Abstract
The production of recombinant proteins is important in academic research to identify protein functions. Moreover, recombinant enzymes are used in the food and chemical industries, and high-quality proteins are required for diagnostic, therapeutic, and pharmaceutical applications. Though many recombinant proteins are produced by microbial or mammalian cell-based expression systems, plants have been promoted as alternative, cost-effective, scalable, safe, and sustainable expression systems. The development and improvement of transient expression systems have significantly reduced the period of protein production and increased the yield of recombinant proteins in plants. In this review, we consider the importance of plant-based expression systems for recombinant protein production and as genetic engineering tools.
Keywords: ascorbic acid, deconstructed vector, necrosis, transient expression, Tsukuba system
Introduction
Recombinant proteins are used for both the treatment and prevention of diseases in humans and animals. The production of recombinant proteins in academic research is also important to identify protein functions and elucidate protein structure. Human insulin was the first therapeutic recombinant protein; it was produced in Escherichia coli in 1978 by Dr. David Goeddel and his colleagues (Goeddel et al. 1979) and was approved by FDA in 1982, then sold as ‘Humulin’ (Quianzon and Cheikh 2012). Since then, more than 170 recombinant protein drugs have come to the market and hundreds are currently being developed for the treatment of diseases, such as arthritis and cancer (Batta et al. 2020; Margolin et al. 2018; Marsian and Lomonossoff 2016; Pham 2018; Torre and Albericio 2021). Most of the approved recombinant biopharmaceuticals are generated in mammalian cell lines (Lalonde and Durocher 2017). Currently, biologics are produced in bacteria, yeast, mammalian cells, insects, and plant systems.
In contrast to chemical compounds, recombinant proteins are very large and complex molecules with sophisticated and specific mechanisms. Because of their size and complexity, the chemical synthesis of proteins is incredibly difficult; thus, recombinant proteins are biologically produced using the protein synthesis machinery (Puetz and Wurm 2019). All production systems have their own advantages and disadvantages, such as production time, operating costs, protein yield, potential contamination from pathogenic microorganisms, posttranslational modifications, and regulatory approval.
Plant expression systems have several potential advantages over conventional expression systems. Besides, the reliability of such systems for the production of highly valuable proteins has been demonstrated (Lindsay et al. 2018; Malm et al. 2019; Ward et al. 2021). The first recorded example of biopharming in plants was the production of a chimeric human growth hormone via transgenic tobacco and sunflower callus tissue in 1986 (Barta et al. 1986). In addition, the first genetically modified plant-derived therapeutic approved by the FDA consisted of taliglucerase alfa produced by transgenic carrot cell suspension cultures; it was commercialized by Protalix Biotherapeutics for the treatment of Gaucher disease (Zimran et al. 2011). The production system is based on bioreactors, and the company claims to have lower initial investment and running costs compared to mammalian-based systems (https://protalix.com/technology/procellex-platform/).
Among several plant expression systems, Nicotiana benthamiana is the current core production host used by many companies, including Medicago Inc. (https://www.medicago.com), Kentucky BioProcessing (https://kentuckybioprocessing.com), Icon Genetics (https://www.icongenetics.com), iBio (https://www.ibioinc.com/), and UniBio (https://unibio-jp.com/en/). N. benthamiana grows naturally in the Australian desert; apparently, it adopted a life strategy of sacrificing pathogen defenses to an accelerated reproduction cycle. Thus, this plant exhibits remarkable susceptibility to pathogens and is an excellent host for transient gene expression (Bally et al. 2015). For this kind of experiments, N. benthamiana plants are usually grown for 4–7 weeks and then infected with Agrobacterium tumefaciens harboring the genes of interest (GOI). The GOI product typically reaches a peak level 3–7 days after infection.
Recently, it was demonstrated that the crude extracts of N. benthamiana leaves transiently expressing the influenza virus hemagglutinin (H5) trimers prevented the spread of avian influenza (H5N1) when injected into chickens (Phan et al. 2020). Furthermore, the first randomized phase 3 trials revealed that a quadrivalent virus-like particle of the influenza vaccine produced in N. benthamiana by Medicago Inc. (Quebec, Canada) demonstrated efficacy, safety, and immunogenicity in humans (Tregoning 2020; Ward et al. 2020). This is an important step in plant-derived biologics. As these next-generation vaccines start to enter the market, this new technology promises more accurate protection and a broader range of production options. In this article, we summarize the current status, importance, and prospects of plant expression systems for the cost-effective production of recombinant proteins. We also introduce our transient expression system, the “Tsukuba system”, and its applications.
Benefits of using plants for the production of recombinant proteins
It became apparent that plants offer several potential benefits for the production of recombinant proteins (Table 1). Plants are completely different from animals; thus, the risk of contamination and replication of human pathogens within the system is low (Commandeur and Twyman 2004). Because a sterile environment is not necessary, the cultivation of plants is simple, and fertilizer solutions are inexpensive (∼0.002 €/L) (Buyel and Fischer 2012). Besides, expensive media are required for the culture of mammalian cells, and more than 50 €/L is required (Xu et al. 2017). Furthermore, the cost of purifying the protein of interest and the cost for testing virus-free are reduced in plant expression system. By using A. tumefaciens and/or viral vector-mediated transient expression, recombinant protein expression in plants can be achieved approximately 8 weeks after obtaining the corresponding DNA sequence (Gleba et al. 2014; Shoji et al. 2012). In addition, plant expression systems have the advantage of producing intrinsically disordered proteins, including the anti-cancer mistletoe lectin viscumin (Gengenbach et al. 2019), which are not synthesized efficiently in mammalian cells or prokaryotes due to their toxicity or complex structure, respectively. In addition, much larger recombinant proteins can be produced in a plant expression system than in a bacterial expression system.
Table 1. Advantage and disadvantage of protein expression systems (modified from Shanmugaraj et al. 2020).
| Expression system | Advantage | Disadvantage |
|---|---|---|
| Bacteria | Easy to manipulate | Improper folding |
| Low cost | Endotoxin accumulation | |
| High expression | ||
| Existing regulatory approval | ||
| Mammalian cells | Proper folding | High production cost |
| High yields | Risk of human pathogen contamination | |
| Existing regulatory approval | Expensive media | |
| Yeast | Rapid growth and scalable | Difficulty in cell disruption |
| Easy to manipulate | Limited glycosylation capacity | |
| Scalable | ||
| Existing regulatory approval | ||
| Insect cells | High expression levels | High cost and time consuming |
| Proper folding | Expensive media | |
| Scalable | Nonhuman glycosylation | |
| Plant | Less pathogen and bacterial toxin contamination | Regulatory compliance |
| Economical | Limited glycosylation capacity |
In some cases, the biological activity of recombinant proteins depends on protein folding. In prokaryotic expression systems, proper protein folding is sometimes not achieved because bacterial protein processing complexes and posttranslational modification capacities are limited (Sahdev et al. 2008). In contrast, plants have the capacity to assemble and perform posttranslational modifications of large multimeric proteins. However, the glycosylation mechanisms differ between species. This is a major concern for every non-human expression system. Since humans are constantly exposed to plant glycoproteins in the diet, glycosylated plant-made proteins may be acceptable for topical and oral administration (Gomord et al. 2005). The treatment of Gaucher’s disease patients with taligulcerase alfa, which is an enzyme produced in genetically engineered carrot cells and keeps plant-specific α1,3-fucose and β1,2-xylose residues, has been well tolerated in multiple clinical trials. And no report has been announced about anti-glycan effects during clinical development or post-authorization (Zimran et al. 2018a, b). Beside N. benthamiana, N. tabacum is used for the stable and transient expression of recombinant proteins. Furthermore, several crops, fruits, and vegetables, such as rice, maize, lettuce, tomato, and potato, have also been evaluated for the production of recombinant proteins (Shanmugaraj et al. 2020).
Transient expression systems used to increase recombinant protein production yield in plants
The maximum yield of each system is a major consideration because downstream processing becomes significantly more expensive when proteins are purified from more diluted mixtures. To produce transgenic plants, it takes approximately 4–6 months, or even more. In contrast, transient expression allows rapid production of large amounts of recombinant proteins. For example, the purified end product of an influenza vaccine may be produced 3 weeks after the sequence is received by Medicago Inc., a company that uses plant-based transient expression systems for recombinant protein production (D’Aoust et al. 2010).
There are two types of transient protein expression systems in plants. One is based on plant viruses such as the tobacco mosaic virus (TMV); the other is the Agrobacterium-mediated transient gene expression system or agroinfiltration (Burnett and Burnett 2020). The virus-based system poses the risk of viral vectors infecting plants in the ecosystem because plant viruses are replicated autonomously. In the agroinfiltration method, a suspension of A. tumefaciens is introduced into plant leaves either by injection or vacuum infiltration, where the bacteria transfer the GOI into the nucleus of host plant cells via delivery of T-DNA. Generally, the expression of recombinant proteins via agroinfiltration is higher and more efficient than that obtained through traditional plant transformations.
Searching for new tools for obtaining higher yields of recombinant protein production by agroinfiltration, “deconstructed” viral vectors have been developed. Because the viral genome contains several components to replicate the virus itself, such as those for capsid production, not all components are essential for the expression of recombinant proteins. Therefore, unnecessary components are eliminated, while the viral replication system and transcription elements are maintained in each deconstructed viral vector. Because of elimination of several components, infection to plants and delivery of T-DNA to plant cell nucleus are performed by Agrobacterium, and intracellular gene amplification and expression is done in plant cell nucleus with help of virus replication system. Consequently, the size of the deconstructed viral vectors is reduced, and the insertion of large transgenes is accommodated (Chen et al. 2013). On the other hand, a limited size of transgenes is inserted into the viral vector. The viral replication systems of the TMV, potato virus X (PVX), geminivirus bean yellow dwarf virus (BeYDV), cowpea mosaic virus (CPMV), and other viruses are used to produce recombinant proteins.
The most famous deconstructed viral vector is the TMV-derived magnICON system. Transient expression of model proteins such as green fluorescent protein (GFP) in the magnICON system resulted in a yield of 4 mg/g fresh mass (FM) in N. benthamiana within one week (Marillonnet et al. 2005). If it is assumed that 1 l of fermentation volume is roughly equivalent to 1 kg of plant biomass, the productivity of plant-based expression systems is in the similar order of productivity (approximately 5 g/L) as Chinese hamster ovary cells (Reinhart et al. 2015).
A BeYDV-derived deconstructed vector was developed (Moon et al. 2014), but the expression level of recombinant proteins achieved with such vector was two to three times below that obtained with the magnICON system (Moon et al. 2014; Yamamoto et al. 2018a). We revealed that the combination of the BeYDV replication system and a double terminator improved the expression levels of recombinant proteins. The geminiviral replication system ensures rolling circle replication (Rizvi et al. 2015) and a double terminator may have the function of reducing readthrough and terminating transcription efficiently. The expression level of GFP with our vector reached a yield of approximately 4 mg/g FM in 3 days (Yamamoto et al. 2018a). Thus, at 3 days post-agroinfiltration, GFP expression was significantly higher using our vector than with the magnICON system (Yamamoto et al. 2018a). We named our expression system, “Tsukuba system”. Using the Tsukuba system, recombinant proteins can be obtained more rapidly and in larger quantities than with other systems.
Vectors for the Tsukuba system
We developed the pBYR2HS (Figure 1) vector for the Tsukuba system; it contains a geminiviral replication system and a double terminator composed of a heat shock protein and extensin terminators. The yield of GFP using pBYR2HS was 4 mg/g FM in N. benthamiana (Yamamoto et al. 2018a). When the Agrobacterium solution is diluted, many plants may be agroinfiltrated from one culture. For example, Agrobacterium strain GV3101 harboring pBYR2HS-EGFP was infiltrated into N. benthamiana leaves at OD600=0.01, 0.02, 0.05, 0.1, 0.2, and 0.5. No significant difference in GFP emission was observed between plants infiltrated with GV3101 at OD600=0.1, 0.2, and 0.5 (Figure 2A). Similarly, in a previous study, no significant difference in GUS activity in N. benthamiana leaves agroinfiltrated with pEAQ-GSN was observed at OD600=0.1, 0.5, and 1.5 (Norkunas et al. 2018). These results suggest that Agrobacterium cultures at OD600=0.1 or higher are effective.
Figure 1. Schematic representation of the T-DNA regions of plasmids pBYR2HS, pTKB2, and pTKB3. pBYR2HS is a previously produced vector (Yamamoto et al. 2018a). 35S-p×2, CaMV 35S promoter with double-enhanced element; AtADH5′, 5′-untranslated region (UTR) of Arabidopsis thaliana alcohol dehydrogenase gene; TMV Ω, 5′-leader sequence of the tobacco mosaic virus; HSPter, terminator of a heat shock protein gene; Ext3′, tobacco extensin gene 3′ element; 35Ster, terminator of CaMV 35S; NOSter, NOS terminator; LIR, long intergenic region of the bean yellow dwarf virus (BeYDV) genome; SIR, short intergenic region of the BeYDV genome; C1/C2, BeYDV ORFs C1 and C2 encoding for replication initiation protein (Rep) and RepA, respectively; LB and RB, the left and right borders of the T-DNA region, respectively; Nos-p and Nos-t, NOS promoter and terminator, respectively; p19, a gene-silencing suppressor gene from the tomato bushy stunt virus; GOI, gene of interest.
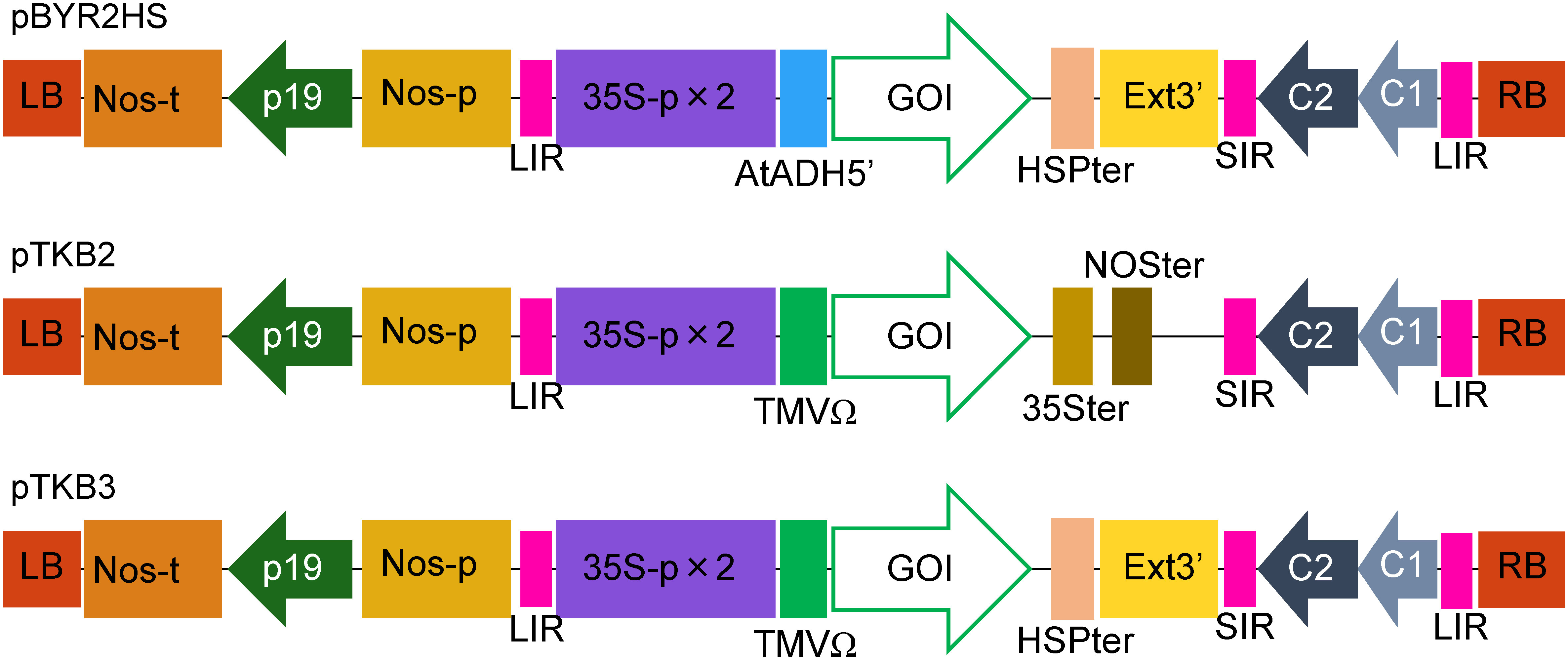
Figure 2. Agrobacterium culture conditions and concentrations for achieving efficient agroinfiltration and protein expression levels in N. benthamiana. (A) Agrobacterium carrying pBYR2HS-EGFP was agroinfiltrated (with 1-ml syringes) at different OD600 into N. benthamiana. GFP emission was observed 3 days post agroinfiltration (dpa) with an ultraviolet-absorbing filter, Fujifilm SC-52. (B) Agrobacterium carrying pBYR2HS-EGFP was resuspended either in agroinfiltration MES buffer (10 mM MES, pH 5.6, 10 mM MgCl2, 100 μM acetosyringone; MES) or Phosphate buffer (10 mM potassium phosphate, pH 5.6, 10 mM MgCl2, 100 μM acetosyringone; P buf.) at OD600=0.5, and used to agroinfiltrate N. benthamiana. GFP emission was observed with an ultraviolet-absorbing filter, Fujifilm SC-52, 3 dpa. (C) Agrobacterium resuspensions containing either pBYR2HS-EGFP, pTKB2-EGFP, or pTKB3-EGFP were adjusted to OD600=0.5 or 0.1 and agroinfiltrated into N. benthamiana. GFP emission was detected at 3 dpa. (D) Total soluble proteins, which were extracted from 0.2 mg FW N. benthamiana leaves agroinfiltrated with Agrobacterium carrying pBYR2HS-EGFP, pTKB2-EGFP, or pTKB3-EGFP, were separated by SDS-PAGE, and stained with Coomassie Brilliant Blue (CBB) dye. (E) The relative amount of GFP was calculated based on protein band intensities in CBB-stained gels using ImageJ software. Data represent means±SE (n=4–6).

Agrobacterium is generally resuspended in agroinfiltration MES buffer (10 mM MES, pH 5.6, 10 mM MgCl2, and 100 μM acetosyringone). MES is a stable Good’s buffer, that is relatively expensive (∼15,000 yen/250 g). Interestingly, we observed that replacing MES buffer with Phosphate (P) buffer (10 mM potassium phosphate buffer, pH 5.6, 10 mM MgCl2, and 100 μM acetosyringone) did not affect GFP emission levels (Figure 2B). P buffer consists of potassium dihydrogen phosphate (∼1,500 yen/500 g) and di-potassium hydrogen phosphate (∼2,000 yen/500 g). Thus, the use of P buffer reduces the cost.
We suspected that the 5′ UTR sequence affected the expression level of the recombinant protein. As AtADH 5′ UTR was used in pBYR2HS, it was substituted by the TMVΩ sequence, and the resulting vector was named pTKB3 (Figure 1). In addition, we constructed plasmid vector pTKB2, in which the double terminator was composed of 35S and NOS terminators (Figure 1). Agrobacterium strain GV3101 harboring pBYR2HS-EGFP, pTKB2-EGFP, or pTKB3-EGFP was agroinfiltrated into N. benthamiana leaves at an OD600 of 0.5 and 3 days after agroinfiltration, similar GFP emissions were observed (Figure 2C). To confirm these results, total soluble proteins were separated by SDS-PAGE (Figure 2D) and the intensity of the corresponding band was measured using ImageJ software (Figure 2E). No significant differences in GFP expression levels between leaves agroinfiltrated with pBYR2HS-EGFP and pTKB3-EGFP were detected. In addition, a slight but not significant reduction in GFP expression in leaves agroinfiltrated with pTKB2-EGFP was detected (Figure 2E). These results indicate that both AtADH 5′UTR and TMVΩ sequence similarly contribute expression of recombinant proteins in Tsukuba system.
Prevention of necrosis increases the accumulation of transiently produced proteins
Dehydration and/or necrosis of leaves is observed when certain recombinant proteins, such as hemagglutinin (Matsuda et al. 2012), hepatitis B (Huang et al. 2006, 2008), human growth hormone (Gils et al. 2005), and tumor associated MUC1 peptide (Pinkhasov et al. 2011), are transiently expressed. Leaf necrosis is sometimes prevented by changes in the subcellular localization of a recombinant protein. No or moderate necrosis was observed when a recombinant human growth hormone was expressed in the chloroplast or cytoplasm, respectively (Gils et al. 2005). Endoplasmic reticulum (ER) targeting frequently increases the accumulation level of proteins of interest, because the ER contains molecular chaperones to fold and properly assemble proteins and has few proteolytic enzymes (Benchabane et al. 2008). Some recombinant proteins causes necrosis, even when a recombinant protein is expressed in the ER. Thus, simple methods to prevent dehydration and/or necrosis are required. Recently, we demonstrated that the application of ascorbate (AsA) at high concentrations (100 mM or higher) via foliar spraying suppresses the necrosis of N. benthamiana leaves caused by the high production of exogenous proteins (Figure 3) (Nosaki et al. 2021).
Figure 3. Necrosis of leaves is suppressed by foliar spraying of high concentrations of sodium ascorbate (AsA). After expressing either human cullin protein, hCul1, or F-box protein hFbxw7 in agroinfiltrated leaves, tissue necrosis (gray area) was observed (A), but not when leaves were sprayed with 200 mM AsA (B). AsA was applied by foliar spray (C).

Proteins are synthesized and folded within the ER, wherein the prolonged accumulation of misfolded or unfolded proteins can trigger ER stress, which in turn initiates a cascade of reactions referred to as the unfolded protein response (UPR), causing a perturbation of cellular homeostasis. UPR suppresses protein synthesis and induces the activity of molecular chaperones that play roles in refolding misfolded proteins. Furthermore, UPR stimulates protein degradation via autophagy and enhances the clearance of unfolded proteins through a process termed ER-associated degradation (Wu and Rapoport 2018). However, failure to resolve ER stress can result in induction of apoptosis. Studies have shown that neither the application of ER stress inhibitors nor chemical chaperones suppress necrosis in N. benthamiana (Nosaki et al. 2021). The release of high amounts of reactive oxygen species (ROS) that accumulate during ER stress results in plant cell death, which manifests as necrosis. In Arabidopsis, the respiratory burst oxidase homologs RBOHD and RBOHF play roles in the production of ROS during ER stress (Angelos and Brandizzi 2018). High concentrations of ROS can lead to both cellular and DNA damage and to the promotion of signaling pathways associated with necrosis, autophagy, and programmed cell death (Huang et al. 2019). High concentrations of AsA may function as antioxidants to suppress ROS accumulation, leading to the prevention of necrosis.
Transient expression system applications other than recombinant protein production
The Tsukuba system is also applicable and enhances expression levels in several crops, such as tomatoes, eggplants, hot peppers, melons, orchids, soybeans, common beans, and radishes, even though the expression level of GFP in these crops is not high compared to that in N. benthamiana (Hoshikawa et al. 2019; Kitajima et al. 2020; Suzaki et al. 2019; Yamamoto et al. 2018a). This is also a unique feature compared to the magnICON system, which can primarily work in tobacco, but not in lettuce and tomatoes (Hoshikawa et al. 2019; Lai et al. 2012). Because magnICON system is a TMV-based viral vector, TMV replication system primarily works in tobacco. On the other hand, Tsukuba system is a geminivirus-based viral vector. Because BeYDV has a broad host range in dicotyledonous plants (Hefferon 2014), the geminiviral replication system can work in several species.
This feature can be applied to genome-editing technology. The CRISPR/Cas9 (clustered regularly interspaced short palindromic repeats/CRISPR-associated endonuclease 9) system is an RNA-based DNA-targeting system. In recent years, genome editing technologies, such as CRISPR/Cas9, have been used for functional genetic research in plant physiology and plant breeding (Ezura and Miura 2018; Yamamoto et al. 2018b). For genome editing in plants, increasing the expression of genome-editing components may improve efficiency (Liu et al. 2019). For example, transient expression of genome editing enzymes and guide RNA (gRNA) using the Tsukuba system improved base editing in tomatoes (Yuan et al. 2021). The conventional way to produce genome editing crops is to produce transgenic plants harboring genome editing enzymes; after genome editing, foreign genes are removed by segregation. However, by using the Tsukuba system, genome editing enzymes are transiently expressed and foreign genes are not integrated into the plant genome; thus, it is not necessary to follow any further methods to obtain null-segregants.
Recently, several viral expression vectors have been developed to expand the range of plant hosts (LeBlanc et al. 2021). For example, the foxtail mosaic virus vector improved monocot transformation, increased product yields, and showed greater delivery of the insert over the traditional barley strip mosaic virus- and wheat streak mosaic virus-based systems (Bouton et al. 2018). These viral vectors have the potential to produce genome editing crops via the transient expression of a genome editing enzyme.
Prospective and future directions
The potential market for therapeutic proteins and recombinant proteins for basic sciences is huge. Protein expression systems in plants have several unique advantages and may not compete with existing microbial and mammalian systems. In terms of good manufacturing practice and regulatory approval in an industrial setting, plant expression systems are disadvantageous compared to well-established microbial and mammalian systems. Several companies, such as Medicago Inc. and Icon Genetics, set up regulatory approval processes to produce recombinant proteins in plants. The benefits of plant expression systems include low cost and high scalability. Plants are considered to be safer than other expression systems because plants do not constitutively produce endotoxins or support the growth of viruses or prions that infect humans (Moustafa et al. 2016).
Because of the high regulatory burden of therapeutic proteins, the development of veterinary vaccines, non-pharmaceutical diagnostics, cosmetic products, and industrial enzymes in plants is a way to move forward. Considering the low costs and scalability of plant production systems, commercialization of non-pharmaceutical proteins is straightforward because of lower regulatory challenges. The understanding of recombinant protein expression systems and their limitations provides information about the expression system that is ideal for producing a specific recombinant protein. Plants are sustainable production platforms and many complex proteins are produced in plants. Plant expression systems will be, with no doubt, universally used expression systems.
Acknowledgments
We thank Ms. Yuriko Nagai at the University of Tsukuba for technical support. This work was partly supported by the Program on Open Innovation Platform with Enterprise, Research Institute and Academia, Japan Science and Technology Agency (JST-OPERA, JPMJOP1851) and a Cooperative Research Grant of the Plant Transgenic Design Initiative (PTraD) by the Gene Research Center, Tsukuba-Plant Innovation Research Center (T-PIRC), University of Tsukuba.
References
- Angelos E, Brandizzi F (2018) NADPH oxidase activity is required for ER stress survival in plants. Plant J 96: 1106–1120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bally J, Nakasugi K, Jia F, Jung H, Ho SYW, Wong M, Paul CM, Naim F, Wood CC, Crowhurst RN, et al. (2015) The extremophile Nicotiana benthamiana has traded viral defence for early vigour. Nat Plants 1: 15165. [DOI] [PubMed] [Google Scholar]
- Barta A, Sommergruber K, Thompson D, Hartmuth K, Matzke MA, Matzke AJ (1986) The expression of a nopaline synthase—Human growth hormone chimaeric gene in transformed tobacco and sunflower callus tissue. Plant Mol Biol 6: 347–357 [DOI] [PubMed] [Google Scholar]
- Batta A, Kalra B, Khirasaria R (2020) Trends in FDA drug approvals over last 2 decades: An observational study. J Family Med Prim Care 9: 105–114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benchabane M, Goulet C, Rivard D, Faye L, Gomord V, Michaud D (2008) Preventing unintended proteolysis in plant protein biofactories. Plant Biotechnol J 6: 633–648 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouton C, King RC, Chen H, Azhakanandam K, Bieri S, Hammond-Kosack KE, Kanyuka K (2018) Foxtail mosaic virus: A viral vector for protein expression in cereals. Plant Physiol 177: 1352–1367 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burnett MJB, Burnett AC (2020) Therapeutic recombinant protein production in plants: Challenges and opportunities. Plants, People. Planet 2: 121–132 [Google Scholar]
- Buyel JF, Fischer R (2012) Predictive models for transient protein expression in tobacco (Nicotiana tabacum L.) can optimize process time, yield, and downstream costs. Biotechnol Bioeng 109: 2575–2588 [DOI] [PubMed] [Google Scholar]
- Chen Q, Lai H, Hurtado J, Stahnke J, Leuzinger K, Dent M (2013) Agroinfiltration as an effective and scalable strategy of gene delivery for production of pharmaceutical proteins. Adv Tech Biol Med 1: 103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Commandeur U, Twyman RM (2004) Biosafety aspects of molecular farming in plants. In: Fisher R, Schillberg S (eds) Molecular Farming: Plant-Made Pharmaceuticals and Technical Proteins. Wiley-VCH Verlag GmbH & Co. KGaA, Weinheim, pp 251–266
- D’Aoust MA, Couture MM, Charland N, Trépanier S, Landry N, Ors F, Vézina LP (2010) The production of hemagglutinin-based virus-like particles in plants: A rapid, efficient and safe response to pandemic influenza. Plant Biotechnol J 8: 607–619 [DOI] [PubMed] [Google Scholar]
- Ezura H, Miura K (2018) Genome editing technologies for plant physiology. Plant Physiol Biochem 131: 1. [DOI] [PubMed] [Google Scholar]
- Gengenbach BB, Keil LL, Opdensteinen P, Müschen CR, Melmer G, Lentzen H, Bührmann J, Buyel JF (2019) Comparison of microbial and transient expression (tobacco plants and plant-cell packs) for the production and purification of the anticancer mistletoe lectin viscumin. Biotechnol Bioeng 116: 2236–2249 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gils M, Kandzia R, Marillonnet S, Klimyuk V, Gleba Y (2005) High-yield production of authentic human growth hormone using a plant virus-based expression system. Plant Biotechnol J 3: 613–620 [DOI] [PubMed] [Google Scholar]
- Gleba YY, Tusé D, Giritch A (2014) Plant viral vectors for delivery by Agrobacterium. Curr Top Microbiol Immunol 375: 155–192 [DOI] [PubMed] [Google Scholar]
- Goeddel DV, Kleid DG, Bolivar F, Heyneker HL, Yansura DG, Crea R, Hirose T, Kraszewski A, Itakura K, Riggs AD (1979) Expression in Escherichia coli of chemically synthesized genes for human insulin. Proc Natl Acad Sci USA 76: 106–110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomord V, Chamberlain P, Jefferis R, Faye L (2005) Biopharmacertical production in plants: Problems, solutions and opportunities. Trends Biotechnol 23: 559–565 [DOI] [PubMed] [Google Scholar]
- Hefferon KL (2014) DNA virus vectors for vaccine production in plants: Spotlight on geminiviruses. Vaccines (Basel) 2: 642–653 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoshikawa K, Fujita S, Renhu N, Ezura K, Yamamoto T, Nonaka S, Ezura H, Miura K (2019) Efficient transient protein expression in tomato cultivars and wild species using agroinfiltration-mediated high expression system. Plant Cell Rep 38: 75–84 [DOI] [PubMed] [Google Scholar]
- Huang H, Ullah F, Zhou D-X, Yi M, Zhao Y (2019) Mechanisms of ROS regulation of plant development and stress responses. Front Plant Sci 10: 800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang Z, LePore K, Elkin G, Thanavala Y, Mason HS (2008) High-yield rapid production of hepatitis B surface antigen in plant leaf by a viral expression system. Plant Biotechnol J 6: 202–209 [Google Scholar]
- Huang Z, Santi L, LePore K, Kilbourne J, Arntzen CJ, Mason HS (2006) Rapid, high-level production of hepatitis B core antigen in plant leaf and its immunogenicity in mice. Vaccine 24: 2506–2513 [DOI] [PubMed] [Google Scholar]
- Kitajima S, Miura K, Yasuda J (2020) Radish sprouts as an efficient and rapidly available host for an agroinfiltration-based transient gene expression system. Plant Biotechnol 37: 89–92 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lai H, He J, Engle M, Diamond MS, Chen Q (2012) Robust production of virus-like particles and monoclonal antibodies with geminiviral replicon vectors in lettuce. Plant Biotechnol J 10: 95–104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lalonde ME, Durocher Y (2017) Therapeutic glycoprotein production in mammalian cells. J Biotechnol 251: 128–140 [DOI] [PubMed] [Google Scholar]
- LeBlanc Z, Waterhouse P, Bally J (2021) Plant-based vaccines: The way ahead? Viruses 13: 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindsay BJ, Bonar MM, Costas-Cancelas IN, Hunt K, Makarkov AI, Chierzi S, Krawczyk CM, Landry N, Ward BJ, Rouiller I (2018) Morphological characterization of a plant-made virus-like particle vaccine bearing influenza virus hemagglutinins by electron microscopy. Vaccine 36: 2147–2154 [DOI] [PubMed] [Google Scholar]
- Liu Y, Zeng J, Yuan C, Guo Y, Yu H, Li Y, Huang C (2019) Cas9-PF, an early flowering and visual selection marker system, enhances the frequency of editing event occurrence and expedites the isolation of genome-edited and transgene-free plants. Plant Biotechnol J 17: 1191–1193 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malm M, Diessner A, Tamminen K, Liebscher M, Vesikari T, Blazevic V (2019) Rotavirus VP6 as an adjuvant for bivalent norovirus vaccine produced in Nicotiana benthamiana. Pharmaceutics 11: 229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Margolin E, Chapman R, Williamson AL, Rybicki EP, Meyers AE (2018) Production of complex viral glycoproteins in plants as vaccine immunogens. Plant Biotechnol J 16: 1531–1545 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marillonnet S, Thoeringer C, Kandzia R, Klimyuk V, Gleba Y (2005) Systemic Agrobacterium tumefaciens-mediated transfection of viral replicons for efficient transient expression in plants. Nat Biotechnol 23: 718–723 [DOI] [PubMed] [Google Scholar]
- Marsian J, Lomonossoff GP (2016) Molecular pharming—VLPs made in plants. Curr Opin Biotechnol 37: 201–206 [DOI] [PubMed] [Google Scholar]
- Matsuda R, Tahara A, Matoba N, Fujiwara K (2012) Virus vector-mediated rapid protein production in Nicotiana benthamiana: Effects of temperature and photosynthetic photon flux density on hemagglutinin accumulation. Environ Control Biol 50: 375–381 [Google Scholar]
- Moon KB, Lee J, Kang S, Kim M, Mason HS, Jeon JH, Kim HS (2014) Overexpression and self-assembly of virus-like particles in Nicotiana benthamiana by a single-vector DNA replicon system. Appl Microbiol Biotechnol 98: 8281–8290 [DOI] [PubMed] [Google Scholar]
- Moustafa K, Makhzoum A, Trémouillaux-Guiller J (2016) Molecular farming on rescue of pharma industry for next generations. Crit Rev Biotechnol 36: 840–850 [DOI] [PubMed] [Google Scholar]
- Norkunas K, Harding R, Dale J, Dugdale B (2018) Improving agroinfiltration-based transient gene expression in Nicotiana benthamiana. Plant Methods 14: 71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nosaki S, Kaneko MK, Tsuruta F, Yoshida H, Kato Y, Miura K (2021) Prevention of necrosis caused by transient expression in Nicotiana benthamiana by application of ascorbic acid. Plant Physiol 186: 832–835 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pham PV (2018) Medical biotechnology: Techniques and applications. In: Barh D, Azevedo V (eds) Omics Technologies and Bio-Engineering. Academic Press, Massachusetts, USA, pp 449–469
- Phan HT, Pham VT, Ho TT, Pham NB, Chu HH, Vu TH, Abdelwhab EM, Scheibner D, Mettenleiter TC, Hanh TX, et al. (2020) Immunization with plant-derived multimeric H5 hemagglutinins protect chicken against highly pathogenic avian influenza virus H5N1. Vaccines (Basel) 8: 593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinkhasov J, Alvarez ML, Rigano MM, Piensook K, Larios D, Pabst M, Grass J, Mukherjee P, Gendler SJ, Walmsley AM, et al. (2011) Recombinant plant-expressed tumour-associated MUC1 peptide is immunogenic and capable of breaking tolerance in MUC1.Tg mice. Plant Biotechnol J 9: 991–1001 [DOI] [PubMed] [Google Scholar]
- Puetz J, Wurm FM (2019) Recombinant Proteins for Industrial versus Pharmaceutical Purposes: A Review of Process and Pricing. Processes (Basel) 7: 476 [Google Scholar]
- Quianzon CC, Cheikh I (2012) History of insulin. J Community Hosp Intern Med Perspect 2: 18701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reinhart D, Damjanovic L, Kaisermayer C, Kunert R (2015) Benchmarking of commercially available CHO cell culture media for antibody production. Appl Microbiol Biotechnol 99: 4645–4657 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rizvi I, Choudhury NR, Tuteja N (2015) Insights into the functional characteristics of geminivirus rolling-circle replication initiator protein and its interaction with host factors affecting viral DNA replication. Arch Virol 160: 375–387 [DOI] [PubMed] [Google Scholar]
- Sahdev S, Khattar SK, Saini KS (2008) Production of active eukaryotic proteins through bacterial expression systems: A review of the existing biotechnology strategies. Mol Cell Biochem 307: 249–264 [DOI] [PubMed] [Google Scholar]
- Shanmugaraj B, Bulaon CJI, Phoolcharoen W (2020) Plant molecular farming: A viable platform for recombinant biopharmaceutical production. Plants 9: 842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shoji Y, Farrance CE, Bautista J, Bi H, Musiychuk K, Horsey A, Park H, Jaje J, Green BJ, Shamloul M, et al. (2012) A plant-based system for rapid production of influenza vaccine antigens. Influenza Other Respir Viruses 6: 204–210 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzaki T, Tsuda M, Ezura H, Day B, Miura K (2019) Agroinfiltration-based efficient transient protein expression in leguminous plants. Plant Biotechnol 36: 119–123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torre BG, Albericio F (2021) The pharmaceutical industry in 2020. An analysis of FDA drug approvals from the perspective of molecules. Molecules 26: 627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tregoning JS (2020) First human efficacy study of a plant-derived influenza vaccine. Lancet 396: 1464–1465 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ward BJ, Makarkov A, Séguin A, Pillet S, Trépanier S, Dhaliwall J, Libman MD, Vesikari T, Landry N (2020) Efficacy, immunogenicity, and safety of a plant-derived, quadrivalent, virus-like particle influenza vaccine in adults (18–64 years) and older adults (≥65 years): Two multicentre, randomised phase 3 trials. Lancet 396: 1491–1503 [DOI] [PubMed] [Google Scholar]
- Ward BJ, Séguin A, Couillard J, Trépanier S, Landry N (2021) Phase III: Randomized observer-blind trial to evaluate lot-to-lot consistency of a new plant-derived quadrivalent virus like particle influenza vaccine in adults 18–49 years of age. Vaccine 39: 1528–1533 [DOI] [PubMed] [Google Scholar]
- Wu X, Rapoport TA (2018) Mechanistic insights into ER-associated protein degradation. Curr Opin Cell Biol 53: 22–28 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu S, Gavin J, Jiang R, Chen H (2017) Bioreactor productivity and media cost comparison for different intensified cell culture processes. Biotechnol Prog 33: 867–878 [DOI] [PubMed] [Google Scholar]
- Yamamoto T, Hoshikawa K, Ezura K, Okazawa R, Fujita S, Takaoka M, Mason HS, Ezura H, Miura K (2018a) Improvement of the transient expression system for production of recombinant proteins in plants. Sci Rep 8: 4755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto T, Kashojiya S, Kamimura S, Kameyama T, Ariizumi T, Ezura H, Miura K (2018b) Application and development of genome editing technologies to the Solanaceae plants. Plant Physiol Biochem 131: 37–46 [DOI] [PubMed] [Google Scholar]
- Yuan S, Kawasaki S, Abdellatif IMY, Nishida K, Kondo A, Ariizumi T, Ezura H, Miura K (2021) Efficient base editing in tomato using a highly expressed transient system. Plant Cell Rep 40: 667–676 [DOI] [PubMed] [Google Scholar]
- Zimran A, Brill-Almon E, Chertkoff R, Petakov M, Blanco-Favela F, Muñoz ET, Solorio-Meza SE, Amato D, Duran G, Giona F, et al. (2011) Pivotal trial with plant cell-expressed recombinant glucocerebrosidase, taliglucerase alfa, a novel enzyme replacement therapy for Gaucher disease. Blood 118: 5767–5773 [DOI] [PubMed] [Google Scholar]
- Zimran A, Gonzalez-Rodriguez DE, Abrahamov A, Cooper PA, Varughese S, Girado P, Petakov M, Tan E, Sm Chertkoff R (2018a) Long-term safety and efficacy of taliglucerase alfa in pediatric Gaucher disease patients who were treatment-native or previously treated with imiglucerase. Blood Cells Mol Dis 68: 163–172 [DOI] [PubMed] [Google Scholar]
- Zimran A, Wajnrajch M, Hernandez B, Pastores GM (2018b) Taliglucerase alfa safety and efficacy across 6 clinical studies in adults and children with Gaucher disease. Orphanet J Rare Dis 13: 36. [DOI] [PMC free article] [PubMed] [Google Scholar]


