Abstract
In order to understand autoimmune phenomena contributing to the pathophysiology of COVID-19 and post-COVID syndrome, we have been profiling autoantigens (autoAgs) from various cell types. Although cells share numerous autoAgs, each cell type gives rise to unique COVID-altered autoAg candidates, which may explain the wide range of symptoms experienced by patients with autoimmune sequelae of SARS-CoV-2 infection. Based on the unifying property of affinity between autoantigens (autoAgs) and the glycosaminoglycan dermatan sulfate (DS), this paper reports 140 candidate autoAgs identified from proteome extracts of human Jurkat T-cells, of which at least 105 (75%) are known targets of autoantibodies. Comparison with currently available multi-omic COVID-19 data shows that 125 (89%) of DS-affinity proteins are altered at protein and/or RNA levels in SARS-CoV-2-infected cells or patients, with at least 94 being known autoAgs in a wide spectrum of autoimmune diseases and cancer. Protein alterations by ubiquitination and phosphorylation in the viral infection are major contributors of autoAgs. The autoAg protein network is significantly associated with cellular response to stress, apoptosis, RNA metabolism, mRNA processing and translation, protein folding and processing, chromosome organization, cell cycle, and muscle contraction. The autoAgs include clusters of histones, CCT/TriC chaperonin, DNA replication licensing factors, proteasome and ribosome proteins, heat shock proteins, serine/arginine-rich splicing factors, 14-3-3 proteins, and cytoskeletal proteins. AutoAgs such as LCP1 and NACA that are altered in the T cells of COVID patients may provide insight into T-cell responses in the viral infection and merit further study. The autoantigen-ome from this study contributes to a comprehensive molecular map for investigating acute, subacute, and chronic autoimmune disorders caused by SARS-CoV-2.
Keywords: Autoantigens, autoantibodies, autoimmunity, SARS-Cov-2, COVID-19, long COVID
Introduction
The COVID-19 pandemic has been devastating. After initial recovery from acute SARS-CoV-2 infection, many people continue to suffer from lingering health problems (so called “long COVID” or post-COVID syndrome), such as fatigue, shortness of breath, joint pain, chest pain, muscle pain, loss of smell or taste, and other neurological problems. Although the underlying causes are far from clear, autoimmune effects are likely important contributors to chronic post-COVID disorders. To understand how SARS-CoV-2 infection may induce autoimmune responses, we are establishing a comprehensive COVID autoantigen atlas, i.e., all possible endogenous autoantigens (autoAgs) that may be rendered immunogenic by the viral infection. Because different tissues or cells may give rise to distinct pools of autoAgs, we have been profiling autoAgs from multiple human tissues and cell types, including human lung fibroblast HFL1 cells, human lung epithelial-like A549 cells, and B-lymphoblast HS-Sultan cells [1–4]. In this study, we report an autoantigen-ome identified from human Jurkat T-lymphoblast cells.
Our autoAg discovery is based a unifying mechanism of autoantigenicity that we have uncovered [5–8]. AutoAgs are the targets of autoantibodies (autoAbs) and T-cell autoimmune responses. Typically, self-molecules are naturally tolerated by the immune system and do not provoke autoimmune responses. However, certain self-molecules transform into autoAgs and become targets of autoimmune attacks. Thus far, hundreds of autoAgs with seemingly no obvious structural or functional commonality have been identified across various autoimmune diseases and cancers. Our studies have demonstrated that autoAgs do, in fact, share common properties. AutoAgs are commonly released by apoptotic cells, and we found that the glycosaminoglycan dermatan sulfate (DS) has peculiar affinity to apoptotic cells and their autoAgs [5, 7, 8]. DS and autoAgs can form affinity complexes and cooperatively stimulate autoreactive B1 cells and autoantibody production [5, 7, 8]. Based on autoAg-DS affinity, we have identified several hundred autoAgs from various cells and tissues [1–3, 9–11].
A variety of autoantibodies have been identified in COVID-19 patients [12–22]. Children infected with SARS-CoV-2 who develop the rare multisystem inflammatory syndrome show multiple autoAbs, including classical antinuclear antigen (ANA) autoAbs and specific autoAbs recognizing endothelial, gastrointestinal, or immune cell autoAgs [12, 13]. ANA autoAbs are also frequently detected in COVID-19 patients with acute respiratory syndrome or other critical conditions [14–16] and in COVID patients with no previous clinical record of autoimmune diseases [17]. A high frequency of cerebrospinal fluid autoAbs is found in COVID patients with neurological symptoms [18]. New-onset autoAbs were detected in a significant proportion of hospitalized COVID-19 patients and were positively correlated with immune responses to SARS-CoV-2 proteins [20]. Overall, an increasing number of observations suggest a positive correlation between emergence of autoAbs and an adverse clinical course of COVID-19.
As revealed by our prior studies, SARS-CoV-2 infection may induce numerous molecular changes in the host and transform naturally non-antigenic self-molecules to antigenic autoAgs [1–3]. In order to better understand the possible extent of autoimmune disorders caused by SARS-CoV-2, we are building a comprehensive catalog of all possible intrinsic autoAgs across cell and tissue types related to the viral infection. Herein, we report a profile of autoAgs identified from human Jurkat T-cells using our DS-affinity enrichment approach, which will provide valuable molecular targets for understanding the diverse autoimmune sequelae of COVID-19.
Results and Discussion
Autoantigen-ome of Jurkat cells identified by DS-affinity
Total proteins were extracted from Jurkat T-cells and fractionated in a DS-Sepharose affinity column. Proteins with increasing DS-affinity were eluted from the column with increasing ionic strength of salt. Fractions eluted with 0.4, 0.6, and 1.0 M NaCl correspond to proteins with intermediate, strong, and very strong DS-affinity, respectively. Mass spectrometry sequencing identified a total of 140 proteins from these three DS-affinity fractions (Table 1). The majority of proteins (120/140) were eluted with 0.4 M NaCl, 31 proteins were found in the 0.6 M NaCl elution, and 11 proteins were identified in the 1.0 M NaCl elution. Three proteins were detected redundantly in all three fractions (HIST4H4, H2AC1, RPLP2), 1H2BC1 was detected in both 0.6 and 1.0 M fractions, C1QBP was detected in both 0.4 and 1.0 M NaCl fractions, and 13 proteins were detected in both 0.4 and 0.6 M fractions.
Table 1.
DS-affinity autoantigens from Jurkat T-cells and their alterations in SARS-CoV-2 infection
| Symbol | Protein Name | DS-affinity | SARS-CoV-2 effect | Ref. | ||||
|---|---|---|---|---|---|---|---|---|
| VS | S | M | Up | Dn | Interact | |||
| ACTC1 | Actin, alpha 1, skeletal muscle | 2 | 6 | u | d | [1] | ||
| ACTG1 | Actin, cytoplasmic 2 | 4 | u | d | [2] | |||
| ACTN1 | Alpha-actinin-1, f-actin cross linking protein | 8 | u | d | [3] | |||
| ALDH18A1 | Delta 1-pyrroline-5-carboxylate synthetase | 2 | d | |||||
| ANP32A | Acidic leucine-rich nuclear phosphoprotein 32 family member a | 9 | u | d | ||||
| ANP32B | Acidic leucine-rich nuclear phosphoprotein 32 family member b | 6 | d | [4] | ||||
| ANXA6 | Annexin a6 (chromobindin-20) | 9 | u | d | [5] | |||
| ATP5F1B | ATP synthase subunit beta, mitochondrial precursor | 7 | u | d | Nsp6 | [6] | ||
| BZW2 | Basic leucine zipper and W2 domain-containing protein 2 | 2 | M | |||||
| C1QBP | Complement component 1 q subcomponent-binding protein | 2 | 2 | d | [7] | |||
| CALM1 | Calmodulin-1 | 4 | d | [8] | ||||
| CALM3 | Calmodulin-3 | 2 | u | [9] | ||||
| CALR | Calreticulin precursor | 11 | u | d | [10] | |||
| CAND1 | Cullin-associated nedd8-dissociated protein 1, TIP120 | 6 | ||||||
| CAPRIN1 | Membrane component chromosome 11 surface marker 1 | 3 | d | |||||
| CAPZA1 | F-actin capping protein alpha-1 subunit | 2 | d | [11] | ||||
| CCT2 | T-complex protein 1 subunit beta | 8 | d | [12] | ||||
| CCT3 | T-complex protein 1 subunit gamma | 12 | u | [13] | ||||
| CCT4 | T-complex protein 1 subunit delta (stimulator of tar rna-binding) | 3 | u | [13] | ||||
| CCT5 | T-complex protein 1 subunit epsilon | 7 | u | d | [12] | |||
| CCT6A | T-complex protein 1 subunit zeta | 5 | u | d | [12] | |||
| CCT7 | T-complex protein 1 subunit eta | 9 | [12] | |||||
| CCT8 | T-complex protein 1 subunit theta | 18 | u | d | [13] | |||
| CDC37 | Hsp90 chaperone protein kinase-targeting subunit | 6 | u | d | ||||
| DDB1 | Damage-specific DNA-binding protein 1 | 2 | u | d | [14] | |||
| DDX39A | ATP-dependent RNA helicase ddx39 | 7 | u | d | ||||
| DDX39B | Spliceosome RNA helicase bat1 | 2 | d | |||||
| DHX15 | Pre-mRNA-splicing factor atp-dependent rna helicase | 2 | d | |||||
| EEF1B2 | Elongation factor 1-beta | 2 | d | |||||
| EEF1G | Elongation factor 1-gamma | 5 | u | d | ||||
| EIF4A1 | Eukaryotic initiation factor 4A-I | 14 | u | d | ||||
| EIF5A2 | Eukaryotic translation initiation factor 5a isoform 2 | 2 | d | [15] | ||||
| FASN | Fatty acid synthase | 5 | u | d | [16] | |||
| HDGF | Hepatoma-derived growth factor | 3 | u | d | [17] | |||
| HIST1H1A | Histone h1.1, H1–1 | 3 | 2 | u | d | [18] | ||
| HIST1H1B | Histone h1.5 (histone h1a), H1–5 | 5 | 3 | u | d | [19] | ||
| HIST1H1C | Histone h1.2 (histone h1d) , H1–2 | 3 | 3 | u | d | [20] | ||
| HIST1H2AA | Histone h2a type 1-a, H2AC1, H2AFR | 3 | 2 | 2 | [20] | |||
| HIST1H2BA | Histone h2b type 1-a (testis-specific histone h2b), H2BC1 | 5 | 4 | [18] | ||||
| HIST1H2BB | Histone h2b type 1-b (h2b.f) H2BC3 | 2 | [21] | |||||
| HIST3H3 | Histone h3.4, H3–4 | 3 | [18] | |||||
| HIST4H4 | Histone h4, H4C1 | 5 | 6 | 8 | u | [21] | ||
| HMGB1 | High mobility group protein 1-like 10 (hmg-1l10) | 10 | d | [17] | ||||
| HMGCS1 | Hydroxymethylglutaryl-coa synthase | 2 | u | d | ||||
| HNRNPA1 | hnRNP core protein A1 | 2 | u | d | [22] | |||
| HNRNPCL1 | hnRNP core protein C-like 1 | 2 | [23] | |||||
| HNRNPK | hnRNP K | 3 | u | [24] | ||||
| HNRNPU | hnRNP U (scaffold attachment factor a) | 2 | u | d | [25] | |||
| HSP90AA1 | Heat shock protein hsp 90-alpha (hsp 86) | 2 | 38 | u | d | [26] | ||
| HSP90AB1 | Heat shock protein hsp 90-beta (hsp 84) (hsp 90) | 16 | u | d | [27] | |||
| HSP90B1 | Heat shock protein 90 kda beta member 1 (grp94) | 23 | u | d | [28] | |||
| HSPA4 | Heat shock 70 kda protein 4 | 14 | u | d | [29] | |||
| HSPA5 | GRP78, BiP | 8 | u | d | Nsp2 Nsp4 |
[30] | ||
| HSPD1 | Hsp60 (mitochondrial matrix protein p1) | 30 | u | d | [31] | |||
| HSPH1 | Heat-shock protein 105 kda | 13 | u | [32] | ||||
| HYOU1 | Hypoxia up- regulated 1, ORP150 | 2 | u | Orf8 | [33] | |||
| IPO5 | Importin beta-3, ranbp5 | 7 | [34] | |||||
| KPNB1 | Importin beta-1 subunit (nuclear factor p97) | 5 | [34] | |||||
| LCP1 | Plastin-2 | 8 | u | [35] | ||||
| LMNB1 | Lamin-b1 | 2 | u | d | [36] | |||
| LSM8 | U6 snRNA-associated Sm-like protein LSm8 | 2 | ||||||
| MAPRE1 | Microtubule-associated protein rp/eb family member 1 | 3 | Orf3 | |||||
| MCM2 | DNA replication licensing factor mcm2 | 6 | d | [37] | ||||
| MCM3 | DNA replication licensing factor mcm3 | 7 | u | d | [37] | |||
| MCM4 | DNA replication licensing factor mcm4, CDC21 | 5 | u | d | [37] | |||
| MCM5 | DNA replication licensing factor mcm5, CDC46 | 3 | u | d | [37] | |||
| MCM6 | DNA replication licensing factor mcm6 | 9 | u | d | [37] | |||
| MYL6 | Myosin light polypeptide 6 | 2 | u | [38] | ||||
| NACA | Nascent polypeptide-associated complex subunit alpha | 3 | u | d | [39] | |||
| NASP | Nuclear autoantigenic sperm protein | 4 | u | d | [40] | |||
| NCL | Nucleolin | 23 | u | d | [41] | |||
| NPM1 | Nucleophosmin | 6 | 6 | u | d | [42] | ||
| NUDT5 | ADP-sugar pyrophosphatase | 2 | d | |||||
| P4HB | Protein disulfide-isomerase precursor (thyroid hormone-binding protein) | 7 | u | d | [43] | |||
| PABPC3 | Polyadenylate-binding protein 3 | 3 | d | |||||
| PCNA | Proliferating cell nuclear antigen | 8 | u | d | [44] | |||
| PDIA4 | Protein disulfide-isomerase a4 precursor | 12 | u | d | [45] | |||
| PDIA6 | Protein disulfide-isomerase a6 precursor | 4 | u | d | [43] | |||
| PFDN3 | Prefoldin subunit 3, VBP1 | 3 | d | |||||
| POTEKP | Putative beta-actin-like protein 3, kappa actin, ACTBL3 | 2 | 2 | u | ||||
| PPP1R7 | Protein phosphatase 1 regulatory subunit 7 | 2 | u | |||||
| PPP2R1A | Serine/threonine-protein phosphatase 2a (pp2a) regulatory subunit A | 7 | d | [46] | ||||
| PRKCSH | Glucosidase 2 subunit beta (protein kinase c substrate heavy chain) | 4 | d | Orf3 | ||||
| PRMT1 | Protein arginine n-methyltransferase 1 | 3 | d | [43] | ||||
| PSMA1 | Proteasome subunit alpha type 1 | 3 | u | [47] | ||||
| PSMA2 | Proteasome subunit alpha type 2 | 2 | d | |||||
| PSMA3 | Proteasome subunit alpha type 3 | 2 | u | d | [48] | |||
| PSMA5 | Proteasome subunit alpha type 5 | 5 | u | [49] | ||||
| PSMA7 | Proteasome subunit alpha type 7 | 2 | u | d | [50] | |||
| PSMA8 | Proteasome subunit alpha type 7-like | 2 | [50] | |||||
| PSMB3 | Proteasome subunit beta type 3 | 2 | d | [48] | ||||
| PSMB4 | Proteasome subunit beta type 4 | 3 | ||||||
| PSMB7 | Proteasome subunit beta type 7 (subunit z) | 2 | d | [48] | ||||
| PSMC1 | 26s Proteasome regulatory subunit 4 | 2 | d | |||||
| PSME3 | Proteasome activator complex subunit 3 | 3 | d | [51] | ||||
| PTGES3 | Prostaglandin E synthase 3 | 2 | d | |||||
| PTMA | Prothymosin alpha | 4 | u | d | [52] | |||
| RBBP7 | Histone-binding protein rbbp7 | 3 | u | d | ||||
| RPA3 | Replication protein A 14 kda subunit | 2 | [53] | |||||
| RPL22 | 60s ribosomal protein L22 (heparin-binding protein hbp15) | 2 | d | [54] | ||||
| RPL5 | 60s ribosomal protein L5 | 5 | d | [55] | ||||
| RPL6 | 60s ribosomal protein L6 | 4 | u | d | [37] | |||
| RPL7 | 60s ribosomal protein L7 | 3 | u | d | [54] | |||
| RPLP0 | 60s acidic ribosomal protein P0 | 3 | u | d | [56] | |||
| RPLP2 | 60s acidic ribosomal protein P2 (ny-ren-44 antigen) | 2 | 2 | 2 | u | d | [57] | |
| RPS3A | Ribosomal protein S3a | 2 | u | d | ||||
| RPS7 | Ribosomal protein S7 | 2 | u | d | ||||
| SET | Protein SET | 4 | u | d | [58] | |||
| SF3B3 | Splicing factor 3b subunit 3, SAP130 | 3 | u | |||||
| SNRNP70 | U1 snRNP 70 kda | 3 | u | d | [59] | |||
| SNRPD2 | Small nuclear ribonucleoprotein D2 polypeptide | 3 | 2 | d | [60] | |||
| SNRPD3 | Small nuclear ribonucleoprotein sm d3 | 2 | d | [61] | ||||
| SRRT | Arsenite-resistance protein 2 | 2 | d | |||||
| SRSF1 | Splicing factor, arginine/serine-rich 1 | 5 | u | d | [62] | |||
| SRSF3 | Serine/arginine-rich splicing factor 3, SFRS3 | 2 | [63] | |||||
| SRSF5 | Serine/arginine-rich splicing factor 5, SRP40 | 2 | u | d | [64] | |||
| SRSF7 | Splicing factor, arginine/serine-rich 7 (9g8) | 2 | u | |||||
| SRSF8 | Serine/arginine-rich splicing factor 8 | 2 | d | |||||
| SSB | Lupus La protein (Sjogren syndrome type b antigen) | 3 | 5 | u | d | [65] | ||
| ST13 | Hsc70-interacting protein (suppression of tumorigenicity protein 13) | 6 | u | [66] | ||||
| SYNCRIP | hnRNP Q (synaptotagmin-binding, cytoplasmic rna-interacting protein) | 3 | d | |||||
| TCP1 | T-complex protein 1 subunit alpha | 7 | d | [12] | ||||
| TPM1 | Tropomyosin 1 alpha chain | 3 | u | d | [67] | |||
| TPM3 | Tropomyosin alpha-3 chain | 5 | u | d | [68] | |||
| TPM4 | Tropomyosin alpha-4 chain | 5 | u | d | [69] | |||
| TUBA1C | Tubulin alpha-6 chain | 2 | 2 | u | d | [70] | ||
| TUBA3C | Tubulin alpha-2 chain | 3 | 10 | |||||
| TUBB | Beta-tubulin | 2 | 7 | u | d | [71] | ||
| UBA1 | Ubiquitin-activating enzyme E1 | 2 | u | d | [72] | |||
| VCP | Transitional endoplasmic reticulum ATPase | 14 | u | d | [73] | |||
| VIM | Vimentin | 4 | 10 | u | d | [74] | ||
| VPS35 | Vacuolar protein sorting 35 | 2 | u | d | [75] | |||
| XRCC5 | ATP-dependent dna helicase 2 subunit 2 (lupus ku86) | 8 | d | [76] | ||||
| XRCC6 | ATP-dependent dna helicase 2 subunit 1 (lupus ku70) | 6 | 11 | u | d | [77] | ||
| YWHAB | 14-3-3 protein beta/alpha | 12 | u | d | ||||
| YWHAE | 14-3-3 protein epsilon | 8 | u | d | [78] | |||
| YWHAG | 14-3-3 protein gamma | 5 | u | [78] | ||||
| YWHAH | 14-3-3 protein eta | 3 | d | [79] | ||||
| YWHAQ | 14-3-3 protein theta | 3 | u | d | [80] | |||
| YWHAZ | 14-3-3 protein zeta/delta | 3 | u | d | [81] | |||
Abbreviations from left to right: VS (very strong DS-affinity, eluted with 1.0 M NaCl), S (strong DS-affinity, eluted with 0.6 M NaCl), M (medium DS-affinity, eluted with 0.4 M NaCl), Up (up-regulated in SARS-CoV-2 infection), Dn (down-regulated in SARS-CoV-2 infection), Interact (found in the protein interactomes of listed SARS-CoV-2 viral proteins), Ref. (representative literature references in which autoantibodies to specific autoAgs are reported)
References for Table 1
D. Chatterjee, M. Pieroni, M. Fatah, F. Charpentier, K. S. Cunningham, D. A. Spears et al. An autoantibody profile detects Brugada syndrome and identifies abnormally expressed myocardial proteins. European heart journal, 2020;41:2878–90.
E. Vainio, G. M. Lenoir, R. M. Franklin. Autoantibodies in three populations of Burkitt’s lymphoma patients. Clinical and experimental immunology, 1983;54:387–96.
S. Pandey, I. Dioni, D. Lambardi, F. Real-Fernandez, E. Peroni, G. Pacini et al. Alpha actinin is specifically recognized by Multiple Sclerosis autoantibodies isolated using an N-glucosylated peptide epitope. Molecular & cellular proteomics : MCP, 2013;12:277–82.
M. C. Pott, N. Frede, J. Wanders, L. Hammarström, E. O. Glocker, C. Glocker et al. Autoantibodies against BAFF, APRIL or IL21 - an alternative pathogenesis for antibody-deficiencies? BMC immunology, 2017;18:34.
Y. Seko, A. Matsumoto, T. Fukuda, Y. Imai, T. Fujimura, H. Taka et al. A case of neonatal lupus erythematosus presenting delayed dilated cardiomyopathy with circulating autoantibody to annexin A6. Int Heart J, 2007;48:407–15.
J. Creaney, I. M. Dick, D. Yeoman, S. Wong, B. W. Robinson. Auto-antibodies to β-F1-ATPase and vimentin in malignant mesothelioma. PloS one, 2011;6:e26515.
V. M. Beutgen, C. Schmelter, N. Pfeiffer, F. H. Grus. Autoantigens in the trabecular meshwork and glaucoma-specific alterations in the natural autoantibody repertoire. Clinical & translational immunology, 2020;9:e01101.
C. N. Gruber, R. S. Patel, R. Trachtman, L. Lepow, F. Amanat, F. Krammer et al. Mapping Systemic Inflammation and Antibody Responses in Multisystem Inflammatory Syndrome in Children (MIS-C). Cell, 2020;183:982–95.e14.
Y. Ikeda, G. Toda, N. Hashimoto, T. Maruyama, H. Oka. Antibody that recognizes conformations of calmodulin in the serum from patient with chronic active hepatitis. Biochemical and biophysical research communications, 1987;144:191–7.
J. Boehm, T. Orth, P. Van Nguyen, H. D. Soling. Systemic lupus erythematosus is associated with increased auto-antibody titers against calreticulin and grp94, but calreticulin is not the Ro/SS-A antigen. Eur J Clin Invest, 1994;24:248–57.
K. Matsuo, Y. Xiang, H. Nakamura, K. Masuko, K. Yudoh, K. Noyori et al. Identification of novel citrullinated autoantigens of synovium in rheumatoid arthritis using a proteomic approach. Arthritis research & therapy, 2006;8:R175.
S. I. Yokota, D. Hirata, S. Minota, T. Higashiyama, M. Kurimoto, H. Yanagi et al. Autoantibodies against chaperonin CCT in human sera with rheumatic autoimmune diseases: comparison with antibodies against other Hsp60 family proteins. Cell Stress Chaperones, 2000;5:337–46.
K. Hirai, H. Maeda, K. Omori, T. Yamamoto, S. Kokeguchi, S. Takashiba. Serum antibody response to group II chaperonin from Methanobrevibacter oralis and human chaperonin CCT. Pathog Dis, 2013;68:12–9.
J. H. Rho, W. Zhang, M. Murali, M. H. Roehrl, J. Y. Wang. Human proteins with affinity for dermatan sulfate have the propensity to become autoantigens. Am J Pathol, 2011;178:2177–90.
C. Pagaza-Straffon, L. A. Marchat, L. Herrera, J. Díaz-Chávez, M. G. Avante, Y. P. Rodríguez et al. Evaluation of a panel of tumor-associated antigens in breast cancer. Cancer biomarkers : section A of Disease markers, 2020;27:207–11.
C. K. Heo, M. K. Woo, D. Y. Yu, J. Y. Lee, J. S. Yoo, H. S. Yoo et al. Identification of autoantibody against fatty acid synthase in hepatocellular carcinoma mouse model and its application to diagnosis of HCC. Int J Oncol, 2010;36:1453–9.
A. M. Rosenberg, D. M. Cordeiro. Relationship between sex and antibodies to high mobility group proteins 1 and 2 in juvenile idiopathic arthritis. J Rheumatol, 2000;27:2489–93.
C. Stemmer, N. Tuaillon, A. M. Prieur, S. Muller. Mapping of B-cell epitopes recognized by antibodies to histones in subsets of juvenile chronic arthritis. Clinical immunology and immunopathology, 1995;76:82–9.
J. Wesierska-Gadek, E. Penner, H. Lindner, E. Hitchman, G. Sauermann. Autoantibodies against different histone H1 subtypes in systemic lupus erythematosus sera. Arthritis and rheumatism, 1990;33:1273–8.
Y. S. Kwon, J. Chung, G. T. Shin, S. Y. Lee, Y. J. Jang. Variable region genes of human monoclonal autoantibodies to histones H2A and H2B from a systemic lupus erythematosus patient. Molecular immunology, 2005;42:311–7.
J. Dieker, J. H. Berden, M. Bakker, J. P. Briand, S. Muller, R. Voll et al. Autoantibodies against Modified Histone Peptides in SLE Patients Are Associated with Disease Activity and Lupus Nephritis. PloS one, 2016;11:e0165373.
G. C. Astaldi Ricotti, M. Bestagno, A. Cerino, C. Negri, R. Caporali, F. Cobianchi et al. Antibodies to hnRNP core protein A1 in connective tissue diseases. J Cell Biochem, 1989;40:43–7.
D. Stanek, J. Vencovsky, J. Kafkova, I. Raska. Heterogenous nuclear RNP C1 and C2 core proteins are targets for an autoantibody found in the serum of a patient with systemic sclerosis and psoriatic arthritis. Arthritis and rheumatism, 1997;40:2172–7.
J. Y. Zhang, E. K. Chan, X. X. Peng, E. M. Tan. A novel cytoplasmic protein with RNA-binding motifs is an autoantigen in human hepatocellular carcinoma. J Exp Med, 1999;189:1101–10.
S. Britton, C. Froment, P. Frit, B. Monsarrat, B. Salles, P. Calsou. Cell nonhomologous end joining capacity controls SAF-A phosphorylation by DNA-PK in response to DNA double-strand breaks inducers. Cell Cycle, 2009;8:3717–22.
L. Harlow, I. O. Rosas, B. R. Gochuico, T. R. Mikuls, P. F. Dellaripa, C. V. Oddis et al. Identification of citrullinated hsp90 isoforms as novel autoantigens in rheumatoid arthritis-associated interstitial lung disease. Arthritis and rheumatism, 2013;65:869–79.
A. Pashov, A. Kenderov, S. Kyurkchiev, I. Kehayov, S. Hristova, S. Lacroix-Desmazes et al. Autoantibodies to heat shock protein 90 in the human natural antibody repertoire. Int Immunol, 2002;14:453–61.
H. Y. Qin, J. L. Mahon, M. A. Atkinson, P. Chaturvedi, E. Lee-Chan, B. Singh. Type 1 diabetes alters anti-hsp90 autoantibody isotype. Journal of autoimmunity, 2003;20:237–45.
M. Tishler, Y. Shoenfeld. Anti-heat-shock protein antibodies in rheumatic and autoimmune diseases. Semin Arthritis Rheum, 1996;26:558–63.
S. Blass, A. Union, J. Raymackers, F. Schumann, U. Ungethum, S. Muller-Steinbach et al. The stress protein BiP is overexpressed and is a major B and T cell target in rheumatoid arthritis. Arthritis Rheum, 2001;44:761–71.
L. Horvath, L. Cervenak, M. Oroszlan, Z. Prohaszka, K. Uray, F. Hudecz et al. Antibodies against different epitopes of heat-shock protein 60 in children with type 1 diabetes mellitus. Immunol Lett, 2002;80:155–62.
M. Minohara. [Heat shock protein 105 in multiple sclerosis]. Nippon Rinsho, 2003;61:1317–22.
T. Kobayashi, T. Yura, H. Yanagi. The increment of anti-ORP150 autoantibody in initial stages of atheroma in high-fat diet fed mice. The Journal of veterinary medical science, 2002;64:177–80.
T. O. Ola, P. A. Biro, M. I. Hawa, J. Ludvigsson, M. Locatelli, M. A. Puglisi et al. Importin beta: a novel autoantigen in human autoimmunity identified by screening random peptide libraries on phage. Journal of autoimmunity, 2006;26:197–207.
K. Ueda, T. Nakanishi, A. Shimizu, T. Takubo, N. Matsuura. Identification of L-plastin autoantibody in plasma of patients with non-Hodgkin’s lymphoma using a proteomics-based analysis. Ann Clin Biochem, 2008;45:65–9.
J. L. Senecal, J. Rauch, T. Grodzicky, J. P. Raynauld, I. Uthman, A. Nava et al. Strong association of autoantibodies to human nuclear lamin B1 with lupus anticoagulant antibodies in systemic lupus erythematosus. Arthritis and rheumatism, 1999;42:1347–53.
G. Frampton, S. Moriya, J. D. Pearson, D. A. Isenberg, F. J. Ward, T. A. Smith et al. Identification of candidate endothelial cell autoantigens in systemic lupus erythematosus using a molecular cloning strategy: a role for ribosomal P protein P0 as an endothelial cell autoantigen. Rheumatology (Oxford, England), 2000;39:1114–20.
D. A. Bledzhyants, R. M. Muratov, R. R. Movsesyan, Z. A. Podlubnaya. Autoantibodies to myosin light chains in the blood as early marker of myocardial injury after aortocoronary bypass surgery. Bull Exp Biol Med, 2007;144:241–5.
R. Mossabeb, S. Seiberler, I. Mittermann, R. Reininger, S. Spitzauer, S. Natter et al. Characterization of a novel isoform of alpha-nascent polypeptide-associated complex as IgE-defined autoantigen. The Journal of investigative dermatology, 2002;119:820–9.
I. N. Batova, R. T. Richardson, E. E. Widgren, M. G. O’Rand. Analysis of the autoimmune epitopes on human testicular NASP using recombinant and synthetic peptides. Clinical and experimental immunology, 2000;121:201–9.
Z. Qin, B. Lavingia, Y. Zou, P. Stastny. Antibodies against nucleolin in recipients of organ transplants. Transplantation, 2011;92:829–35.
D. B. Ulanet, M. Torbenson, C. V. Dang, L. Casciola-Rosen, A. Rosen. Unique conformation of cancer autoantigen B23 in hepatoma: a mechanism for specificity in the autoimmune response. Proc Natl Acad Sci U S A, 2003;100:12361–6.
S. Nagayama, T. Yokoi, H. Tanaka, Y. Kawaguchi, T. Shirasaka, T. Kamataki. Occurrence of autoantibody to protein disulfide isomerase in patients with hepatic disorder. J Toxicol Sci, 1994;19:163–9.
Y. Takasaki, K. Kaneda, M. Matsushita, H. Yamada, M. Nawata, R. Matsudaira et al. Glyceraldehyde 3-phosphate dehydrogenase is a novel autoantigen leading autoimmune responses to proliferating cell nuclear antigen multiprotein complexes in lupus patients. Int Immunol, 2004;16:1295–304.
J. Gut, U. Christen, N. Frey, V. Koch, D. Stoffler. Molecular mimicry in halothane hepatitis: biochemical and structural characterization of lipoylated autoantigens. Toxicology, 1995;97:199–224.
Z. Mojtahedi, A. Safaei, Z. Yousefi, A. Ghaderi. Immunoproteomics of HER2-positive and HER2-negative breast cancer patients with positive lymph nodes. Omics : a journal of integrative biology, 2011;15:409–18.
I. Mayo, J. Arribas, P. Villoslada, R. Alvarez DoForno, S. Rodriguez-Vilarino, X. Montalban et al. The proteasome is a major autoantigen in multiple sclerosis. Brain, 2002;125:2658–67.
E. Feist, U. Kuckelkorn, T. Dörner, H. Dönitz, S. Scheffler, F. Hiepe et al. Autoantibodies in primary Sjögren’s syndrome are directed against proteasomal subunits of the alpha and beta type. Arthritis and rheumatism, 1999;42:697–702.
C. Bohring, W. Krause. Characterization of spermatozoa surface antigens by antisperm antibodies and its influence on acrosomal exocytosis. Am J Reprod Immunol, 2003;50:411–9.
K. Sugimoto, T. Hiwasa, K. Shibuya, S. Hirano, M. Beppu, S. Isose et al. Novel autoantibodies against the proteasome subunit PSMA7 in amyotrophic lateral sclerosis. Journal of neuroimmunology, 2018;325:54–60.
M. Roessler, W. Rollinger, L. Mantovani-Endl, M. L. Hagmann, S. Palme, P. Berndt et al. Identification of PSME3 as a novel serum tumor marker for colorectal cancer by combining two-dimensional polyacrylamide gel electrophoresis with a strictly mass spectrometry-based approach for data analysis. Mol Cell Proteomics, 2006;5:2092–101.
P. G. Vlachoyiannopoulos, S. Frillingos, A. G. Tzioufas, K. Seferiadis, H. M. Moutsopoulos, O. Tsolas. Circulating antibodies to prothymosin alpha in systemic lupus erythematosus. Clinical immunology and immunopathology, 1989;53:151–60.
Y. Yamasaki, S. Narain, L. Hernandez, T. Barker, K. Ikeda, M. S. Segal et al. Autoantibodies against the replication protein A complex in systemic lupus erythematosus and other autoimmune diseases. Arthritis research & therapy, 2006;8:R111.
J. A. Luna Coronell, K. Sergelen, P. Hofer, I. Gyurján, S. Brezina, P. Hettegger et al. The Immunome of Colon Cancer: Functional In Silico Analysis of Antigenic Proteins Deduced from IgG Microarray Profiling. Genomics, proteomics & bioinformatics, 2018;16:73–84.
A. Guialis, M. Patrinou-Georgoula, N. Tsifetaki, V. Aidinis, C. E. Sekeris, H. M. Moutsopoulos. Anti-5S RNA/protein (RNP) antibody levels correlate with disease activity in a patient with systemic lupus erythematosus (SLE) nephritis. Clinical and experimental immunology, 1994;95:385–9.
R. Gerli, L. Caponi. Anti-ribosomal P protein antibodies. Autoimmunity, 2005;38:85–92.
K. Elkon, E. Bonfa, R. Llovet, W. Danho, H. Weissbach, N. Brot. Properties of the ribosomal P2 protein autoantigen are similar to those of foreign protein antigens. Proceedings of the National Academy of Sciences of the United States of America, 1988;85:5186–9.
Z. Chai, B. Sarcevic, A. Mawson, B. H. Toh. SET-related cell division autoantigen-1 (CDA1) arrests cell growth. J Biol Chem, 2001;276:33665–74.
D. Hof, K. Cheung, D. J. de Rooij, F. H. van den Hoogen, G. J. Pruijn, W. J. van Venrooij et al. Autoantibodies specific for apoptotic U1–70K are superior serological markers for mixed connective tissue disease. Arthritis Res Ther, 2005;7:R302–9.
M. T. McClain, P. A. Ramsland, K. M. Kaufman, J. A. James. Anti-sm autoantibodies in systemic lupus target highly basic surface structures of complexed spliceosomal autoantigens. Journal of immunology (Baltimore, Md : 1950), 2002;168:2054–62.
H. Brahms, J. Raymackers, A. Union, F. de Keyser, L. Meheus, R. Luhrmann. The C-terminal RG dipeptide repeats of the spliceosomal Sm proteins D1 and D3 contain symmetrical dimethylarginines, which form a major B-cell epitope for anti-Sm autoantibodies. The Journal of biological chemistry, 2000;275:17122–9.
H. Imai, E. K. Chan, K. Kiyosawa, X. D. Fu, E. M. Tan. Novel nuclear autoantigen with splicing factor motifs identified with antibody from hepatocellular carcinoma. The Journal of clinical investigation, 1993;92:2419–26.
N. Iizuka, K. Okamoto, R. Matsushita, M. Kimura, K. Nagai, M. Arito et al. Identification of autoantigens specific for systemic lupus erythematosus with central nervous system involvement. Lupus, 2010;19:717–26.
K. Overzet, T. J. Gensler, S. J. Kim, M. E. Geiger, W. J. van Venrooij, K. M. Pollard et al. Small nucleolar RNP scleroderma autoantigens associate with phosphorylated serine/arginine splicing factors during apoptosis. Arthritis and rheumatism, 2000;43:1327–36.
P. Gordon, M. A. Khamashta, E. Rosenthal, J. M. Simpson, G. Sharland, A. Brucato et al. Anti-52 kDa Ro, anti-60 kDa Ro, and anti-La antibody profiles in neonatal lupus. J Rheumatol, 2004;31:2480–7.
A. Cortini, S. Bembich, L. Marson, E. Cocco, P. Edomi. Identification of novel non-myelin biomarkers in multiple sclerosis using an improved phage-display approach. PloS one, 2019;14:e0226162.
X. Geng, L. Biancone, H. H. Dai, J. J. Lin, N. Yoshizaki, A. Dasgupta et al. Tropomyosin isoforms in intestinal mucosa: production of autoantibodies to tropomyosin isoforms in ulcerative colitis. Gastroenterology, 1998;114:912–22.
S. P. Mahesh, Z. Li, R. Buggage, F. Mor, I. R. Cohen, E. Y. Chew et al. Alpha tropomyosin as a self-antigen in patients with Behcet’s disease. Clinical and experimental immunology, 2005;140:368–75.
A. Kimura, T. Sakurai, M. Yamada, A. Koumura, Y. Hayashi, Y. Tanaka et al. Anti-endothelial cell antibodies in patients with cerebral small vessel disease. Curr Neurovasc Res, 2012;9:296–301.
X. Zhao, Y. Cheng, Y. Gan, R. Jia, L. Zhu, X. Sun. Anti-tubulin-alpha-1C autoantibody in systemic lupus erythematosus: a novel indicator of disease activity and vasculitis manifestations. Clinical rheumatology, 2018;37:1229–37.
A. Kimura, N. Yoshikura, A. Koumura, Y. Hayashi, Y. Kobayashi, I. Kobayashi et al. Identification of target antigens of naturally occurring autoantibodies in cerebrospinal fluid. Journal of proteomics, 2015;128:450–7.
Z. E. Betteridge, H. Gunawardena, H. Chinoy, J. North, W. E. Ollier, R. G. Cooper et al. Clinical and human leucocyte antigen class II haplotype associations of autoantibodies to small ubiquitin-like modifier enzyme, a dermatomyositis-specific autoantigen target, in UK Caucasian adult-onset myositis. Annals of the rheumatic diseases, 2009;68:1621–5.
K. Miyachi, H. Hosaka, N. Nakamura, H. Miyakawa, T. Mimori, M. Shibata et al. Anti-p97/VCP antibodies: an autoantibody marker for a subset of primary biliary cirrhosis patients with milder disease? Scand J Immunol, 2006;63:376–82.
H. Bang, K. Egerer, A. Gauliard, K. Luthke, P. E. Rudolph, G. Fredenhagen et al. Mutation and citrullination modifies vimentin to a novel autoantigen for rheumatoid arthritis. Arthritis Rheum, 2007;56:2503–11.
J. Mao, J. Ladd, E. Gad, L. Rastetter, M. M. Johnson, E. Marzbani et al. Mining the pre-diagnostic antibody repertoire of TgMMTV-neu mice to identify autoantibodies useful for the early detection of human breast cancer. Journal of translational medicine, 2014;12:121.
J. Wen, M. Yaneva. Mapping of epitopes on the 86 kDa subunit of the Ku autoantigen. Mol Immunol, 1990;27:973–80.
S. Hoa, M. Hudson, Y. Troyanov, S. Proudman, J. Walker, W. Stevens et al. Single-specificity anti-Ku antibodies in an international cohort of 2140 systemic sclerosis subjects: clinical associations. Medicine (Baltimore), 2016;95:e4713.
A. Kistner, M. B. Bigler, K. Glatz, S. B. Egli, F. S. Baldin, F. A. Marquardsen et al. Characteristics of autoantibodies targeting 14-3-3 proteins and their association with clinical features in newly diagnosed giant cell arteritis. Rheumatology (Oxford, England), 2017;56:829–34.
M. H. van Beers-Tas, A. Marotta, M. Boers, W. P. Maksymowych, D. van Schaardenburg. A prospective cohort study of 14-3-3eta in ACPA and/or RF-positive patients with arthralgia. Arthritis research & therapy, 2016;18:76.
J. Qiu, G. Choi, L. Li, H. Wang, S. J. Pitteri, S. R. Pereira-Faca et al. Occurrence of autoantibodies to annexin I, 14-3-3 theta and LAMR1 in prediagnostic lung cancer sera. Journal of clinical oncology : official journal of the American Society of Clinical Oncology, 2008;26:5060–6.
R. Chakravarti, K. Gupta, M. Swain, B. Willard, J. Scholtz, L. G. Svensson et al. 14-3-3 in Thoracic Aortic Aneurysms: Identification of a Novel Autoantigen in Large Vessel Vasculitis. Arthritis & rheumatology (Hoboken, NJ), 2015;67:1913–21.
Remarkably, among the 140 DS-affinity proteins identified from Jurkat T-cells, at least 105 (75%) are known autoAgs, i.e., the existence of specific autoantibodies against these proteins has been reported in the literature (see references in Table 1). These autoantibody/autoAg pairs are found in a wide spectrum of autoimmune diseases as well as a variety of cancers. Although 36 of the DS-affinity proteins have not yet been reported as autoAgs, we suspect that most, if not all, are putative autoAgs awaiting serological confirmation. For example, 6 serine/arginine-rich splicing factors were identified by DS-affinity, but only 3 of them (SRSF1, SRSF3, SRSF5) have thus far been individually reported as autoAgs (Table 1). A serine/arginine-rich repeating octapeptide of Arg-Ser-Arg-Ser-Arg(Lys)-Glu(Asp)-Arg-Lys(Arg) has been found in several nuclear autoAgs such as U2AF 35- and 65-kDa splicing factors and 70-KDa U1 snRNP [23], and many other splicing factors have been reported as autoAgs, such as SF3B1 and SRSF2. Therefore, we suspect that the other 3 splicing factors (SRSF3B3, SRSF7, and SRSF8) identified by DS-affinity in this study are likely true autoAgs that are yet to be confirmed.
Proteins eluted with 1.0 M NaCl possess the strongest DS-affinity and, strikingly, 10/13 (90.9%) are known autoAgs (Table 1), indicating that increasing affinity to DS increases the propensity of a protein to be an autoAg, consistent with our prior findings [1–3, 5, 6, 9–11]. These include histones (H4, H2B types 1-a and 1-b, H2A type 1-a), 60S ribosomal proteins (P0, P2, L6, L7), ACTC1 (skeletal muscle actin), and C1QBP, and PABPC3 (polyadenylate-binding protein 3). Histones and ribosomal P proteins are hallmark autoAgs used in routine clinical tests of autoimmune diseases. Histone autoAbs are nearly always present in drug-induced systemic lupus erythematosus, and ribosomal P autoantibodies are tested for to aid in the differential diagnosis of lupus patients with neuropsychiatric symptoms. C1QBP has been repeatedly identified as a putative autoAg in several of our prior studies [1, 2, 9, 10] and was recently confirmed as an autoAg in the neurodegenerative disorder primary open-angle glaucoma [24]. Poly(A)-binding proteins bind the poly(A) tail of messenger RNAs and control mRNA stability and translation initiation. Although PABPC3 has not yet been reported as an autoAg, its paralog PABPC1 has been found to be an autoAg [25].
Proteins eluted with 0.6 M NaCl possess strong DS-affinity and, remarkably, 26/31 (83.9%) are known autoAgs (Table 1). Several well-known autoAgs are identified in this strong DS-affinity fraction, including 6 histone autoAgs, SSB (lupus La autoAg), XRCC6 (lupus Ku70 autoAg), 3 snRNP autoAgs (Sm D2, Sm D3, U1 70kD). Other autoAgs identified with strong DS-affinity include ANP32B, nucleolin, nucleophosmin, SET, HNRNPCL1, HSP90AA1, 3 ribosomal proteins (L22, L5, S3a), 3 serine/arginine-rich splicing factors, 3 tropomyosin subunits, prothymosin alpha, 3 tubulin subunits, vimentin, and T-complex protein 1 alpha. A few have not yet been confirmed as autoAgs, including ANP32A, kappa actin, and ribosomal protein 3A.
The 140 candidate autoAgs identified from Jurkat T-cells are not a random collection but are highly enriched in a few groups of proteins. Among them, there are 11 proteasomal proteins, 8 ribosomal proteins, 8 histones, 8 T-complex protein (CCT/TriC) subunits, 7 heat shock proteins, 6 splicing factors, 6 14-3-3 proteins, 5 DNA replication licensing factors (or minichromosome maintenance proteins), 5 DNA or RNA helicases, and 4 hnRNPs.
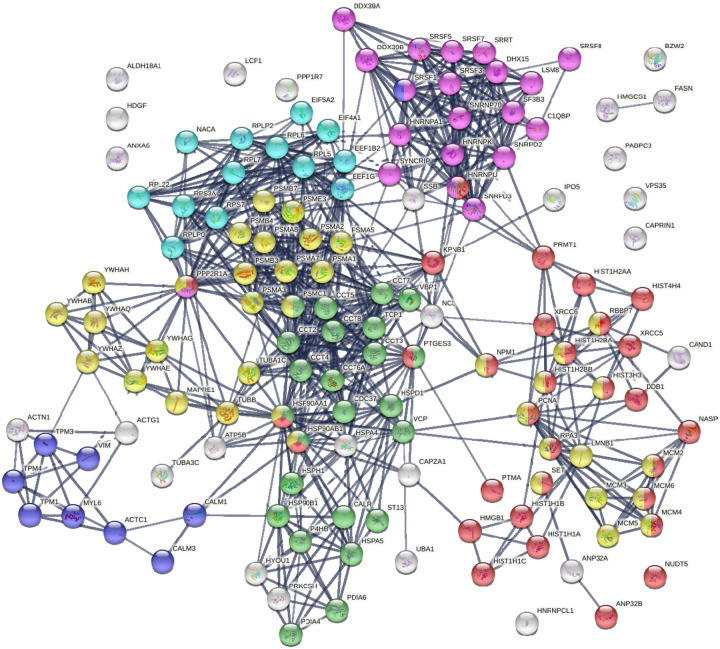
Protein-protein interaction network analysis by STRING [26] reveals that the DS-affinity autoantigen-ome is highly connected (Fig. 1). There are 787 interactions at high confidence level (vs. 284 expected; enrichment p-value <1.0e-16). These DS-affinity proteins are enriched in several clusters and significantly associated with the cell cycle, protein folding, chromosome organization, RNA splicing, translation, and muscle contraction (Fig. 1). There are 36 DS-affinity proteins associated with the cell cycle, particularly the G2/M checkpoints (26 proteins), the G2/M DNA damage checkpoint, and the G1/S and G2/M transitions.
Fig. 1.
The autoantigen-ome from Jurkat T-cells identified by DS affinity. Lines represent protein-protein interactions at high confidence levels. Marked proteins are associated with cell cycle (37 proteins, yellow), chromosome organization (31 proteins, red), RNA splicing (20 proteins, pink), translation (13 proteins, aqua), protein folding (24 proteins, green), and muscle contraction (9 proteins, blue).
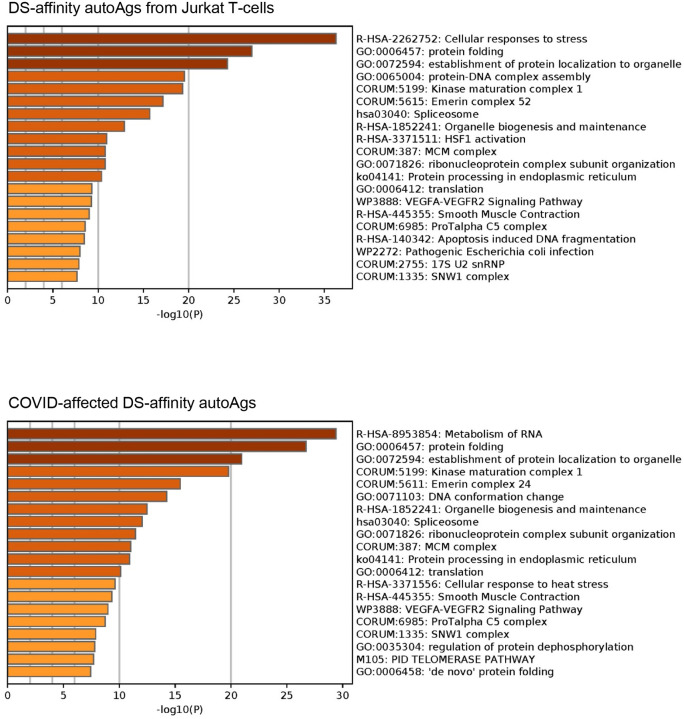
Pathway and process enrichment analyses by Metascape [27] also reveal that proteins of the DS-affinity autoantigen-ome are significantly associated with cellular response to stress, protein folding, and protein localization to organelles (Fig. 2A). In addition, they are associated with kinase maturation complex 1, spliceosome, HSF1 activation (activates gene expression in response to a variety of stresses), protein processing in the endoplasmic reticulum, VEGFA-VEGFR2 signaling (major pathway that activates angiogenesis), apoptosis-induced DNA fragmentation, and 17S U2 snRNP.
Fig. 2.
Top 20 enriched pathways and processes among COVID-altered DS-affinity proteins. Top chart: 140 proteins identified by DS-affinity from Jurkat T-cells. Bottom chart: 125 DS-affinity proteins that are altered in SARS-CoV-2 infection.
DS-affinity autoantigen-ome related to COVID-19
To find out how many of the DS-affinity autoAgs identified from Jurkat T-cells are affected by in SARS-CoV-2 infection, we searched for them in a multi-omic COVID database compiled by Coronascape [27–47]. Among the 140 DS-affinity proteins identified in our study, 125 (89.3%) are affected by SARS-CoV-2 infection, and at least 94 (of the 125; 75.2%) are known autoAgs (Table 1 and Supplemental Table 1). Among the COVID-altered DS-affinity proteins, 17 are up-regulated only, 35 are down-regulated only, and 71 are altered (up or down depending on study conditions) at protein and/or RNA levels in SARS-CoV-2 infected cells. The COVID database was assembled from different cell and patient tissue types by multiple research laboratories using different technologies, including proteomics, phosphoproteomics, ubiquitinomics, and bulk and single-cell RNA sequencing.
Six DS-affinity proteins are found in the interactomes of SARS-CoV-2 viral proteins, i.e., these host proteins interact directly or indirectly with the viral proteins [29, 40, 44]. Specifically, HSPA5 (GRP78/BiP) interacts with Nsp2 and Nsp4, HYOU1 interacts with Orf8, PRKCSH and MAPRE1 interact with Orf3, and BZW2 interacts with the viral M protein. HSPA5/BiP (binding immunoglobulin protein) has been consistently identified by DS-affinity in our previous studies, and we have also recently reported that DS-BiP association plays important roles in regulating precursor autoreactive B1 cells [8]. HYOU1 (hypoxia up-regulated protein 1) was also found overexpressed at protein level in the urine of COVID-19 patients and up-regulated at mRNA level in B cells from 4 patients out of a cohort of 7 hospitalized COVID-19 patients [33, 48]. HYOU1 belongs to the heat shock protein 70 family, accumulates in the endoplasmic reticulum under hypoxic conditions, and has been shown to be up-regulated in tumors. PRKCSH (glucosidase 2 subunit beta) is an N-linked glycan processing enzyme in the endoplasmic reticulum, and mutations of this gene have been associated with the autosomal dominant polycystic liver disease. MAPRE1 (microtubule-associated protein RP/EB family member 1) binds the plus-end of microtubules and regulates microtubule cytoskeleton dynamics. BZW2 (basic leucine zipper and W2 domain 2) may be involved in neuronal differentiation and is associated with congenital hypomyelinating neuropathy.
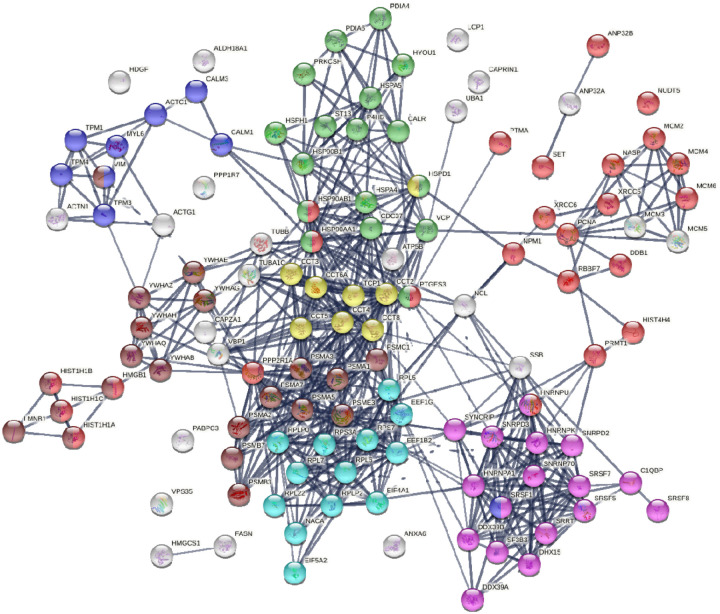
Similar to the 140 DS-affinity protein autoantigen-ome, the 125 COVID-altered DS-affinity proteins are most significantly associated with RNA metabolism and protein folding (Fig. 2B). In addition, they are associated with establishment of protein localization to organelles, kinase maturation complex 1, emerin complex 24, DNA conformation change, spliceosome, cellular response to heat stress, smooth muscle contraction, VEGFA-VESFR2 signaling pathway, prothymosin alpha C5 complex, regulation of protein dephosphorylation, and telomerase pathway (Fig. 2B). Protein-protein interaction network analysis also confirms that the COVID-altered DS-affinity protein network is strongly associated with mRNA processing, translation, chromosome organization, protein processing in the endoplasmic reticulum, CCT/TriC chaperonin, and apoptosis (Fig. 3).
Fig. 3.
DS-affinity proteins that are altered by SARS-CoV-2 infection. Lines represent protein-protein interactions at high confidence levels. Marked proteins are associated with chromosome organization (25 proteins, red), mRNA processing (17 proteins, pink), translation (13 proteins, aqua), protein processing in endoplasmic reticulum (green, 17 proteins), muscle contraction (9 proteins, blue), TCP-1/cpn60 chaperonin (yellow, 8 proteins), and apoptosis (21 proteins, brown).
Nine COVID-altered DS-affinity proteins are associated with muscle contraction, including ACTC1, CALM1, CALM3, MYL6, TPM1, TPM3, TPM4, SRSF1, and VIM. All of these proteins are known autoAgs (Table 1). CALM1 has recently been identified as one of the autoAgs in multisystem inflammatory syndrome in children from SARS-CoV-2 infection [13]. Six 14-3-3 proteins are identified, all of which are autoAgs. Presence of 14-3-3 proteins in cerebrospinal fluid, a marker of ongoing neurodegeneration, has been detected in COVID-19 patients [49].
AutoAgs from altered phosphorylation and ubiquitination
Thirty-eight of the 125 COVID-affected DS-affinity proteins have phosphorylation changes in SARS-CoV-2 infection (Fig. 4). Their molecular functions include histone binding (6 proteins), RNA binding (10 proteins), helicase activity (5 proteins), ATP binding (12 proteins), DNA binding (14 proteins), and hydrolase activity (11 proteins). These COVID-altered phosphoproteins are significantly associated with gene expression, chromosome organization, and mRNA metabolism. Chromosome-associated proteins are particularly related to DNA conformation change (XRCC6, SET, NPM1, HIST1H1C, HIST1H1B, RBBP7, NASP, MCMs) and DNA replication (MCM2, MCM3, MCM4, NASP, RBBP7, and SET). mRNA-associated proteins are related to mRNA splicing (SRSF1, SRSF7, SRRT, HNRNPA1, HNRPNK, HNRNPU, DDX39A) and RNA 3’-end processing (DDX39A, SRSF7, SRSF1, SSB). In addition, nuclear matrix protein lamin-B1, nucleolar protein nucleolin, vacuolar protein sorting-associated protein VPS35, vimentin, fatty acid synthetase FASN, protein phosphatase 1 regulatory subunit PPP1R7, and HDGF (hepatoma-derived growth factor) are altered by phosphorylation.
Fig. 4.
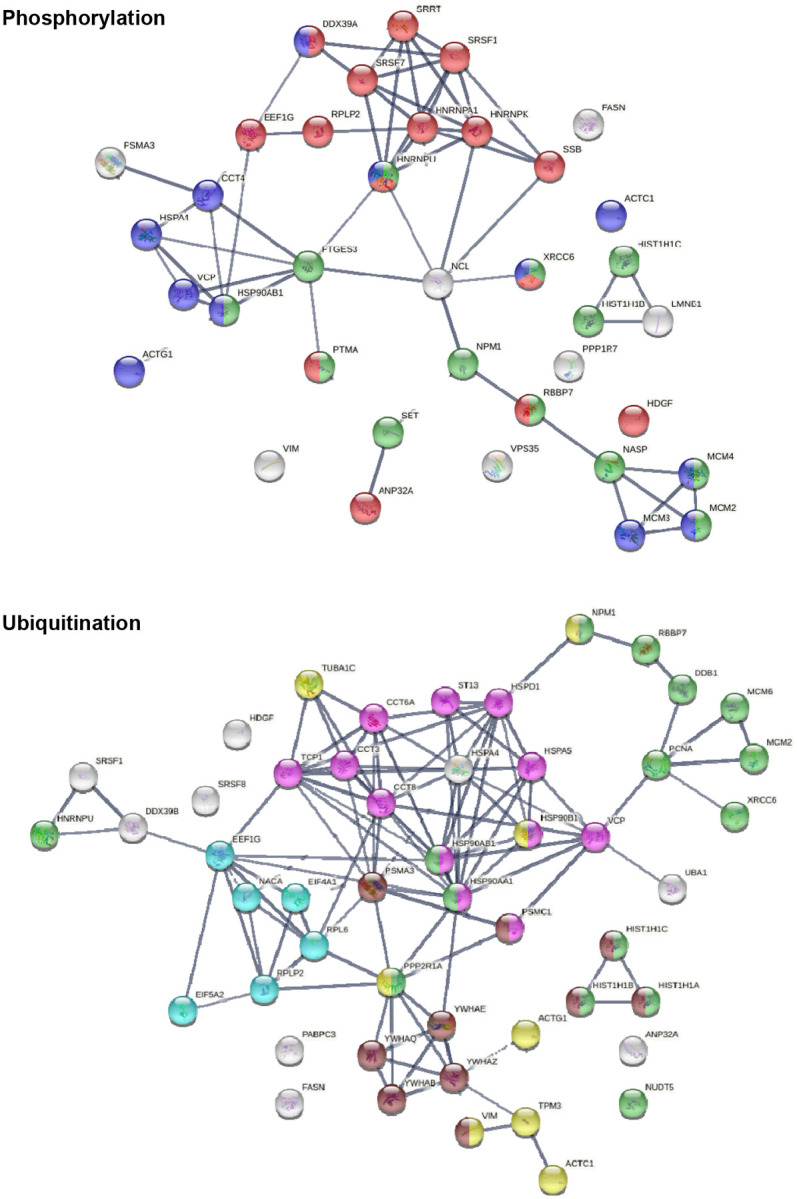
DS-affinity proteins that show changes in phosphorylation or ubiquitination in SARS-CoV-2 infection. Phosphorylation: marked proteins are associated with gene expression (15 proteins, red), chromosome organization (13 proteins, green), and ATP binding (12 proteins, blue). Ubiquitination: marked proteins are associated with protein folding (12 proteins, pink), chromosome organization (15 protein, green), translation (6 protein, aqua), cytoskeleton (8 proteins, yellow), and apoptosis (10 proteins, brown).
Among the 125 COVID-affected DS-affinity proteins, 50 are altered by ubiquitination in SARS-CoV-2 infection (Fig. 4). These proteins are associated with apoptosis, chromosome organization, protein folding, translation, cell cycle, and cytoskeleton. Proteins related to apoptosis include linker histones (HIST1H1A, HIST1H1B, HIST1H1C), 14-3-3 proteins (YWHAB, YWHAE, YWHAQ, YWHAZ), and proteasome proteins (PSMA3, PSMC1). Proteins related to the cell cycle include PNCA, MCM2, MCM6, and 14-3-3 proteins. Five heat shock proteins and 4 subunits of chaperonin CCT/TriC are ubiquitinated. Other interesting ubiquitinated proteins include NACA (nascent polypeptide-associated complex subunit alpha), DDB1 (DNA damage-binding protein 1), NUDT5 (ADP-sugar pyrophosphatase), and NPM1 (nucleophosmin). Ubiquitination is typically the “kiss of death” modification that marks proteins destined for degradation by the proteasome, although ubiquitination may also modulate protein interaction and activity. Intriguingly, we identified UBA1 (ubiquitin-like modifier-activating enzyme 1), which catalyzes the first step in ubiquitin conjugation and plays a central role in ubiquitination, as a ubiquitination-altered DS-affinity autoAg, which is consistent with our previous studies [1, 2].
DS-affinity proteins altered in T cells of COVID-19 patients
Because Jurkat cells were established from human T-cell lymphoblastic leukemia, we searched for DS-affinity proteins that were altered in T cells of seven COVID-19 patients [33]. Five proteins (LCP1, CALR, HSPA5, HSP90AA1, HSP90AB1) were up-regulated in CD4+ T cells, and 13 proteins (LCP1, CALR, HSPA5, HSP90AA1, HSP90AB1, HSPD1, HSPH1, MCM4, VIM, PTMA, TUBB, H1–2, LMNB1) were up-regulated in CD8+ T cells of COVID-19 patients. Three proteins (ACTG1, EEF1B2, SRSF5) were down-regulated in the CD4+ T cells, and 3 proteins (ATCG1, EEF1B2, NACA) were down-regulated in CD8+ T cells. Remarkably, all up-regulated DS-affinity proteins are known autoAgs (Table 1). NACA, ACTG1, and SRSF5, which were down-regulated at the mRNA level, are also known autoAgs. EEF1B2 (or EEF1B, elongation factor 1-beta) has not been identified as an autoAg, although other similar elongation factors such as EEF1A and EF2 are known autoAgs (see references in Table 1).
Among up-regulated proteins, LCP1 was up-regulated in CD4+ T cells of 2 patients (out of 4 patients with available data) and in CD8+ T cells of 2 patients (out of 5 patients with available data), with one of the patients having LCP1 up in both CD4+ and CD8+ T cells. Up-regulation of heat shock proteins, particularly HSPA5 and HSP90AA1, was detected in CD4+ T cells of 2 patients and CD8+ T cells of 1 patient. MCM4 up-regulation was detected in CD8+ T cells of 3 out of 6 patients. Among down-regulated proteins, NACA was detected in CD4+ T cells of 1 patient and CD8+ T cells of all 3 patients whose data were available. EEF1B2 was down in CD4+ T cells of 3 patients (out of 5 with available data) and down in CD8+ T cells of 2 out of 3 patients. ACTG1 down-regulation was detected in CD4+ T cells of 2 patients and CD8+ T cells of 1 patient. SRSF5 was down in CD4+ T cells of 3 out of 5 patients.
Among these T-cell-altered proteins, LCP1 and NACA are perhaps most interesting. LCP1 (plastin-2, an actin binding protein) has been found to play a significant role in T cell activation in response to co-stimulation through TCR/CD3 and regulates the stability of the immune synapse of naïve and effector T cells [50]. NACA (nascent polypeptide-associated complex subunit alpha) binds to newly synthesized polypeptide chains as they emerge from the ribosome, blocks their interaction with the signal recognition particle, and prevents inappropriate targeting of non-secretory polypeptides to the endoplasmic reticulum. NACA is an IgE autoAg in atopic dermatitis patients with chronic skin manifestations [51]. The significance of these T-cell proteins in COVID-19 and autoimmunity merits further study.
Conclusion
In order to establish a comprehensive COVID-19 autoantigen-ome, we have been profiling autoAgs from different cell and tissue types. Compared to other cells we have examined, Jurkat T-cells contain relatively fewer DS-affinity autoAgs than HFL1 lung fibroblasts, A549 lung epithelial cells, HS-Sultan B-lymphoblasts, and HEp-2 fibroblasts. Although cells share numerous autoAgs, each cell type gives rise to unique COVID-altered autoAg candidates, which may explain the wide range of symptoms experienced by patients with autoimmune sequelae of SARS-CoV-2 infection. We believe that our effort of discovering autoAgs across different cell types provides a comprehensive and valuable autoAg database for better understanding of autoimmune diseases and post-COVID-19 health problems.
Materials and Methods
Jurkat T-cell culture
The human T lymphoblast Jurkat cell line was obtained from the ATCC (Manassas, VA) and cultured in complete RPMI-1640 medium. The growth medium was supplemented with 10% fetal bovine serum and a penicillin-streptomycin-glutamine mixture (Thermo Fisher). The cells were grown at 37 °C in a CO2 incubator.
Protein extraction
Protein extraction was performed as previously described [6]. In brief, Jurkat cells were lysed with 50 mM phosphate buffer (pH 7.4) containing the Roche Complete Mini protease inhibitor cocktail and then homogenized on ice with a microprobe sonicator until the turbid mixture turned nearly clear with no visible cells left. The homogenate was centrifuged at 10,000 g at 4 °C for 20 min, and the total protein extract in the supernatant was collected. Protein concentration was measured by absorbance at 280 nm using a NanoDrop UV-Vis spectrometer (Thermo Fisher).
DS-Sepharose resin preparation
The DS-affinity resins were synthesized as previously described [6, 9]. In brief, 20 ml of EAH Sepharose 4B resins (GE Healthcare Life Sciences) were washed with distilled water three times and mixed with 100 mg of DS (Sigma-Aldrich) in 10 ml of 0.1 M MES buffer, pH 5.0. About 100 mg of N-(3-dimethylaminopropyl)-N’-ethylcarbodiimide hydrochloride (Sigma-Aldrich) powder was added, and another 100 mg was added after 8 h of reaction. The reaction proceeded by mixing on a rocker at 25 °C for 16 h. The coupled resins were washed with water and equilibrated with 0.5 M NaCl in 0.1 M acetate (pH 5.0) and 0.5 M NaCl in 0.1 M Tris (pH 8.0).
DS-affinity fractionation
The total proteomes extracted from Jurkat cells were fractionated in a DS-Sepharose column [6]. About 40 mg of proteins in 40 ml of 10 mM phosphate buffer (pH 7.4; buffer A) were loaded onto the DS-affinity column at a rate of 1 ml/min. Unbound and weakly bound proteins were removed with 60 ml of buffer A and then 40 ml of 0.2 M NaCl in buffer A. The remaining bound proteins were eluted in step gradients of 40 ml each of 0.4 M, 0.6 M, and 1.0 M NaCl in buffer A. Fractions were desalted and concentrated with 5-kDa cut-off Vivaspin centrifugal filters (Sartorius). Fractionated proteins were separated in 1-D SDS-PAGE in 4–12% Bis-Tris gels, and each gel lane was divided into two or three sections for sequencing.
Mass spectrometry sequencing
Protein sequencing was performed at the Taplin Biological Mass Spectrometry Facility at Harvard Medical School. Proteins in gels were digested with sequencing-grade trypsin (Promega) at 4 °C for 45 min. Tryptic peptides were separated in a nano-scale C18 HPLC capillary column and analyzed in an LTQ linear ion-trap mass spectrometer (Thermo Fisher). Peptide sequences and protein identities were assigned by matching the measured fragmentation pattern with proteins or translated nucleotide databases using Sequest. All data were manually inspected. Proteins with ≥2 peptide matches were considered positively identified.
COVID data comparison
DS-affinity proteins were compared with currently available COVID-19 multi-omic data compiled in the Coronascape database (as of 02/22/2021) [27–48]. These data have been obtained with proteomics, phosphoproteomics, interactome, ubiquitome, and RNA-seq techniques. Up- and down-regulated proteins or gene transcripts were identified by comparing cells infected vs. uninfected by SARS-CoV-2 or COVID-19 patients vs. healthy controls. Similarity searches were conducted to identify DS-affinity proteins that are up- and/or down-regulated in viral infection at any omic level.
Protein network analysis
Protein-protein interactions were analyzed by STRING [26]. Interactions include both direct physical interaction and indirect functional associations, which are derived from genomic context predictions, high-throughput lab experiments, co-expression, automated text mining, and previous knowledge in databases. Each interaction is annotated with a confidence score from 0 to 1, with 1 being the highest, indicating the likelihood of an interaction to be true. Pathways and processes enrichment were analyzed with Metascape [27], which utilizes various ontology sources such as KEGG Pathway, GO Biological Process, Reactome Gene Sets, Canonical Pathways, CORUM, TRRUST, and DiGenBase. All genes in the genome were used as the enrichment background. Terms with a p-value <0.01, a minimum count of 3, and an enrichment factor (ratio between the observed counts and the counts expected by chance) >1.5 were collected and grouped into clusters based on their membership similarities. The most statistically significant term within a cluster was chosen to represent the cluster.
Autoantigen literature text mining
Every DS-affinity protein identified in this study was searched for specific autoantibodies reported in the PubMed literature. Search keywords included the MeSH keyword “autoantibodies”, the protein name or its gene symbol, or alternative names and symbols. Only proteins for which specific autoantibodies are reported in PubMed-listed journal articles were considered “confirmed” or “known” autoAgs in this study.
Acknowledgements
We are grateful to Dr. Jung-hyun Rho for his technical assistance to the study. We thank Ross Tomaino and the Taplin Mass Spectrometry Facility of Harvard Medical School for expert service with protein sequencing.
Funding Statement
This work was partially supported by Curandis, the US NIH, and a Cycle for Survival Innovation Grant (to MHR). MHR acknowledges NIH/NCI R21 CA251992 and MSKCC Cancer Center Support Grant P30 CA008748. The funding bodies were not involved in the design of the study and the collection, analysis, and interpretation of data.
Competing interest statement
JYW is the founder and Chief Scientific Officer of Curandis. MHR is a member of the Scientific Advisory Boards of Trans-Hit, Proscia, and Universal DX, but these companies have no relation to the study.
References
- [1].Wang J. Y., Zhang W., Roehrl M. W., Roehrl V. B., Roehrl M. H.. An Autoantigen Atlas from Human Lung HFL1 Cells Offers Clues to Neurological and Diverse Autoimmune Manifestations of COVID-19. bioRxiv, 2021:2021.01.24.427965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Wang J. Y., Zhang W., Roehrl M. W., Roehrl V. B., Roehrl M. H.. An Autoantigen Profile of Human A549 Lung Cells Reveals Viral and Host Etiologic Molecular Attributes of Autoimmunity in COVID-19. bioRxiv, 2021:2021.02.21.432171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Wang J. Y., Zhang W., Roehrl V. B., Roehrl M. W., Roehrl M. H.. An Autoantigen-ome from HS-Sultan B-Lymphoblasts Offers a Molecular Map for Investigating Autoimmune Sequelae of COVID-19. bioRxiv, 2021:2021.04.05.438500. [Google Scholar]
- [4].Wang J. Y., Zhang W., Roehrl M. W., Roehrl V. B., Roehrl M. H.. An autoantigen profile of human A549 lung cells reveals viral and host etiologic molecular attributes of autoimmunity in COVID-19. Journal of autoimmunity, 2021;120:102644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Wang J. Y., Lee J., Yan M., Rho J. H., Roehrl M. H.. Dermatan sulfate interacts with dead cells and regulates CD5(+) B-cell fate: implications for a key role in autoimmunity. Am J Pathol, 2011;178:2168–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Rho J. H., Zhang W., Murali M., Roehrl M. H., Wang J. Y.. Human proteins with affinity for dermatan sulfate have the propensity to become autoantigens. Am J Pathol, 2011;178:2177–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Lee J., Rho J.-h., Roehrl M. H., Wang J. Y.. Dermatan Sulfate Is a Potential Master Regulator of IgH via Interactions with Pre-BCR, GTF2I, and BiP ER Complex in Pre-B Lymphoblasts. bioRxiv, 2021:2021.01.18.427153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Lee J., Rho J. H., Roehrl M. H., Wang J. Y.. Dermatan Sulfate Is a Potential Regulator of IgH via Interactions With Pre-BCR, GTF2I, and BiP ER Complex in Pre-B Lymphoblasts. Frontiers in immunology, 2021;12:680212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Wang J. Y., Zhang W., Rho J. H., Roehrl M. W., Roehrl M. H.. A proteomic repertoire of autoantigens identified from the classic autoantibody clinical test substrate HEp-2 cells. Clinical proteomics, 2020;17:35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Zhang W., Rho J. H., Roehrl M. H., Wang J. Y.. A comprehensive autoantigen-ome of autoimmune liver diseases identified from dermatan sulfate affinity enrichment of liver tissue proteins. BMC immunology, 2019;20:21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Zhang W., Rho J. H., Roehrl M. W., Roehrl M. H., Wang J. Y.. A repertoire of 124 potential autoantigens for autoimmune kidney diseases identified by dermatan sulfate affinity enrichment of kidney tissue proteins. PloS one, 2019;14:e0219018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Consiglio C. R., Cotugno N., Sardh F., Pou C., Amodio D., Rodriguez L. et al. The Immunology of Multisystem Inflammatory Syndrome in Children with COVID-19. Cell, 2020;183:968–81.e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Gruber C. N., Patel R. S., Trachtman R., Lepow L., Amanat F., Krammer F. et al. Mapping Systemic Inflammation and Antibody Responses in Multisystem Inflammatory Syndrome in Children (MIS-C). Cell, 2020;183:982–95.e14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Gagiannis D., Steinestel J., Hackenbroch C., Schreiner B., Hannemann M., Bloch W. et al. Clinical, Serological, and Histopathological Similarities Between Severe COVID-19 and Acute Exacerbation of Connective Tissue Disease-Associated Interstitial Lung Disease (CTD-ILD). Frontiers in immunology, 2020;11:587517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Zhou Y., Han T., Chen J., Hou C., Hua L., He S. et al. Clinical and Autoimmune Characteristics of Severe and Critical Cases of COVID-19. Clinical and translational science, 2020;13:1077–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Fujii H., Tsuji T., Yuba T., Tanaka S., Suga Y., Matsuyama A. et al. High levels of anti-SSA/Ro antibodies in COVID-19 patients with severe respiratory failure: a case-based review : High levels of anti-SSA/Ro antibodies in COVID-19. Clinical rheumatology, 2020;39:3171–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Sacchi M. C., Tamiazzo S., Stobbione P., Agatea L., De Gaspari P., Stecca A. et al. SARS-CoV-2 Infection as a trigger of autoimmune response. Clinical and translational science, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Franke C., Ferse C., Kreye J., Reincke S. M., Sanchez-Sendin E., Rocco A. et al. High frequency of cerebrospinal fluid autoantibodies in COVID-19 patients with neurological symptoms. Brain, behavior, and immunity, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Zuo Y., Yalavarthi S., Navaz S., Hoy C., Shi H., Harbaugh A. et al. Autoantibodies stabilize neutrophil extracellular traps in COVID-19. medRxiv : the preprint server for health sciences, 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Chang S. E., Feng A., Meng W., Apostolidis S. A., Mack E., Artandi M. et al. New-Onset IgG Autoantibodies in Hospitalized Patients with COVID-19. medRxiv : the preprint server for health sciences, 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Bastard P., Rosen L. B., Zhang Q., Michailidis E., Hoffmann H. H., Zhang Y. et al. Autoantibodies against type I IFNs in patients with life-threatening COVID-19. Science (New York, NY), 2020;370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Guilmot A., Maldonado Slootjes S., Sellimi A., Bronchain M., Hanseeuw B., Belkhir L. et al. Immune-mediated neurological syndromes in SARS-CoV-2-infected patients. J Neurol, 2020:1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Imai H., Chan E. K., Kiyosawa K., Fu X. D., Tan E. M.. Novel nuclear autoantigen with splicing factor motifs identified with antibody from hepatocellular carcinoma. The Journal of clinical investigation, 1993;92:2419–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Beutgen V. M., Schmelter C., Pfeiffer N., Grus F. H.. Autoantigens in the trabecular meshwork and glaucoma-specific alterations in the natural autoantibody repertoire. Clinical & translational immunology, 2020;9:e01101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Becker A., Ludwig N., Keller A., Tackenberg B., Eienbroker C., Oertel W. H. et al. Myasthenia gravis: analysis of serum autoantibody reactivities to 1827 potential human autoantigens by protein macroarrays. PloS one, 2013;8:e58095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Szklarczyk D., Gable A. L., Lyon D., Junge A., Wyder S., Huerta-Cepas J. et al. STRING v11: protein-protein association networks with increased coverage, supporting functional discovery in genome-wide experimental datasets. Nucleic Acids Res, 2019;47:D607–D13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Zhou Y., Zhou B., Pache L., Chang M., Khodabakhshi A. H., Tanaseichuk O. et al. Metascape provides a biologist-oriented resource for the analysis of systems-level datasets. Nat Commun, 2019;10:1523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Zhang J. Y., Wang X. M., Xing X., Xu Z., Zhang C., Song J. W. et al. Single-cell landscape of immunological responses in patients with COVID-19. Nature immunology, 2020;21:1107–18. [DOI] [PubMed] [Google Scholar]
- [29].Davies J. P., Almasy K. M., McDonald E. F., Plate L.. Comparative Multiplexed Interactomics of SARS-CoV-2 and Homologous Coronavirus Nonstructural Proteins Identifies Unique and Shared Host-Cell Dependencies. ACS infectious diseases, 2020;6:3174–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Klann K., Bojkova D., Tascher G., Ciesek S., Münch C., Cinatl J.. Growth Factor Receptor Signaling Inhibition Prevents SARS-CoV-2 Replication. Molecular cell, 2020;80:164–74.e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Sun J., Ye F., Wu A., Yang R., Pan M., Sheng J. et al. Comparative Transcriptome Analysis Reveals the Intensive Early Stage Responses of Host Cells to SARS-CoV-2 Infection. Frontiers in microbiology, 2020;11:593857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Bojkova D., Klann K., Koch B., Widera M., Krause D., Ciesek S. et al. Proteomics of SARS-CoV-2-infected host cells reveals therapy targets. Nature, 2020;583:469–72. [DOI] [PubMed] [Google Scholar]
- [33].Wilk A. J., Rustagi A., Zhao N. Q., Roque J., Martínez-Colón G. J., McKechnie J. L. et al. A single-cell atlas of the peripheral immune response in patients with severe COVID-19. Nature medicine, 2020;26:1070–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Lieberman N. A. P., Peddu V., Xie H., Shrestha L., Huang M. L., Mears M. C. et al. In vivo antiviral host transcriptional response to SARS-CoV-2 by viral load, sex, and age. PLoS biology, 2020;18:e3000849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Riva L., Yuan S., Yin X., Martin-Sancho L., Matsunaga N., Pache L. et al. Discovery of SARS-CoV-2 antiviral drugs through large-scale compound repurposing. Nature, 2020;586:113–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Bouhaddou M., Memon D., Meyer B., White K. M., Rezelj V. V., Correa Marrero M. et al. The Global Phosphorylation Landscape of SARS-CoV-2 Infection. Cell, 2020;182:685–712.e19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Blanco-Melo D., Nilsson-Payant B. E., Liu W. C., Uhl S., Hoagland D., Møller R. et al. Imbalanced Host Response to SARS-CoV-2 Drives Development of COVID-19. Cell, 2020;181:1036–45.e9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Shen B., Yi X., Sun Y., Bi X., Du J., Zhang C. et al. Proteomic and Metabolomic Characterization of COVID-19 Patient Sera. Cell, 2020;182:59–72.e15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Lamers M. M., Beumer J., van der Vaart J., Knoops K., Puschhof J., Breugem T. I. et al. SARS-CoV-2 productively infects human gut enterocytes. Science (New York, NY), 2020;369:50–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Gordon D. E., Jang G. M., Bouhaddou M., Xu J., Obernier K., White K. M. et al. A SARS-CoV-2 protein interaction map reveals targets for drug repurposing. Nature, 2020;583:459–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Xiong Y., Liu Y., Cao L., Wang D., Guo M., Jiang A. et al. Transcriptomic characteristics of bronchoalveolar lavage fluid and peripheral blood mononuclear cells in COVID-19 patients. Emerging microbes & infections, 2020;9:761–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Vanderheiden A., Ralfs P., Chirkova T., Upadhyay A. A., Zimmerman M. G., Bedoya S. et al. Type I and Type III Interferons Restrict SARS-CoV-2 Infection of Human Airway Epithelial Cultures. Journal of virology, 2020;94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Appelberg S., Gupta S., Svensson Akusjärvi S., Ambikan A. T., Mikaeloff F., Saccon E. et al. Dysregulation in Akt/mTOR/HIF-1 signaling identified by proteo-transcriptomics of SARS-CoV-2 infected cells. Emerging microbes & infections, 2020;9:1748–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Stukalov A., Girault V., Grass V., Bergant V., Karayel O., Urban C. et al. Multi-level proteomics reveals host-perturbation strategies of SARS-CoV-2 and SARS-CoV. bioRxiv, 2020:2020.06.17.156455. [Google Scholar]
- [45].Emanuel W., Kirstin M., Vedran F., Asija D., Theresa G. L., Roberto A. et al. Bulk and single-cell gene expression profiling of SARS-CoV-2 infected human cell lines identifies molecular targets for therapeutic intervention. bioRxiv, 2020:2020.05.05.079194. [Google Scholar]
- [46].Liao M., Liu Y., Yuan J., Wen Y., Xu G., Zhao J. et al. Single-cell landscape of bronchoalveolar immune cells in patients with COVID-19. Nature medicine, 2020;26:842–4. [DOI] [PubMed] [Google Scholar]
- [47].Laurent E. M. N., Sofianatos Y., Komarova A., Gimeno J.-P., Tehrani P. S., Kim D.-K. et al. Global BioID-based SARS-CoV-2 proteins proximal interactome unveils novel ties between viral polypeptides and host factors involved in multiple COVID19-associated mechanisms. bioRxiv, 2020:2020.08.28.272955. [Google Scholar]
- [48].Li Y., Wang Y., Liu H., Sun W., Ding B., Zhao Y. et al. Urine proteome of COVID-19 patients. Urine (Amst), 2020;2:1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Alexopoulos H., Magira E., Bitzogli K., Kafasi N., Vlachoyiannopoulos P., Tzioufas A. et al. Anti-SARS-CoV-2 antibodies in the CSF, blood-brain barrier dysfunction, and neurological outcome: Studies in 8 stuporous and comatose patients. Neurology(R) neuroimmunology & neuroinflammation, 2020;7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Wabnitz G., Balta E., Samstag Y.. L-plastin regulates the stability of the immune synapse of naive and effector T-cells. Advances in biological regulation, 2017;63:107–14. [DOI] [PubMed] [Google Scholar]
- [51].Mossabeb R., Seiberler S., Mittermann I., Reininger R., Spitzauer S., Natter S. et al. Characterization of a novel isoform of alpha-nascent polypeptide-associated complex as IgE-defined autoantigen. The Journal of investigative dermatology, 2002;119:820–9. [DOI] [PubMed] [Google Scholar]