Abstract
Substantial racial and ethnic disparities in COVID-19 mortality have been observed at the state and national level. However, less is known about how race and neighborhood-level disadvantage may intersect in COVID-19 and excess mortality during the pandemic. To assess this, we link death certificate data from Minnesota from 2017 through 2020 (N = 186,595 deaths) to the Area Deprivation Index to examine hyperlocal disparities in mortality. Black, Indigenous, and people of color (BIPOC) standardized COVID-19 mortality was 459 deaths per 100,000 population in the most disadvantaged neighborhoods compared to 126 per 100,000 in the most advantaged. Total mortality increased in 2020 by 14% for whites and 41% for BIPOC. Statistical decompositions show that most of this growth in racial disparity is associated with mortality gaps between whites and communities of color within the same levels of area disadvantage, rather than with the fact that whites live in more advantaged areas.
Keywords: COVID-19, excess mortality, neighborhood disadvantage, Area Deprivation Index, race, ethnicity
Background
An aphorism in disaster planning is that populations that are the worst off prior to a disaster will inexorably be the worst off during response and recovery phases.(1) There has similarly long been recognition that the sequelae of structural racism disadvantage certain populations and advantage others, leading to disparate outcomes in morbidity and mortality across the United States. (2) Within the space of public health, the concept of inequality has become a core tenet of public health practice. National frameworks, like Public Health 3.0, the National Prevention Strategy, Healthy People 2030, and the revised 10 Essential Public Health Services all have equity at their core. There is recognition that to achieve “an optimal and equitable level of health,” (3) structural improvements must be made to the social determinants of health. Yet despite attestations within the field, COVID-19 has shown, concretely, that disparities persist. (4, 5) The identification of drivers of disparities is less well developed. A lack of available data has made it challenging to examine the intersection between socioeconomic status and other sociodemographic characteristics as drivers of COVID-19 mortality.
Socioeconomic inequalities may lead to higher risk of COVID-19 mortality through heightened risk of exposure. (6, 7) Socioeconomically advantaged individuals may have more opportunity to socially distance through access to white-collar remote work, less crowded housing and transportation, and other resources such as the ability to pay for delivery services for essential commodities. At the same time, socioeconomically disadvantaged individuals are more likely to be essential workers, live in crowded housing and take public transportation, and lack other resources necessary for social distancing and thus are at heightened risk of exposure to COVID-19.(6, 8) Residential segregation, often racial and socioeconomic, also increases infectious disease risk through isolation and concentration: isolation confines infections to segregated areas and prevents transmission to the rest of the population, and high-density areas increase the probability of disease transmission within the segregated group.(7) Overall, COVID-19 mortality is higher among socioeconomically disadvantaged populations.(9–16)
The higher mortality rates among Black, Indigenous and other People of Color (BIPOC) during the pandemic has been the subject of much public concern. Nationally, the age-standardized mortality rates for Black and Hispanic Americans are more than triple the rates for non-Hispanic whites.(17, 18) Other research has shown higher rates of COVID-19 mortality among American Indians.(19) Socioeconomic status (20) also interacts with race and ethnicity to affect mortality risk. (21)
Geography also is a well-established correlate of inequity. Institutionalized racial discrimination in housing, employment, credit markets, and consumer interactions created wide racial and ethnic disparities in SES that manifest geographically.(22) As a result, BIPOC are more likely to live in socioeconomically disadvantaged neighborhoods.(23) Examining the intersection of neighborhood disadvantage and race/ethnicity serves as one alternative to attributing the disparities to specious differences in biology in a way that can prevent meaningful policy solutions.(24)
To understand how systemic racism interacts with other forms of inequity in the pandemic to manifest in elevated mortality, we characterize the association of neighborhood socioeconomic (dis)advantage with pre-2020 mortality, COVID-19 mortality, and 2020 excess mortality in the state of Minnesota in the United States using death certificate data. We then examine how these associations intersect with race.
Minnesota is of particular interest due to its rapid publication of death certificates electronically, because it became a “hot spot” for COVID-19 late in 2020, and because Minnesota has some of highest pre-COVID racial and socioeconomic disparities in the country. (25) These extreme disparities occur in the context of an overwhelmingly white state that has also been a major destination for Hmong and, especially, East African refugees. Minnesota is about 80% white and its remaining population is approximately evenly divided between Black, Asian-American, and Hispanic populations, with a smaller Native American population. About half of Minnesota’s residents live in the Minneapolis/St. Paul metro area, and the other half live in rural areas and smaller cities in what we call “Outstate Minnesota” using local parlance (26). Black and Asian-American, and to a lesser extent Hispanic, populations disproportionately live in the Metro area, while white and Native American populations are disproportionately Outstate.
Methods
Geographic Measures
We linked geographic data to the complete set of death certificates from the Minnesota Department of Health (MDH) Office of Vital Records for deaths occurring in Minnesota from January 1, 2017 through December 31, 2020 (n=186,595). For the linkage, we geocoded all decedent residential addresses in Minnesota to obtain Block Group and linked to the Area Deprivation Index (ADI), which is a measure of neighborhood-level socioeconomic (dis)advantage derived from data in the American Community Survey (ACS). ADI is a stronger measure of neighborhood socioeconomic (dis)advantage than unidimensional measures (such as poverty rate) because it combines multiple measures of disadvantage that more accurately reflect the multidimensional character of a neighborhood’s socioeconomic position.(27) ADI rankings were constructed using a combination of income, education, employment and housing quality measures to generate rankings. The methodology used to create the ADI data are described elsewhere.(28–30) The range of ADI rankings is from one to ten where a ranking of one indicates the lowest level of disadvantage within the state of Minnesota and ten indicates the highest level of disadvantage within the state; these scores represent deciles of the socioeconomic index across block groups in the state. While much prior work on COVID-19 has examined counties, (10, 31–34) ADI measures, used here at the block group and tract level, capture very localized distinctions. See Appendix A for more information on the ADI.(35)
We also distinguished between mortality in the 7-County Minneapolis/St. Paul “Twin Cities” Metro area and all other Census tracts and block groups outside of the 7-county Metro area, which we refer to as “Outstate” Minnesota. Each of these two regions comprises roughly half of the state’s total population. While COVID-19 mortality increased in rural areas over the course of 2020, the majority of COVID-19 mortality remained concentrated in urban and suburban areas (36).
In neighborhood level analyses of mortality, we excluded mortality in long-term care facilities (LTCFs), or to residents of such facilities when they died elsewhere (e.g., in a hospital), from residential household mortality in order to better reflect neighborhood characteristics. ADI does not serve as a meaningful proxy for neighborhood (dis)advantage among institutionalized populations in the same way as residential households.(11) However, because LTCFs were a large portion of the state’s total pandemic mortality—accounting for 53% of confirmed COVID-19 deaths in 2020—this exclusion could plausibly affect the results. To examine whether and how our results were affected by excluding LTCFs, we completed the same analysis including LTCFs. After excluding LTCF deaths, our data include 122,617 total and 2,733 COVID-19 deaths.
Selecting a deprivation index
This analysis utilizes the ADI instead of other aerial deprivation indices, such as the Social Vulnerability Index (SVI). While the SVI is being used by many states for COVID-19 vaccine allocation, the ADI is used here to explore how the structural sequelae of racism interacts with socio-spatial inequalities because the SVI includes direct measures of race/ethnicity in its construction and so would confound the comparison. Because the ADI is defined at the Census Block Group level, we also use it to explore how deprivation at the Census Tract level relates to more micro scales (Appendix A, Appendix B).
Population Measures
Population size estimates for Census Block Groups and Census Tracts are from the IPUMS National Historic Geographic Information System for 2014–2018 (37). These estimates are adjusted to 2019 population sizes (see Appendix A for detailed approach and equations). The 2019 population sizes are based on the 2019 American Community Survey.(38)
Mortality Measures
We calculated excess mortality—or the difference between observed and expected mortality—in 2020 by subtracting the average mortality from 2017 – 2019 from mortality in 2020 for the entire year. Excess mortality incorporates deaths from COVID-19 and other causes indirectly attributable to the pandemic through pathways like averted medical care.(39) For COVID-19 mortality, we characterized a death as a COVID-19 death if it was listed as “confirmed” COVID-19 on the death certificate (in any cause of death line) using an International Classification of Disease (ICD-10) code of U07.1 assigned by the National Center for Health Statistics (n = 5,803 in 2020). This designation was not necessarily based on COVID-19 laboratory tests.
Race/Ethnicity Measures
To examine whether mortality patterns vary by race and ethnicity, we conducted a race-stratified analysis using race and ethnicity from the death certificate data. We used race-bridging algorithms(40) to match population denominators to the racial classifications on death certificates (see Appendix A for more information). In order to use the best-available race-specific population estimates, we aggregated race-specific analyses up to the Census tract level (see Appendix A for details). Furthermore, because extremely few BIPOC Minnesotans live in ADI 1 or ADI 2 (highly advantaged) tracts in Outstate Minnesota (N~1,200 in ADI 1, N~7,000 in ADI 2), we combined ADI levels 1–3 in the main race-specific analyses stratified by region (see Appendix C for raw rates that retain all ten ADI categories).
Due to the small geographic unit of analysis—even after aggregating into ADI deciles—we were unable to examine distinct racial and ethnic groups. Thus, we stratified the analysis with non-Hispanic white individuals in one group and BIPOC—which includes Black, American Indian or Alaskan Native, Asian or Pacific Islander, and Hispanic/Latino—in another group.(41) The majority of COVID-19 deaths were among white Minnesotans (n=5,010) and rest were distributed among BIPOC (n=793). The BIPOC group combines populations with distinctive histories and experiences. It has different racial/ethnic composition in the Metro (44% Black, 29% Asian, 24% Hispanic, 2% Native American) compared to Outstate (39% Hispanic, 29% Black, 17% Asian, 14% Native American). Previous analyses showed that, in Minnesota, the primary racial divide in COVID mortality is between non-Hispanic whites and all other racial groups.(42) This differs from the national pattern, where Asian and Asian-American populations often have similar mortality to non-Hispanic whites, and may reflect that Minnesota’s Asian-American population is predominantly Southeast Asian in origin—an ethnic group experiencing substantial systemic racism in the United States.(43, 44)
Decomposition Analyses
To further characterize geographic disparities associated with COVID-19 by race/ethnicity, we decomposed the absolute BIPOC/white mortality disparity into “neighborhood disadvantage” and “residual disadvantage” components. The “neighborhood disadvantage” component reflects the mortality disparity associated with racial differences in the ADI deciles, i.e., the fact that white people live in more advantaged neighborhoods on average. The “residual disadvantage” component captures the extent to which BIPOC have higher (or lower) mortality than white populations living in the same ADI decile. This component is functionally an ADI-standardized racial disparity. The decomposition equations are given in Appendix A.
While some of the descriptive results present unadjusted mortality in order to describe the overall risk for each population, other results, including the decomposition analysis, use mortality estimates adjusted for age and sex in order to summarize the interaction of racial and place-based socioeconomic stratification, net of the other major demographic drivers of COVID-19 risk. The “standard” age and sex distribution is that of the 2019 population of Minnesota.
Limitations
A limitation of this study is its reliance on death certificate data, which introduces the possibility of misclassification of key information, particular race/ethnicity and cause of death. Misclassification rates for race and ethnicity on death certificate data are low for the non-Hispanic white, non-Hispanic Black, Hispanic/Latino, and Asian or Pacific Islander populations.(45) Misclassification is higher, however, for the American Indian or Alaska Native population (40% in one study).(45) Since we group the BIPOC population, the racial misclassification that would be consequential would be white being misclassified as non-white or vice-versa. In particular, misclassification will bias our results if, for example, it is more likely that Hispanic whites would be misclassified as non-Hispanic white depending on their neighborhood. To the extent that Hispanic whites have higher mortality than non-Hispanic whites, such misclassification would tend to inflate white mortality estimates, and thus is likely to be conservative for the size of racial disparities.
Meanwhile, undercounting of COVID-19 deaths is a concern, and is likely to be differential by race and geography.(18, 31, 42, 46) To mitigate these concerns, we examine multiple outcomes: COVID-19 mortality, excess mortality, and total mortality in 2020. To the extent that COVID-19 was differentially underdiagnosed in BIPOC populations, the racial disparities in COVID-19 mortality reported here will be underestimates. Any such underestimates will also bias our neighborhood analyses to the extent that diagnosis patterns also differed by neighborhood. In contrast with COVID-19 mortality, excess mortality is less likely to be underreported, yet is harder to interpret as a direct reflection of the pandemic. Excess mortality is likely to reflect a mixture of diagnosed and undiagnosed COVID-19 deaths, mortality indirectly attributable to the pandemic, and the suppression of some causes of death (e.g., influenza) due to social distancing. Although neither certified COVID-19 nor excess mortality is a perfect measure of the intensity of the pandemic, examining the two together offers a more complete picture of the pandemic’s mortality burden.
Results
Total mortality in 2020 outside long term care facilities increased 17% from reference years of 2017–2019 in Minnesota, with much of the increase directly attributable to the COVID-19 pandemic. This increase was 14% for whites and 41% for BIPOC.
In the reference period (2017–2019), mortality statewide across Minnesota was concentrated in more disadvantaged neighborhoods compared to advantaged neighborhoods (Appendix B). Excluding deaths among residents of long-term care facilities, the crude 2017–2019 mortality rate was 635 deaths per 100,000 people in the most disadvantaged neighborhoods (ADI of 10) compared to 341 deaths per 100,000 in the most advantaged neighborhoods (ADI of 1). The 2020 COVID-19 mortality rate was 75 deaths per 100,000 people in the most disadvantaged neighborhoods compared to 23 deaths per 100,000 in the most advantaged neighborhoods, and the 2020 excess mortality rate grew to 120 deaths per 100,000 people in the most disadvantaged compared to 53 deaths per 100,000 people in the most advantaged neighborhoods.
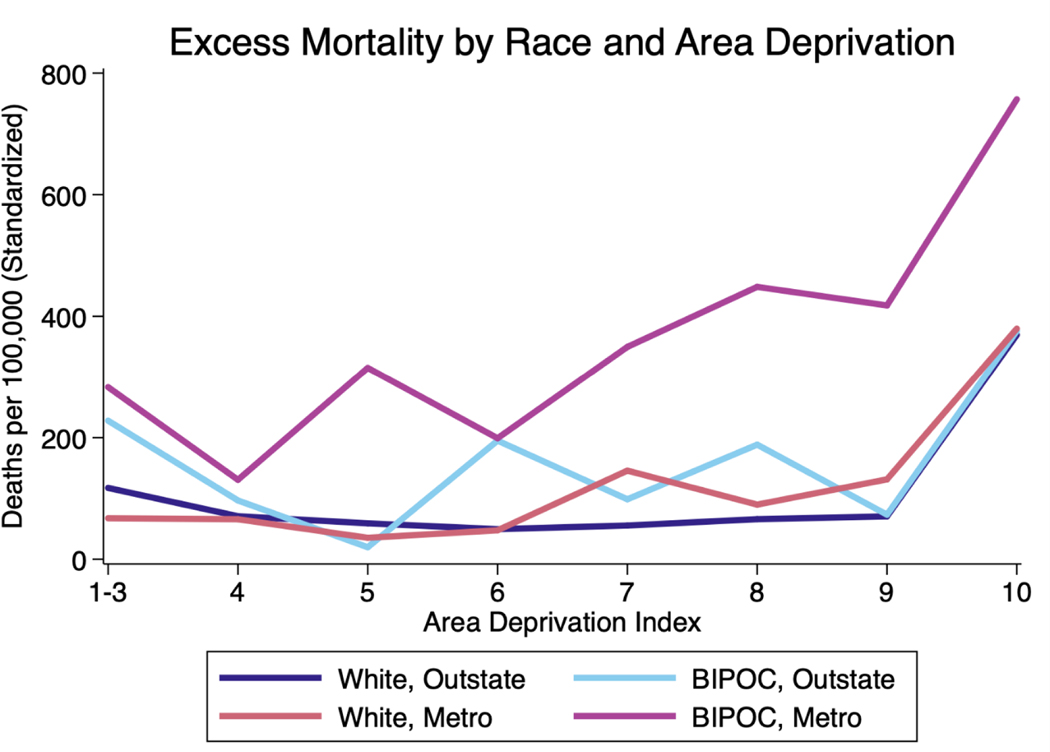
In 2020 in Minnesota, COVID-19 and excess mortality rates were substantially higher for BIPOC living in the Metro region than for all other race-region combinations (Exhibit 1; Appendix C). The elevated mortality for Metro BIPOC coincided with two other patterns: higher mortality in Metro vs. Outstate Minnesota (i.e., areas outside the Twin Cities Metro Area) for whites at relatively high deprivation levels (ADI 7+), and, for all groups, notably higher excess mortality and COVID-19 mortality rates in the most disadvantaged neighborhoods (ADI 10) compared to all others. The excess mortality rate, standardized for age and sex, was 639 deaths per 100,000 people for BIPOC in the most disadvantaged neighborhoods (statewide), compared to 229 per 100,000 people in the most advantaged neighborhoods; the same rates for whites were 437 and 152 per 100,000, respectively. Those rates were as high as 734 per 100,000 for Metro BIPOC, compared with 563 per 100,000 for Metro whites. Because relatively few BIPOC live in Outstate Minnesota, excess mortality estimates for this population are imprecisely estimated; estimates for other populations and for other outcomes are more precise (Appendix D).
Exhibit 1 (figure):
2020 Excess Mortality (Adjusted for Age and Sex) in Minnesota by Race, Region, and Area Deprivation. Data Sources: Death Certificates from the Minnesota Department of Health (MDH) Office of Vital Records linked to the Area Deprivation Index (ADI) data.
Notes: An ADI ranking of one indicates the lowest level of disadvantage within the state of Minnesota and ten indicates the highest level of disadvantage within the state of Minnesota. Excess mortality was calculated by subtracting the average all-cause mortality from 2017–2019 from mortality in 2020. Twin Cities Metro includes all deaths among residents of the 7-County Twin Cities Metro area (Anoka, Carver, Dakota, Hennepin, Ramsey, Scott, and Washington counties). BIPOC includes all people of color who did not identify their race as white. Death rates in this exhibit are standardized for age and sex (details in Appendix A).
These differences in mortality rates interact with differences in where white and BIPOC people live. For example, among BIPOC in the Metro area, 8% live in Census tracts with ADI 1, while 6% live in tracts with average ADI 10; among whites in the Metro area, 18% are in ADI 1 and less than 1% in ADI 10. In Outstate, few people of any race live in advantaged tracts, and the population is generally more likely to reside in deprived areas (Appendix C). Mean tract-level ADI is 5.5 for Metro BIPOC, 6.9 for Outstate BIPOC, 3.7 for Metro whites, and 6.2 for Outstate whites. Thus, while deprived Metro neighborhoods had elevated risk for whites as well as BIPOC, Metro whites live in notably more advantaged neighborhoods than other populations in the state. These population distributions raise the question of how differences in neighborhood deprivation interact with mortality differences within neighborhood.
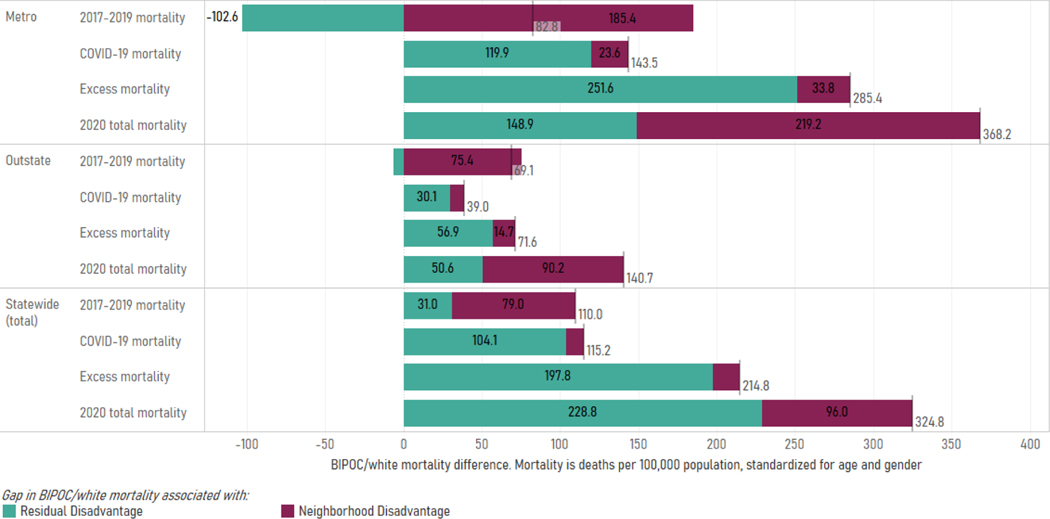
The racial disparity in age- and gender-standardized mortality outside LTCFs grew substantially in 2020, from 110 deaths per 100,000 in 2017–2019 to 325 per 100,000 in 2020—increasing by a factor of nearly 3. Alongside this increase in racial disparity, the relationship of mortality disparity to the racial patterning of neighborhood disadvantage also shifted. Racial disparities in mortality before the pandemic tended to be driven by whites living in more advantaged geographies than BIPOC populations on average (neighborhood disadvantage), while aggregate disparities during the pandemic were driven by higher BIPOC mortality net of geographic disadvantage (residual disadvantage). For total mortality in Minnesota, 72% of the racial disparity in 2017–2019—but only 30% of the 2020 disparity—was driven by BIPOC populations tending to live in more disadvantaged neighborhoods. However, these results obscure a “Simpson’s Paradox”(47) in 2020 mortality: neighborhood disadvantage statistically accounts for a majority of the racial disparity in both Metro (60%) and Outstate (64%) regions, despite accounting for only half of that amount statewide, indicating that geographic sorting at both the regional and neighborhood levels contributed to racial disparities in 2020 mortality.
Underscoring the importance of geographic sorting, in 2017–2019, racial disparities in mortality in Outstate and, especially, Metro Minnesota were fully statistically accounted for by neighborhood disadvantage: Within ADI deciles in that time period, on average, the white population had higher age- and gender-adjusted mortality than the BIPOC population (reflected in the negative sign of the residual disadvantage measure). This negative residual disadvantage was driven by large racial disparities, in the direction of higher white mortality, in highly disadvantaged neighborhoods (Appendix Table E2).
Against this backdrop of pre-pandemic mortality heavily associated with neighborhood disadvantage, the pandemic has moderately increased the mortality disparities due to BIPOC living in more disadvantaged neighborhoods than whites on average, while radically increasing the residual mortality disparities. Residual disparities, reflecting BIPOC disadvantage compared to whites in similar-quality neighborhoods, were much larger in the Metro area compared to Outstate Minnesota. For example, residual disadvantage in 2020 excess mortality was 252 deaths per 100,000 in the Metro, compared with 57 deaths per 100,000 in Outstate Minnesota.
In total mortality in 2020, the largest disparities within ADI deciles were in highly advantaged neighborhoods in the Metro and, to a lesser extent, middle-ADI neighborhoods in Outstate Minnesota. Combining the within-ADI (residual disadvantage) and between-ADI (neighborhood disadvantage) disparities, the neighborhoods contributing the most total disparity in 2020 were primarily highly advantaged neighborhoods in the Metro (where BIPOC are substantially underrepresented, overall mortality is notably low, and mortality is highly unequal across racial groups) (Appendix Table E2).
As a sensitivity analysis, we repeated the analysis including LTCFs. The results indicated that mortality is less disproportionately concentrated in disadvantaged neighborhoods when LTCFs are included, and thus indicated that including LTCFs masked socioeconomic disparities in COVID-19 and excess mortality (Appendix F). This finding may be because the SES of long-term care residents are different from the SES of the neighborhood as a whole.(11)
Discussion
In 2020, COVID-19 mortality and excess mortality in Minnesota were concentrated in disadvantaged neighborhoods. This is particularly meaningful, as BIPOC across the state live in less advantaged neighborhoods than whites, on average, although whites in the Twin Cities Metro Area also live in significantly more advantaged areas, on average, compared to whites in Outstate Minnesota (i.e., outside of the Metro). In a race- and geography-stratified analysis, we found that COVID-19 mortality and excess mortality were substantially higher for Metro-area BIPOC than Metro-area whites living in similarly disadvantaged neighborhoods. We also found that, for both racial groups, both regions, and all types of mortality, the most disadvantaged neighborhoods had substantially higher mortality than slightly less disadvantaged neighborhoods.
A primary take-away from the results is that, before the pandemic, BIPOC/white mortality disparities were largely driven by neighborhood disadvantage, yet COVID-19 and excess mortality were primarily driven by what we called “residual disadvantage” (i.e., disparities net of neighborhood disadvantage). Thus, while pre-pandemic BIPOC/white mortality disparity reflects BIPOC living in more highly deprived neighborhoods, we found that pandemic-associated mortality stratifies similarly disadvantaged BIPOC and white residents. This shift in the overall disparity to largely reflect residual disadvantage does not reflect a reduction in neighborhood disadvantage for BIPOC. Indeed, neighborhood disadvantage increased somewhat in 2020 compared to prior years. Rather, it reflects a far larger increase in the racial disparity in mortality even for BIPOC and whites at similar levels of neighborhood deprivation. We found that, prior to the COVID-19 pandemic, within broad regions, whites on average had higher pre-pandemic mortality than BIPOC in similar neighborhoods. This disparity may reflect neighborhood selection processes(48) such that whites living in highly deprived areas may be likely to lead highly disordered lives compared to BIPOC for whom systemic racism produces more pathways into such neighborhoods. During the pandemic, this residual disadvantage reversed, reflecting substantially greater BIPOC risk net of neighborhood disadvantage. This large residual disadvantage may reflect systematic inequalities that have led BIPOC to be at greater risk of COVID-19 exposure due to occupational risk to unprotected essential workers and their families,(48–50) denser housing,(50, 51) and increased likelihood of living in multigenerational households;(52) along with greater vulnerability to mortality due to increased likelihood of comorbidities(53, 54) and worse treatment by some medical providers.(55) Residual disadvantage may also reflect segregation processes at an even more micro level than Census tracts, such as at the level of individual city blocks.
The high rates of COVID-19 mortality in disadvantaged neighborhoods in all parts of the state warrant further investigation and remediation. During 2020, COVID-19 incidence increased dramatically in rural areas, and by the end of the year, rural areas reported the most new COVID-19 cases (56). Understanding the local nature of these mortality disparities can illuminate the ways in which current public health infrastructure and investments in infrastructure can ameliorate or exacerbate health inequalities during public health crises. Prioritizing social investment by area deprivation prior to a disaster may help ameliorate the disparities that otherwise would present during the disaster. At the same time, the notably higher COVID-19 and excess mortality for Metro-area BIPOC, as well as the heightened mortality for the most highly deprived neighborhoods throughout the state—even compared with only slightly less deprived neighborhoods—suggests that highly targeted pandemic responses—such as testing and, more recently, vaccination—directed at highly deprived neighborhoods might have paid disproportionate dividends.
Understanding whether neighborhood disadvantage is a risk factor for COVID-19, and whether race affects the strength of this association, can improve our understanding of the social determinants of health. COVID-19 shows that it is insufficient to be concerned about high-level inequalities observed across geographies, but that we must embrace the call of Public Health 3.0 (3) to utilize geographically acute, actionable data in decision-making and policy setting. County and even city-level analyses may obfuscate the very disparities and social determinants that public health frameworks increasingly attend to. Indices like the ADI or SVI that operate at tract or block group levels provide far deeper insight into the structural issues facing localities than broader regional residential patterns considered alone. Yet the differential patterns of racial and neighborhood-level disadvantage in the Metro vs. Outstate Minnesota regions—and the fact that area deprivation is a better predictor of mortality within these geographic regions than in the state as a whole—also suggest the necessity of considering multiple scales of geography simultaneously in order to accurately characterize intersectional risks.
Neighborhood-level analysis of 2020 mortality in Minnesota showed that the age- and sex-adjusted gap between BIPOC and whites is attributable to (a) BIPOC populations dying at higher rates than whites who live in the same geographic level of disadvantage and (b) BIPOC populations being much more likely to live in disadvantaged communities compared to whites. Efforts to improve the social determinants, then, must seek to ameliorate both types of disparities, mortality gaps observed among populations living in similar levels of geographic disadvantage and the gap from unequal geographic disadvantage, in order to improve both the aggregate level of population health and the distribution of health between groups. Interventions that consider neighborhood context are more likely to be effective in reducing COVID-19 mortality than universal and indiscriminate intervention approaches. (57)
Supplementary Material
Exhibit 2 and Exhibit 3:
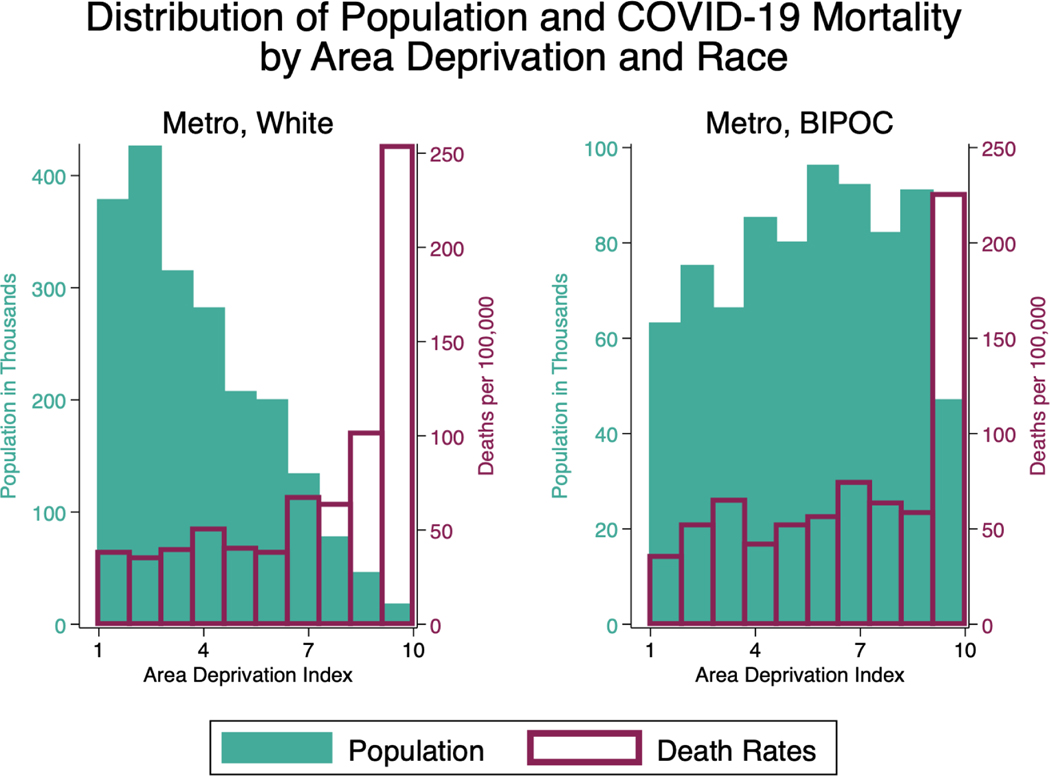
Exhibit 2 (figure): Distribution of Population and COVID-19 Death Rates by Area Deprivation (White Metro Population). Data Sources: Death Certificates from the Minnesota Department of Health (MDH) Office of Vital Records linked to the Area Deprivation Index (ADI) data.
Notes: The range of ADI ranking of one indicates the lowest level of disadvantage within the state of Minnesota and ten indicates the highest level of disadvantage within the state of Minnesota. COVID-19 mortality includes COVID-19 (ICD U07.1) on the death certificate. Twin Cities Metro includes all deaths among residents of the 7-County Twin Cities Metro area (Anoka, Carver, Dakota, Hennepin, Ramsey, Scott, and Washington counties). BIPOC includes all people of color who did not identify their race as white. Death rates in these exhibits are not adjusted for age or sex in order to highlight descriptive distributions of populations and deaths.
Exhibit 3 (figure): Distribution of Population and COVID-19 Death Rates by Area Deprivation (BIPOC Metro Population). Data Sources: Death Certificates from the Minnesota Department of Health (MDH) Office of Vital Records linked to the Area Deprivation Index (ADI) data.
Notes: The range of ADI ranking of one indicates the lowest level of disadvantage within the state of Minnesota and ten indicates the highest level of disadvantage within the state of Minnesota. COVID-19 mortality includes COVID-19 (ICD U07.1) on the death certificate. Twin Cities Metro includes all deaths among residents of the 7-County Twin Cities Metro area (Anoka, Carver, Dakota, Hennepin, Ramsey, Scott, and Washington counties). BIPOC includes all people of color who did not identify their race as white. Death rates in these exhibits are not adjusted for age or sex in order to highlight descriptive distributions of populations and deaths.
Exhibit 4 (figure):
Gap in BIPOC/White Mortality, by Type. Data Sources: Death Certificates from the Minnesota Department of Health (MDH) Office of Vital Records linked to the Area Deprivation Index (ADI) data.
Notes: Pre-2020 mortality reflects the average all-cause mortality from 2017–2019. COVID-19 mortality includes lab-confirmed COVID-19 (ICD U07.1) on the death certificate. Excess mortality was calculated by subtracting the average all-cause mortality from 2017–2019 from mortality in 2020 for the same calendar months. 2020 total mortality includes all-cause mortality (including COVID-19 mortality) during 2020. The gap in BIPOC/white mortality associated with ‘neighborhood disadvantage’ indicates how much of the mortality gap is associated with whites living in more advantaged areas on average, whereas ‘residual disadvantage’ is the gap between BIPOC/whites that live in the same level of disadvantage. The vertical line on each bar indicates the total BIPOC/white disparity and is the sum of neighborhood disadvantage and residual disadvantage.
Contributor Information
Elizabeth Wrigley-Field, Department of Sociology and the Minnesota Population Center, University of Minnesota, Twin Cities, in Minneapolis, Minnesota..
Sarah Garcia, Department of Sociology, University of Minnesota, Twin Cities..
Jonathon P. Leider, Division of Health Policy and Management, University of Minnesota School of Public Health, in Minneapolis, Minnesota, and an associate faculty member at the Johns Hopkins Bloomberg School of Public Health, in Baltimore, Maryland..
David Van Riper, Institute for Social Research and Data Innovation, University of Minnesota, Twin Cities..
References
- 1.DeBruin D, Liaschenko J, Marshall MF. Social justice in pandemic preparedness. Am J Public Health. 2012. April;102(4):586–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Williams DR, Lawrence JA, Davis BA. Racism and Health: Evidence and Needed Research. Annu Rev Public Health. 2019. April 1;40:105–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.DeSalvo KB, Wang YC, Harris A, Auerbach J, Koo D, O’Carroll P. Public Health 3.0: A Call to Action for Public Health to Meet the Challenges of the 21st Century. Prev Chronic Dis. 2017. September 7;14:E78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Siegel M, Critchfield-Jain I, Boykin M, Owens A. Actual Racial/Ethnic Disparities in COVID-19 Mortality for the Non-Hispanic Black Compared to Non-Hispanic White Population in 35 US States and Their Association with Structural Racism. Journal of racial and ethnic health disparities. 2021:1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mackey K, Ayers CK, Kondo KK, Saha S, Advani SM, Young S, et al. Racial and ethnic disparities in COVID-19–related infections, hospitalizations, and deaths: a systematic review. Ann Intern Med. 2021;174(3):362–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yancy CW. COVID-19 and African Americans. JAMA. 2020. May 19;323(19):1891–2. [DOI] [PubMed] [Google Scholar]
- 7.Acevedo-Garcia D. Residential segregation and the epidemiology of infectious diseases. Soc Sci Med. 2000. October;51(8):1143–61. [DOI] [PubMed] [Google Scholar]
- 8.Laster Pirtle WN. Racial Capitalism: A Fundamental Cause of Novel Coronavirus (COVID-19) Pandemic Inequities in the United States. Health Educ Behav. 2020. August;47(4):504–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Drefahl S, Wallace M, Mussino E, Aradhya S, Kolk M, Branden M, et al. A population-based cohort study of socio-demographic risk factors for COVID-19 deaths in Sweden. Nat Commun. 2020. October 9;11(1):5097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Feldman JM, Bassett MT. The relationship between neighborhood poverty and COVID-19 mortality within racial/ethnic groups (Cook County, Illinois). medRxiv. 2020. [Google Scholar]
- 11.Blaser M, Cailas MD, Canar J, COOPER B, GERACI P, OSIECKI K, et al. Analyzing COVID-19 Mortality Within the Chicagoland Area: Data Limitations and Solutions: University of Illinois-Chicago Policy, Practice and Prevention Center 2020. [Google Scholar]
- 12.KC M, Oral E, Straif-Bourgeois S, Rung AL, Peters ES. The effect of area deprivation on COVID-19 risk in Louisiana. PLoS One. 2020;15(12):e0243028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bryan MS, Sun J, Jagai J, Horton DE, Montgomery A, Sargis R, et al. Coronavirus disease 2019 (COVID-19) mortality and neighborhood characteristics in Chicago. Ann Epidemiol. 2021;56:47–54. e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Khazanchi R, Beiter ER, Gondi S, Beckman AL, Bilinski A, Ganguli I. County-level association of social vulnerability with COVID-19 cases and deaths in the USA. J Gen Intern Med. 2020;35(9):2784–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Karmakar M, Lantz PM, Tipirneni R. Association of social and demographic factors with COVID-19 incidence and death rates in the US. JAMA network open. 2021;4(1):e2036462-e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Adhikari S, Pantaleo NP, Feldman JM, Ogedegbe O, Thorpe L, Troxel AB. Assessment of community-level disparities in coronavirus disease 2019 (COVID-19) infections and deaths in large US metropolitan areas. JAMA network open. 2020;3(7):e2016938-e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gross CP, Essien UR, Pasha S, Gross JR, Wang SY, Nunez-Smith M. Racial and Ethnic Disparities in Population-Level Covid-19 Mortality. J Gen Intern Med. 2020. October;35(10):3097–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mackey K, Ayers CK, Kondo KK, Saha S, Advani SM, Young S, et al. Racial and Ethnic Disparities in COVID-19-Related Infections, Hospitalizations, and Deaths : A Systematic Review. Ann Intern Med. 2021. March;174(3):362–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wrigley-Field E, Garcia S, Leider JP, Robertson C, Wurtz R. Racial Disparities in COVID-19 and Excess Mortality in Minnesota. Socius. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nersesian WS, Petit MR, Shaper R, Lemieux D, Naor E. Childhood death and poverty: a study of all childhood deaths in Maine, 1976 to 1980. Pediatrics. 1985;75(1):41–50. [PubMed] [Google Scholar]
- 21.Adler NE, Rehkopf DH. US disparities in health: descriptions, causes, and mechanisms. Annu Rev Public Health. 2008;29:235–52. [DOI] [PubMed] [Google Scholar]
- 22.Pager D, Shepherd H. The Sociology of Discrimination: Racial Discrimination in Employment, Housing, Credit, and Consumer Markets. Annu Rev Sociol. 2008. January 1;34:181–209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Massey DS. Segregation and stratification. Biosocial Theories of Crime. 2017:49. [Google Scholar]
- 24.McLaren J. Racial Disparity in COVID-19 Deaths: Seeking Economic Roots with Census data. 2020. [Google Scholar]
- 25.Fust R, Webster MJ. How did Minn. become one of the most racially inequitable states? Star Tribune. 2019. [Google Scholar]
- 26.Census Bureau US. QuickFacts: Minnesota. https://Census.gov 2019; Available from: https://www.census.gov/quickfacts/MN. [Google Scholar]
- 27.Messer LC, Kaufman JS. Using census data to approximate neighborhood effects. Methods in social epidemiology. 2006:209–36. [Google Scholar]
- 28.Kind AJ, Jencks S, Brock J, Yu M, Bartels C, Ehlenbach W, et al. Neighborhood socioeconomic disadvantage and 30-day rehospitalization: a retrospective cohort study. Ann Intern Med. 2014. December 2;161(11):765–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Singh GK. Area deprivation and widening inequalities in US mortality, 1969–1998. Am J Public Health. 2003. July;93(7):1137–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Knighton AJ, Savitz L, Belnap T, Stephenson B, VanDerslice J. Introduction of an Area Deprivation Index Measuring Patient Socioeconomic Status in an Integrated Health System: Implications for Population Health. EGEMS (Wash DC). 2016;4(3):1238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Stokes AC, Lundberg DJ, Elo IT, Hempstead K, Bor J, Preston SH. COVID-19 and excess mortality in the United States: A county-level analysis. PLoS Med. 2021. May;18(5):e1003571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Stokes AC, Lundberg DJ, Hempstead K, Elo IT, Preston SH. Assessing the Impact of the Covid-19 Pandemic on US Mortality: A County-Level Analysis. medRxiv. 2020. September 2. [Google Scholar]
- 33.Ackley CA, Lundberg DJ, Elo IT, Preston SH, Stokes AC. County-Level Estimates of Excess Mortality associated with COVID-19 in the United States. medRxiv. 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Traub E, Amoon AT, Rollin-Alamillo L, Haddix M, Poortinga K, Ibrahim M, et al. Excess Mortality Associated With the COVID-19 Pandemic-Los Angeles County, March-September 2020. J Public Health Manag Pract. 2021. May-June 01;27(3):233–9. [DOI] [PubMed] [Google Scholar]
- 35.To access the appendix, click on the Details tab of the article online.
- 36.McMinn S, Talbot R, Eng J. America’s 200,000 COVID-19 Deaths: Small Cities And Towns Bear A Growing Share. National Public Radio. 2020. [Google Scholar]
- 37.Manson S, Schroeder J, Van Riper D, Kugler T, Ruggles S. IPUMS National Historical Geographic Information System: Version 15.0 [dataset]. In: IPUMS, editor. Minneapolis, MN2020. [Google Scholar]
- 38.Ruggles S, Flood S, Goeken R, Grover J, Meyer E, Pacas J, et al. IPUMS USA: Version 10.0 [dataset]. In: IPUMS, editor. Minneapolis, MN2020. [Google Scholar]
- 39.Lange SJ, Ritchey MD, Goodman AB, Dias T, Twentyman E, Fuld J, et al. Potential indirect effects of the COVID-19 pandemic on use of emergency departments for acute life-threatening conditions - United States, January-May 2020. Am J Transplant. 2020. September;20(9):2612–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Liebler CA, Halpern-Manners A. A practical approach to using Multiple-Race response data: A bridging method for publicuse microdata. Demography. 2008;45(1):143–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Qizilbash N, Gregson J, Johnson ME, Pearce N, Douglas I, Wing K, et al. BMI and risk of dementia in two million people over two decades: a retrospective cohort study. Lancet Diabetes Endocrinol. 2015. June;3(6):431–6. [DOI] [PubMed] [Google Scholar]
- 42.Wrigley-Field E, Garcia S, Leider JP, Robertson C, Wurtz R. Racial Disparities in COVID-19 and Excess Mortality in Minnesota. Socius. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Council on Asian Pacific Minnesotans. Annual Report. St. Paul2020.
- 44.Rao M. Asian-Americans in Minnesota face insults, hostility during virus outbreak. Star Tribune. 2020. 3/28. [Google Scholar]
- 45.Arias E, Schauman WS, Eschbach K, Sorlie PD, Backlund E. The validity of race and Hispanic origin reporting on death certificates in the United States. Vital Health Stat 2. 2008. October(148):1–23. [PubMed] [Google Scholar]
- 46.Zhang CH, Schwartz GG. Spatial disparities in coronavirus incidence and mortality in the United States: an ecological analysis as of May 2020. The Journal of Rural Health. 2020;36(3):433–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hernan MA, Clayton D, Keiding N. The Simpson’s paradox unraveled. Int J Epidemiol. 2011. Jun;40(3):780–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Elwert F, Winship C. Endogenous Selection Bias: The Problem of Conditioning on a Collider Variable. Annu Rev Sociol. 2014. July;40:31–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Rogers TN, Rogers CR, VanSant-Webb E, Gu LY, Yan B, Qeadan F. Racial Disparities in COVID-19 Mortality Among Essential Workers in the United States. World medical & health policy. 2020;12(3):311–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Chen Y-H, Glymour M, Riley A, Balmes J, Duchowny K, Harrison R, et al. Excess mortality associated with the COVID-19 pandemic among Californians 18–65 years of age, by occupational sector and occupation: March through November 2020. PLoS One. 2021;16(6):e0252454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.van Ingen T, Brown KA, Buchan SA, Akingbola S, Daneman N, Smith BT. Neighbourhood-level risk factors of COVID-19 incidence and mortality. medRxiv. 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Nafilyan V, Islam N, Ayoubkhani D, Gilles C, Katikireddi SV, Mathur R, et al. Ethnicity, household composition and COVID-19 mortality: a national linked data study. J R Soc Med. 2021. April;114(4):182–211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Gupta R, Agrawal R, Bukhari Z, Jabbar A, Wang D, Diks J, et al. Higher comorbidities and early death in hospitalized African-American patients with Covid-19. BMC Infect Dis. 2021. January 18;21(1):78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Bello-Chavolla OY, Gonzalez-Diaz A, Antonio-Villa NE, Fermin-Martinez CA, Marquez-Salinas A, Vargas-Vazquez A, et al. Unequal Impact of Structural Health Determinants and Comorbidity on COVID-19 Severity and Lethality in Older Mexican Adults: Considerations Beyond Chronological Aging. J Gerontol A Biol Sci Med Sci. 2021. February 25;76(3):e52–e9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Fiscella K, Sanders MR. Racial and Ethnic Disparities in the Quality of Health Care. Annu Rev Public Health. 2016;37(1):375–94. [DOI] [PubMed] [Google Scholar]
- 56.MPR. Nov. 14 update on COVID-19 in MN: Record 8,703 new cases; 35 more deaths. MPR News. 2020. [Google Scholar]
- 57.Rasmussen SA, Khoury MJ, Del Rio C. Precision public health as a key tool in the COVID-19 response. JAMA. 2020;324(10):933–4. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.