Abstract
Objective
To study the characteristics of point-by-point destruction of white matter tracts in patients using automated fiber tract quantification (AFQ).
Methods
Thirty-four classic trigeminal neuralgia (CTN) patients and 34 healthy control (HC) subjects underwent 3.0 T diffusion tensor magnetic resonance imaging and T1-weighted imaging. The fractional anisotropy (FA) and mean diffusivity (MD) of 100 nodes of 20 fiber tracts were analyzed by AFQ, and the correlations of the FA and MD with the visual analogue scale (VAS) pain score were assessed.
Results
The FA values of the left thalamic radiation (middle segment), left corticospinal tract, callosum forceps minor, and right uncinate fasciculus were significantly lower in CTN patients than in the HC group. The MD of the left thalamic tract (middle segment), left corticospinal tract, right superior longitudinal fasciculus, and left superior longitudinal fasciculus (anterior segment) were significantly higher in the CTN group. Additionally, the VAS pain score in CTN patients was positively correlated with FA and negatively correlated with MD.
Conclusion
Specific fiber tract nodes were damaged in CTN patients, which was related to the VAS pain score. Multi-node quantitative studies of fiber tract damage are valuable for understanding the white matter tract damage pattern in CTN patients.
Keywords: Classic trigeminal neuralgia, automated fiber tract quantification, fractional anisotropy, mean diffusivity, visual analog scale, magnetic resonance imaging
Introduction
Classic trigeminal neuralgia (CTN) is a typical type of neuropathic pain that refers to episodic discharges or pinprick pain limited to the trigeminal nerve distribution.1,2 The pain is significantly different from other neuropathic pain and is characterized by the absence of significant sensory deficits and the presence of intermittent pain. 3 The annual incidence of CTN is 4 to 5 per 100,000 people, and it is estimated that 1 in 15,000 to 20,000 people worldwide are affected by CTN. 4 CTN is mainly caused by neurovascular compression of the trigeminal nerve root in the brain region, and microvascular decompression surgery is the most effective method to relieve this type of neuralgia.5,6 However, the peripheral nerve injury caused by neurovascular compression does not fully explain the persistent long-term pain of CTN patients. 7
Studies have shown that pain, attention, and mood changes in CTN patients are associated with structural changes in the brain.8,9 At present, a growing number of researchers are using diffusion tensor imaging (DTI) to visualize white matter fibers in the brain. 10 Previous studies of white matter abnormalities caused by chronic pain and peripheral nerve injury in CTN patients have mainly focused on the analysis of fractional anisotropy (FA), mean diffusivity (MD), and other parameters.11,12 However, a study by Zhang et al. showed that only one segment of the white matter tract may be damaged, while the other parts remain intact. 13 Therefore, an ideal method is needed to examine specific characteristics along each fiber tract at the individual level to provide more detailed information about white matter abnormalities.
Automated fiber quantification (AFQ) is a new analysis method that applies a deterministic tractography approach to recreate whole-brain white matter tracts and estimate point-wise diffusion parameters of specific tracts. 14 In addition to quickly and reliably tracing white matter tracts within the context of an individual's brain anatomy without manual intervention, AFQ segments each white matter fascicle into several individual segments for quantitative analysis across the spatial extent of the white matter tract. 15 In recent years, AFQ has been successfully applied to the study of several neuropsychiatric disorders.14,16,17 Consequently, AFQ may provide a promising strategy to investigate whether the microstructural integrity of white matter is abnormal along the entire tract or at a specific location in a tract during the progression of CTN.
Because AFQ is a relatively new method, only a few reports have been published using this method to examine schizophrenia, 18 development and aging, 19 depression, 20 and attention-deficit/hyperactivity disorder. 21 This method has not been studied in CTN patients. In the present study, we aimed to analyze DTI data of CTN patients using AFQ technology and explore imaging markers of structural damage in the brain.
Methods
Participants
All patients included in this study had right trigeminal neuralgia. The study adhered to the Declaration of Helsinki and was approved by the ethics committee of Jining No. 1 People’s Hospital. Written informed consent was obtained from all individual participants included in the study. The reporting of this study conformed to the STROBE guidelines. 22
Screening was performed according to the International Classification of Headache Diseases (third edition), 23 and high-resolution imaging was performed to exclude secondary causes of CTN. The following diagnostic criteria were used: 1) pain occurred in one or more branches of the trigeminal nerve distribution area, with no pain outside the distribution area of the trigeminal nerve; 2) at least three episodes of unilateral pain of the face, lasting from a fraction of a second to 2 minutes, were reported. The pain was described as clicking, shooting, or stabbing; 3) patients had no neurological deficits.
The inclusion criteria of CTN patients were as follows: 1) age >18 years old; 2) right-handed; 3) unilateral pain involving one or more branches of the trigeminal nerve; 4) patients did not use psychotropic substances or have a substance abuse problem; 5) no contraindications to magnetic resonance imaging; 6) patients did not have head trauma or neurological problems. The inclusion criteria for healthy control (HC) subjects were as follows: 1) age >18 years old; 2) right-handed; 3) subjects did not use psychotropic substances or have a substance abuse problem; 4) no contraindications to magnetic resonance imaging.
The exclusion criteria were as follows: 1) other headaches; 2) chronic pain in other body parts; 3) previous trigeminal nerve surgery; 4) untreated hypertension or diabetes; 5) left-handed; 6) subjects used psychotropic substances or had a substance abuse problem; 7) contraindications to magnetic resonance imaging.
Magnetic resonance imaging and DTI
DTI data were collected with a 3.0 T magnetic resonance scanner (Achieva; Philips Healthcare, Best, the Netherlands) with a 12-channel head coil, and tight foam padding was used to limit head movement. All subjects underwent DTI and three-dimensional (3D) T1-weighted imaging examinations. The data acquisition parameters for DTI were as follows: repetition time (TR)/echo time (TE) = 10,000 ms/91 ms; field of view (FOV) = 256 mm ×256 mm; voxel dimension = 2 mm × 2 mm ×2 mm, and b = 1000 s/mm2 collected from 30 directions. Meanwhile, a b = 0 s/mm2 image was collected, and the data scanning time was 5 minutes and 27 s.
The 3D T1-weighted imaging parameters were as follows: TR/TE = 1900 ms/2.52 ms; FOV = 256 mm × 256 mm; voxel dimension = 1 mm × 1 mm × 1 mm; 176 continuous axial slices with a thickness of 1 mm.
Data preprocessing
First, the image quality was checked using MRIcron (Achieva; Philips Healthcare, Best, the Netherlands). If the scanned images had obvious defects such as artifacts, cross-layers, and other defects, they were removed. Then, Dcm2nii.exe was used to convert files in the Dicom format to the NIfTI format, which is recognized by Fsl software (FSL 4.1, FMRIB Software Library, www.fmrib.ox.ac.uk/fsl). Finally, head movement, eddy current correction, T1 and DTI denudation, dispersion of FA, MD and other indicators were calculated using Fsl software.
Automated fiber tract quantification
Briefly, the fiber bundle was first tracked. When the tracked fiber FA value was less than 0.2 or the angle was greater than 35°, the fiber was considered to have terminated. Second, the regions of interest of the average DTI image of each group were defined in Montreal Neurological Institute standard space, and the fiber tracts within the space were equidistantly segmented. Finally, in the quantitative analysis of fiber tracts, the average parameters of 100 points in each tract segmentation were estimated, mainly including the FA and MD. The 20 fiber tracts defined included the bilateral thalamic tracts, bilateral corticospinal tracts, bilateral cingulate cortex, bilateral tracts connecting the cingulate cortex and the hippocampus and the genu and splenium of the corpus callosum, bilateral inferior frontal occipital tracts, bilateral inferior longitudinal tracts, bilateral upper longitudinal tracts, bilateral uncinate fasciculus tracts, and bilateral arcuate tracts.
Assessment of clinical parameters
All patients with CTN were assessed using the International Headache Disease Classification (3rd version) and the visual analog scale (VAS) to determine the intensity and frequency of pain. In addition, all subjects underwent emotional assessment and self-scoring using a self-rated anxiety scale (SAS) and a self-rated depression scale (SDS).
Statistical analyses
SPSS 23.0 (IBM Corp., Armonk, NY, USA) was used to analyze all data. The Wilcoxon signed rank test was used to compare differences in FA and MD between groups. Pearson correlation analysis was conducted to examine the correlations of FA and MD values of fibers that were significantly different between the two groups with the pain intensity according to the VAS, pain course, SAS score, and SDS score. Values of P < 0.05 were considered statistically significant.
Results
Demographic data and clinical characteristics
Thirty-four patients (age 53.53 ± 11.0 years; 22 women and 12 men) with CTN and 34 HC subjects (age 49.12 ± 10.7 years; 22 women and 12 men) admitted to Jining First People's Hospital from August 2018 to April 2019 met the inclusion and exclusion criteria and were selected for this study. The demographic and clinical characteristics of each group are summarized in Table 1. There were no statistically significant differences in gender, mean age (53.53 ± 11.0 vs. 49.12 ± 10.7 years), education level (6.14 ± 5.1 vs. 6.48 ± 5.3 years), SAS scores (37.46 ± 8.3 vs. 38.86 ± 8.1), and SDS scores (38.36 ± 5.7 vs. 37.64 ± 6.9) between the CTN and HC groups.
Table 1.
Demographic and clinical characteristics of patients with CTN and HC subjects.
| Variable | CTN | HC | T value | P-value |
|---|---|---|---|---|
| Sex (female/male) | 34 (22/12) | 34 (22/12) | – | – |
| Age (years) | 53.53 ± 11.0 | 49.12 ± 10.7 | 1.59 | 0.12 |
| Education level (years) | 6.14 ± 5.1 | 6.48 ± 5.3 | −1.78 | 0.08 |
| VAS | 6.26 ± 2.5 | NA | – | – |
| Medical history (years) | 5.91 ± 7.4 | NA | – | – |
| SAS | 37.46 ± 8.3 | 38.86 ± 8.1 | −1.79 | 0.08 |
| SDS | 38.36 ± 5.7 | 37.64 ± 6.9 | 1.96 | 0.06 |
CTN, classic trigeminal neuralgia; HC, healthy control; VAS, visual analog scale; SAS, self-rating anxiety scale; SDS, self-rating depression scale; “–” No data.
Comparison of FA values between the CTN and HC groups on the basis of AFQ analysis
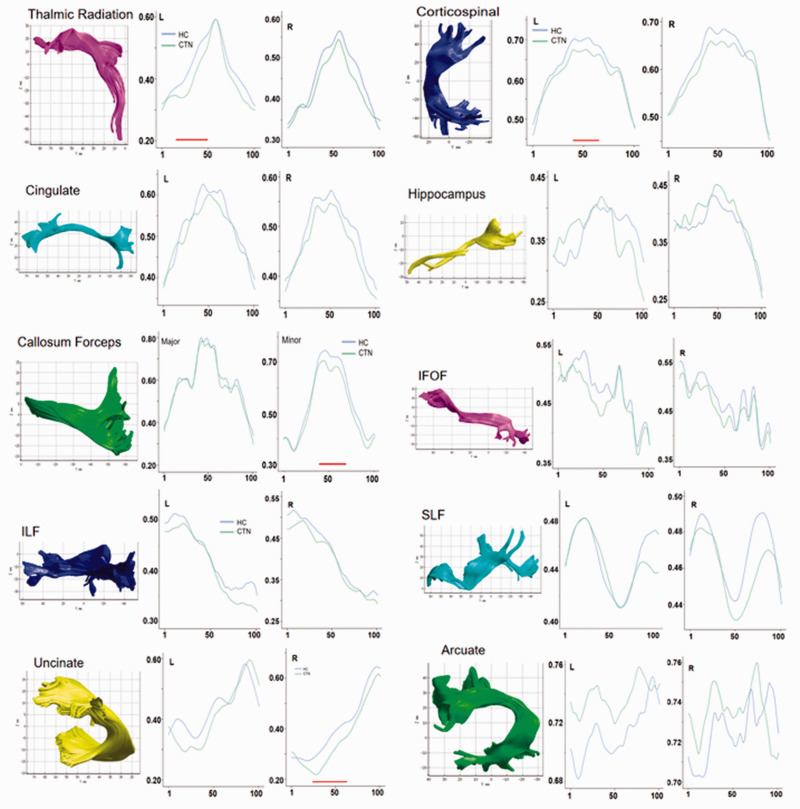
In CTN patients, the FA values of the middle segment of the left thalamic radiation (nodes 16–47, t = 17.76, P˂0.001), middle segment of the left corticospinal tract (nodes 40–61, t = 11.45, P˂0.001), middle segment of the corpus callosum forceps minor (nodes 40–67, t = 18.16, P˂0.001), and middle segment of the right uncinate fasciculus (nodes 30–61, t = 18.435, P˂0.001) were significantly lower than those of the HC group (Figure 1 and Table 2).
Figure 1.
The FA value analysis of 100 nodes on 20 nerve fiber tracts in CTN patients and HC subjects. Green represents the CTN group, and blue represents the HC group. The horizontal axis represents 100 equipartitioned nodes of the fiber tracts, and the vertical axis represents the FA values of different fiber tracts. The red horizontal lines below the curves represent nodes in a fiber tract with a statistically significant difference in the FA value between groups (P < 0.001).
FA, fractional anisotropy; CTN, classic trigeminal neuralgia; HC, healthy control; IFOF, inferior fronto-occipital fasciculus; ILF, inferior longitudinal fascicle; SLF, superior longitudinal fascicle.
Table 2.
Comparison of FA values of 100 nodes between the CTN and HC groups.
| Fiber tract | FA |
|||
|---|---|---|---|---|
| CTN | HC | T value | P-value | |
| Left thalamic radiation (nodes 16–47) | 0.42 ± 0.09 | 0.45 ± 0.08 | 17.76 | <0.001 |
| Left corticospinal tract | 0.61 ± 0.06 | 0.63 ± 0.07 | 11.45 | <0.001 |
| (nodes 40–61) | ||||
| Corpus callosum forceps | 0.52 ± 0.13 | 0.54 ± 0.14 | 18.16 | <0.001 |
| (nodes 40–67) | ||||
| Right uncinate fasciculus | 0.38 ± 0.06 | 0.41 ± 0.06 | 18.44 | <0.001 |
| (nodes 30–61) | ||||
CTN, classic trigeminal neuralgia; HC, healthy control; FA, fractional anisotropy.
Comparison of MD values between the CTN and HC groups on the basis of AFQ analysis
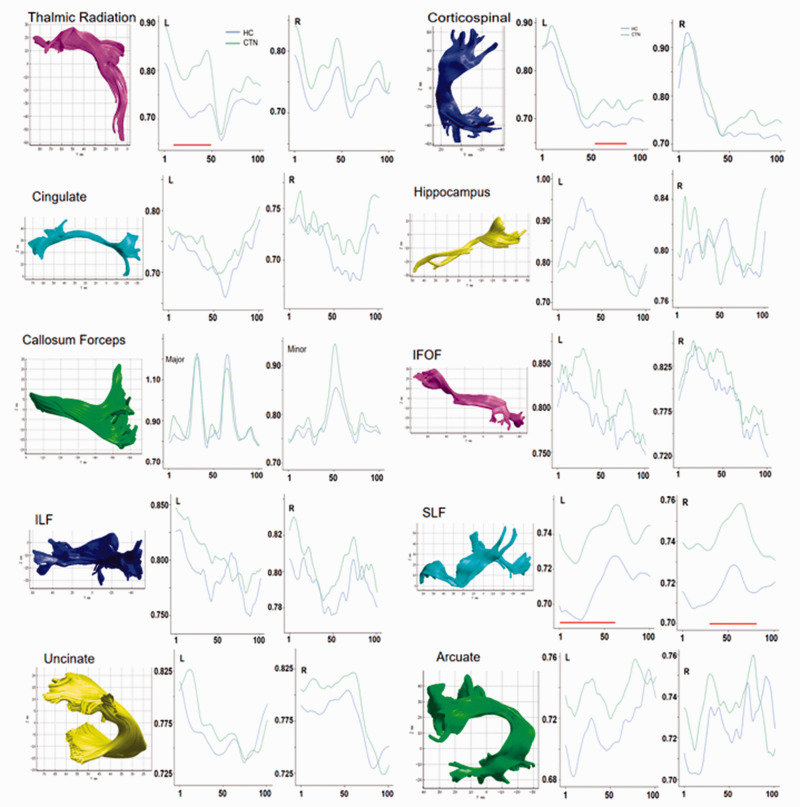
In CTN patients, the MD values of the middle segment of the left thalamic radiation (nodes 10–50, t = −18.91, P˂0.001), middle segment of the left corticospinal tract (nodes 52–79, t = −28.35, P˂0.001), anterior segment of the left superior longitudinal fasciculus (nodes 1–61, t = −47.63, P˂0.001), and middle segment of the right superior longitudinal fasciculus (nodes 36–80, t = −41.79, P˂0.001) were significantly higher than those of the HC group (Figure 2 and Table 3).
Figure 2.
The MD analysis of 100 nodes of 20 nerve fiber tracts in CTN patients and HC subjects. Green represents the CTN group, and blue represents the HC group. The horizontal axis represents 100 equipartitioned nodes of the fiber tracts, and the vertical axis represents the MD values of different fiber tracts. The red horizontal lines below the curves represent nodes in a fiber tract with a statistically significant difference in MD between groups (P < 0.001).
MD, mean diffusivity; CTN, classic trigeminal neuralgia; HC, healthy control; IFOF, inferior fronto-occipital fasciculus; ILF, inferior longitudinal fascicle; SLF, superior longitudinal fascicle.
Table 3.
Comparison of MD values of 100 nodes between the CTN and HC groups.
| Fiber tract | MD |
|||
|---|---|---|---|---|
| CTN | HC | T value | P-value | |
| Left thalamic radiation (nodes 10–50) | 0.78 ± 0.05 | 0.73 ± 0.03 | −18.91 | <0.001 |
| Left corticospinal tract | 0.76 ± 0.06 | 0.73 ± 0.06 | −28.35 | <0.001 |
| (nodes 52–79) | ||||
| Superior left longitudinal tract | 0.74 ± 0.01 | 0.71 ± 0.01 | −47.63 | <0.001 |
| (nodes 1–61) | ||||
| Superior right longitudinal tract | 0.74 ± 0.08 | 0.72 ± 0.06 | −41.79 | <0.001 |
| (nodes 36–80) | ||||
CTN, classic trigeminal neuralgia; HC, healthy control; MD, mean diffusivity.
Correlation analysis of altered DTI metrics and clinical variables
As presented in Figure 3, the FA value of the middle segment of the left thalamic tract was positively correlated with the VAS pain assessment (r = 0.454, P = 0.007), while the MD value of the middle segment of the left thalamic tract was negatively correlated with the VAS pain assessment (r = −0.0371, P = 0.031).
Figure 3.
Correlation analysis between of the FA and MD and the VAS.
FA, fractional anisotropy; MD, mean diffusivity; VAS, visual analog scale.
Discussion
AFQ is a relatively novel method for automatic quantification of white matter tracts and can be used to quantify the diffusion parameters of multiple fiber tract nodes. In this study, a quantitative analysis of 100 nodes of 20 fiber tracts was performed in the brains of CTN patients. The results suggested that the middle segment of the left thalamic radiation, middle segment of the left corticospinal tract, middle segment of the corpus callosum forceps minor, middle segment of the right uncinate fasciculus, anterior segment of the left superior longitudinal fasciculus, and middle posterior segment of the right superior longitudinal fasciculus were damaged to a certain extent. Moreover, the FA and MD of damaged fiber tracts were related to pain.
FA and MD are two common quantitative metrics used to evaluate the structural integrity of white matter on DTI. FA is a scalar value representing anisotropic water diffusion that reflects the degree of directionality of the anisotropy of the diffusion process. In addition, higher FA values reflect greater white matter integrity. 24 MD is a scalar value representing the mean water diffusion at each voxel. Therefore, the MD is lower in regions where white matter complexity is increased. 25 In this study, we found that the FA value of the left thalamic radiation and left corticospinal tract were decreased, and the MD value was increased, indicating that the fibers in this area are damaged in CTN patients. The results were supported by data from previous studies. Briefly, Jin et al. found that the 18 main white matter tracts in Alzheimer's disease patients generally had decreased FA and increased MD, which may reflect the diffuse abnormality of myelin and/or fiber axons in Alzheimer's disease. 26 Mito and colleagues revealed significant white matter loss at both the microstructural and macrostructural levels in patients with Alzheimer's disease, which was evident in specific fiber pathways associated with the default mode network node. 27 Taken together, the findings indicated that FA and MD can be used as early predictors of the development of CTN.
Clinically, the changes in FA and MD of the left thalamic radiation and the left corticospinal tract, which are associated with sensory input and motor output, suggested projection fiber damage. 28 No obvious movement disorder was found in patients, which may be related to the strong compensatory ability of sensorimotor cortex function during the pain interval. Furthermore, the damaged projection fibers are on the left side, supporting damage to the commissural fiber, which is consistent with the damage to the middle segment of the corpus callosum found in the study. The corpus callosum is part of the brain's highest-order, latest-to-mature neural network. At different times, it is highly susceptible to negative impacts, and central nervous system plasticity is most likely to occur because of peripheral trigeminal nerve injury.29,30
In terms of fiber composition, the concentration of small, unmyelinated axons was highest in the corpus callosum forceps. It was speculated that early myelination and the unique fiber composition of the anterior corpus callosum may increase its likelihood of causing pain in patients with CTN. 31 The right uncinate fasciculus, right superior longitudinal fasciculus, and left superior longitudinal fasciculus play important roles in connecting various brain regions in the ipsilateral hemisphere. 32 The increase in the FA value and decrease in the MD value of these fibers suggested that the connection efficiency between relevant brain regions decreases after fiber injury, which may lead to the occurrence of relevant clinical symptoms, especially the sensation of pain. In this study, the degree of pain was positively correlated with the FA value and negatively correlated with the MD value, indicating that with disease progression, white matter damage becomes more serious, and pain sensation becomes more serious with the aggravation of white matter damage.
In this study, specific nodes of the damaged fiber tracts were located and quantitatively analyzed, which is a more accurate method than that used in a previous study examining the average FA value of whole fiber tracts. However, AFQ has some limitations. The present study based on the AFQ method is limited to major white matter tracts, and some small but useful tracts may have been overlooked. Another limitation of AFQ is that fiber tracts may not follow the true brain information transfer pathways because of limitations of tractography parameters. 15 In the future, more advanced algorithms will be incorporated to increase the accuracy of the AFQ method. In addition, the sample size of the study was small, and additional subjects need to be enrolled to verify the stability of the study results. Furthermore, in this study, only the FA and MD values of the white matter tracts were analyzed. Other quantitative parameters, such as radial diffusion and axial diffusion will be further evaluated in subsequent studies.
Conclusion
Using AFQ technology to analyze the DTI data of CTN patients, we found that some specific nodes of fiber tracts were damaged, and this was related to the pain scale score. More importantly, this multi-node quantitative study of the degree of damage of fiber tracts revealed more detailed information regarding changes in fiber tracts than when the average value of the entire fiber tract was examined.
Footnotes
Declaration of conflicting interest: The authors declare that there is no conflict of interest.
Funding: This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
ORCID iD: Guangbin Wang https://orcid.org/0000-0001-7410-5540
References
- 1.Haanpää M, Attal N, Backonja M, et al. NeuPSIG guidelines on neuropathic pain assessment. Pain 2011; 152: 14–27. DOI: 10.1016/j.pain.2010.07.031. [DOI] [PubMed] [Google Scholar]
- 2.Headache Classification Committee of the International Headache Society (IHS). The International Classification of Headache Disorders, 3rd edition (beta version). Cephalalgia 2013; 33: 629–808. DOI: 10.1177/0333102413485658. [DOI] [PubMed] [Google Scholar]
- 3.Cruccu G, Finnerup NB, Jensen TS, et al. Trigeminal neuralgia: New classification and diagnostic grading for practice and research. Neurology 2016; 87: 220–228. DOI: 10.1212/wnl.0000000000002840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mueller D, Obermann M, Yoon MS, et al. Prevalence of trigeminal neuralgia and persistent idiopathic facial pain: a population-based study. Cephalalgia 2011; 31: 1542–1548. DOI: 10.1177/0333102411424619. [DOI] [PubMed] [Google Scholar]
- 5.Wanke I, Dietrich U, Oppel F, et al. Endovascular treatment of trigeminal neuralgia caused by arteriovenous malformation: is surgery really necessary? Zentralbl Neurochir 2005; 66: 213–216. DOI: 10.1055/s-2005-836601. [DOI] [PubMed] [Google Scholar]
- 6.Liu Y, Li J, Butzkueven H, et al. Microstructural abnormalities in the trigeminal nerves of patients with trigeminal neuralgia revealed by multiple diffusion metrics. Eur J Radiol 2013; 82: 783–786. DOI: 10.1016/j.ejrad.2012.11.027. [DOI] [PubMed] [Google Scholar]
- 7.Fahlström A Laurell K andEricson H. [ Trigeminal neuralgia]. Lakartidningen 2014; 111: 2295–2298. [PubMed] [Google Scholar]
- 8.Zhu J, Zhuo C, Qin W, et al. Performances of diffusion kurtosis imaging and diffusion tensor imaging in detecting white matter abnormality in schizophrenia. Neuroimage Clin 2015; 7: 170–176. DOI: 10.1016/j.nicl.2014.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hardan AY, Pabalan M, Gupta N, et al. Corpus callosum volume in children with autism. Psychiatry Res 2009; 174: 57–61. DOI: 10.1016/j.pscychresns.2009.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Stěpán-Buksakowska I, Keller J, Laczó J, et al. Diffusion tensor imaging in Alzheimer disease and mild cognitive impairment. Neurol Neurochir Pol 2012; 46: 462–471. DOI: 10.5114/ninp.2012.31357. [DOI] [PubMed] [Google Scholar]
- 11.Wang Y, Li D, Bao F, et al. Microstructural abnormalities of the trigeminal nerve correlate with pain severity and concomitant emotional dysfunctions in idiopathic trigeminal neuralgia: A randomized, prospective, double-blind study. Magn Reson Imaging 2016; 34: 609–616. DOI: 10.1016/j.mri.2015.11.013. [DOI] [PubMed] [Google Scholar]
- 12.DeSouza DD Hodaie M andDavis KD.. Abnormal trigeminal nerve microstructure and brain white matter in idiopathic trigeminal neuralgia. Pain 2014; 155: 37–44. DOI: 10.1016/j.pain.2013.08.029. [DOI] [PubMed] [Google Scholar]
- 13.Zhang X, Sun Y, Li W, et al. Characterization of white matter changes along fibers by automated fiber quantification in the early stages of Alzheimer's disease. Neuroimage Clin 2019; 22: 101723. DOI: 10.1016/j.nicl.2019.101723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chen H, Sheng X, Qin R, et al. Aberrant white matter microstructure as a potential diagnostic marker in Alzheimer's disease by automated fiber quantification. Front Neurosci 2020; 14: 570123. DOI: 10.3389/fnins.2020.570123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yeatman JD, Dougherty RF, Myall NJ, et al. Tract profiles of white matter properties: automating fiber-tract quantification. PLoS One 2012; 7: e49790. DOI: 10.1371/journal.pone.0049790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Deng F, Wang Y, Huang H, et al. Abnormal segments of right uncinate fasciculus and left anterior thalamic radiation in major and bipolar depression. Prog Neuropsychopharmacol Biol Psychiatry 2018; 81: 340–349. DOI: 10.1016/j.pnpbp.2017.09.006. [DOI] [PubMed] [Google Scholar]
- 17.Huber E, Donnelly PM, Rokem A, et al. Rapid and widespread white matter plasticity during an intensive reading intervention. Nat Commun 2018; 9: 2260. DOI: 10.1038/s41467-018-04627-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sun H, Lui S, Yao L, et al. Two patterns of white matter abnormalities in medication-naive patients with first-episode schizophrenia revealed by diffusion tensor imaging and cluster analysis. JAMA Psychiatry 2015; 72: 678–686. DOI: 10.1001/jamapsychiatry.2015.0505. [DOI] [PubMed] [Google Scholar]
- 19.Yeatman JD Wandell BA andMezer AA.. Lifespan maturation and degeneration of human brain white matter. Nat Commun 2014; 5: 4932. DOI: 10.1038/ncomms5932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sacchet MD, Prasad G, Foland-Ross LC, et al. Structural abnormality of the corticospinal tract in major depressive disorder. Biol Mood Anxiety Disord 2014; 4: 8. DOI: 10.1186/2045-5380-4-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lin Q, Bu X, Wang M, et al. Aberrant white matter properties of the callosal tracts implicated in girls with attention-deficit/hyperactivity disorder. Brain Imaging Behav 2020; 14: 728–735. DOI: 10.1007/s11682-018-0010-2. [DOI] [PubMed] [Google Scholar]
- 22.Von Elm E, Altman DG, Egger M, et al. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. PLoS Med 2007; 4: e296. DOI: 10.1371/journal.pmed.0040296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zung WW. A self-rating depression scale. Arch Gen Psychiatry 1965; 12: 63–70. DOI: 10.1001/archpsyc.1965.01720310065008. [DOI] [PubMed] [Google Scholar]
- 24.Dou X, Yao H, Feng F, et al. Characterizing white matter connectivity in Alzheimer's disease and mild cognitive impairment: An automated fiber quantification analysis with two independent datasets. Cortex 2020; 129: 390–405. DOI: 10.1016/j.cortex.2020.03.032. [DOI] [PubMed] [Google Scholar]
- 25.Xie Y, Zhang Y, Qin W, et al. White matter microstructural abnormalities in type 2 diabetes mellitus: a diffusional kurtosis imaging analysis. AJNR Am J Neuroradiol 2017; 38: 617–625. DOI: 10.3174/ajnr.A5042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jin Y, Huang C, Daianu M, et al. 3D tract-specific local and global analysis of white matter integrity in Alzheimer's disease. Hum Brain Mapp 2017; 38: 1191–1207. DOI: 10.1002/hbm.23448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mito R, Raffelt D, Dhollander T, et al. Fibre-specific white matter reductions in Alzheimer's disease and mild cognitive impairment. Brain 2018; 141: 888–902. DOI: 10.1093/brain/awx355. [DOI] [PubMed] [Google Scholar]
- 28.Obermann M, Rodriguez-Raecke R, Naegel S, et al. Gray matter volume reduction reflects chronic pain in trigeminal neuralgia. Neuroimage 2013; 74: 352–358. DOI: 10.1016/j.neuroimage.2013.02.029. [DOI] [PubMed] [Google Scholar]
- 29.Taylor KS Anastakis DJ andDavis KD.. Cutting your nerve changes your brain. Brain 2009; 132: 3122–3133. DOI: 10.1093/brain/awp231. [DOI] [PubMed] [Google Scholar]
- 30.Davis KD Taylor KS andAnastakis DJ.. Nerve injury triggers changes in the brain. The Neuroscientist: a review journal bringing neurobiology, Neuroscientist 2011; 17: 407–422. DOI: 10.1177/1073858410389185. [DOI] [PubMed] [Google Scholar]
- 31.Innocenti GM Caminiti R andHof PR.. Fiber composition in the planum temporale sector of the corpus callosum in chimpanzee and human. Brain Struct Funct 2010; 215: 123–128. DOI: 10.1007/s00429-010-0274-9. [DOI] [PubMed] [Google Scholar]
- 32.Bhatia K, Henderson L, Yim M, et al. Diffusion tensor imaging investigation of uncinate fasciculus anatomy in healthy controls: description of a subgenual stem. Neuropsychobiology 2017; 75: 132–140. DOI: 10.1159/000485111. [DOI] [PubMed] [Google Scholar]