Abstract
Objectives:
Worldwide, smokeless tobacco products vary greatly in their formulations and chemical composition. Understanding of toxic and carcinogenic constituent variations in such products can provide valuable insights for the development of effective tobacco control policies. In this study, we applied a standardized protocol to collect and analyze smokeless products sold in Mumbai, India.
Methods:
Tobacco products were purchased at three markets in Mumbai, using standardized protocol for sample collection, labeling, and storage. Moisture content, pH, total and unprotonated nicotine, and five tobacco-specific N-nitrosamines (TSNA) were analyzed by validated methods.
Results:
We purchased 39 samples representing eight varieties of manufactured and vendor-made smokeless tobacco products. Total nicotine ranged from 5.3 to 57.8 mg/g dry weight. Unprotonated nicotine content varied from 0.13% to 99.8% of total nicotine. Total TSNA content ranged from 0.17 to 81.0 μg/g dry weight. When expressed per wet weight of product, unprotonated nicotine varied more than 300-fold and TSNA content varied more than 650-fold across the products. Substantial vendor-to-vendor variations were also observed.
Conclusions:
Our findings emphasize the critical need for systematic smokeless tobacco surveillance in India, to improve understanding of exposures and cancer risks in users of these products.
Keywords: Smokeless tobacco, constituents, surveillance, nicotine, tobacco-specific nitrosamines
INTRODUCTION
Close to a third of the Indian population uses various forms of smokeless tobacco products.1,2 As a result, India is home to over 80% of all smokeless tobacco users worldwide.3 The widespread use of smokeless tobacco in India persists despite several important policy and legislative measures that have been introduced in India since the 1990’s, including bans on advertising, introduction of graphic warnings, and bans of select smokeless product types, like gutkha.4–6 Overall, the use of smokeless tobacco has been identified as a cause of oral, pancreatic, and esophageal cancer.7,8 In India, the association of smokeless tobacco use with oral cancer is particularly striking.9–12 The rates of oral cancer in India are among the highest in the world, and it is the leading cause of cancer-related death in that country.12,13 Characterization of the carcinogenic potential of smokeless products is an important element for the development of tobacco control policies and can serve as an effective tool in the education of healthcare professionals and consumers about the harms of smokeless tobacco use.
There is an abundant diversity of smokeless tobacco products in India, ranging from unprocessed tobacco to cured flavored tobacco with lime, to complex mixes containing various non-tobacco ingredients, such as in betel quid.10,14 Multiple manufactured brands of the same product type can be found in various parts of the country, and some products are cottage-made or prepared by vendors at the place of purchase, altogether adding to the diversity of the smokeless tobacco market in India.10,14 This diversity is accompanied by substantial variations in the chemical composition across individual product types.14 For instance, the most recent report on the chemical composition of several products purchased in India showed that the levels of unprotonated nicotine, the biologically available form of the main known addictive constituent in tobacco, ranged from 0.05 to 4.68 mg/g product, while the sum of several tobacco-specific N-nitrosamines (TSNA), the major group of carcinogenic constituents in smokeless tobacco, ranged from 0.264 to 23.9 μg/g product.15
In addition to variations across product types, some tobacco constituents can vary within the same brand due to subtle variations in product composition, differences of storage duration and conditions, or other factors. For instance, the levels of unprotonated nicotine are drastically affected by even slight changes in product pH,16–18 while TSNA can be formed via the nitrosation of tobacco alkaloids during product storage.19,20 We have previously demonstrated that the levels of unprotonated nicotine and TSNA in novel smokeless tobacco products marketed in the U.S. varied regionally and over time within the same brand of product.21–23 Monitoring such within-brand variations of toxicant and carcinogen levels in Indian smokeless products can provide valuable insights for the evaluation of addictive and carcinogenic potential of these products and inform effective regulatory and preventive measures.
The goal of this study was to apply a standardized protocol to collect and analyze multiple samples of several manufactured and vendor-made Indian smokeless tobacco products purchased in a defined location in India. We present levels of nicotine, unprotonated nicotine, TSNA, and moisture content in manufactured and vendor-made products purchased at three different markets in Mumbai.
METHODS
Tobacco samples
Sample collection was carried out at three street markets in Mumbai, India, following our standardized sampling and labeling procedures.21,23,24 A pilot survey of the most popular smokeless tobacco products was conducted by the Indian collaborators prior to the start of the study, in order to compose a representative list of samples to be collected and transported to the University of Minnesota for analyses. The list was further confirmed with each vendor at the time of purchase. In order to evaluate the potential variations within the same product brand/type, six samples of each manufactured product variety were obtained, with duplicate samples being purchased from three different markets. The content of manufactured products was assessed by inspecting the information printed on the packages. For the products made by the vendor at the time of purchase, one sample per vendor was obtained and the list of ingredients has been recorded. At all locations, products and/or ingredients used in product preparation were exposed to high ambient temperatures and humidity, similar to the routine handling of other smokeless tobacco products on Indian markets. After the purchase, samples were labeled and handled according to our standardized sampling and labeling procedures.21,24 In the laboratory, samples were sealed in plastic sleeves and stored at 4 °C until analysis.
Constituent analyses
Samples were prepared according to our routine validated methods.21,23,24 Negative control (extraction solvent) and positive control (reference tobacco CRP1 and CRP2) were included to monitor for potential contamination and analytical accuracy.
Moisture content and pH were measured via the difference in weight of a tobacco sample before and after its drying for 3 h at 99 °C, and pH was measured in aqueous tobacco extracts as described.21,25
TSNA.
Five commonly measured TSNA – Nʹ-nitrosonornicotine (NNN), 4-(methylnitrosamino)-1-(3-pyridyl)-butanone (NNK), 4-(methylnitrosamino)-1-(3-pyridyl)-butanol (NNAL), Nʹ-nitrosoanatabine (NAT), and Nʹ-nitrosoanabasine (NAB) – were analyzed in single analytical procedure by using our standard liquid chromatography-tandem mass-spectrometry (LC-MS/MS) protocol.24,26 Briefly, tobacco samples were extracted with 15 mM ammonium acetate, the extracts were purified on ChemElut cartridges (Agilent, Santa Clara, CA), and the purified samples were analyzed by LC-MS/MS in positive ion electrospray mode with selected reaction monitoring for m/z 178 → 148 for NNN, m/z 208 → 178 for NNK, m/z 210 → 190 for NNAL, m/z 190 → 160 for NAT, and m/z 192 → 162 for NAB. Corresponding transitions were monitored for 13C6NNN, [pyridine-D4]NNK, and 13C6NNAL, which were used as internal standards.
Nicotine and unprotonated nicotine.
These were measured by our standard laboratory procedures as previously described.21,27 Briefly, tobacco was extracted with 15 mM ammonium acetate, an aliquot was mixed with [CD3]nicotine internal standard, and the sample was diluted and analyzed by LC-MS/MS, monitoring m/z 163 → 130 for nicotine and m/z 166 → 130 for [CD3]nicotine.27,28 The amount of unprotonated nicotine was calculated using the Henderson-Hasselbalch equation, using the measured nicotine, pH, and pKa of 8.02.29
RESULTS
A total of 39 samples of smokeless tobacco products were purchased at Belapur, Nerul, and Sanpada stations in Mumbai (Table 1). Based on the content, these products can be separated in three groups: (i) manufactured tobacco that consumers mix with lime before use (Sandeep Pandharpuri, Om Special Pandharpuri, and Tambakhu Gai Chhap); (ii) manufactured alkaline tobacco (Miraj and Chaini Khaini); and (iii) vendor-made mixed products containing tobacco, areca nut, and other ingredients (mawa and betel quid) (Figure 1). In the case of manufactured products, two samples were purchased at each of the three markets to a total of 6 samples per product. In the case of vendor-made mawa and betel quid, one sample was purchased at each of the three markets, or 3 samples per product.
Table 1.
Products and samples analyzed in this study
| Product | Contents | Number of samples |
|---|---|---|
|
| ||
|
Manufactured, pre-packaged products
|
||
| Pandharpuri Sandeep | Tobacco, intended for mixing with lime before use | 6 |
| Om Special Pandharpuri | Tobacco, intended for mixing with lime before use | 6 |
| Tambakhu Gai Chhap | Tobacco, intended for mixing with lime before use | 6 |
| Miraj Tobacco | Tobacco, lime stone paste | 6 |
| Chaini Khaini | Moist tobacco in pouches, marketed as ‘snus’ | 6 |
|
| ||
|
Vendor-made products, prepared at the time of purchase
|
||
| Mawa ‘120–300’ | Tobacco varieties ‘120’ and ‘300’, areca nut, lime, khiwam, small scented green leaves, clove | 3 |
| Mawa ‘Bhola’ | Tobacco Bhola and Sagar tobacco, areca nut, lime, khiwam, small scented green leaves, clove | 3 |
| Betel Quid /Banarasi Paan | Tobacco, areca nut, catechu, lime, khiwam, small scented green leaves, clove | 3 |
|
| ||
| Total samples | 39 | |
Figure 1.
Betel quid prepared by a vendor in Mumbai; the mix is prepared at the time of purchase and wrapped before being given to a consumer.
Moisture content, pH, and total and unprotonated nicotine levels in all samples are presented in Table 2. Levels of TSNA in all products are presented in Table 3. Moisture content varied significantly across the tested brands; therefore, levels of constituents in Tables 2 and 3 are expressed per gram dry weight. Nicotine levels in all samples ranged from 5.3 to 57.8 mg/g dry weight, with the levels being generally lower in vendor-made mawa and betel quid than in manufactured products (Table 2). Product pH ranged from 5.14 to 10.82, resulting in unprotonated nicotine variation from 0.13% to 99.8% of total nicotine content. The highest unprotonated nicotine content was found in Miraj and Chaini Khaini products: 23.0 ± 4.0 and 12.9 ± 3.0 mg/g dry weight, respectively.
Table 2.
Moisture content, pH, and nicotine content in select Indian smokeless tobacco products purchased in Mumbaia
| Product | Market | Moisture, % | pH | Total nicotine, mg/g dry weight | Unprotonated nicotine |
|
|---|---|---|---|---|---|---|
| % of total | mg/g dry weight | |||||
|
| ||||||
| Manufactured, pre-packaged products | ||||||
| Pandharpuri Sandeep | Belapur Station | 18.0 ± 0.69 | 5.25 ± 0.05 | 49.3 ± 5.5 | 0.17 ± 0.02 | 0.08 ± 0.001 |
| Nerul Station | 17.9 ± 0.13 | 5.23 ± 0.004 | 44.3 ± 6.2 | 0.16 ± 0.001 | 0.07 ± 0.01 | |
| Sanpada Station | 17.0 ± 0.08 | 5.19 ± 0.02 | 50.4 ± 7.1 | 0.15 ± 0.01 | 0.07 ± 0.01 | |
| Average ± SD for Pandharpuri Sandeep | 17.6 ± 0.60 | 5.22 ± 0.04 | 48.0 ± 5.7 | 0.16 ± 0.01 | 0.08 ± 0.01 | |
|
| ||||||
| Om Special Pandharpuri | Belapur Station | 16.7 ± 1.4 | 5.21 ± 0.00 | 53.4 ± 6.2 | 0.15 ± 0.00 | 0.08 ± 0.01 |
| Nerul Station | 19.0 ± 0.17 | 5.23 ± 0.07 | 46.7 ± 0.23 | 0.16 ± 0.03 | 0.08 ± 0.01 | |
| Sanpada Station | 17.9 ± 0.47 | 5.17 ± 0.04 | 45.6 ± 2.9 | 0.14 ± 0.01 | 0.06 ± 0.001 | |
| Average ± SD for Om Special Pandharpuri | 17.9 ± 1.2 | 5.20 ± 0.05 | 48.6 ± 4.9 | 0.15 ± 0.02 | 0.07 ± 0.01 | |
|
| ||||||
| Tambakhu Gai Chhap | Belapur Station | 14.2 ± 0.73 | 6.25 ± 0.06 | 34.8 ± 0.03 | 1.7 ± 0.23 | 0.59 ± 0.08 |
| Nerul Station | 17.0 ± 0.33 | 6.22 ± 0.004 | 35.3 ± 0.67 | 1.6 ± 0.01 | 0.55 ± 0.01 | |
| Sanpada Station | 15.0 ± 0.23 | 6.23 ± 0.01 | 30.9 ± 1.4 | 1.6 ± 0.03 | 0.49 ± 0.03 | |
| Average ± SD for Tambakhu Gai Chhap | 15.4 ± 1.3 | 6.24 ± 0.03 | 33.7 ± 2.3 | 1.6 ± 0.12 | 0.54 ± 0.06 | |
|
| ||||||
| Miraj Tobacco | Belapur Station | 42.3 ± 0.22 | 10.29 ± 0.01 | 28.0 ± 0.39 | 99.5 ± 0.02 | 27.8 ± 0.40 |
| Nerul Station | 43.5 ± 0.02 | 10.75 ± 0.10 | 19.4 ± 2.1 | 99.8 ± 0.04 | 19.3 ± 2.1 | |
| Sanpada Station | 44.5 ± 0.21 | 10.53 ± 0.30 | 22.0 ± 0.05 | 99.7 ± 0.22 | 22.0 ± 0.10 | |
| Average ± SD for Miraj Tobacco | 43.4 ± 1.0 | 10.53 ± 0.25 | 23.1 ± 4.1 | 23.1 ± 4.1 | 23.0 ± 4.0 | |
|
| ||||||
| Chaini Khaini | Belapur Station | 26.1 ± 0.01 | 9.10 ± 0.13 | 10.9 ± 0.33 | 92.2 ± 2.2 | 10.0 ± 0.06 |
| Nerul Station | 23.2 ± 2.5 | 9.49 ± 0.14 | 17.2 ± 1.0 | 96.7 ± 1.1 | 16.6 ± 1.2 | |
| Sanpada Station | 24.0 ± 1.6 | 9.71 ± 0.30 | 12.6 ± 0.57 | 97.8 ± 1.4 | 12.3 ± 0.4 | |
| Average ± SD for Chaini Khaini | 24.4 ± 1.9 | 9.43 ± 0.32 | 13.6 ± 2.9 | 95.5 ± 2.9 | 13.0 ± 3.0 | |
| Vendor-made products, prepared at the time of purchase | ||||||
|
| ||||||
| Mawa ‘120–300’ | Belapur Station | 16.3 | 6.58 | 5.9 | 3.5 | 0.21 |
| Nerul Station | 20.7 | 7.55 | 7.7 | 25.3 | 1.9 | |
| Sanpada Station | 16.3 | 6.82 | 10.2 | 5.9 | 0.61 | |
| Average ± SD for mawa ‘120–130’ | 17.8 ± 2.6 | 6.98 ± 0.51 | 7.9 ± 2.2 | 11.6 ± 12.0 | 0.91 ± 0.88 | |
|
| ||||||
| Mawa ‘Bhola’ | Belapur Station | 12.8 | 5.95 | 12.1 | 0.83 | 0.10 |
| Nerul Station | 20.0 | 7.07 | 5.8 | 10.0 | 0.58 | |
| Sanpada Station | 15.2 | 6.15 | 5.3 | 1.3 | 0.07 | |
| Average ± SD for mawa ‘Bhola’ | 16.0 ± 3.7 | 6.39 ± 0.60 | 7.7 ± 3.8 | 4.1 ± 5.1 | 0.25 ± 0.29 | |
|
| ||||||
| Betel Quid /Banarasi Paan | Belapur Station | 29.6 | 6.78 | 7.4 | 5.4 | 0.40 |
| Nerul Station | 45.4 | 6.35 | 8.4 | 2.1 | 0.18 | |
| Sanpada Station | 50.0 | 6.50 | 6.7 | 2.9 | 0.20 | |
| Average ± SD for betel quid | 41.7 ± 10.7 | 6.54 ± 0.22 | 7.5 ± 0.88 | 3.5 ± 1.7 | 0.26 ± 0.12 | |
Arithmetic means ± standard deviations are shown for products for which 2 or more samples were available. Otherwise, results of single sample analyses are shown.
Table 3.
Levels of tobacco-specific N−nitrosamines in select Indian smokeless tobacco products purchased in Mumbaia
| Product | Market | μg/g dry weight |
|||||
|---|---|---|---|---|---|---|---|
| NNN | NNK | NNAL | NAT | NAB | Total TSNAb | ||
|
| |||||||
| Pandharpuri Sandeep | Belapur Station | 6.37 ± 0.21 | 2.79 ± 0.45 | 0.221 ± 0.05 | 5.51 ± 0.04 | 1.55 ± 0.04 | 16.4 ± 0.62 |
| Nerul Station | 5.90 ± 0.27 | 4.57 ± 2.8 | 0.326 ± 0.14 | 5.08 ± 0.70 | 1.33 ± 0.14 | 17.2 ± 3.8 | |
| Sanpada Station | 5.24 ± 0.23 | 2.21 ± 0.11 | 0.333 ± 0.03 | 4.50 ± 0.55 | 1.39 ± 0.37 | 13.7 ± 1.2 | |
| Average ± SD for Pandharpuri Sandeep | 5.84 ± 0.54 | 3.19 ± 1.7 | 0.293 ± 0.09 | 5.03 ± 0.60 | 1.43 ± 0.20 | 15.8 ± 2.5 | |
|
| |||||||
| Om Special Pandharpuri | Belapur Station | 3.66 ± 0.02 | 1.71 ± 0.28 | 0.185 ± 0.04 | 3.63 ± 0.06 | 1.24 ± 0.10 | 10.4 ± 0.18 |
| Nerul Station | 3.74 ± 0.44 | 1.93 ± 0.22 | 0.160 ± 0.06 | 3.69 ± 0.55 | 1.27 ± 0.15 | 10.8 ± 1.4 | |
| Sanpada Station | 4.62 ± 0.12 | 2.39 ± 0.16 | 0.263 ± 0.02 | 4.67 ± 0.49 | 1.45 ± 0.16 | 13.4 ± 0.63 | |
| Average ± SD for Om Special Pandharpuri | 4.01 ± 0.52 | 2.01 ± 0.36 | 0.203 ± 0.06 | 4.00 ± 0.62 | 1.32 ± 0.15 | 11.5 ± 1.6 | |
|
| |||||||
| Tambakhu Gai Chhap | Belapur Station | 16.2 ± 0.69 | 2.62 ± 0.33 | 1.48 ± 0.07 | 22.6 ± 2.5 | 5.66 ± 0.11 | 48.5 ± 3.5 |
| Nerul Station | 16.5 ± 0.19 | 3.39 ± 0.13 | 1.34 ± 0.34 | 24.8 ± 0.80 | 6.49 ± 0.47 | 52.5 ± 1.9 | |
| Sanpada Station | 14.2 ± 0.05 | 2.31 ± 0.05 | 1.45 ± 0.07 | 20.0 ± 1.8 | 5.05 ± 0.14 | 43.0 ± 1.6 | |
| Average ± SD for Tambakhu Gai Chhap | 15.6 ± 1.2 | 2.77 ± 0.52 | 1.42 ± 0.17 | 22.5 ± 2.6 | 5.73 ± 0.68 | 48.0 ± 4.7 | |
|
| |||||||
| Miraj Tobacco | Belapur Station | 2.74 ± 0.06 | 0.282 ± 0.03 | 0.167 ± 0.03 | 2.47 ± 0.04 | 1.05 ± 0.27 | 6.72 ± 0.31 |
| Nerul Station | 2.61 ± 0.10 | 0.109 ± 0.02 | 0.225 ± 0.10 | 1.20 ± 0.11 | 0.821 ± 0.05 | 4.97 ± 0.33 | |
| Sanpada Station | 2.93 ± 0.02 | 0.211 ± 0.01 | 0.276 ± 0.04 | 1.94 ± 0.01 | 0.950 ± 0.09 | 6.31 ± 0.09 | |
| Average ± SD for Miraj Tobacco | 2.76 ± 0.16 | 0.201 ± 0.08 | 0.223 ± 0.07 | 1.87 ± 0.57 | 0.942 ± 0.17 | 6.00 ± 0.84 | |
|
| |||||||
| Chaini Khaini | Belapur Station | 24.9 ± 2.0 | 3.17 ± 0.11 | 3.11 ± 0.06 | 7.46 ± 0.34 | 10.0 ± 0.14 | 48.7 ± 2.3 |
| Nerul Station | 37.0 ± 3.1 | 4.51 ± 2.7 | 6.88 ± 0.53 | 14.2 ± 1.7 | 16.8 ± 0.55 | 79.5 ± 2.2 | |
| Sanpada Station | 34.1 ± 0.81 | 3.56 ± 1.5 | 6.87 ± 0.38 | 8.16 ± 0.38 | 14.5 ± 0.30 | 67.1 ± 2.0 | |
| Average ± SD for Chaini Khaini | 32.0 ± 5.9 | 3.75 ± 1.5 | 5.62 ± 2.0 | 9.95 ± 3.4 | 13.8 ± 3.1 | 65.1 ± 14.0 | |
|
| |||||||
| Vendor-made products, prepared at the time of purchase | |||||||
|
| |||||||
| Mawa ‘120–300’ | Belapur Station | 6.77 | 2.97 | 0.634 | 5.25 | 0.723 | 16.3 |
| Nerul Station | 0.457 | 0.143 | 0.145 | 0.319 | 0.076 | 1.14 | |
| Sanpada Station | 0.641 | 0.288 | 0.096 | 0.708 | 0.088 | 1.82 | |
| Average ± SD for mawa ‘120–130’ | 2.62 ± 3.59 | 1.13 ± 1.6 | 0.292 ± 0.30 | 2.09 ± 2.7 | 0.296 ± 0.37 | 6.44 ± 8.6 | |
|
| |||||||
| Mawa ‘Bhola’ | Belapur Station | 0.709 | 0.291 | 0.191 | 0.613 | 0.088 | 1.89 |
| Nerul Station | 0.727 | 0.258 | 0.285 | 0.587 | 0.086 | 1.94 | |
| Sanpada Station | 0.666 | 0.248 | 0.050 | 0.517 | 0.063 | 1.54 | |
| Average ± SD for mawa ‘Bhola’ | 0.701 ± 0.03 | 0.265 ± 0.02 | 0.175 ± 0.12 | 0.572 ± 0.05 | 0.079 ± 0.01 | 1.79 ± 0.22 | |
|
| |||||||
| Betel Quid /Banarasi Paan | Belapur Station | 0.299 | 0.059 | 0.158 | 0.246 | 0.034 | 0.796 |
| Nerul Station | 0.068 | 0.010 | 0.039 | 0.048 | 0.007 | 0.171 | |
| Sanpada Station | 0.767 | 0.101 | 0.320 | 0.816 | 0.088 | 2.09 | |
| Average ± SD for betel quid | 0.378 ± 0.36 | 0.057 ± 0.05 | 0.172 ± 0.14 | 0.370 ± 0.40 | 0.043 ± 0.04 | 1.02 ± 0.98 | |
Arithmetic means ± standard deviations are shown for products for which 2 or more samples were available. Otherwise, results of single sample analyses are shown.
Total TSNA, sum of NNN, NNK, NNAL, NAT, and NAB
The sum of all five measured TSNA ranged from 0.17 μg/g dry weight in a sample of betel quid to 81.0 μg/g dry weight in Chaini Khaini (Table 3). Overall, levels of TSNA were higher in manufactured than in vendor-made products. The sum of the three carcinogenic TSNA – NNN, NNK, and NNAL – was the highest in Chaini Khaini (41.4±8.2 μg/g dry weight), followed by Tambakhu Gai Chhap (19.8±1.6 μg/g dry weight), and the lowest levels were found in betel quid (0.61±0.54 μg/g dry weight).
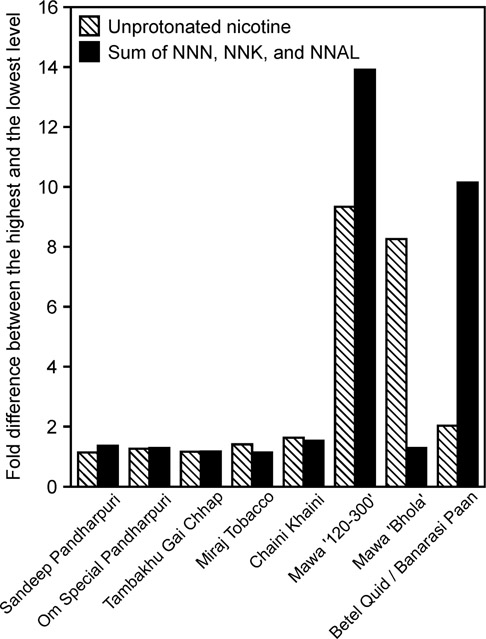
Vendor-to-vendor comparison of constituent levels in individual products showed the largest variation for the vendor-made products (Figure 2). Levels of unprotonated nicotine in mawa ‘120–300’ varied more than 9-fold among the three vendors, ranging from 0.2 to 1.9 mg/g dry weight. The sum of NNN, NNK, and NNAL in mawa ‘120–300’ and in Banarasi Paan betel quid varied 14-fold and 10-fold, respectively, across the vendors. Among the manufactured products, the variation of unprotonated nicotine and carcinogenic TSNA was slightly higher in Chaini Khaini and Miraj tobacco than in other products.
Figure 2.
Variation of unprotonated nicotine and the sum of carcinogenic TSNA in products purchased from various vendors.
DISCUSSION
This study reports the results of chemical analyses performed on a range of manufactured and vendor-made smokeless tobacco products purchased in Mumbai, India. To provide insights into the potential vendor-to-vendor variation in the chemical composition of tested products, samples of each product variety were purchased at three different markets. We found more than 300-fold difference in unprotonated nicotine content, and more than 650-fold difference in the total TSNA content, across eight tobacco products that are commonly used in Mumbai. Vendor-to-vendor variations in constituent levels have been also observed for some products.
Effect of product type on constituent levels
Both the similarities and the differences of constituent profiles among the individual products within the same categories provide important insights into the addictive and carcinogenic potential of various product types.
(i). Manufactured tobacco that consumers mix with lime before use.
These products contained higher levels of total nicotine than other products analyzed in this study, ranging from 30.1 to 53.4 mg/g dry weight. The relatively low pH of these products (range, 5.13 – 6.25) leads to only small proportion of total nicotine being present in the unprotonated form (Table 2). However, the amount of lime that a user may add to these products can potentially increase the pH above 10 and thus convert up to 100% of nicotine into unprotonated, biologically available form that is rapidly absorbed into the bloodstream and is associated with addiction.16 Therefore, consumers using the same product type may be exposed to unprotonated nicotine at levels that range from as low as 0.05 mg/g to more than 40 mg/g product (as is, or “wet” weight). Such elasticity suggests that this category of products may be appealing to a wide range of users, from young people who initiate tobacco use to established users who are highly dependent and require high doses of biologically available nicotine.16,30 This category of products may also serve as a flexible option for the “graduation” from low to high nicotine intake in the same user.31 Two of the three products in this category – Sandeep Pandharpuri and Om Special Pandharpuri – had similar levels of TSNA (Table 3). The relative ratios between carcinogenic TSNA were similar in these products as well, suggesting that similar tobacco type and processing method was used in the preparation of both products. Total TSNA content in the third product, Tambakhu Gai Chhap, is 3 to 4 fold higher, and the relative content of various TSNA is different, than in the Pandharpuri varieties, suggesting different tobacco processing and/or type. These observations suggest that there could be substantial variations in carcinogenic potential across tobacco products that may be perceived as very similar based on their packaging, nicotine content, and the intended mode of use.
(ii). Manufactured alkaline tobacco.
Overall, Miraj and Chaini Khaini had different constituent profiles than samples discussed above. These products are ready-to-use formulations with high pH (ranging from 9.10 to 10.75) resulting in high unprotonated nicotine content (Table 2). Such products can be highly addictive,16,32 and this perhaps explains the reason for prevalence of khaini use in India being by far the highest (11.6% vs. the next group, gutka – 8.2%).2 Moisture content in these products was higher than that of other manufactured products in this study, with Miraj tobacco containing moisture at levels similar to the U.S. moist snuff (43.4±1.1 %). The levels of TSNA were drastically different between Miraj and Chaini Khaini, and different from other manufactured products. The sum of NNN, NNK, and NNAL in Miraj tobacco was the lowest among the manufactured brands, 3.18±0.23 μg/g dry weight, and in Chaini Khaini it was the highest, 41.4±8.2 μg/g dry weight (Table 3). We previously reported these extremely high levels of TSNA in Chaini Khaini,24 these results are consistent with other reports on TSNA levels in khaini tobacco products.15,33 Both the total nicotine content (Table 2) and the relative amounts of individual TSNA (Table 3) are different between Miraj and Chaini Khaini, again indicating that different tobacco types and/or tobacco processing methods are used in the preparation of these products.
(iii). Vendor-made mixed products containing tobacco, areca nut, and other ingredients.
In these products, tobacco accounts for only a small proportion of total content (Table 1, Figure 1). Therefore, the levels of tobacco constituents in these products are relatively low as compared to manufactured products in which tobacco is the primary ingredient (Tables 2 and 3). It should be noted, however, that these products contain areca nut, which contains additional addictive and carcinogenic constituents, such as areca alkaloids and areca-specific nitrosamines.11,34–36 The use of areca nut-containing products, even without tobacco, has been strongly associated with non-malignant and carcinogenic effects, particularly with oral and esophageal cancers.11,36 It is not known how the combined exposures to tobacco- and areca-derived toxicants and carcinogens affect the addictive and carcinogenic potential of these products.
Constituent variations by place of purchase
Variations in nicotine and TSNA levels were observed for both the manufactured and the vendor-made products. Among the manufactured products, vendor-to-vendor differences in levels of nicotine were larger for the more complex products Miraj and Chaini Khaini than for the products that contain only tobacco. This could be due to the potentially higher batch-to-batch variations in complex product preparations. Some variations in TSNA levels were observed across all manufactured products, with levels of individual TSNA being generally similar in duplicate samples purchased from the same vendor. The levels of TSNA in manufactured tobacco products can be affected by both the batch differences and the storage conditions. Overall, the variation of constituent levels was modest in manufactured products: when expressed per gram product (wet weight), maximum unprotonated nicotine variation was 1.65-fold, and maximum variation in the sum of NNN, NNK, and NNAL was 1.55-fold (both in Chaini Khaini) (Figure 2). It is possible, however, that larger variations would be observed in the same products purchased in different seasons or in different parts of India.
Vendor-made products, such as mawa and betel quid, are prepared by various vendors who are mixing ingredients immediately prior to selling the product to individual customers. Our results demonstrate that this results in high variability of constituent levels across different preparations of the same product (Figure 2). There is a need to better characterize the ranges of important toxic and carcinogenic constituents in such products, and resulting exposures and cancer risk in their users. Longitudinal biomarker-based studies would be critical in understanding the levels of tobacco constituent exposures in users of vendor-made products, and whether the potential day-to-day variability in their tobacco toxicant and carcinogen intake affects product use and cancer risk.
IMPLICATIONS FOR TOBACCO REGULATION
Nicotine is the major known addictive constituent in tobacco, and tobacco-specific N-nitrosamines are the most prevalent strong carcinogens in smokeless tobacco, widely believed to play significant causative role in oral, esophageal, and pancreatic cancer in people who use these products.37–43 Over the years, isolated studies have reported on the levels of these important constituents in specific smokeless products marketed in India.15,33,38,44–49 However, differences in analytical methodologies and the lack of a standardized approach to collection and analysis of product samples did not allow for an accurate assessment of inter- and intra-product variability of these constituents. Our study addresses this gap and offers a snapshot of the extent of nicotine and TSNA variation in products commonly used in Mumbai, showing several hundred-fold variations in the levels of these constituents across different products types, as well as substantial vendor-to-vendor variation within some product categories. Our findings emphasize the importance of systematic product surveillance in India, in order to better understand exposures and product-associated cancer risks in users, and to identify effective approaches to tobacco product regulation.
Acknowledgements.
The authors thank Bob Carlson for editorial assistance. The study was supported by NCI Contract HHSN261201200392P and by the startup funds from the Masonic Cancer Center, University of Minnesota to IS. Mass-spectrometry analyses were carried out in the Analytical Biochemistry Shared Resource of the Masonic Cancer Center, supported in part by grant CA-77598 from the NCI.
Footnotes
Conflict of Interest Disclosure
All authors of this article declare they have no conflicts of interest.
References
- 1.Bhawna G Burden of smoked and smokeless tobacco consumption in India - results from the Global Adult Tobacco Survey India (GATS-India) - 2009–2011. Asian Pac J Cancer Prev. 2013;14:3323–3329. [DOI] [PubMed] [Google Scholar]
- 2.WHO. Global Adult Tobacco Survey. GATS India 2009–2010. 2010. New Delhi, India, Ministry of Health and Family Welfare, Government of India. [Google Scholar]
- 3.NCI/CDC. Smokeless Tobacco and Public Health: A Global Perspective. Rockville, MD: U.S. Department of Health and Human Services, National Cancer Institute and Centers for Disease Control and Prevention; 2014. [Google Scholar]
- 4.Arora M and Madhu R. Banning smokeless tobacco in India: Policy analysis. Indian J Cancer. 2012;49:336–341. [DOI] [PubMed] [Google Scholar]
- 5.Dhumal GG and Gupta PC. Assessment of gutka ban in Maharashtra: Findings from a focus group discussion. Int J Head Neck Surg. 2013;4:115–118. [Google Scholar]
- 6.Pimple S, Gunjal S, Mishra GA, et al. Compliance to gutka ban and other provisions of COTPA in Mumbai. Indian J Cancer. 2014;51:s60–s66. [DOI] [PubMed] [Google Scholar]
- 7.International Agency for Research on Cancer. Smokeless tobacco and tobacco-specific nitrosamines. In IARC Monographs on the Evaluation of Carcinogenic Risks to Humans, v. 89. Lyon, FR: IARC; 2007. [PMC free article] [PubMed] [Google Scholar]
- 8.Zhou J, Michaud DS, Langevin SM, et al. Smokeless tobacco and risk of head and neck cancer: Evidence from a case-control study in New England. Int J Cancer. 2013;132:1911–1917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.International Agency for Research on Cancer. Tobacco habits other than smoking: betel quid and areca nut chewing and some related nitrosamines. In IARC Monographs on the Evaluation of the Carcinogenic Risk of Chemicals to Humans, vol. 37. Lyon, FR: IARC; 1985. p. 37–202. [PubMed] [Google Scholar]
- 10.Gupta PC and Ray CS. Smokeless tobacco and health in India and South Asia. Respirology. 2003;8:419–431. [DOI] [PubMed] [Google Scholar]
- 11.International Agency for Research on Cancer. Betel-quid and areca-nut chewing and some arece-nut-derived nitrosamines. In IARC Monographs on the Evaluation of Carcinogenic Risks to Humans, vol. 85. Lyon, FR: IARC; 2004. [PMC free article] [PubMed] [Google Scholar]
- 12.Reddy KS, Shah B, Varghese C, Ramadoss A. Responding to the threat of chronic diseases in India. Lancet. 2005;366:1746–1751. [DOI] [PubMed] [Google Scholar]
- 13.Moore SR, Johnson NW, Pierce AM, Wilson DF. The epidemiology of mouth cancer: a review of global incidence. Oral Dis. 2000;6:65–74. [DOI] [PubMed] [Google Scholar]
- 14.Stanfill S and Stepanov I. Chapter 3: A Global View of Smokeless Tobacco Products. In U.S.Department of Health and Human Services CfDCaPaNIoHNCI (ed.), Smokeless Tobacco and Public Health: A Global Perspective. Bethesda, MD: 2014. [Google Scholar]
- 15.Stanfill SB, Connoly GN, Zhang L, et al. Global surveillance of oral tobacco products: total nicotine, unionized nicotine and tobacco-specific N-nitrosamines. Tob Control. 2011;20:e2 doi: 10.1136/tc.2010.037465. [DOI] [PubMed] [Google Scholar]
- 16.Tomar SL and Henningfield JE. Review of the evidence that pH is a determinant of nicotine dosage from oral use of smokeless tobacco. Tob Control. 1997;6:219–225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Djordjevic MV, Hoffmann D, Glynn T, Connolly GN. US Commercial brands of moist snuff, 1994. I. Assessment of nicotine, moisture and pH. Tob Control. 1995;4:62–66. [Google Scholar]
- 18.Richter P, Hodge K, Stanfill S, et al. Surveillance of moist snuff: total nicotine, moisture, pH, unionized nicotine, and tobacco-specific nitrosamines. Nicotine Tobacco Res. 2008;10:1645–1652. [DOI] [PubMed] [Google Scholar]
- 19.Andersen RA, Burton HR, Fleming PD, Hamilton-Kemp TR. Effect of storage conditions on nitrosated, acylated, and oxidized pyridine alkaloid derivatives in smokeless tobacco products. Cancer Res. 1989;49:5895–5900. [PubMed] [Google Scholar]
- 20.Djordjevic MV, Fan J, Bush LP, et al. Effects of storage conditions on levels of tobacco-specific N-nitrosamines and N-nitrosamino acids in U.S. moist snuff. J Agric Food Chem. 1993;41:1790–1794. [Google Scholar]
- 21.Stepanov I, Biener L, Knezevich A, et al. Monitoring tobacco-specific N-nitrosamines and nicotine in novel Marlboro and Camel smokeless tobacco products: Findings from Round I of the New Product Watch. Nicotine Tobacco Res. 2012;14:274–281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Stepanov I, Jensen J, Biener L, et al. Increased pouch sizes and resulting changes in the amounts of nicotine and tobacc-specific N-nitrosamines in single pouches of Camel Snus and Marlboro Snus. Nicotine Tobacco Res. 2012;14:1241–1245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Stepanov I, Biener L, Yershova K, et al. Monitoring tobacco-specific N-nitrosamines and nicotine in novel smokeless tobacco products: Findings from Round 2 of the New Product Watch. Nicotine Tobacco Res. 2014;16:1070–1078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Stepanov I, Gupta PC, Dhumal G, et al. High levels of tobacco-specific N-nitrosamines and nicotine in Chaini Khaini, a product marketed as snus. Tob Control. 2014;24:e271–e274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Stepanov I, Jensen J, Hatsukami D, Hecht SS. New and traditional smokeless tobacco: comparison of toxicant and carcinogen levels. Nicotine Tobacco Res. 2008;10:1773–1782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Stepanov I, Knezevich A, Zhang L, et al. Carcinogenic tobacco-specific N-nitrosamines in US cigarettes: three decades of remarkable neglect by the tobacco industry. Tob Control. 2012;21:44–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yershova K, Yuan JM, Wang R, et al. Tobacco-specific N-nitrosamines and polycyclic aromatic hydrocarbons in cigarettes smoked by the participants of the Shanghai Cohort Study. Int J Cancer. 2016;139:1261–1269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Murphy SE, Villalta P, Ho SW, von Weymarn LB. Analysis of [3’,3’-d(2)]-nicotine and [3’,3’-d(2)]-cotinine by capillary liquid chromatography-electrospray tandem mass spectrometry. J Chromatogr B Analyt Technol Biomed Life Sci. 2007;857:1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Richter P and Spierto FW. Surveillance of smokeless tobacco nicotine, pH, moisture, and unprotonated nicotine content. Nicotine Tobacco Res. 2003;5:885–889. [DOI] [PubMed] [Google Scholar]
- 30.Henningfield JE, Fant RV, Tomar SL. Smokeless tobacco: An addicting drug. Advances in Dental Research. 1997;11:330–335. [DOI] [PubMed] [Google Scholar]
- 31.Alpert HR, Koh H, Connolly GN. Free nicotine content and strategic marketing of moist snuff tobacco products in the United States: 2000–2006. Tob Control. 2008;17:332–338. [DOI] [PubMed] [Google Scholar]
- 32.Hatsukami DK and Severson HH. Oral spit tobacco: addiction, prevention and treatment. Nicotine Tobacco Res. 1999;1:21–44. [DOI] [PubMed] [Google Scholar]
- 33.Stepanov I, Hecht SS, Ramakrishnan S, Gupta PC. Tobacco-specific nitrosamines in smokeless tobacco products marketed in India. Intl J Cancer. 2005;116:16–19. [DOI] [PubMed] [Google Scholar]
- 34.Nair J, Ohshima H, Friesen M, et al. Tobacco-specific and betel nut-specific N-nitroso compounds: occurrence in saliva and urine of betel quid chewers and formation in vitro by nitrosation of betel quid. Carcinogenesis. 1985;6:295–303. [DOI] [PubMed] [Google Scholar]
- 35.Stich HF, Rosin MP, Brunnemann KD. Oral lesions, genotoxicity and nitrosamines in betel quid chewers with no obvious increase in oral cancer risk. Cancer Lett. 1986;31:15–25. [DOI] [PubMed] [Google Scholar]
- 36.International Agency for Research on Cancer. Personal habits and indoor combusion: Betel quid and areca nut. In IARC Monographs on the Evaluation of Carcinogenic Risks to Humans, Vol. 100E. Lyon, France: 2012. [Google Scholar]
- 37.Hecht SS and Hoffmann D. The relevance of tobacco-specific nitrosamines to human cancer. Cancer Surv. 1989;8:273–294. [PubMed] [Google Scholar]
- 38.Hecht SS and Hoffmann D. Tobacco-specific nitrosamines, an important group of carcinogens in tobacco and tobacco smoke. Carcinogenesis. 1988;9:875–884. [DOI] [PubMed] [Google Scholar]
- 39.Hecht SS. Tobacco carcinogens, their biomarkers, and tobacco-induced cancer. Nature Rev Cancer. 2003;3:733–744. [DOI] [PubMed] [Google Scholar]
- 40.Magee PN. The experimental basis for the role of nitroso compounds in human cancer. Cancer Surv. 1989;8:207–239. [PubMed] [Google Scholar]
- 41.Preston-Martin S and Correa P. Epidemiological evidence for the role of nitroso compounds in human cancer. Cancer Surv. 1989;8:459–473. [PubMed] [Google Scholar]
- 42.Magee PN. Nitrosamines and human cancer: introduction and overview. Eur J Cancer Prev. 1996;5:7–10. [DOI] [PubMed] [Google Scholar]
- 43.Bartsch H and Spiegelhalder B. Environmental exposure to N-nitroso compounds (NNOC) and precursors: an overview. Eur J Cancer Prev. 1996;5:11–18. [PubMed] [Google Scholar]
- 44.Hoffmann D, Brunnemann KD, Prokopczyk B, Djordjevic MV. Tobacco-specific N-nitrosamines and areca-derived N-nitrosamines: chemistry, biochemistry, carcinogenicity, and relevance to humans. J Toxicol Environ Health. 1994;41:1–52. [DOI] [PubMed] [Google Scholar]
- 45.Hoffmann D, Djordjevic MV, Fan J, et al. Five leading U.S. commercial brands of moist snuff in 1994 - Assessment of carcinogenic N-nitrosamines. J Natl Cancer Inst. 1995;87:1862–1869. [DOI] [PubMed] [Google Scholar]
- 46.Nair J, Pakhale SS, Bhide SV. Carcinogenic tobacco-specific nitrosamines in Indian tobacco products. Food Chem Toxicol. 1989;27:751–753. [DOI] [PubMed] [Google Scholar]
- 47.Brunnemann KD, Genoble L, Hoffmann D. N-Nitrosamines in chewing tobacco: an international comparison. J Agric Food Chem. 1985;33:1178–1181. [Google Scholar]
- 48.Tricker AR and Preussmann R. The occurrence of N-nitroso compounds [corrected] in zarda tobacco. Cancer Lett. 1988;42:113–118. [DOI] [PubMed] [Google Scholar]
- 49.Tricker AR and Preussmann R. The occurrence of N-nitroso compounds in kiwam tobacco. Cancer Lett. 1989;46:221–224. [DOI] [PubMed] [Google Scholar]