Abstract
Blood leukocyte differentials can be useful for understanding changes associated with bovine respiratory disease (BRD) progression. By improving turnaround time, point-of-care leukocyte differential assays (PCLD) may provide logistical advantages to laboratory-based assays. Our objective was to assess BRD progression in steers challenged with bovine herpesvirus 1 and Mannheimia haemolytica using point-of-care and laboratory-based blood leukocyte differentials. Thirty Holstein steers (average body weight of 211 kg + 2.4 kg) were inoculated intranasally on day 0 with bovine herpesvirus 1 and intrabronchially on day 6 with Mannheimia haemolytica. Blood leukocytes differentials were measured using both assays from study days 0 to 13. Linear mixed models were fitted to evaluate the associations between: (1) the type of assay (laboratory-based or PCLD) with respect to leukocyte, lymphocyte, and neutrophil concentrations; (2) study day with cell concentrations; and (3) cell concentrations with lung consolidation measured at necropsy. Point-of-care leukocyte, lymphocyte, and neutrophil concentrations were significantly associated (P < 0.05) with the respective cell concentrations obtained from the laboratory-based leukocyte differential. Cell concentrations reported by both assays differed significantly (P < 0.05) over time, indicating shifts from healthy to viral and bacterial disease states. Lymphocyte concentrations, lymphocyte/neutrophil ratios obtained from both assays, and band neutrophil concentrations from the laboratory-based assay were significantly associated (P < 0.05) with lung consolidation, enhancing assessments of disease severity. The PCLD may be a useful alternative to assess BRD progression when laboratory-based leukocyte differentials are impractical.
Keywords: bovine respiratory disease complex, leukocyte differentials, point, of, care diagnostics
INTRODUCTION
In the United States, cattle are commonly affected by bovine respiratory disease (BRD), a multifactorial disease complex that causes an estimated burden of 3 billion USD annually (Griffin, 1997). In feedlots, the economic impact associated with BRD may include costs associated with negative performance (e.g., average daily gain) and carcass characteristics (e.g., hot carcass weight and quality grade), treatment and prevention, and death (Gardner et al., 1999; Engler et al., 2014; Johnson and Pendell, 2017). In addition to BRD significant economic effect on the feedlot industry, the dairy, backgrounding, and cow–calf industries are also significantly impacted (Brooks et al., 2011; Guterbock, 2014).
A common practice for BRD diagnosis consists of assessing clinical signs of individual animals and measuring rectal temperature (Apley, 2006). This practice, however, has poor diagnostic sensitivity and specificity, both estimated at ~60% (White and Renter, 2009). At the individual level, refining case definition and diagnosis for BRD is necessary to improve the animal’s health and well-being as well as promote the judicious use of antimicrobials. At the population level, improving risk definitions for cohorts of animals could aid the implementation of more targeted metaphylaxis practices. Although reports of diagnostic strategies for BRD are common in the literature, determining the onset of disease is still challenging due to the lack of benchmark ante-mortem diagnostic tests. To address this issue, several authors have used challenge studies to evaluate the performance of diagnostic assays (Hanzlicek et al., 2010; Fraser et al., 2013; Carlos-Valdez et al., 2016).
Although several combinations of pathogens can cause BRD, one of the most important combinations observed on the field consists of a viral infection with BoHV-1 followed by lung colonization with Mh (Griffin et al., 2010; Caswell, 2014). Blood leukocyte differentials have been used to aid diagnostics, clinical assessment, monitoring of disease or therapeutic actions, and in particular, to assess BRD in challenge studies (Burciaga-Robles et al., 2010; Hanzlicek et al., 2010; Fraser et al., 2013; Molina et al., 2013; Carlos-Valdez et al., 2016). However, blood leukocyte differentials have not been used to evaluate the progression of disease following a BoHV-1/Mh challenge.
Consistent with acute infectious processes, prior research has demonstrated an increase in leukocyte and neutrophil concentrations and a decrease in lymphocyte concentration upon infection with BRD causative pathogens (Burciaga-Robles et al., 2010; Carlos-Valdez et al., 2016). Early determination of blood leukocyte differentials could help veterinarians and producers mitigate the impacts of BRD, but it is yet unclear how these data change as the disease process progresses and lung consolidation increases. Leukocyte differentials are usually measured by submitting blood samples to trained personnel in a clinical pathology laboratory, which can create logistical challenges for field case management. Point-of-care systems have been developed to provide a more rapid, chute-side measurement alternative (Gonçalves et al., 2017). The objective of this study was to assess BRD progression in steers challenged with bovine herpesvirus 1 and Mannheimia haemolytica using point-of-care and laboratory-based blood leukocyte differentials.
MATERIALS AND METHODS
This study was conducted from May 11 (study day 0) to May 24, 2017, at the Veterinary Biomedical and Research Center facility (VBRC), in Manhattan, Kansas. The study protocol was approved by the VBRC Institutional Animal Care and Use Committee (approval number VAC17053B).
Study Design and Study Subjects
Information regarding study design, challenge characteristics, and management of study subjects for this study has been reported previously for a concurrent study (Baruch et al., 2019). Briefly, 30 Holstein steers with a mean average body weight of 211 kg (± 2.4 kg) were enrolled in the study and observed for over 14 d (study days 0 to 13). Upon study initiation, all steers tested negative to Mh and BoHV-1 antibodies, based on microagglutination and virus neutralization tests, respectively, which were performed at the Texas A&M Veterinary Medical Diagnostic Laboratory. Negative cutpoint values corresponded to titers less than or equal to 1:4 and 1:2 for Mh and BoHV-1, respectively. Animals received a 7-way clostridial vaccine in their first week of life, had no prior history of BRD treatment or clinical signs, were weaned and castrated prior to arrival, and were individually housed and on a grain-based diet prior to arrival. A veterinarian inspected the animals upon arrival to the study facility (study day −6), and for the following 6 d after arrival, to ensure animals were healthy before trial initiation. Animals were housed at the VBRC research facility in a single feedlot-size pen (30 m wide × 38 m long with 26 m of concrete feed bunks and a 1.8-m concrete waterer) and were fed a growing ration ad libitum. The ration contained 59% wet distiller’s grain, 39.6% forage, 1.4% protein, and a mineral supplement with sodium monensin. Animals were observed, and clinical illness scores assigned (Baruch et al., 2019), daily or twice a day on study days 6 and 7. Animals were randomly selected to be sequentially euthanized on study days 6 to 13, and lung consolidation scores were assigned to lung lesions by a veterinarian during necropsy (Baruch et al., 2019). Moribund animals were euthanized outside the randomization process, but lung consolidation was also recorded during necropsy (Baruch et al., 2019).
On study day 0, animals were restrained in a hydraulic chute without sedation and the BoHV-1 inoculum was administered into each nasal passage (Baruch et al., 2019) using an model 163 atomizer (De Vilbiss Healthcare, LLC; Port Washington, New York). On study day 6, after blood samples were collected (see Sampling below), animals were restrained and inoculated with the Mh isolate (serotype 1). The Mh inoculum was administrated while animals were restrained in the chute with their heads stabilized (Baruch et al., 2019). An endoscope was introduced into the right nasal passage until reaching the first bronchial bifurcation where the Mh inoculum was administrated (Baruch et al., 2019). In summary, the study timeline was as follows: animals were acclimated at the VBRC facility through study day 0, the viral challenge was administered on study day 0, the bacterial challenge on study day 6, and animals were monitored up to study day 13 (Baruch et al., 2019).
Sampling
On study days 0, 1, 2, 4, 6, 6.5, 7, 7.5, 9, 11, and 13, animals were restrained in the chute and their heads were haltered to collect blood samples by means of jugular venipuncture using 20-gauge needles and EDTA vacutainer tubes. Samples were placed on ice and transported to the Kansas State Veterinary Diagnostic Laboratory (KSVDL) within 3 h of the first animal being sampled. After arrival to the KSVDL, samples were processed for a complete blood count with leukocyte differential, including segmented neutrophil, band neutrophil, lymphocyte, monocyte, eosinophil, and basophil concentrations, by the clinical pathology laboratory (laboratory-based leukocyte differential, LLD) measured by a medical technologist who was blinded to the animal’s disease status. Complete blood counts were analyzed using a flow cytometry-based analysis (Harris et al., 2005).
A second blood sample was obtained, by means of jugular venipuncture, using a specialized EDTA tube/needle—provided by the instrument’s manufacturer—and immediately aliquoted to a single slide deck and inserted in the point-of-care leukocyte differential (PCLD) instrument (QScout BLD test; Advanced Animal Diagnostics; Morrisville, NC). Samples evaluated by the PCLD were analyzed using the manufacturer’s settings, the results were obtained after 2 min, and the data were stored. The PCLD data included total leukocyte, total neutrophil, and lymphocyte concentrations.
Data Analyses
Statistical analyses were performed in SAS 9.4 (SAS Institute Inc., Carry, NC) and STATA 12 (Stata Corp., College Station, TX) software. Descriptive statistics (mean, median, standard deviation, and range) were computed for all outcomes by study day.
Linear mixed models (LMMs) were fitted using the GLIMMIX procedure in SAS to account for repeated measures when evaluating the association between the blood parameters reported by the 2 assays with study day. Leukocyte, lymphocyte, segmented neutrophil, total neutrophil/lymphocyte ratio, basophils, eosinophil, band neutrophil, monocyte, spun hematocrit, hematocrit calculated, hemoglobin, cellular hemoglobin, fibrinogen, and plasma protein were modeled as continuous outcomes using a Gaussian distribution, identity link, and a residual pseudo-likelihood (RSPL) estimation technique. Study day (with categories from days 0 to 13) was included as a fixed effect in all models. A random residual with a heterogeneous first-order autoregressive covariance structure at the animal level was included. Models were checked for outliers by using a ≥|2| SD cut-off in the studentized residuals. Homoscedasticity and normality assumptions were assessed via visual appraisal of scatterplots and histograms of standardized residuals. Leukocyte, lymphocyte, segmented neutrophil, total neutrophil/lymphocyte ratio, and monocyte concentrations were log 10 transformed to improve normality and model fit and back-transformed for interpretation. After attempting several transformations, outcomes for which the residuals did not meet the normality assumption, namely basophil, eosinophil, and band neutrophil concentrations, were transformed into categorical variables. Based on the reference intervals provided by the KSVDL, variables were categorized as follows: basophils concentration “within reference interval” <0.2 × 109/L) and “outside of reference interval” >0.2 × 109/L), eosinophil concentration “within reference interval” <1.6 × 109/L) and “outside of reference interval” > 1.6 × 109/L), and band neutrophil concentration “within reference interval” < 0.2 × 109/L) and “outside of reference interval” > 0.2 × 109/L). These outcomes were modeled using a binary distribution, logit link, and an RSPL estimation technique. A random residual term with an autoregressive moving average covariance structure at the animal level was fitted to account for repeated measures. P-values were adjusted for multiple comparisons between study days using a Tukey–Kramer method.
Leukocyte, lymphocyte, and neutrophil concentrations were reported for both LLD and PCLD assays. Therefore, 3 LMMs were fitted using the GLIMMIX procedure in SAS to evaluate the association between the results from the PCLD and the LLD. Outcomes for each of the 3 models, respectively, were leukocyte, lymphocyte, and segmented neutrophil concentrations reported by LLD modeled with a Gaussian distribution, identity link, and an RSPL estimation technique. Fixed effects included leukocyte, lymphocyte, and total neutrophil concentrations reported from the PCLD and study day. An interaction term between the 2 main effects (cell count and study day) also was included in each of the 3 models. A random residual with a heterogeneous first-order autoregressive covariance structure at the animal level was used for each model. Model fit was evaluated and multiple comparisons adjustments were performed as described above.
Eleven generalized LMMs were fitted to assess the associations between leukocyte, lymphocyte, neutrophil, total neutrophil/lymphocyte ratio, plasma protein, and fibrinogen obtained from the LLD and the PCLD with lung consolidation scores obtained at necropsy. For the LLD, band and segmented neutrophil concentration were used in different models, whereas for the PCLD—where segmented and band neutrophil concentrations were not differentiated—total neutrophils were used. Plasma protein and fibrinogen were only reported by the LLD. In each model, lung consolidation scores, recorded as the proportion of consolidated tissue in the lung, were modeled as the outcome using a beta distribution, logit link, and an RSPL estimation technique using the GLIMMIX procedure in SAS. Estimated lung consolidation proportions were then converted to percentages for reporting and interpretation purposes. Fixed effects corresponded to leukocyte, lymphocyte, segmented neutrophil, band neutrophil, total neutrophil/lymphocyte ratio, plasma protein, and fibrinogen obtained from the LLD, and leukocyte, lymphocyte, total neutrophil, and total neutrophil/lymphocyte ratio obtained from the PCLD, for the 11 models, respectively. Fixed effects on each model were categorized into 3 categories (33% of observations on each category) for the PCLD and LLD. Categories were defined as follows: leukocytes <8.1 × 109/L; 8.1–12.2 × 109/L; >12.2 × 109/L), lymphocytes <2.9 × 109/L; 2.9–5.1 × 109/L; >5.1 × 109/L), segmented or total neutrophils <4.4 × 109/L; 4.4–6.5 × 109/L; >6.5 × 109/L), band neutrophils <0.2 × 109/L; 0.2–0.7 × 109/L; >0.7 × 109/L), total neutrophil/lymphocyte ratio < 0.9; 0.9–2.3; >2.3), plasma protein <7.0 g/L; 7.0–7.6 g/L; >7.6 g/L), and fibrinogen <700 g/L; 700–900 g/L; >900 g/L).
RESULTS AND DISCUSION
All study calves tested negative for BoHV-1 and Mh upon enrollment, and BRD was successfully induced with all animals showing clinical signs of progressive severity up to study termination. Clinical signs, necropsy findings, and results from other diagnostic methods have been reported in detail elsewhere (Baruch et al., 2019). Briefly, up to study day 6, animals did not present clinical signs of respiratory disease or presented mild depression (Baruch et al., 2019). After study day 6, animals presented moderate and severe depression, with some animals being moribund (Baruch et al., 2019). On study day 6, fibrinous to mucopurulent tracheitis with almost no lung consolidation was observed at necropsy. After study day 6, the fibrinous to mucopurulent tracheitis decreased, but lung consolidation increased to an average maximum of 55.4% on study day 10 (Baruch et al., 2019). Descriptive statistics (mean, standard deviation, and range) for blood parameters during the study period are depicted in Supplementary Tables S1 and S2.
Associations of LLD-Blood Parameters by Study Day
Model-adjusted mean leukocyte concentrations were outside the reference interval (5.0–10.0 × 109/L) during study days 0, 1, 2, and 4, which could be attributed to the age of the study animals. Study animals were 6- to 7-mo-old calves and leukocyte concentrations are known to decrease as cattle mature (Jones and Allison, 2007). Similarly, higher leukocyte concentrations can be explained by a catecholamine-induced response associated with the periodic handling of animals (Stockham and Scott, 2013). As the viral phase of the study progressed (study day 0 or 1 vs. 6), all white blood cells decreased (P < 0.05; Table 1). Similarly, Molina et al. (2013) described a slight decrease in leukocyte concentration by study day 4, after BoHV-1 inoculation.
Table 1.
Model-adjusted means (and standard error of the mean) of clinical pathology parameters reported by a laboratory-based assay in calves inoculated with BoHV-1 on day 0 and Mannheimia haemolytica on day 6, by study day
| Study day | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 0 | 1 | 2 | 4 | 6 | 6.5 | 7 | 7.5 | 9 | 11 | 13 | P 3 | ||
| Parameter, unit1 | Int2 | Model-adjusted mean (SEM) | |||||||||||
| Leuk 109/L | 5.0–10.0 | 11.9 a | 11.9 a | 10.5 bd | 10.2 bcd | 9.4de | 9.1de | 11.4 ab | 9.1df | 9.2abdf | 6.7cef | 6.2abdf | <0.01 |
| (1.03) | (1.04) | (1.04) | (1.04) | (1.05) | (1.06) | (1.06) | (1.06) | (1.08) | (1.14) | (1.26) | |||
| Lymp 109/L | 2.5–7.5 | 5.5a | 4.7bc | 4.6ab | 4.8ac | 5.4ab | 2.7d | 3.5f | 3.1df | 2.6df | 2.5df | 2.9abdf | <0.01 |
| (1.04) | (1.05) | (1.06) | (1.05) | (1.05) | (1.07) | (1.06) | (1.08) | (1.12) | (1.12) | (1.24) | |||
| Seg 109/L | 1.0–5.0 | 4.9ace | 5.2 ae | 4.7ace | 4.0ac | 2.7b | 4.1acd | 5.6 e | 4.1abd | 3.1bda | 2.0bc | 0.8 abe | <0.01 |
| (1.07) | (1.07) | (1.06) | (1.08) | (1.08) | (1.09) | (1.08) | (1.12) | (1.21) | (1.32) | (2.04) | |||
| Neut/lymp | No.of ref. | 0.9ae | 1.1ace | 1.0ae | 0.8 ae | 0.5b | 1.7cd | 1.8d | 1.4ade | 1.6de | 1.1abde | 0.5ab | <0.01 |
| (1.08) | (1.10) | (1.08) | (1.08) | (1.09) | (1.11) | (1.10) | (1.17) | (1.24) | (1.34) | (1.43) | |||
| Mono 109/L | 0.0–0.8 | 1.1 abdg | 1.3 de | 0.8cgh | 1.0 abdh | 0.9 abdh | 1.3 ebi | 1.1 abdh | 0.8ac | 0.8abhi | 0.3f | 0.6abfh | <0.01 |
| (1.09) | (1.09) | (1.08) | (1.09) | (1.09) | (1.11) | (1.11) | (1.09) | (1.14) | (1.21) | (1.26) | |||
| Hct spun3 | 0.26–0.42 | 0.35ai | 0.35ai | 0.34adi | 0.33beh | 0.32cdfg | 0.32cgh | 0.34aef | 0.34aef | 0.33efgi | 0.36ae | 0.38a | <0.01 |
| (0.006) | (0.006) | (0.005) | (0.006) | (0.006) | (0.006) | (0.007) | (0.012) | (0.012) | (0.012) | (0.013) | |||
| Hct calc3 | 0.24–0.46 | 0.32abc | 0.32ac | 0.31b | 0.29de | 0.29de | 0.28e | 0.30abd | 0.31abd | 0.31abde | 0.33abcd | 0.36c | <0.01 |
| (0.006) | (0.005) | (0.005) | (0.005) | (0.005) | (0.005) | (0.006) | (0.006) | (0.012) | (0.013) | (0.014) | |||
| Hemo, g/L | 80–150 | 125ac | 123acd | 118be | 116be | 116bde | 113bf | 119ae | 121aeg | 121aefg | 128aeg | 133cg | <0.01 |
| (2.0) | (2.0) | (2.0) | (2.0) | (2.0) | (2.0) | (2.0) | (2.0) | (4.0) | (5.0) | (4.0) | |||
| C.hemo, g/L | No. of ref. | 124ae | 122ae | 118bf | 114cdg | 114cf | 110d | 116afg | 119afg | 119adfg | 127aefg | 136e | <0.01 |
| (2.0) | (2.0) | (2.0) | (2.0) | (2.0) | (2.0) | (2.0) | (2.0) | (4.0) | (5.0) | (4.0) | |||
| Fibri, g/L | 3.0–7.0 | 4.2d | 4.6cd | 4.4d | 4.5d | 5.5cb | 5.6cb | 6.1b | 5.8bc | 7.6 a | 8.1 a | 8.7 a | <0.01 |
| (0.26) | (0.26) | (0.26) | (0.26) | (0.26) | (0.28) | (0.28) | (0.30) | (0.30) | (0.37) | (0.39) | |||
| P. prot, g/L | 70–90 | 76a | 74bcd | 75ab | 74bde | 72cf | 70ghi | 69 gi | 69 hi | 71ceg | 74acej | 76adf | <0.01 |
| (1.0) | (1.0) | (1.0) | (1.0) | (1.0) | (1.0) | (1.0) | (1.0) | (1.0) | (1.0) | (1.0) |
1Param = Parameters, Leuk = Leukocyte, Lymp = Lymphocyte, Seg = Segmented neutrophils, Neut = Neutrophils, Mono = Monocytes, Hct = Hematocrit, Hct calc = Hematocrit calculated, Hemo = Hemoglobin, C.hemo = Cellular hemoglobin, Fibri = Fibrinogen, P. prot = Plasma protein.
2Reference intervals, as provided by the Kansas State Veterinary Diagnostic Laboratory.
3Statistical difference among study days.
3Proportion of 1.
Different superscript letters within a row indicate statistically significant differences (P < 0.05). Values in bold indicate concentrations outside of the reference intervals. Calves were randomly assigned to be euthanized on days 6 to 13.
During the bacterial phase of the study (study days 6.5 to 13), mean leukocyte concentration increased on study day 6.5 when compared with study day 6 (when animals were inoculated with Mh; P < 0.05; Table 1). Mean leukocyte concentration increased on study day 7 when compared with study day 6.5 (P < 0.05); however, concentrations on study days 7.5, 9, 11, and 13 did not differ when compared to study day 6.5 (P-value > 0.05; Table 1). In our study, leukocyte concentration increased 24 h after the bacterial inoculation but decreased after 36 h. Previous research by Burciaga-Robles et al. (2010) and Hanzlicek et al. (2010) also indicated an increase in leukocyte concentration 24 h after Mh inoculation. Cattle have a small reserve pool of neutrophils in the bone marrow (Stockham and Scott, 2013), thus, in times of acute inflammation, the leukocyte count will transiently increase during the release of neutrophils from the reserve pool (Stockham and Scott, 2013). Similar to what it is observed in an acute inflammation process, leukocyte concentrations might transitionally increase due to the hypothalamic–pituitary–adrenal axis activation and subsequent catecholamine excretion (Chen et al., 2015). Once the neutrophil storage pool is depleted, the leukocyte count will decrease as the tissue demand overwhelms the bone marrow’s ability to respond, which is consistent with the leukocyte count decrease 36 h after the start of the bacterial phase of the study (after study day 7.5).
Throughout the study, mean lymphocyte concentrations remained within the reference interval (2.5–5.0 × 109/L; Table 1). Although lymphocyte concentrations did not differ (P > 0.05; Table 1) during the viral phase of the study (study days 1 to 6), mean lymphocyte concentrations decreased when comparing study day 0 to each of the study days 6.5, 7, 7.5, 9, and 11 (P < 0.05; Table 1). Lymphopenia is commonly observed in acute inflammatory disease processes as observed with BRD (Jones and Allison, 2007). During our study, the significant decrease on lymphocyte concentrations 12 h after bacterial infection was likely in response to the Mh inoculation on already immunosuppressed animals (Jones and Allison, 2007). Possibly, in healthy calves, exposure to low doses of Mh may be eliminated naturally; however, in sick calves, a large inoculation dose of Mh would produce an acute inflammatory process that explains the observed lymphopenia (Rice et al., 2007). Contrary to our results, another study by Hanzlicek et al. (2010) reported no differences in lymphocyte concentrations in Mh challenged animals; however, in their study, animals were only inoculated with Mh, and likely the disease process was not as acute or severe as the one observed after a BoHV-1/Mh challenge.
Means for segmented neutrophil concentrations were within the reference interval (1.0–5.0 × 109/L) except for study days 1, 7, and 13 when mean concentrations were outside the reference interval (Table 1). Mean segmented neutrophil concentration decreased from study day 0 compared with study day 6 (P < 0.05; Table 1). Infections with BoHV-1 cause inflammation in the upper-respiratory-tract and neutropenia is commonly observed 48 h after an initial inflammatory process (Jones and Allison, 2007). In our study, an increase of upper-respiratory-tract inflammation due to the inoculation with BoHV-1 was observed upon necropsy (Baruch et al., 2019), potentially explaining the decrease in segmented neutrophils. Others have also described a decrease in neutrophil concentrations 4 d after inoculation with BoHV-1 (Molina et al., 2013). However, 12 h after the bacterial inoculation (study day 6.5), the mean segmented neutrophil concentration increased when compared to study day 6 (P < 0.05; Table 1). Segmented neutrophil concentrations decreased after study day 7. Specifically, the concentration decreased on study days 7.5, 9, and 11 when compared with study day 7 (P < 0.05; Table 1). When animals experience large inflammatory processes, such as when bacterial infection occurs, neutrophilia can also be observed (Jones and Allison, 2007). Similarly, an increase in segmented neutrophils 24 h after a Mh inoculation on animals exposed to bovine viral diarrhea virus has been described previously (Burciaga-Robles et al., 2010; Carlos-Valdez et al., 2016), suggesting that combinations of virus and bacteria might cause an increase in segmented neutrophil concentrations.
The mean ratio between total neutrophils and lymphocytes increased from study days 6 to 6.5 (P < 0.05; Table 1). Specifically, mean lymphocyte concentration was double the neutrophil concentration on study day 6, whereas on study day 6.5, neutrophil concentrations were 70.0% greater than lymphocyte concentrations (Table 1). The mean ratio of neutrophils/lymphocytes remained above 1 from study day 6.5 until study day 13, indicating that total neutrophils were the predominant cells. Overall, variations in the neutrophil and lymphocyte ratio were observed within hours after calves, already infected with BoHV-1, were inoculated with Mh. Thus, measuring changes in this ratio could contribute to strategies for assessing disease progression relative to viral and bacterial involvement.
Model-adjusted mean hematocrit values (both calculated and spun values) and hemoglobin decreased from study day 0 to study day 6 (P < 0.05; Table 1). Mean hematocrit values significantly increased on study days 11 and 13 when compared with study day 6 (P < 0.05; Table 1). Erythrocytes transport oxygen in the blood, and an increase in the number of erythrocytes frequently occurs in chronic respiratory diseases as part of a mechanism to compensate for respiratory failure (Jones and Allison, 2007). However, there are apparently no changes in erythrocyte-related parameters upon the initial (nonchronic) stages of BRD (Hanzlicek et al., 2010). In our study, hematocrit and hemoglobin concentrations decreased during the viral infection (until study day 6) and then increased after the inoculation with Mh, but the mean values were never outside of the reference interval, likely preventing the usefulness of hematocrit and hemoglobin parameters for making diagnostic or prognostic decisions. Moreover, severely ill cattle also may eat and drink less, resulting in a higher hematocrit due to dehydration and subsequent hemoconcentration.
Mean fibrinogen values increased throughout the study, reaching its peak on the last day of the study. On day 6, animals presented increased fibrinogen compared with day 0 (P < 0.05), and fibrinogen was higher on each of the last 3 study days when compared with all other study days (P < 0.05; Table 1). Large amounts of serofibrin are commonly observed in the lungs of animals that died or were euthanized due to BRD (Hanzlicek et al., 2010; Baruch et al., 2019), and hence, fibrinogen has been studied in detail as a marker for acute response to inflammatory processes (Jones and Allison, 2007). Given that fibrinogen is a positive acute-phase protein, and its production is upregulated when inflammatory processes are generated, it is not surprising that fibrinogen increased during the bacterial phase of the study. Similarly, increased fibrinogen values have been observed previously in Mycoplasma bovis or Mh challenge studies (Hanzlicek et al., 2010; Fraser et al., 2013).
Plasma protein values decreased from study day 0 to study days 4 and 6 (P < 0.05; Table 1). However, plasma protein values increased from study days 6.5 to 11 and 13 (P < 0.05; Table 1). The increase in plasma protein that occurred later in the study could be associated with the increases in fibrinogen or hemoconcentration correlating to the concurrent increase in hematocrit. Similar to a previous study (Guterbock, 2014), these results are consistent with an increase in the animals’ inflammatory process due to BRD progression. Although fibrinogen and plasma proteins seem to be appropriate indicators for monitoring disease progression over time, limited diagnostic accuracy has been previously described (Abdallah et al., 2016).
The probability of having basophil concentrations outside the reference interval (>0.2 × 109/L) did not differ between study days 0, 1, and 2 (P < 0.05; Table 2). However, from study day 4 to study termination, no basophils concentrations were observed outside the reference interval. Animals did not present eosinophils concentrations outside the reference interval (>1.6 × 109/L) during the study period; therefore, no models were fitted, and only descriptive results are depicted in Supplementary Table S1.
Table 2.
Model-adjusted probabilities (and standard errors) of animals presenting basophil and band neutrophil concentrations outside of the reference intervals reported by a laboratory-based assay in calves inoculated with BoHV-1 on day 0 and Mannheimia haemolytica on day 6, by study day
| Study day | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 0 | 1 | 2 | 4 | 6 | 6.5 | 7 | 7.5 | 9 | 11 | 13 | P 1 | |
| Cell type | Mean-adjusted probability (SE) | |||||||||||
| Baso outside vs. within ref intervals2 | 0.20 | 0.26 | 0.13 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.33 |
| (0.073) | (0.079) | (0.061) | — | — | — | — | — | — | — | — | ||
| Band neut outside vs. within ref intervals3 | 0.00 | 0.00 | 0.00 | 0.00 | 0.03 a | 0.59 b | 0.79 b | 0.46 ab | 0.85 b | 0.76 b | 0.42 ab | <0.01 |
| — | — | — | — | (0.031) | (0.102) | (0.079) | (0.111) | (0.076) | (0.121) | (0.212) |
1Statistical difference among study days.
2Basophils outside of reference intervals >0.2 × 109/L) vs. within reference intervals <0.2 × 109/L).
3Band neutrophils outside of reference intervals >0.2 × 109/L) vs. within reference intervals <0.2 × 109/L).
Different superscript letters within a row indicate statistically significant differences (P < 0.05). Calves were randomly assigned to be euthanized on days 6 to 13.
Prior to study day 6, no animals had band neutrophil concentrations outside the reference interval (>0.2 × 109/L). On study day 6.5, the mean probability of animals having band neutrophil concentrations outside the reference interval increased from 0.03 to 0.59 (P < 0.05; Table 2), 12 h after animals were challenged with Mh. From study days 6.5 to study day 13, the probability of animals having band neutrophil concentrations outside the reference interval ranged from 0.42 to 0.85, with no differences between study days 6.5, 7, 7.5, 9, 11, and 13 (P > 0.05; Table 2). This sudden increase in band neutrophils concentration above the reference interval, which was sustained until study termination, could be attributed to the acute inflammatory process produced by the bacterial challenge. Band neutrophils are released from the bone marrow when an animal experiences an acute inflammatory process, as observed in BRD progression (Whiteley et al., 1992; Jones and Allison, 2007). Contrary to our results, another study reported no differences over time in band neutrophil concentration in Mh challenged animals (Hanzlicek et al., 2010). Thus, in our study, the inflammatory stimulus and tissue demand may have been sufficiently severe to utilize the storage pool of neutrophils and mobilize band neutrophils from the bone marrow, (Jones and Allison, 2007) making band neutrophils potentially useful indicators of disease progression.
Associations of PCLD-Blood Parameters by Study Day
Model-adjusted mean leukocyte concentrations from the PCLD decreased through the first 6 d of the study (P < 0.05; Table 3). However, when comparing study day 6 with study day 7, the mean concentrations increased (P < 0.05; Table 3). Mean lymphocyte concentrations did not vary by study day during the first 6 d of the study (P > 0.05), but 12 h after the inoculation with Mh (by study day 6.5), the mean concentration decreased when compared with study day 6 (P < 0.05; Table 3). Mean lymphocyte concentrations decreased on study days 9, 11, and 13 when compared with study days 0, 1, 2, and 6 (P < 0.05; Table 3). Mean total neutrophil concentrations were lower on study days 2, 4, and 6 when compared with the mean concentration on study day 0 (P < 0.05; Table 3). Mean total neutrophil concentration increased 12 h after the Mh inoculation (study day 6.5 compared with study day 6; P < 0.05; Table 3). However, mean total neutrophil concentrations did not differ between study days 7.5, 9, 11, and 13 (P > 0.05). The mean ratio between total neutrophils and lymphocytes increased from study days 6 to 6.5 (P < 0.05). On study day 6, lymphocytes were the predominant cells, whereas on study day 6.5 (12 h after the Mh inoculation), neutrophils predominated. During the rest of the study, the predominant cells continued to be neutrophils, except for study day 9, in which lymphocytes were more common. The patterns observed for leukocyte, lymphocyte, and neutrophil concentrations were similar to those obtained using the LLD, and therefore the biological mechanisms and comparisons to previous literature used to explain the LLD results also apply to the PCLD results. A detailed analysis depicting the associations between each cell concentration obtained by the PCLD and LLD are described in the following section.
Table 3.
Model-adjusted means (and standard error of the means) of blood leukocyte differentials measured by the point-of-care leukocyte differential assay in calves inoculated with BoHV-1 on day 0 and Mannheimia haemolytica on day 6, by study day
| Study day | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 0 | 1 | 2 | 4 | 6 | 6.5 | 7 | 7.5 | 9 | 11 | 13 | P 1 | |
| Cell type, unit | Model-adjusted mean (SEM) | |||||||||||
| Leukocytes, 109/L | 14.1a | 14.2ag | 12.9ae | 11.9beg | 10.7df | 10.4bdfg | 13.6ae | 10.6bcd | 10.8ade | 7.9cf | 7.7adef | <0.01 |
| (1.04) | (1.05) | (1.04) | (1.05) | (1.05) | (1.06) | (1.06) | (1.06) | (1.09) | (1.13) | (1.26) | ||
| Lymphocytes, 109/L | 7.0a | 6.8a | 6.6ac | 6.4ac | 6.1ac | 4.5b | 5.3cd | 3.9e | 3.9be | 2.4f | 2.8bdef | <0.01 |
| (1.05) | (1.05) | (1.05) | (1.04) | (1.04) | (1.06) | (1.06) | (1.06) | (1.11) | (1.13) | (1.26) | ||
| Total neutrophils, 109/L | 6.7ad | 6.8af | 5.6bd | 4.7b | 3.9c | 5.2ab | 7.4df | 5.9abd | 6.2abd | 4.9abcd | 3.6abcd | <0.01 |
| (1.05) | (1.07) | (1.06) | (1.06) | (1.06) | (1.07) | (1.08) | (1.09) | (1.10) | (1.16) | (1.27) | ||
| Neutrophil/lymphocyte | 1.0ab | 1.0abf | 0.9ac | 0.7c | 0.6h | 1.4bd | 1.6de | 2.0def | 0.9dg | 1.2eg | 1.5abcf | <0.01 |
| (1.11) | (1.12) | (1.10) | (1.04) | (1.02) | (1.11) | (1.31) | (1.11) | (1.11) | (1.10) | (1.14) |
1Statistical difference among study days.
Different superscript letters within a row indicate statistically significant differences (P < 0.05). Calves were randomly assigned to be euthanized on days 6 to 13.
Associations Between PCLD and LLD Results
Leukocyte, segmented neutrophil, and lymphocyte concentrations reported by the PCLD assay were associated with leukocyte, total neutrophil, and lymphocyte concentrations measured by the LLD assay, respectively (P < 0.05; Table 4). In addition, results from all 3 models included significant interaction terms between cell types and study day (P < 0.05), indicating that the associations for each cell type (i.e., leukocyte, lymphocyte, or neutrophil) as recorded by the different assays depended on the time of the measurement (P < 0.05 Table 4). Although our study was not designed specifically to validate the PCLD and LLD assays, the strong linear associations between cell types reported by the 2 assays indicate that the instruments provide similar results. The PCLD has the advantage of improving turnaround time by providing results within minutes, reducing logistical problems of shipping samples and mixing sick and healthy animals while awaiting results. Similarly, reducing logistical problems improves animal well-being by decreasing the time between diagnosis and treatment, in turn, potentially increasing treatment success. However, these advantages need to be assessed directly along with a cost–benefit analysis, which was beyond the scope of this study.
Table 4.
Model-adjusted means (× 109/L) and standard error of the mean (SEM) for the 3 models evaluating the associations between (1) leukocytes, (2) neutrophils, and (3) lymphocytes reported by a point-of-care leukocyte differential (PCLD) and a laboratory-based leukocyte differential (LLD; outcome) assays while accounting for study day and an interaction between its respective cell count and study day
| LLD-leukocytes | LLD-neutrophils | LLD-lymphocytes | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Mean | SEM | P 1 | Mean | SEM | P 1 | Mean | SEM | P 1 | |||
| PCLD-Leuk2 | 1.1 | 1.0 | <0.01 | PCLD-Neut2 | 1.2 | 1.0 | <0.01 | PCDL-Lymph2 | 1.1 | 1.0 | <0.01 |
| Study day | <0.01 | Study day | <0.01 | Study day | <0.01 | ||||||
| 0 | Ref | Ref | 0 | Ref | Ref | 0 | Ref | Ref | |||
| 1 | 1.0 | 1.10 | 1 | 1.5 | 1.18 | 1 | 0.8 | 1.19 | |||
| 2 | 0.9 | 1.14 | 2 | 1.9 | 1.17 | 2 | 0.6 | 1.18 | |||
| 4 | 0.9 | 1.12 | 4 | 1.0 | 1.20 | 4 | 0.6 | 1.20 | |||
| 6 | 0.9 | 1.11 | 6 | 0.9 | 1.28 | 6 | 0.8 | 1.19 | |||
| 6.5 | 1.0 | 1.13 | 6.5 | 1.3 | 1.21 | 6.5 | 0.4 | 1.28 | |||
| 7 | 1.3 | 1.11 | 7 | 2.0 | 1.24 | 7 | 0.7 | 1.22 | |||
| 7.5 | 0.9 | 1.11 | 7.5 | 1.4 | 1.23 | 7.5 | 0.9 | 1.31 | |||
| 9 | 0.8 | 1.10 | 9 | 0.7 | 1.48 | 9 | 0.4 | 1.32 | |||
| 11 | 0.6 | 1.12 | 11 | 0.5 | 1.51 | 11 | 0.4 | 1.32 | |||
| 13 | 0.4 | 1.18 | 13 | 0.0 | 2.89 | 13 | 0.3 | 1.39 | |||
| PCLD*day | <0.01 | PCLD*day | <0.01 | PCLD*day | 0.04 | ||||||
| Leuk*0 | Ref | Ref | Neut*0 | Ref | Ref | Lymp*0 | Ref | Ref | |||
| Leuk*1 | 1.0 | 1.01 | Neut*1 | 0.9 | 1.01 | Lymp*1 | 1.0 | 1.01 | |||
| Leuk*2 | 1.0 | 1.01 | Neut*2 | 0.9 | 1.00 | Lymp*2 | 1.1 | 1.01 | |||
| Leuk*4 | 1.0 | 1.02 | Neut*4 | 1.0 | 1.01 | Lymp*4 | 1.1 | 1.00 | |||
| Leuk*6 | 1.0 | 1.02 | Neut*6 | 1.0 | 1.00 | Lymp*6 | 1.1 | 1.02 | |||
| Leuk*6.5 | 1.0 | 1.01 | Neut*6.5 | 1.0 | 1.04 | Lymp*6.5 | 1.1 | 1.03 | |||
| Leuk*7 | 1.0 | 1.04 | Neut*7 | 0.9 | 1.03 | Lymp*7 | 1.0 | 1.02 | |||
| Leuk*7.5 | 1.0 | 1.03 | Neut*7.5 | 0.9 | 1.03 | Lymp*7.5 | 1.0 | 1.10 | |||
| Leuk*9 | 1.0 | 1.04 | Neut*9 | 1.0 | 1.12 | Lymp*9 | 1.1 | 1.12 | |||
| Leuk*11 | 1.0 | 1.00 | Neut*11 | 1.0 | 1.12 | Lymp*11 | 1.1 | 1.08 | |||
| Leuk*13 | 1.1 | 1.00 | Neut*13 | 2.3 | 1.38 | Lymp*13 | 1.2 | 1.13 |
1Statistical significance of a variable.
2Continuous fixed effects included in the model: Leuk = Leukocytes × 109/L, Neut = Neutrophils × 109/L, Lymp = lymphocytes × 109/L.
Ref = reference category. Calves were inoculated with BoHV-1 on day 0 and Mannheimia haemolytica on day 6. Calves were randomly assigned to be euthanized on days 6 to 13.
Associations Between PCLD and LLD Results with Lung Consolidation
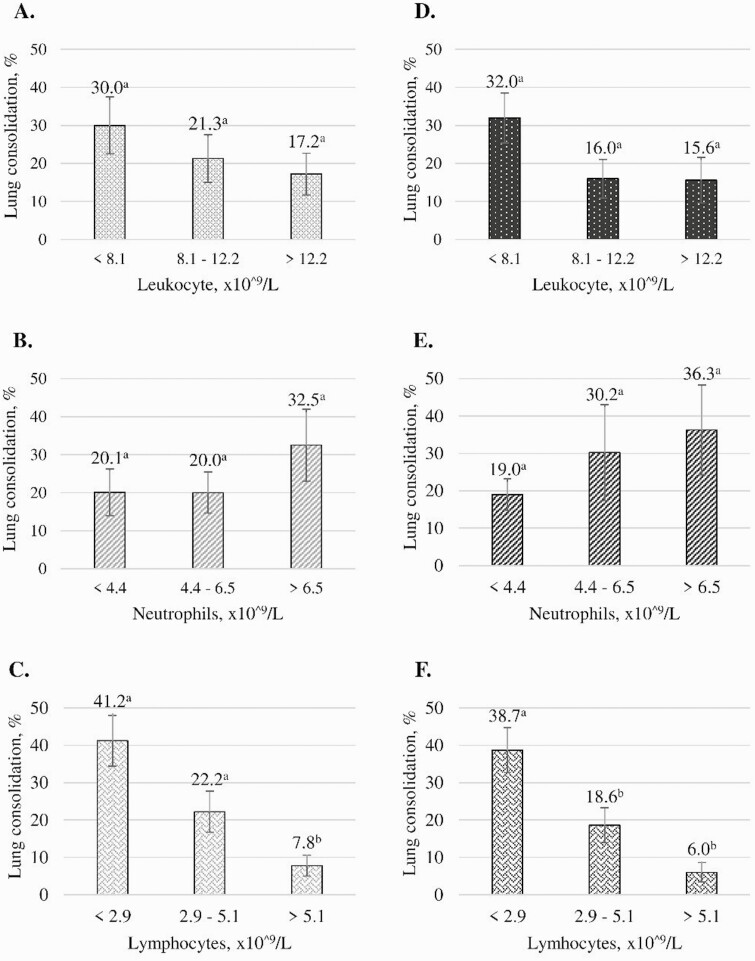
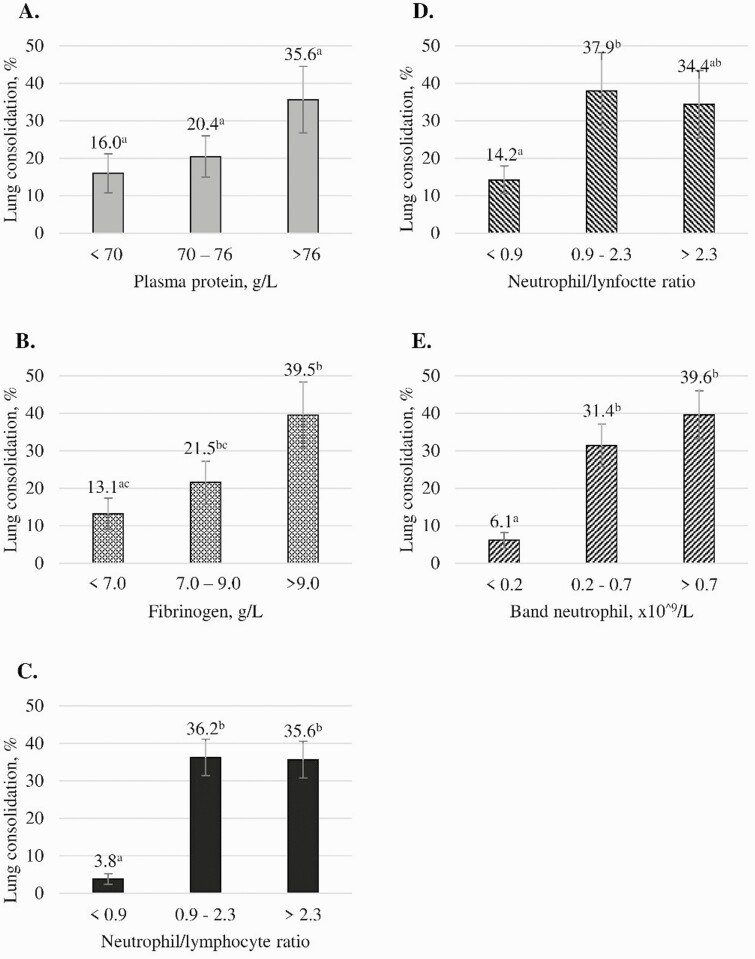
Given that moribund animals were euthanized early, our sample size was reduced to 27 for evaluating the associations between blood parameters and lung consolidation. Mean leukocyte concentrations (on the day of euthanasia) measured by the LLD and the PCLD assays were not associated (P = 0.10 and P = 0.36, respectively; Fig. 1) with the proportion of lung consolidation. Similarly, segmented neutrophil and total neutrophil concentrations measured by the LLD and the PCLD assays, respectively, were not associated (P = 0.26 and P = 0.42, respectively; Fig. 1) with the proportion of lung consolidation. Conversely, band neutrophil concentration measured by the LLD, lymphocyte concentrations, and total neutrophil/lymphocyte ratio from both assays were associated with the proportion of lung consolidation (P < 0.05; Figs. 1 and 2). Plasma protein levels were not associated with the proportion of lung consolidation (P = 0.14), but fibrinogen levels were (P = 0.03; Fig. 2). As lymphocyte concentrations (measured by both assays) increased, the proportion of lung consolidation decreased (P < 0.05).
Figure 1.
Graphs depicting associations between blood leukocyte differentials measured by a laboratory-based blood leukocyte differential (LLD) and a point-of-care blood leukocyte differential (PCLD) assays with lung consolidation, in %, measured during necropsy. Calves were inoculated with BoHV-1 on day 0 and Mannheimia haemolytica on day 6. Calves were randomly assigned to be euthanized on days 6 to 13. (A) Leukocytes concentration from PCLD, (B) Total neutrophils concentration from PCLD. (C) Lymphocyte concentration from PCLD. (D) Leukocytes concentration from LLD. (E) Segmented neutrophils concentration from LLD. (F) Lymphocyte concentration from the LLD. Error bars represent standard error of the mean. Different letter superscripts within a graph indicate statistically significant differences (P < 0.05).
Figure 2.
Graphs depicting associations between blood parameters measured by a laboratory-based blood leukocyte differential (LLD) and a point-of-care blood leukocyte differential (PCLD) assays with lung consolidation, in %, measured during necropsy. Calves were inoculated with BoHV-1 on day 0 and Mannheimia haemolytica on day 6. Calves were randomly assigned to be euthanized on days 6 to 13. (A) Plasma proteins from LLD. (B) Fibrinogen from LLD. (C) Neutrophil/lymphocyte ratio from PCLD. (D) Neutrophil/lymphocyte ratio from LLD. (E) Band neutrophil concentration from LLD. Error bars represent standard error of the mean. Different letter superscripts within a graph indicate statistically significant differences (P < 0.05).
In contrast, as band neutrophil and fibrinogen levels measured by the LLD assay and the total neutrophil/lymphocyte ratio obtained from both methods increased, the proportion of lung consolidation increased (P < 0.05). Specifically, when band neutrophil concentrations were within the reference interval, consolidation was significantly lower than when concentration exceeded that interval, which is consistent with a release of band neutrophils from the bone marrow due to the acute inflammatory process associated with lung consolidation (Jones and Allison, 2007). Band neutrophils, however, were only measured by the LLD assay. Higher total neutrophil/lymphocyte ratios (measured by LLD and PCLD) and fibrinogen (measured by LLD) association with higher lung consolidation is consistent with increasingly acute inflammation and an accumulation of fibrin in the lungs.
Although researchers commonly use challenge studies to reproduce disease in a naïve population, challenge studies do not capture the variation that occurs under natural field conditions, compromising external validity. However, when the onset of the disease is hard to identify, which is the case for BRD, a challenge study can reduce misclassification bias and enable researchers to assess disease progression. Because hematological profiles of cattle can vary based on physiological (e.g., age, sex, diet, stress, reproductive status, and hydration) and environmental characteristics (e.g., ambient temperature and wind), minimizing variability in demographic factors and environmental exposures is advantageous to reduce unexplained variation and more accurately estimate the magnitude of associations.
Stress related to transportation and animal handling can alter blood leukocyte differentials by increasing neutrophil concentrations (Ishizaki and Kariya, 2010). However, the increase in neutrophils is normally transitory and stabilizes 48 h after an initial stressful event (Ishizaki and Kariya, 2010). Although handling remained throughout the study and its potential effect cannot be ignored, acclimation of animals for 6 d prior to study initiation helped minimized stress associated with transportation, as well as changes in feed, housing and management.
Given that controls were not incorporated into our study, predictions related to disease severity based on initial screening on study day 0 were not possible. Investigating the potential prediction capabilities of blood leukocyte differentials to enable targeted antimicrobial interventions, including metaphylaxis, is warranted.
The fact that total neutrophils (reported by PCLD), segmented neutrophils (reported by LLD), leukocyte (reported by LLD and PCLD), and plasma proteins (reported by LLD) were not significantly associated with the percentage of lung consolidation could be due to the limited sample size available for these analyses. Whenever possible, future studies should utilize a greater number of animals in which both leukocyte differentials and lung consolidation scores are recorded.
CONCLUSIONS
The results of this study indicate that lymphocytes, total neutrophils, and neutrophil/lymphocyte ratios differed between pre- and postbacterial challenge period, whereas band neutrophils and fibrinogen increased over time. These changes, together with the associations with lung consolidation, may be useful indicators of BRD progression and differentiating between viral and bacterial infections. Because changes in blood leukocyte differentials in cattle can be associated with diseases other than BRD, diagnostic or prognostic evaluations should consider hematological results in combination with clinical assessment and application of additional diagnostic procedures. Despite its lack of specificity, leukocyte differentials could contribute valuable information toward diagnosis and/or prognosis, and to decisions on interventions, such as administration of antimicrobials. Except for a few assay differences (i.e., differentiation of band and segmented neutrophils, and assessment of plasma protein and fibrinogen by the LLD), the performance of both assays was comparable in terms of magnitude and direction of associations between blood cells recorded by either assay, over time, and with respect to changes in lung consolidation. Further evaluation of the PCLD or similar systems is justified given the relevance of BRD and the need for point-of-care diagnostic assays with a fast turnaround time to aid in early disease diagnosis, prediction, and management.
ACKNOWLEDGMENTS
Funding for this study was provided by Merck Animal Health, with additional support from the College of Veterinary Medicine, Kansas State University, the United States Department of Agriculture, National Institute of Food and Agriculture, Agriculture, the Food Research Initiative Competitive Grant no. 2015-67015-23079, the hatch multistate project no. 1018845, and Contribution no. 22-013-J from the Kansas Agricultural Experiment Station.
Conflict of interest statement. The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
LITERATURE CITED
- Abdallah, A., Hewson J., Francoz D., Selim H., and Buczinski S.. . 2016. Systematic review of the diagnostic accuracy of Haptoglobin, Serum Amyloid A, and Fibrinogen versus clinical reference standards for the diagnosis of bovine respiratory disease. J. Vet. Intern. Med. 30:1356–1368. doi: 10.1111/jvim.13975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Apley, M. 2006. Bovine respiratory disease: pathogenesis, clinical signs, and treatment in lightweight calves. Vet. Clin. North Am. Food Anim. Pract. 22:399–411. doi: 10.1016/j.cvfa.2006.03.009. [DOI] [PubMed] [Google Scholar]
- Baruch, J., Cernicchiaro N., Cull C. A., Lechtenberg K. F., Nickell J. S., and Renter D. G.. . 2019. Performance of multiple diagnostic methods in assessing the progression of bovine respiratory disease in calves challenged with infectious bovine rhinotracheitis virus and Mannheimia haemolytica. J. Anim. Sci. 97: 1–11. doi: 10.1093/jas/skz107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brooks, K. R., Raper K. C., and Ward C. E.. . 2011. Economic effects of bovine respiratory disease on feedlot cattle during backgrounding and finishing phases. Prof. Anim. Sci. 27:195–203. doi: 10.15232/S1080-7446(15)30474-5. [DOI] [Google Scholar]
- Burciaga-Robles, L. O., Step D. L., Krehbiel C. R., Holland B. P., Richards C. J., Montelongo M. A., Confer A. W., and Fulton R. W.. . 2010. Effects of exposure to calves persistently infected with bovine viral diarrhea virus type 1b and subsequent infection with Mannheima haemolytica on clinical signs and immune variables: model for bovine respiratory disease via viral and bacterial interacti. J. Anim. Sci. 88:2166–2178. doi: 10.2527/jas.2009-2005. [DOI] [PubMed] [Google Scholar]
- Carlos-Valdez, L., Wilson B. K., Burciaga-Robles L. O., Step D. L., Holland B. P., Richards C. J., Montelongo M. A., Confer A. W., Fulton R. W., and Krehbiel C. R.. . 2016. Effect of timing of Mannheimia haemolytica challenge following short-term natural exposure to bovine viral diarrhea virus type 1b on animal performance and immune response in beef steers. J. Anim. Sci. 94:4799–4808. doi: 10.2527/jas.2016-0712. [DOI] [PubMed] [Google Scholar]
- Caswell, J. L. 2014. Failure of respiratory defenses in the pathogenesis of bacterial pneumonia of cattle. Vet. Pathol. 51:393–409. doi: 10.1177/0300985813502821. [DOI] [PubMed] [Google Scholar]
- Chen, Y., Arsenault R., Napper S., and Griebel P.. . 2015. Models and methods to investigate acute stress responses in cattle. Animals (Basel). 5:1268–1295. doi: 10.3390/ani5040411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engler, M., Defoor P., King C., and Gleghorn J.. . 2014. The impact of bovine respiratory disease: the current feedlot experience. Anim. Health Res. Rev. 15:126–129. doi: 10.1017/S1466252314000139. [DOI] [PubMed] [Google Scholar]
- Fraser, B. C., Anderson D. E., White B. J., Miesner M. D., Lakritz J., Amrine D., and Mosier D. A.. . 2013. Associations of various physical and blood analysis variables with experimentally induced Mycoplasma bovis pneumonia in calves. Am. J. Vet. Res. 74:6–11. [DOI] [PubMed] [Google Scholar]
- Gardner, B. A., Dolezal H. G., Bryant L. K., Owens F. N., and Smith R. A.. . 1999. Health of finishing steers: effects on performance, carcass traits, and meat tenderness. J. Anim. Sci. 77:3168–3175. doi: 10.2527/1999.77123168x. [DOI] [PubMed] [Google Scholar]
- Gonçalves, J. L., Lyman R. L., Hockett M., Rodriguez R., Dos Santos M. V., and Anderson K. L.. . 2017. Using milk leukocyte differentials for diagnosis of subclinical bovine mastitis. J. Dairy Res. 84:309–317. doi: 10.1017/S0022029917000267. [DOI] [PubMed] [Google Scholar]
- Griffin, D. 1997. Economic impact associated with respiratory disease in beef cattle. Vet. Clin. North Am. Food Anim. Pract. 13:367–377. doi: 10.1016/s0749-0720(15)30302-9. [DOI] [PubMed] [Google Scholar]
- Griffin, D., Chengappa M. M., Kuszak J., and McVey D. S.. . 2010. Bacterial pathogens of the bovine respiratory disease complex. Vet. Clin. North Am. Food Anim. Pract. 26:381–394. doi: 10.1016/j.cvfa.2010.04.004. [DOI] [PubMed] [Google Scholar]
- Guterbock, W. M. 2014. The impact of BRD: the current dairy experience. Anim. Health Res. Rev. 15:130–134. doi: 10.1017/S1466252314000140. [DOI] [PubMed] [Google Scholar]
- Hanzlicek, G. A., White B. J., Mosier D., Renter D. G., and Anderson D. E.. . 2010. Serial evaluation of physiologic, pathological, and behavioral changes related to disease progression of experimentally induced Mannheimia haemolytica pneumonia in postweaned calves. Am. J. Vet. Res. 71:359–369. doi: 10.2460/ajvr.71.3.359. [DOI] [PubMed] [Google Scholar]
- Harris, N., Kunicka J., and Kratz A.. . 2005. The ADVIA 2120 hematology system: flow cytometry-based analysis of blood and body fluids in the routine hematology laboratory. Lab. Hematol. 11:47–61. doi: 10.1532/LH96.04075. [DOI] [PubMed] [Google Scholar]
- Ishizaki, H., and Kariya Y.. . 2010. Road transportation stress promptly increases bovine peripheral blood absolute NK cell counts and cortisol levels. J. Vet. Med. Sci. 72:747–753. doi: 10.1292/jvms.09-0441. [DOI] [PubMed] [Google Scholar]
- Johnson, K. K., and Pendell D. L.. . 2017. Market impacts of reducing the prevalence of bovine respiratory disease in United States beef cattle feedlots. Front. Vet. Sci. 4:189. doi: 10.3389/fvets.2017.00189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones, M. L., and Allison R. W.. . 2007. Evaluation of the ruminant complete blood cell count. Vet. Clin. North Am. Food Anim. Pract. 23:377–402, v. doi: 10.1016/j.cvfa.2007.07.002. [DOI] [PubMed] [Google Scholar]
- Molina, V., Risalde M. A., Sánchez-Cordón P. J., Pedrera M., Romero-Palomo F., Luzzago C., and Gómez-Villamandos J. C.. . 2013. Effect of infection with BHV-1 on peripheral blood leukocytes and lymphocyte subpopulations in calves with subclinical BVD. Res. Vet. Sci. 95:115–122. doi: 10.1016/j.rvsc.2013.02.018. [DOI] [PubMed] [Google Scholar]
- Rice, J. A., Carrasco-Medina L., Hodgins D. C., and Shewen P. E.. . 2007. Mannheimia haemolytica and bovine respiratory disease. Anim. Health Res. Rev. 8:117–128. doi: 10.1017/S1466252307001375. [DOI] [PubMed] [Google Scholar]
- Stockham, S. L., and Scott M. A.. . 2013. Fundamentals of veterinary clinical pathology. Hobeken, New Jersey: John Wiley & Sons. [Google Scholar]
- White, B. J., and Renter D. G.. . 2009. Bayesian estimation of the performance of using clinical observations and harvest lung lesions for diagnosing bovine respiratory disease in post-weaned beef calves. J. Vet. Diagn. Invest. 21:446–453. doi: 10.1177/104063870902100405. [DOI] [PubMed] [Google Scholar]
- Whiteley, L. O., Maheswaran S. K., Weiss D. J., Ames T. R., and Kannan M. S.. . 1992. Pasteurella haemolytica A1 and bovine respiratory disease: pathogenesis. J. Vet. Intern. Med. 6:11–22. doi: 10.1111/j.1939-1676.1992.tb00980.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.