Abstract
Background
A year following the onset of the COVID-19 pandemic, new infections and deaths continue to increase in Europe. Serological studies, through providing evidence of past infection, can aid understanding of the population dynamics of SARS-CoV-2 infection.
Objectives
This systematic review of SARS-CoV-2 seroprevalence studies in Europe was undertaken to inform public health strategies including vaccination, that aim to accelerate population immunity.
Methods
We searched the databases Web of Science, MEDLINE, EMBASE, SCOPUS, Cochrane Database of Systematic Reviews and grey literature sources for studies reporting seroprevalence of SARS-CoV-2 antibodies in Europe published between 01/12/2019–30/09/20. We provide a narrative synthesis of included studies. Studies were categorized into subgroups including healthcare workers (HCWs), community, outbreaks, pregnancy and children/school. Due to heterogeneity in other subgroups, we only performed a random effects meta-analysis of the seroprevalence amongst HCWs stratified by their country.
Results
115 studies were included spanning 17 European countries, that estimated the seroprevalence of SARS-CoV-2 from samples obtained between November 2019 –August 2020. A total of 54/115 studies included HCWs with a reported seroprevalence among HCWs ranging from 0.7% to 45.3%, which did not differ significantly by country. In community studies significant heterogeneity was reported in the seroprevalence between different age groups and the majority of studies reported there was no significant difference by gender.
Conclusion
This review demonstrates a wide heterogeneity in reported seroprevalence of SARS-CoV-2 antibodies between populations. Continued evaluation of seroprevalence is required to understand the impact of public health measures and inform interventions including vaccination programmes.
Introduction
On 11th March 2020 the World Health Organization (WHO) declared the spread of the novel SARS-CoV-2 virus as a pandemic [1]. SARS-CoV-2 is thought to spread mainly by respiratory droplets, airborne transmission and some evidence also suggests spread via fomites [2–4]. SARS-CoV-2 causes varying degrees of illness from mild symptoms including fatigue and myalgia to acute respiratory failure and death [5]. As the pandemic unfolded evidence emerged that a large number of individuals are asymptomatic with SARS-CoV-2 infection [6, 7]. It has been suggested that asymptomatic transmission of SARS-CoV-2 could account for at least a third and up to 59% of transmission across the world [4].
In order to control the spread of SARS-CoV-2 it is important to understand the extent to which different populations have already been exposed to the virus, especially as a large number of infections are asymptomatic and therefore may not have been tested for SARS-CoV-2 at the time of infection. Many countries, organizational bodies and facilities have turned to mass testing to estimate the spread of infection and inform public health measures [8, 9]. One such testing strategy is by nasal and throat swabbing to detect viral RNA which has been piloted in England [10]. A further method is mass testing of the population for antibodies against SARS-CoV-2 [11]. Several tests for immunoglobulin G (IgG), immunoglobulin A(IgA) and immunoglobulin M(IgM) antibodies against SARS-CoV-2 have recently been developed. These broadly include enzyme linked immunosorbent assays, chemiluminescence immunoassays (CLIA) and point of care lateral flow assays [12].
Seroprevalence studies have been used in the past to help with outbreak responses. During a recent Ebola outbreak, seroprevalence studies were performed to gain further information on the immune response and immune protection [13]. Seroprevalence studies have also been used for infections such as rubella, mumps and measles to map resurgence and to gain further information on how public health strategies can target high risk populations [14]. Moreover, seroprevalence studies provide valuable information for vaccination strategies as they help to quantify how much of a population has been exposed to the virus helping to achieve herd immunity and helping to identify populations who may be at greater risk of infection.
By estimating the seroprevalence in different populations we can use the results to understand transmission dynamics, herd immunity and the immune response over time. These studies can help to guide the public health response to ultimately help prevent the spread of SARS-CoV-2. Here we present the results of a systematic review on the seroprevalence of SARS-CoV-2 in Europe.
Methods
Search strategy and selection criteria
This systematic review and meta-analysis adheres to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines [15]. The protocol was registered on the University of York database for Prospectively Registered Systematic Reviews (PROSPERO: 2020 CRD42020212149). We systematically searched electronic data sources from (01/12/2019) until (30/09/20) using search terms on seroprevalence and SARS-CoV-2. We searched the following electronic databases: Web of Science, MEDLINE, EMBASE, SCOPUS and Cochrane Database of Systematic Reviews. We also searched database search engine EBSCO to search the following databases: EBSCOhost e-book collection, biomedical reference collection, CINAHL plus and MEDLINE Complete. We conducted a secondary search by searching the reference lists of included studies for relevant articles.
Furthermore, we searched the grey literature. Firstly, we searched pre-print articles in the electronic database search engine EPMC to search pre-print databases including MedRXIV and BioRXIV. Secondly, we then used the database OpenGrey to search research reports, doctoral dissertations, conference papers and other forms of grey literature. Thirdly we searched the websites of national and international health agencies for reports relating to the seroprevalence of SARS-CoV-2 (World Health Organization, European Centres for Disease Control, Public Health England, Department of Health and Social Care in UK). Finally, we conducted a google search for further government reports.
Search terms were developed alongside a health science librarian (Table 1).
Table 1. Search strategy.
| Search Strategy | |
|---|---|
| 1 | COVID-19 OR SARS-CoV-2 |
| 2 | antibod* OR immun* |
| 3 | Seroprevalence |
| 4 | EUROPE |
| 5 | 1 AND 2 AND 3 AND 4 |
| 6 | animals NOT humans |
| 7 | 4 NOT 5 |
Studies were included if they were written in English and published between 1/12/19–30/09/20 and were cross-sectional or cohort studies conducted in Europe. We defined European countries as those listed on the WHO regional office for Europe website [16]. Vaccine evaluations and randomised controlled trials were excluded.
Data extraction
Titles and abstracts of retrieved studies were de-duplicated and screened independently by two reviewers to identify if they met the inclusion criteria. Screened references then underwent full text review by two reviewers independently. Any disagreement between them over the eligibility of studies was resolved through discussion with a third reviewer.
A standardised data extraction form was used. Data was extracted on the characteristics of the study (country, date, setting, selection method, funder), antibody assay employed, specificity and sensitivity of the assay, sample characteristics (age, gender, ethnicity, co-morbidities) and prevalence. Data extraction was carried out autonomously by the reviewers and consensus was sought between the team. Data was recorded on the type of funding the studies received; this included government funding, research grants with some studies receiving no external funding.
Assessment of the methodological quality of included studies
We assessed the risk of bias using an adapted version of the Hoy et al modified Risk of Bias Tool, as used by Nguyen et al [17, 18]. This is a tool designed to assess the risk of bias in population-based prevalence studies. It uses a scoring system to assess the external and internal validity of the study. Studies that score 0–3 points are classified as low risk, 4–6 medium risk and 7–9 high risk. Two reviewers independently applied the criteria. Disagreements were resolved through discussion. After reviewing the relevant literature, we did not perform traditional asymmetry tests and funnel plots for assessing publication bias, as the meta-analysis we conducted was a summary of proportions and not a comparison of treatments/interventions [19, 20].
Data analysis
We provide a narrative synthesis of the findings of all included studies, study population characteristics, antibody assays used and seroprevalence estimates for each study. A narrative synthesis aims to summarise the findings of multiple studies in the form of words and text [21]. Studies were categorized into subgroups including those that examined health care workers (HCWs), community studies, outbreaks, seroprevalence in children/schools and seroprevalence studies performed in pregnant women. We included studies in the subgroup outbreak if they investigated the seroprevalence following a sudden increase in SARS-CoV-2 cases related to time and place in a particular population [22]. We used Metaprop in STATA version 14, statistical software (Stata Corp. College Station, TX, USA), package to perform a random–effects meta-analysis of seroprevalence amongst health care workers (HCWs) stratified by country. The random effect model used the DerSimonian and Laird method with study heterogeneity estimated from the inverse-variance fixed-effect model. The pooled effect estimate was calculated after Freeman-Tukey Double Arcsine Transformation and Wilson score 95% confidence intervals were calculated for individual studies. We also performed a random-effects meta-analysis of seroprevalence amongst health care workers (HCWs) stratified by their risk of exposure to SARS-CoV-2 infected patients. HCWs were categorised as high risk if they worked with patients with known SARS-CoV-2 infection, medium risk if they had patient contact but without known SARS-CoV-2 infection and low risk if they had no patient contact (e.g., laboratory staff and administrative staff). If studies included participants from a mixture of risk groups they were categorised as medium risk. Heterogeneity was measured using the I2 statistic which describes the percentage of total variation due to inter-study heterogeneity. Tests of heterogeneity were undertaken within the sub-groups and for the overall meta-analysis. Sensitivity analysis was done by removing those studies with a moderate risk of bias score. This had no effect of the I2 value, so these studies were included in the final meta-analysis.
Results
The literature search yielded 2126 articles. After removing duplicates and excluding studies based on their abstract or through full text examination 115 studies were identified as eligible (Fig 1). The 115 articles spanned 17 countries in Europe and estimated the seroprevalence of SARS-CoV-2 from serum samples dated from November 2019 –August 2020. The 115 articles reviewed the seroprevalence in approximately 516,361 samples; among included studies 59.92% of subjects were females and the overall age range of participants was 0–90+ years (Table 2).
Fig 1. PRISMA flow chart.
Table 2. Included studies, dates of sampling, population studied and overall seroprevalence.
| Country | Author, year | Time period | Population | Total Seroprevalence (95% CI) |
|---|---|---|---|---|
| Orth-Höller et al 2020[23] | 20th-27th March | Primary and Secondary care physicians in Tyrol | 5% (3.3–7.7) | |
| Knabl et al 2020[24] | April 21st- April 27th | Residents in Ischgl/Tyrol | 42.4% (39.8–44.7) | |
| Austria | Fuereder et al 2020 [25] | 21st March - 4th June | HCWs and patients the Division of Oncology, Medical University of Vienna, Austria between 1 April and 4 June 2020. | HCWs—3.2% (0.4–11.2%) and patients 2.4% (0.3–8.3%) |
| Reiter et al 2020[26] | Not recorded but published on medRxiv in July | Staff members of the Division of Nephrology and Dialysis, Department of Medicine III, at the Medical University of Vienna, Austria. | 25.5% (20.4–31.5) | |
| Herzog et al 2020[27] | 30th March -5th July | Residual sera from ten private diagnostic laboratories in Belgium. | 30 March– 5 April 2.90% (2.3–3.4%) .20–26 April 6% (5.1–7.1%). 18–25 May 6.9% (5.9–8). 8–13 June 5.5% (4.7–6.5%). 29 June– 4 July 4.5%(3.7–5.4%) | |
| Berardis et al 2020[28] | 16th April 16 and 19th May | Cystic Fibrosis (CF) patients followed in the CF reference centre of the Cliniques universitaires Saint-Luc (Brussels). | 2.70% | |
| Belgium | Steensels et al 2020[29] | 22nd April - 30th April | HCWs at Hospital East-Limburg | 6.40% |
| Martin et al 2020[30] | 15thApril-18th May | HCWs on COVID wards in Centre Hospitalier Universitaire Saint-Pierre (in Brussels | 11–12% | |
| Desombere et al[31] | 6th - 10th May | HCWs in Belgium | 8.40% | |
| Blairon et al 2020[32] | 25th May 25–19th June | HCWs at network of Iris hospitals South, Brussels, Belgium | 14.60% | |
| Jerković et al 2020[33] | 23rd - 28th April | Factory employees living in the Split-Dalmatia and Šibenik-Knin County | 1.27% (0.77–1.98%) | |
| Croatia | Vilibic-Cavlek et al 2020[34] | 25th April - 24th May | HCWs and allied professions | 2.7% based on IgG |
| Iversen et al 2020 [35] | 15th April and 23rd April | HCWs and blood donors in the Capital Region of Denmark | HCWs—4.04% (3·82–4·27). Blood Donors 3.04% (2.58–3.57) | |
| Erikstrup et al 2020[36] | 6th April to 3rd May | Blood Donors in Denmark | 2% (1.8–2.2) | |
| Egerup et al 2020 [37] | 4th April– 3rd July | Women giving birth, their partners and newborns at University Hospital Hvidovre Obstetric and Gynaceology Unit | Women giving birth 2.85% (1.87%-4.25%) Partners 3.8% (2.6%-5.5% Newborns 1.4% | |
| Denmark | Jespersen et al 2020[38] | May 18th until June 19th | HCWs and administrative staff that work in hospitals in the Central Denmark Region | 3.7% (3.5%–4.0%) |
| Laursen et al 2020[39] | 22nd June to 10th August | Emergency and non-emergency HCWs employed by Falck in Sweden and Denmark | Denmark—2.8%. Sweden 8.3% | |
| Petersen et al 2020[40] | 27th April– 1st May | Residents of the Faroe Islands | 0.6% (0.2%–1.2%) | |
| Germain et al 2020[41] | 1st November 2019 to 16th March 2020 | All tissue donors at Lille Tissue bank | 1.70% | |
| Solodky et al 2020[42] | 1st March -16th April | HCWs and cancer patients | HCWs 5.4%. Cancer patients 5.9% | |
| Grzelak et al 2020[43] | 20th March | A cohort of pauci-symptomatic individuals in Crepy-en-Valois. Blood donors from the Etablissement Français du Sang in Lille (France) | Blood donors mean of 3%. Pauci symptomatic cohort mean of 32%. | |
| Fontanet et al 2020 [44] | 30th March -4th April | Students, teachers and non-teaching staff in a high school following an outbreak | 25.86% (22.6%-29.4%) | |
| Carrat et al 2020[45] | 1st April - 27th May | Residents from Ile-de-France or Grand Estor Nouvelle-Acquitaine | 6.70% | |
| France | Gallian et al 2020[46] | Last week of March to the first week of April | Blood Donors | 2.70% |
| Sermet et al 2020[47] | 1st April - 1st June | Non COVID paediatric patients consulting or hospitalized in a paediatric tertiary health care department of the Assistance Publique-Hoôpitaux de Paris | 22.19% | |
| Lisandru et al 2020[48] | 16th April– 15th June | Residual serum samples in Corsica | 5.27% (4.33%-6.35%) | |
| Fontanet et al 2020[49] | 28th - 30th April | Pupils, their parents and relatives, and staff of primary schools exposed to SARS-CoV-2 in February and March 2020 in a city north of Paris, France | 10.40% | |
| Mattern et al 2020[50] | 4th May - 31st May | Patients admitted to the delivery room at Antoine Béclère Hospital maternity ward (Paris area, France) | 8.00% | |
| Péré et al 2020[51] | 2nd May - 26th June | HCWs at Hôpital Européen Georges Pompidou | 12.20% | |
| Simon et al 2020[52] | February—April 2020 | Patients on immunomodulatory imide drugs (IMIDs) with and without continuous cytokine blockade. HCWs and a cohort of healthy participants unrelated to health care | Healthy participants 2.27% HCWs 4.2%. IMIDs on cytokine inhibition 0.75%. IMIDs not on cytokine inhibition 3.08% | |
| Fischer et al 2020[53] | 9th March - 3rd June | Blood donors in three German federal states North Rhine-Westphalia, Lower Saxony and Hesse | 0.91% (0.58–1.24%) | |
| Brandstetter et al 2020[54] | 20th March | HCWs at university children’s and maternity hospital in Regensburg | 16.90% | |
| Brehm et al 2020[55] | 20th March - 24th July | Employees of University Medical Center Hamburg-Eppendorf | 1.8% (1–2.5%) | |
| Behrens et al 2020[56] | 23rd March - 17th April | HCWs involved in COVID-19 patient care | 0.90% | |
| Korth et al 2020[57] | 25th March - 21st April | HCWs at University Hospital Essen | 1.60% | |
| Streeck et al 2020[58] | 31st March - 6th April | Residents of Gangelt | 13.6% based on IgG | |
| Germany | Krähling et al 2020[59] | 6th April - 14th April | Employees of Infraserv Höchst, a large industrial site operator in Frankfurt am Main | 0.50% |
| Schmidt et al 2020[60] | 20th April - 30th April | Employees BDH-Clinic Hessisch Oldendorf | 2.86% | |
| Aziz et al 2020[61] | 24th April -30th June | Individuals enrolled in the Rhineland Study an ongoing community- based prospective cohort study in people aged 30 years and above in the city of Bonn, Germany. Group I all living participants who had been enrolled in the Rhineland Study until March 18, 2020.Group II individuals who were eligible for but had not yet participated in the Rhineland Study. | Group I: 0.97% (0·72−1·30). Group II: 1.94% (0·84−4·42) | |
| Weis et al 2020[62] | 12th May - 22nd May | Residents in Neustadtam Rennsteig | 8.40% | |
| Bahrs et al 2020[63] | 19 th May– 20th May | HCWs in a University Hospital | 2.7% (1.6% -4.3%) | |
| Armann et al 2020[64] | 25th May - 30th June | Students grade 8–11 and their teachers in 13 secondary schools in eastern Saxony | 0.60% | |
| Epstude et al 2020[65] | 15th June - 30th June | HCWs and housekeeping staff in an oncology unit | 3.10% | |
| Hoffmann et al 2020[66] | 20th July | HCWs in a standard care hospital in Oberspreewald-Lausitz | 1.30% | |
| Bogogiannidou et al 2020[67] | March and April | Individuals who visited the laboratories for a check-up, chronic disease follow-up or other reasons unrelated to COVID-19 in the whole of Greece | March: 0.24% (0.03–0.45%). April 0.42% (0.23–0.61%) | |
| Greece | Psichogiou et al 2020[68] | 30th April - 15th May | HCWs across two hospitals in Greece | 1.00% |
| Tsitsilonis et al 2020[69] | June-July 2020 | Student and staff at National and Kapodistrian University of Athens | 1.00% | |
| Iceland | Gudbjartsson et al 2020[70] | January—July | Two groups of qPCR-positive Icelanders and six groups of Icelanders who had not been qPCR-tested or who had been tested and had received a negative result | 4.22% |
| Plebani et al 2020[71] | 22nd February - 29th May | HCWs in several Structures of the National Healthcare Service of the Veneto Region | 4.6% (4.1–5.0%) | |
| Valenti et al 2020[72] | 24th February - 8th April | Blood donors in Milan | 5.07% | |
| Pancrazzi et al 2020[73] | 17th - 21st March | Patients in the Emergency Room and from subjects undergoing health surveillance by territorial and hospital prevention departments in Tuscany | 13.00% | |
| Vena et al 2020 [74] | March and April | Residents living in Liguria and Lombardi regions | 11.00% | |
| Norsa et al 2020[75] | March- July | Patients with IBD on biologic treatment | 21.10% | |
| Percivalle et al 2020[76] | 18th March - 6th April | Registered blood donors in Lodi Italy | 23.00% | |
| Lahner et al 2020[77] | 18th March–27th April | HCWs in a teaching hospital in Rome | 0.70% | |
| Fusco et al 2020[78] | 23rd March - 2nd April | HCWs working in a specialist infectious disease setting, the ‘D. Cotugno’ hospital in Naples, Italy. | 1.70% | |
| Sotgiu et al 2020[79] | 2nd - 16th April | HCWs at San Paulo University General Hospital | 7.4% based on IgG | |
| Amendola et al 2020[80] | 15th April | HCWs in Buzzi Hospital | 5.13% | |
| Italy | Carozzi et al 2020 [81] | 20th April | HCWs across 6 health facilities in Tuscany | 2.4% only including positive results and not borderline results |
| Comar et al 2020[82] | Published in medRxiv in April | All employees of the Mother and Child Research Hospital Burlo Garofolo | 7.2% just including positive and not borderline results. | |
| Cosma et al 2020[83] | 16th April - 4th June | Pregnant women attending for first trimester screening (11‐13 weeks of gestation) at Sant’Anna Hospital, Turin, Piedmont | 10.10% | |
| Sandri et al 2020[84] | 28th April - 16th May | Employees of 7 different hospitals, located across the Lombardy region | 11.21% | |
| Fiore et al 2020[85] | 1st May - 31st May | Blood donors in Apulia region, South Eastern Italy | 0.99% | |
| Pagani et al 2020[86] | 18th May - 7th June | Residents of Castiglione D’Adda | 22.6% (17.2–29.1%) | |
| Paderno et al 2020[87] | No date recorded | All staff working in a COVID-19-free Otolaryngology Department in Italy | 6.90% | |
| Tosato et al 2020[88] | Not recorded but published on medRxiv in May | HCWs working in the Department of Laboratory Medicine | 4.50% | |
| Netherlands | Westerhuis et al 2020[89] | 2nd March - 3rd April | Patients of the Erasmus Medical Centre in Rotterdam | March: 0.7%. April: 3% |
| Slot et al 2020[90] | 1st - 15th April | Plasma donations | 2.60% | |
| Luxembourg | Snoeck et al 2020[91] | 15th April - 5th May | Population of Luxembourg | 1.97% |
| Portugal | Figueiredo-Campos et al 2020[92] | 6th April - 10th July | Hospitalised patients and HCWs who tested positive for SARS-CoV-2 by PCR, healthy post-COVID-19 volunteers and staff of the University of Lisbon | Patients: 51%, HCWs 100%, plasma donations 88%, University staff 1.5% |
| Dacosta-Urbieta et al 2020[93] | March—April | HCWs of the Paediatric Department of the Hospital Clínico Universitario de Santiago de Compostela | 4.00% | |
| Garcia-Basteiro et al 2020[94] | 28th March - 9th April | HCWs at Hospital Clínic of Barcelona | 9.3% (7.1–12%) | |
| Valdivia et al 2020[95] | 13th - 30th April | HCWs at Hospital Clínico Universitario of Valencia | 3.50% | |
| Galán et al 2020[96] | 14th -27th April | All HCWs Hospital Universitario Fundación Alcorcón | 31.60% | |
| Crovetto et al 2020[97] | 14th April-5th May | Pregnant women consecutively attending first trimester screening or delivery | 14.30% | |
| Garralda Fernandez et al 2020[98] | 14th April - 13th May | HCWs at Hospital Universitario de Fuenlabrada | 16.9% based on IgG | |
| Spain | Olalla et al 2020[99] | 15th - 25th April | HCWs of the Costa del Sol Hospital in Marbella of the units involved in patient care with COVID-19 | 2.20% |
| Martín et al 2020[100] | 20th April | General practitioners (GP) and primary care nurses in the Healthcare Area of León, who worked in health centres or nursing homes. | 5.90% | |
| Montenegro et al 2020[101] | 21 April to 24 April 2020 (Study population A) and from 29 April to 5 May 2020 (Study population B) | Population A: individuals registered in a primary health care centre, from a community area of Barcelona, Spain. Population B: Patients from GPs in Barcelona presenting with mild-moderate symptoms of COVID but no diagnosis of COVID | Population A: 5.47% (3.44–8.58%). Population B: 38.49% (34.78%-42.33%) | |
| Moncunill et al 2020[102] | April—May | HCWs from Hospital Clínic de Barcelona | 14.50% | |
| Soriano et al 2020[103] | April—May | Asymptomatic adults in Madrid | 13.80% | |
| Pollan et al 2020[104] | 27th April - 11th May | Spanish households | 5% (4.7–5.4%) based on POC testing | |
| Martínez et al 2020[105] | 15th April– 15TH June | Essential workers in Madrid who were known to have exposure to SARS-CoV-2 contacts or had reported COVID-19 compatible symptoms | 33.69% (29.27%– 38.21%) | |
| Barallat et al 2020[106] | 4th - 22nd May | HCWs of the Catalan Institute of Health (ICS) Northern Metropolitan Area of Barcelona | 10.30% | |
| Cabezón-Gutiérrez et al 2020[107] | 1st- 19th June | Oncology outpatients who attended the medical oncology consultation of the University Hospital of Torrejón | 31.40% | |
| Castro Dopico et al 2020[108] | 30th March - 23rd August | Blood Donors and pregnant women in Stockholm | 13.7% (9.5–19.3%) estimated by ENS Learner | |
| Rudberg et al 2020[109] | 24th April - 8th May | HCWs at Danderyd Hospital | 19.10% | |
| Lindahl et al 2020[110] | 20th April | Employees of elderly care homes situated in Stockholm city and its suburbs | 23% (20.4–25.7%) | |
| Sweden | Roxhed et al 2020[111] | 20th April | Households in Stockholm | 4.4% (2.4%-6.3%) for IgG |
| Lidström et al 2020[112] | 27th May– 25TH June | All healthcare staff including support staff, in the Region Uppsala | 6.60% | |
| Lundkvist et al 2020[113] | 17th-18th June | Residents of Norra Djurgårdsstaden and Tensta in Stockholm | 15.00% | |
| Laursen et al 2020[39] | 22nd June to 10th August | Emergency and nonemergency HCWs employed by Falck in Sweden and Denmark | Denmark—2.8%. Sweden 8.3% | |
| Emmenegger et al 2020[114] | February—July | Patients entering the University Hospital of Zurich and healthy blood donors in Zurich | Hospitalised patients: March 2020 0.3% (0.1% - 0.5%). April 2020 1.4% (1.0%-1.7%). Blood donors: April 1.2% (0.7%-1.8%). May 1.6% (1.0%-2.3%) estimated by quadratic discriminant analysis | |
| Switzerland | Stringhini et al 2020[115] | 6th April - 9th May | Residents from the Canton of Geneva | 7.90% |
| Ulyte et al 2020[116] | June-July | Schools in the Canton of Zurich | 2.8% (1.4–6.1%) | |
| Turkey | Alkurt et al 2020[117] | 30th May - 6th June | HCWs in the University of Health Sciences Umraniye Teaching and Research Hospital (UEAH), Istanbul University-Cerrahpasa, Cerrahpasa Medical Faculty Hospital (Cerrahpasa), Darica Farabi Teaching and Research Hospital (Farabi) | 12.30% |
| Thompson et al 2020[118] | 17th March - 19th May 2020 | Blood donors in Scotland | 3.17% | |
| Houlihan et al 2020[119] | 26th March - 8th April | HCWs at University College London Hospitals (UCLH) who work in accident and emergency (A&E), COVID ward, acute medical unit (AMU), intensive care (ICU) or haematology | 45.30% | |
| Pallett et al 2020[120] | 8th April - 12th June | HCWs in two hospitals in London | 39.30% | |
| Waterfield et al 2020[121] | 16th April - 3rd July | Children of healthcare workers, aged between 2 and 15 years | 6.90% | |
| Eyre et al 2020[122] | 23rd April - 8th June | Hospital staff at Oxford University Hospitals NHS Foundation Trust | 10.70% | |
| Shields et al 2020[123] | 24th -25th April | HCWs at University of Birmingham and University Hospitals Birmingham NHS Foundation Trust | 24.40% | |
| UK | Clarke et al 2020[124] | 27th April - 7th May | Patients receiving in centre haemodialysis (ICHD) within two units affiliated with Imperial College Renal and Transplant Centre | 36.20% |
| Wells et al 2020[125] | 27th April - 2nd June | Participants of the Twins UK Cohort Study | 12.00% | |
| The Government of Jersey 2020[126] | 29th April - 5th May | Residents in Jersey over 16 years of age | 3.1% (+/- 1.3%) | |
| Poulikakos et al 2020[127] | 4th-6th May | HCWs from renal and biochemistry department in a tertiary hospital in the northwest of England | 6.00% | |
| Khalil et al 2020[128] | 15th - 28th May | HCWs at Portland Hospital for Women and Children | 22.00% | |
| Grant et al 2020[129] | 15th May - 5th June | HCWs at Whittington Health | 31.64% | |
| Ladhani et al 2020[130] | 20th May | Residents and staff of the six care homes following an outbreak of COVID-19 | 77.9% (73.6–81.7%) | |
| Biobank 2020[131] | 27th May - 14th August | UK Biobank participants | 8.2% (7.9%-8.7%) | |
| Public Health England 2020[132] | May | Healthy adult blood donors, supplied by the NHS Blood and Transplant (NHS BT) | 8.5% (6.9–10%) this is adjusted for the sensitivity and specificity of the Euroimmune assay | |
| Mulchandani et al 2020 [133] | 1st June - 26th June | Police and Fire and Rescue services, healthcare workers and healthcare workers with previously positive for SARS-CoV-2 | Police and Fire and Rescue services: 10.6%. HCWs: 23.3% | |
| Nsn et al 2020[134] | 20th June | Residents in 4 nursing homes in UK where a covid-19 outbreak happened | 71.80% | |
| Favara et al 2020[135] | June—July | HCWs with direct patient contact working in an oncology unit in either of the following hospitals the Queen Elizabeth Hospital Kings Lynn NHS Foundation Trust (QEH), North West Anglia NHS Foundation Trust (Peterborough City Hospital, NWA), and Cambridge University Hospitals NHS Foundation Trust (CUH). | 13.3% using Luminex assay on day 28 | |
| Ladhani et al 2020[136] | June—July | Teachers and students in 131 schools across England | 11.7% (10.5–13.3%) | |
| Ward et al 2020 [137] | 20th June - 13th July | Residents in England over the age of 18 years | 6% (5.8–6.1%) adjusted for test performance and re-weighted for sampling |
Abbreviations: A&E–accident and emergency; AMU–acute medical unit
CF—cystic fibrosis; HCWs–health care workers; IBD—inflammatory bowel disease; ICHD–in centre haemodialysis; IMIDs - immunomodulatory imide drugs; ITU—intensive care unit; NHS–national health service; qPCR–quantitative polymerase chain reaction; UK–United Kingdom.
Assessment of the methodological quality of included studies
Each study underwent a risk of bias assessment using the modified Hoy et al risk of bias tool [18]. Twenty nine of the 115 studies were deemed to be at moderate risk of bias [31, 34, 42, 44, 47, 54, 56, 57, 67, 69, 73, 74, 88, 95, 97, 100, 107, 108, 111, 116, 118, 121, 122, 125, 127, 128, 132, 134, 136]. This was often due to lack of information about the sampling frame, selection process and non-response bias. No studies scored high risk (Table 3).
Table 3. Assessment of the methodological quality of included studies.
| Country | Author, year | Overall Risk of Study: high, moderate or low | Reason if moderate/high risk |
|---|---|---|---|
| Orth-Höller et al 2020[23] | Low risk | ||
| Knabl et al 2020[24] | Low risk | ||
| Austria | Fuereder et al 2020 [25] | Low risk | |
| Reiter et al 2020[26] | Low risk | ||
| Herzog et al 2020[27] | Low risk | ||
| Berardis et al 2020[28] | Low risk | ||
| Belgium | Steensels et al 2020[29] | Low risk | |
| Martin et al 2020[30] | Low risk | ||
| Desombere et al [31] | Moderate risk | Not enough information given about the sampling frame, definition of a positive result, selection process, type of test used, if the same test was used on all participants and non-response bias. | |
| Blairon et al 2020[32] | Low risk | ||
| Jerković et al 2020[33] | Low risk | ||
| Croatia | Vilibic-Cavlek et al 2020[34] | Moderate risk | Not enough information given about the sampling frame, selection process and non-response bias. |
| Iversen et al 2020 [35] | Low risk | ||
| Erikstrup et al 2020[36] | Low risk | ||
| Egerup et al 2020 [37] | Low risk | ||
| Denmark | Jespersen et al 2020[38] | Low risk | |
| Laursen et al 2020[39] | Low risk | ||
| Petersen et al 2020[40] | Low risk | ||
| Germain et al 2020[41] | Low risk | ||
| Solodky et al 2020[42] | Moderate risk | Not enough information given about the sampling frame, selection process and non-response bias. | |
| Grzelak et al 2020[43] | Low risk | ||
| Fontanet et al 2020 [44] | Moderate risk | Not enough information on case definition, sampling frame and non -response bias | |
| Carrat et al 2020[45] | Low risk | ||
| France | Gallian et al 2020[46] | Low risk | |
| Sermet et al 2020[47] | Moderate risk | Not enough information given about the sampling frame, selection process and non-response bias. | |
| Lisandr et al 2020 [48] | Low risk | ||
| Fontanet et al 2020[49] | Low risk | ||
| Mattern et al 2020[50] | Low risk | ||
| Péré et al 2020[51] | Low risk | ||
| Simon et al 2020[52] | Low risk | ||
| Fischer et al 2020[53] | Low risk | ||
| Brandstetter et al 2020[54] | Moderate risk | Not enough information given about the sampling frame, selection process and non-response bias. | |
| Brehm et al 2020[55] | Low risk | ||
| Behrens et al 2020[56] | Moderate risk | Not enough information given about the sampling frame, selection process and non-response bias. | |
| Korth et al 2020[57] | Moderate risk | Not enough information given about the sampling frame, selection process and non-response bias. | |
| Streeck et al 2020[58] | Low risk | ||
| Germany | Krähling et al 2020[59] | Low risk | |
| Schmidt et al 2020[60] | Low risk | ||
| Aziz et al 2020[61] | Low risk | ||
| Weis et al 2020[62] | Low risk | ||
| Bahrs et al 2020 [63] | Low risk | ||
| Armann et al 2020[64] | Low risk | ||
| Epstude et al 2020[65] | Low risk | ||
| Hoffmann et al 2020[66] | Low risk | ||
| Bogogiannidou et al 2020[67] | Moderate risk | Not enough information given about definition of a positive result, non-responder bias and discrepancies in the tables. | |
| Greece | Psichogiou et al 2020[68] | Low risk | |
| Tsitsilonis et al 2020[69] | Moderate risk | Not enough information given about the sampling frame, selection process and non-response bias. | |
| Iceland | Gudbjartsson et al 2020[70] | Low risk | |
| Plebani et al 2020[71] | Low risk | ||
| Valenti et al 2020[72] | Low risk | ||
| Pancrazzi et al 2020[73] | Moderate risk | Not enough information given about the sampling frame, selection process and non-response bias. | |
| Vena et al 2020 [74] | Moderate risk | Not enough information given about the sampling frame, selection process and non-response bias. | |
| Norsa et al 2020[75] | Low risk | ||
| Percivalle et al 2020[76] | Low risk | ||
| Lahner et al 2020[77] | Low risk | ||
| Fusco et al 2020[78] | Low risk | ||
| Sotgiu et al 2020[79] | Low risk | ||
| Amendola et al 2020[80] | Low risk | ||
| Italy | Carozzi et al 2020 [81] | Low risk | |
| Comar et al 2020[82] | Low risk | ||
| Cosma et al 2020[83] | Low risk | ||
| Sandri et al 2020[84] | Low risk | ||
| Fiore et al 2020[85] | Low risk | ||
| Pagani et al 2020[86] | Low risk | ||
| Paderno et al 2020[87] | Low risk | ||
| Tosato et al 2020[88] | Moderate risk | Not enough information given about the sampling frame, selection process and non-response bias. | |
| Netherlands | Westerhuis et al 2020[89] | Low risk | |
| Slot et al 2020[90] | Low risk | ||
| Luxembourg | Snoeck et al 2020[91] | Low risk | |
| Portugal | Figueiredo-Campos et al 2020[92] | Low risk | |
| Dacosta-Urbieta et al 2020[93] | Low risk | ||
| Garcia-Basteiro et al 2020[94] | Low risk | ||
| Valdivia et al 2020[95] | Moderate risk | Not enough information given about the sampling frame, selection process and non-response bias. | |
| Galán et al 2020[96] | Low risk | ||
| Crovetto et al 2020[97] | Moderate risk | Not enough information given about the sampling frame, selection process and non-response bias. | |
| Garralda Fernandez et al 2020[98] | Low risk | ||
| Spain | Olalla et al 2020[99] | Low risk | |
| Martín et al 2020[100] | Moderate risk | High non-response rate and not enough information given about the sampling frame and selection process. | |
| Montenegro et al 2020[101] | Low risk | ||
| Moncunill et al 2020[102] | Low risk | ||
| Soriano et al 2020[103] | Low risk | ||
| Pollan et al 2020[104] | Low risk | ||
| Martínez et al 2020[105] | Low risk | ||
| Barallat et al 2020[106] | Low risk | ||
| Cabezón-Gutiérrez et al 2020[107] | Moderate risk | Not enough information given about the sampling frame, definition of a positive result, selection process and non-response bias. | |
| Castro Dopico et al 2020[108] | Moderate risk | Not enough information given about the sampling frame, definition of a positive result, selection process and non-response bias. | |
| Rudberg et al 2020[109] | Low risk | ||
| Lindahl et al 2020[110] | Low risk | ||
| Sweden | Roxhed et al 2020[111] | Moderate risk | Not enough information given about the sampling frame, definition of a positive result, selection process and non-response bias. |
| Lidstrom et al 2020 [112] | Low risk | ||
| Lundkvist et al 2020[113] | Low risk | ||
| Laursen et al 2020[39] | Low risk | ||
| Emmenegger et al 2020[114] | Low risk | ||
| Switzerland | Stringhini et al 2020[115] | Low risk | |
| Ulyte et al 2020[116] | Moderate risk | Not enough information given about the sampling frame, definition of a positive result, selection process and non-response bias. | |
| Turkey | Alkurt et al 2020[117] | Low risk | |
| Thompson et al 2020[118] | Moderate risk | Not enough information given about the sampling frame, definition of a positive result, selection process and non-response bias. | |
| Houlihan et al 2020[119] | Low risk | ||
| Pallett et al 2020[120] | Low risk | ||
| Waterfield et al 2020[121] | Moderate risk | Not enough information given about the sampling frame, definition of a positive result, selection process and non-response bias. | |
| Eyre et al 2020[122] | Moderate risk | High non-response rate and not enough information given if the same test was used on all participants and definition of a positive result. | |
| Shields et al 2020[123] | Low risk | ||
| UK | Clarke et al 2020[124] | Low risk | |
| Wells et al 2020[125] | Moderate risk | High non-response rate and not enough information given about the sampling frame and selection process. | |
| The Government of Jersey 2020[126] | Low risk | ||
| Poulikakos et al 2020[127] | Moderate risk | Not enough information given about the sampling frame, selection process and non-response bias. | |
| Khalil et al 2020[128] | Moderate risk | Not enough information given about the sampling frame, selection process and non-response bias. | |
| Grant et al 2020[129] | Low risk | ||
| Ladhani et al 2020[130] | Low risk | ||
| Biobank 2020[131] | Low risk | ||
| Public Health England 2020[132] | Moderate risk | Not enough information given about the sampling frame, selection process and non-response bias. | |
| Mulchandani et al 2020 [133] | Low risk | ||
| Nsn et al 2020[134] | Moderate risk | Not enough information given about the selection process and non-response bias. | |
| Favara et al 2020[135] | Low risk | ||
| Ladhani et al 2020[136] | Low risk | ||
| Ward et al 2020 [137] | Low risk |
Results by population subgroups
Health care workers
54 studies included seroprevalence data among HCWs. These studies included data from 13 countries in Europe and were conducted between February 2020 and August 2020.
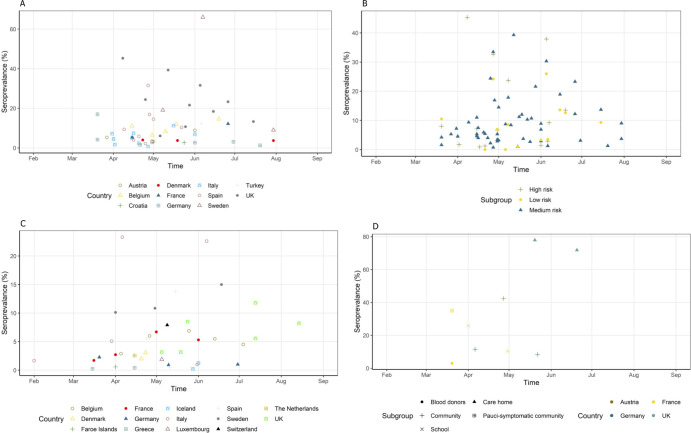
The lowest seroprevalence was seen in a teaching hospital in Rome, Italy during the months of March–April 2020, reporting a seroprevalence of 0.7% based on IgG antibodies and 0% based on IgM [77]. The highest seroprevalence (45.3%) was reported in March–April 2020 in a University Hospital in London [119]. Fig 2A shows the seroprevalence of HCWs by country over time. The majority of studies report a seroprevalence < 10% between March–August 2020. A few studies predominately based in the UK report a seroprevalence 20–45% among HCWs during this time period [26, 96, 119, 120, 123, 128, 129, 133].
Fig 2. Seroprevalence of subgroups over time.
(A): the seroprevalence of HCWs by country over time. (B): the seroprevalence of HCWs over time stratified by risk group. (C): the seroprevalence of community studies over time by country. (D): the seroprevalence of outbreak studies over time stratified by country and subgroup.
Fig 2B shows the seroprevalence of HCWs categorised by their risk of exposure to SARS-CoV-2 patients over time. All subgroups with a seroprevalence of >30% belonged to either medium or high risk. The majority (67/79) of the subgroups had a seroprevalence of less than 20% regardless of their risk.
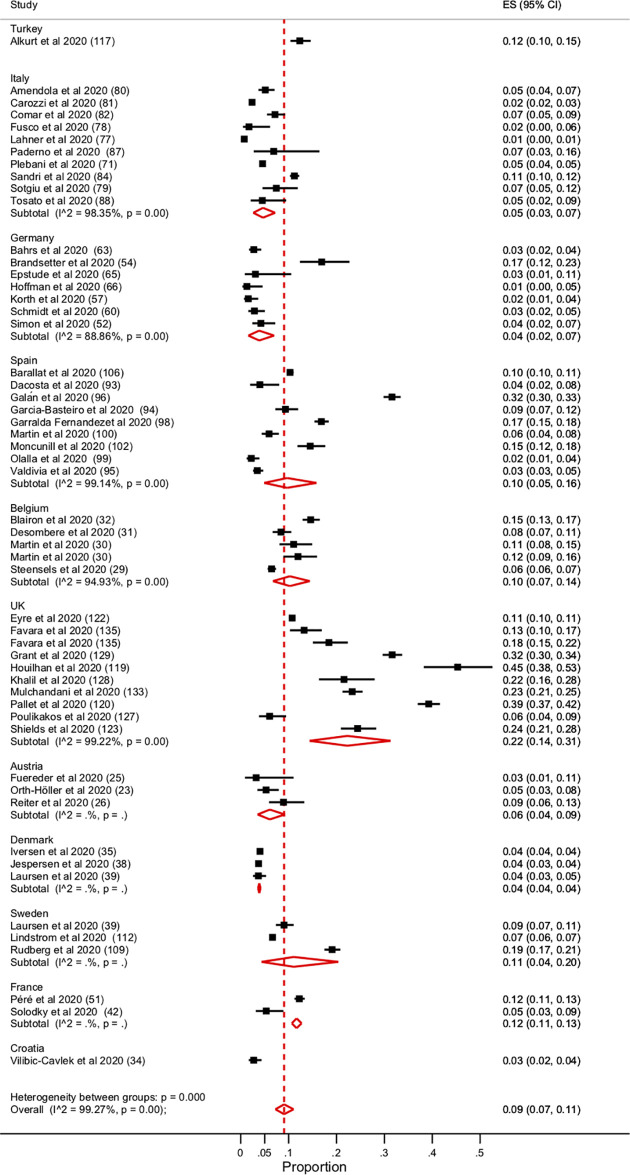
There was no significant difference in seroprevalence amongst HCWs when stratified by country (Fig 3). There is a large amount of heterogeneity between the studies (I2 value = 99.27%, p = 0.00). There was no reduction in heterogeneity when moderate risk of bias studies were removed. Similarly, when the seroprevalence amongst HCWs was stratified by their risk of exposure to SARS-CoV-2 patients there was no significant difference (S1 Fig).
Fig 3. Forest plot of the seroprevalence among HCWs stratified by country.
Community studies
In total 34 studies were set in the community, spanning 13 countries. The studies collected data between February 2020—August 2020. Ten of these studies collected samples from blood donors; five studies used residual serum samples from clinics, laboratories and hospital facilities; one study used tissues samples and the remaining studies were randomised population-based studies.
The overall lowest seroprevalence was reported in Greece in March 2020 of 0.24% (0.03–0.45%) [67]. The same study reported an increase in seroprevalence in April of 0.42% (0.23–0.61%). The overall highest seroprevalence was reported in Lodi, Italy during the months of March and April at 23% [76]. The majority of studies reported an overall seroprevalence of less than 10% during the months of February–August 2020 (Fig 2C).
Age. Many of the community studies report the seroprevalence among different age groups. There is significant heterogeneity between the results. In general, lower seroprevalences were reported at the extremes of age. Several studies report a higher seroprevalence among the over 50 age group [40, 52, 74, 85, 86, 104]. In contrast some studies report a higher seroprevalence in the less than 30 years age group, these include studies from Switzerland, the Netherlands, Denmark, France, Luxembourg and the UK [45, 90, 91, 115, 131, 137].
Gender. In the majority of community studies there was no significant difference identified by gender. However, two studies reported a significantly higher number of female participants having antibodies against SARS-CoV-2 [45, 74]. Carrat et al investigated the seroprevalence in three administrative regions of France Ile-de-France (IDF), Grand Est (GE) and Nouvelle-Aquitaine (NA), and reported a significant association of antibodies associated with the female gender only in Nouvelle-Aquitaine [45]. Similarly, Vena et al report a significantly higher seroprevalence among female participants in five administrative regions in Italy [74].
Blood donors. Many studies report the seroprevalence in blood donors as they are usually healthy individuals who represent the general population. There was a large variation in seroprevalence among blood donors between countries and over time.
The lowest seroprevalence in blood donors was reported in Germany between March and June 2020 of 0.91% (0.58–1.24%) [53]. In contrast Percivalle et al reported the highest seroprevalence amongst Italian blood donors in April, living in the Lodi Red Zone of 23.3% [76]. The Lombardi Red Zone is an area of 10 municipalities that were put in total social and commercial lockdown from the 23rd February 2020 [76].The same study reports a seroprevalence of 1.67% in February 2020. In addition a study in the South East of Italy reports a seroprevalence on 0.99% in May 2020 [85].
Similar variations of estimates of seroprevalence were reported in blood donors in the UK. A study conducted in Scotland reported a seroprevalence of 3.17% between the months of March–May 2020 [118]. A seroprevalence of 8.5% (6.9–10%) was reported in blood donors across England in May [132].
Children/ Schools. Seven studies investigated the seroprevalence in school/university settings or among children only, across 5 different countries [49, 64, 69, 116, 121, 136]. Four of the studies examined the seroprevalence in schools [49, 64, 116, 136]. The lowest seroprevalence was reported in Germany; 0.6% among students in grade 8–11 and their teachers in 13 secondary schools in eastern Saxony between the months of May–June 2020 [64]. The highest seroprevalence of 11.7% was reported in students and teachers across schools in England between June-July 2020 (10.5–13.3%) [136].
Fontanet et al investigate the seroprevalence in a high school following an outbreak [44]. They report an overall seroprevalence of 25.86% among pupils, staff and parents of high school pupils [44]. Following the original outbreak Fontanet et al then investigated the seroprevalence among pupils and teachers in primary schools in the local area and reported an overall seroprevalence of 10.4% [49]. They noted that 41.4% of infected children had asymptomatic infection compared to 9.9% of seropositive adults [49].
One study examined the seroprevalence among university students and staff in Greece [69]. They reported an overall seroprevalence of 1%, with no significant difference by age, gender, school or position [69].
Outbreaks. Eight studies across four countries investigated the seroprevalence following an outbreak of SARS-CoV2 [24, 43, 44, 49, 58, 62, 130, 134]. Two of the studies were conducted in the UK and reported a high prevalence of antibodies against SARS-CoV-2 in residents and staff in care homes/nursing homes where there had been a recent SARS-CoV-2 outbreak [130, 134]. They report a high prevalence of antibodies against SARS-CoV-2. Ladhani et al estimated a seroprevalence of 77.9% (73.6–81.7%) and Nsn et al report a seroprevalence of 71.8% [130, 134].
Four studies report seroprevalences following outbreaks among blood donors or communities [24, 43, 58, 62]. They report much lower rates of seroprevalence compared to nursing home outbreaks. Grzelak et al investigated the seroprevalence of pauci-symptomatic individuals in Crepy-en-Valois France and blood donors in the surrounding region following an outbreak; they reported a seroprevalence of 3% in blood donors and 32% in the pauci-symptomatic individuals [43]. Similarly, studies in Germany following community outbreaks report low rates of seroprevalence among residents. Streeck et al reported a prevalence of SARS-CoV-2 antibodies of 13.6% and Weis et al reported a seroprevalence of 8.4% [58, 62]. Fig 2D shows the seroprevalence of these outbreak studies over time.
Pregnancy
Four studies examined the seroprevalence of SARS-CoV-2 in pregnant women [37, 50, 83, 97]. Two of these studies conducted in Italy between April–June 2020 reported a prevalence of SARS-CoV-2 antibodies of 10.1% and 14.3% in pregnant women in their first trimester screening or at delivery [83, 97]. Mattern et al estimated a seroprevalence of 8% among pregnant women admitted to the delivery room in France in May 2020 [50]. Mattern et al found that the seroprevalence among pregnant women was similar to that of the general public [50].
Egerup et al investigate the seroprevalence among pregnant women, their partners and newborn babies [37]. They report a seroprevalence of 2.85% among parturient women, 3.8% among partners and 1.4% among newborn babies [37]. They report no association between COVID-19 and obstetric or neonatal complications [37].
Assays
In total 47 different commercial assays and 22 in-house assays were used. The majority of studies used more than one assay. Of the commercial assays 11 were enzyme-linked immunosorbent assay (ELISA), seven were chemiluminescent microparticle immunoassays, two were based on flow cytometry and 27 were point of care tests (POC). The most used commercial assay was the SARS-CoV-2 (IgA/IgG) ELISA EUROIMMUN Medizinische Labordiagnostik, Lübeck, Germany (Table in S2 Table).
Discussion
Our systematic review demonstrates a large variation in the seroprevalence of SARS-CoV-2 antibodies throughout Europe in the first half of 2020.
HCWs in the UK had a much higher seroprevalence compared to HCWs in the rest of Europe during the months of March and August 2020. There are nine studies which took place in UK and six of them reported a seroprevalence of more 20% among HCWs [119, 120, 123, 128, 129, 133]. In contrast, Italy reports a low seroprevalence among HCWs. Of 10 studies among HCWs in Italy, nine reported a seroprevalence of SARS-CoV-2 antibodies of less than 10% [71, 77–82, 87, 88]. Both countries included studies from a mixture of high, medium and low risk HCWs and during this time both countries had high numbers of SARS-CoV-2 infections.
In health care settings, the risk of HCWs of SARS CoV-2 exposure was determined by the COVID-19 caseload coming though the facility and the application of infection control measures. Infection control practices in relation to personal protective equipment (PPE) may in part explain some of the differences.
Between European countries there are differences in the recommended PPE. The UK government guidelines on PPE include the use of eye/face protection, filtering facepiece class 3 (FFP3) respirator, disposable fluid-repellent coverall, and disposable gloves for aerosol-generating procedures and higher-risk acute care areas. For all inpatient ward settings eye/face protection, fluid-resistant (type IIR) surgical mask (FRSM), disposable plastic apron and disposable gloves are recommended [138].
In comparison, the National Institute of Health in Italy recommended that all HCWs wear a full-length gown with long sleeves, hairnet, goggles, gloves and surgical mask in the case of low-risk patients, and hairnet, googles or face-shield, FFP3 mask, water-resistant gown with long sleeves, and two pairs of gloves (second one covering the wrist of gown sleeves) for high risk patients and SARS-CoV-2 positive patients [77].
The main difference in the PPE provided in both countries was that Italy recommended that for all HCWs a full-length gown should be worn, a hairnet and an FFP3 mask when caring for all high-risk patients and all SARS-CoV-2 positive patients. As evidence now supports aerosol spread of SARS-CoV-2, reviews have been conducted investigating the protectiveness of surgical face masks and FFP3 masks. One such study concluded that surgical face masks provide no protection against aerosol particles, in comparison FFP3 masks provided adequate protection [139].
Although the availability and differences in PPE across European countries may partly explain the difference in seroprevalence seen in HCWs, there are other factors that require consideration. For example, differences in public health strategies and the time of their implementation such as the public wearing face masks, closure of educational settings and other public facilities. Furthermore, differences in adherence to infection control measures such as hand hygiene and social distancing could also explain the difference in seroprevalence seen among HCWs across Europe.
Our systematic review found that in the majority of studies in Europe there was no difference in seroprevalence between female and male participants. Our findings are in keeping with a meta-analysis which showed there was no difference in the proportion of males and females with confirmed COVID-19 [140].
Throughout the current pandemic there has been debate on the role of children in the transmission of SARS-CoV-2 and the need for school closure to slow the pandemic. In this review three studies were conducted in schools not involved in an outbreak of SARS-CoV-2. A study in Germany reported the lowest seroprevalence among students and teachers in a school of 0.6% considered by the authors to be in keeping with local surveillance data of the surrounding community [64]. Ulyte et al reported that seroprevalence is inversely related to age in their school study [116]. They conclude this could be due to the lack of social distancing among young children and differences in immune response [116]. In contrast Ladhani et al reported no significant difference between the seroprevalence in students compared to staff [136]. However all studies concluded that there was no major transmission in schools and that the majority of children were asymptomatic or had mild symptoms [64, 116, 136].
However, since these studies have been conducted there has been the introduction of the SARS-CoV-2 variants; B 1.1.7 and B 1.617.2. An increase in these variants have been seen among children and young adolescents with outbreaks occurring in schools and child-care facilities [141, 142].
As new variants emerge it would be reasonable for more school-based studies investigating the seroprevalence among staff and students to be conducted to fully understand transmission dynamics and immune response over time. Viewed within the context of local community data this will help to inform public health strategies to both protect children and to minimise transmission in the wider community.
In studies conducted during local outbreaks, there was a noticeable difference between those conducted in care/nursing homes compared to community and school settings. Those that took place in care/ nursing homes reported a seroprevalence as high as 77.9%, whereas those in a community setting reported a seroprevalence ranging from 3% - 42.4% and those in a school reported a seroprevalence between 10.4% and 25.86% [24, 43, 44, 49, 58, 62, 130] This large discrepancy could be attributable to the close proximity of care/nursing home residents, shared living spaces and the intimate care and handling of residents by staff.
Limitations
This systematic review and meta-analysis had several limitations. Firstly, of the 115 studies included in this review, not all of them could be included in sub-analysis as complete data sets could not be retrieved from every study and data quality was heterogeneous. As this review only included studies performed in Europe, the results of this review can only be applied to European countries. By only including papers written in English we may have overlooked important seroprevalence studies conducted in Europe but published in another language. In addition, most studies were performed either in the UK, Italy, Spain or Germany. There was a large gap in studies being performed in Eastern European countries. Those studies performed in the UK predominately took place in the South of England. Therefore, our review maybe bias towards countries and regions where there is more funding and resources available to conduct seroprevalence studies. In this review the studies used a variety of different assays, with different sensitivities and specificities which could lead to an under or overestimation of the true prevalence reported. Furthermore, many of the studies were pre-print articles that had not undergone peer-review; however, study quality was assessed and subgroup analysis based on risk of bias was performed.
Conclusion
This systematic review and metanalysis highlights substantial heterogeneity between countries, within countries, among professions, and among settings. This heterogeneity, in addition to indicating the general trajectory of the pandemic in different regions, might have been driven by a variety of other factors including governmental policies and restrictions, local guidelines and restrictions, availability of PPE, the time period when the study was conducted and serological test performance. Nevertheless, seroprevalence studies yield large amounts of useful, locally relevant information and should be regularly repeated as the pandemic evolves and local guidelines and restrictions change. As testing standardises and new studies are reported they will also help identify different national experiences across Europe and provide a means to distil best pandemic control practices for the future. As mass vaccinations have been launched in many countries seroprevalence studies will be able to provide valuable information on the immune response over time following vaccination and the need for booster vaccinations, help understand if herd immunity has been achieved and identify populations at risk of transmission and therefore need for vaccination. Finally, as new variants of SARS-CoV-2 now emerge, and many countries prepare for future waves it is vital that regular seroprevalence studies are conducted to aid control by informing public health measures.
Supporting information
(XLSX)
In some cases, the manufacture’s specificity and sensitivity were unable to be found, so evaluation study data was used instead.
(PDF)
(TIFF)
Acknowledgments
NC and DH are affiliated to the NIHR Health Protection Research Unit (HPRU) in Gastrointestinal Infections at University of Liverpool, in partnership with Public Health England (PHE), in collaboration with University of Warwick. NC and DH are based at The University of Liverpool. NF and DH are affiliated to the NIHR HPRU Emerging and Zoonotic Infections, a partnership between PHE, the University of Liverpool in collaboration with the Liverpool School of Tropical Medicine and the University of Oxford. The views expressed are those of the author(s) and not necessarily those of the NIHR, the Department of Health and Social Care, HPRU or PHE.
Data Availability
All relevant data are within the manuscript and its Supporting Information files.
Funding Statement
The authors received no specific funding for this work.
References
- 1.World Health Organization. WHO Director-General’s opening remarks at the media briefing on COVID-19–11 March 2020 [Internet]. [cited 2020 Nov 18]. Available from: https://www.who.int/director-general/speeches/detail/who-director-general-s-opening-remarks-at-the-media-briefing-on-covid-19—11-march-2020
- 2.Kutti-Sridharan G, Vegunta R, Vegunta R, Mohan BP, Rokkam VRP. SARS-CoV2 in Different Body Fluids, Risks of Transmission, and Preventing COVID-19: A Comprehensive Evidence-Based Review. Int J Prev Med [Internet]. 2020. [cited 2020 Nov 18];11:97. Available from: http://www.ncbi.nlm.nih.gov/pubmed/33042494 doi: 10.4103/ijpvm.IJPVM_255_20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Anderson EL, Turnham P, Griffin JR, Clarke CC. Consideration of the Aerosol Transmission for COVID-19 and Public Health. Risk Anal [Internet]. 2020. [cited 2020 Nov 18];40(5):902–7. Available from: http://www.ncbi.nlm.nih.gov/pubmed/32356927 doi: 10.1111/risa.13500 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Greenhalgh T, Jimenez JL, Prather KA, Tufekci Z, Fisman D, Schooley R. Ten scientific reasons in support of airborne transmission of SARS-CoV-2. Lancet (London, England) [Internet]. 2021. May 1 [cited 2021 Jun 25];397(10285):1603–5. Available from: http://www.ncbi.nlm.nih.gov/pubmed/33865497 doi: 10.1016/S0140-6736(21)00869-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Guan W, Ni Z, Hu Y, Liang W, Ou C, He J, et al. Clinical Characteristics of Coronavirus Disease 2019 in China. N Engl J Med [Internet]. 2020. Apr 30 [cited 2020 Nov 18];382(18):1708–20. Available from: http://www.nejm.org/doi/10.1056/NEJMoa2002032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Day M. Covid-19: four fifths of cases are asymptomatic, China figures indicate. BMJ [Internet]. 2020. Apr 2 [cited 2020 Nov 18];369:m1375. Available from: http://www.ncbi.nlm.nih.gov/pubmed/32241884 doi: 10.1136/bmj.m1375 [DOI] [PubMed] [Google Scholar]
- 7.Zhou J, Tan Y, Li D, He X, Yuan T, Long Y. Observation and analysis of 26 cases of asymptomatic SARS-COV2 infection. J Infect [Internet]. 2020. Jul [cited 2020 Nov 18];81(1):e69–70. Available from: http://www.ncbi.nlm.nih.gov/pubmed/32251687 doi: 10.1016/j.jinf.2020.03.028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.European Centre for Disease Prevention and control. Population-wide testing of SARS-CoV-2: country experiences and potential approaches in the EU/EEA and the United Kingdom European Commission request Definition [Internet]. 2020 [cited 2020 Oct 1]. Available from: https://www.ecdc.europa.eu/sites/default/files/documents/covid-19-population-wide-testing-country-experiences.pdf
- 9.World Health Organization. COVID-19 Strategy Update April 2020 [Internet]. 2020 [cited 2020 Nov 20]. Available from: https://www.who.int/docs/default-source/coronaviruse/covid-strategy-update-14april2020.pdf?sfvrsn=29da3ba0_19
- 10.Iacobucci G. Covid-19: Mass population testing is rolled out in Liverpool. BMJ [Internet]. 2020. Nov 3 [cited 2020 Nov 18];371:m4268. Available from: http://www.ncbi.nlm.nih.gov/pubmed/33144291 doi: 10.1136/bmj.m4268 [DOI] [PubMed] [Google Scholar]
- 11.Burgess S, Ponsford MJ, Gill D. Are we underestimating seroprevalence of SARS-CoV-2? BMJ [Internet]. 2020. Sep 3 [cited 2020 Nov 18];370:m3364. Available from: http://www.ncbi.nlm.nih.gov/pubmed/32883673 doi: 10.1136/bmj.m3364 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Watson J, Richter A, Deeks J. Testing for SARS-CoV-2 antibodies. BMJ [Internet]. 2020. Sep 8 [cited 2020 Nov 19];370:m3325. Available from: http://www.ncbi.nlm.nih.gov/pubmed/32900692 doi: 10.1136/bmj.m3325 [DOI] [PubMed] [Google Scholar]
- 13.Mafopa NG, Russo G, Wadoum REG, Iwerima E, Batwala V, Giovanetti M, et al. Seroprevalence of Ebola virus infection in Bombali District, Sierra Leone. J Public Health Africa [Internet]. 2017. Dec 31 [cited 2020 Nov 20];8(2):732. Available from: http://www.ncbi.nlm.nih.gov/pubmed/29456826 doi: 10.4081/jphia.2017.732 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Béraud G, Abrams S, Beutels P, Dervaux B, Hens N. Resurgence risk for measles, mumps and rubella in France in 2018 and 2020. Euro Surveill [Internet]. 2018. [cited 2020 Nov 20];23(25). Available from: http://www.ncbi.nlm.nih.gov/pubmed/29945697 doi: 10.2807/1560-7917.ES.2018.23.25.1700796 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ [Internet]. 2021. Mar 29 [cited 2021 Jun 24];n71. Available from: https://www.bmj.com/lookup/doi/10.1136/bmj.n71 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.World Health Organization (WHO). European Countries [Internet]. World Health Organisation. World Health Organization; 2021 [cited 2021 Jun 25]. Available from: https://www.euro.who.int/en/countries
- 17.Hoy D, Brooks P, Woolf A, Blyth F, March L, Bain C, et al. Assessing risk of bias in prevalence studies: modification of an existing tool and evidence of interrater agreement. J Clin Epidemiol [Internet]. 2012. Sep [cited 2021 Jul 2];65(9):934–9. Available from: http://www.ncbi.nlm.nih.gov/pubmed/22742910 doi: 10.1016/j.jclinepi.2011.11.014 [DOI] [PubMed] [Google Scholar]
- 18.Nguyen K, Peer N, Mills E, Kengne A. A Meta-Analysis of the Metabolic Syndrome Prevalence in the Global HIV-Infected Population. PLoS One [Internet]. 2016. [cited 2020 Dec 28];11(3). Available from: https://pubmed.ncbi.nlm.nih.gov/27008536/ doi: 10.1371/journal.pone.0150970 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wang N. How to Conduct a Meta-Analysis of Proportions in R: A Comprehensive Tutorial [Internet]. 2018. [cited 2021 Mar 30]. Available from: https://www.researchgate.net/publication/325486099_How_to_Conduct_a_Meta-Analysis_of_Proportions_in_R_A_Comprehensive_Tutorial [Google Scholar]
- 20.Hunter JP, Saratzis A, Sutton AJ, Boucher RH, Sayers RD, Bown MJ. In meta-analyses of proportion studies, funnel plots were found to be an inaccurate method of assessing publication bias. J Clin Epidemiol [Internet]. 2014. Aug 1 [cited 2021 Mar 30];67(8):897–903. Available from: http://www.ncbi.nlm.nih.gov/pubmed/24794697 doi: 10.1016/j.jclinepi.2014.03.003 [DOI] [PubMed] [Google Scholar]
- 21.Popay J, Roberts H, Sowden A, Petticrew M, Arai L, Rodgers M, et al. Guidance on the Conduct of Narrative Synthesis in Systematic Reviews A Product from the ESRC Methods Programme Peninsula Medical School, Universities of Exeter and Plymouth [Internet]. 2006. [cited 2021 Jun 26]. Available from: https://www.lancaster.ac.uk/media/lancaster-university/content-assets/documents/fhm/dhr/chir/NSsynthesisguidanceVersion1-April2006.pdf [Google Scholar]
- 22.Reintjes R, Zanuzdana A. Outbreak Investigations. In: Modern Infectious Disease Epidemiology [Internet]. Nature Publishing Group; 2009. [cited 2021 Jun 24]. p. 159–76. Available from: http://link.springer.com/10.1007/978-0-387-93835-6_9 [Google Scholar]
- 23.Orth-Höller D, Eigentler A, Weseslindtner L, Möst J. Antibody kinetics in primary- and secondary-care physicians with mild to moderate SARS-CoV-2 infection. Emerg Microbes Infect [Internet]. 2020;9(1):1692–4. Available from: http://europepmc.org/abstract/MED/32654611 doi: 10.1080/22221751.2020.1793690 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Knabl L, Mitra T, Kimpel J, Roessler A, Volland A, Walser A, et al. High SARS-CoV-2 Seroprevalence in Children and Adults in the Austrian Ski Resort Ischgl. medRxiv [Internet]. 2020. Jan 1;2020.08.20.20178533. Available from: http://medrxiv.org/content/early/2020/08/22/2020.08.20.20178533.abstract [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fuereder T, Berghoff AS, Heller G, Haslacher H, Perkmann T, Strassl R, et al. SARS-CoV-2 seroprevalence in oncology healthcare professionals and patients with cancer at a tertiary care centre during the COVID-19 pandemic. ESMO open [Internet]. 2020. Sep;5(5). Available from: 10.1136/esmoopen-2020-000889 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Reiter T, Pajenda S, Wagner L, Gaggl M, Atamaniuk J, Holzer B, et al. Covid-19 serology in nephrology health care workers. medRxiv [Internet]. 2020. Jan 1;2020.07.21.20136218. Available from: http://medrxiv.org/content/early/2020/07/26/2020.07.21.20136218.abstract [Google Scholar]
- 27.Herzog S, De Bie J, Abrams S, Wouters I, Ekinci E, Patteet L, et al. Seroprevalence of IgG antibodies against SARS coronavirus 2 in Belgium: a prospective cross-sectional nationwide study of residual samples. medRxiv [Internet]. 2020. Jan 1;2020.06.08.20125179. Available from: http://medrxiv.org/content/early/2020/07/30/2020.06.08.20125179.abstract [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Berardis S, Verroken A, Vetillart A, Struyf C, Gilbert M, Gruson D, et al. SARS-CoV-2 seroprevalence in a Belgian cohort of patients with cystic fibrosis. J Cyst Fibros [Internet]. 2020. Aug; Available from: https://europepmc.org/articles/PMC7418700 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Steensels D, Oris E, Coninx L, Nuyens D, Delforge ML, Vermeersch P, et al. Hospital-Wide SARS-CoV-2 Antibody Screening in 3056 Staff in a Tertiary Center in Belgium. JAMA—J Am Med Assoc [Internet]. 2020;324(2):195–7. Available from: https://www.scopus.com/inward/record.uri?eid=2-s2.0-85086874531&doi=10.1001%2Fjama.2020.11160&partnerID=40&md5=963394d58512419685c8fb2d69f16e62 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Martin C, Montesinos I, Dauby N, Gilles C, Dahma H, Van Den Wijngaert S, et al. Dynamics of SARS-CoV-2 RT-PCR positivity and seroprevalence among high-risk healthcare workers and hospital staff. J Hosp Infect [Internet]. 2020. [cited 2020 Dec 23];106(1):102. Available from: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7316468/ doi: 10.1016/j.jhin.2020.06.028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Desombere I, Mortgat L, Duysburgh E. COVID-19 study: 8,4% of Belgian health workers have antibodies to SARS-COV-2 [Internet]. Sciensano press release. 2020. Available from: https://www.sciensano.be/en/press-corner/covid-19-study-84-belgian-health-workers-have-antibodies-sars-cov-2 [Google Scholar]
- 32.Blairon L, Mokrane S, Wilmet A, Dessilly G, Kabamba-Mukadi B, Beukinga I, et al. Large-scale, molecular and serological SARS-CoV-2 screening of healthcare workers in a 4-site public hospital in Belgium after COVID-19 outbreak. J Infect [Internet]. 2020; Available from: http://europepmc.org/abstract/MED/32739485 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Jerković I, Ljubić T, Bašić Ž, Kružić I, Kunac N, Bezić J, et al. SARS-CoV-2 Antibody Seroprevalence in Industry Workers in Split-Dalmatia and Šibenik-Knin County, Croatia. J Occup Environ Med [Internet]. 2020. Sep 8 [cited 2020 Dec 21]; Available from: https://journals.lww.com/10.1097/JOM.0000000000002020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Vilibic-Cavlek T, Stevanovic V, Tabain I, Betica-Radic L, Sabadi D, Peric L, et al. Severe acute respiratory syndrome coronavirus 2 seroprevalence among personnel in the healthcare facilities of Croatia, 2020. Rev Soc Bras Med Trop [Internet]. 2020;53:e20200458. Available from: https://europepmc.org/articles/PMC7451497 doi: 10.1590/0037-8682-0458-2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Iversen K, Bundgaard H, Hasselbalch RB, Kristensen JH, Nielsen PB, Pries-Heje M, et al. Risk of COVID-19 in health-care workers in Denmark: an observational cohort study. Lancet Infect Dis [Internet]. 2020. Aug; Available from: https://europepmc.org/articles/PMC7398038 doi: 10.1016/S1473-3099(20)30589-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Erikstrup C, Hother CE, Pedersen OBV, Mølbak K, Skov RL, Holm DK, et al. Estimation of SARS-CoV-2 infection fatality rate by real-time antibody screening of blood donors. medRxiv [Internet]. 2020. Jan 1;2020.04.24.20075291. Available from: http://medrxiv.org/content/early/2020/04/28/2020.04.24.20075291.abstract [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Egerup P, Olsen LF, Christiansen A-MH, Westergaard D, Severinsen ER, Hviid KVR, et al. Impact of SARS-CoV-2 antibodies at delivery in women, partners and newborns. medRxiv [Internet]. 2020. Sep 15 [cited 2021 Jun 27];2020.09.14.20191106. Available from: https://www.medrxiv.org/content/10.1101/2020.09.14.20191106v1 [Google Scholar]
- 38.Jespersen S, Mikkelsen S, Greve T, Kaspersen KA, Tolstrup M, Boldsen JK, et al. SARS-CoV-2 seroprevalence survey among 18,000 healthcare and administrative personnel at hospitals, pre-hospital services, and specialist practitioners in the Central Denmark Region. medRxiv [Internet]. 2020. Jan 1;2020.08.10.20171850. Available from: http://medrxiv.org/content/early/2020/08/12/2020.08.10.20171850.abstract [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Laursen J, Petersen J, Didriksen M, Iversen KK, Ullum H. Prevalence of SARS-CoV-2 IgG/IgM antibodies among Danish and Swedish Falck emergency and non-emergency healthcare workers [Internet]. medRxiv; 2020. Available from: http://europepmc.org/abstract/PPR/PPR219363 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Petersen MS, Strom M, Christiansen DH, Fjallsbak JP, Eliasen EH, Johansen M, et al. Seroprevalence of SARS-CoV-2-Specific Antibodies, Faroe Islands. Emerg Infect Dis [Internet]. 2020;26(11). Available from: http://europepmc.org/search?query=(DOI:10.3201/eid2611.202736) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Germain N, Herwegh S, Hatzfeld AS, Bocket L, Prevost B, Danze PM, et al. Retrospective study of COVID-19 seroprevalence among tissue donors at the onset of the outbreak before implementation of strict lockdown measures in France [Internet]. medRxiv; 2020. Available from: http://europepmc.org/abstract/PPR/PPR213447 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Solodky ML, Galvez C, Russias B, Detourbet P, N’Guyen-Bonin V, Herr A-L, et al. Lower detection rates of SARS-COV2 antibodies in cancer patients versus health care workers after symptomatic COVID-19. Ann Oncol [Internet]. 2020. [cited 2020 Dec 21];31(8):1087. Available from: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7252166/ doi: 10.1016/j.annonc.2020.04.475 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Grzelak L, Temmam S, Planchais C, Demeret C, Tondeur L, Huon C, et al. A comparison of four serological assays for detecting anti-SARS-CoV-2 antibodies in human serum samples from different populations. Sci Transl Med [Internet]. 2020. Aug 17 [cited 2020 Dec 21];12(559). Available from: https://europepmc.org/article/MED/32817357 doi: 10.1126/scitranslmed.abc3103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Fontanet A, Tondeur L, Madec Y, Grant R, Besombes C, Jolly N, et al. Cluster of COVID-19 in northern France: A retrospective closed cohort study. medRxiv [Internet]. 2020. Jan 1;2020.04.18.20071134. Available from: http://medrxiv.org/content/early/2020/04/23/2020.04.18.20071134.abstract [Google Scholar]
- 45.Carrat F, de Lamballerie X, Rahib D, Blanche H, Lapidus N, Artaud F, et al. Seroprevalence of SARS-CoV-2 among adults in three regions of France following the lockdown and associated risk factors: a multicohort study [Internet]. medRxiv; 2020. Available from: http://europepmc.org/abstract/PPR/PPR215593 [Google Scholar]
- 46.Gallian P, Pastorino B, Morel P, Chiaroni J, Ninove L, de Lamballerie X. Lower prevalence of antibodies neutralizing SARS-CoV-2 in group O French blood donors. Antiviral Res [Internet]. 2020. Sep;181:104880. Available from: https://europepmc.org/articles/PMC7362788 doi: 10.1016/j.antiviral.2020.104880 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.sermet isabelle, temmam sarah, huon christele, behillil sylvie, gadjos vincent, bigot thomas, et al. Prior infection by seasonal coronaviruses does not prevent SARS-CoV-2 infection and associated Multisystem Inflammatory Syndrome in children. medRxiv [Internet]. 2020. Jan 1;2020.06.29.20142596. Available from: http://medrxiv.org/content/early/2020/06/30/2020.06.29.20142596.abstract [Google Scholar]
- 48.Lisandru C, Nazli A, Shirley M, Jean C, Stéphane P, Helene SM, et al. Seroprevalence of SARS-CoV-2 IgG antibodies, in Corsica (France), April and June 2020. medRxiv [Internet]. 2020. Sep 30 [cited 2021 Jun 27];2020.09.29.20201368. Available from: https://www.medrxiv.org/content/10.1101/2020.09.29.20201368v1 [Google Scholar]
- 49.Fontanet A, Grant R, Tondeur L, Madec Y, Grzelak L, Cailleau I, et al. SARS-CoV-2 infection in primary schools in northern France: A retrospective cohort study in an area of high transmission. medRxiv [Internet]. 2020. Jan 1;2020.06.25.20140178. Available from: http://medrxiv.org/content/early/2020/06/29/2020.06.25.20140178.1.abstract [Google Scholar]
- 50.Mattern J, Vauloup-Fellous C, Zakaria H, Benachi A, Carrara J, Letourneau A, et al. Post lockdown COVID-19 seroprevalence and circulation at the time of delivery, France. Spradley FT, editor. PLoS One [Internet]. 2020. Oct 15 [cited 2020 Dec 21];15(10):e0240782. Available from: doi: 10.1371/journal.pone.0240782 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Péré H, Wack M, Védie B, Guinet ND, Najiby KC, Janot L, et al. Sequential SARS-CoV-2 IgG assays as confirmatory strategy to confirm equivocal results: Hospital-wide antibody screening in 3,569 staff health care workers in Paris. J Clin Virol [Internet]. 2020. Sep; Available from: https://europepmc.org/articles/PMC7470734 doi: 10.1016/j.jcv.2020.104617 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Simon D, Tascilar K, Krönke G, Kleyer A, Zaiss MM, Heppt F, et al. Patients with immune-mediated inflammatory diseases receiving cytokine inhibitors have low prevalence of SARS-CoV-2 seroconversion. Nat Commun [Internet]. 2020. Jul;11(1):3774. Available from: https://europepmc.org/articles/PMC7382482 doi: 10.1038/s41467-020-17703-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Fischer B, Knabbe C, Vollmer T. SARS-CoV-2 IgG seroprevalence in blood donors located in three different federal states, Germany, March to June 2020. Euro Surveill [Internet]. 2020. Jul;25(28). Available from: https://europepmc.org/articles/PMC7376847 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Brandstetter S, Roth S, Harner S, Buntrock‐Döpke H, Toncheva AA, Borchers N, et al. Symptoms and immunoglobulin development in hospital staff exposed to a SARS‐CoV‐2 outbreak. Kalaycı Ö, editor. Pediatr Allergy Immunol [Internet]. 2020. Oct 18 [cited 2020 Dec 21];31(7):841–7. Available from: https://onlinelibrary.wiley.com/doi/10.1111/pai.13278 [DOI] [PubMed] [Google Scholar]
- 55.Brehm TT, Schwinge D, Lampalzer S, Schlicker V, Kuechen J, Thompson M, et al. High effectiveness of multimodal infection control interventions in preventing SARS−CoV−2 infections in healthcare professionals: a prospective longitudinal seroconversion study. medRxiv [Internet]. 2020. Jan 1;2020.07.31.20165936. Available from: http://medrxiv.org/content/early/2020/08/02/2020.07.31.20165936.abstract [Google Scholar]
- 56.Behrens GMN, Cossmann A, Stankov M V, Witte T, Ernst D, Happle C, et al. Perceived versus proven SARS-CoV-2-specific immune responses in health-care professionals. Infection [Internet]. 2020. Aug;48(4):631–634. Available from: https://europepmc.org/articles/PMC7286418 doi: 10.1007/s15010-020-01461-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Korth J, Wilde B, Dolff S, Anastasiou OE, Krawczyk A, Jahn M, et al. SARS-CoV-2-specific antibody detection in healthcare workers in Germany with direct contact to COVID-19 patients. J Clin Virol [Internet]. 2020. Jul;128:104437. Available from: https://europepmc.org/articles/PMC7219425 doi: 10.1016/j.jcv.2020.104437 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Streeck H, Schulte B, Kuemmerer B, Richter E, Hoeller T, Fuhrmann C, et al. Infection fatality rate of SARS-CoV-2 infection in a German community with a super-spreading event. medRxiv [Internet]. 2020. Jan 1;2020.05.04.20090076. Available from: http://medrxiv.org/content/early/2020/06/02/2020.05.04.20090076.abstract [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Krähling V, Kern M, Halwe S, Müller H, Rohde C, Savini M, et al. Title: Epidemiological study to detect active SARS-CoV-2 infections and seropositive persons in a selected cohort of employees in the Frankfurt am Main metropolitan area. [cited 2020 Dec 21]; Available from: 10.1101/2020.05.20.20107730 [DOI] [Google Scholar]
- 60.Schmidt SB, Grüter L, Boltzmann M, Rollnik JD. Prevalence of serum IgG antibodies against SARS-CoV-2 among clinic staff. Adrish M, editor. PLoS One [Internet]. 2020. Jun 25 [cited 2020 Dec 21];15(6):e0235417. Available from: https://dx.plos.org/10.1371/journal.pone.0235417 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Aziz A, Corman V, Echterhoff AKC, Richter A, Schmandke A, Schmidt ML, et al. Seroprevalence and correlates of SARS-CoV-2 neutralizing antibodies: Results from a population-based study in Bonn, Germany [Internet]. medRxiv; 2020. Available from: 10.1101/2020.08.24.20181206 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Weis S, Scherag A, Baier M, Kiehntopf M, Kamradt T, Kolanos S, et al. Seroprevalence of SARS-CoV-2 antibodies in an entirely PCR-sampled and quarantined community after a COVID-19 outbreak—the CoNAN study. medRxiv [Internet]. 2020. Jan 1;2020.07.15.20154112. Available from: http://medrxiv.org/content/early/2020/07/17/2020.07.15.20154112.abstract [Google Scholar]
- 63.Bahrs C, Kimmig A, Weis S, Ankert J, Hagel S, Maschmann J, et al. Seroprevalence of SARS CoV-2 antibodies in healthcare workers and administration employees: a prospective surveillance study at a 1,400-bed university hospital in Germany. medRxiv [Internet]. 2020. Sep 29 [cited 2021 Jun 26];2020.09.29.20203737. Available from: https://www.medrxiv.org/content/10.1101/2020.09.29.20203737v1 [Google Scholar]
- 64.Armann JP, Unrath M, Kirsten C, Lueck C, Dalpke A, Berner R. Anti-SARS-CoV-2 IgG antibodies in adolescent students and their teachers in Saxony, Germany (SchoolCoviDD19): very low seropraevalence and transmission rates. medRxiv [Internet]. 2020. Jan 1;2020.07.16.20155143. Available from: http://medrxiv.org/content/early/2020/07/28/2020.07.16.20155143.abstract [Google Scholar]
- 65.Epstude J, Harsch IA. Seroprevalence of COVID-19 antibodies in the cleaning and oncological staff of a municipal clinic. GMS Hyg Infect Control [Internet]. 2020;15:Doc18. Available from: https://europepmc.org/articles/PMC7376972 doi: 10.3205/dgkh000353 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Hoffmann S, Spallek J, Gremmels H-D, Schiebel J, Hufert F. Testing the backbone of the healthcare system: a prospective serological-epidemiological cohort study of healthcare workers in rural Germany [Internet]. Research Square; 2020. Available from: http://europepmc.org/abstract/PPR/PPR220081 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Bogogiannidou Z, Vontas A, Dadouli K, Kyritsi MA, Soteriades S, Nikoulis DJ, et al. Repeated leftover serosurvey of SARS-CoV-2 IgG antibodies, Greece, March and April 2020. Euro Surveill [Internet]. 2020. Aug;25(31). Available from: https://europepmc.org/articles/PMC7459271 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Psichogiou M, Karabinis A, Pavlopoulou ID, Basoulis D, Petsios K, Roussos S, et al. Antibodies against SARS-CoV-2 among health care workers in a country with low burden of COVID-19. medRxiv [Internet]. 2020. Jan 1;2020.06.23.20137620. Available from: http://medrxiv.org/content/early/2020/06/23/2020.06.23.20137620.abstract [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Tsitsilonis OE, Paraskevis D, Lianidou E, Pierros V, Akalestos A, Kastritis E, et al. Seroprevalence of antibodies against SARS-CoV-2 among the personnel and students of the national and kapodistrian university of athens, greece: A preliminary report. Life [Internet]. 2020;10(9):1–8. Available from: https://www.scopus.com/inward/record.uri?eid=2-s2.0-85091318842&doi=10.3390%2Flife10090214&partnerID=40&md5=3d9928b21aede787d8edb6606006e92a doi: 10.3390/life10090214 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Gudbjartsson DF, Norddahl GL, Melsted P, Gunnarsdottir K, Holm H, Eythorsson E, et al. Humoral Immune Response to SARS-CoV-2 in Iceland. N Engl J Med [Internet]. 2020; Available from: http://europepmc.org/abstract/PMC/PMC7494247 doi: 10.1056/NEJMoa2026116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Plebani Padoan, Fedeli Schievano, Vecchiato Lippi, et al. SARS-CoV-2 serosurvey in health care workers of the Veneto Region. Clin Chem Lab Med [Internet]. 2020. [cited 2021 Jan 1];58(12). Available from: https://pubmed.ncbi.nlm.nih.gov/32845861/ doi: 10.1515/cclm-2020-1236 [DOI] [PubMed] [Google Scholar]
- 72.Valenti L, Bergna A, Pelusi S, Facciotti F, Lai A, Tarkowski M, et al. SARS-CoV-2 seroprevalence trends in healthy blood donors during the COVID-19 Milan outbreak. medRxiv [Internet]. 2020. Jan 1;2020.05.11.20098442. Available from: http://medrxiv.org/content/early/2020/05/31/2020.05.11.20098442.abstract [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Pancrazzi A, Magliocca P, Lorubbio M, Vaggelli G, Galano A, Mafucci M, et al. Comparison of serologic and molecular SARS-CoV 2 results in a large cohort in Southern Tuscany demonstrates a role for serologic testing to increase diagnostic sensitivity. Clin Biochem [Internet]. 2020;84:87–92. Available from: https://www.scopus.com/inward/record.uri?eid=2-s2.0-85088807240&doi=10.1016%2Fj.clinbiochem.2020.07.002&partnerID=40&md5=fdf2066950d4259b654f1f1c5086359e [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Vena A, Berruti M, Adessi A, Blumetti P, Brignole M, Colognato R, et al. Prevalence of Antibodies to SARS-CoV-2 in Italian Adults and Associated Risk Factors. J Clin Med [Internet]. 2020. Aug;9(9). Available from: 10.3390/jcm9092780 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Norsa L, Cosimo P, Indriolo A, Sansotta N, D’Antiga L, Callegaro A. Asymptomatic SARS-CoV-2 infection in patients with inflammatory bowel disease under biologic treatment. Gastroenterology [Internet]. 2020. Aug; Available from: https://europepmc.org/articles/PMC7448738 doi: 10.1053/j.gastro.2020.08.046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Percivalle E, Cambiè G, Cassaniti I, Nepita EV, Maserati R, Ferrari A, et al. Prevalence of SARS-CoV-2 specific neutralising antibodies in blood donors from the Lodi Red Zone in Lombardy, Italy, as at 06 April 2020. Euro Surveill [Internet]. 2020. Jun;25(24). Available from: https://europepmc.org/articles/PMC7315724 doi: 10.2807/1560-7917.ES.2020.25.24.2001031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Lahner E, Dilaghi E, Prestigiacomo C, Alessio G, Marcellini L, Simmaco M, et al. Prevalence of Sars-Cov-2 Infection in Health Workers (HWs) and Diagnostic Test Performance: The Experience of a Teaching Hospital in Central Italy. Int J Environ Res Public Health [Internet]. 2020. [cited 2020 Dec 20];17(12). Available from: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7345358/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Fusco FM, Pisaturo M, Iodice V, Bellopede R, Tambaro O, Parrella G, et al. COVID-19 among healthcare workers in a specialist infectious diseases setting in Naples, Southern Italy: results of a cross-sectional surveillance study. J Hosp Infect [Internet]. 2020. Aug;105(4):596–600. Available from: https://europepmc.org/articles/PMC7301109 doi: 10.1016/j.jhin.2020.06.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Sotgiu G, Barassi A, Miozzo M, Saderi L, Piana A, Orfeo N, et al. SARS-CoV-2 specific serological pattern in healthcare workers of an Italian COVID-19 forefront hospital. BMC Pulm Med [Internet]. 2020. Jul;20(1):203. Available from: https://europepmc.org/articles/PMC7388425 doi: 10.1186/s12890-020-01237-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Amendola A, Tanzi E, Folgori L, Barcellini L, Bianchi S, Gori M, et al. Low seroprevalence of SARS-CoV-2 infection among healthcare workers of the largest children hospital in Milan during the pandemic wave. Infect Control Hosp Epidemiol [Internet]. 2020. Aug;1–2. Available from: https://europepmc.org/articles/PMC7438626 doi: 10.1017/ice.2020.401 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Carozzi FM, Cusi MG, Pistello M, Galli L, Bartoloni A, Anichini G, et al. Detection of asymptomatic SARS-CoV-2 infections among healthcare workers: results from a large-scale screening program based on rapid serological testing. medRxiv [Internet]. 2020. Jan 1;2020.07.30.20149567. Available from: http://medrxiv.org/content/early/2020/08/04/2020.07.30.20149567.abstract [Google Scholar]
- 82.Comar M, Brumat M, Concas MP, Argentini G, Bianco A, Bicego L, et al. COVID-19 experience: first Italian survey on healthcare staff members from a Mother-Child Research Hospital using combined molecular and rapid immunoassays test. medRxiv [Internet]. 2020. Apr 22 [cited 2020 Dec 21];2020.04.19.20071563. Available from: https://www.medrxiv.org/content/10.1101/2020.04.19.20071563v1 [Google Scholar]
- 83.Cosma S, Borella F, Carosso A, Sciarrone A, Cusato J, Corcione S, et al. The “scar” of a pandemic: Cumulative incidence of COVID-19 during the first trimester of pregnancy. J Med Virol [Internet]. 2020. Jul; Available from: https://europepmc.org/articles/PMC7361535 doi: 10.1002/jmv.26267 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Sandri MT, Azzolini E, Torri V, Carloni S, Tedeschi M, Castoldi M, et al. IgG serology in health care and administrative staff populations from 7 hospital representative of different exposures to SARS-CoV-2 in Lombardy, Italy. medRxiv [Internet]. 2020. Jan 1;2020.05.24.20111245. Available from: http://medrxiv.org/content/early/2020/05/26/2020.05.24.20111245.abstract [Google Scholar]
- 85.Fiore JR, Centra M, De Carlo A, Granato T, Rosa A, Sarno M, et al. Results from a survey in healthy blood donors in South Eastern Italy indicate that we are far away from herd immunity to SARS-CoV-2. J Med Virol [Internet]. 2020. Aug; Available from: https://europepmc.org/articles/PMC7436723 doi: 10.1002/jmv.26425 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Pagani G, Conti F, Giacomelli A, Bernacchia D, Rondanin R, Prina A, et al. Seroprevalence of SARS-CoV-2 significantly varies with age: Preliminary results from a mass population screening. J Infect [Internet]. 2020. Dec 1 [cited 2020 Dec 21];81(6):e10–2. Available from: https://www.journalofinfection.com/article/S0163-4453(20)30629-0/fulltext doi: 10.1016/j.jinf.2020.09.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Paderno A, Fior M, Berretti G, Schreiber A, Mattavelli D, Deganello A, et al. SARS-CoV-2 infection in healthcare workers: cross-sectional analysis of an Otolaryngology Unit 2 [Internet]. [cited 2020 Dec 21]. Available from: https://www.entnet.org/sites/default/files/uploads/paderno2_sars-cov-2_infection_in_healthcare_workers.pdf [DOI] [PubMed] [Google Scholar]
- 88.Tosato F, Pelloso M, Gallo N, Giraudo C, Llanaj G, Cosma C, et al. Severe Acute Respiratory Syndrome Coronavirus 2 Serology in Asymptomatic Healthcare Professionals: Preliminary Experience of a Tertiary Italian Academic Center. medRxiv [Internet]. 2020. Jan 1;2020.04.27.20073858. Available from: http://medrxiv.org/content/early/2020/05/01/2020.04.27.20073858.abstract [Google Scholar]
- 89.Westerhuis BM, de Bruin E, Chandler FD, Ramakers CRB, Okba NMA, Li W, et al. Homologous and heterologous antibodies to coronavirus 229E, NL63, OC43, HKU1, SARS, MERS and SARS-CoV-2 antigens in an age stratified cross-sectional serosurvey in a large tertiary hospital in The Netherlands. medRxiv [Internet]. 2020. Jan 1;2020.08.21.20177857. Available from: http://medrxiv.org/content/early/2020/08/24/2020.08.21.20177857.abstract [Google Scholar]
- 90.Slot E, Hogema B, Reusken CBEM, Reimerink J, Molier M, Karregat JHM, et al. Herd immunity is not a realistic exit strategy during a COVID-19 outbreak [Internet]. Research Square; 2020. Available from: 10.21203/rs.3.rs-25862/v1 [DOI] [Google Scholar]
- 91.Snoeck CJ, Vaillant M, Abdelrahman T, Satagopam VP, Turner JD, Beaumont K, et al. Prevalence of SARS-CoV-2 infection in the Luxembourgish population: the CON-VINCE study. medRxiv [Internet]. 2020. Jan 1;2020.05.11.20092916. Available from: http://medrxiv.org/content/early/2020/05/18/2020.05.11.20092916.abstract [Google Scholar]
- 92.Figueiredo-Campos P, Blankenhaus B, Mota C, Gomes A, Serrano M, Ariotti S, et al. Seroprevalence of anti-SARS-CoV-2 antibodies in COVID-19 patients and healthy volunteers [Internet]. medRxiv; 2020. Available from: doi: 10.1002/eji.202048970 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Dacosta-Urbieta A, Rivero-Calle I, Pardo-Seco J, Redondo-Collazo L, Salas A, Gomez-Rial J, et al. Seroprevalence of SARS-CoV-2 Among Pediatric Healthcare Workers in Spain. Front Pediatr [Internet]. 2020;8. Available from: http://europepmc.org/search?query=(DOI:10.3389/fped.2020.00547) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Garcia-Basteiro AL, Moncunill G, Tortajada M, Vidal M, Guinovart C, Jimenez A, et al. Seroprevalence of antibodies against SARS-CoV-2 among health care workers in a large Spanish reference hospital. medRxiv [Internet]. 2020. Jan 1;2020.04.27.20082289. Available from: http://medrxiv.org/content/early/2020/05/02/2020.04.27.20082289.abstract [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Valdivia A, Torres I, Huntley D, Alcaraz MJ, Albert E, de la Asunción C, et al. Caveats in interpreting SARS-CoV-2 IgM+ /IgG- antibody profile in asymptomatic health care workers. J Med Virol [Internet]. 2020. Aug; Available from: https://europepmc.org/articles/PMC7436589 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Galan I, Velasco M, Casas ML, Goyanes MJ, Rodriguez-Caravaca G, Losa JE, et al. SARS-CoV-2 SEROPREVALENCE AMONG ALL WORKERS IN A TEACHING HOSPITAL IN SPAIN: UNMASKING THE RISK. medRxiv [Internet]. 2020. Jan 1;2020.05.29.20116731. Available from: http://medrxiv.org/content/early/2020/05/29/2020.05.29.20116731.abstract [Google Scholar]
- 97.Crovetto F, Crispi F, Llurba E, Figueras F, Gómez-Roig MD, Gratacós E. Seroprevalence and presentation of SARS-CoV-2 in pregnancy. Lancet [Internet]. 2020;396(10250):530–1. Available from: https://www.scopus.com/inward/record.uri?eid=2-s2.0-85089518432&doi=10.1016%2FS0140-6736%2820%2931714-1&partnerID=40&md5=d1a1beb365755cc3668ff54d05bc525b [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Garralda Fernandez J, Molero Vilches I, Bermejo Rodriguez A, Cano de Torres I, Colino Romay EI, Garcia-Arata I, et al. Impact of SARS-CoV-2 pandemic among health care workers in a secondary teaching hospital in Spain. medRxiv [Internet]. 2020. Jan 1;2020.07.26.20162529. Available from: http://medrxiv.org/content/early/2020/07/29/2020.07.26.20162529.abstract [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Olalla J, Correa AM, Martin-Escalante MD, Hortas ML, Martin-Sendarrubias MJ, Fuentes V, et al. Search for asymptomatic carriers of SARS-CoV-2 in healthcare workers during the pandemic: a Spanish experience. medRxiv [Internet]. 2020. Jan 1;2020.05.18.20103283. Available from: http://medrxiv.org/content/early/2020/05/20/2020.05.18.20103283.abstract doi: 10.1093/qjmed/hcaa238 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Martín V, Fernández-Villa T, Lamuedra Gil de Gomez M, Mencía-Ares O, Rivero Rodríguez A, Reguero Celada S, et al. Prevalence of SARS-CoV-2 infection in general practitioners and nurses in primary care and nursing homes in the Healthcare Area of León and associated factors. Semergen [Internet]. 2020;46 Suppl 1:35–9. Available from: http://europepmc.org/abstract/MED/32646731 doi: 10.1016/j.semerg.2020.05.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Montenegro P, Brotons C, Fernandez D, Ichazo B, Moral I, Pitarch M, et al. Community seroprevalence of COVID-19 in probable and possible cases at primary health care centres in Spain. Fam Pract [Internet]. 2020; Available from: https://academic.oup.com/fampra/advance-article-pdf/doi/10.1093/fampra/cmaa096/33734322/cmaa096.pdf [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Moncunill G, Mayor A, Santano R, Jimenez A, Vidal M, Tortajada M, et al. SARS-CoV-2 infections and antibody responses among health care workers in a Spanish hospital after a month of follow-up. medRxiv [Internet]. 2020. Jan 1;2020.08.23.20180125. Available from: http://medrxiv.org/content/early/2020/08/25/2020.08.23.20180125.abstract [Google Scholar]
- 103.Soriano V, Meiriño R, Corral O, Guallar MP. SARS-CoV-2 antibodies in adults in Madrid, Spain. Clin Infect Dis [Internet]. 2020. Jun; Available from: https://europepmc.org/articles/PMC7337724 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Pollán M, Pérez-Gómez B, Pastor-Barriuso R, Oteo J, Hernán MA, Pérez-Olmeda M, et al. Prevalence of SARS-CoV-2 in Spain (ENE-COVID): a nationwide, population-based seroepidemiological study. Lancet (London, England) [Internet]. 2020. Aug;396(10250):535–544. Available from: https://europepmc.org/articles/PMC7336131 doi: 10.1016/S0140-6736(20)31483-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Martínez PT. IgG seroprevalence against SARS-CoV-2 in a cohort of 449 non-hospitalized, high-risk exposure individuals. Res Sq [Internet]. 2020. Aug 18 [cited 2021 Jun 27]; Available from: https://www.researchsquare.com/article/rs-53747/v1 [Google Scholar]
- 106.Barallat J, Fernández-Rivas G, Quirant-Sánchez B, Martinez-Caceres, Pina M, Matllo J, et al. Seroprevalence of SARS-CoV-2 IgG Specific Antibodies among Healthcare Workers in the Northern Metropolitan Area of Barcelona, Spain, after the first pandemic wave. [cited 2020 Dec 21]; Available from: doi: 10.1371/journal.pone.0244348 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Cabezón-Gutiérrez L, Custodio-Cabello S, Palka-Kotlowska M, Oliveros-Acebes E, José García-Navarro M, Khosravi-Shahi P. Seroprevalence of SARS-CoV-2–specific antibodies in cancer outpatients in Madrid (Spain): A single center, prospective, cohort study and a review of available data. Cancer Treat Rev [Internet]. 2020. Sep; Available from: https://europepmc.org/articles/PMC7462449 doi: 10.1016/j.ctrv.2020.102102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Castro Dopico X, Hanke L, Sheward DJ, Christian M, Muschiol S, Grinberg NF, et al. Antibody phenotypes and probabilistic seroprevalence estimates during the emergence of SARS-CoV-2 in Sweden. medRxiv [Internet]. 2020. Jan 1;2020.07.17.20155937. Available from: http://medrxiv.org/content/early/2020/08/25/2020.07.17.20155937.abstract [Google Scholar]
- 109.Rudberg A-S, Havervall S, Manberg A, Jernbom Falk A, Aguilera K, Ng H, et al. SARS-CoV-2 exposure, symptoms and seroprevalence in health care workers. medRxiv [Internet]. 2020. Jan 1;2020.06.22.20137646. Available from: http://medrxiv.org/content/early/2020/06/23/2020.06.22.20137646.abstract [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Lindahl JF, Hoffman T, Esmaeilzadeh M, Olsen B, Winter R, Amer S, et al. High seroprevalence of SARS-CoV-2 in elderly care employees in Sweden [Internet]. Vol. 10, Infection ecology & epidemiology. 2020. p. 1789036. Available from: http://europepmc.org/abstract/MED/32939231 doi: 10.1080/20008686.2020.1789036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Roxhed N, Bendes A, Dale M, Mattsson C, Hanke L, Dodig-Crnkovic T, et al. A translational multiplex serology approach to profile the prevalence of anti-SARS-CoV-2 antibodies in home-sampled blood. medRxiv [Internet]. 2020. Jan 1;2020.07.01.20143966. Available from: http://medrxiv.org/content/early/2020/07/02/2020.07.01.20143966.abstract [Google Scholar]
- 112.Lidström A-K, Sund F, Albinsson B, Lindbäck J, Westman G. Work at inpatient care units is associated with an increased risk of SARS-CoV-2 infection; a cross-sectional study of 8679 healthcare workers in Sweden. Ups J Med Sci [Internet]. 2020. Nov [cited 2021 Jun 27];125(4):305–10. Available from: http://www.ncbi.nlm.nih.gov/pubmed/32684119 doi: 10.1080/03009734.2020.1793039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Lundkvist Å, Hanson S, Olsen B. Pronounced difference in Covid-19 antibody prevalence indicates cluster transmission in Stockholm, Sweden [Internet]. Vol. 10, Infection ecology & epidemiology. 2020. p. 1806505. Available from: http://europepmc.org/abstract/MED/32944166 doi: 10.1080/20008686.2020.1806505 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Emmenegger M, De Cecco E, Lamparter D, Jacquat RPB, Ebner D, Schneider MM, et al. Early peak and rapid decline of SARS-CoV-2 seroprevalence in a Swiss metropolitan region. medRxiv [Internet]. 2020. Jan 1;2020.05.31.20118554. Available from: http://medrxiv.org/content/early/2020/08/07/2020.05.31.20118554.abstract [Google Scholar]
- 115.Stringhini S, Wisniak A, Piumatti G, Azman AS, Lauer SA, Baysson H, et al. Seroprevalence of anti-SARS-CoV-2 IgG antibodies in Geneva, Switzerland (SEROCoV-POP): a population-based study. Lancet (London, England) [Internet]. 2020. Aug;396(10247):313–319. Available from: https://europepmc.org/articles/PMC7289564 doi: 10.1016/S0140-6736(20)31304-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Ulyte A, Radtke T, Abela I, Haile S, Blankenberger J, Jung R, et al. Variation in SARS-CoV-2 seroprevalence in school-children across districts, schools and classes [Internet]. medRxiv; 2020. Available from: http://europepmc.org/abstract/PPR/PPR215541 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.ALKURT G, MURT A,AYDIN Z, TATLI O, AGAOGLU NB, IRVEM A, et al. Seroprevalence of Coronavirus Disease 2019 (COVID-19) Among Health Care Workers from Three Pandemic Hospitals of Turkey. medRxiv [Internet]. 2020. Jan 1;2020.08.19.20178095. Available from: http://medrxiv.org/content/early/2020/08/22/2020.08.19.20178095.abstract [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Thompson CP, Grayson N, Paton R, Bolton JS, Lourenço J, Penman B, et al. Detection of neutralising antibodies to SARS coronavirus 2 to determine population exposure in Scottish blood donors between March and May 2020. medRxiv [Internet]. 2020. Jan 1;2020.04.13.20060467. Available from: http://medrxiv.org/content/early/2020/07/29/2020.04.13.20060467.abstract doi: 10.2807/1560-7917.ES.2020.25.42.2000685 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Houlihan C, Vora N, Byrne T, Lewer D, Heaney J, Moore D. SARS-CoV-2 virus and antibodies in front-line Health Care Workers in an acute hospital in London: preliminary results from a longitudinal study. medRxiv. 2020; [Google Scholar]
- 120.Pallett SJC, Rayment M, Patel A, Fitzgerald-Smith SAM, Denny SJ, Charani E, et al. Point-of-care serological assays for delayed SARS-CoV-2 case identification among health-care workers in the UK: a prospective multicentre cohort study. Lancet Respir Med [Internet]. 2020. Sep;8(9):885–894. Available from: https://europepmc.org/articles/PMC7380925 doi: 10.1016/S2213-2600(20)30315-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Waterfield T, Watson C, Moore R, Ferris K, Tonry C, Watt A, et al. Seroprevalence of SARS-CoV-2 antibodies in children: a prospective multicentre cohort study. Arch Dis Child [Internet]. 2020. Nov 23 [cited 2020 Dec 21]; Available from: https://adc.bmj.com/content/early/2020/11/22/archdischild-2020-320558 [DOI] [PubMed] [Google Scholar]
- 122.Eyre DW, Lumley SF, O'Do nell D, Cam bell M, Sims E, L wsoet al. t al. Differential occupational risks to healthcare workers from SARS-CoV-2: A prospective observational study. medRxiv [Internet]. 2020. Jan 1;2020.06.24.20135038. Available from: http://medrxiv.org/content/early/2020/06/29/2020.06.24.20135038.1.abstract [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Shields A, Faustini SE, Perez-Toledo M, Jossi S, Aldera E, Allen JD, et al. SARS-CoV-2 seroprevalence and asymptomatic viral carriage in healthcare workers: a cross-sectional study. Thorax [Internet]. 2020. Aug; Available from: https://europepmc.org/articles/PMC7462045 doi: 10.1136/thoraxjnl-2020-215414 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Clarke C, Prendecki M, Dhutia A, Ali MA, Sajjad H, Shivakumar O, et al. High Prevalence of Asymptomatic COVID-19 Infection in Hemodialysis Patients Detected Using Serologic Screening. J Am Soc Nephrol [Internet]. 2020. Sep;31(9):1969–1975. Available from: doi: 10.1681/ASN.2020060827 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Wells PM, Doores KJ, Couvreur S, Nunez RM, Seow J, Graham C, et al. Estimates of the rate of infection and asymptomatic COVID-19 disease in a population sample from SE England. J Infect [Internet]. 2020. Dec 1 [cited 2020 Dec 21];81(6):931–6. Available from: https://www.journalofinfection.com/article/S0163-4453(20)30653-8/fulltext doi: 10.1016/j.jinf.2020.10.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.The Government of Jersey. SARS-CoV-2: Prevalence of antibodies in Jersey. Jersey, UK, 2020. [Internet]. 2020 [cited 2020 Dec 21]. Available from: www.gov.je/statistics
- 127.Poulikakos D, Sinha S, Kalra PA. SARS-CoV-2 antibody screening in healthcare workers in a tertiary centre in North West England. J Clin Virol [Internet]. 2020. Aug;129:104545. Available from: https://europepmc.org/articles/PMC7338856 doi: 10.1016/j.jcv.2020.104545 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Khalil A, Hill R, Wright A, Ladhani S, O’Brien P. SARS-CoV-2-specific antibody detection in healthcare workers in a UK maternity Hospital: Correlation with SARS-CoV-2 RT-PCR results. Clin Infect Dis [Internet]. 2020. Aug; Available from: https://europepmc.org/articles/PMC7454457 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Grant JJ, Wilmore SMS, McCann NS, Donnelly O, Lai RWL, Kinsella MJ, et al. Seroprevalence of SARS-CoV-2 antibodies in healthcare workers at a London NHS Trust. Infect Control Hosp Epidemiol [Internet]. 2020. Aug;1–3. Available from: https://europepmc.org/articles/PMC7438618 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Ladhani SN, Jeffery-Smith AJ, Patel M, Janarthanan R, Fok J, Crawley-Boevey E, et al. High prevalence of SARS-CoV-2 antibodies in care homes affected by COVID-19; a prospective cohort study in England. medRxiv [Internet]. 2020. Jan 1;2020.08.10.20171413. Available from: http://medrxiv.org/content/early/2020/08/12/2020.08.10.20171413.abstract [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.UK Biobank. UK Biobank SARS-CoV2 Serology Study (Weekly Report—21 July 2020) [Internet]. 2020 [cited 2020 Dec 21]. Available from: https://www.ukbiobank.ac.uk/media/s3af0k5q/ukb_serologystudy_month1_report.pdf
- 132.Public Health England. Weekly Coronavirus Disease 2019 (COVID-19) Surveillance Report London: PHE; 2020 [Internet]. 2020 [cited 2020 Dec 21]. Available from: https://assets.publishing.service.gov.uk/government/uploads/system/uploads/attachment_data/file/889981/Weekly_COVID19_Epidemiological_Summary_w23.pdf
- 133.Mulchandani R, Taylor-Phillips S, Jones H, Ades T, Borrow R, Linley E, et al. Self assessment overestimates historical COVID-19 disease relative to sensitive serological assays: cross sectional study in UK key workers. medRxiv [Internet]. 2020. Jan 1;2020.08.19.20178186. Available from: http://medrxiv.org/content/early/2020/08/22/2020.08.19.20178186.abstract [Google Scholar]
- 134.Nsn G, C J, R M, P R, N L, Sn L, et al. High rates of SARS-CoV-2 seropositivity in nursing home residents. J Infect [Internet]. 2020. Aug; Available from: https://europepmc.org/articles/PMC7449890 doi: 10.1016/j.jinf.2020.08.040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Favara D, McAdam K, Cooke A, Bordessa-Kelly A, Budriunaite I, Bossingham S, et al. SARS-CoV-2 antigen and antibody prevalence among UK staff working with cancer patients during the COVID-19 pandemic [Internet]. medRxiv; 2020. Available from: http://europepmc.org/abstract/PPR/PPR217237 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Ladhani S. Prospective active national surveillance of preschools and primary schools for SARS-CoV-2 infection and transmission in England, June 2020 (sKIDs COVID-19 surveillance in school KIDs) [Internet]. 2020. [cited 2020 Dec 21]. Available from: https://assets.publishing.service.gov.uk/government/uploads/system/uploads/attachment_data/file/914700/sKIDs_Phase1Report_01sep2020.pdf [Google Scholar]
- 137.Ward H, Atchison CJ, Whitaker M, Ainslie KEC, Elliott J, Okell LC, et al. Antibody prevalence for SARS-CoV-2 in England following first peak of the pandemic: REACT2 study in 100,000 adults. medRxiv [Internet]. 2020. Jan 1;2020.08.12.20173690. Available from: http://medrxiv.org/content/early/2020/08/21/2020.08.12.20173690.abstract [Google Scholar]
- 138.Mantelakis A, Spiers HVM, Lee CW, Chambers A, Joshi A. Availability of Personal Protective Equipment in NHS Hospitals During COVID-19: A National Survey. Ann Work Expo Heal [Internet]. [cited 2020 Dec 28]; Available from: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7499547/ doi: 10.1093/annweh/wxaa087 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Douglas JDM, McLean N, Horsley C, Higgins G, Douglas CM, Robertson E. COVID-19: smoke testing of surgical mask and respirators. Occup Med (Lond) [Internet]. 2020. [cited 2021 Jun 26];70(8):556–63. Available from: http://www.ncbi.nlm.nih.gov/pubmed/33150448 doi: 10.1093/occmed/kqaa167 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Peckham H, de Gruijter NM, Raine C, Radziszewska A, Ciurtin C, Wedderburn LR, et al. Male sex identified by global COVID-19 meta-analysis as a risk factor for death and ITU admission. Nat Commun [Internet]. 2020. Dec 9 [cited 2020 Dec 28];11(1):6317. Available from: http://www.nature.com/articles/s41467-020-19741-6 doi: 10.1038/s41467-020-19741-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Gurdasani D, Hisham Z, Reicher S, Mckee M. Covid-19 and the delta variant—we need an urgent focus on mitigations in schools— The BMJ [Internet]. 2021. [cited 2021 Jun 26]. Available from: https://blogs.bmj.com/bmj/2021/06/11/covid-19-and-the-delta-variant-we-need-an-urgent-focus-on-mitigations-in-schools/ [Google Scholar]
- 142.Loenenbach A, Markus I, Lehfeld A-S, An der Heiden M, Haas W, Kiegele M, et al. SARS-CoV-2 variant B.1.1.7 susceptibility and infectiousness of children and adults deduced from investigations of childcare centre outbreaks, Germany, 2021. Euro Surveill [Internet]. 2021. [cited 2021 Jun 26];26(21). Available from: http://www.ncbi.nlm.nih.gov/pubmed/34047274 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(XLSX)
In some cases, the manufacture’s specificity and sensitivity were unable to be found, so evaluation study data was used instead.
(PDF)
(TIFF)
Data Availability Statement
All relevant data are within the manuscript and its Supporting Information files.