Abstract
The aim of these studies was to elucidate a role for epidermal growth factor (EGF) signaling in the transcriptional regulation of the glycoprotein hormone α subunit gene, a subunit of chorionic gonadotropin. Studies examined the effects of EGF and the adenylate cyclase activator forskolin on the expression of a transfected α subunit reporter gene in a human choriocarcinoma cell line (JEG3). At maximal doses, administration of EGF resulted in a 50% increase in a subunit reporter activity; forskolin administration induced a fivefold activation; the combined actions of EGF and forskolin resulted in synergistic activation (greater than eightfold) of the α subunit reporter. Mutagenesis studies revealed that the cyclic AMP response elements (CRE) were required and sufficient to mediate EGF-forskolin-induced synergistic activation. The combined actions of EGF and forskolin resulted in potentiated activation of extracellular signal-regulated kinase (ERK) enzyme activity compared with EGF alone. Specific blockade of ERK activation was sufficient to block EGF-forskolin-induced synergistic activation of the α subunit reporter. Pretreatment of JEG3 cells with a p38 mitogen-activated protein kinase inhibitor did not influence activation of the α reporter. However, overexpression of c-Jun N-terminal kinase (JNK)-interacting protein 1 as a dominant interfering molecule abolished the synergistic effects of EGF and forskolin on the α subunit reporter. CRE binding studies suggested that the CRE complex consisted of CRE binding protein and EGF-ERK-dependent recruitment of c-Jun–c-Fos (AP-1) to the CRE. A dominant negative form of c-Fos (A-Fos) that specifically disrupts c-Jun–c-Fos DNA binding inhibited synergistic activation of the α subunit. Thus, synergistic activation of the α subunit gene induced by EGF-forskolin requires the ERK and JNK cascades and the recruitment of AP-1 to the CRE binding complex.
Chorionic gonadotropin (CG) is a heterodimeric glycoprotein hormone consisting of an α subunit common to other glycoprotein hormone family members noncovalently linked to a CG-specific β subunit (58). CG is synthesized and secreted by placental syncytiotrophoblast cells during the first trimester of pregnancy in women and nonhuman primates. CG is a luteotropin required for maintenance of progesterone production by the ovarian corpus luteum in early gestation. An important factor in the establishment of early pregnancy appears to be the timing and rate of increase in the secretion of CG that is highly correlated with a rise in progesterone levels in peripheral circulation (42). Insufficient progesterone production during early pregnancy is correlated with the potential for early or recurrent pregnancy loss in women (6, 7, 27, 47). Thus, endocrine mechanisms that potentiate the synthesis of CG subunits and CG secretion are essential for the establishment of pregnancy. Despite the clear importance of CG to early pregnancy, the specific ligands and signaling mechanisms that regulate the expression of CG subunit genes in placental cells have not been fully elucidated.
The α subunit of the glycoprotein hormones is a unique and useful transcriptional model for the study of tissue-specific gene expression because the α subunit gene is expressed in placental and pituitary cells, albeit by various mechanisms. Analysis of the architecture of the α subunit promoter revealed the presence of multiple promoter elements that are required for transcriptional regulation. These include the pituitary glycoprotein hormone basal element, which binds members of the LIM class of homeobox-containing proteins (67, 71, 72); the α basal element (32); the gonadotrope-specific element, which binds steroidogenic factor 1 (9, 36); the upstream regulatory element (URE) (12, 26, 38, 59); the GATA element, which binds several GATA factors (75); the dual tandem cyclic AMP (cAMP) response elements (CREs), which bind CRE binding protein (CREB) (5, 10, 12, 13, 22, 25, 32, 35, 52, 59, 74); the junctional regulatory element (JRE) (4, 12); and a unique CAAT box (12, 40). Extensive mutagenesis studies have begun to unravel the specific requirements for different combinations of cis-acting elements in cell-specific α subunit expression. In pituitary cells, for example, analysis of the human α subunit promoter revealed that pituitary-specific expression required the pituitary glycoprotein hormone basal element, α basal element, gonadotrope-specific element, CREs, and URE (32, 71). In cells of placental origin, the URE, GATA, dual CREs, JRE, and a unique CAAT box all contribute to basal trophoblast-specific promoter regulation to the human α subunit (4, 12, 22, 32). However, the absolute contribution of each of these five regulatory elements varies in level of transcriptional importance. For example, mutations within the dual CREs render the α subunit promoter essentially silent in placental cells, providing evidence for the absolute importance of the CREs. Interestingly, mutations within the URE or the JRE/CAAT box result in 80 to 90% loss in promoter activity despite the presence of a wild-type CRE (12). Thus, the importance of the CREs in regulating basal α subunit gene expression appears to be within the context of other critical cis elements within the α subunit promoter (12). The emerging model for cell-specific transcriptional regulation of the α subunit gene is that the appropriate combinatorial code of cis elements and their cognate binding proteins is required to regulate expression of the gene (56).
Despite advances in our understanding of cell-specific regulation of the α subunit, our understanding of the mechanisms of endocrine-inducible regulation of α subunit expression in placental cells is less comprehensive. In placental trophoblasts, studies of cell signaling have primarily elucidated the effects of cAMP in increasing activation of protein kinase A (PKA) and the subsequent phosphorylation-dependent activation of CREB. Elevated levels of cAMP increase CRE-dependent expression of the genes encoding the α and β CG peptides in a coordinated but distinct manner (2, 5, 11, 13, 19, 52). Interestingly, combined activation of the cAMP/PKA and phorbol ester/protein kinase C (PKC) signaling pathways results in potentiated activation of the α subunit gene. However, the mechanism(s) for this transcriptional response has not been elucidated (5, 21). The dual tandem CREs of the α subunit likely play a prominent role in inducible regulation by multiple signaling cascades since this cis element has been shown to bind CREB and other inducible factors such as c-Jun (25, 33).
In addition to regulation by PKA and PKC, trophoblasts express receptors for epidermal growth factor (EGF) on their cell surface as well as EGF itself (3), suggesting the possibility for autocrine regulation by this growth peptide. EGF receptor binding and subsequent activation alter trophoblast differentiation (18, 55) and increase the synthesis and secretion of CG from placental cells (8, 14, 37, 55, 64, 65). Mechanistically, the effects of EGF on CG subunit synthesis appear to be mediated primarily via stabilization of CG subunit mRNA rather than a direct increase in the rate of transcription in human trophoblast cells (14). Recent evidence (51) derived from a rat trophoblast cell model (Rcho-1 cells) suggests that the human α subunit is directly regulated by EGF signaling through CREB phosphorylation. However, similar studies have not been conducted in human cell lines in which the α subunit gene is expressed endogenously. In the present study, we sought to examine the role of EGF stimulation on induction of the glycoprotein hormone α subunit promoter in the human choriocarcinoma cell line JEG3. We demonstrate that unlike the case in Rcho-1 cells, acute EGF administration alone only modestly enhances α subunit gene activation. However, concurrent administration of EGF and activation of the cAMP/PKA pathway resulted in synergistic activation of the α subunit gene.
MATERIALS AND METHODS
Plasmids, hormones, agonists, and reagents.
All plasmids were prepared by two cycles through cesium chloride by standard methods. Human α −880 luciferase and the CRE(−) α subunit luciferase reporters were a gift from Richard A. Maurer (Oregon Health Sciences University, Portland) and contained nucleotides −846 to +42 of the 5′-flanking region of the α subunit gene. The human α-204 luciferase reporter was prepared by PCR with the α −846 luciferase reporter as a template. The −204 deletion was confirmed by nucleotide sequence analysis and essentially contained the URE, the CREs, the JRE, and the CCAAT box. Mutations in the individual CREs within the α subunit promoter were accomplished by oligonucleotide-directed mutagenesis as described before (66, 67). Each individual CRE was replaced with a NotI restriction site (5′GCGGCCGC3′), generating hα-204-CRE1-MUT-luc and hα-204-CRE2-MUT-luc. Mutagenesis was confirmed by nucleotide sequence analysis. The human α CRE-Prl-luciferase reporter was constructed by cloning annealed oligonucleotides for the dual tandem CRE from the α subunit gene into the SmaI site in the reporter Prl-luciferase (a gift from D. Duval and C. Clay, Colorado State University, Fort Collins). The Prl-luciferase reporter contains the TATAA box from the prolactin gene cloned upstream of the luciferase coding sequence in the luciferase reporter pGL3 (Promega, Madison, Wis.). The synthesized CRE oligonucleotides were purchased from Gibco-BRL (Gaithersburg, Md.). The nucleotide sequences for the oligonucleotides were 5′GGGATTGACGTCATGGTAAAAATTGACGTCATG3′ and 5′CATGACGTCAATTTTTACCATGACGTCAATCCC3′. The orientation of the annealed CRE oligonucleotide in Prl-luciferase was confirmed by restriction analysis; the proper orientation of the CRE relative to luciferase reestablishes a SmaI restriction site. The expression vector for Raf-CAAX was a gift from Linda VanAelst (Cold Spring Harbor Laboratory, Cold Spring Harbor, N.Y.). The expression vector for cytomegalovirus JNK-interacting protein (CMV-JIP) was a gift from Roger Davis (University of Massachusetts, Worcester). The expression vectors for c-Jun and c-Fos were gifts from Paul Dobner (University of Massachusetts, Worcester) and Michael Greenberg (Harvard Medical School, Boston, Mass.). The expression vector for A-Fos and CMV/500 were a gift from Charles Vinson (National Cancer Institute, Bethesda, Md.).
Forskolin was purchased from Sigma (St. Louis, Mo.) and resuspended at a stock concentration of 1 mM in dimethyl sulfoxide (DMSO). EGF was purchased from Gibco-BRL and resuspended at a stock concentration of 1 mg/ml in Dulbecco's phosphate-buffered saline. PD98059 was purchased from New England Biolabs (Beverly, Mass.) and resuspended at a stock concentration of 50 mM in DMSO. SB203580 was purchased from Calbiochem (La Jolla, Calif.) and resuspended at a stock concentration of 20 mM in DMSO. All tissue culture media were purchased from Sigma. Fetal bovine and horse sera were purchased from Gibco-BRL. Luciferin and the transcription and translation reticulocyte lysate kit were purchased from Promega.
Cell culture, transient transfection, and luciferase assay.
The human choriocarcinoma cell line JEG3 was cultured in monolayers with Dulbecco's modified Eagle's medium (DMEM) supplemented with 10% fetal bovine serum. The rat lactotrope cell line GH3 was cultured in monolayers with DMEM supplemented with 15% horse serum and 2.5% fetal bovine serum. Prior to all studies, cell cultures were split to fresh medium and cultured to approximately 50% confluence. All transient-transfection studies were conducted as described previously (65, 66). In transfection studies requiring agonist administration, cells were treated with forskolin and/or EGF at the concentrations noted for individual experiments for 6 h prior to collection (18 h following electroporation) except in Fig. 1. In Fig. 1, transfected cells received control solution, EGF alone, forskolin alone, or the combination of forskolin and EGF, all administered at time zero. Time zero was designated as the starting point of a 6-h period of agonist administration. Some cells received a 4-h pretreatment (administered at −4 h relative to time zero) with EGF. This was followed by administration of control solution or forskolin at time zero. All cells in these studies were collected 6 h later. PD98059 (50 μM) or SB203580 (20 μM) was used in some experiments and administered to cells 15 min prior to agonist administration. Following cell collection, lysates were prepared by three freeze-thaw cycles and clarified by centrifugation, and luciferase activity was determined as described before (23, 66, 67). All transfection studies were conducted in triplicate on at least three separate occasions with similar results. Data shown are reported as means (n = 3) ± standard errors of the mean and were analyzed by analysis of variance to detect treatment interactions.
FIG. 1.
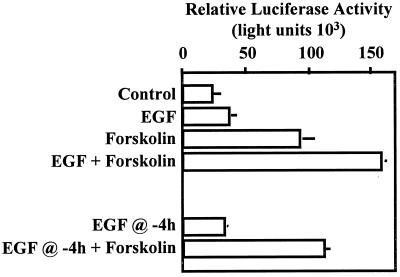
EGF and forskolin synergistically activate the glycoprotein hormone α subunit gene. JEG3 cells were transfected by electroporation with 500 ng of a reporter gene containing nucleotides −846 to +42 of 5′-flanking sequences from the human glycoprotein hormone α subunit gene fused upstream of a luciferase reporter. Approximately 18 h following transfection, cells received control solution, EGF (50 ng/ml), forskolin (1 μM), or the combination of EGF and forskolin (time zero). Six hours later, cells were scraped, and lysates were prepared by three freeze-thaw cycles and assayed for luciferase activity. Some transfected cells received EGF 14 h following transfection (EGF @ −4h). These cells received either control solution or forskolin at time zero (EGF @ −4h + Forskolin). Cells were collected 6 h following time zero. Luciferase activity is reported as relative activity ± standard error of the mean from a single representative experiment (n = 3 independent electroporations per treatment). All transfection studies were conducted on at least three separate occasions (in triplicate) with similar results.
Preparation of nuclear extracts and CRE pull-down assays.
JEG3 cells were serum starved for 2 h prior to preparation of nuclear extracts. Nuclear extracts were prepared following administration of either control solution, EGF, forskolin, or the combination of EGF and forskolin as described for the transient-transfection studies. Hormones were administered for a total of 2 h. Plates were placed on an ice bed, and cells were washed with HEPES-buffered saline (HBS; 10 mM HEPES [pH 7.4]–150 mM NaCl). Cells were collected by scraping into ice-cold HBS and pelleted by centrifugation (2,000 rpm for 15 min). Cells were lysed in a Dounce homogenizer, and nuclei were isolated using the sucrose cushion method described previously (66, 67). Nuclei were resuspended in a buffer containing 10 mM HEPES (pH 7.4), 2.5 mM MgCl2, 3.6 mM KCl, 150 mM NaCl, 10% glycerol, 1 mM dithiothreitol, 5 mM benzamadine, and 0.2 mM phenylmethylsulfonyl fluoride (PMSF). This buffer is referred to as binding buffer. Nuclear proteins were extracted by adding additional NaCl (in binding buffer) to a final concentration of 450 mM and incubating the extract for 30 min at 4°C with constant rocking. Nuclear debris was removed by centrifugation, and protein concentration was determined by the Bradford assay. Nuclear extracts were aliquoted and stored at −80°C until later use.
CRE oligonucleotides were synthesized as described above except that the sense strand was biotinylated at the 5′ termini during the synthesis reaction. Equal amounts of each oligonucleotide were resuspended in binding buffer, heated to 100°C for 10 min, and allowed to cool slowly to room temperature. Streptavidin-agarose (SA) beads (Sigma) were washed four times in binding buffer, resuspended in binding buffer containing annealed CRE oligonucleotides, and allowed to batch bind for 1 h with constant rocking at 4°C. Bound CRE-SA beads were washed four times in binding buffer and resuspended at a final approximate CRE concentration of 2 pmol/μl of SA beads. CRE binding reactions were carried out in binding buffer containing 50 pmol of bound CRE, 10 μg of sonicated salmon sperm DNA, and 100 μg of nuclear protein in a total volume of 500 μl. Binding reactions were carried out for 2 h at 4°C with constant rocking. Bound complexes were washed six times (1 ml each) in binding buffer and resuspended in 75 μl of 2× sodium dodecyl sulfate (SDS) loading buffer (100 mM Tris [pH 6.8], 4% SDS, 5% glycerol, 0.01% bromophenol blue). The constituents of the binding complexes were examined by immunoblot analysis, described below. These experiments were carried out with three separate nuclear extracts prepared on separate occasions. Results were similar with all three nuclear extracts.
Antibodies, immunoblot analysis, and immunoprecipitation (IP) kinase assays.
CREB antibody was generously supplied by R. A. Maurer (Oregon Health Sciences University) and used at a titer of 1:5,000 with nonfat dry milk (5%) as a blocking agent. Antibodies for c-Jun, c-Fos, JNK1, ERK2, p38, all secondary antibodies coupled to horseradish peroxidase, and protein A/G plus agarose were purchased from Santa Cruz Biotechnology (Santa Cruz, Calif.). Phosphorylation-specific extracellular signal-regulated kinase (ERK) and p38 antibodies were purchased from New England Biolabs and used per the manufacturer's instructions.
For studies of the effects of agonist administration on cell signaling components, JEG3 cells were plated at approximately 50% confluence and serum starved for 2 h prior to agonist administration. In some experiments, PD98059 was administered 15 min prior to agonist administration. Agonists were administered for the specified times (see individual experiments), plates were placed on ice, and cells were washed with ice-cold HBS. Cells were lysed in a radio- immunoprecipitation assay (RIPA) buffer containing 20 mM Tris (pH 8.0), 137 mM NaCl, 10% glycerol, 1% NP-40, 0.1% SDS, 0.5% deoxycholate, 2 mM EDTA, 5 mM sodium vanadate, 5 mM benzamadine, and 0.2 mM PMSF. Debris from cell lysates was cleared by centrifugation, and lysates were suspended in an equal volume of 2× SDS loading buffer. All protein samples were boiled for 5 min and chilled briefly on ice prior to loading on gels. For Western blotting, proteins were resolved by SDS-polyacrylamide gel electrophoresis (PAGE) and transferred to polyvinylidene difluoride membranes by electroblotting. The membranes were blocked, and antibodies were applied at titers specific to each antibody (per the manufacturer). Proteins were visualized by chemiluminescence with reagents purchased from NEN-DuPont (Boston, Mass.).
Analysis of JNK activity was accomplished by IP followed by an in vitro kinase assay. Cell lysates were prepared in RIPA buffer as described above. JNK antibody (0.5 μg/200 μl of cell lysate) was added to the cell lysate with protein A/G-agarose and mixed by rocking for 2 h at 4°C. Protein A/G-agarose beads containing JNK activity were washed twice (1 ml each) with ice-cold RIPA buffer. Beads were then washed twice (1 ml each) in an ice-cold buffer containing 20 mM Tris (pH 8.0), 137 mM NaCl, 10% glycerol, 1% NP-40, 2 mM EDTA, 5 mM sodium vanadate, 5 mM benzamadine, and 0.2 mM PMSF. The beads were finally washed in 1 ml of ice-cold kinase buffer containing 20 mM HEPES (pH 7.4), 20 mM MgCl2, 25 mM β-glycerol phosphate, 100 μM sodium vanadate, 20 μM ATP, and 2 mM dithiothreitol. The beads were then resuspended in kinase buffer (50 μl) containing 5 μCi of [γ-32P]ATP and approximately 1 μg of bacterially expressed and partially purified glutathione-S-transferase (GST)–ATF2 as a substrate. Incubation of the kinase reaction mixture was carried out for 30 min at 30°C with frequent mixing. The kinase reaction was terminated by the addition of 50 μl of 2× SDS loading buffer. Samples were boiled, resolved by SDS-PAGE, and visualized by autoradiography. All of the studies were repeated on at least three separate occasions with similar results.
Preparation of AP-1 in reticulocyte lysates and EMSA.
Expression vectors for c-Fos and c-Jun were used in a coupled transcription and translation synthesis reaction in reticulocyte lysates. Individual preparations were mixed and incubated at 37°C for 60 min to allow heterodimer formation. Electrophoretic mobility shift assays (EMSAs) were conducted essentially as described before (67). Briefly, activator protein 1 (AP-1) binding activity from reticulocyte lysates was mixed with binding buffer, 1 μg of poly(dI-dC), and specific antisera (c-Fos, c-Jun, and Ets antibodies; Santa Cruz Biotechnology) and maintained at room temperature for 45 min. CRE oligonucleotides were radiolabeled with polynucleotide kinase and [γ-32P]ATP. Labeled CRE probe (approximately 0.06 pmol) was then added and incubated further for 30 min at room temperature. The binding reactions were resolved on native polyacrylamide gels. The gels were dried, and DNA-protein complexes were visualized by autoradiography.
RESULTS
Combined actions of EGF and forskolin induce synergistic activation of the glycoprotein hormone α subunit gene in JEG3 cells.
We initially conducted dose-response studies with EGF and forskolin to determine the conditions for maximum biological response to agonists, using activation of a glycoprotein hormone α subunit-luciferase reporter as an endpoint in JEG3 cell transfection studies. Transfections were carried out by electroporation. The transfected cells were administered increasing doses of EGF (0, 10, 25, 50, and 100 ng/ml) or forskolin (0, 0.1, 1, and 10 μM), and cell lysates were examined for luciferase activity. At the dose of EGF that resulted in maximal response (50 ng/ml), α subunit reporter expression was induced 1.53 (±0.25)-fold. At the dose of forskolin that resulted in maximal response (1 μM), α subunit reporter expression was induced 5.34 (±1.1)-fold. All remaining studies were conducted with these doses of agonists. We examined the effects of each agonist alone and in combination on α subunit reporter gene activity (Fig. 1). The response to EGF and forskolin administration was consistent with the preliminary dose-response studies, in which EGF alone induced an approximately 1.5-fold increase in α subunit promoter activity, while forskolin induced reporter gene activity approximately fivefold (Fig. 1). When both agonists were administered concurrently, the combined effects of EGF and forskolin induced α subunit reporter gene activation approximately 8.5-fold. Analysis of variance of these data revealed a statistically significant (P = 0.0018) interaction between the main effects of EGF and forskolin. The combined effects of EGF and forskolin were more than additive, representing synergistic activation. We then examined whether concurrent administration of both agonists was required to induce synergistic activation of the α subunit gene. Administration of forskolin 4 h after EGF application did not result in synergistic activation of the α reporter (Fig. 1). These studies support the conclusion that synergistic activation of the α subunit reporter requires concurrent activation of both the EGF- and forskolin-inducible signaling cascades.
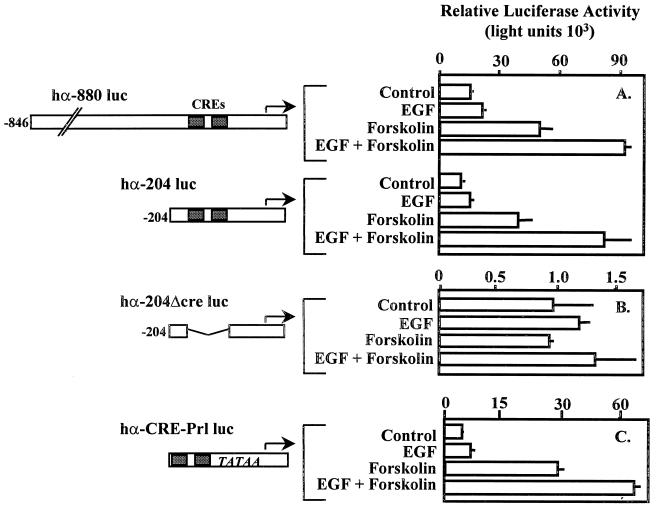
To determine the DNA sequences required for EGF-forskolin-induced expression of the α subunit, deletion mutagenesis experiments were performed. We compared the effects of EGF and forskolin on reporters containing either nucleotides −846 to +42 or −204 to +42 of the 5′-flanking region of the α subunit gene (Fig. 2). The results from transient-transfection studies revealed that sequences upstream of −204 within the 5′-flanking region of the α subunit gene were not required for synergistic activation by EGF and forskolin (Fig. 2A). Since the effect(s) of EGF appeared to augment the effect(s) of forskolin, likely through activation of PKA and subsequent CREB activation, we examined the activity of an α subunit reporter construct in which the CREs had been deleted within the context of the −204 to +42 promoter region. The results of these studies reveal that in the absence of the dual tandem CREs, α subunit promoter activity was reduced by approximately 95% and the reporter was no longer responsive to the actions of forskolin or EGF and forskolin (hα-204Δcre luc; Fig. 2B). We examined the possibility that the dual tandem CREs alone were sufficient to mediate synergistic transcriptional activity. A single copy of the dual tandem CREs was cloned immediately upstream of the prolactin minimal promoter fused to the luciferase gene (hαCRE-Prl-Luc; Fig. 2C). The dual tandem CRE-luciferase reporter was clearly sufficient to mediate synergistic transcriptional activation consistent with the −846 and −204 α subunit promoter fragments. In the absence of the CRE, the prolactin minimal promoter coupled to luciferase was essentially silent and was not affected by the administration of EGF and forskolin (data not shown). The results of these studies support the conclusion that the α subunit CREs were required and sufficient to mediate the combined effects of EGF and forskolin.
FIG. 2.
Dual tandem CREs within the α subunit promoter are minimally required for synergistic transcriptional activation by EGF and forskolin. JEG3 cells were transfected by electroporation with 500 ng of an α subunit reporter containing (A) nucleotides −846 to +42 (hα-880 luc) or −204 to +42 (hα-204 luc) of 5′-flanking sequence coupled to a luciferase reporter; (B) hα-204luc with dual tandem CREs deleted (hα-204Δcre luc); or (C) the dual tandem CREs fused upstream of a minimal promoter from the prolactin gene coupled to a luciferase reporter (hα-CRE-Prl luc). Agonists were administered for a 6-h period as described in the legend to Fig. 1, and luciferase activity was assayed. Luciferase activity is reported as relative activity ± standard error of the mean from a single representative experiment (n = 3 independent electroporations per treatment). All transfection studies were conducted on at least three separate occasions (in triplicate) with similar results.
Synergistic activation of the α subunit by EGF and forskolin is cell specific.
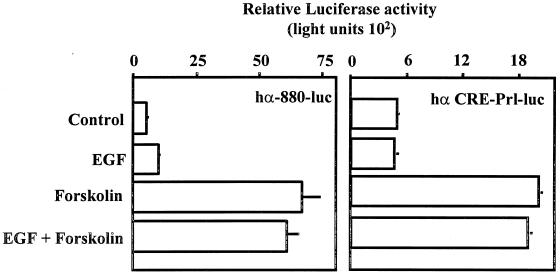
The data presented support the conclusion that EGF- and forskolin-induced cell signaling together are required for synergistic activation of the α subunit gene in the JEG3 placental cell line. We examined the possibility that the α subunit or the CRE-Prl-luciferase reporters would be induced in a similar manner in a heterologous cell type, since the components of EGF- and forskolin-induced cell signaling cascades are likely ubiquitous. The rat pituitary lactotrope cell line GH3 was an ideal heterologous cell model, since GH3 cells are EGF responsive (76, 85) and numerous studies of CREB activity have been carried out with this cell model (77–79). GH3 cells were transfected with the α subunit or the CRE-Prl-luciferase reporter, and luciferase activity was examined following administration of control solution, EGF, forskolin, or the combination of EGF and forskolin. In GH3 cells, neither reporter was EGF responsive. Both reporters were clearly induced by forskolin administration to a similar magnitude as observed in JEG3 cells (four- to sixfold) (Fig. 3). Interestingly, in GH3 cells, the combined effect(s) of EGF and forskolin on these reporters was not different from that of forskolin treatment alone. The GH3 cells used for these studies were EGF responsive, based upon studies examining changes in tyrosine phosphorylation of intracellular proteins by immunoblot analysis (data not shown). Similar results were observed with HeLa cells (data not shown). These studies support the conclusion that the ability of EGF and forskolin to induce synergistic activation of the α subunit is cell type specific.
FIG. 3.
Activation of the α subunit by EGF and forskolin is cell type specific. GH3 cells were transfected by electroporation with 500 ng of an α subunit reporter (hα-880-luc; 500 ng) or the dual tandem CREs fused upstream of a minimal promoter from the prolactin gene coupled to a luciferase reporter (hα-CRE-Prl-luc). Transfected cells were cultured for 18 h. Agonists were administered for a 6-h period as described in the legend to Fig. 1, and luciferase activity was assayed. Luciferase activity is reported as relative activity ± standard error of the mean from a single representative experiment (n = 3 independent electroporations per treatment). All transfection studies were conducted on at least three separate occasions (in triplicate) with similar results.
EGF and forskolin administration results in potentiated ERK activation in JEG3 cells.
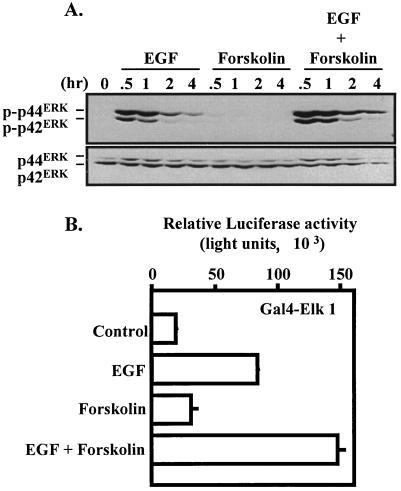
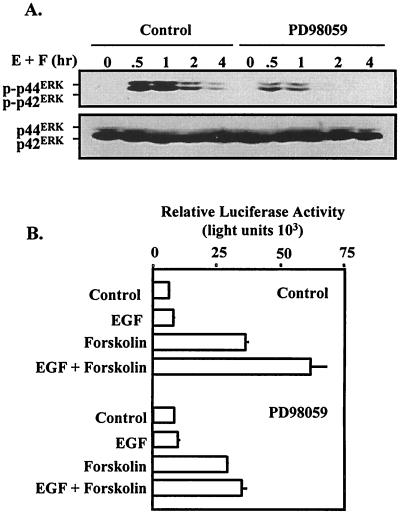
One of the principal signaling pathways induced by EGF receptor activation is the ERK cascade (45). The magnitude and duration of EGF-induced ERK activation can vary with cell type. In an effort to gain insight into the signaling mechanisms involved in the synergistic induction of the α subunit gene, an extended time course for EGF-induced ERK activation was examined (Fig. 4A). ERK activity was measured by immunoblot analysis with a phosphorylation-specific antibody that recognizes the dual phosphorylated forms of p42 and p44 ERK. As expected, EGF induced robust ERK activation that peaked at 30 min and remained measurable for up to 4 h following treatment. Forskolin administration did not induce any apparent activation of the ERK pathway. Interestingly, the combined actions of EGF and forskolin resulted in potentiated activation of the ERK cascade. The levels of ERKs (regardless of phosphorylation) were similar in each lane (Fig. 4A). Potentiation of ERK activation by EGF and forskolin was also observed in an in vivo ERK activation assay in which a Gal4-Elk1 fusion protein was expressed in combination with a Gal4-dependent luciferase reporter (Fig. 4B) (66). These studies revealed that the level of Elk1 transcriptional activity was increased by EGF treatment but not by forskolin administration. Consistent with biochemical studies of ERK phosphorylation, the combined actions of EGF and forskolin resulted in a synergistic activation of the Gal4-Elk1 fusion protein.
FIG. 4.
Influence of EGF and forskolin on induction of the ERK pathway in JEG3 cells. (A) JEG3 cells were plated at approximately 60% confluence and serum starved for 2 h. Cells were administered control solution, EGF, forskolin, or EGF plus forskolin for 0, 0.5, 1, 2, or 4 h. Whole-cell lysates were prepared and resolved by SDS-PAGE. Western blot analysis was conducted with a phosphorylation-specific ERK (p-p42ERK and p-p44ERK) antibody. The blot was then stripped and reprobed with an ERK antibody to determine equivalent loading of each lane (p42ERK and p44ERK). (B) JEG3 cells were cotransfected by electroporation with a luciferase reporter (1 μg) containing five Gal4 binding sites coupled to the E1B minimal promoter and the luciferase coding sequence and an expression vector for Gal4-Elk1 transactivation domain fusion (Gal4-Elk1, 1 μg). Eighteen hours later, cells were administered control solution, EGF, forskolin, or the combination of EGF plus forskolin. Luciferase activity was determined 6 h later. Luciferase activity is reported as relative activity ± standard error of the mean from a single representative experiment (n = 3 independent electroporations per treatment). All transfection studies were conducted on at least three separate occasions (in triplicate) with similar results.
Activation of the ERK pathway required but not sufficient for synergistic activation of the α subunit.
Thus far, our studies have implicated EGF-induced activation of the ERK pathway in potentially contributing to the activation of the α subunit gene. We sought to disrupt EGF signaling to the ERK cascade to examine the requirement for the ERK pathway for synergistic activation of the α subunit reporter. Initial studies confirmed that pretreatment of JEG3 cells with PD98059 reduced the magnitude and duration of EGF-induced ERK activation (Fig. 5A). In parallel experiments, administration of PD98059 was sufficient to block the synergistic activation of the α subunit reporter induced by concurrent administration of EGF and forskolin (Fig. 5B). Administration of PD98059 did not influence forskolin-induced α subunit reporter activity, suggesting that the pharmacological actions of this MEK-1 inhibitor were specific. These studies confirm that EGF-induced ERK activation was required for synergistic activation of the α subunit promoter.
FIG. 5.
Blockade of the ERK cascade abolishes synergistic activation of the α subunit by EGF and forskolin. (A) JEG3 cells were plated at approximately 60% confluence and serum starved for 2 h. Cells were administered EGF plus forskolin (E + F) for 0, 0.5, 1, 2, or 4 h in the absence (control) or presence of PD98059 (50 μM). Whole-cell lysates were prepared and resolved by SDS-PAGE. Western blot analysis was conducted with a phosphorylation-specific ERK (p-p42ERK and p-p44ERK) antibody. The blot was then stripped and reprobed with an ERK antibody to determine equivalent loading of each lane (p42ERK and p44ERK). (B) JEG3 cells were transfected by electroporation with hα-880-luc as described in the legend to Fig. 1. Fifteen minutes prior to administration of agonists, some cells received PD98059 (50 μM). Luciferase activity was determined 6 h following agonist administration. Luciferase activity is reported as relative activity ± standard error of the mean from a single representative experiment (n = 3 independent electroporations per treatment). All transfection studies were conducted on at least three separate occasions (in triplicate) with similar results.
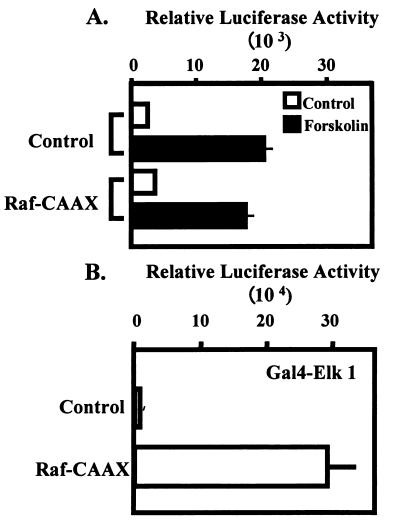
We next examined the possibility that activation of the ERK pathway by overexpression of a constitutively active form of Raf kinase (Raf-CAAX) was sufficient (in combination with forskolin) to mediate synergistic activation of the α subunit. JEG3 cells were cotransfected with the α subunit reporter and a control plasmid or the Raf-CAAX expression vector. The transfected cells were cultured overnight and then stimulated with control solution or forskolin for a 6-h period. Luciferase activity was then determined. The addition of expression vector for Raf-CAAX did not potentiate the effects of forskolin on the α subunit reporter (Fig. 6A). In a control study, the same dose of Raf-CAAX expression vector cotransfected with the Gal4-Elk1 expression vector and the Gal4-dependent luciferase reporter was sufficient to induce robust activation of the Elk1 fusion protein, demonstrating the efficacy of the Raf-CAAX expression vector (Fig. 6B). These studies provide evidence that ERK activation alone is not sufficient to mediate synergistic activation of the α subunit.
FIG. 6.
ERK activation by overexpression of Raf-CAAX is not sufficient to mediate synergistic activation of the α subunit. (A) JEG3 cells were cotransfected by electroporation with an α subunit reporter (hα-880-luc; 500 ng) and 2.5 μg of control plasmid (pcDNA3) or expression vector for Raf-CAAX, a constitutively active form of Raf kinase. Eighteen hours later, control solution (open bars) or forskolin (solid bars) was administered for a 6-h period as described in the legend to Fig. 1, and luciferase activity was determined. (B) JEG3 cells were cotransfected by electroporation with a luciferase reporter (1 μg) containing five Gal4 binding sites coupled to the E1B minimal promoter and the luciferase coding sequence with an expression vector (1 μg) encoding a Gal4 DNA-binding domain-Elk1 transactivation domain fusion. Some cells were also transfected with control plasmid (pcDNA3; 2.5 μg) or Raf-CAAX (2.5 μg). Twenty-four hours later, cells were lysed, and luciferase activity was determined. Luciferase activity is reported as relative activity ± standard error of the mean from a single representative experiment (n = 3 independent electroporations per treatment). All transfection studies were conducted on at least three separate occasions (in triplicate) with similar results.
EGF induces the p38 MAPK and JNK pathways in JEG3 cells.
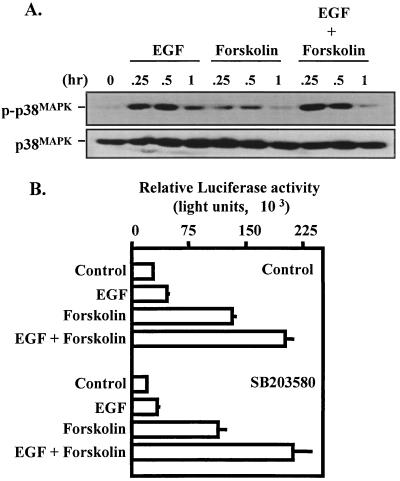
For many tyrosine kinase and serpentine receptors, ligand-mediated activation of multiple mitogen-activated protein kinase (MAPK) cascades appears to be the rule rather than the exception (16, 29, 30, 69). We investigated the possibility that EGF receptor activation resulted in activation of multiple MAPK pathways in placental cells. EGF administration resulted in transient activation of the p38 MAPK (Fig. 7A) and JNK (Fig. 8A) pathways. The activity of both enzymes peaked at 30 min and was reduced greatly by 1 h following hormone administration. The combined effects of EGF and forskolin did not appear to potentiate activation of either the p38 MAPK or JNK signaling pathway (Fig. 7A and 8A). Specific disruption of the p38 MAPK cascade is possible with the pyridinyl imidazole SB203580 (17). We have recently demonstrated that administration of SB203580 at the doses used in the present experiment was sufficient to attenuate gonadotropin-releasing hormone/p38 MAPK-induced c-fos reporter activity in αT3-1 cells (68). In JEG3 cells, pretreatment with 20 μM SB203580 did not alter the effects of EGF, forskolin, or the combined action of EGF and forskolin on the α subunit reporter (Fig. 7B). Thus, despite the fact that EGF receptor activation results in a marked increase in p38 MAPK activity in JEG3 cells, these studies do not provide any evidence that p38 MAPK is required for synergistic activation of the α subunit by EGF and forskolin.
FIG. 7.
Influence of EGF and forskolin on activation of the p38 MAPK cascade. (A) JEG3 cells were plated at approximately 60% confluence and serum starved for 2 h. Cells were administered control solution, EGF, forskolin, or EGF and forskolin for 0, 0.25, 0.5, or 1 h. Whole-cell lysates were prepared and resolved by SDS-PAGE. Western blot analysis was conducted with a phosphorylation-specific p38 antibody (p-p38MAPK). The blot was then stripped and reprobed with a p38 antibody (p38MAPK) to determine equivalent loading in each lane. (B) In separate studies, JEG3 cells were transfected by electroporation with an α subunit reporter (hα-880-luc; 500 ng) and cultured for 18 h. Fifteen minutes prior to agonist administration, some cells received control solution (DMSO) or SB203580 (20 μM). Agonists were administered for a 6-h period as described in the legend to Fig. 1, and luciferase activity was assayed. Luciferase activity is reported as relative activity ± standard error of the mean from a single representative experiment (n = 3 independent electroporations per treatment). All transfection studies were conducted on at least three separate occasions (in triplicate) with similar results.
FIG. 8.
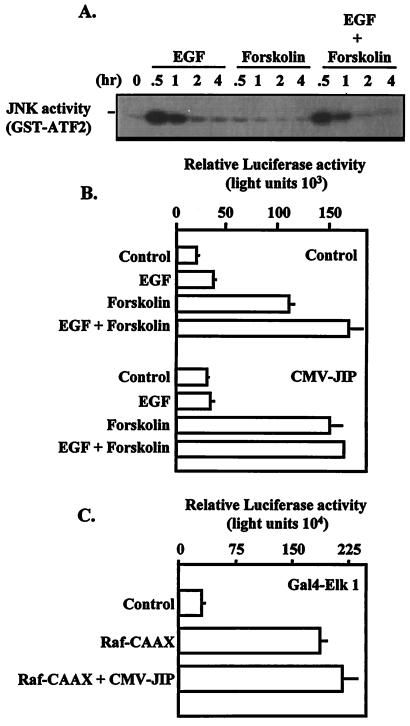
JNK cascade required for synergistic activation of the α subunit. (A) JEG3 cells were plated at approximately 60% confluence and serum starved for 2 h. Cells were administered control solution, EGF, forskolin, or EGF and forskolin for 0, 0.5, 1, 2, or 4 h. Whole-cell lysates were prepared, and equal amounts of lysate were subjected to IP with a JNK antibody and protein A-agarose. JNK activity was determined by an in vitro kinase assay with GST-ATF2 as a substrate. The kinase reaction products were resolved by SDS-PAGE and visualized by autoradiography. (B) JEG3 cells were cotransfected by electroporation with an α subunit reporter (hα-880-luc; 500 ng) and 10 μg of control plasmid (pcDNA3) or expression vector for JIP (CMV-JIP). Eighteen hours later, agonists were administered for a 6-h period as described in the legend to Fig. 1, and luciferase activity was assayed. (C) JEG3 cells were cotransfected by electroporation with a luciferase reporter (1 μg) containing five Gal4 binding sites coupled to the E1B minimal promoter and the luciferase coding sequence with an expression vector (1 μg) encoding a Gal4 DNA-binding domain-Elk1 transactivation domain fusion. Some cells were also transfected with control plasmid (pcDNA3; 12.5 μg), a constitutively active form of Raf kinase (Raf-CAAX; 2.5 μg) plus pcDNA3 (10 μg) or the combination of Raf-CAAX (2.5 μg) and an expression vector for JIP-1 (CMV-JIP; 10 μg). Twenty-four hours later, cells were lysed, and luciferase activity was determined. Luciferase activity is reported as relative activity ± standard error of the mean from a single representative experiment (n = 3 independent electroporations per treatment). All transfection studies were conducted on at least three separate occasions (in triplicate) with similar results.
Specific pharmacological inhibition of the JNK signaling cascade is problematic due to the lack of reagents that specifically inhibit this enzyme. Recently, JIP-1, a cytoplasmic inhibitor of the JNK pathway, was cloned and characterized (24, 43). We used overexpression of this putative dominant negative expression vector to disrupt JNK signaling to determine a potential role for this signaling cascade in the synergistic activation of the α subunit reporter. Transfection of the JIP-1 expression plasmid in JEG3 cells resulted in an 85% increase in basal α subunit reporter activity with a minimal effect on induction of the reporter by forskolin (forskolin induced a 5.4-fold versus 4.7-fold induction for control versus JIP, respectively) (Fig. 8B). Furthermore, overexpression of the JIP-1 expression vector attenuated the synergistic effects of EGF and forskolin on the α subunit reporter (EGF combined with forskolin induced an 8.1-fold increase for controls versus 5.0-fold induction for JIP-overexpressing cells) (Fig. 8B). Statistical analysis of these data revealed no significant treatment interaction. Similar results were observed at lower doses of JIP expression vector (data not shown), suggesting that the dose used induced maximal inhibition. The effects of the JIP-1 expression vector were specific, since this putative dominant negative expression vector did not interfere with Raf kinase-induced activation of the Gal4-Elk1 fusion protein (Fig. 9C). Additional studies confirmed that JIP overexpression did not interfere with EGF-mediated induction of ERK activation examined by immunoblot analysis with the phosphorylation-specific ERK antibody (data not shown). These data implicate a potential role for EGF-induced JNK activation in the synergistic activation of the α subunit.
FIG. 9.
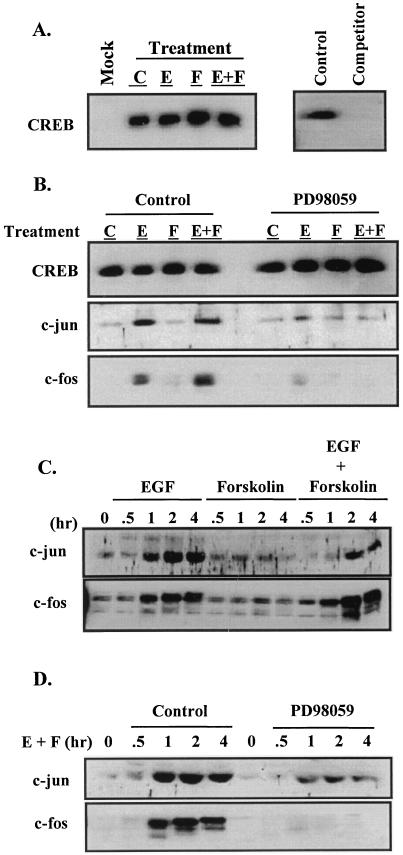
EGF treatment recruits AP-1 to the CRE binding complex. JEG3 cells were plated at approximately 60% confluence and cultured overnight. The cells were then serum starved for 2 h and received control solution (0.1% DMSO; lane C), EGF (50 ng/ml; lane E), forskolin (1 μM; lane F), or the combination of agonists (lane E+F) for a 2-h treatment period. Some cells received PD98059 (50 μM) for 15 min prior to administration of agonists. Nuclei were isolated, and nuclear extracts were prepared. Nuclear extract (200 μg) was mixed in a binding reaction with biotinylated CRE oligonucleotides coupled to SA beads in a CRE pull-down experiment (see Materials and Methods). CRE binding complexes were washed, subjected to SDS-PAGE, and probed by Western blot analysis. (A) Control studies initially conducted for analysis of CREB within the CRE binding complex. In some binding reactions, SA beads were used in the absence of biotinylated CRE (lane Mock). Some binding reactions were conducted in the absence (lane control) or presence of a 50-fold molar excess of nonbiotinylated CRE oligonucleotide added to the reaction mixture as a competitor (lane Competitor). (B) Western analysis was initially conducted with CREB antiserum followed by c-Jun and then c-Fos antiserum; the blot was stripped between antibodies. (C) Whole-cell lysates from JEG3 cells treated with various agonists over the time course denoted were examined for expression of Jun and Fos oncoproteins by Western blot analysis. (D) JEG3 cells were treated with control solution (DMSO) or PD98059 (50 μM) for 15 min prior to administration of agonists. Whole-cell lysates were then subjected to Western blot analysis for c-Jun and c-Fos. All of these studies were repeated at least three times with independently prepared nuclear extracts or whole-cell lysates with essentially identical results.
Binding complex associated with the α subunit dual CREs is altered by EGF and forskolin administration.
We examined the hypothesis that the CRE binding complex contained factors that were induced by or would facilitate the response to signaling pathways induced by EGF. We sought to examine the components of the CRE binding complex using a biotinylated CRE “pull-down” method followed by immunoblot analysis (Fig. 9). Biotinylated dual tandem CRE oligonucleotides were bound to SA beads and mixed with nuclear extracts derived from JEG3 cells following a 2-h administration of control solution, EGF, forskolin, or the combination of EGF and forskolin. The CRE binding complexes were resolved by SDS-PAGE and subjected to Western blot analysis. Initial control studies revealed that CREB was present in a JEG3 cell nuclear extract regardless of agonist treatment (Fig. 9A). Furthermore, nuclear extract from cells treated with EGF and forskolin and subjected to the pull-down assay in the absence of a biotinylated CRE failed to pull down CREB in the binding complex (Fig. 9A, Mock). Binding reactions conducted in the presence of a 50-fold molar excess of unbiotinylated CRE as a competitor showed that CRE was effective in competing for CREB binding, suggesting that the binding activity was specific (Fig. 9A). In the absence of agonists and after treatment with forskolin alone, the dual tandem CRE binding complex was predominantly composed of CREB (Fig. 9B). EGF stimulation of JEG3 cells resulted in the recruitment of c-Jun to the CRE binding complex. The presence of c-Jun in the CRE binding complex has been found in previous studies (33). Surprisingly, c-Fos was also recruited to the CRE binding complex, and the combined effects of EGF and forskolin-stimulated signaling resulted in an approximately twofold increase in c-Fos present in the CRE complex. In addition to c-Jun and c-Fos, we also examined whether other members of the Jun/Fos family of transcription factors were present in the CRE complex following EGF and/or forskolin stimulation. Western blotting analysis in the pull-down assay for FosB, JunB, or JunD revealed that none of these basic leucine zipper proteins were present in the α subunit CRE binding complex (data not shown). Additional competition studies revealed that a 50-fold molar excess of unbiotinylated CRE was effective at competing for c-Jun and c-Fos binding (data not shown). These studies provide support for the specificity of the CRE pull-down studies and clear evidence that the composition of the CRE binding complex was altered during agonist stimulation of JEG3 cells.
In the presence of EGF and/or EGF and forskolin stimulation, the CRE binding complex consisted minimally of CREB and AP-1 heterodimers. To determine whether EGF-induced ERK activation was required for the recruitment of AP-1 to the CRE binding complex, identical nuclear extracts were prepared following treatment with PD98059. Blockade of the ERK pathway with PD98059 resulted in a failure to recruit AP-1 to the CRE binding complex as accessed by the CRE pull-down assay (Fig. 9B). We then examined a more extensive time course for c-Jun and c-Fos activation following treatment with EGF, forskolin, and the combination of agonists (Fig. 9C). EGF administration was clearly the primary stimulus for c-Jun and c-Fos oncoprotein accumulation in JEG3 cells. Consistent with the CRE pull-down studies, the combined effects of EGF and forskolin induced elevated levels of c-Fos but not c-Jun. Pretreating the cells with PD98059 greatly reduced the induction of both oncoproteins by the combined actions of EGF and forskolin (Fig. 9D). These studies support the conclusion that AP-1 is recruited to the CRE binding complex and that EGF induction of the ERK pathway is required for upregulation of c-Jun and c-Fos oncoproteins.
Dual tandem CREs are capable of binding AP-1, and AP-1 binding is required for synergistic activation of the α subunit promoter.
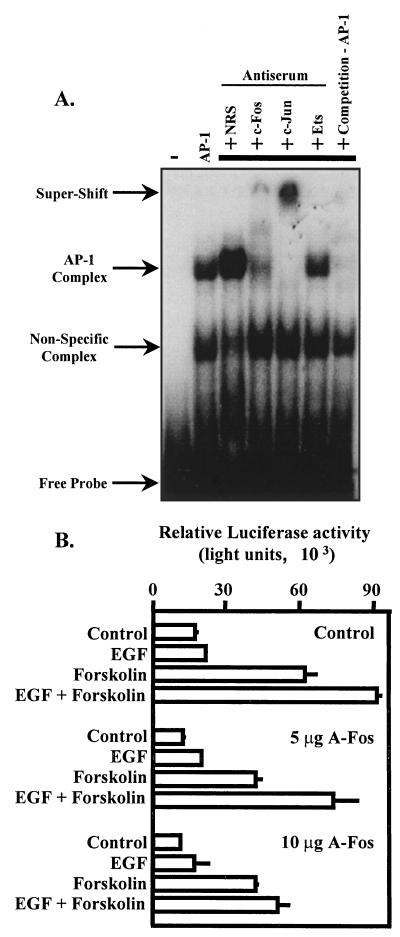
The CRE pull-down studies could not specifically resolve the possibility that the AP-1 present in the CRE binding complex was a result of direct binding of AP-1 to the dual CREs or that AP-1 in the CRE binding complex resulted from interaction of AP-1 with potential coactivators independent of DNA binding. To resolve this issue, c-Fos and c-Jun were prepared using a coupled transcription and translation reaction in reticulocyte lysates. c-Fos and c-Jun were mixed and incubated at 37°C to allow heterodimer formation. AP-1 binding activity was used in an EMSA with radiolabeled dual tandem CREs as a probe. Specific DNA-protein interactions were visualized by autoradiography (Fig. 10A). Use of extracts from the reticulocyte lysate alone (expressing a control luciferase plasmid) in the EMSA reactions resulted in the formation of a nonspecific complex. Addition of AP-1 binding activity to the EMSA resulted in the formation of a specific DNA-protein complex (AP-1 complex). AP-1 binding to the dual CREs was enhanced by the addition of normal rabbit serum. In contrast, the AP-1–CRE binding complex was attenuated with the addition of an antibody to c-Fos. Furthermore, addition of a c-Jun antibody resulted in a pronounced “supershift” of the CRE complex. Addition of an antibody for the DNA-binding domain of Ets1 and -2 did not interfere with the AP-1–CRE complex, providing evidence for the specificity of the antibodies. Addition of an unlabeled, consensus AP-1 oligonucleotide (at 50-fold molar excess) greatly reduced CRE binding to AP-1 (competition −AP-1). Taken together, these DNA-binding studies support the hypothesis that c-Jun–c-Fos heterodimers can interact directly with the dual tandem CREs of the α subunit promoter.
FIG. 10.
Dual tandem CREs are capable of binding AP-1, and AP-1 binding is required for synergistic activation of the α subunit promoter. The oncoproteins c-Fos and c-Jun were synthesized in a coupled transcription and translation reaction with reticulocyte lysates. AP-1 binding activity was then used in EMSAs. (A) The dual CRE probe was radiolabeled with polynucleotide kinase (free probe). An apparent nonspecific binding activity was present in reticulocyte lysates expressing a control luciferase plasmid and is indicated by an arrow. In some binding reactions, 2 μg of normal rabbit serum (NRS), c-Fos, c-Jun, or Ets antiserum was added to perturb potential DNA-protein complexes. This is indicated in part by an arrow labeled Supershift. Unlabeled consensus AP-1 oligonucleotides (3 pmol) were added to some binding reaction mixtures at an approximately 50-fold molar excess (competition—AP-1) to disrupt CRE–AP-1 interactions. −, binding reaction without reticulocyte lysate. (B) JEG3 cells were cotransfected by electroporation with an α subunit reporter (hα-880-luc; 500 ng) and 10 μg of control plasmid (CMV/500) or 5 or 10 μg of expression vector for A-Fos. Eighteen hours later, agonists were administered for a 6-h period as described in the legend to Fig. 1, and luciferase activity was assayed. Luciferase activity is reported as relative activity ± standard error of the mean from a single representative experiment (n = 3 independent electroporations per treatment). All transfection studies were conducted on at least three separate occasions (in triplicate) with similar results.
To assess the role of elevated c-Fos and c-Jun–c-Fos heterodimers on synergistic activation of the α subunit gene, we overexpressed a putative dominant negative inhibitor to c-Fos (A-Fos) developed by David Ginty and Charles Vinson (1). A-Fos is a fusion of an acidic amphipathic peptide extension onto the amino terminus of the basic leucine zipper region of c-Fos that disrupts c-Jun–c-Fos DNA binding with remarkable specificity (1). In those studies, A-Fos did not alter the DNA binding of CREB homodimers. The aim of our studies was to putatively “knock down” c-Jun–c-Fos heterodimer binding to the α subunit CREs by overexpression of A-Fos. We cotransfected increasing doses of expression vector for A-Fos with the α subunit reporter and treated transfected cells with control solution, EGF, forskolin, or the combination of EGF and forskolin. Transfected cells overexpressing the parental vector (control) and treated with EGF and forskolin demonstrated synergistic activation of the α subunit reporter (Fig. 10B). Overexpression of the A-Fos expression vector resulted in a decrease (25 to 35%) in basal levels of α subunit expression and a dose-dependent decrease in synergistic activation of the α reporter. At the highest dose of A-Fos used, the effects of EGF and forskolin on the α subunit reporter were similar to those with forskolin administered alone. Overexpression of the A-Fos expression vector did not disrupt the effects of forskolin, suggesting that the effects of A-Fos overexpression were specific. Similar results were obtained with the hα-CRE-Prl-luciferase reporter (not shown). Consistent with the observations of others (1), our results suggest that A-Fos overexpression attenuated synergistic activation of the α subunit reporter, presumably by disrupting AP-1 binding of c-Jun–A-Fos rather than c-Jun–c-Fos complexes. These studies support the conclusion that direct participation of AP-1 in the CRE binding complex is essential for synergistic activation of the α subunit reporter by EGF and forskolin.
Two functional CREs are required for synergistic activation of the α subunit by EGF and forskolin.
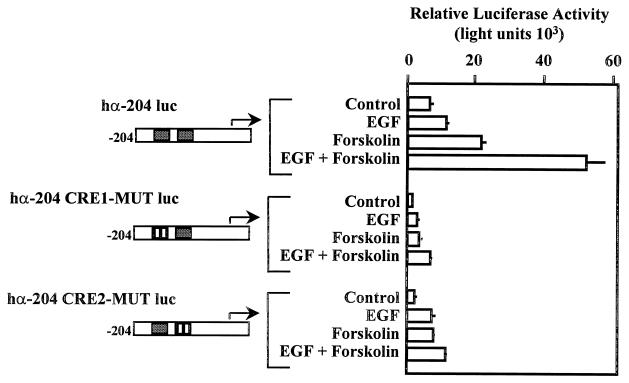
One potential hypothesis explaining the synergistic activation of the α subunit by EGF and forskolin is that CREB dimers bind to one CRE while AP-1 binds to the other CRE. This hypothesis suggests a requirement for two functional CREs within the α subunit promoter to facilitate responses to EGF and forskolin. In order to examine this possibility, we tested whether two functional CREs were required for synergistic activation of the α subunit promoter. Individual CREs were replaced with NotI restriction sites by site-directed mutagenesis. Mutant α subunit promoter fragments (in the context of the human α luciferase −204 to +42 promoter fragment) were cloned into a luciferase reporter and transfected into JEG3 cells (Fig. 11). Transfected cells were administered EGF, forskolin, and the combination of EGF and forskolin. The results of these studies provide evidence that two functional CREs were required for synergistic activation of the α subunit by EGF and forskolin. A significant treatment interaction (P = 0.0038) of the main effects of EGF and forskolin treatment was observed for the wild-type α subunit promoter. This statistical interaction was not detected with either of the CRE mutations. Basal expression of individual CRE mutant reporters and response to forskolin (fold induction) were reduced 45 to 58% and 28 to 38%, respectively, compared with the results with the wild-type α subunit. The results of these studies provide evidence that two functional CREs within the α subunit promoter were required for maintenance of basal levels of expression, response to forskolin, and synergistic activation by EGF and forskolin.
FIG. 11.
Two functional CREs are required for synergistic activation of the α subunit by EGF and forskolin. JEG3 cells were transfected by electroporation with 500 ng of an α subunit reporter containing hα-204 luc, hα-204 luc with the 5′ CRE mutated (hα-204 CRE1-MUT-luc), or hα-204 luc with the 3′ CRE mutated (hα-204 CRE2-MUT-luc). Agonists were administered for a 6-h period as described in the legend to Fig. 1, and luciferase activity was assayed. Luciferase activity is reported as relative activity ± standard error of the mean from a single representative experiment (n = 3 independent electroporations per treatment). All transfection studies were conducted on at least three separate occasions (in triplicate) with similar results.
DISCUSSION
Studies of corpus luteum function during early pregnancy in women reveal that one important determinant of adequate progesterone production is the rate and/or timing of the increase in CG secretion from cells of the trophoblast lineage (6, 7, 27, 42, 47). Thus, mechanisms that contribute to maximal synthesis of CG subunits are important determinants in the establishment of luteotropic support and maintenance of early pregnancy. In the present studies, we describe the novel synergistic activation of the glycoprotein hormone α subunit gene by interaction between two key intracellular signaling pathways, the MAPK and PKA cascades. We have used a choriocarcinoma cell model to examine the role of EGF and the adenylate cyclase activator forskolin in the regulation of the α subunit gene. Cells of the trophoblast lineage express the EGF receptor, and EGF is a critical growth peptide involved in the differentiation of trophoblasts and the regulation of CG subunit mRNA stability and CG secretion (8, 14, 18, 37, 55, 65). In the present studies, α subunit reporter gene activity was only modestly increased by EGF receptor activation. This is consistent with studies by Rao and others (14), demonstrating that EGF receptor activation increases the stability of α subunit mRNA in placental cells rather than altering the rate of transcription. In contrast, EGF receptor activation in rat placental cells (Rcho cells) results in two- to threefold induction of an α subunit reporter gene in a PKC/CRE/CREB-dependent mechanism (51). The differences between studies may reflect variation in the individual cell models. In JEG3 cells, EGF appears to play a more prominent role in α subunit gene activation when administered concurrently with forskolin, when the combined actions of the agonists induce synergistic activation of the α subunit gene. The effect(s) of EGF and forskolin on expression of the α subunit gene is closely coordinated with CG secretion, since cAMP-induced CG secretion is markedly potentiated by the addition of EGF (63–65).
As seen in the studies of others (31, 44, 45, 60), EGF receptor activation in JEG3 cells results in activation of all three MAPK pathways. Of the three MAPK pathways, the combined actions of EGF and forskolin potentiated the activation of the ERK cascade (increased magnitude of enzyme activity for a longer duration). Potentiated ERK activation was demonstrated by examining ERK phosphorylation (Fig. 4A), induction of a Gal4-Elk1 fusion protein (Fig. 4B), and the c-fos proto-oncogene (Fig. 9C), which requires Elk1 in the formation of a ternary complex at the c-fos serum response element (34, 49, 61, 73, 81, 82). Potentiated ERK activation induced by EGF and forskolin in JEG3 cells is reminiscent of the situation in some prostate cancer cell lines, in which the combined actions of EGF and inducers of cAMP such as forskolin result in similar ERK potentiation (15, 62). However, this is not the case in all cell types. Administration of forskolin or cAMP attenuates EGF-induced ERK signaling in hepatocytes (83), Rat-1 fibroblasts (84), and adenocarcinoma cell lines (46). Inhibition of EGF-induced ERK signaling by cAMP appears to occur upstream of ERK in the signaling pathway, likely through phosphorylation of the regulatory domain of Raf1, which reduces its affinity for Ras binding (84). The cellular mechanism(s) leading to potentiated ERK activation following combined stimulation with EGF and forskolin in JEG3 and other cell types is not presently clear.
In our studies, blockade of EGF-forskolin-induced ERK activation with a selective inhibitor (PD98059) reduced the magnitude and duration of ERK activity and was sufficient to block synergistic transcriptional activation of the α subunit gene. These studies provide clear evidence that ERK activation is absolutely required for synergistic transcriptional activation of the α subunit gene. It is reasonable to suggest that potentiated ERK activation induced by the combined actions of EGF and forskolin is required for regulation of the α subunit in trophoblast cells. Prolonged or potentiated ERK activation in some cell types leads to expression of a differentiated phenotype, such as in neurite outgrowth in PC12 cells (50, 80). Furthermore, prolonged ERK phosphorylation results in increased nuclear retention of activated ERK (41, 70). Thus, it is plausible that potentiated activation of the ERK cascade in JEG3 cells leads to prolonged nuclear retention of activated ERK, which leads to maximal expression of the α subunit gene, one marker of trophoblast differentiation.
EGF induced both the JNK and p38 MAPK cascades in JEG3 cells. Activation of additional signaling pathways is likely critical to synergistic activation of the α subunit, since direct activation of the ERK cascade by Raf-CAAX overexpression was not sufficient (in combination with forskolin) to induce transcriptional synergy. A role for the JNK cascade was demonstrated by JIP-1 overexpression. JIP is a putative intracellular anchoring protein that, when overexpressed, specifically retains JNK in the cytoplasmic compartment, thus restricting JNK-dependent gene activation (24). These studies support the conclusion that the EGF-induced JNK cascade leading to c-Jun activation is presumably required for synergistic activation of the α subunit. JNK is the only known kinase that is capable of c-Jun association, phosphorylation, and transcriptional activation (20, 39, 53, 54). A caveat to interpreting these studies with putative dominant negative signaling molecules is that overexpression likely influences or shifts the stoichiometry of the endogenous pathways, possibly leading to altered function of other potential effector molecules. In defense of these studies, overexpression of JIP-1 did not interfere with Raf kinase-induced Gal4-Elk1 transcriptional activation, which, within the context of our control studies, was ERK dependent. Furthermore, JIP-1 overexpression did not interfere with EGF-induced ERK phosphorylation or forskolin-induced signaling to the α subunit, providing additional evidence for the specific action of the JIP-1 expression vector. In contrast to the JNK cascade, pharmacological blockade of the p38 MAPK pathway by administration of SB203580 at a maximal dose (17, 68) did not alter α subunit gene activation. Thus, EGF-induced p38 MAPK likely is not required, while the JNK cascade appears to contribute to synergistic activation of the α subunit gene in placental cells. It is reasonable to hypothesize that both combinatorial MAPK activation (ERK and JNK activation) and relative changes in the magnitude and duration of ERK signaling contribute to α subunit gene expression in placental cells.
The CRE from the α subunit has been shown previously to bind CREB and c-Jun by EMSA (25, 33, 57). Our studies extend these findings in several ways. First, our studies confirm the presence of CREB and c-Jun in the CRE binding complex in an immunoblot analysis to provide evidence for the specificity of antibodies, which is not possible in an EMSA. Second, our studies identify c-Fos as a component of the α subunit CRE binding complex. c-Fos was not detected in CRE binding complexes when assayed by EMSA (33), probably because of blocked epitopes within the CRE complex. Furthermore, AP-1 binding activity prepared in vitro was capable of forming a complex with the α subunit CREs independent of other potential binding partners (Fig. 10A). Mutagenesis studies revealed that two functional CREs were required for basal, forskolin-, and EGF- and forskolin-induced activation of the α subunit gene. These studies suggest the possibility that concurrent CREB and AP-1 dimer binding to the α subunit promoter via dual CREs may be required for synergistic activation of the α subunit gene. Based upon our understanding of the growing complexity of the CRE binding complex, additional studies will be necessary to clearly resolve this compelling issue.
The importance of c-Jun–c-Fos within the CRE binding complex is supported by A-Fos overexpression studies. Putative knockdown of AP-1 binding by overexpression of A-Fos was effective at blocking synergistic activation of the α subunit (Fig. 11). This dominant interfering mutant has been shown previously to disrupt c-Jun–c-Fos heterodimer binding to DNA in a remarkably specific manner (1). Our interpretation of these collective studies is that the recruitment of AP-1 to the α subunit CREs requires ERK and JNK catalytic activity and is essential for synergistic activation of the α subunit by EGF and forskolin. Since CREB and c-Jun can both physically interact with CREB binding protein (CBP) (for a review, see reference 28), it is reasonable to predict that recruitment of CBP to the CRE binding complex may also be critical to ERK-JNK-dependent synergistic activation of the α subunit. Recent evidence suggests that transcriptional activation by subdomains of CBP can be regulated by nerve growth factor in an ERK-dependent manner (48), providing one potential mechanism for the actions of EGF-ERK and forskolin on the α subunit.
It is interesting to note that the effect(s) of EGF- and forskolin-induced signaling on the α subunit promoter was cell type specific. Since the PKA and MAPK cascades are likely ubiquitously expressed, we speculated that the combined effect of EGF and forskolin would induce synergistic activation of the α subunit in a heterologous cell type. However, this was not the case. In several cell types, including GH3 and HeLa cells, activation of the α subunit by the combined agonists was not different from that by forskolin alone. This putative cell specificity may reflect several possibilities. From the cell-specific effects of EGF and cAMP on the ERK pathway described earlier, it may not be a surprise that the combined effects of these two agonists were not sufficient to induce synergistic activation of the α subunit in heterologous cell types. However, inhibition of EGF-induced ERK activation by cAMP was not necessarily a causal factor in these studies. Comparative analysis of signaling pathways downstream of the EGF receptor revealed marked cell-specific variation in MAPK signaling. For example, in GH3 and HeLa cells, EGF-induced ERK activation was markedly shorter in duration than that observed in JEG3 cells. In both heterologous cell lines, EGF-induced activation of the ERK pathway peaked at 15 min following hormone application and returned to baseline by 1 h (M. S. Roberson, unpublished observations). Furthermore, EGF-induced JNK activity was greatly reduced in GH3 cells compared with EGF action on the JNK cascade in JEG3 cells (not shown). This cell-specific response to EGF alone (independent of forskolin treatment) may account for the differences observed between JEG3 cells and other EGF-responsive cell types. These studies also cannot discount the possibility that other tissue-specific factors may be playing an important role in placenta-specific regulation of the α subunit gene by EGF and forskolin in JEG3 cells. Additional mutagenesis studies will be required to identify other sequences within the promoter that are required for synergistic regulation of the α subunit.
The present studies identify a novel mechanism for synergistic activation of the glycoprotein α subunit gene in placental cells. The dual tandem CREs appear to be sufficient to mediate the effects of concurrent EGF- and forskolin-induced cell signaling. The ERK and JNK cascades are required for this activation via an AP-1-dependent mechanism. While EGF receptor stimulation resulted in p38 MAPK activation, this pathway does not appear to contribute to synergistic activation of the α subunit gene in JEG3 cells. Based upon extensive contributions from John Nilson and his colleagues and other laboratories, there is clear evidence for the hypothesis that discrete combinations of transcriptional regulators are required for placenta-specific α subunit gene activation. Our studies add further complexity to cell-specific α subunit regulation by suggesting that combinatorial signal transduction pathways contribute to induction of the α subunit gene in cells of the trophoblast lineage. Furthermore, it may not be sufficient to merely identify the signaling molecules necessary within specific signaling cascades; it may also be necessary to examine issues of magnitude and duration of enzyme activation that may contribute to the specificity of a given response. The combined effects of the PKA and MAPK signaling cascades facilitate maximal levels of α subunit gene activation through integration of a higher-order, multifactor CRE binding complex that reflects a changing endocrine milieu.
ACKNOWLEDGMENTS
We thank Richard A. Maurer (Oregon Health Sciences University, Portland), Colin Clay (Colorado State University, Fort Collins), Roger Davis (University of Massachusetts, Worcester), Linda VanAelst (Cold Spring Harbor Laboratory, Cold Spring Harbor, N.Y.), David Ginty (Johns Hopkins University, Baltimore, Md.), Paul Dobner (University of Massachusetts, Worcester), Michael Greenberg (Harvard Medical School, Boston, Mass.), and Charles Vinson (National Cancer Institute, Bethesda, Md.) for generously sharing valuable reagents. Special thanks go to Jane Reusch (University of Colorado, Denver) for helpful discussions during the course of these studies. We thank Joanne Fortune (Cornell University, Ithaca, N.Y.) and Colin Clay (Colorado State University, Fort Collins) for helpful criticisms during the preparation of the manuscript.
This work was supported by a Consolidated Research Grant from the College of Veterinary Medicine, Cornell University.
REFERENCES
- 1.Ahn S, Olive M, Aggarwal S, Krylov D, Ginty D D, Vinson C. A dominant-negative inhibitor of CREB reveals that it is a general mediator of stimulus-dependent transcriptional of c-fos. Mol Cell Biol. 1998;18:967–977. doi: 10.1128/mcb.18.2.967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Albanese C, Kay T W, Troccoli N M, Jameson J L. Novel cyclic adenosine 3′, 5′-monophosphate response element in the human chorionic gonadotropin beta-subunit gene. Mol Endocrinol. 1991;5:693–702. doi: 10.1210/mend-5-5-693. [DOI] [PubMed] [Google Scholar]
- 3.Amemiya K, Kurachi H, Adachi H, Morishige K I, Adachi K, Imai T, Miyake A. Involvement of epidermal growth factor (EGF)/EGF receptor autocrine and paracrine mechanism in human trophoblast cells: functional differentiation in vitro. J Endocrinol. 1994;143:291–301. doi: 10.1677/joe.0.1430291. [DOI] [PubMed] [Google Scholar]
- 4.Andersen B, Kennedy G C, Nilson J H. A cis-acting element located between the cAMP response elements and CCAAT box augments cell-specific expression of the glycoprotein hormone alpha subunit gene. J Biol Chem. 1990;265:21874–21880. [PubMed] [Google Scholar]
- 5.Andersen B, Milsted A, Kennedy G, Nilson J H. Cyclic AMP and phorbol esters interact synergistically to regulate expression of the chorionic gonadotropin genes. J Biol Chem. 1988;263:15578–15583. [PubMed] [Google Scholar]
- 6.Baird D D, Weinberg C R, Wilcox A J, McConnaughey D R, Musey P I, Collins D C. Hormonal profiles of natural conception cycles ending in early, unrecognized pregnancy loss. J Clin Endocrinol Metab. 1991;72:793–800. doi: 10.1210/jcem-72-4-793. [DOI] [PubMed] [Google Scholar]
- 7.Baird D D, Wilcox A J, Weinberg C R, Kamel F, McConnaughey D R, Musey P I, Collins D C. Preimplantation hormonal differences between the conception and nonconception menstrual cycles of 32 normal women. Hum Reprod. 1997;12:2607–2613. doi: 10.1093/humrep/12.12.2607. [DOI] [PubMed] [Google Scholar]
- 8.Barnea E R, Feldman D, Kaplan M, Morrish D W. The dual effect of epidermal growth factor upon human chorionic gonadotropin secretion by the first trimester placenta in vitro. J Clin Endocrinol Metab. 1990;71:923–928. doi: 10.1210/jcem-71-4-923. [DOI] [PubMed] [Google Scholar]
- 9.Barnhart K M, Mellon P L. The orphan nuclear receptor, steroidogenic factor-1, regulates the glycoprotein hormone alpha-subunit gene in pituitary gonadotropes. Mol Endocrinol. 1994;8:878–885. doi: 10.1210/mend.8.7.7527122. [DOI] [PubMed] [Google Scholar]
- 10.Bokar J A, Keri R A, Farmerie T A, Fenstermaker R A, Andersen B, Hamernik D L, Yun J, Wagner T, Nilson J H. Expression of the glycoprotein hormone alpha-subunit gene in the placenta requires a functional cyclic AMP response element, whereas a different cis-acting element mediates pituitary-specific expression. Mol Cell Biol. 1989;9:5113–5122. doi: 10.1128/mcb.9.11.5113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bokar J A, Roesler W J, Vandenbark G R, Kaetzel D M, Hanson R W, Nilson J H. Characterization of the cAMP responsive elements from the genes for the alpha-subunit of glycoprotein hormones and phosphoenolpyruvate carboxykinase (GTP). Conserved features of nuclear protein binding between tissues and species. J Biol Chem. 1988;263:19740–19747. [PubMed] [Google Scholar]
- 12.Budworth P R, Quinn P G, Nilson J H. Multiple characteristics of a pentameric regulatory array endow the human α-subunit glycoprotein hormone promoter with trophoblast specificity and maximal activity. Mol Endocrinol. 1997;11:1669–1680. doi: 10.1210/mend.11.11.0007. [DOI] [PubMed] [Google Scholar]
- 13.Burrin J M, Jameson J L. Regulation of transfected glycoprotein hormone alpha-gene expression in primary pituitary cell cultures. Mol Endocrinol. 1989;3:1643–1651. doi: 10.1210/mend-3-10-1643. [DOI] [PubMed] [Google Scholar]
- 14.Cao H, Lei Z M, Rao C V. Transcriptional and posttranscriptional mechanisms in epidermal growth factor regulation of human chorionic gonadotropin (hCG) subunits and hCG receptor gene expression in human choriocarcinoma cells. Endocrinology. 1994;135:962–970. doi: 10.1210/endo.135.3.8070393. [DOI] [PubMed] [Google Scholar]
- 15.Chen T, Cho R W, Stork P J, Weber M J. Elevation of cyclic adenosine 3′,5′-monophosphate potentiates activation of mitogen-activated protein kinase by growth factors in LNCaP prostate cancer cells. Cancer Res. 1999;59:213–218. [PubMed] [Google Scholar]
- 16.Cobb M H. MAP kinase pathways. Prog Biophys Mol Biol. 1999;71:479–500. doi: 10.1016/s0079-6107(98)00056-x. [DOI] [PubMed] [Google Scholar]
- 17.Cuenda A, Rouse J, Doza Y N, Meier R, Cohen P, Gallagher T F, Young P R, Lee J C. SB203580 is a specific inhibitor of the MAP kinase homologue which is stimulated by cellular stress and interleukin-1. FEBS Lett. 1995;362:229–233. doi: 10.1016/0014-5793(95)00357-f. [DOI] [PubMed] [Google Scholar]
- 18.Dakour J, Li H, Chen H, Morrish D W. EGF promotes development of a differentiated trophoblast phenotype having c-myc and junB proto-oncogene activation. Placenta. 1999;20:119–126. doi: 10.1053/plac.1998.0336. [DOI] [PubMed] [Google Scholar]
- 19.Delegeane A M, Ferland L H, Mellon P L. Tissue-specific enhancer of the human glycoprotein hormone alpha-subunit gene: dependence on cyclic AMP-inducible elements. Mol Cell Biol. 1987;7:3994–4002. doi: 10.1128/mcb.7.11.3994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Derijard B, Hibi M, Wu I H, Barrett T, Su B, Deng T, Karin M, Davis R J. JNK1: a protein kinase stimulated by UV light and Ha-Ras that binds and phosphorylates the c-Jun activation domain. Cell. 1994;76:1025–1037. doi: 10.1016/0092-8674(94)90380-8. [DOI] [PubMed] [Google Scholar]
- 21.Deutsch P J, Hoeffler J P, Jameson J L, Habener J F. Cyclic AMP and phorbol ester-stimulated transcription mediated by similar DNA elements that bind distinct proteins. Proc Natl Acad Sci USA. 1988;85:7922–7926. doi: 10.1073/pnas.85.21.7922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Deutsch P J, Jameson J L, Habener J F. Cyclic AMP responsiveness of human gonadotropin-alpha gene transcription is directed by a repeated 18-base pair enhancer. Alpha-promoter receptivity to the enhancer confers cell-preferential expression. J Biol Chem. 1987;262:12169–12174. [PubMed] [Google Scholar]
- 23.deWet J R, Wood K V, DeLuca M, Helinski D R, Subramani S. Firefly luciferase gene: structure and expression in mammalian cells. Mol Cell Biol. 1987;7:725–737. doi: 10.1128/mcb.7.2.725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dickens M, Rogers J S, Cavanagh J, Raitano A, Xia Z, Halpern J R, Greenberg M E, Sawyers C L, Davis R J. A cytoplasmic inhibitor of the JNK signal transduction pathway. Science. 1997;277:693–696. doi: 10.1126/science.277.5326.693. [DOI] [PubMed] [Google Scholar]
- 25.Drust D S, Troccoli N M, Jameson J L. Binding specificity of cyclic adenosine 3′,5′-monophosphate-responsive element (CRE)-binding proteins and activating transcription factors to naturally occurring CRE sequence variants. Mol Endocrinol. 1991;5:1541–1551. doi: 10.1210/mend-5-10-1541. [DOI] [PubMed] [Google Scholar]
- 26.Fenstermaker R A, Farmerie T A, Clay C M, Hamernik D L, Nilson J H. Different combinations of regulatory elements may account for expression of the glycoprotein hormone alpha-subunit gene in primate and horse placenta. Mol Endocrinol. 1990;4:1480–1487. doi: 10.1210/mend-4-10-1480. [DOI] [PubMed] [Google Scholar]
- 27.France J T, Keelan J, Song L, Liddell H, Zanderigo A, Knox B. Serum concentrations of human chorionic gonadotrophin and immunoreactive inhibin in early pregnancy and recurrent miscarriage: a longitudinal study. Aust NZ J Obstet Gynaecol. 1996;36:325–330. doi: 10.1111/j.1479-828x.1996.tb02722.x. [DOI] [PubMed] [Google Scholar]
- 28.Goldman P S, Tran V K, Goodman R H. The multifunctional role of the co-activator CBP in transcriptional regulation. Recent Prog Horm Res. 1997;52:103–119. [PubMed] [Google Scholar]
- 29.Gutkind J S. Cell growth control by G protein-coupled receptors: from signal transduction to signal integration. Oncogene. 1998;17:1331–1342. doi: 10.1038/sj.onc.1202186. [DOI] [PubMed] [Google Scholar]
- 30.Gutkind J S. The pathways connecting G protein-coupled receptors to the nucleus through divergent mitogen-activated protein kinase cascades. J Biol Chem. 1998;273:1839–1842. doi: 10.1074/jbc.273.4.1839. [DOI] [PubMed] [Google Scholar]
- 31.Hashimoto A, Kurosaki M, Gotoh N, Shibuyi M, Kurosaki T. Shc regulates epidermal growth factor-induced activation of the JNK signaling pathway. J Biol Chem. 1999;274:20139–20143. doi: 10.1074/jbc.274.29.20139. [DOI] [PubMed] [Google Scholar]
- 32.Heckert L L, Schultz K, Nilson J H. Different composite regulatory elements direct expression of the human α subunit gene to pituitary and placenta. J Biol Chem. 1995;270:26497–26504. doi: 10.1074/jbc.270.44.26497. [DOI] [PubMed] [Google Scholar]
- 33.Heckert L L, Schultz K, Nilson J H. The cAMP response elements of the α subunit gene bind similar proteins in trophoblasts and gonadotropes but have distinct functional sequence requirements. J Biol Chem. 1996;271:31650–31656. doi: 10.1074/jbc.271.49.31650. [DOI] [PubMed] [Google Scholar]
- 34.Hill C S, Treisman R. Transcriptional regulation by extracellular signals: mechanisms and specificity. Cell. 1995;80:199–211. doi: 10.1016/0092-8674(95)90403-4. [DOI] [PubMed] [Google Scholar]
- 35.Hoeffler J P, Meyer T E, Yun Y, Jameson J L, Habener J F. Cyclic AMP-responsive DNA-binding protein: structure based on a cloned placental cDNA. Science. 1988;242:1430–1433. doi: 10.1126/science.2974179. [DOI] [PubMed] [Google Scholar]
- 36.Horn F, Windle J J, Barnhart K M, Mellon P L. Tissue-specific gene expression in the pituitary: the glycoprotein hormone alpha-subunit gene is regulated by a gonadotrope-specific protein. Mol Cell Biol. 1992;12:2143–2153. doi: 10.1128/mcb.12.5.2143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ilekis J, Benveniste R. Effects of epidermal growth factor, phorbol myristate acetate, and arachidonic acid on choriogonadotropin secretion by cultured human choriocarcinoma cells. Endocrinology. 1985;116:2400–2409. doi: 10.1210/endo-116-6-2400. [DOI] [PubMed] [Google Scholar]
- 38.Jameson J L, Albanese C, Habener J F. Distinct adjacent protein-binding domains in the glycoprotein hormone alpha gene interact independently with a cAMP-responsive enhancer. J Biol Chem. 1989;264:16190–16196. [PubMed] [Google Scholar]
- 39.Karin M. The regulation of AP-1 activity by mitogen-activated protein kinases. J Biol Chem. 1995;270:16483–16486. doi: 10.1074/jbc.270.28.16483. [DOI] [PubMed] [Google Scholar]
- 40.Kennedy G C, Andersen B, Nilson J H. The human alpha subunit glycoprotein hormone gene utilizes a unique CCAAT binding factor. J Biol Chem. 1990;265:6279–6285. [PubMed] [Google Scholar]
- 41.Khokhlatchev A V, Canagarajah B, Wilsbacher J, Robinson M, Atkinson M, Goldsmith E, Cobb M H. Phosphorylation of the MAP kinase ERK2 promotes its homodimerization and nuclear translocation. Cell. 1998;93:605–615. doi: 10.1016/s0092-8674(00)81189-7. [DOI] [PubMed] [Google Scholar]
- 42.Kratzer P G, Taylor R N. Corpus luteum function in early pregnancies is primarily determined by the rate of change of human chorionic gonadotropin levels. Am J Obstet Gynecol. 1990;163:1497–1502. doi: 10.1016/0002-9378(90)90613-c. [DOI] [PubMed] [Google Scholar]
- 43.Kumahara E, Ebihara T, Steffen D. Nerve growth factor induces zif268 gene expression via MAPK-dependent and independent pathways in PC12D cells. J Biochem (Tokyo) 1999;125:541–553. doi: 10.1093/oxfordjournals.jbchem.a022319. [DOI] [PubMed] [Google Scholar]
- 44.Lange C A, Richer J K, Horwitz K B. Hypothesis: progesterone primes breast cancer cells for cross-talk with proliferative and antiproliferative signals. Mol Endocrinol. 1999;13:829–836. doi: 10.1210/mend.13.6.0290. [DOI] [PubMed] [Google Scholar]
- 45.Lange C A, Richer J K, Shen T, Horwitz K B. Convergence of progesterone and epidermal growth factor signaling in breast cancer. J Biol Chem. 1998;273:31308–31316. doi: 10.1074/jbc.273.47.31308. [DOI] [PubMed] [Google Scholar]
- 46.Leiberman M D, Paty P, Li X K, Naama H, Evoy D, Daly J M. Elevation of intracellular cyclic adenosine monophosphate inhibits the epidermal growth factor signal transduction pathway and cellular growth in pancreatic adenocarcinoma cell lines. Surgery. 1996;120:354–359. doi: 10.1016/s0039-6060(96)80309-6. [DOI] [PubMed] [Google Scholar]
- 47.Liu H C, Pyrgiotis E, Davis O, Rosenwaks Z. Active corpus luteum function at pre-, peri- and postimplantation is essential for a viable pregnancy. Early Pregnancy. 1995;1:281–287. [PubMed] [Google Scholar]
- 48.Liu Y, Chrivia J C, Latchman D S. Nerve growth factor up-regulates the transcriptional activity of CBP through activation of the p42/p44MAPK cascade. J Biol Chem. 1998;273:32400–32407. doi: 10.1074/jbc.273.49.32400. [DOI] [PubMed] [Google Scholar]
- 49.Marais R, Wynne J, Treisman R. The SRF accessory protein Elk-1 contains a growth factor-regulated transcriptional activation domain. Cell. 1993;73:381–393. doi: 10.1016/0092-8674(93)90237-k. [DOI] [PubMed] [Google Scholar]
- 50.Marshall C J. Specificity of receptor tyrosine kinase signaling: transient versus sustained extracellular signal-regulated kinase activation. Cell. 1995;80:179–185. doi: 10.1016/0092-8674(95)90401-8. [DOI] [PubMed] [Google Scholar]
- 51.Matsumoto K, Yamamoto T, Kurachi H, Nishio Y, Takeda T, Homma H, Morishige K I, Miyake A, Murata Y. Human chorionic gonadotropin-α gene is transcriptionally activated by epidermal growth factor through cAMP response element in trophoblast cells. J Biol Chem. 1998;273:7800–7806. doi: 10.1074/jbc.273.14.7800. [DOI] [PubMed] [Google Scholar]
- 52.Milsted A, Cox R P, Nilson J H. Cyclic AMP regulates transcription of the genes encoding human chorionic gonadotropin with different kinetics. DNA. 1987;6:213–219. doi: 10.1089/dna.1987.6.213. [DOI] [PubMed] [Google Scholar]
- 53.Minden A, Lin A, Claret F X, Abo A, Karin M. Selective activation of the JNK signaling cascade and c-Jun transcriptional activity by the small GTPases Rac and Cdc42Hs. Cell. 1995;81:1147–1157. doi: 10.1016/s0092-8674(05)80019-4. [DOI] [PubMed] [Google Scholar]
- 54.Minden A, Lin A, Smeal T, Derijard B, Cobb M, Davis R, Karin M. c-Jun N-terminal phosphorylation correlates with activation of the JNK subgroup but not the ERK subgroup of mitogen-activated protein kinases. Mol Cell Biol. 1994;14:6683–6688. doi: 10.1128/mcb.14.10.6683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Morrish D W, Bhardwaj D, Dabbagh L K, Marusyk H, Siy O. Epidermal growth factor induces differentiation and secretion of human chorionic gonadotropin and placental lactogen in normal human placenta. J Clin Endocrinol Metab. 1987;65:1282–1290. doi: 10.1210/jcem-65-6-1282. [DOI] [PubMed] [Google Scholar]
- 56.Nilson J H, Bokar J A, Clay C M, Farmerie T A, Fenstermaker R A, Hamernik D L, Keri R A. Different combinations of regulatory elements may explain why placenta-specific expression of the glycoprotein hormone alpha-subunit gene occurs only in primates and horses. Biol Reprod. 1991;44:231–237. doi: 10.1095/biolreprod44.2.231. [DOI] [PubMed] [Google Scholar]
- 57.Pestell R G, Hollenberg A N, Albanese C, Jameson J L. c-jun represses transcription of the human chorionic gonadotropin α and β genes through distinct types of CREs. J Biol Chem. 1994;269:31090–31096. [PubMed] [Google Scholar]
- 58.Pierce J G, Parsons T F. Glycoprotein hormones: structure and function. Annu Rev Biochem. 1981;50:465–495. doi: 10.1146/annurev.bi.50.070181.002341. [DOI] [PubMed] [Google Scholar]
- 59.Pittman R H, Clay C M, Farmerie T A, Nilson J H. Functional analysis of the placenta-specific enhancer of the human glycoprotein hormone alpha subunit gene. Emergence of a new element. J Biol Chem. 1994;269:19360–19368. [PubMed] [Google Scholar]
- 60.Pomerance M, Multon M C, Parker F, Venot C, Blondeau J P, Tocque B, Schweighoffer F. Grb2 interaction with MEK-kinase 1 is involved in regulation of jun-kinase activities in response to epidermal growth factor. J Biol Chem. 1998;273:24301–24304. doi: 10.1074/jbc.273.38.24301. [DOI] [PubMed] [Google Scholar]
- 61.Price M A, Rogers A E, Treisman R. Comparative analysis of the ternary complex factors Elk-1, SAP-1a and SAP-2 (ERP/NET) EMBO J. 1995;14:2589–2601. doi: 10.1002/j.1460-2075.1995.tb07257.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Putz T, Culig Z, Eder I E, Nessler-Menardi C, Bartsch G, Grunicke H, Uberall F, Klocker H. Epidermal growth factor (EGF) receptor blockade inhibits the action of EGF, insulin-like growth factor I, and a protein kinase A activator on the mitogen-activated protein kinase pathway in prostate cancer cell lines. Cancer Res. 1999;59:227–233. [PubMed] [Google Scholar]
- 63.Ritvos O, Butzow R, Jalkanen J, Stenman U H, Huhtaniemi I, Ranta T. Differential regulation of hCG and progesterone secretion by cholera toxin and phorbol ester in human cytotrophoblasts. Mol Cell Endocrinol. 1988;56:165–169. doi: 10.1016/0303-7207(88)90021-4. [DOI] [PubMed] [Google Scholar]
- 64.Ritvos O, Jalkanen J, Huhtaniemi I, Stenman U H, Alfthan H, Ranta T. 12-O-Tetradecanoyl phorbol-13-acetate potentiates adenosine 3′,5′-monophosphate-mediated chorionic gonadotropin secretion by culture human choriocarcinoma cells. Endocrinology. 1987;120:1521–1526. doi: 10.1210/endo-120-4-1521. [DOI] [PubMed] [Google Scholar]
- 65.Ritvos O, Jalkanen J, Pekonen F, Stenman U H, Ranta T. Epidermal growth factor but not insulin-like growth factor-I potentiates adenosine 3′,5′-monophosphate-mediated chorionic gonadotropin secretion by cultured human choriocarcinoma cells. Endocrinology. 1988;123:859–865. doi: 10.1210/endo-123-2-859. [DOI] [PubMed] [Google Scholar]
- 66.Roberson M S, Misra-Press A, Laurance M E, Stork P J, Maurer R A. A role for mitogen-activated protein kinase in mediating activation of the glycoprotein hormone alpha-subunit promoter by gonadotropin-releasing hormone. Mol Cell Biol. 1995;15:3531–3539. doi: 10.1128/mcb.15.7.3531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Roberson M S, Schoderbek W E, Tremml G, Maurer R A. Activation of the glycoprotein hormone alpha-subunit promoter by a LIM-homeodomain transcription factor. Mol Cell Biol. 1994;14:2985–2993. doi: 10.1128/mcb.14.5.2985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Roberson M S, Zhang T, Li H L, Mulvaney J M. Activation of p38 mitogen-activated protein kinase pathway by gonadotropin-releasing hormone. Endocrinology. 1999;140:1310–1318. doi: 10.1210/endo.140.3.6579. [DOI] [PubMed] [Google Scholar]
- 69.Robinson M J, Cobb M H. Mitogen-activated protein kinase pathways. Curr Opin Cell Biol. 1997;9:180–186. doi: 10.1016/s0955-0674(97)80061-0. [DOI] [PubMed] [Google Scholar]
- 70.Robinson M J, Stippec S A, Goldsmith E, White M A, Cobb M H. A constitutively active and nuclear form of the MAP kinase ERK2 is sufficient for neurite outgrowth and cell transformation. Curr Biol. 1998;8:1141–1150. doi: 10.1016/s0960-9822(07)00485-x. [DOI] [PubMed] [Google Scholar]
- 71.Schoderbek W E, Kim K E, Ridgway E C, Mellon P L, Maurer R A. Analysis of DNA sequences required for pituitary-specific expression of the glycoprotein hormone alpha-subunit gene. Mol Endocrinol. 1992;6:893–903. doi: 10.1210/mend.6.6.1379672. [DOI] [PubMed] [Google Scholar]
- 72.Schoderbek W E, Roberson M S, Maurer R A. Two different DNA elements mediate gonadotropin releasing hormone effects on expression of the glycoprotein hormone alpha-subunit gene. J Biol Chem. 1993;268:3903–3910. [PubMed] [Google Scholar]
- 73.Seternes O M, Sorensen R, Johansen B, Loennechen T, Aarbakke J, Moens U. Synergistic increase in c-fos expression by simultaneous activation of the ras/raf/map kinase- and protein kinase A signaling is mediated by the c-fos AP-1 and SRE sites. Biochim Biophys Acta. 1998;1395:345–360. doi: 10.1016/s0167-4781(97)00189-9. [DOI] [PubMed] [Google Scholar]
- 74.Silver B J, Bokar J A, Virgin J B, Vallen E A, Milsted A, Nilson J H. Cyclic AMP regulation of the human glycoprotein hormone alpha-subunit gene is mediated by an 18-base-pair element. Proc Natl Acad Sci USA. 1987;84:2198–2202. doi: 10.1073/pnas.84.8.2198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Steger D J, Hecht J H, Mellon P L. GATA-binding proteins regulate the human gonadotropin alpha-subunit gene in the placenta and pituitary gland. Mol Cell Biol. 1994;14:5592–5602. doi: 10.1128/mcb.14.8.5592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Stepan V M, Dickinson C J, del Valle J, Matsushima M, Todisco A. Cell-type specific requirement of the MAPK pathway for growth factor action of gastrin. Am J Physiol. 1999;276:G1363–G1372. doi: 10.1152/ajpgi.1999.276.6.G1363. [DOI] [PubMed] [Google Scholar]
- 77.Sun P, Enslen H, Myung P S, Maurer R A. Differential activation of CREB by Ca2+/calmodulin-dependent protein kinases type II and type IV involves phosphorylation of a site that negatively regulates activity. Genes Dev. 1994;8:2527–2539. doi: 10.1101/gad.8.21.2527. [DOI] [PubMed] [Google Scholar]
- 78.Sun P, Maurer R A. An inactivating point mutation demonstrates that interaction of cAMP response element binding protein (CREB) with the CREB binding protein is not sufficient for transcriptional activation. J Biol Chem. 1995;270:7041–7044. doi: 10.1074/jbc.270.13.7041. [DOI] [PubMed] [Google Scholar]
- 79.Sun P, Schoderbek W E, Maurer R A. Phosphorylation of cyclic adenosine 3′,5′-monophosphate (cAMP) response element-binding protein isoforms by the cAMP-dependent protein kinase. Mol Endocrinol. 1992;6:1858–1866. doi: 10.1210/mend.6.11.1480175. [DOI] [PubMed] [Google Scholar]
- 80.Traverse S, Gomez N, Paterson H, Marshall C, Cohen P. Sustained activation of the mitogen-activated protein (MAP) kinase cascade may be required for differentiation of PC12 cells. Comparison of the effects of nerve growth factor and epidermal growth factor. Biochem J. 1992;288:351–355. doi: 10.1042/bj2880351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Treisman R. The serum response element. Trends Biochem Sci. 1992;17:423–426. doi: 10.1016/0968-0004(92)90013-y. [DOI] [PubMed] [Google Scholar]
- 82.Treisman R. Ternary complex factors: growth factor regulated transcriptional activators. Curr Opin Genet Dev. 1994;4:96–101. doi: 10.1016/0959-437x(94)90097-3. [DOI] [PubMed] [Google Scholar]
- 83.Ulrich R G, Cramer C T, Adams L A, Kletzein R F. Activation and glucagon regulation of mitogen-activated protein kinases (MAPK) by insulin and epidermal growth factor in cultured rat and human hepatocytes. Cell Biochem Funct. 1998;16:77–85. doi: 10.1002/(SICI)1099-0844(199806)16:2<77::AID-CBF769>3.0.CO;2-U. [DOI] [PubMed] [Google Scholar]
- 84.Wu J, Dent P, Jelinek T, Wolfman A, Weber M J, Sturgill T W. Inhibition of the EGF-activated MAP kinase signaling pathway by adenosine 3′,5′-monophosphate. Science. 1993;262:1065–1069. doi: 10.1126/science.7694366. [DOI] [PubMed] [Google Scholar]
- 85.Yoshinaga N, Murayama T, Nomura Y. Death by a dopaminergic neurotoxin, 1-methyl-4-phenylpyridinium ion (MPP+) and protection by EGF in GH3 cells. Brain Res. 1998;794:137–142. doi: 10.1016/s0006-8993(98)00225-x. [DOI] [PubMed] [Google Scholar]