Abstract
Background:
Fatigue is a common and expected side effect of cancer treatment. However, most studies have focused on average levels of fatigue, which may obscure important individual differences in the severity and course of fatigue over time. The current study was designed to identify distinct trajectories of fatigue from diagnosis into survivorship in a longitudinal study of women with early-stage breast cancer.
Methods:
Women with Stage 0-IIIA breast cancer (n=270) were recruited before (neo)adjuvant therapy with radiation, chemotherapy, and/or endocrine therapy and completed assessments at baseline, post-treatment, and 6, 12, and 18-month follow-ups. Growth mixture modeling was used to identify trajectories of fatigue, and differences among the trajectory groups on demographic, medical, and psychosocial variables were examined.
Results:
Five distinct trajectories of fatigue were identified: Stable Low (66%), with low levels of fatigue across assessments; Stable High (13%), with high fatigue across assessments; Decreasing (4%), with high fatigue at baseline that resolved over time; Increasing (9%), with low fatigue at baseline that increased over time; and Reactive (8%), with increased fatigue after treatment that resolved over time. Both psychological and treatment-related factors were associated with fatigue trajectories, with psychological factors most strongly linked to high fatigue at the beginning and over the course of treatment.
Conclusion:
There is considerable variability in the experience of fatigue among women with early-stage breast cancer. Although most women report relatively low fatigue, those with a history of depression and elevated psychological distress may be at risk for more severe and persistent fatigue.
Introduction
Fatigue is a well-known side effect of cancer diagnosis and treatment and has a negative impact on all aspects of quality of life 1,2. On average, cancer-related fatigue tends to increase during cancer treatment and remit within a year after treatment completion 3-6. However, these averages mask considerable individual variability in the severity and course of fatigue. There is growing evidence that fatigue may be elevated even before treatment onset in some patients 7, and that a subset of survivors continues to experience fatigue one or more years after successful treatment 8,9. In contrast, some patients may experience very little fatigue, despite intensive treatment. Few longitudinal studies have examined individual differences in the course of fatigue from diagnosis into survivorship to identify distinct profiles of fatigue over time, despite important implications for developing a more precise understanding of cancer-related fatigue and implementing personalized interventions.
Research on psychological adjustment to stressful life events has increasingly used data-driven modeling approaches to identify distinct profiles or trajectories of distress over time 10. This work has highlighted the diversity of psychological responses to stress and challenged the notion that distress is ubiquitous in the aftermath of major life events, including cancer diagnosis and treatment. For example, in 460 breast cancer patients who were assessed longitudinally for 12 months after invasive breast cancer diagnosis, four distinct trajectories of depressive symptoms were identified, including two “resilient” groups with low (32%) or very low depressive symptoms (11%), a “recovery” group with high symptoms that declined over time (20%), and a “chronic” group with persistent high levels of depression (38%) 11. Interestingly, the most common profile here, as in other studies of psychological responses, is the “resilient” group characterized by low distress over time.
A number of studies have applied this data-driven approach to identify trajectories of fatigue during and after cancer treatment. Based on longitudinal studies documenting overall increases in fatigue during treatment 3-6, it might be expected that most patients would show increases in fatigue during treatment that resolve after treatment completion. Indeed, two studies conducted with breast cancer patients undergoing chemotherapy identified groups that showed increases in fatigue after treatment onset 12,13. However, these groups varied considerably in their pre-treatment fatigue levels as well as the extent and duration of the increase in fatigue during treatment. Importantly, both studies also identified groups with low levels of fatigue across the assessment period, which included between one-quarter 12 and one-half of patients 13. Further, both studies identified groups with high fatigue at pre-treatment that remained elevated over time. Two additional studies assessed breast cancer patients before and for 6-8 months after surgery, when many were receiving adjuvant therapy14,15. Both studies identified two fatigue groups, one with high fatigue and one with low fatigue, although the percentage of women classified in each group varied across the two reports. Of note, both studies found that women exposed to chemotherapy were more likely to be in the high fatigue group.
Another two studies focused on post-treatment fatigue trajectories in women with early-stage breast cancer. One report assessed women after treatment completion and at 2, 4, and 6-month follow-ups and identified two distinct trajectory groups, one with high fatigue (67%) and one with low fatigue (33%) across the assessment period 16. In a study of breast cancer survivors assessed over a longer (4 year) post-treatment period, we identified five distinct trajectories of fatigue, including very low, low, high, recovery, and late fatigue groups, indicating that more diverse fatigue profiles may emerge in the year after treatment completion 17. Importantly, neither of these studies included a pre-treatment assessment, so it is unclear to what extent fatigue at post-treatment was influenced by baseline characteristics or by treatment exposures. This is important because patients with high levels of fatigue at treatment onset may differ considerably from patients whose fatigue is driven primarily by adjuvant therapies.
The current study was designed to identify trajectories of fatigue from diagnosis into survivorship in women with breast cancer and to characterize patients in each trajectory. Data came from the RISE study, a longitudinal, observational study of women with early-stage breast cancer. Drawing upon an empirically-based biobehavioral model, we examined demographic, medical, and psychosocial factors that have been linked with fatigue in previous research 18. Of particular interest were childhood adversity and depression, which have emerged as significant predictors of fatigue during and after cancer treatment17. Indeed, initial analyses in the RISE sample found links between childhood adversity, depression history, and fatigue at the pre-treatment assessment 19. We also assessed medical and psychosocial factors that are correlated with fatigue and may drive its onset and persistence. Previous studies of cancer-related fatigue trajectories have shown that medical comorbidities4,15, body mass index16,17, physical activity14,16, sleep disturbance17, and cancer-related stress20 are each associated with fatigue and were included in the current report.
METHODS
Patients and Procedures:
Patients were recruited from oncology practices in Los Angeles to participate in a longitudinal, observational study of cancer-related fatigue (RISE study). Women were eligible if they had been recently diagnosed with Stage 0-IIIA breast cancer, had not yet started adjuvant or neoadjuvant therapy with radiation, chemotherapy, or endocrine therapy, and were English proficient. Primary recruitment sites were UCLA and Cedars Sinai Medical Center (CSMC).
Participants completed assessments at baseline, end of treatment (for those who received radiation and/or chemotherapy), and at 6, 12, and 18-month post-treatment follow-ups. The majority of women (91%) had completed surgery (lumpectomy or mastectomy) at the time of enrollment; the remaining 9% were enrolled before neoadjuvant therapy and thus had not had surgery at baseline. The Institutional Review Boards at UCLA and CSMC approved the study, and all participants provided written informed consent.
Measures:
Data were collected through self-report questionnaires, interviews, blood collection, and medical chart review.
Fatigue was assessed with the General Fatigue subscale of the Multidimensional Fatigue Symptom Inventory-Short Form, 21,22, which assesses the degree to which respondents felt tired, worn out, sluggish, fatigued, run down, and pooped in the past week.
Demographic characteristics were obtained from self-report at baseline and included age, race/ethnicity, marital status, income, education, employment status, and presence of children at home.
Disease and treatment-related information was obtained from medical record abstraction and included cancer stage, type of surgery received (mastectomy or lumpectomy), and type of adjuvant therapy (radiation, chemotherapy, and/or endocrine therapy).
Pre-cancer medical co-morbidities were assessed with a questionnaire version of the Charlson Co-morbidity Scale 23. Height and weight were measured at baseline for determination of body mass index. Physical activity was assessed using the Godin Leisure Time Exercise Questionnaire, which assesses the number of times per week an individual performs strenuous, moderate, and mild forms of exercise24.
History of childhood maltreatment was assessed with the Childhood Trauma Questionnaire 25, which includes questions about physical, emotional, and sexual abuse as well as physical and emotional neglect that occurred during childhood. Women were categorized into one of three maltreatment groups using a scoring algorithm with established sensitivity and specificity 26, and analyses combined the two maltreatment groups to compare those with vs. without a history of maltreatment.
History of major depressive disorder (MDD) prior to cancer diagnosis was determined using the Structured Clinical Interview for DSM-IV-TR Axis I Disorders (SCID). The SCID was administered by trained interviewers and interviews were reviewed and scored by a consensus panel, led by a psychiatrist with expertise in depression (MI).
Depressive symptoms were assessed with the Center for Epidemiologic Studies - Depression Scale (CES-D),27 with scores of 16 or above suggesting clinically significant depressive symptoms.
Sleep quality was assessed with the Pittsburgh Sleep Quality Index (PSQI)28, with scores above 5 suggesting clinically significant sleep disturbance.
Cancer-related distress was assessed with the Intrusions subscale of the Impact of Event Scale (IES)29, which assesses the frequency of intrusive thoughts and feelings about breast cancer in the last week.
Statistical Analysis
Growth mixture modeling, also called latent class mixed models for Gaussian longitudinal outcomes, was conducted using the R package lcmm 30, with the MFSI General scale as the dependent variable. A latent class mixed model assumes that the population is composed of a certain number of latent classes of subjects, with each class having a characteristic mean trajectory. The mean trajectories are modeled using linear mixed models. The number of latent classes, M, as well as the mean trajectories of each latent class are not known in advance. Modeling proceeds by fitting a series of models specifying varying numbers of latent classes and varying linear mixed model complexity and comparing model fit indices. We fit models specifying 1 to 5 latent classes and linear mixed model complexity ranging from intercept and slope parameters varying within class to intercept, slope, quadratic and cubic parameters varying within class. Because the timing of assessments was anchored to specific events, time was modeled as discrete. When selecting among models, we restricted attention to models for which the smallest class had at least 10 participants to avoid solutions with very small class sizes. Model fit was compared using AIC, BIC, sample size-adjusted BIC (SABIC) and entropy. Once the final model was selected, each participant was assigned to the latent class for which they had the highest class-membership probability, based on posterior calculations.
After identifying the best fitting latent class model and assigning each participant to a latent class, we tested for differences among the latent classes on demographic, disease, treatment and psychosocial variables. Fisher exact tests (categorical variables) and analysis of variance (continuous variables) were used to obtain omnibus p-values for bivariate associations between each variable and latent class membership. Fisher exact tests (categorical variables) and t-tests (continuous variables) were used to obtain p-values for pairwise comparisons of latent classes for variables with omnibus p-values of p ≤ 0.10. Pairwise comparisons with p-values < 0.05 are reported.
RESULTS
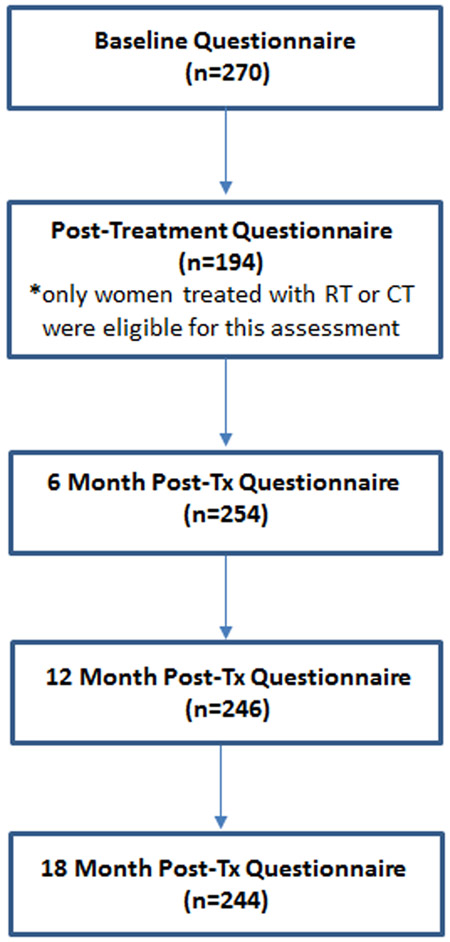
Enrollment began in January 2013 and ended in July 2015. Over this period, 409 women were screened, 302 of whom met initial eligibility criteria and consented for participation. Thirty-two women were later determined to be ineligible (n=5) or failed to complete the baseline questionnaire (n=27). Thus, the full analytic sample included 270 at baseline. The number of women completing each of the follow-up visits is shown in Figure 1. Ten women were lost to follow-up over the study period and 11 women asked to be withdrawn from the study. In total, 244 women completed the final (18 month) post-treatment questionnaire, a 90% retention rate.
Figure 1.
Flow chart indicating the number of patients who completed each assessment. CT indicates chemotherapy; RT, radiotherapy; Tx, treatment.
Identifying Fatigue Trajectories
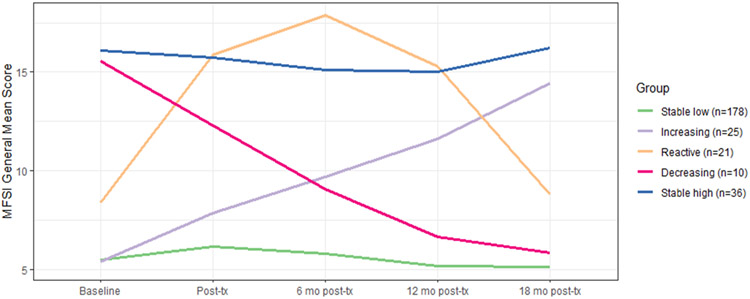
Model comparison identified a 5-class solution as the best fitting model based on AIC, sample size-adjusted BIC and entropy (see Supplemental Tables S1, S2 and S3 and Supplemental Figure S1 for details). The mean MFSI-SF General Fatigue trajectories for the five latent classes are displayed in Figure 2. The groups were labeled Stable Low (66% of sample), Stable High (13% of sample), Decreasing (high initial fatigue that declined over time; 4%), Reactive (increasing then decreasing fatigue; 8%), and Increasing (initial low fatigue that increased over time; 9%). The posterior classification probabilities indicated that the model had very good discriminant ability, with participants classified into specific latent classes with minimal ambiguity (see Supplementary material). Mean (SD) fatigue levels on the MFSI-general subscale for each trajectory group at the baseline assessment were as follows: Stable Low, 5.6 (4.0); Stable High, 17.0 (3.1); Decreasing, 17.2 (4.0); Reactive, 8.3 (5.0); and Increasing, 4.9 (3.3). For reference, in the validation study for the MFSI, the mean score on the MFSI-general subscale was 5.06 in non-cancer controls and 7.28 in a combined group of breast cancer patients undergoing treatment and survivors who had completed treatment21.
Figure 2. Mean trajectories of MFSI General Fatigue scores for the five latent classes.
Mean scores on the MFSI-General Fatigue scale for the latent trajectory groups at each study assessment: baseline (before onset of adjuvant therapy), post-treatment (after completion of radiation and/or chemotherapy, if received); and 6-mo, 12-mo, and 18-mo post-treatment follow-ups. Five trajectory groups were identified, including Stable Low (the largest group; 66% of sample), Decreasing (4%), Reactive (8%), Increasing (9%), and Stable High (13% of sample).
Characterizing Fatigue Trajectory Groups
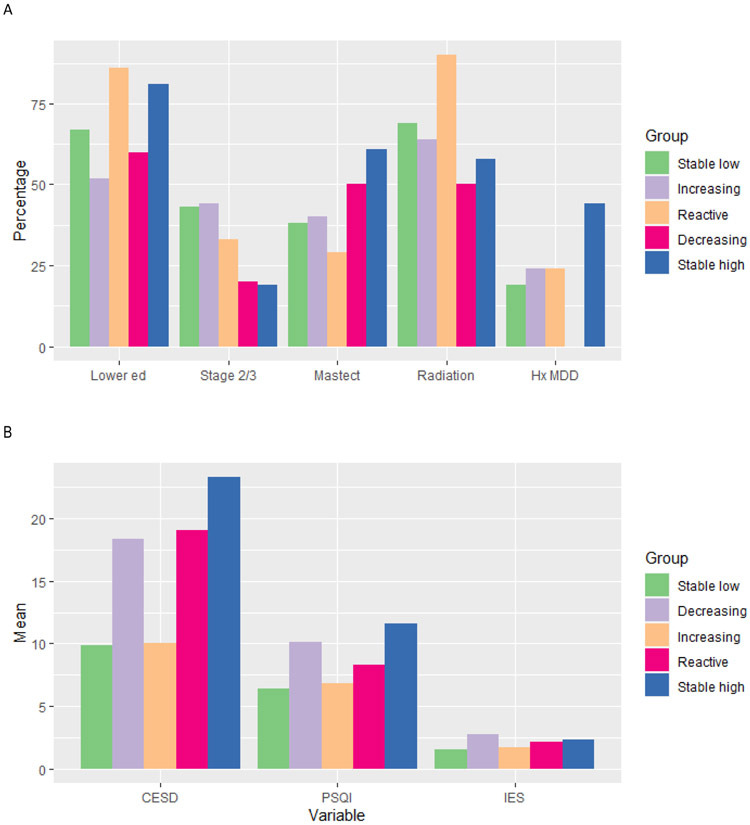
Table 1 shows descriptive statistics for potential predictors by latent trajectory group along with tests for bivariate relations. There were significant or marginally significant associations between fatigue group and education, disease stage, type of surgery, receipt of radiation therapy, history of depression, and psychosocial risk factors (all omnibus ps ≤ .10). Figure 3 provides covariate profiles for the five groups, visually characterizing each group on all variables with omnibus ps<0.10.
Table 1.
Bivariate associations of patient factors with latent trajectory classes (N = 270)
| Overall N = 270 |
1: Stable Low N = 178 (66%) |
2: Increasing N = 25 (9%) |
3: Reactive N = 21 (8%) |
4: Decreasing N = 10 (4%) |
5: Stable High N = 36 (13%) |
Omnibus p-value |
Pairwise comparisons with p<0.05 |
|
|---|---|---|---|---|---|---|---|---|
| Demographic and general health | ||||||||
| Age: mean (SD) | 56 (11) | 57 (11) | 55 (13) | 55 (13) | 51 (11) | 54 (9) | .24 | |
| BMI: mean (SD) | 24.4 (5.8) | 25.4 (6.0) | 25.6 (5.1) | 24.5 (5.5) | 24.5 (7.1) | 26.1 (5.5) | .89 | |
| Race/ethnicity | .37 | |||||||
| White | 203 (75) | 133 (75) | 18 (72) | 16 (76) | 8 (80) | 28 (78) | ||
| Black | 12 (4) | 6 (4) | 4 (16) | 1 (5) | 1 (10) | 0 (0) | ||
| Asian | 30 (11) | 23 (13) | 2 (8) | 2 (10) | 0 (0) | 3 (8) | ||
| Other | 25 (9) | 16 (9) | 1 (4) | 2 (10) | 1 (10) | 5 (14) | ||
| Income | .80 | |||||||
| Under $60K | 67 (25) | 39 (22) | 7 (29) | 7 (35) | 3 (33) | 11 (31) | ||
| $60-100K | 53 (20) | 34 (19) | 5 (21) | 3 (15) | 2 (22) | 9 (24) | ||
| $100K or more | 146 (55) | 104 (59) | 12 (50) | 10 (50) | 4 (44) | 16 (44) | ||
| Education | .063 | 2 vs 3 (p=.026) | ||||||
| College or less | 186 (69) | 120 (67) | 13 (52) | 18 (86) | 6 (60) | 29 (81) | 2 vs 5 (p=.025) | |
| Postgraduate | 84 (31) | 58 (33) | 12 (48) | 3 (14) | 4 (40) | 7 (19) | ||
| Employed | 161 (60) | 110 (62) | 16 (64) | 11 (52) | 4 (40) | 20 (56) | .58 | |
| Partnered | 174 (64) | 115 (65) | 12 (48) | 14 (67) | 8 (80) | 25 (69) | .35 | |
| Children at home | 189 (70) | 124 (70) | 15 (60) | 16 (76) | 8 (80) | 26 (72) | .71 | |
| Charlson Co-morbidity Scale: mean (SD) | 0.31 (0.62) | 0.30 (0.63) | 0.28 (0.46) | 0.24 (0.44) | 0.10 (0.32) | 0.47 (0.81) | .41 | |
| Godin Physical Activity Scale: median (IQR) | 21 (9-38) | 25 (13-41) | 21 (12-35) | 14 (6-29) | 14.5 (0-36) | 18 (9-37) | .18 | |
| Menopausal status at baseline | .44 | |||||||
| Pre- or perimenopausal | 84 (31) | 49 (28) | 9 (36) | 11 (52) | 4 (40) | 11 (31) | ||
| Postmenopausal | 170 (63) | 119 (67) | 14 (56) | 9 (43) | 6 (60) | 22 (61) | ||
| Hysterectomy | 16 (6) | 10 (6) | 2 (8) | 1 (5) | 0 (0) | 3 (8) | ||
| Disease and treatment-related | ||||||||
| Stage: | .06 | 1 vs 5 (p=.008) | ||||||
| 0 or I | 160 (62) | 95 (57) | 14 (56) | 14 (67) | 8 (80) | 29 (81) | 2 vs 5 (p=.049) | |
| II or III | 99 (38) | 72 (43) | 11 (44) | 7 (33) | 2 (20) | 7 (19) | ||
| Surgery, eventual | .08 | 1 vs 5 (p=.015) | ||||||
| None or lumpectomy | 159 (59) | 110 (62) | 15 (60) | 15 (71) | 5 (50) | 14 (39) | 3 vs 5 (p=.028) | |
| Mastectomy | 110 (41) | 67 (38) | 10 (40) | 6 (29) | 5 (50) | 22 (61) | ||
| Receipt of chemotherapy | .16 | |||||||
| None | 172 (64) | 109 (62) | 14 (56) | 13 (62) | 8 (80) | 28 (78) | ||
| Adjuvant | 73 (27) | 51 (29) | 11 (44) | 6 (29) | 1 (10) | 4 (11) | ||
| Neoadjuvant | 25 (9) | 17 (10) | 0 (0) | 2 (10) | 1 (10) | 4 (11) | ||
| Receipt of radiation | 183 (69) | 122 (69) | 16 (64) | 19 (90) | 5 (50) | 21 (58) | .08 | 1 vs 3 (p=.043) 2 vs 3 (p=.045) 3 vs 4 (p=.022) 3 vs 5 (p=.015) |
| Receipt of endocrine therapy | 168 (62) | 112 (63) | 20 (80) | 10 (48) | 6 (60) | 20 (56) | .20 | |
| Psychosocial | ||||||||
| Childhood maltreatment | .42 | |||||||
| None | 163 (60) | 109 (61) | 17 (68) | 13 (62) | 6 (60) | 18 (50) | ||
| Non-sexual | 75 (28) | 53 (30) | 4 (16) | 6 (29) | 2 (20) | 10 (28) | ||
| Sexual | 32 (12) | 16 (9) | 4 (16) | 2 (10) | 2 (20) | 8 (22) | ||
| History of major depressive disorder | 60 (23) | 33 (19) | 6 (24) | 5 (24) | 0 (0) | 16 (44) | .01 | 1 vs 5 (p=.002) 4 vs 5 (p=.009) |
| CES-D, baseline: mean (SD) | 12.7 (10.4) | 10.0 (8.0) | 8.4 (7.9) | 19.1 (13.5) | 19.6 (11.2) | 23.2 (11.2) | <.0001 | 1 vs 3 (p<.0001) 1 vs 4 (p=.0004) 1 vs 5 (p<.0001) 2 vs 3 (p=.002) 2 vs 4 (p=.002) 2 vs 5 (p<.0001) |
| PSQI, baseline: mean (SD) | 7.4 (4.0) | 6.5 (3.6) | 6.3 (2.9) | 8.3 (4.0) | 9.9 (3.4) | 11.5 (3.7) | <.0001 | 1 vs 3 (p=.037) 1 vs 4 (p=.004) 1 vs 5 (p<.0001) 2 vs 4 (p=.004) 2 vs 5 (p<.0001) 3 vs 5 (p=.003) |
| Clinical sleep disturbance (PSQI>5) | 166 (61) | 97 (55) | 13 (52) | 13 (62) | 8 (80) | 35 (97) | <.0001 | 1 vs 5 (p<.0001) 2 vs 5 (p<.0001) 3 vs 5 (p=.001) |
| IES, baseline: mean (SD) | 1.7 (1.3) | 1.5 (1.2) | 1.6 (1.3) | 2.1 (1.3) | 2.6 (1.4) | 2.3 (1.3) | .002 | 1 vs 4 (p=.011) 1 vs 5 (p=.001) 2 vs 5 (p=.048) |
Omnibus p-values are from Fisher exact tests (categorical variables) and analysis of variance (continuous variables). P-values for pairwise comparisons of latent classes are from Fisher exact tests (categorical variables) and t-tests (continuous variables). For the Godin, the Kruskal-Wallis rank test was used for omnibus and pairwise tests. CES-D, Center for Epidemiologic Studies Depression Scale; IES, impact of Events scale; PSQI, Pittsburgh Sleep Quality Index.
Figure 3. Covariate profiles for the five latent classes.
A. Categorical predictors of fatigue trajectory groups. Graphs represent percentage of patients in each trajectory class with lower educational status (college or less vs. post grad), stage 2 or 3 (versus 0 or 1), treated with mastectomy, radiation therapy, and with history of major depressive disorder (MDD).
B. Continuous predictors of fatigue trajectory groups. Graphs represent mean scores of patients in each trajectory class on depressive symptoms (CES-D), sleep disturbance (PSQI), and cancer-related distress (IES).
Two of the trajectory groups reported elevated fatigue at the pre-treatment assessment: Stable High and Decreasing. Women in the Stable High group had the highest rate of depression history (44%), significantly higher than the Stable Low and Decreasing groups. They also had the highest levels of depressive symptoms and sleep disturbance, with scores significantly higher than the Stable Low and Increasing fatigue groups (for CES-D) and the Stable Low, Increasing, and Reactive fatigue groups (for PSQI). In addition, they were significantly more likely to have had a mastectomy than the Stable Low or Reactive groups, and had lower stage disease than the Stable Low or Increasing groups. Women in the Decreasing group had the highest levels of cancer-related distress in the sample and also reported high levels of depressive symptoms and sleep disturbance, significantly higher than the Stable Low group (for all measures) and the Increasing group (for CES-D and PSQI). In contrast to the Stable High group, none of the 10 women in the Decreasing group had a history of depression.
Three of the trajectory groups reported relatively low fatigue at the baseline assessment: Stable Low, Reactive, and Increasing. Women in the Stable Low group were characterized by low levels of psychological distress, with significantly lower scores on the CES-D and IES than the Stable High and Decreasing groups and lower scores on the CES-D than the Reactive group. They also had lower scores on the PSQI than the Stable High, Decreasing, and Reactive groups, though their mean score was in the clinically-significant range (>5) on this measure. In general, the Stable Low group did not differ from the others on demographic, medical, or disease-related risk factors, though they were less likely to have received a mastectomy than women in the Stable High group and had higher stage disease.
The most notable feature of the Reactive group was treatment-related. This group was more likely to have received radiation therapy than any other group, with 90% undergoing radiation. Of note, among all women who received radiation, only 10% fell into the Reactive group and the majority (69%) were in the Stable Low fatigue trajectory, indicating that radiation did not inevitably induce fatigue. The Reactive group also reported higher levels of depressive symptoms at baseline than did the Stable Low and Increasing groups, and higher levels of sleep disturbance than the Stable Low group.
DISCUSSION
Fatigue is a common and expected side effect of cancer diagnosis and treatment. However, results from this study highlight considerable variability in fatigue severity and persistence among women with early-stage breast cancer. We identified five distinct trajectories of fatigue that represented very different patient experiences from diagnosis through the first 18 months of survivorship. Importantly, our findings indicate that many women with early-stage breast cancer experience relatively minimal fatigue over this period (Stable Low group; 66% of sample). Indeed, levels of fatigue in the Stable Low group were comparable to non-cancer controls 21. This is consistent with research on profiles of psychological responses to stress, where the majority of individuals demonstrate a “resilient” profile characterized by low distress 10.
Two previous studies of fatigue trajectories in post-treatment breast cancer survivors similarly identified groups with stable low fatigue, which comprised between 33% 16 to 44% 17 of the sample. It is possible that a higher percentage of women in the current study (66%) reported low fatigue due in part to changes in adjuvant therapies over time, with less use of anthracyclines and avoidance of chemotherapy due to genomic classifiers. Women in the low fatigue group also reported low levels of psychological distress, including depressive symptoms and cancer-related distress. Of note, although this group had lower levels of sleep disturbance than several other groups, 55% reported clinically-significant sleep disturbance, indicating that this is a common problem even among women with low fatigue.
Results also identified a high-risk group of women with elevated levels of fatigue across the assessment period (Stable High group; 13% of sample). Although this was not a large group, they reported very high levels of fatigue even before adjuvant therapy, with scores roughly three times higher than the Stable Low fatigue group (and non-cancer controls 21). This group also reported high levels of psychological distress and sleep disturbance; almost half (44%) had an interview-based history of major depressive disorder prior to their breast cancer diagnosis, and almost all (97%) had clinically significant sleep disturbance. There was also a small group of women with high fatigue at the pre-treatment assessment that declined over the assessment period (Decreasing group; 4% of sample). These women were quite distressed at baseline, with the highest levels of cancer-specific distress, but did not have a history of depression. Thus, a previous psychiatric history, in combination with high levels of behavioral symptoms at treatment onset, may set the stage for persistent fatigue across the cancer trajectory and help to identify vulnerable women for early intervention.
Previous studies support the importance of psychosocial factors as predictors of fatigue trajectories during and after treatment. In a large, representative sample of cancer patients undergoing chemotherapy (n=1332), the two high fatigue groups reported more lifetime stress exposure as well as higher levels of cancer-related stress20. In our previous study with post-treatment breast cancer survivors, psychological factors emerged as the strongest predictors of membership in the high fatigue groups, particularly depressive symptoms and childhood adversity 17.
Disease and treatment-related factors were also associated with fatigue trajectories, albeit to a lesser extent. Women in the Stable High fatigue group had the highest rate of mastectomy, and unexpectedly had lower stage disease than the Stable Low and Increasing groups. Further, radiation therapy was associated with the “reactive” fatigue profile. Previous studies have documented increases in mean levels of fatigue over the course of radiation therapy 31,32; the current findings suggest that there may be patients who are particularly vulnerable to radiation-induced fatigue, perhaps those who also have high levels of depressive symptoms (which were elevated in the Reactive group relative to the Stable Low and Increasing groups). In contrast, chemotherapy did not differentiate among the fatigue groups. We have previously shown that chemotherapy is associated with high initial post-treatment fatigue that decreases over time17, and other groups have similarly documented a link between chemotherapy exposure and high fatigue during and in the early aftermath of breast cancer treatment14,15. Our sample had relatively low levels of chemotherapy exposure, which may have contributed to differences with earlier reports.
These findings shed light on individual variability in the experience of fatigue among women with early stage breast cancer, but should be interpreted in light of several factors. First, the current sample is predominantly White and well-educated, reflecting the demographics of the cancer centers from which they were recruited, and may not generalize to other samples. Second, women in the RISE study had lower rates of chemotherapy than earlier samples investigated by our group 33-35 and others15, reflecting shifts in treatment recommendations for women with early stage disease as well as use of genomic classifiers that allow avoidance of chemotherapy. Third, it is possible that different trajectories would emerge over a longer follow-up period. This may be particularly relevant for capturing effects of endocrine therapy, which many women were still receiving at the 18-month follow-up. Fourth, we did not evaluate diurnal patterns of fatigue, which may have unique predictors20. Fifth, we focused on general fatigue, which includes feelings of being tired, worn out, and sluggish; other dimensions of fatigue (e.g., physical fatigue, cognitive fatigue) may have unique trajectories and predictors 19. Finally, because there was no comparison group of individuals without cancer, we cannot confirm that the observed trajectories were specific to breast cancer.
Summary and clinical recommendations:
Although cancer-related fatigue is typically thought to be a ubiquitous response to cancer treatment, findings from the current study strongly suggest that fatigue is instead highly variable across individual patients, particularly in the post-treatment period. If a patient with early-stage breast cancer reports elevated fatigue before adjuvant therapy, the odds that she will remain fatigued are high; in this sample, 78% of women with elevated fatigue at baseline did not spontaneously recover within 18 months after treatment, and the odds of persisting fatigue increased with pre-existing history of depression as well as elevated symptoms of depression, distress, and sleep disturbance. These symptoms are amenable to intervention 1,36,37, and connecting patients with appropriate treatments has the potential to improve fatigue and associated behavioral comorbidities. Among those with low fatigue before adjuvant therapy, prospects are good, with 80% expected to continue reporting low fatigue. These women would benefit from general recommendations to maintain physical activity and sleep during cancer treatment 1, but may not require more intensive or directed interventions. However, 20% of such individuals are likely to show increasing fatigue in the future - either a transient peak (9%) or a late increase in fatigue between 12 and 18 months (11%). Advising patients about these different trajectories, and conducing regular assessments of fatigue at clinic visits, can help identify vulnerable patients and intervene with those most in need, ultimately enhancing quality of life in the growing population of breast cancer survivors.
Supplementary Material
Financial support:
This work was supported by National Institutes of Health/National Cancer Institute R01 CA160427. Crespi and Hurvitz were also supported by P30 CA16042.
Footnotes
Declaration of interests: None
References:
- 1.Bower JE, Bak K, Berger AM, et al. : Screening, Assessment and Management of Fatigue in Adult Survivors of Cancer: An American Society of Clinical Oncology Clinical Practice Guideline Adaptation J Clin Oncol, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Curt GA, Breitbart W, Cella D, et al. : Impact of cancer-related fatigue on the lives of patients: new findings from the Fatigue Coalition. Oncologist. 5:353–360, 2000 [DOI] [PubMed] [Google Scholar]
- 3.Donovan KA, Jacobsen PB, Andrykowski MA, et al. : Course of fatigue in women receiving chemotherapy and/or radiotherapy for early stage breast cancer. J Pain Symptom.Manage 28:373–380, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dhruva A, Dodd M, Paul SM, et al. : Trajectories of fatigue in patients with breast cancer before, during, and after radiation therapy. Cancer Nurs. 33:201–212, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jacobsen PB, Hann DM, Azzarello LM, et al. : Fatigue in women receiving adjuvant chemotherapy for breast cancer: characteristics, course, and correlates. J.Pain Symptom.Manage 18:233–242, 1999 [DOI] [PubMed] [Google Scholar]
- 6.Nieboer P, Buijs C, Rodenhuis S, et al. : Fatigue and relating factors in high-risk breast cancer patients treated with adjuvant standard or high-dose chemotherapy: a longitudinal study. J Clin Oncol 23:8296–8304, 2005 [DOI] [PubMed] [Google Scholar]
- 7.Ancoli-Israel S, Liu L, Rissling M, et al. : Sleep, fatigue, depression, and circadian activity rhythms in women with breast cancer before and after treatment: a 1-year longitudinal study. Support Care Cancer 22:2535–45, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bower JE, Ganz PA, Desmond K, et al. : Fatigue in long-term breast cancer survivors: A longitudinal investigation. Cancer, 2005 [DOI] [PubMed] [Google Scholar]
- 9.Cella D, Davis K, Breitbart W, et al. : Cancer-related fatigue: prevalence of proposed diagnostic criteria in a United States sample of cancer survivors. J.Clin.Oncol 19:3385–3391, 2001 [DOI] [PubMed] [Google Scholar]
- 10.Galatzer-Levy IR, Huang SH, Bonanno GA: Trajectories of resilience and dysfunction following potential trauma: A review and statistical evaluation. Clin Psychol Rev 63:41–55, 2018 [DOI] [PubMed] [Google Scholar]
- 11.Stanton AL, Wiley JF, Krull JL, et al. : Depressive episodes, symptoms, and trajectories in women recently diagnosed with breast cancer. Breast Cancer Res Treat 154:105–15, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Junghaenel DU, Cohen J, Schneider S, et al. : Identification of distinct fatigue trajectories in patients with breast cancer undergoing adjuvant chemotherapy. Support Care Cancer 23:2579–87, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Whisenant M, Wong B, Mitchell SA, et al. : Distinct Trajectories of Fatigue and Sleep Disturbance in Women Receiving Chemotherapy for Breast Cancer. Oncol Nurs Forum 44:739–750, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bodtcher H, Bidstrup PE, Andersen I, et al. : Fatigue trajectories during the first 8 months after breast cancer diagnosis. Qual Life Res 24:2671–9, 2015 [DOI] [PubMed] [Google Scholar]
- 15.Kober KM, Smoot B, Paul SM, et al. : Polymorphisms in Cytokine Genes Are Associated With Higher Levels of Fatigue and Lower Levels of Energy in Women After Breast Cancer Surgery. J Pain Symptom Manage 52:695–708 e4, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Donovan KA, Small BJ, Andrykowski MA, et al. : Utility of a cognitive-behavioral model to predict fatigue following breast cancer treatment. Health Psychol 26:464–472, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bower JE, Wiley J, Petersen L, et al. : Fatigue after breast cancer treatment: Biobehavioral predictors of fatigue trajectories. Health Psychol 37:1025–1034, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bower JE: The role of neuro-immune interactions in cancer-related fatigue: Biobehavioral risk factors and mechanisms. Cancer 125:353–364, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bower JE, Asher A, Garet D, et al. : Testing a biobehavioral model of fatigue before adjuvant therapy in women with breast cancer. Cancer 125:633–641, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wright F, Kober KM, Cooper BA, et al. : Higher levels of stress and different coping strategies are associated with greater morning and evening fatigue severity in oncology patients receiving chemotherapy. Support Care Cancer, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Stein KD, Martin SC, Hann DM, et al. : A multidimensional measure of fatigue for use with cancer patients. Cancer Pract. 6:143–152, 1998 [DOI] [PubMed] [Google Scholar]
- 22.Stein KD, Jacobsen PB, Blanchard CM, et al. : Further validation of the multidimensional fatigue symptom inventory-short form. J Pain Symptom Manage 27:14–23, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Katz JN, Chang LC, Sangha O, et al. : Can comorbidity be measured by questionnaire rather than medical record review? Med.Care 34:73–84, 1996 [DOI] [PubMed] [Google Scholar]
- 24.Godin G, Shephard RJ: A simple method to assess exercise behavior in the community. Can.J Appl.Sport Sci 10:141–146, 1985 [PubMed] [Google Scholar]
- 25.Bernstein DPF, L. A. : Childhood Trauma Questionnaire Manual. San Antonio, The Psychological Corporation, 1998 [Google Scholar]
- 26.Walker EA, Unutzer J, Rutter C, et al. : Costs of health care use by women HMO members with a history of childhood abuse and neglect. Arch Gen Psychiatry 56:609–13, 1999 [DOI] [PubMed] [Google Scholar]
- 27.Radloff LS: The CES-D Scale: A self-report depression scale for research in the general population. Appl Psychol Measur 1:385–401, 1977 [Google Scholar]
- 28.Buysse DJ, Reynolds CF III, Monk TH, et al. : Quantification of subjective sleep quality in healthy elderly men and women using the Pittsburgh Sleep Quality Index (PSQI). Sleep 14:331–338, 1991 [PubMed] [Google Scholar]
- 29.Sundin EC, Horowitz MJ: Impact of Event Scale: psychometric properties. Br.J Psychiatry 180:205–209, 2002 [DOI] [PubMed] [Google Scholar]
- 30.Proust-Lima C, Philipps V, Liquet B: Estimation of Extended Mixed Models Using Latent Classes and Latent Processes: The R Package lcmm. 2017. 78:56, 2017 [Google Scholar]
- 31.Bower JE, Ganz PA, Belin TR, et al. : Inflammation and fatigue during radiation therapy for breast and prostate cancer. Psychsom Med, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Irvine DM, Vincent L, Graydon JE, et al. : Fatigue in women with breast cancer receiving radiation therapy. Cancer Nurs. 21:127–135, 1998 [DOI] [PubMed] [Google Scholar]
- 33.Bower JE, Ganz PA, Desmond KA, et al. : Fatigue in breast cancer survivors: occurrence, correlates, and impact on quality of life. J.Clin.Oncol 18:743–753, 2000 [DOI] [PubMed] [Google Scholar]
- 34.Bower JE, Ganz PA, Irwin MR, et al. : Inflammation and behavioral symptoms after breast cancer treatment: do fatigue, depression, and sleep disturbance share a common underlying mechanism? J Clin Oncol 29:3517–3522, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ganz PA, Kwan L, Stanton AL, et al. : Quality of life at the end of primary treatment of breast cancer: first results from the moving beyond cancer randomized trial. J Natl.Cancer Inst 96:376–387, 2004 [DOI] [PubMed] [Google Scholar]
- 36.Andersen BL, DeRubeis RJ, Berman BS, et al. : Screening, Assessment, and Care of Anxiety and Depressive Symptoms in Adults With Cancer: An American Society of Clinical Oncology Guideline Adaptation. Journal of Clinical Oncology 32:1605–1619, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Irwin MR, Olmstead R, Carrillo C, et al. : Tai Chi Chih Compared With Cognitive Behavioral Therapy for the Treatment of Insomnia in Survivors of Breast Cancer: A Randomized, Partially Blinded, Noninferiority Trial. J Clin Oncol 35:2656–2665, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.