/D2 receptor-expressing neurons; EIAV, equine infectious anemia lenti virus; EnvA, avian tumor virus envelope A protein; fDIO, Flp-dependent, double-floxed, inverted open reading frame; GPe, globus pallidus; hM4DI, human muscarinic M4 receptor exclusively activated by a designer drug (CNO); oChlEF, channelrhodopsin variant; 6-OHDA, 6-hydroxydopamine; oPBG, optimized Pasteur rabies strain G and B19G chimeric protein; PF, parafascicular nucleus; PV, parvalbumin; RG, rabies glycoprotein; RVΔG, glycoprotein (G)-deleted rabies virus; SNr, substantia nigra pars reticulata; SPN, spiny projection neurons; STR, striatum; TVA, avian tumor vims receptor A.
To investigate the effects of these neural pathways on behavior, oChlEF (a fast channelrhodopsin variant) was expressed in all PV-GPe neurons, but stimulation was driven by optical fibers implanted into either the SNr or the PF. Stimulation of PV-GPe axons in the SNr drove an increase in spontaneous locomotion in an open field, whereas stimulation of axons in the PF did not. Conversely, stimulation of PV-GPe axons in the PF impaired reversal learning on a context-based food-foraging task, whereas stimulation of PV-SNr neurons did not.
These results suggest that PVGPe-SNr neurons participate in circuits that impact on locomotion, whereas PVGPe-PF neurons participate in circuits that govern cognitive flexibility. Based on these findings, and on the role of the GPe as a physiological node for basal ganglia dysfunction in Parkinson's disease, the authors hypothesized that PVGPe-SNr and PVGPe-PF neurons might represent cellular targets to differentially treat motor versus non-motor behavioral deficits in dopamine-depleted mice. Synaptic electrophysiology recordings in acute brain slices revealed that, following dopamine depletion with 6-hydroxydopamine (6-OHDA), PVGPe-SNr synapses were weakened, whereas PVGPe-PF synapses were strengthened. This led the authors to hypothesize that either stimulating the PVGPe-SNr pathway or inhibiting the PVGPe-PF pathway would be therapeutic in dopamine-depleted mice.
Stimulation of PV-GPe cell bodies has previously been shown to restore locomotion in dopamine-depleted mice [5], and the authors hypothesized that this effect is driven through the motor PVGPe-SNr pathway. To test this hypothesis, they attempted to rescue movement in dopamine-depleted mice by activating the terminals of PVGPe-SNr neurons with optical fibers implanted in the SNr, and found that this manipulation was sufficient to restore locomotion in dopamine-depleted mice, with locomotion persisting well beyond the end of stimulation, consistent with the long-lasting results seen following cell body stimulation [5] (Figure 1). These results are consistent with the interpretation that the direct actions of PVGPe-SNr neurons on basal ganglia output are sufficient to induce this rescue, but the authors did not rule out the possibility that axon stimulation antidromically activates the cell bodies of PVGPe-SNr neurons, which could in turn impact on the activity of other GPe subpopulations via the actions of inhibitory collaterals within the nucleus.
To test whether inhibiting the pathologically strengthened PVGPe-PF pathway might restore cognitive flexibility in dopamine-depleted mice, the authors chemogenetically inhibited this pathway by infusing clozapine N-oxide (CNO). A context-based food-foraging task was used to assess behavioral flexibility. In this task, dopamine-intact mice rapidly learn to change their behavior when the contexts associated with food reward are reversed, but dopamine-depleted mice struggle to change their behavior when the rewarded contexts are reversed. Interestingly, this cognitive deficit is present in mice with only 50% dopamine loss, rather than the >70% dopamine loss needed to evoke motor deficits, allowing the experimenters to run these experiments under depleted conditions that still enable locomotion, consistent with findings that cognitive deficits often emerge well before the onset of motor deficits [3,4]. Chemogenetic inhibition of the PVGPe-PF pathway improved the cognitive flexibility of dopamine-depleted mice, enabling them to reverse their behavior when food rewards were switched at rates comparable with those seen in dopamine-intact mice (Figure 1).
The finding of Lilascharoen et al. highlight the intertwined nature of cellular pathways in the basal ganglia, and underscore the importance of developing cell type-specific strategies for therapeutic interventions in disease. Far from being a passive motor relay nucleus in the basal ganglia, the GPe is a site of active information processing for both motor and non-motor behaviors.
Acknowledgments
A.G. is supported by National Institutes of Health (NIH) grants R01NS101016, R01NS104835, and R01NS117058.
Footnotes
Declaration of interests
The authors declare no competing interests.
References
- 1.Cox J and Witten IB (2019) Striatal circuits for reward learning and decision-making. Nat. Rev. Neurosci 20, 482–494 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tecuapetla F et al. (2016) Complementary contributions of striatal projection pathways to action initiation and execution. Cell 166, 703–715 [DOI] [PubMed] [Google Scholar]
- 3.McGregor MM and Nelson AB (2019) Circuit mechanisms of Parkinson’s disease. Neuron 101, 1042–1056 [DOI] [PubMed] [Google Scholar]
- 4.Obeso JA et al. (2017) Past, present, and future of Parkinson’s disease: a special essay on the 200th Anniversary of the shaking palsy. Mov. Disord 32, 1264–1310 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mastro KJ et al. (2017) Cell-specific pallidal intervention induces long-lasting motor recovery in dopamine-depleted mice. Nat. Neurosci 20, 815–823 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Simmons DNV et al. (2020) Indirect pathway control of firing rate and pattern in the substantia nigra pars reticulata. J. Neurophysiol 123, 800–814 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Saga Y et al. (2017) Roles of multiple globus pallidus territories of monkeys and humans in motivation, cognition and action: an anatomical, physiological and pathophysiological review. Front. Neuroanat 11, 1–12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lilascharoen V et al. (2021) Divergent pallidal pathways underlying distinct Parkinsonian behavioral deficits. Nat. Neurosci 24, 504–515 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mastro KJ et al. (2014) Transgenic mouse lines subdivide external segment of the globus pallidus (GPe) neurons and reveal distinct GPe output pathways. J. Neurosci 34, 2087–2099 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Brown HD et al. (2010) The parafascicular thalamic nucleus concomitantly influences behavioral flexibility and dorsomedial striatal acetylcholine output in rats. J. Neurosci 30, 14390–14398 [DOI] [PMC free article] [PubMed] [Google Scholar]