Abstract
Objective:
The mortality rate for Black women with endometrial cancer (EC) is double that of White women, although the incidence rate is lower among Black women. Unequal access to care may contribute to this racial disparity. This study aimed to assess whether survival varied between non-Hispanic Black (NHB) and non-Hispanic White (NHW) women with EC in the Military Health System (MHS) which provides equal access care to its beneficiaries despite racial/ethnic background.
Methods:
The study was conducted using data from the U.S. Department of Defense’s (DoD) Automated Central Tumor Registry (ACTUR). Study subjects included NHB and NHW women with histologically confirmed and surgically managed EC diagnosed between 1988 and 2013. The study outcome was all-cause death. Overall survival between NHB and NHW women was compared using multivariable Cox modeling.
Results:
The study included 144 NHB and 1,439 NHW women with EC. Kaplan-Meier curves showed NHB women had worse survival than NHW women (log-rank P<0.0001). The disparity in survival between NHB and NHW women persisted after adjusting for age, diagnosis period, tumor stage, tumor histology/grade, and adjuvant treatment (HR=1.64, 95% CI=1.19 to 2.27). Multivariable analyses stratified by tumor features or treatment showed that the racial disparity was confined to women with low-risk features (stage I/II disease or low-grade EC) or no adjuvant treatment.
Conclusion:
There were racial differences in overall survival between NHB and NHW women with EC in the MHS equal access healthcare system, suggesting that factors other than access to care may be related to this racial disparity.
Introduction
Cancer of the uterine corpus is the most common gynecological malignancy diagnosed among women in the United States, with 65,620 estimated new cases and 12,590 deaths in 2020 (1). Research has found that the mortality rate of endometrial cancer among Black women is nearly double that among White women, although the incidence is higher among White women (2). These racial disparities may be related to later tumor stage at diagnosis, higher tumor grade, and more aggressive histology types among Black women (3–7). However, some studies showed that survival remains less favorable among Black women than White women with tumor of similar stage, grade, and histology (6–9).
While racial disparities in endometrial cancer survival are likely multifactorial, inequities in access to medical care and insurance between racial groups in the U.S. general population may be important factors (10, 11). In the United States, Black women are less likely to have medical insurance than White women (11). Lack of health insurance or access to care may delay the detection of cancer and negatively affect the timing and quality of cancer treatments, which may result in worse clinical outcomes and premature death. Prior research has reported that compared to White women, Black women were more likely to present with later stage tumors, aggressive histology, and poor tumor grade; were less likely to receive hysterectomies; and had shorter survival (8).
Conducting research within an equal healthcare access system may help elucidate the factors that contribute to the racial disparities. If racial disparities are not observed in an equal access system, it suggests that unequal access to healthcare may play a significant role in observed disparities in the general population. On the other hand, an observed racial difference in an equal access system may suggest the potential effects of factors other than access to care. In early studies in the Henry Ford Healthcare System, a managed health care organization that provides care to all its members, Black women with endometrial cancer had worse survival than White women, but the racial difference became non-significant after adjustment for age and tumor variables (12) or tumor stage and other pathologic features (13).
The United States Department of Defense’s (DoD’s) Military Health System (MHS) provides its beneficiaries with equal access to care regardless of race, ethnicity or socioeconomic factors and is therefore an important resource for investigating racial disparities in cancer health outcomes. In a previous study based on the DoD cancer registry data, Kost et al. showed that Black women diagnosed with endometrial cancer from 1988 to 1995 had worse survival compared to White women, but the difference disappeared after adjustment for tumor stage (14), which was later among Black women than White women in the population (15). The purpose of this study was to investigate whether there were racial differences in survival among NHB and NHW women diagnosed with endometrial cancer of the uterine corpus in the DoD cancer registry data, using the data from a longer period (1988 to 2013) and adjusting for not only tumor stage but also adjuvant therapy.
Methods
Data Source
This study was conducted using data from the DoD’s Automated Central Tumor Registry (ACTUR). The ACTUR was initiated in 1986 for the collection of information on all DoD beneficiaries including active-duty members, retirees and their dependents who are diagnosed with cancer or receive cancer treatment at military treatment facilities. Local registrars review and verify all cases reported to ACTUR and follow all cases until death. The ACTUR data contain information on age at diagnosis, sex, race, ethnicity, primary site, tumor stage, histology, diagnosis date, diagnostic confirmation, cancer treatment, recurrence, last contact date, death date, and vital status. The use of the data for research was approved by the institutional review boards of Walter Reed National Military Medical Center and the National Institutes of Health.
Study Subjects
Non-Hispanic White and non-Hispanic Black women histologically diagnosed with endometrial cancer of the uterine corpus between 1988 and 2013 were eligible for the study. Endometrial cancer was defined using International Classification for Oncology (ICD-O) topography codes (C540-C543 and C548-C549) and histologic codes (see appendix) (16–17). Histologic types were dichotomized into endometrioid or non-endometrioid groups. Endometrioid cancers were divided into low grade, high grade, and ungraded. Non-endometrioid cancers under consideration in this study included serous carcinoma, clear cell carcinoma, and mixed epithelial adenocarcinomas, and carcinosarcoma. Other rare histologic types including sarcomas with a high variety in prognosis were excluded from this investigation. Tumor stage was defined following the American Joint Committee on Cancer (AJCC) staging system (18–23). Women who had a previous or current diagnosis of another cancer or did not have a surgical procedure for the treatment of their endometrial cancer were excluded from the analysis.
Statistical Analysis
As the first step of data analysis, we compared differences in the distribution of demographic and tumor characteristics by race using Chi-square test. The primary clinical endpoint for this study was all-cause survival as cause of death attributions were not consistently available. Survival times were calculated from date of diagnosis to date of death or to date of last contact or December 31, 2014, whichever occurred first for censored cases. Survival curves were generated using Kaplan-Meier method and compared using log-rank test. Finally, Cox regression, for which the proportional hazards assumption was confirmed, was used to calculate hazard ratios (HRs) and 95% confidence intervals (CIs) to estimate the relationship between race and survival while adjusting for potential confounding factors including age at diagnosis, diagnosis period, tumor stage, histology, and receipt of adjuvant treatment (none, radiation, chemotherapy, or combination). All analyses were performed using SAS software version 9.4 (SAS Institute, Inc., Cary, NC).
Results
The analysis included a total of 1,583 women, 1,439 NHW and 144 NHB women. Table 1 shows the diagnosis period, tumor features, treatment, or survival status by race in this cohort. NHB women were more likely than NHW women to be diagnosed during the later period (2005 to 2013; 56.3% vs. 35.3%), have high grade endometrioid EC (18.1% vs. 10%) and have non-endometrioid histology (20.8% vs. 5.1%), respectively. Treatment with adjuvant chemotherapy was more common in NHB women vs. NHW women (18.1% vs. 8.8%).
Table 1.
Clinical characteristics in surgically managed non-Hispanic Black and non-Hispanic White women with endometrial cancer in ACTUR diagnosed between 1988–2013
| Non-Hispanic White (N=1,439) | Non-Hispanic Black (N=144) | p-value | |||
|---|---|---|---|---|---|
| Cases | % | Cases | % | ||
| Age group | 0.3573 | ||||
| <45 | 132 | 9.2 | 20 | 13.9 | |
| 45–54 | 303 | 21.1 | 24 | 16.7 | |
| 55–64 | 608 | 42.3 | 60 | 41.7 | |
| 65–74 | 267 | 18.6 | 28 | 19.4 | |
| 75+ | 129 | 9.0 | 12 | 8.3 | |
| Diagnosis period | <0.0001 | ||||
| 1988–1994 | 303 | 21.1 | 13 | 9.0 | |
| 1995–2004 | 628 | 43.6 | 50 | 34.7 | |
| 2005–2013 | 508 | 35.3 | 81 | 56.3 | |
| Stage | 0.0524 | ||||
| Stage I | 1,101 | 76.5 | 95 | 66.0 | |
| Stage II | 81 | 5.6 | 9 | 6.3 | |
| Stage III | 132 | 9.2 | 21 | 14.6 | |
| Stage IV | 44 | 3.1 | 8 | 5.6 | |
| Unknown | 81 | 5.6 | 11 | 7.6 | |
| Histology | <0.0001 | ||||
| Endometrioid | |||||
| Low grade | 1,104 | 76.7 | 78 | 54.2 | |
| High grade | 144 | 10.0 | 26 | 18.1 | |
| Ungraded | 118 | 8.2 | 10 | 6.9 | |
| Non-endometrioid | 73 | 5.1 | 30 | 20.8 | |
| Radiation | 0.1345 | ||||
| No | 1,051 | 73.0 | 99 | 68.8 | |
| Yes | 349 | 24.3 | 37 | 25.7 | |
| Unknown | 39 | 2.7 | 8 | 5.6 | |
| Chemotherapy | 0.0015 | ||||
| No | 1,297 | 90.1 | 116 | 80.6 | |
| Yes | 127 | 8.8 | 26 | 18.1 | |
| Unknown | 15 | 1.0 | 2 | 1.4 | |
Figure 1 shows that NHB women had worse survival than NHW women (log-rank P<0.0001). Table 2 indicates that NHB women had an adjusted 64% increased risk of death over NHW women (95% CI=1.19 to 2.27) after controlling for age, diagnosis period, stage, histology/grade, and adjuvant treatment. Further Cox analyses stratified by age, diagnosis period, tumor stage or histology, and adjuvant treatment demonstrated that the overall difference in risk of death between NHB and NHW women persisted within the subset with stage I/II disease (HR=1.81, 95% CI=1.17 to 2.79), low grade endometrioid carcinoma (HR=2.47, 95% CI=1.55 to 3.95), non-endometrioid cancers (HR=2.05, 95% CI=1.08 to 3.90) and those who did not receive adjuvant treatment (HR=2.15, 95% CI=1.31 to 3.53).
Figure 1.
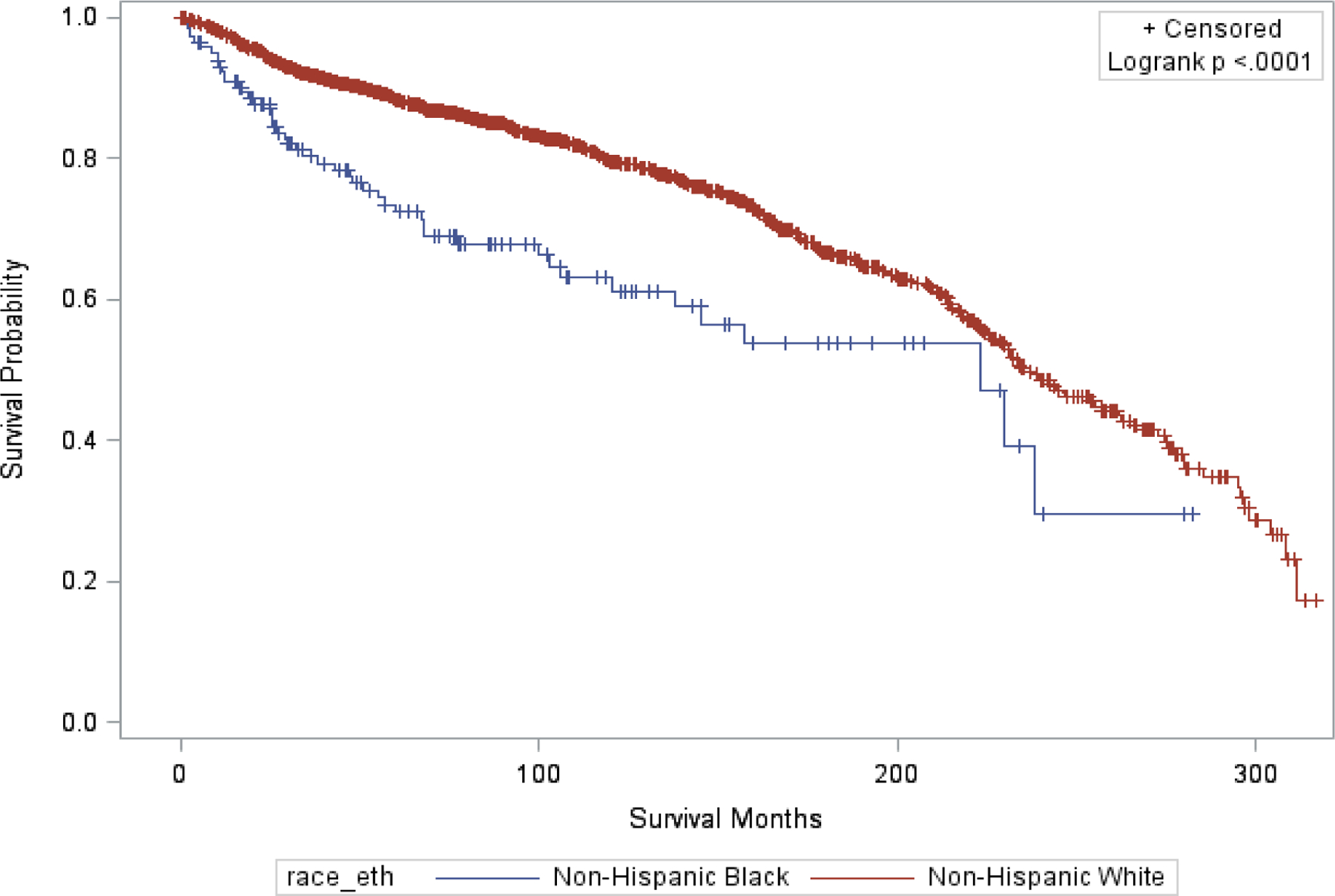
Survival distribution for non-Hispanic Black vs. non-Hispanic White women in ACTUR with surgically managed endometrial cancer
Table 2.
Stratified analysis of survival in surgically managed non-Hispanic Black vs. non-Hispanic White women with endometrial cancer
| Multivariable NHB vs. NHWa | |||
|---|---|---|---|
| HR | 95% CI | p | |
| Overall | 1.64 | 1.19 to 2.27 | 0.0024 |
| By age group | |||
| <65 | 1.30 | 0.81 to 2.07 | 0.2795 |
| 65+ | 1.36 | 0.86 to 2.15 | 0.1925 |
| By diagnosis period | |||
| 1988–1994 | 1.04 | 0.41 to 2.62 | 0.9331 |
| 1995–2004 | 1.68 | 1.07 to 2.65 | 0.0241 |
| 2005–2013 | 1.41 | 0.75 to 2.68 | 0.2899 |
| By stage | |||
| Stage I/II | 1.81 | 1.17 to 2.79 | 0.0076 |
| Stage III/IV | 1.05 | 0.59 to 1.86 | 0.8614 |
| Unknown | 0.88 | 0.22 to 3.52 | 0.8551 |
| By histology | |||
| Endometrioid low grade | 2.47 | 1.55 to 3.95 | 0.0002 |
| Endometrioid high grade | 0.45 | 0.21 to 0.97 | 0.0424 |
| Endometrioid ungraded | 1.03 | 0.12 to 8.60 | 0.9788 |
| Non-endometrioid | 2.05 | 1.08 to 3.90 | 0.0277 |
| By adjuvant treatment | |||
| None | 2.15 | 1.31 to 3.53 | 0.0026 |
| Radiation alone | 1.40 | 0.76 to 2.59 | 0.2780 |
| Chemotherapy alone | 0.83 | 0.36 to 1.93 | 0.6622 |
| Chemotherapy and radiation | 0.68 | 0.13 to 3.62 | 0.6498 |
| Unknown | 1.14 | 0.12 to 10.1 | 0.9098 |
Hazard Ratios (HRs) for risk of death for NHB vs. NHW women adjusted for age at diagnosis (continuous), diagnosis period, tumor stage, histology, and receipt of adjuvant treatment; HRs for subgroup analysis were not adjusted for the corresponding stratified variable.
Discussion
This study showed that even in an equal access healthcare system, non-Hispanic Black women had significantly shorter survival than did non-Hispanic White women. This racial difference was observed in women with low risk features (stage I/II disease or low-grade) of endometrioid carcinoma, non-endometroid carcinoma, or no adjuvant treatment.
Several other studies similarly found worse survival for NHB women than their NHW counterparts. Using the SEER data, two previous studies in the general population observed worse survival among NHB women compared to NHW women even after matching on prognostic variables and stratifying by tumor characteristics such as stage and histology (24–25). Using the SEER data, Tarney et al. conducted analysis stratified by age, tumor stage and grade, and demonstrated worse survival for Black woman compared with White women with either endometrioid carcinoma or non-endometrioid carcinoma (7). The similar findings were obtained in a study based on the National Cancer Database after controlling for factors associated with survival and stratifying by stage (26). However, previous studies conducted within the Henry Ford Healthcare System, in which members have similar access to care, found no racial differences in either all-cause or endometrial cancer-specific survival. These studies adjusted for comorbidities and biological variables unavailable in our registry data (12–13). Compared with Kost’s study based on the DoD Cancer Registry data (14), which found worse cancer-free survival in Black women than White women in univariate analysis but not in analysis with adjustment for tumor stage, our study adjusted for not only tumor stage but also radiation and chemotherapy. In a multi-site study (27), in which all the study participants received surgery, racial differences in recurrence of endometrial carcinoma were observed for low-grade or early-stage tumors but not for high-grade or non-endometrial tumors. These results were similar to our findings, although the study outcome was recurrence rather than death.
Several factors might explain a poorer survival among Black women than White women when access to care is equal. First, histologic subtype may vary among racial/ethnic groups. Previous studies have reported that Black women were more likely to present with non-endometroid histologic subtypes including serous and clear cell carcinoma, which are more aggressive and have higher risks of recurrence, progression, and mortality (3, 8). This was also observed in our study, although the numbers of women with these types were too small (data not shown). Nevertheless, we adjusted for histologic type in our analysis. Second, other tumor characteristics that were not considered may have differed by race. Previous studies have suggested that the observed racial disparities could be attributed to differences in molecular features of tumors (e.g., PTEN, HER2/neu, p53 mutations, copy number variant high subtype, mitotic molecular subtype or transcript cluster 4 subtype) (7, 28–32). This information is not included in our data and could not be evaluated. Third, our study used all-cause mortality as the study outcome which included death from causes other than endometrial cancer, such as comorbid conditions. Previous research has found that comorbid conditions affect overall survival among women with endometrial cancer (12). Because Black women are more likely to have comorbidities than White women (33–36), they may be more likely to die of these medical conditions and thus have shorter overall survival. A study conducted using SEER data found that women diagnosed with endometrial cancer are more likely to die from cardiovascular disease than endometrial cancer (37). In the study by Tarney et al. there was worse non-cancer-related mortality among Black women with endometrial carcinoma younger than 65 years than their White counterparts, further confirming the possible effects of comorbidities (7). In our study, the observed racial differences among women with early stage or low-grade endometrial cancer and women without adjuvant chemotherapy, who have less aggressive cancer and thus may be less likely to die of endometrial cancer itself, might also imply the effects of comorbid diseases. However, the overlapping confidence intervals of HRs due to the small sample sizes in the stratified analyses prevent us from ascertaining this conjecture. Nevertheless, a separate study showed that a higher prevalence of comorbidities among Black women may not fully account for the higher mortality compared to White women (38). Fourth, treatment effectiveness may vary between racial groups. Research has shown that while 43% of White women with endometrial cancer responded to doxorubicin and cisplatin, only 35% of Black women responded (39). Finally, Black and White women might differ in treatment intensity and completeness and therefore survival. Although our multivariable models adjusted for whether a woman received treatment, we cannot exclude the possible effects of racial differences in treatment timing, intensity, and duration.
Endometroid carcinoma constitutes a majority of endometrial cancer as also shown in our study. As described above, in the study by Felix et al., the racial differences in recurrence were observed only in women with low-risk features of endometroid carcinoma (stage I/II disease or low-grade endometrioid carcinoma) among patients who all received surgery (27). Several factors may contribute to the racial difference in these low risk patients. In addition to the potential effects of comorbidities described above, molecular features that may reflect more aggressiveness of tumors may be a factor. One of our previous studies by Dubil et al. (32) showed that Black women were more likely than White women to exhibit the somatic copy number alteration (SCNA)-based cluster 4 subtype (‘serous-like tumor’) among patients with low-grade (16.7% vs. 1.4%) or early-stage (29.6% vs. 10%) tumors. This finding might also account for why the racial difference was observed only among patients with no adjuvant treatment. Patients who received surgery but not adjuvant treatment usually have low-stage or low-grade tumors and thus the racial difference among these patients may reflect that in early-stage or low-grade tumors. Nevertheless, in the study by Dubil et al., the racial differences in these molecular features were also observed in high-grade or late-stage tumors (32). While it is not clear why the racial differences in survival were not shown in high-risk tumors assuming more aggressive tumors features in Black patients, it might result from complex effects of multiple factors such as tumor-risk related molecular features, utilization of medical care, and family support. One of the factors might be no or minimal financial barriers to and thus wide utilization of adjuvant treatment, which is more widely used for high-grade and late-stage tumors, in the Military Health System that provides universal care. As a result, racial differences in survival might be mitigated among patients with high-grade or late-stage disease or who received adjuvant treatment.
Factors related to the racial difference in non-endometroid carcinoma are unclear. Non-endometroid carcinoma consists of different histologic types varying in tumor aggressiveness. Small numbers of patients for both races prevented us from analysis by histologic subtype, tumor stage, or treatment, which may be associated with the identified racial differences.
The strength of this study is to assess racial differences in survival in an equal-access health care system. This largely minimized the impact from unequal access on survival disparity and the research findings imply potential effects of factors other than access to care. However, some limitations should be kept in mind. First, the outcome was all-cause mortality. It reflects not only endometrial cancer-specific death but also deaths of other causes, as stated above. Second, this study was based on cancer registry data, which do not contain information on lifestyle and anthropometric factors related to survival, such as obesity (40), we do not exclude the possibility of residual confounding. Third, there were relatively small numbers of women for stratified analysis, which led to low study power for identifying the racial difference in subgroups.
In conclusion, this study suggests NHB women with endometrial cancer had poorer survival than NHW women in an equal access healthcare system, further supporting that factors other than access to care may be related to the racial disparity in survival.
Supplementary Material
S1: Appendix. ICD-O-3 Histology Codes of Endometrial Cancer
Acknowledgements
This project was supported by John P. Murtha Cancer Center Research Program, Department of Surgery, Uniformed Services University of the Health Sciences and Walter Reed National Military Medical Center under the auspices of the Henry M. Jackson Foundation for the Advancement of Military Medicine. The authors thank the Joint Pathology Center for providing the ACTUR data. The authors would also like to thank Drs. Chad A. Hamilton and Shelia Hoar Zahm for their comments on the initial draft of this manuscript.
Footnotes
Conflict of Interest
Dr. Casablanca reports other from Pfizer, other from Regeneron, outside the submitted work.
Publisher's Disclaimer: Disclaimer
Publisher's Disclaimer: The contents of this publication are the sole responsibility of the author(s) and do not necessarily reflect the views, opinions, or policies of Uniformed Services University of the Health Sciences (USUHS), the Henry M. Jackson Foundation for the Advancement of Military Medicine, Inc., the Department of Defense (DoD) or the Departments of the Army, Navy, or Air Force. Mention of trade names, commercial products, or organizations does not imply endorsement by the U.S. Government.
REFERENCES
- 1.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2020. CA Cancer J Clin. 2020. January;70(1):7–30. [DOI] [PubMed] [Google Scholar]
- 2.U.S. Cancer Statistics Working Group. U.S. Cancer Statistics Data Visualizations Tool, based on November 2018 submission data (1999–2016): U.S. Department of Health and Human Services, Centers for Disease Control and Prevention and National Cancer Institute; www.cdc.gov/cancer/dataviz, June 2019.
- 3.Smotkin D, Nevadunsky NS, Harris K, Einstein MH, Yu Y, Goldberg GL. Histopathologic differences account for racial disparity in uterine cancer survival. Gynecologic Oncology 2012;127(3):616–619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Oliver KE, Enewold LR, Zhu K, Conrads TP, Rose GS, Maxwell GL, et al. Racial disparities in histopathologic characteristics of uterine cancer are present in older, not younger blacks in an equal-access environment. Gynecologic Oncology 2011;123(1):76–81. [DOI] [PubMed] [Google Scholar]
- 5.Sud S, Holmes J, Eblan M, Chen R, Jones E. Clinical characteristics associated with racial disparities in endometrial cancer outcomes: A surveillance, epidemiology and end results analysis. Gynecologic Oncology 2018;148(2):349–356. [DOI] [PubMed] [Google Scholar]
- 6.Baskovic M, Lichtensztajn DY, Nguyen T, Karam A, English DP. Racial disparities in outcomes for high‐grade uterine cancer: A California cancer registry study. Cancer Med. 2018;7(9):4485–4495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tarney CM, Tian C, Wang G, Dubil EA, Bateman NW, Chan JK, et al. Impact of age at diagnosis on racial disparities in endometrial cancer patients. Gynecol Oncol. 2018;149:12–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sherman ME, Devesa SS. Analysis of racial differences in incidence, survival, and mortality for malignant tumors of the uterine corpus. Cancer 2003;98(1):176–186. [DOI] [PubMed] [Google Scholar]
- 9.Wright JD, Fiorelli J, Schiff PB, Burke WM, Kansler AL, Cohen CJ, et al. Racial disparities for uterine corpus tumors: changes in clinical characteristics and treatment over time. Cancer 2009;115(6):1276–85. [DOI] [PubMed] [Google Scholar]
- 10.Gottwald L, Pluta P, Piekarski J, Spych M, Hendzel K, Topczewska-Tylinska K, et al. Long-term survival of endometrioid endometrial cancer patients. Arch Med Sci 2010;6(6):937–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.DeNavas-Walt CPB, Smith JC. Income, Poverty, and Health Insurance Coverage in the United States: 2010. In: Current Population Reports. Washington D.C.: U.S. Government Printing Office; 2011. p. 60–239. [Google Scholar]
- 12.Elshaikh MA, Munkarah AR, Robbins JR, Laser BS, Bhatt N, Cogan C, et al. The impact of race on outcomes of patients with early stage uterine endometrioid carcinoma. Gynecol Oncol 2013;128(2):171–4. [DOI] [PubMed] [Google Scholar]
- 13.Hicks ML, Kim W, Abrams J, Johnson CC, Blount AC, Parham GP. Racial differences in surgically staged patients with endometrial cancer. J Natl Med Assoc 1997;89(2):134–40. [PMC free article] [PubMed] [Google Scholar]
- 14.Kost ER, Hall KL, Hines JF, Farley JH, Nycum LR, Rose GS, et al. Asian-Pacific Islander race independently predicts poor outcome in patients with endometrial cancer. Gynecol Oncol 2003;89(2):218–26. [DOI] [PubMed] [Google Scholar]
- 15.Oliver KE, Enewold LR, Zhu K, Conrads TP, Rose GS, Maxwell GL, Farley JH. Racial disparities in histopathologic characteristics of uterine cancer are present in older, not younger blacks in an equal-access environment. Gynecol Oncol. 2011. October;123(1):76–81. [DOI] [PubMed] [Google Scholar]
- 16.Kurman Robert J; Ellenson Lora Hedrick; Ronnett BM. Blaustein’s Pathology of the Female Genital Tract 6th Edition: Springer; 2011. [Google Scholar]
- 17.World Health Organization. (2013). International classification of diseases for oncology (ICD-O) – 3rd edition, 1st revision, 3rd ed. https://apps.who.int/iris/handle/10665/96612. [Google Scholar]
- 18.American Joint Committee on Cancer: Manual for Staging of Cancer, second edition. New York, JB Lippincott, 1983. [Google Scholar]
- 19.American Joint Committee on Cancer: Manual for Staging of Cancer, third edition. New York, JB Lippincott, 1988. [Google Scholar]
- 20.Beahrs O, Henson DE, Hutter RVP, Kennedy BJ, editors. American Joint Committee on Cancer: manual for staging of cancer. 4th ed. Philadelphia: JB Lippincott; 1992. [Google Scholar]
- 21.American Joint Committee on Cancer: AJCC Cancer Staging Manual, 5th ed. Fleming ID, Cooper JS, Henson DE et al. (Eds.). Philadelphia: Lippincott-Raven, 1977. [Google Scholar]
- 22.American Joint Committee on Cancer. AJCC Cancer Staging Manual. 6th ed. New York: Springer-Verlag; 2002. [Google Scholar]
- 23.Edge SB, Byrd DR, Compton CC, Fritz AG, Greene FL, Trotti A, editors. AJCC cancer staging manual (7th ed). New York, NY: Springer; 2010. [Google Scholar]
- 24.Wright JD, Fiorelli J, Schiff PB, Burke WM, Kansler AL, Cohen CJ, & Herzog TJ. Racial disparities for uterine corpus tumors: changes in clinical characteristics and treatment over time. Cancer 2009;115(6), 1276–1285. [DOI] [PubMed] [Google Scholar]
- 25.Cote ML, Ruterbusch JJ, Olson SH, Lu K, Ali-Fehmi R. The Growing Burden of Endometrial Cancer: A Major Racial Disparity Affecting Black Women. Cancer Epidemiol Biomarkers Prev. 2015. September;24(9):1407–15. [DOI] [PubMed] [Google Scholar]
- 26.Fader AN, Habermann EB, Hanson KT, Lin JF, Grendys EC, Dowdy SC. Disparities in treatment and survival for women with endometrial cancer: A contemporary national cancer database registry analysis. Gynecol Oncol. 2016;143(1):98–104. [DOI] [PubMed] [Google Scholar]
- 27.Felix AS, Brasky TM, Cohn DE, Mutch DG, Creasman WT, Thaker PH, et al. Endometrial carcinoma recurrence according to race and ethnicity: An NRG Oncology/Gynecologic Oncology Group 210 Study. Int J Cancer. 2018;142(6):1102–1115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Santin AD, Bellone S, Siegel ER, Palmieri M, Thomas M, Cannon MJ, et al. Racial differences in the overexpression of epidermal growth factor type II receptor (HER2/neu): a major prognostic indicator in uterine serous papillary cancer. Am J Obstet Gynecol 2005;192(3):813–8. [DOI] [PubMed] [Google Scholar]
- 29.Maxwell GL, Risinger JI, Hayes KA, Alvarez AA, Dodge RK, Barrett JC, et al. Racial disparity in the frequency of PTEN mutations, but not microsatellite instability, in advanced endometrial cancers. Clin Cancer Res 2000;6(8):2999–3005. [PubMed] [Google Scholar]
- 30.Maxwell GL, Allard J, Gadisetti CV, Litzi T, Casablanca Y, Chandran U, et al. Transcript expression in endometrial cancers from Black and White patients. Gynecol Oncol 2013;130(1):169–73. [DOI] [PubMed] [Google Scholar]
- 31.Yap OW, Matthews RP. Racial and ethnic disparities in cancers of the uterine corpus. J Natl Med Assoc 2006;98(12):1930–3. [PMC free article] [PubMed] [Google Scholar]
- 32.Dubil EA, Tian C, Wang G, Tarney CM, Bateman NW, Levine DA, et al. Racial disparities in molecular subtypes of endometrial cancer. Gynecol Oncol 2018;149(1):106–116. [DOI] [PubMed] [Google Scholar]
- 33.Esnaola NF, Hall BL, Hosokawa PW, Ayanian JZ, Henderson WG, Khuri SF, et al. Race and surgical outcomes: it is not all black and white. Ann Surg 2008;248(4):647–55. [DOI] [PubMed] [Google Scholar]
- 34.Guggina P, Flahive J, Hooven FH, Watts NB, Siris ES, Silverman S, et al. Characteristics associated with anti-osteoporosis medication use: data from the Global Longitudinal Study of Osteoporosis in Women (GLOW) USA cohort. Bone 2012;51(6):975–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Nicholas Z, Hu N, Ying J, Soisson P, Dodson M, Gaffney DK. Impact of Comorbid Conditions on Survival in Endometrial Cancer. Am J Clin Oncol 2012. [DOI] [PubMed] [Google Scholar]
- 36.Ostchega Y, Dillon CF, Hughes JP, Carroll M, Yoon S. Trends in hypertension prevalence, awareness, treatment, and control in older U.S. adults: data from the National Health and Nutrition Examination Survey 1988 to 2004. J Am Geriatr Soc 2007;55(7):1056–65. [DOI] [PubMed] [Google Scholar]
- 37.Ward KK, Shah NR, Saenz CC, McHale MT, Alvarez EA, Plaxe SC. Cardiovascular disease is the leading cause of death among endometrial cancer patients. Gynecol Oncol 2012;126(2):176–9. [DOI] [PubMed] [Google Scholar]
- 38.Ruterbusch JJ, Ali-Fehmi R, Olson SH, Sealy-Jefferson S, Rybicki BA, Hensley-Alford S, et al. The influence of comorbid conditions on racial disparities in endometrial cancer survival. Am J Obstet Gynecol 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Farley JH, Tian C, Rose GS, Brown CL, Birrer M, Risinger JI, et al. Chemotherapy intensity and toxicity among black and white women with advanced and recurrent endometrial cancer: a Gynecologic Oncology Group Study. Cancer 2010;116(2):355–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Chia VM, Newcomb PA, Trentham-Dietz A, Hampton JM. Obesity, diabetes, and other factors in relation to survival after endometrial cancer diagnosis. Int J Gynecol Cancer 2007;17(2):441–6. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
S1: Appendix. ICD-O-3 Histology Codes of Endometrial Cancer


