Summary
Background
While the world is experiencing another wave of COVID-19 pandemic, global vaccination program is hampered by an evident shortage in the supply of licensed vaccines. In an effort to satisfy vaccine demands we developed a new single-dose vaccine based on recombinant adenovirus type 26 (rAd26) vector carrying the gene for severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) spike (S) glycoprotein – “Sputnik Light”.
Methods
We conducted an open label, prospective, non-randomised phase 1/2 trial aimed to assess safety, tolerability, and immunogenicity of “Sputnik Light” vaccine in a single center in Russia. Primary outcome measures were antigen-specific humoral immunity (Anti-RBD-SARS-CoV-2 antibodies measured by ELISA on days 1, 10, 28, and 42) and safety (number of participants with adverse events monitored throughout the study). Secondary outcome measures were antigen-specific cellular immunity (measured by antigen-dependent CD4+ and CD8+ T-cell proliferation, number of antigen-specific interferon-γ-producing cells as well as interferon-γ concentration upon antigen restimulation) and change in neutralizing antibodies (measured in SARS-CoV-2 neutralization assay).
Findings
Most of the solicited adverse reactions were mild (66·4% from all vaccinees), few were moderate (5·5%). No serious adverse events were detected. Assessment of Anti-RBD-SARS-CoV-2 antibodies revealed a group with pre-existing immunity to SARS-CoV-2. Upon this finding we separated all safety and immunogenicity data based on pre-existing immunity to SARS-CoV-2. There were notable differences in the vaccine effects on immunogenicity by the groups. Vaccination of seropositive (N=14) volunteers rapidly boosted RBD-specific IgGs from reciprocal geometric mean titer (GMT) 594·4 at a baseline up to 26899 comparing to 29·09 in seronegative group (N=96) by day 10. By day 42 seroconversion rate reached 100% (93/93) in seronegative group with GMT 1648. At the same time, in the seropositive group, seroconversion rate by day 42 was 92·9% (13/14) with GMT 19986. Analysis of neutralizing antibodies to SARS-CoV-2 showed 81·7% (76/93) and 92·9% (13/14) seroconversion rates by day 42 with median reciprocal GMT 15·18 and 579·7 in the seronegative and seropositive groups, respectively. Antigen-specific T cell proliferation, formation of IFNy-producing cells, and IFNy secretion were observed in 96·7% (26/27), 96% (24/25), and 96% (24/25) of the seronegative group respectively and in 100% (3/3), 100% (5/5), and 100% (5/5) of the seropositive vaccinees, respectively.
Interpretation
The single-dose rAd26 vector-based COVID-19 vaccine “Sputnik Light” has a good safety profile and induces a strong humoral and cellular immune responses both in seronegative and seropositive participants.
Funding
Russian Direct Investment Fund.
Research in context.
Evidence before this study
Despite the tremendous progress in vaccine development and manufacturing, the existing demand for vaccines against COVID-19 calls for new effective vaccines that are easy to manufacture, distribute, and administer in order to accelerate global control of the ongoing pandemic. In order to develop a highly immunogenic vaccine with a good safety profile that is both cost-effective and has efficient manufacturing, logistics and administration; we focused on development of a single-dose non-replicating adenoviral vaccine.
We searched ClinicalTrials.gov and PubMed up to June 23, 2021, using different combinations of the terms “COVID-19” OR “SARS-CoV-2” AND “vaccine” AND “clinical trial” AND “single-dose” AND “non-replicating virus”, with no date or language restrictions. We identified published clinical trial data on single-dose COVID-19 vaccine candidates based on two non-replicating human recombinant adenoviruses type 5 (Ad5) and type 26 (Ad26) both used at dose of 1 × 10¹¹ viral particles, developed by CanSino Biologics and Janssen Pharmaceuticals, correspondingly. Additionally, ChAdOx1 nCoV-19 vaccine produced by Oxford–AstraZeneca based on non-replicating chimpanzee adenovirus (ChAd) was studied in single-dose regimen (5 × 10¹0 viral particles). Shown to be safe and immunogenic all vaccines have been already approved in a number of countries.
The composition of “Sputnik Light” vaccine is equal to the first component of two-dose “Sputnik V” vaccine (Gam-Covid-Vac), for which safety and immunogenicity were assessed in number of completed and ongoing clinical trials. In the Phase 1 study of Sputnik V vaccination including 9 participants with one dose of rAd26-S at 1 × 10¹¹ vp dose resulted in 66·7% seroconversion rate on day 28. No serious adverse events were registered.
A separate phase 1/2 clinical trial of a single-dose rAd26-based vaccine “Sputnik Light” was organized to accumulate convincing data regarding its safety and immunogenic properties.
Added value of this study
Owing to easier manufacturing (compared to heterologous prime-boost “Sputnik-V” vaccine) and its single-dose regimen, we believe “Sputnik Light” vaccine could contribute towards accelerating the pace of vaccination in Russia as well as in other countries that are lacking sufficient vaccine supply.
Here we report preliminary results (up to day 42 post-vaccination) of safety, reactogenicity, and immunogenicity of “Sputnik Light” vaccine in 110 healthy volunteers aged 18–59 years. The results show that the vaccine was well tolerated and produced both humoral and cellular immune responses in both seronegative and seropositive healthy adults. Single immunization of naïve volunteers is sufficient for rapid induction of immune responses against SARS-CoV-2 (100% seroconversion rate reached by day 42). Interestingly, “Sputnik Light” swiftly induced (by day 10) a more prominent immune response in the seropositive group of volunteers compared to the seronegative as well as the convalescents.
No correlations of antigen-specific IgG or neutralizing antibodies with age nor with pre-existing neutralizing antibodies to Ad26 have been registered.
Implications of all the available evidence
Single-shot vaccines against SARS-CoV-2 with simple and easy administration will compensate for the lack of vaccine supply, allowing to reach population immunity in a shorter time frame, therefore contributing to prevention of further waves of COVID-19 worldwide.
Our findings indicate that “Sputnik Light” vaccine is safe and immunogenic in both seronegative and seropositive healthy adults. Thus “Sputnik Light” might be considered not only for primary vaccination, but also could be useful as an efficient tool for further revaccination or vaccination after previous COVID-19 infection. This data encouraged us to initiate an international multicenter, randomised, double-blind, placebo-controlled phase 3 study to further evaluate the efficacy of the vaccine (NCT04741061).
Alt-text: Unlabelled box
Introduction
Ever since the COVID-19 pandemic was declared on March 11, 2020 more than 179 million cases and 3·8 million disease-associated deaths have been reported worldwide and these numbers are still continuously growing.1 Sudden emergence of SARS-CoV-2 virus and its rapid spread with high morbidity and mortality called for extraordinary efforts in the field of vaccine development that led to the registration of several vaccines licensed for emergency use by the end of 2020.2 Currently, we have encouraging examples, which prove that rapid rollout of mass vaccines is the most efficient way to prevent the spread of SARS-CoV-2 and halt economic damages and increasing deaths. Having vaccinated approximately 60% of their entire population with at least with one dose of the vaccine, and by May 26, 2021; Israel minimized the incidence of newly detected COVID-19 cases significantly (22 cases in almost 9 million population).3 However, despite such local successes, the global vaccination program is still at its initial stage and is already experiencing problems. To date only about 23.7% of world population received at least one dose of COVID-19 vaccine, thereby exhibiting an enormous discrepancy among different regions4 While the high-income countries have reasonable chances to complete vaccination by the end of 2021, the low-income countries that received just 1% of all vaccinations remain defenseless against the incoming new waves of COVID-19.5
There are discernible limitations to scaling up the vaccine manufacturing such as complicated manufacturing process, fragile supply chains, and storage facilities requiring ultra-low temperatures. To add to these limitations, the two-dose regimen of many licensed vaccines makes it additionally difficult to ensure full vaccine compliance and also increases the cost relative to single-dose vaccines.
In order to increase the rate of global vaccinations to fight the SARS-CoV-2 infection, a new COVID-19 vaccine has been developed. Here, we present interim results of a phase 1/2 clinical trial regarding the safety, tolerability, and immunogenicity of the new “Sputnik Light” vaccine that is based on a human adenoviral vector containing full length SARS-CoV-2 spike insert. We believe that a single-dose COVID-19 vaccine will broaden the portfolio of licensed COVID-19 vaccines thereby contributing to the vaccine supply needed to reach population immunity at a national and global level.
Methods
Study design and participants
We conducted an open label, prospective, non-randomised phase 1/2 trial to evaluate safety, reactogenicity, and immunogenicity of Ad26-vectored COVID-19 candidate vaccine (“Sputnik Light”) at a single clinical site “Eco-Safety” Medical Center located in Saint-Petersburg, Russia. Written informed consent was obtained from each participant before screening. Screening procedure included physical examination wherein the medical history, demographic, and anthropometric data were collected; and the relevant vital functions were assessed (e.g., blood pressure, heart rate, and axillar temperature). Volunteers were assessed by their risk category of contracting the COVID-19 infection (high, medium, and general) and underwent laboratory testing: complete blood count and biochemistry, testing for infections (HIV, hepatitis, and syphilis), COVID-19 diagnostics (PCR, qualitative IgM / IgG ELISA) and urine testing for drugs, alcohol, and pregnancy (in women). Volunteers were considered eligible if they were deemed healthy after screening procedures, had no history of COVID-19 or prior contact with patients with COVID-19 within 14 days of participation in the study, did not receive any other vaccinations within 30 days, had not undergone therapy with steroids, immunoglobulins, or any other blood-derived products within 30 days, had not consumed any immunosuppressive drugs for more than 3 months and had no allergy to immunobiological preparations including any vaccine component. A complete list of inclusion and exclusion criteria is available in the protocol. First 110 eligible volunteers were enrolled in the study (appendix p1).
The trial was approved by the local ethics committee and was conducted with the approval of the Ministry of Health of Russian Federation in compliance with International Conference on Harmonization and National Good Clinical Practice guidelines and Declaration of Helsinki. The trial is registered at ClinicalTrials.gov, NCT04713488.
Procedures
“Sputnik Light” vaccine candidate was developed and manufactured by N. F. Gamaleya National Research Centre for Epidemiology and Microbiology (Moscow, Russia) according to Good Manufacturing Practice. The vaccine candidate comprises recombinant adenovirus type 26 (rAd26) carrying full-length glycoprotein S gene of SARS-CoV-2 (rAd26-S). The vaccine was manufactured as a liquid formulation containing 10¹¹ vp per 0·5 mL/dose. In the combined Phase 1 and 2 clinical protocol all 110 participants received full dose administered intramuscularly in a single-dose schedule and were assessed for safety, reactogenicity (reported here up to day 28) and immunogenicity (reported here up to day 42) over the whole period of investigation (180 days). No randomization or special stratification was carried out in this study. Screening procedure began from the moment when informed consent was given and lasted no more than 7 days before a participant was determined as eligible and included in the study. Participants underwent physical examination at screening, on vaccination day (day 1) and on days 10, 28, and 42. Blood (complete blood count, erythrocyte sedimentation rate, alanine aminotransferase, aspartate aminotransferase, protein, bilirubin, total cholesterol, lactate dehydrogenase, alkaline phosphatase, prothrombin index, glucose, urea, and creatinine) and urine (pH, clarity, protein, glucose, ketones, cellular content, crystals) laboratory analyses were performed at the screening and on day 28. General immune parameters (absolute and relative numbers of CD3, CD4, CD8, CD16, CD19, CD4/8 cells, total IgM, IgG, IgA, IgE) were analyzed at screening and on day 28. PCR COVID-19 test was done at screening and on day 1, 10, and 28. On vaccination day volunteers were given subject diaries for self-reporting of adverse events which were checked at each visit (day 10, 28, 42). COVID-19-specific immune reactions were assessed using methods described in the appendix (pp 2–3). In brief, anti-S/N IgM and anti-S IgG antibodies were assessed during screening period at local laboratory of investigative site by semi-quantitative ELISA kits (D-5502 and D-5501, Vector-Best, Russia) to assess pre-existing immunity of volunteers. Post-vaccination humoral immune response was examined by measuring anti-RBD antibodies and neutralizing antibodies at a central laboratory. For measuring titers of RBD-specific antibodies on day 1,10, 28, and 42; a quantitative ELISA kit was developed and manufactured at N. F. Gamaleya National Research Centre for Epidemiology and Microbiology (registration for clinical use in Russia: P3H 2020/10393 2020-05-18). Neutralizing antibody responses were detected before vaccination on day 1 (baseline) and on day 28 and 42 with microneutralization assay using SARS-CoV-2 (hCoV-19/Russia/ Moscow_PMVL-1/2020) virus. Post-vaccination humoral immune response (Anti-RBD-SARS-CoV-2 IgG as well as virus neutralizing antibodies) was compared to the response that occurred naturally after infection with SARS-CoV-2. 56 plasma samples from convalescent donors were obtained from N.V. Sklifosovsky Research Institute for Emergency Medicine of the Moscow Healthcare Department.
Cell-mediated immune response was studied before vaccination on day 1 (baseline) and on day 10 by detecting of antigen-specific proliferating CD4+ and CD8+ T cells by flow cytometry; and by quantification of interferon-γ release of peripheral blood mononuclear cells (PBMCs) upon antigen restimulation using ELISA and ELISpot methods.
Outcomes
Primary outcome measures were safety, reactogenicity, and immunogenicity of the “Sputnik Light” vaccine candidate. The primary outcome measures for safety were the number and features of adverse events during the whole study. The primary outcome measure for immunogenicity was the change from baseline in antigen-specific antibody levels on days 10, 28, 42, 90, and 180 measured by ELISA. Secondary immunogenicity outcome measures were changes from baseline in virus neutralizing antibody titers (on days 28 and 42) and determination of antigen-specific cellular immunity (antigen-specific proliferation of CD4+ and CD8+ T-cells, quantity of interferon-γ producing cells measured by ELISPOT as well as production of interferon-γ by PBMCs measured by ELISA) on day 10.
Statistical analysis
All statistical calculations were done in GraphPad Prism 8 (GraphPad Software, USA). Normality of the data distribution was assessed with the d'Agostino-Pearson test. We used the paired Wilcoxon test to compare antibody titers, % of proliferating CD4 and CD8 cells as well as the increase in concentrations of interferon-γ within the same group of volunteers at different timepoints (e.g., before and after vaccination). We used the Mann–Whitney U test to compare results obtained in different groups (e.g., seronegative/seropositive, vaccinees/convalescents). To ensure the statistical significance of the ELISPOT assay, two-way ANOVA with Tukey's multiple comparisons post-hoc test was performed to compare the spot forming cell number between unstimulated and antigen-stimulated samples obtained before and after immunization. Correlation analysis was done with Spearman's test; the correlation coefficient r shows interactions between two datasets and takes values either from 0 to 1 (in the case of a positive correlation) or from -1 to 0 (in the case of a negative correlation).
Role of the funding source
The funder of the study had no role in study design, data collection, data analysis, data interpretation, or writing of the report. All authors had full access to data in the study and had final responsibility for the decision to submit for publication.
Results
Between January 14 and 27, 2021 150 participants were screened, and 110 were enrolled. Demographic characteristics of the enrolled participants are presented in Table 1.
Table 1.
Baseline characteristics.
| Sex | |
| Male | 55 (50·0%) |
| Female | 55 (50·0%) |
| Height, m | 1.7 (0·10) |
| Bodyweight, kg | 71.4 (14·86) |
| Body-mass index*, kg/m² | 23.9 (3·81) |
| Age, | |
| Mean (SD) | 35.4 (14·84) |
| ≥60 y.o. | 16 (14·5%) |
| Ethnicity † | |
| White | 109 (99·1%) |
| Asian | 1 (0·9%) |
| Concomitant diseases (diabetes, hypertension, ischaemic heart disease, obesity) | |
| No | 102 (92·7%) |
| Yes | 8 (7·3%) |
| Risk of infection in volunteers# | |
| Medium | 7 (6·4%) |
| General | 103 (93·6%) |
Data are n (%) or mean (SD).
Calculation was based on the bodyweight and height measured at the time of screening.
Race or ethnic group was reported by the participants.
Medium risk is those who have professional contact with a large number of people, such as general practitioners, social workers, and shop assistants; and general risk denotes those with no additional risks associated with their professional activities.
Of the 40 initially screened individuals who were excluded, 24 were positive for SARS-CoV-2 by nucleic acid test or serology, 1 had abnormal laboratory values, 1 was withdrawn by physician, 8 voluntarily withdrew their consent to participate. Owing to competitive recruitment, 6 individuals who were screened and found to be eligible were not enrolled (Fig. 1).
Figure 1.
Trial profile. *Due to competitive recruitment, all sites were screening participants individually; therefore, there was an excess of eligible participants who were not enrolled because the recruitment target was met. # Two participants quit the trial between day 28 and day 42 on personal request. † One participant has missed visit on day 42, but was not excluded from the study.
Two participants that received the vaccination, have completed their visit and screening on day 28, but had to withdraw consent owing to personal reasons before their visit on day 42. One participant missed the visit on day 42 but continued to participate in the study.
The safety analyses contained registration of solicited local and systemic adverse reactions within the first 28 days after an injection as well as changes in safety-related laboratory parameters. In general, adverse events were in line with the ones registered in clinical trials of the same type of vaccines based on recombinant viral vectors (e.g., “Sputnik V”).6
Overall incidence of solicited adverse reactions was 74 (67·2%) of 110 participants (Table 2). Some volunteers had several adverse events of different degrees of severity. Most of the registered, solicited systemic and local adverse reactions were mild (73 [66·4%]). Only 6 participants (5·5%) had adverse events of moderate grade in severity. No serious adverse events were reported.
Table 2.
Systemic and local solicited adverse events within 28 days after single vaccine dose (110 participants).
| Total | Grade 1 | Grade 2 | |
|---|---|---|---|
| Any symptom | 74 (67·2) | 73 (66·4) | 6 (5·5) |
| Any injection-site symptoms | 7 (6·4) | 6 (5·5) | 1 (0·9) |
| Pain in injection site | 6 (5·5) | 5 (4·5) | 1 (0·9) |
| Redness | 1 (0·9) | 1 (0·9) | 0 |
| Any systemic symptoms | 72 (65·5) | 71 (64·5) | 5 (4·5) |
| Flu-like syndrome | 54 (49·1) | 51 (46·4) | 3 (2·7) |
| Fatigue | 6 (5·5) | 6 (5·5) | 0 |
| Headache | 5 (4·5) | 5 (4·5) | 0 |
| Muscle and joint pain | 5 (4·5) | 5 (4·5) | 0 |
| Hyperthermia | 5 (4·5) | 3 (2·7) | 2 (1·8) |
| Chills | 5 (4·5) | 5 (4·5) | 0 |
| Decreased appetite | 4 (3·6) | 4 (3·6) | 0 |
| Rash | 3 (2·7) | 3 (2·7) | 0 |
| Hidrosis | 3 (2·7) | 3 (2·7) | 0 |
| Dizziness | 2 (1·8) | 2 (1·8) | 0 |
| Diarrhoea | 1 (0·9) | 1 (0·9) | 0 |
| Abdominal pain | 1 (0·9) | 1 (0·9) | 0 |
| Raised blood pressure | 1 (0·9) | 1 (0·9) | 0 |
| Lymphadenopathy | 1 (0·9) | 1 (0·9) | 0 |
| Insomnia | 1 (0·9) | 1 (0·9) | 0 |
| Drowsiness | 1 (0·9) | 1 (0·9) | 0 |
| Nasal congestion | 1 (0·9) | 1 (0·9) | 0 |
| Hypaesthesia | 1 (0·9) | 1 (0·9) | 0 |
The table shows the total number (%) of volunteers who developed solicited adverse events, based on the severity: mild [grade 1], moderate [grade 2], no serious [grade 3] adverse events were reported. Some volunteers had several adverse events of different degrees of severity.
Local solicited reactions were expressed only by pain at injection site (7 volunteers out of 110 [6·4%]) and redness (1 [0·9%]). The most frequent solicited systemic reaction was flu-like syndrome defined as a complex of more than one symptom including fever, chills, headache, muscle or body aches, cough, sore throat, runny nose, fatigue, nausea, vomiting, or diarrhea (72 [65·5%]). Other frequent (>1%) solicited systemic adverse events included fatigue (6 [5·5%]), headache (5 [4·5%]), muscle and joint pain (5 [4·5%]), hyperthermia (5 [4·5%]), chills (5 [4·5%]), decreased appetite (4 [3·6%]), rash (3 [2·7%]), hidrosis (3 [2·7%]) and dizziness (2 [1·8%]).
Laboratory analysis showed that 11 participants (10%) had mild and transient changes in erythrocyte sedimentation rate (4 [3·5%]), changes in concentration of alanine aminotransferase (2 [1·8%]), aspartate aminotransferase (3 [2·7%]), lactate dehydrogenase (2 [1·8%]), leucocyte count (one increased [0·8%]), lymphocyte count (one increased [0·9%] and one decreased [0·9%]), neutrophil count (one increased [0·9%] and two decreased [1·8%]), increase in total IgE concentration (1 [0·9%]). Also, one participant had proteinuria (0·9%) and one leukocyturia (0·9%) on day 28.
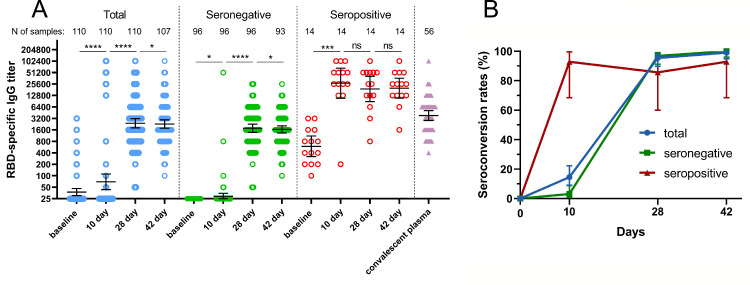
Sera for detection of SARS-CoV-2 RBD-specific antibodies were collected before immunization (on day 1, in addition to conventional SARS-CoV-2 IgM / IgG test that was done at local laboratory during screening) and on days 10, 28, and 42. Analysis was performed at once after receiving samples from the day 42 timepoint. While the protocol criteria excluded subjects with history of COVID-19 by way of negative semi-quantitative SARS-CoV-2 IgM / IgG ELISA test at screening, another quantitative ELISA assay done at vaccine immunogenicity assessment revealed a group of seropositive subjects (N=14) with baseline geometric mean titer (GMT) of 594·4 SARS-CoV-2 RBD-specific antibodies indicating previous COVID-19 infection. Understanding that pre-existing immunity to SARS-CoV-2 has an impact on all safety and immunogenicity data, we present all results including RBD-specific IgG response in all participants vaccinated with “Sputnik Light” as well as separated by pre-existing anti-RBD-SARS-CoV-2 IgG antibody titers at baseline (Fig. 2).
Figure 2.
RBD-specific IgG response in participants vaccinated with “Sputnik Light”. (a) RBD-specific IgG reciprocal titers before vaccination (baseline, day 1) and on days 10, 28, and 42, as measured by ELISA, in all vaccinated participants as well as separated by pre-existing anti-RBD-SARS-CoV-2 IgG antibody titers. Dots show individual data points. Horizontal lines represent geometric mean titers, whiskers are 95% CIs. N shows number of participants in each stratum. Significant differences between different timepoints are indicated by asterisks and lines: * for p<0·05, *** for p<0·001, or **** for p<0·0001. NS- indicates not significant difference. (b) Seroconversion rates (percentage) of participants in different timepoints. Seroconversion was defined as at least a four-fold increase in post-vaccination titer from baseline. Whiskers are 95% CIs calculated by Wilson/Brown method.
Vaccination of all 110 participants with “Sputnik-Light” resulted in increased RBD-specific IgGs as early as on day 10 (GMT 69·39), reaching maximal levels on day 28 (GMT 2395) and day 42 (GMT=2285). Kinetics of RBD-specific IgG titers in seronegative and seropositive groups were different. Seronegative participants showed a minimal (but significant) increase in the RBD-specific titer on day 10 (GMT=29, p<0·05) reaching the plateau between day 28 (GMT=1770) and day 42 (GMT=1648). Having GMT of RBD-specific IgGs 594 at the baseline seropositive participants swiftly responded to vaccination showing great numbers on day 10 (GMT 26899) with non-significant decrease on day 28 (GMT= 19021) and day 42 (GMT=19986). In all time points geometric mean antibody titers of participants with preexisting immunity were significantly higher than those without preexisting immunity. Differences in IgG response kinetics were also reflected in different seroconversion curves (Fig. 2 b). While the total seroconversion rate was 14·55% for all vaccinated participants (16 participants from 110) on day 10 only 3·13% (3/96) seroconverted in seronegative group compared to 92·86% (13/14) in seropositive group at the same time point. On day 42 seroconversion in seronegative group reached 100% (93/93), compared to 92·86% (13/14) in seropositive group, averaging out at 99·07% [106/107]) in all vaccinated participants. Comparison of antibody responses to SARS-CoV-2 after immunization with titers in convalescent plasma from 56 individuals (GMT 3805) showed that post-vaccination ELISA titers in seronegative group were lower than titers after COVID-19 at all time points. At the same time vaccination of seropositive participants with single dose of “Sputnik-Light” resulted in considerably higher titers in all time points comparing to convalescents. Descriptive statistics for RBD-specific IgG titers are presented in the appendix (p 4). Comparing correlation between RBD-specific IgG response and age of vaccinees we have detected no significant correlation between these parameters (appendix p5).
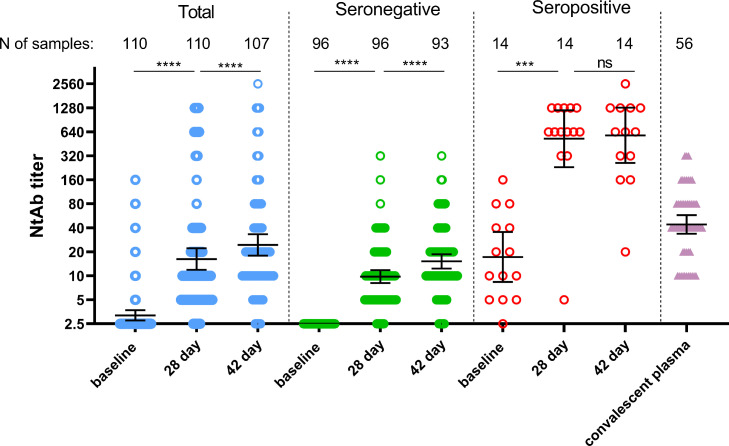
Analysis of neutralizing antibodies to SARS-CoV-2 in the whole group of participants showed 62·7% (69/110) and 83·2% (89/107) seroconversion by day 28 and 42, correspondingly (Fig. 3).
Figure 3.
Neutralizing antibody response in participants vaccinated with “Sputnik Light”. Neutralizing antibodies before immunization (day 1) and on days 28, and 42, as measured by microneutralization assay with 100 TCID50, in all participants vaccinated with “Sputnik Light” as well as separated by pre-existing anti-RBD-SARS-Cov2 IgG antibody titers. Dots represent individual data points. Horizontal lines represent geometric mean titers, whiskers are 95% CIs. N represents number of participants in each group. Significant differences between different timepoints are indicated by asterisks and lines: *** for p<0·001, or **** for p<0·0001. NS, not significant. TCID50=50% tissue culture infective dose.
Among all 110 participants, we identified 13 with elevated titers of neutralizing antibodies to SARS-CoV-2 before vaccination (all correspond to seropositive group according to pre-existing RBD-specific IgGs). Seroconversion in seronegative vaccine recipients were 58·3% (56/96) on day 28 and increased up to 81·7% (76/93) by day 42. In seropositive group seroconversion reached 92·8% (13/14) by day 28 and remained the same on day 42. Geometric mean titers of neutralizing antibodies were continuously growing after immunization reaching 16·25 by day 28 (p<0·0001 compared to baseline) and 24·45 by day 42 (p<0·0001 compared to day 28) in the whole group. In seronegative group neutralizing antibody GMT were also elevating during time: 9·79 (p<0·0001 comparing to baseline) on day 28 and 15·18 (p<0·0001 compared to day 28) on day 42, correspondingly. Vaccination with “Sputnik Light” significantly boosted neutralizing antibody GMT in seropositive vaccinees from 17·24 (before immunization) up to 525·0 (p<0·0001 compared to baseline) by day 28. However, on day 42 there were no further statistically significant elevation in neutralizing antibody titers (GMT 579·7) in this group (p=0·58). Comparing to convalescent plasma (GMT 44·16) single-dose vaccination results in lower neutralizing antibody response at its maximum (day 42) in seronegative group (p<0·0001). However, in seropositive group post-vaccination neutralizing antibody titers were significantly higher on both day 28 (p<0·0001) and day 42 (p<0·0001) than titers after COVID-19 disease. Descriptive statistics for neutralizing antibody titers are presented in the appendix (p6). At evaluation of correlation between neutralizing antibody response and age of vaccinees we have detected no significant correlation between these parameters (appendix p7, table S4). We also analyzed correlation between SARS-CoV-2 RBD ELISA titers and neutralizing antibody titers. In line of previous studies, we noted strong correlation between these variables at different time points (appendix p7, table S5).7
Lastly, with the growing threat of newly emerging lineages of SARS-CoV-2, we studied virus-neutralizing activity of sera from volunteers vaccinated with “Sputnik Light” against two internationally relevant variants of concern (VOC): alpha (B.1.1.7) and beta (B.1.351) with an aim to assess potential vaccine cross-protection, comparing responses to those from the original genetic lineage variant (B.1.1.1) (appendix p8).
Using serum samples obtained on day 28 from seronegative vaccinees (N=96), we detected a slight but statistically significant 1·11- (p<0·05) and 1·99-fold decrease (p<0·0001) in the virus neutralization titers against B.1.1.7 and B.1.1.351 respectively, compared to virus neutralization against the original B.1.1.1 strain. Referring to recently published data using sera obtained from people vaccinated with “Sputnik V” we expect that vaccination with “Sputnik Light” still will be able neutralize B.1.617 VOC but apparently in reduced titer.8
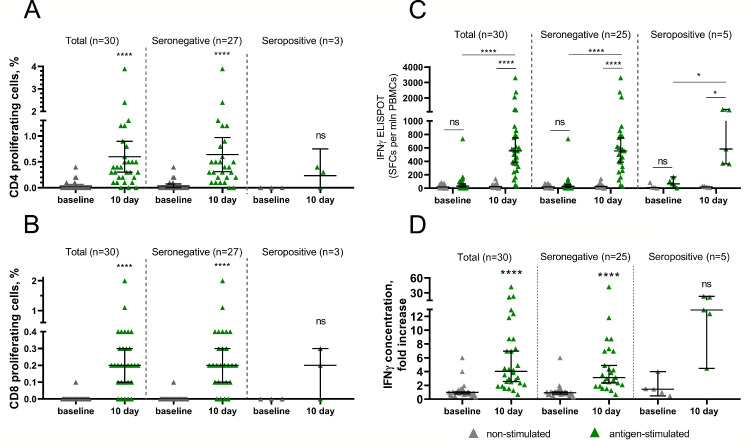
Cellular immune response in vaccinees was evaluated using three independent methods: (i) by T-helper (CD4+) and T-killer (CD8+) proliferative response to antigen-restimulation in vitro, (ii) by changes in IFNγ-producing antigen-specific cells measured by ELISPOT as well as (iii) by changes in interferon-γ secretion in peripheral blood mononuclear cells measured by ELISA. For each method PBMCs from 30 different volunteers were collected before (day 1) and on day 10 after immunization. Seronegative and seropositive volunteers were segregated from total group in each analysis. Further we describe data from seronegative volunteers to present immunogenic properties of “Sputnik Light” used in naïve population. CD4+ or CD8+ proliferative responses to glycoprotein S restimulation were detected in 26 of 27 (96·3%) volunteers on day 10 with medians of 0·40% CD4+ and 0·2% CD8+ cells (Fig. 4A,B).
Figure 4.
Cell-mediated immune response to SARS-CoV-2 glycoprotein in participants vaccinated with “Sputnik Light”. Antigen-specific proliferation of CD4+ (a) and CD8+ (b) T cells and increase in IFNγ-producing antigen-specific cells measured by ELISPOT (c) or interferon-γ secretion in peripheral blood mononuclear cells measured by ELISA (d) in all participants vaccinated with “Sputnik Light” as well as separated by pre-existing anti-RBD-SARS-Cov2 IgG antibody titers. Dots represent individual data points. Horizontal lines represent geometric mean titers, whiskers are 95% CIs. N represents number of participants in each stratum. Significant differences between different timepoints are indicated by asterisks and lines: * for p<0·05, or **** for p<0·0001. NS, not significant.
Formation of antigen-specific PBMCs producing IFN-gamma was detected in 24 from 25 seronegative volunteers (96%) with median 554·3 spots per 1million (mln) of PBMCs upon antigen restimulation (compared to 25·71 spots in non-stimulated cells) by day 10 after immunization (Fig. 4C).
Interferon-γ secretion of PBMCs is reported as fold increase in secretion upon exposure to glycoprotein S of SARS-CoV-2 (Fig. 4D). Antigen-specific interferon-γ secretion was found in 24 from 25 seronegative volunteers (96%) with median 3·122-fold increase over non-stimulated cells by day 10 after immunization. Thus, administration of “Sputnik Light” led to formation of cell-mediated response in 96% of seronegative volunteers, which was shown by each assay. Owing to low numbers of seropositive participants assessed in each test we have not obtained reliable statistical differences in data within this group. However, each test showed that all seropositive participants formed cell-mediated immunity upon single-dose vaccination.
The number of participants with cell-mediated responses to antigen, as well as descriptive statistics are shown in the appendix (pp 9-13). Individual representative ELISPOT data of participants are presented in appendix (pp 14-17).
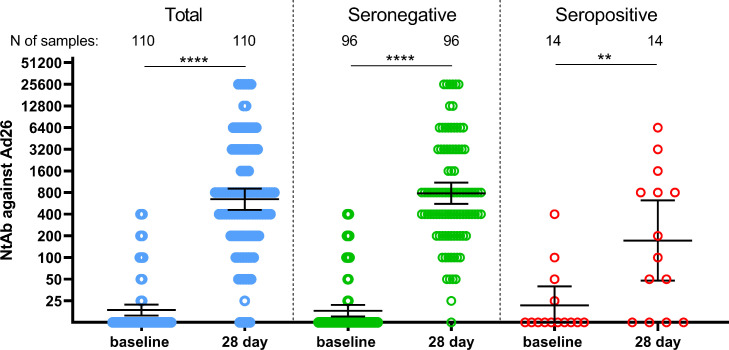
Finally, we have assessed anti-Ad26 humoral response in volunteers before vaccination with “Sputnik Light” and on the day 28. Upon vaccination Ad26 neutralizing GMT were increased from 18·2 up to 782·9 by day 28 (Fig. 5).
Figure 5.
Neutralizing antibody response to rAd26 vector in participants vaccinated with “Sputnik Light”. Neutralizing antibodies to rAd26 vector before immunization (day 1) and on day 28 as measured by microneutralization test using recombinant Ad26-EGFP, in all participants vaccinated with “Sputnik Light” as well as separated by pre-existing anti-RBD-SARS-Cov2 IgG antibody titers. Dots represent individual data points. Horizontal lines represent geometric mean titers, whiskers are 95% CIs. N represents number of participants in each stratum. Significant differences between different timepoints are indicated by asterisks and lines: ** for p<0·05, or **** for p<0·0001.
Descriptive statistics for neutralizing antibodies against rAd26 vector are presented in the appendix (p 18).
We noticed that some volunteers had pre-existing immunity to Ad26 (N=20) which was not correlated with pre-existing immunity to SARS-CoV-2 (appendix p19, table S13). We also estimated whether pre-existing neutralizing antibodies to Ad26 vector could hamper the formation of a humoral response to the vaccine antigen. No significant correlation was noted between the titer of neutralizing antibodies to Ad26 viral vector on day 1 and titers of RBD-specific IgGs in serum samples of participants on days 10, 28, and 42 (appendix p19, table S13). There was an interesting observation of a weak correlation between titers of neutralizing antibodies to Ad26 and age of volunteers (appendix p 19, table S14), indicating that older people have higher chances to catch natural Ad26 infection; which does not affect anti-SARS-CoV-2 immunity upon “Sputnik Light” vaccination.
Discussion
COVID-19 vaccination has been proved to be the most effective measure to bring an end the pandemic. COVID-19 vaccines have been shown to reduce the severity of disease, lower the mortality rate, and also cause a decrease in coronavirus transmission.9 In this light, the price that humanity will eventually pay to end the pandemic mainly depends on the pace of global vaccination campaign. Contributing to global vaccine roll-out we developed new single-dose “Sputnik Light” vaccine based on non-replicating recombinant adenovirus type 26 (Ad26). Here, we report interim results of safety, tolerability, and immunogenicity from an open label, non-randomised phase 1/2 clinical trial. Upon assessment of anti-RBD IgG antibodies in 110 enrolled vaccinees we identified a group of participants with pre-existing immunity to SARS-CoV-2 (N=14). This group enabled us to estimate effects of “Sputnik Light” vaccine as a revaccination regimen. Taking into account that the initial COVID-19 cases were detected more than a year and a half ago (Dec 31, 2019) and that the first set of vaccinated volunteers are approaching the end of the estimated period of vaccine protection (e.g., first vaccination using “Sputnik V” started Sept 07, 2020) revaccination in all likelihood will take place in the near future.10,11 Thus, evaluation of safety and immunogenicity parameters COVID-19 vaccines in pre-immunized groups is of great importance.
In terms of safety outcomes, we found that “Sputnik Light” vaccine was well tolerated both in seronegative and seropositive groups (appendix p20). The most common solicited systemic adverse effect was flu-like syndrome equally found in seronegative (47/96 [49·0%]) and seropositive (7/14 [50·0%]) groups. Interestingly that only participants without immunity to SARS-CoV-2 complained of muscle and joint pain after vaccination (5/96 [5·2%]). Naïve to SARS-CoV-2 participants also showed changes in laboratory variables (10/96 [11·4%]), whereas there were none in seropositive group (0/14 [0%]) (appendix p21). These data show that revaccination may cause milder adverse effects compared to first immunization against COVID-19. It is important to note that most observed solicited adverse effects were mild (73/110 [66·4%]), only 6 participants out of 110 (5·5%) had moderate grade adverse effects. All observed effects were transient. No serious adverse events were reported during the study.
“Sputnik Light” showed to be immunogenic, inducing both binding and neutralizing antibody responses in 100% (93/93) and 81·7% (76/93) of seronegative participants by day 42, respectively. We also found that a single-dose of “Sputnik Light” vaccine formed a faster humoral immune response in seropositive participants, which was at the same time up to 12 times higher in anti-RBD-SARS-CoV-2 IgG antibody titers and up to 54 times higher in neutralizing antibody titers than those of vaccinees without preexisting immunity at the same time points. Compared to convalescents, we showed that immunization with “Sputnik Light” resulted in 2·9-fold lower GMT of SARS-CoV-2 neutralizing antibodies in seronegative group whereas a single dose of vaccine in seropositive group boosted GMT up to 13·1-fold increase over the level observed in convalescents.
It has been recently proven that level of neutralization antibodies is a highly predictive indicator of immune protection against COVID-19.12 Having calculated mean neutralization level (fold of convalescent) in seronegative group (0·34) we derived 63·0% [95% CI = 54·8–71·2%] efficacy upon vaccination with “Sputnik Light” in naïve volunteers. High neutralization titers in seropositive group after injection of “Sputnik Light” (that de facto replicate revaccination regimen) implies neutralization level (13·72) out of the curve values range resulting in theory unprecedented 98·4% initial protection efficacy.13
These data indicate that “Sputnik Light” is a good candidate for the boosting vaccine (after initial vaccination or natural infection) against COVID-19 used in the revaccination regimen. This conclusion is also supported by previously published data showing that second dose of either BNT162b2 [Pfizer] or Sputnik-V vaccine does not result in further increase in antibody titers in seropositive participants and thus only single dose of vectored vaccine is needed for efficient revaccination.14,15
In the context of other single-dose virus vector-based analogs developed by Janssen Pharmaceuticals (99%),16 Oxford–AstraZeneca (100%)17 and CanSino Biologics (97%)18 “Sputnik Light” vaccine showed 100% IgG seroconversion for seronegatives 28-29 days after vaccination. Considering safety interim results, “Sputnik Light” vaccine resulted in lowest percentage of total solicited adverse reactions (67·2%) comparing to Oxford–AstraZeneca (94·6%),19 Janssen Pharmaceuticals (89·9%)16 and CanSino Biologics (72·7%)18 products which make it a promising candidate for vaccine susceptible or hesitant individuals.
Finally, “Sputnik-Light” may be considered also as an effective vaccine in adults above 65 years old. According to recently published results of a study conducted by Argentine scientists the effectiveness of the first component (rAd26) of Gam-COVID-Vac (which is “Sputnik-Light”) was 78·6% [95% CI = 74·8 - 81·7]; and for reducing hospitalizations and deaths was, respectively, 87·6% [95% CI = 80·3 - 92·2] and 84·8% [95% CI = 75·0 - 90·7].20 At the same time reported effectiveness of single-dose vaccine analogues in preventing symptomatic infection was between 51-76%, hospitalisations 66·9-91%, and deaths 85-91% for BNT162b2 mRNA (Pfizer-BioNTech), ChAdOx1 nCoV-19 (Oxford–AstraZeneca), mRNA-1273 (Moderna) and Ad26.COV2.S (Janssen Pharmaceuticals) vaccines.21, 22, 23
The results of this clinical trial have made a basis for provisional vaccine approval for clinical use issued on May 06, 2021 (registration of LP-006993) under the current Decree of the Government of the Russian Federation of April 3, 2020, no 441. Provisional licensure made it possible to start international multicenter randomized, double-blind, placebo-controlled phase 3 clinical trial (NCT04741061) to evaluate efficacy, immunogenicity and safety of the “Sputnik Light” vector vaccine in the parallel assignment of the subjects in prophylactic treatment for SARS-СoV-2 infection. This trial with involvement of 6000 participants (4500 subjects in vaccine group and 1500 in placebo group) would help to further investigate whether single-dose “Sputnik Light” vaccine could be a good choice for revaccination providing effective protection from SARS-CoV-2 infection in persons with pre-existing immunity.
Contributors
AIT wrote the original draft. AIT, IVD, DVS, KAZ, BSN, DYL and ALG edited the manuscript. AIT, IVD, DVS, OVZ, ASD, AVK, DMG, ASE, AGB, FMI, OP, TAO, IBE, IAF, DIZ, DVV, DNS, and ASS collected data. AIT, IVD, DVS and ASD contributed to data analysis. AIT, IVD, DVS, OVZ, EAS, KAZ, VBV, SVB curated data. NLL, MMS, NAN, VAG, SVB, DYL had supervision responsibilities. BSN, DYL, ALG contributed to project conceptualization. NLL, YVS, EAT, EAS, TGZ, VBV, DYL, ALG administrated research and ALG had the final decision to publish. ALG and DYL are responsible for funding acquisition from the Russian Direct Investment Fund. All authors critically reviewed the report and approved the final version.
Declaration of interests
OVZ, TAO, IVD, OP, DVS, DMG, ASD, AIT, DNS, IBE, EAT, AGB, ASE, FMI, NAN, NLL, ASS, SVB, BSN, DYL, ALG report patents for an immunobiological expression vector, pharmaceutical agent, and its method of use to prevent COVID-19. All other authors declare no competing interests.
Acknowledgments
Data sharing
Anonymous participant data will be available upon completion of clinical trials and publication of the results of the completed study upon request to the corresponding author. Proposals will be reviewed and approved by the sponsor, security department, researcher, and staff on the basis of scientific merit and absence of competing interests. Once the proposal has been approved, data can be transferred through a secure online platform after the signing of a data access agreement and a confidentiality agreement.
Acknowledgments
This research was supported by the Russian Direct Investment Fund. We thank the study participants, site research staff, and members of the trial management groups, trial steering committee, and independent data monitoring committee.
Footnotes
Supplementary material associated with this article can be found in the online version at doi:10.1016/j.lanepe.2021.100241.
Contributor Information
Amir I. Tukhvatulin, Email: amir_tukhvatulin@gamaleya.org.
Denis Y. Logunov, Email: ldenisy@gmail.com.
Appendix. Supplementary materials
References
- 1.WHO. Coronavirus (COVID-19) Dashboard. https://covid19whoint (accessed 23 Jun, 2021).
- 2.WHO. Draft landscape of COVID-19 candidate vaccines. 29 Jun, 2021. https://wwwwhoint/publications/m/item/draft-landscape-of-covid-19-candidate-vaccines (accessed 05 Jul, 2021).
- 3.Israel COVID-19 Data Tracker. 25 Jun, 2021. https://datadashboardhealthgovil/COVID-19/general?utm_source=gogovil&utm_medium=referral (accessed 25 Jun, 2021).
- 4.Our World in Data. 02 Jul, 2021. https://ourworldindataorg/covid-vaccinations (accessed 02 Jul, 2021).
- 5.WHO. WHO Director-General's opening remarks at the media briefing on COVID-19. April 09, 2021. https://wwwwhoint/director-general/speeches/detail/director-general-s-opening-remarks-at-the-media-briefing-on-covid-19-9-april-2021 (accessed Jul 05, 2020).
- 6.Logunov DY, Dolzhikova IV, Shcheblyakov DV. Safety and efficacy of an rAd26 and rAd5 vector-based heterologous prime-boost COVID-19 vaccine: an interim analysis of a randomised controlled phase 3 trial in Russia. Lancet. 2021;397(10275):671–681. doi: 10.1016/S0140-6736(21)00234-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mendrone A, Dinardo CL, Ferreira SC. Correlation between SARS-COV-2 antibody screening by immunoassay and neutralizing antibody testing. Transfusion. 2021;61(4):1181–1190. doi: 10.1111/trf.16268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gushchin VA, Dolzhikova IV, Shchetinin AM. Neutralizing Activity of Sera from Sputnik V-Vaccinated People against Variants of Concern (VOC: B.1.1.7, B.1.351, P.1, B.1.617.2, B.1.617.3) and Moscow Endemic SARS-CoV-2 Variants. Vaccines (Basel) 2021;9(7) doi: 10.3390/vaccines9070779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Haghpanah F, Lin G, Levin SA, Klein E. Analysis of the potential impact of durability, timing, and transmission blocking of COVID-19 vaccine on morbidity and mortality. EClinicalMedicine. 2021;35 doi: 10.1016/j.eclinm.2021.100863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Skegg D, Gluckman P, Boulton G. Future scenarios for the COVID-19 pandemic. Lancet. 2021;397(10276):777–778. doi: 10.1016/S0140-6736(21)00424-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Radbruch A, Chang HD. A long-term perspective on immunity to COVID. Nature. 2021 doi: 10.1038/d41586-021-01557-z. [DOI] [PubMed] [Google Scholar]
- 12.Khoury DS, Cromer D, Reynaldi A. Neutralizing antibody levels are highly predictive of immune protection from symptomatic SARS-CoV-2 infection. Nat Med. 2021 doi: 10.1038/s41591-021-01377-8. [DOI] [PubMed] [Google Scholar]
- 13.Olliaro P, Torreele E, Vaillant M. COVID-19 vaccine efficacy and effectiveness-the elephant (not) in the room. Lancet Microbe. 2021 doi: 10.1016/S2666-5247(21)00069-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Krammer F, Srivastava K, Alshammary H. Antibody Responses in Seropositive Persons after a Single Dose of SARS-CoV-2 mRNA Vaccine. N Engl J Med. 2021;384(14):1372–1374. doi: 10.1056/NEJMc2101667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rossi AH, Ojeda DS, Varese A. Sputnik V vaccine elicits seroconversion and neutralizing capacity to SARS-CoV-2 after a single dose. Cell Rep Med. 2021;2(8) doi: 10.1016/j.xcrm.2021.100359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sadoff J, Le Gars M, Shukarev G. Interim Results of a Phase 1-2a Trial of Ad26.COV2.S Covid-19 Vaccine. N Engl J Med. 2021;384(19):1824–1835. doi: 10.1056/NEJMoa2034201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Folegatti PM, Ewer KJ, Aley PK. Safety and immunogenicity of the ChAdOx1 nCoV-19 vaccine against SARS-CoV-2: a preliminary report of a phase 1/2, single-blind, randomised controlled trial. Lancet. 2020;396(10249):467–478. doi: 10.1016/S0140-6736(20)31604-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhu FC, Guan XH, Li YH. Immunogenicity and safety of a recombinant adenovirus type-5-vectored COVID-19 vaccine in healthy adults aged 18 years or older: a randomised, double-blind, placebo-controlled, phase 2 trial. Lancet. 2020;396(10249):479–488. doi: 10.1016/S0140-6736(20)31605-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Riad A, Pokorna A, Mekhemar M. Safety of ChAdOx1 nCoV-19 Vaccine: Independent Evidence from Two EU States. Vaccines (Basel) 2021;9(6) doi: 10.3390/vaccines9060673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Soledad González SO, Salazar Martín, Calabria Ana, Regairaz Lorena, Marín Lupe, Campos Patricia, Varela Teresa, Martínez Veronica V.González, Ceriani Leticia, Garcia Enio, Kreplak Nicolás, Pifano Marina, Estenssoro Elisa, Marsico Franco. Effectiveness of the first component of Gam-COVID-Vac (Sputnik V) on reduction of SARS-CoV-2 confirmed infections, hospitalisations and mortality in patients aged 60-79: a retrospective cohort study in Argentina. EClinicalMedicine. 2021;40(101126) doi: 10.1016/j.eclinm.2021.101126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chung H, He S, Nasreen S. Effectiveness of BNT162b2 and mRNA-1273 covid-19 vaccines against symptomatic SARS-CoV-2 infection and severe covid-19 outcomes in Ontario, Canada: test negative design study. BMJ. 2021;374:n1943. doi: 10.1136/bmj.n1943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Shrotri M, Krutikov M, Palmer T. Vaccine effectiveness of the first dose of ChAdOx1 nCoV-19 and BNT162b2 against SARS-CoV-2 infection in residents of long-term care facilities in England (VIVALDI): a prospective cohort study. Lancet Infect Dis. 2021 doi: 10.1016/S1473-3099(21)00289-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Corchado-Garcia JaP-Z David, Hughes Travis, Cristea-Platon Tudor, Lenehan Patrick, Pawlowski Colin, Bade Sairam, O'Horo John C., Gores Gregory J., Williams Amy W., Badley Andrew D., Halamka John, Virk Abhinash, Swift Melanie D., Wagner Tyler, Soundararajan Venky. Real-World Effectiveness of Ad26.COV2.S Adenoviral Vector Vaccine for COVID-19. medRxiv. 2021 doi: 10.1001/jamanetworkopen.2021.32540. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.