Abstract
Objectives
Policymakers have suggested increasing peritoneal dialysis (PD) would improve end-stage kidney disease (ESKD) outcomes and reduce Medicare spending compared to hemodialysis (HD). We compared mortality, hospitalizations, and Medicare spending between PD and HD among uninsured adults with incident ESKD.
Methods
Using an instrumental variable design, we exploited a natural experiment encouraging PD among the uninsured. Uninsured patients usually receive Medicare at dialysis month four. For those initiating with PD, Medicare covers the first three dialysis months, including pre-dialysis services in the calendar month of dialysis start. Starting dialysis later in a calendar month increases pre-dialysis coverage essential for PD catheter placements. The policy incrementally encourages PD when developing ESKD later in the month. Dialysis start day appears unrelated to patient characteristics and effectively “randomizes patients” to dialysis modality, mitigating selection bias.
Results
Starting dialysis later in the month was associated with increased PD uptake: every week later in the month was associated with an absolute increase of 0.8% (95% CI: 0.6%, 0.9%) at dialysis day 1 and 0.5% (95% CI: 0.3%, 0.7%) at dialysis month 12. We observed no significant absolute difference between PD and HD for 12-month mortality (–0.9%, 95% CI: −3.3%, 0.8%), hospitalizations during months 7–12 (–0.05, 95% CI: −0.20, 0.07), and Medicare spending during months 7–12 (–$702, 95% CI: −$4,004, $2,909).
Conclusions
In an instrumental variable analysis, PD did not result in improved outcomes or lower costs compared to HD.
Precis:
Despite an impetus by policymakers aimed at increasing home dialysis use, policies that promote home dialysis are unlikely to improve outcomes or reduce spending.
INTRODUCTION
The end-stage kidney disease (ESKD) program costs Medicare $36 billion per year or 7% of Medicare’s budget.1,2 Kidney disease experts3–5 and policy makers6 have suggested that home dialysis, which is primarily peritoneal dialysis (PD), improves ESKD outcomes and reduces Medicare spending relative to center-based hemodialysis (HD). On July 10, 2019, the President signed the “Advancing American Kidney Health” Executive Order, which aims to increase home dialysis use and kidney transplantation to 80% of the incident ESKD population by 2025.7 Over 85% of incident patients use center-based HD, so this policy would radically reverse decades of practice.1
Home dialysis momentum continues despite the absence of randomized evidence.8 The Centers for Medicare and Medicaid Services (CMS) has already finalized a mandatory payment model penalizing providers who do not successfully increase PD use in their practice.9 Although observational studies suggest that home dialysis offers similar to improved survival,10–13 selection bias remains a major shortcoming. Some experts espouse potential savings of $10,000–$15,000 per patient when switching from center-based HD to PD,1,3,5,14 but these estimates do not adequately adjust for patient characteristics. Ideally, a randomized-controlled trial (RCT) could compare PD and center-based HD. However, randomizing alternative dialysis modalities is difficult, and past trials have failed to recruit enough patients.15
We compared PD to HD provision by exploiting an idiosyncratic policy encouraging asymmetric PD use in the uninsured based on the calendar day of dialysis start. Medicare eligibility normally begins in the fourth calendar month of dialysis.16 Uninsured patients starting with PD receive Medicare at day one and retroactive pre-dialysis coverage to the beginning of the calendar month. Since PD catheters require two to four weeks of surgical site healing before commencing dialysis,17 initiating dialysis later in the month increases the likelihood of receiving sufficient pre-dialysis coverage for the catheter and in turn increases the likelihood of PD use at dialysis start and thereafter.18 We therefore used the calendar day of dialysis start as a natural experiment to compare mortality, hospitalizations, and costs in uninsured patients starting with PD versus HD.
METHODS
Analytic Strategy
We conducted an instrumental variable (IV) analysis, a quasi-experimental method commonly used to reduce observational study bias (Appendix).19–23 Medicare coverage policies encourage patients starting dialysis at the end of the month to start with PD (Appendix Figure 1). If the calendar day of dialysis start were randomly distributed, we would expect patients have similar characteristics irrespective of start day. Additionally, if starting dialysis later in the calendar month encourages PD use, we may use dialysis start day as an instrument that randomly “assigns” patients to PD versus HD (as a coin flip assigns treatment in an RCT). A caveat of our analysis (and IV analyses in general) is that we estimated the local average treatment effect of uninsured patients who were encouraged to switch from HD to PD due to the Medicare coverage policy based on the calendar day of the month.
Although we cannot definitively prove that dialysis start day is random, we provide corroborating evidence. Specifically, we find no evidence of bunching of dialysis starts or substantive differences in observable patient characteristics based on dialysis start day (Figure 1, Table 1, Appendix Figure 2, Appendix Table 1).
Figure 1: Comparing observed days of the month of dialysis start and predicted.
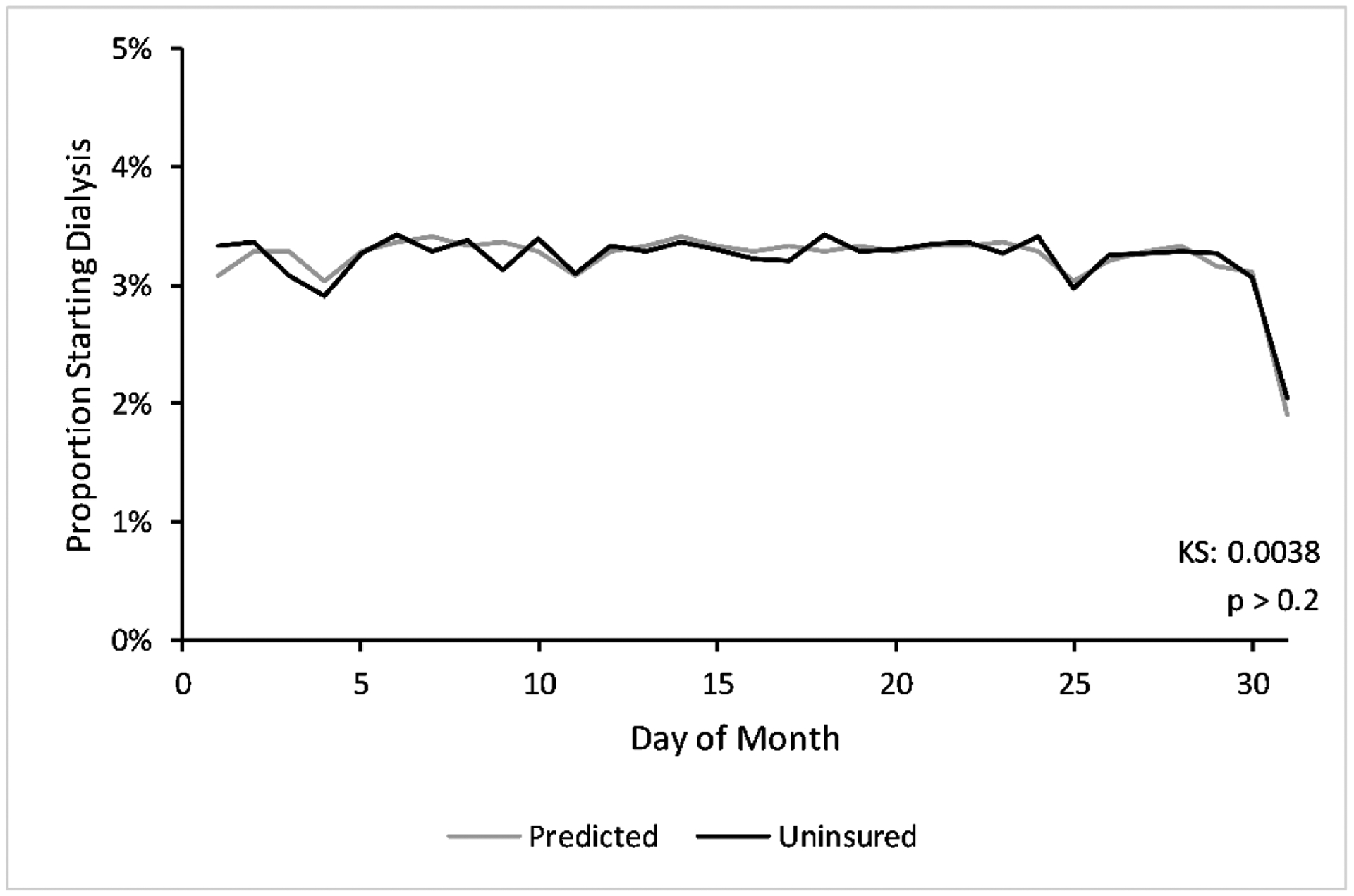
We show the proportion of uninsured patients starting dialysis at each numbered day of the month (in black) against the predicted distribution or the proportion of times the numbered day appears between 1/1/2006 and 12/31/2015 (in gray). Distributions exclude weekends and federal holidays. We show the cumulative distribution in Appendix Figure 2. The one-sample Kolmogorov-Smirnov statistic is the maximum distance between the predicted and observed cumulative distributions (p > 0.2). Abbreviations: KS = Kolmogorov-Smirnov statistic.
Table 1:
Baseline Characteristics of Uninsured Adults, Stratified by Starting Dialysis Modality and by Day of Dialysis Start
| CHARACTERISTIC | Dialysis Modality at Start | Half of Calendar Month at Start | ||||||
|---|---|---|---|---|---|---|---|---|
| HD | PD | First Half | Second Half | |||||
| N | % | N | % | N | % | N | % | |
| Patient | ||||||||
| Age at Day 1 of Dialysis ‡ | ||||||||
| 18–45 | 19,440 | 36% | 1,914 | 40% | 10,447 | 37% | 10,907 | 36% |
| 46–55 | 17,272 | 32% | 1,494 | 31% | 9,067 | 32% | 9,699 | 32% |
| 56–65 | 15,165 | 28% | 1,366 | 28% | 8,070 | 28% | 8,461 | 28% |
| >65 | 1,823 | 3% | 65 | 1% | 929 | 3% | 959 | 3% |
| Sex ‡ | ||||||||
| Male | 34,182 | 64% | 2,688 | 56% | 18,030 | 63% | 18,840 | 63% |
| Female | 19,518 | 36% | 2,151 | 45% | 10,483 | 37% | 11,186 | 37% |
| Race ‡ | ||||||||
| White | 28,274 | 53% | 3,146 | 65% | 15,170 | 53% | 16,250 | 54% |
| Black | 22,548 | 42% | 1,450 | 30% | 11,842 | 42% | 12,156 | 41% |
| Asian | 1,640 | 3% | 154 | 3% | 860 | 3% | 934 | 3% |
| Other | 1,238 | 2% | 89 | 2% | 641 | 2% | 686 | 2% |
| Hispanic Ethnicity ‡ | 12,481 | 23% | 1,039 | 22% | 6,572 | 23% | 6,948 | 23% |
| Employment Status ‡ | ||||||||
| Unemployed | 32,809 | 61% | 2,516 | 52% | 17,204 | 60% | 18,121 | 60% |
| Employed | 5,729 | 11% | 981 | 20% | 3,224 | 11% | 3,486 | 12% |
| Retired-Age | 2,479 | 5% | 215 | 4% | 1,295 | 5% | 1,399 | 5% |
| Disabled (Short- and Long-term) | 10,826 | 20% | 886 | 18% | 5,727 | 20% | 5,985 | 20% |
| Other | 1,857 | 4% | 241 | 5% | 1,063 | 4% | 1,035 | 3% |
| Primary Cause of ESRD ‡ | ||||||||
| Diabetes | 21,723 | 41% | 1,912 | 40% | 11,485 | 40% | 12,150 | 41% |
| Hypertension | 18,054 | 34% | 1,428 | 30% | 9,595 | 34% | 9,887 | 33% |
| Other | 13,923 | 26% | 1,499 | 31% | 7,433 | 26% | 7,989 | 27% |
| Prior Nephrology Care ‡ § | ||||||||
| Yes | 18,513 | 35% | 3,285 | 68% | 10,478 | 37% | 11,320 | 38% |
| No | 28,238 | 53% | 1,281 | 27% | 14,442 | 51% | 15,077 | 50% |
| Unknown | 6,949 | 13% | 273 | 6% | 3,593 | 13% | 3,629 | 12% |
| Comorbid Conditions | ||||||||
| Alcohol Dependence ‡ | 1,969 | 4% | 47 | 1% | 982 | 3% | 1,034 | 3% |
| Amputation † | 1,109 | 2% | 68 | 1% | 571 | 2% | 606 | 2% |
| Atherosclerotic Heart Disease ‡ | 4,446 | 8% | 305 | 6% | 2,339 | 8% | 2,412 | 8% |
| Cancer ‡ | 1,341 | 3% | 76 | 2% | 688 | 2% | 729 | 2% |
| Congestive Heart Failure ‡ | 11,574 | 22% | 658 | 14% | 5,951 | 21% | 6,281 | 21% |
| Chronic Obstructive Pulmondary Disease ‡ | 1,958 | 4% | 126 | 3% | 1,022 | 4% | 1,062 | 4% |
| Prior CVA/TIA * | 2,676 | 5% | 208 | 4% | 1,408 | 5% | 1,476 | 5% |
| Diabetes: Insulin Dependent | 17,102 | 32% | 1,484 | 31% | 9,041 | 32% | 9,545 | 32% |
| Diabetes: Without Medications | 2,914 | 5% | 248 | 5% | 1,547 | 5% | 1,615 | 5% |
| Diabetes: Oral Medications * | 5,845 | 11% | 477 | 10% | 3,049 | 11% | 3,273 | 11% |
| Diabetes: Retinopathy ‡ | 4,277 | 8% | 470 | 10% | 2,286 | 8% | 2,461 | 8% |
| Drug Dependence ‡ | 2,336 | 4% | 48 | 1% | 1,192 | 4% | 1,192 | 4% |
| Hypertension ‡ | 47,192 | 88% | 4,347 | 90% | 25,063 | 88% | 26,476 | 88% |
| Disability ‡ | 3,786 | 7% | 120 | 3% | 1,895 | 7% | 2,011 | 7% |
| Other Cardiac Disease ‡ | 4,838 | 9% | 331 | 7% | 2,479 | 9% | 2,690 | 9% |
| Peripheral Vascular Disease ‡ | 3,195 | 6% | 216 | 5% | 1,693 | 6% | 1,718 | 6% |
| Smoker ‡ | 6,028 | 11% | 458 | 10% | 3,157 | 11% | 3,329 | 11% |
| BMI, kg/m2 ‡ | ||||||||
| <18.5 | 1,770 | 3% | 96 | 2% | 902 | 3% | 964 | 3% |
| 18.5 – <30 | 31,364 | 59% | 2,712 | 57% | 16,549 | 59% | 17,527 | 59% |
| 30 – <40 | 14,838 | 28% | 1,577 | 33% | 8,029 | 28% | 8,386 | 28% |
| 40+ | 5,245 | 10% | 400 | 8% | 2,784 | 10% | 2,861 | 10% |
| Albumin, g/dl ‡ | ||||||||
| <2.5 | 9,097 | 23% | 360 | 10% | 4,594 | 22% | 4,863 | 22% |
| 2.5 – <3 | 9,236 | 23% | 476 | 13% | 4,743 | 22% | 4,969 | 22% |
| 3 – <3.5 | 10,229 | 26% | 867 | 24% | 5,465 | 26% | 5,631 | 25% |
| 3.5+ | 11,587 | 29% | 1,989 | 54% | 6,577 | 31% | 6,999 | 31% |
| Hemoglobin, g/dl ‡ | ||||||||
| <8 | 9,741 | 20% | 471 | 11% | 5,034 | 20% | 5,178 | 19% |
| 8 – <9 | 10,280 | 21% | 691 | 16% | 5,336 | 21% | 5,635 | 21% |
| 9 – <11 | 19,630 | 41% | 1,877 | 44% | 10,410 | 41% | 11,097 | 41% |
| 11+ | 8,782 | 18% | 1,197 | 28% | 4,857 | 19% | 5,122 | 19% |
| Facility | ||||||||
| Hospital-Based ‡ ∥ | 7,909 | 15% | 374 | 8% | 4,165 | 15% | 4,118 | 14% |
| For-Profit Status ‡ § | ||||||||
| For-Profit | 42,236 | 79% | 4,094 | 85% | 22,439 | 79% | 23,891 | 80% |
| Non-Profit | 10,263 | 19% | 681 | 14% | 5,433 | 19% | 5,511 | 18% |
| Unknown | 1,201 | 2% | 64 | 1% | 641 | 2% | 624 | 2% |
| Total facility patients per year ‡ | ||||||||
| 1–50 | 10,004 | 19% | 667 | 14% | 5,307 | 19% | 5,364 | 18% |
| 51–100 | 21,954 | 41% | 1,583 | 33% | 11,385 | 40% | 12,152 | 41% |
| 101–150 | 13,307 | 25% | 1,292 | 27% | 7,062 | 25% | 7,537 | 25% |
| 151+ | 8,435 | 16% | 1,297 | 27% | 4,759 | 17% | 4,973 | 17% |
| Total PD patients per year ‡ ¶ | ||||||||
| 0 | 31,086 | 58% | 69 | 1% | 15,359 | 54% | 15,796 | 53% |
| 1–10 | 9,355 | 17% | 671 | 14% | 4,930 | 17% | 5,096 | 17% |
| 11–25 | 7,687 | 14% | 1,448 | 30% | 4,385 | 15% | 4,750 | 16% |
| 26–50 | 4,246 | 8% | 1,463 | 30% | 2,707 | 10% | 3,002 | 10% |
| 50+ | 1,326 | 3% | 1,188 | 25% | 1,132 | 4% | 1,382 | 5% |
| Patient : nurse Ratio ‡ | ||||||||
| >0–10 | 12,340 | 23% | 990 | 21% | 6,546 | 23% | 6,784 | 23% |
| >10–15 | 17,648 | 33% | 1,857 | 39% | 9,468 | 34% | 10,037 | 34% |
| >15–20 | 13,283 | 25% | 1,145 | 24% | 7,007 | 25% | 7,421 | 25% |
| 20+ | 9,550 | 18% | 753 | 16% | 5,019 | 18% | 5,284 | 18% |
| Patient : Staff Ratio ‡ § | ||||||||
| >0–4 | 5,009 | 10% | 191 | 4% | 2,576 | 9% | 2,624 | 9% |
| >4–5 | 7,313 | 14% | 345 | 7% | 3,796 | 14% | 3,862 | 13% |
| >5–6 | 13,410 | 25% | 789 | 17% | 6,941 | 25% | 7,258 | 25% |
| >6–7 | 13,355 | 25% | 918 | 19% | 7,008 | 25% | 7,265 | 25% |
| >7–8 | 8,004 | 15% | 864 | 18% | 4,204 | 15% | 4,664 | 16% |
| 8+ | 5,757 | 11% | 1,639 | 35% | 3,527 | 13% | 3,869 | 13% |
| Geographic | ||||||||
| Total population in zipcode ‡ | ||||||||
| 0 – <5,000 | 1,976 | 4% | 198 | 4% | 1,059 | 4% | 1,115 | 4% |
| 5,000 – <15,000 | 6,722 | 13% | 661 | 14% | 3,601 | 13% | 3,782 | 13% |
| 15,000 – <25,000 | 12,435 | 23% | 1,263 | 26% | 6,655 | 23% | 7,043 | 24% |
| 25,000 – <50,000 | 24,996 | 47% | 2,285 | 47% | 13,236 | 47% | 14,045 | 47% |
| 50,000+ | 7,512 | 14% | 428 | 9% | 3,930 | 14% | 4,010 | 13% |
| Median income of zipcode ($) ‡ | ||||||||
| 0 – <25,000 | 3,850 | 7% | 369 | 8% | 2,065 | 7% | 2,154 | 7% |
| 25,000 – <50,000 | 32,845 | 62% | 2,733 | 57% | 17,365 | 61% | 18,213 | 61% |
| 50,000 – <75,000 | 12,883 | 24% | 1,370 | 29% | 6,904 | 24% | 7,349 | 25% |
| 75,000 – <100,000 | 2,976 | 6% | 252 | 5% | 1,578 | 6% | 1,650 | 6% |
| 100,000+ | 779 | 2% | 75 | 2% | 407 | 1% | 447 | 2% |
| Portion of zipcode below poverty line ‡ | ||||||||
| 0 – <15 | 16,979 | 32% | 1,768 | 37% | 9,085 | 32% | 9,662 | 32% |
| 15 – <25 | 18,930 | 35% | 1,640 | 34% | 10,109 | 36% | 10,461 | 35% |
| 25 + | 17,540 | 33% | 1,396 | 29% | 9,174 | 32% | 9,762 | 33% |
| Portion of zipcode unemployed (%) ‡ | ||||||||
| 0 – <5 | 13,946 | 26% | 1,528 | 32% | 7,489 | 26% | 7,985 | 27% |
| 5 – <10 | 33,894 | 63% | 2,917 | 60% | 17,974 | 63% | 18,837 | 63% |
| 10 – <15 | 5,343 | 10% | 314 | 7% | 2,761 | 10% | 2,896 | 10% |
| 15 + | 420 | 1% | 71 | 2% | 236 | 1% | 255 | 1% |
| Portion of zipcode without high school diploma ‡ | ||||||||
| 0 – 15 | 22,282 | 42% | 2,579 | 53% | 12,085 | 43% | 12,776 | 43% |
| 15 – 30 | 24,137 | 45% | 1,877 | 39% | 12,657 | 45% | 13,357 | 45% |
| 30 + | 7,184 | 13% | 374 | 8% | 3,718 | 13% | 3,840 | 13% |
| Median rent of zipcode ($) ‡ | ||||||||
| 0 – <750 | 22,265 | 42% | 1,957 | 41% | 11,765 | 42% | 12,457 | 42% |
| 750 – <1000 | 19,667 | 37% | 1,893 | 40% | 10,539 | 37% | 11,021 | 37% |
| 1000 – <1250 | 7,361 | 14% | 662 | 14% | 3,894 | 14% | 4,129 | 14% |
| 1250 + | 3,991 | 8% | 279 | 6% | 2,097 | 7% | 2,173 | 7% |
| Urban (versus Rural) ‡ | 44,915 | 84% | 4,286 | 89% | 23,966 | 84% | 25,235 | 84% |
| Temporal | ||||||||
| Year of Dialysis Start ‡ | ||||||||
| 2005 | 3,032 | 6% | 190 | 4% | 1,547 | 5% | 1,675 | 6% |
| 2006 | 5,744 | 11% | 373 | 8% | 2,995 | 11% | 3,122 | 10% |
| 2007 | 5,803 | 11% | 374 | 8% | 3,043 | 11% | 3,134 | 10% |
| 2008 | 5,860 | 11% | 407 | 8% | 3,064 | 11% | 3,203 | 11% |
| 2009 | 6,098 | 11% | 453 | 9% | 3,219 | 11% | 3,332 | 11% |
| 2010 | 6,101 | 11% | 526 | 11% | 3,241 | 11% | 3,386 | 11% |
| 2011 | 5,900 | 11% | 653 | 14% | 3,191 | 11% | 3,362 | 11% |
| 2012 | 5,221 | 10% | 587 | 12% | 2,789 | 10% | 3,019 | 10% |
| 2013 | 5,607 | 10% | 711 | 15% | 3,027 | 11% | 3,291 | 11% |
| 2014 | 4,334 | 8% | 565 | 12% | 2,397 | 8% | 2,502 | 8% |
Notes:
Abbreviations: ESKD = end-stage kidney disease, HD = hemodialysis, PD = peritoneal dialysis p-values computed using Pearson’s chi-square test
PD versus HD: p < 0.05
PD versus HD: p < 0.01
PD versus HD: p < 0.001
1st half versus 2nd half: p < 0.05
1st half versus 2nd half: p < 0.01
1st half versus 2nd half: p < 0.001
These empirical findings argue against the possibility that nephrologists might “game” the start date of dialysis by delaying dialysis starts for patients interested in PD until the end of the month to maximize retroactive Medicare coverage. Widespread gaming of the coverage policy would mean that dialysis start date is not random and thus not suitable as an instrumental variable. Gaming of dialysis starts would result in bunching of dialysis starts at the end of the calendar month. Additionally, we would expect to observe patients starting dialysis later in the calendar month reflecting characteristics associated with PD: younger in age, healthier, and socioeconomically more advantaged.24 We observe neither result.
Instead, our findings are consistent with dialysis start day being random and suggest that patients starting dialysis at the beginning of the calendar month but interested in PD likely initiate HD with the intention of switching to PD. For patients starting with HD and subsequently receiving a PD catheter, as long as the patient switches to PD before the fourth month of dialysis, the patient will receive retroactive Medicare coverage through the beginning of dialysis, including the PD catheter. Patients at the margin, and their nephrologists, likely have weaker modality preferences and are probably willing to initiate HD with the eventual plan of switching to PD. Simultaneously, because dialysis initiation (PD and HD) is complex and involves multiple steps (e.g., referral to a surgeon, identifying an accepting facility, and coordinating patient and dialysis facility schedules), providers are less able to precisely predict the start date, making gaming of dialysis initiation more difficult.
In addition to providing evidence that dialysis start day is random, we also test whether dialysis start day is associated with differences in PD use. Consistent with prior work18 and what we would predict from the coverage policy, dialysis start day was associated with differences in PD use at dialysis start. Interestingly, differences in modality at dialysis start translated into long-term differences in PD use. These findings suggest that having patients start center-based HD, even with the eventual goal of switching to PD, leads to lower long-term PD uptake. A prior study extensively analyzed the IV’s properties and the mechanism by which it affects long-term PD uptake.18 Briefly, the study observed that patients rarely switch to PD after initiating HD. This inertia explains how dialysis start day, which influences PD use at dialysis start, results in long-term differences in PD use. We therefore exploit differences in dialysis start day to test whether sustained PD uptake yields superior outcomes to HD.
Inferring causality between dialysis modality and outcomes requires that the only mechanism through which the instrument (dialysis start day) affects outcomes is through dialysis modality. Although one can never prove this assumption, we provide two points of corroborative evidence. First, the most plausible alternate mechanism is through long-term differences in Medicare coverage. Patients starting in the end of the month are more likely to choose home dialysis and thus more likely to obtain Medicare at dialysis start. However, coverage differences dissipate by month six (Appendix Table 2). Second, we observe no difference in short-term (6-month) mortality between patients starting dialysis in the first versus second half of the month (Appendix Table 3), suggesting that short-term differences in Medicare coverage are not associated with health.
We initially conducted an “intention-to-treat” (ITT) analysis, comparing patients starting dialysis at the beginning of the month to those starting at the end. Afterwards, we conducted an IV regression to directly compare outcomes between PD and HD.
Data Sources, Population, and Follow-up
From the United States Renal Data System (USRDS),1 a registry of all US patients with ESKD linked to Medicare fee-for-service claims, we identified uninsured adults starting dialysis between 6/1/2005 and 12/31/2014. We used the CMS-2728 Form, submitted by dialysis facilities for all incident patients irrespective of insurance coverage, to identify dialysis start date and patient characteristics at start: insurance, employment status, comorbidities, and laboratory data. We identified facility characteristics from the annual dialysis facility survey (CMS-2744) and sociodemographic characteristics of each facility’s zip code from the 2010 Census and the 2012 American Community Survey. We used Medicare Parts A and B claims to obtain total Medicare spending, acute hospitalizations, outpatient dialysis services, and physician dialysis visits. Medicare payments were inflation adjusted to 2015 dollars.
We excluded patients with more than 31 days of retroactive Medicare before ESKD onset according to the enrollment database, who received a kidney transplant prior to dialysis, or who started dialysis on a federal holiday or weekend because dialysis initiation on these days is usually emergent, and PD is not usually initiated under emergent circumstances. We followed patients for 12 months and did not censor for death, kidney transplantation, or modality switch. We defined one month of follow-up as four weeks (28 days) to standardize months.
Variables
Our main outcomes were 12-month mortality, number of hospitalizations (identified using a previously described algorithm25) per patient during dialysis months 7–12, and Medicare spending per patient during dialysis months 7–12. We modeled hospitalizations and costs as continuous variables. Because costs were right-skewed and to minimize the effect of outliers, we modeled the natural logarithm of cost. In a sensitivity analysis, we modeled non-logarithm transformed costs.
To study mortality, we used the entire uninsured population irrespective of downstream Medicare coverage. We could only observe hospitalizations and spending in patients with Medicare Parts A and B as primary payer. For these outcomes, we required Medicare coverage while alive from months 7–12. We started at month seven because coverage differences dissipate by then and including earlier months would bias results against PD. Patients who died between months 7–12 remained in the sample and contributed zero hospitalizations and zero spending in our base analysis. In a sensitivity analysis, we assessed 24-month outcomes, which required excluding patients starting dialysis in 2014.
The independent variables of interest were dialysis start day (instrument), which we modeled as a continuous variable, and whether the patient started with PD (intervention), which we modeled as a binary variable. We controlled for patient characteristics (age, sex, race, ethnicity, employment status, comorbidities, primary cause of ESKD, pre-ESKD nephrology care, body mass index, serum albumin, and serum hemoglobin), facility characteristics (profit status, whether it was hospital-based, number of patients, and patient to staff ratio), geographic characteristics (population, proportion of residents with high school diploma, median income, and median rent within the facility’s zip code), and temporal characteristics (month and year) at dialysis start (full list in Table 1).
Statistical Analyses
All analyses used robust standard errors. Before conducting the IV regression, we tested the instrument’s properties. First, we assessed whether the dialysis start day bunched near the end of the month. Using a Kolmogorov-Smirnov test, we compared the dialysis start day distribution among the entire uninsured population to a theoretical distribution from pure random chance, the proportion of times a numbered day appeared during the study period. We opted for a Kolmogorov-Smirnov test because other more powerful distributional tests (e.g., Shapiro-Wilk’s or Lilliefors) assume a normal distribution. Second, we compared observable characteristics between patients starting in the first half and second half of the calendar month, using Pearson’s chi-square test. Third, we assessed the instrument’s strength, or its correlation with PD use. Conceptually, the analysis estimates adherence to the “assigned” treatment after randomization. We used a linear probability model to regress the probability of starting with PD on the calendar day. We plotted predicted probabilities as a function of each calendar day of dialysis start. We computed the regression’s F-statistic; in the econometrics literature, an F-statistic of 10 typically indicates that the instrument has sufficient strength.26 Finally, we confirmed that the instrument was associated with sustained differences in 12-month PD use.
The ITT analysis regressed mortality, number of hospitalizations, and costs on whether the patient started dialysis in the first versus second half of the month (i.e., we reclassified the instrument as a binary coin flip). We used logistic regression for mortality and ordinary least squares for hospitalizations and the natural logarithm of costs.
When comparing PD to HD, we first employed “traditional” multivariable regression models, logistic for mortality and ordinary least squares for hospitalization and costs. To demonstrate the effect of selection bias, we successively included covariates in a stepwise manner. Subsequently, we conducted an IV regression using two-stage residual inclusion (2SRI), which is commonly employed with a non-linear first-stage regression and has improved precision over two-stage least squares.27 Unlike maximum likelihood estimation, 2SRI relaxes distributional assumptions. The first-stage probit regression estimated the probability of starting with PD as a function of the day of dialysis start (instrument) and controlling covariates. The second-stage used ordinary least squares (OLS) to regress the outcomes on starting with PD, controlling covariates, and the first-stage generalized residual.28,29 We excluded the day of dialysis start (instrument) from the second-stage regressions. We chose OLS for all outcomes, including death and logarithm-transformed costs, because non-linear second-stage models do not necessarily preserve expectations when incorporating the residual.28 Conversely, our base-case specification is robust to misspecifications of the first-stage probit model with asymptotically valid standard errors. We computed the absolute difference in marginal effects of dialysis modality. For costs, we estimated the average predicted cost for PD and HD by exponentiating regression estimates, then took the absolute difference. We used a non-parametric bootstrap (250 samples) to estimate 95% confidence intervals.
Because a large fraction (~25%) of our population had missing serum albumin and hemoglobin, we used multiple imputation (10 imputations) to account for missing covariates. We nested imputations within each bootstrap draw.30,31
Sensitivity Analyses
We performed sensitivity analyses on complete cases given the computational overhead of the multiple imputation model: we (1) performed a complete case analysis; (2) included patients with missing covariates and omitted those covariates; (3) used a 24-month follow-up window among patients initiating dialysis 6/1/2005 to 12/31/2013; (4) modeled costs directly (not using the natural logarithm); (5) modeled costs using a negative binomial second-stage; (6) modeled daily spending while patients were alive; (7) varied the IV regression’s functional form; (8) modeled mortality using a Cox proportional hazards regression in the second stage; (9) repeated the mortality analysis in patients obtaining Medicare by month 7; (10) repeated the hospitalization and spending analyses in patients surviving through 12 months; (11) excluded patients who received a transplant in the study period; (12) excluded patients who changed dialysis modality; (13) included patients starting dialysis on a weekend or holiday; and (14) omitted facility characteristics in case modality choice drives facility choice (i.e., if facility characteristics are endogenous).
We used SAS version 9.4 (SAS Institute, Cary, NC) to construct our analytical dataset and Stata version 14.0, MP edition (StataCorp, College Station, TX) for all statistical analyses.
IRB Approval
The study was approved by the University of Southern California Institutional Review Board (IRB).
RESULTS
Baseline Characteristics and Randomness of Dialysis Start Day
Of 58,539 uninsured adults meeting inclusion criteria (Appendix Figure 3), 8% started with PD (Table 1). Compared to patients starting with HD, patients starting with PD were younger; more likely female, white, and employed; had fewer comorbidities; and had higher serum albumin and hemoglobin. They were more likely to receive pre-dialysis nephrology care and dialyze at for-profit, free-standing, and urban facilities with large PD populations.
The dialysis start day distribution in the entire population was statistically no different from pure random chance, the proportion of times a numbered day appeared during the study period (Kolmogorov-Smirnov test, p>0.2, Figure 1, Appendix Figure 2).32 Critically, we observed no bunching of dialysis starts at the end of the month, which is what we would observe with delayed PD starts. Additionally, we observed no statistical difference in most observable characteristics between those starting in the first versus second half of the month in the entire study cohort (Table 1) and when restricting to the 42,932 adults obtaining Medicare by the end of month six (Appendix Table 1). Together, these findings support the hypothesis that dialysis start day is random and suggest that providers are not delaying dialysis starts for patients interested in PD.
Instrumental Variable’s Properties
Starting dialysis later in the calendar month was associated with increased PD use after adjusting for confounders: every week later in the month was associated with an absolute increase of 0.8% (95% CI: 0.6%, 0.9%) at dialysis day 1 and 0.5% (95% CI: 0.3%, 0.7%) at dialysis month 12 (Figure 2, Appendix Figure 4). In adjusted analysis, 10.2% (95% CI: 9.7%, 10.6%) of patients starting on the first of the month used PD by dialysis month 12, while 12.3% (95% CI: 11.9%, 12.8%) of patients starting on the last day of the month used PD by dialysis month 12, an absolute increase of 2.2% (95% CI: 1.4%, 3.0%) and a relative increase of 22%. The F-statistic was 62, suggesting more than sufficient strength as an IV.
Figure 2: Unadjusted Probability of Peritoneal Dialysis Use at Month 12.
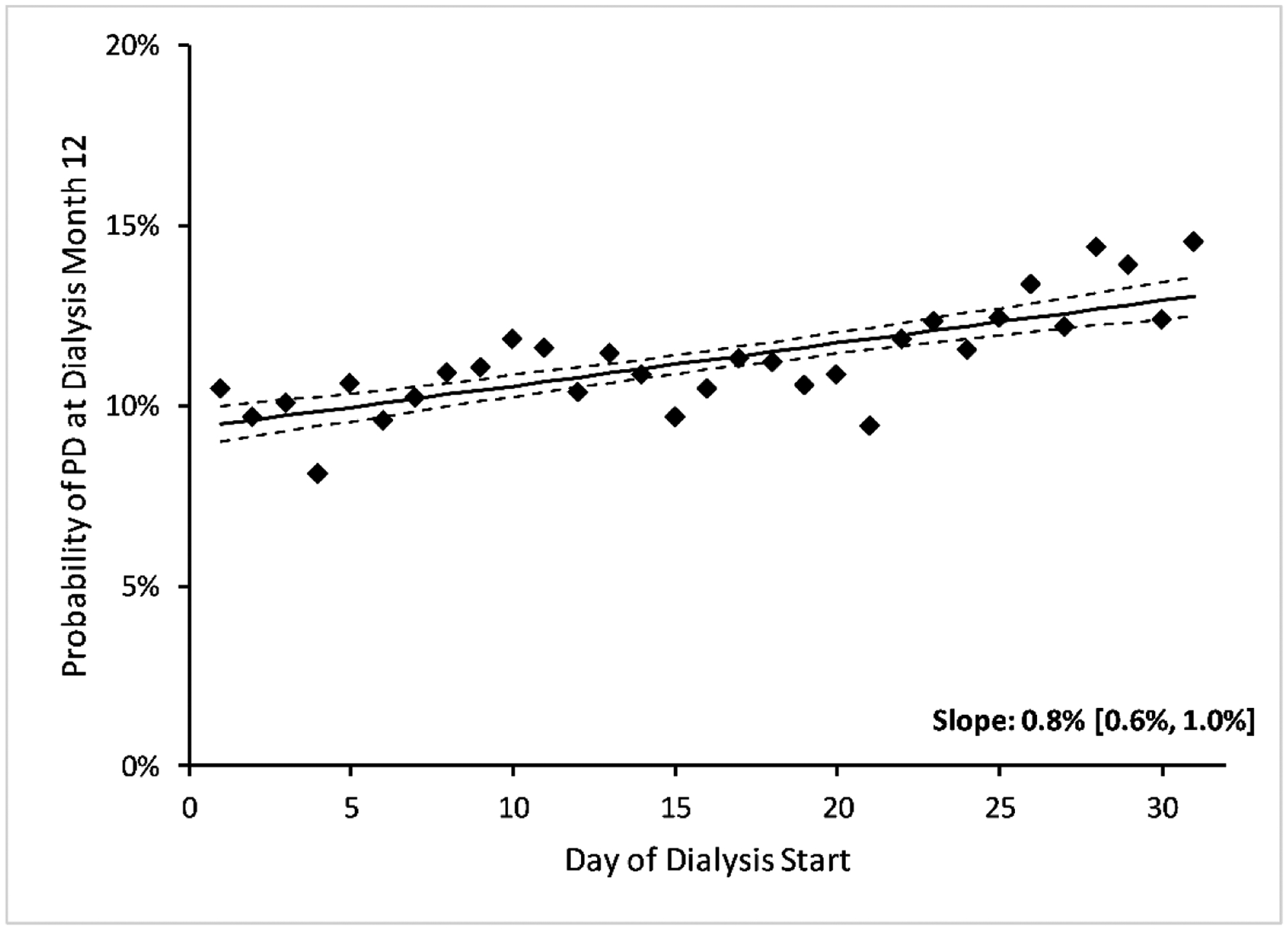
We show the unadjusted and adjusted probabilities of peritoneal dialysis at dialysis start and the adjusted probability of peritoneal dialysis at month 12 in Appendix Figure 4. Abbreviations: PD = peritoneal dialysis.
Intention to Treat Analysis
Patients starting dialysis in the first versus second half of the month had no significant difference in outcomes in unadjusted analysis (Figure 3, Appendix Figure 5). After adjusting for confounders, the odds ratio for mortality was 0.98 (95% CI: 0.92, 1.04), the coefficient for hospitalizations −0.01 (95% CI: −0.03, 0.02), and the risk ratio for total Medicare spending 1.01 (95% CI: 0.96, 1.06) (Appendix Table 4).
Figure 3: Unadjusted changes in mortality and spending by day of dialysis start.
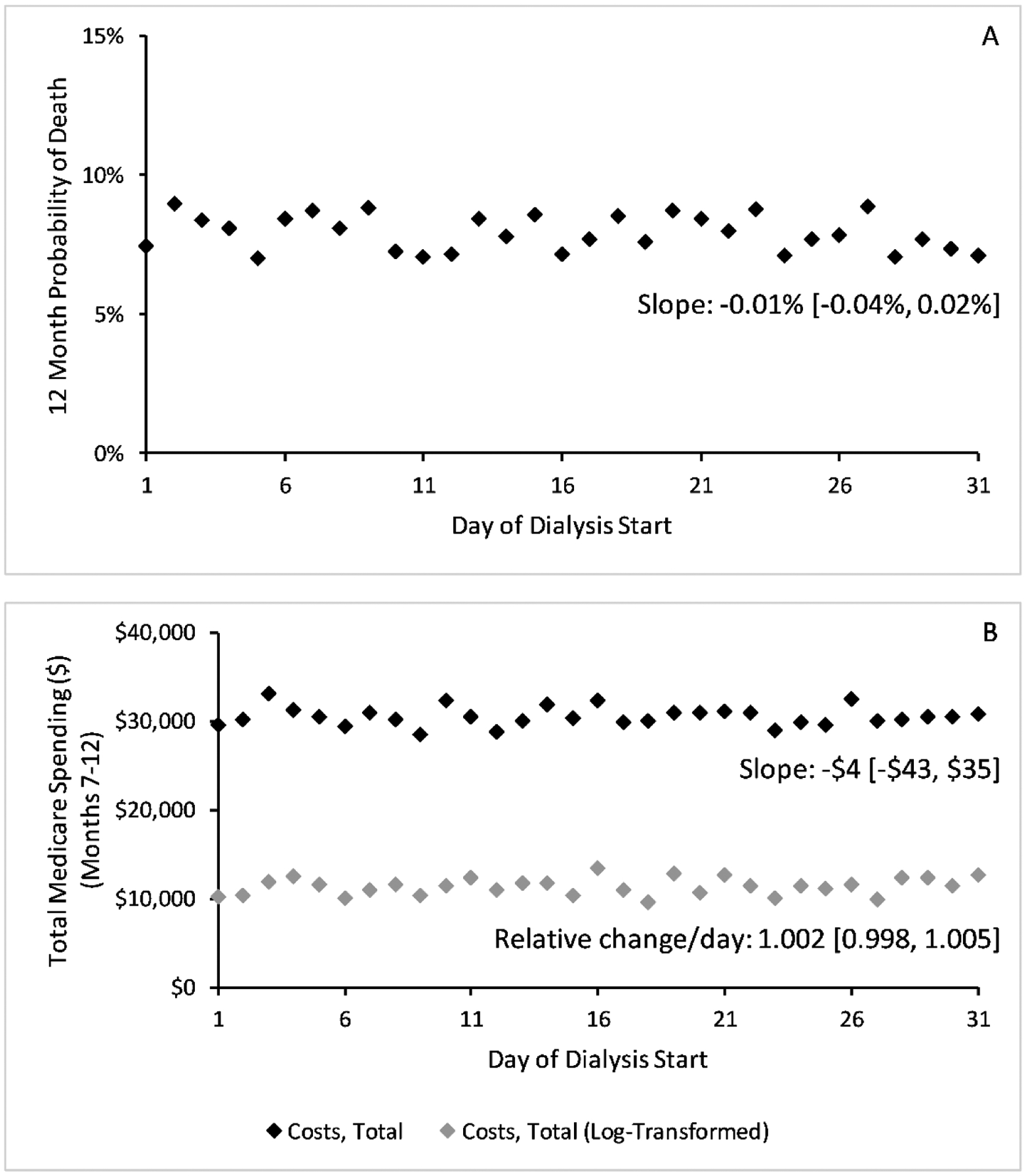
We show unadjusted 12-month probability of death (Panel A) and total Medicare spending per patient in months 7–12 of dialysis (Panel B) by the day of dialysis start. Costs (Panel B) are modeled using log costs (grey dots) and without log transforming (block dots). Trends and slopes were computed using ordinary least squares without adjusting for other covariates. We show differences in hospitalization rates by day of dialysis start in Appendix Figure 5. Abbreviations: HD = hemodialysis, PD = peritoneal dialysis.
Comparing Peritoneal Dialysis and Hemodialysis
Unadjusted, patients starting with PD had significantly higher survival at one year (96% versus 92%, p<0.001), fewer hospitalizations during months 7–12 (0.61 versus 0.69, p<0.001), but higher log-transformed spending during months 7–12 ($15,917 versus $10,622, p<0.001) (Appendix Figure 6). PD was less expensive than HD during months 7–12 when modeling costs directly ($27,386 versus $30,875, p<0.001).
When adjusting for confounders, the “traditional” analysis showed decreased mortality and increased spending between PD and HD (Table 2). Conversely, the IV analysis showed no significant absolute difference between PD and HD for 12-month mortality (−0.9%, 95% CI: −3.3%, 0.8%), hospitalizations during months 7–12 (−0.05, 95% CI: −0.20, 0.07), and Medicare spending during months 7–12 (−$702 (95% CI: −$4,004, $2,909) per patient.
Table 2:
Comparing Mortality, Hospitalizations, and Spending in Patients Starting with Hemodialysis and Peritoneal Dialysis (Instrumental Variable Regression)
| OUTCOME | Traditional Regression | Instrumental Variable Regression | ||||
|---|---|---|---|---|---|---|
| HD | PD | Difference | HD | PD | Difference | |
| Mortality * | ||||||
| Months 1–12 | 8.2% | 6.3% | −1.9% | 8.1% | 7.2% | −0.9% |
| (8.0%, 8.4%) | (5.6%, 6.9%) | (−2.6%, −1.2%) | (7.8%, 8.4%) | (5.2%, 8.8%) | (−3.3%, 0.8%) | |
| # Hospitalizations / Patient † | ||||||
| Months 7–12 | 0.70 | 0.72 | 0.02 | 0.71 | 0.66 | −0.05 |
| (0.69, 0.71) | (0.68, 0.76) | (−0.02, 0.07) | (0.69, 0.72) | (0.52, 0.77) | (−0.20, 0.07) | |
| Total Spending ($) / Patient (Log Model) † | ||||||
| Months 7–12 | $13,847 | $16,508 | $2,661 | $14,208 | $13,506 | −$702 |
| ($13,594, $14,309) | ($15,615, $17,904) | ($1,527, $4,110) | ($13,838, $14,932) | ($10,987, $16,906) | (−$4,004, $2,909) | |
| Inpatient Spending ($) / Patient (Log Model) † | ||||||
| Months 7–12 | $47 | $58 | $11 | $49 | $35 | −$14 |
| ($45, $51) | ($50, $70) | ($1, $23) | ($47, $57) | ($25, $56) | (−$31, $7) | |
| Dialysis Spending ($) / Patient (Log Model) † | ||||||
| Months 7–12 | $6,990 | $8,623 | $1,632 | $7,032 | $8,221 | $1,189 |
| ($6,857, $7,236) | ($8,104, $9,439) | ($1,027, $2,452) | ($6,862, $7,406) | ($6,582, $10,282) | (−$822, $3,418) | |
Notes: We show marginal effects when conditioning the population on starting with hemodialysis and peritoneal dialysis. The difference is the absolute change in estimated outcomes between modalities. All estimates adjusted for patient, facility, geographic, and temporal characteristics. Multiple imputation (10 imputations nested within each bootstrap sample) conducted for missing covariates.
Abbreviations: HD = hemodialysis, PD = peritoneal dialysis
Models estimated in the entire uninsured population irrespective of Medicare coverage. The traditional regression used logistic regression; the instrumental variable regression used probit regression for the first-stage and ordinary least squares for the second-stage with generalized residuals from the first-stage. 95% confidence intervals for both models estimated using non-parametric bootstrap.
Models estimated in uninsured patients with Medicare coverage months 7–12 while alive. The traditional regression used ordinary least squares; the instrumental variable regression used probit regression for the first-stage and ordinary least squares for the second-stage with generalized residuals from the first-stage. 95% confidence intervals for the standard model estimated using the delta method and for the instrumental variable model using non-parametric bootstrap.
To explore the effect of selection bias, we successively added covariates to the traditional model in a complete case analysis. Differences in 12-month mortality and total Medicare spending declined in magnitude but remained significant (Appendix Table 5). When modeling costs directly (i.e., without log-transforming), PD was cheaper than HD in unadjusted analysis but not the adjusted traditional or IV analyses (Appendix Table 6).
Sensitivity Analyses
Results were robust to all sensitivity analyses except when using Pearson residuals (death, costs), when excluding patients who changed dialysis modality (inpatient costs), and when omitting facility characteristics or covariates with missing values (hospitalizations, inpatient costs) (Appendix Table 7).
DISCUSSION
After accounting for selection bias, we found that initiating PD (rather than HD) did not result in statistically significant differences in mortality, hospitalizations, or Medicare spending in uninsured adults with ESKD. Unlike other observational studies, ours used an ostensibly random event, the day of dialysis start, to mitigate bias from unobserved characteristics. We found mortality and cost differences when using traditional observational methods that dissipated with IV regression.
Unlike previous studies associating similar10,12 or improved survival11,13 and decreased costs with PD,3–5,14 our study did not show statistically significant differences in mortality, hospitalization, and costs between PD and HD among uninsured patients.33 In addition to population differences between our analysis and prior studies, there are other key differences. Our log-transformed model showed increased unadjusted costs in PD over HD, while our non-transformed model showed decreased costs (consistent with prior studies). One can reconcile these results by recognizing that the log-transformed model down-weights outliers. That is, the median patient on PD is more expensive than the median patient on HD, but average per patient spending on PD is lower than on HD. To control for confounders, prior studies have used multivariable regression and matching techniques, with annual savings estimates of over $10,000 per patient attributable to PD.3,10–14 However, prior findings are subject to selection bias.
Our study demonstrates the pitfalls of standard observational methods. Selection bias when comparing dialysis modalities is unsurprising, since patients starting with PD are generally younger, healthier, and less socioeconomically disadvantaged than those starting with HD.
Although an RCT could address these shortcomings, none have been successful. In 2003, investigators in the Netherlands designed an RCT to compare PD and HD. They recruited 773 patients, but only 38 patients (fewer than 5%) agreed to participate.15 IV regression mitigates these selection bias concerns.
Generalizability of IV estimates, however, is limited to local average treatment effects20 of uninsured patients who were encouraged by the Medicare coverage policy to switch from PD to HD. We make two observations that suggest our findings may be more widely applicable to the dialysis population. First, the failures of previous RCTs suggest patients have strong preferences for dialysis modality, and patients more likely to switch dialysis modalities due to insurance coverage may be more likely to switch modalities in response to real-world policy. Second, although uninsured patients without ESKD are sicker than the rest of the population, uninsured patients with ESKD are younger and healthier than their Medicare or Medicaid counterparts and are more likely to use peritoneal dialysis than patients with Medicaid.18 Our findings support expert opinion that patients should be allowed to choose between two roughly equivalent dialysis modalities based on personal preferences.
Despite little evidence demonstrating its superiority (at least vis-à-vis conventional outcome metrics), the Administration has made home dialysis the centerpiece of its ESKD reform effort.7 Persuading patients to start with or switch to home dialysis, though, may be challenging. Past randomization failures suggest that many patients have strong dialysis modality preferences by the start of dialysis. CMS implemented financial incentives for home dialysis in 2011, but uptake remains sluggish.24,34 Even in other countries prioritizing home dialysis use, patients using home dialysis usually constitute less than half the ESKD population.35
To further encourage home dialysis use, two new incentives for home dialysis have already taken effect this year: a policy exacting large financial penalties from facilities and nephrologists with low home dialysis uptake and an expanded dialysis transitional add-on payment for home dialysis capital equipment.9,36 Without evidence of improved outcomes or downstream savings, these policies seem likely to penalize providers with little material benefit while potentially adding to already ballooning ESKD costs. Additionally, some experts have suggested that CMS increase reimbursements for pre-dialysis education8,37 or pay for dedicated home dialysis caregivers (“assisted peritoneal dialysis”) with the hope of eventual savings.38,39 Our results suggest that additional funding of large-scale home dialysis efforts would not yield substantial benefits to patients or savings to the Medicare program, and certainly not at the magnitude suggested by policymakers.
Even if PD does not significantly reduce mortality, hospitalizations, or spending, it could yield improved quality of life. Unfortunately, studies investigating quality of life are also fraught with selection bias.40,41 Patients using PD are more likely to have stable housing, more likely employed, and more likely to have strong social supports, characteristics that also contribute to a higher quality of life. Because we could not observe quality of life, we could not formally study this question. Still, based on our findings, we suspect that quality of life studies would see large attenuations in effect sizes if subjected to randomization or quasi-experimental designs.
Our findings have important limitations that may diminish their applicability to the general ESKD population. First, despite using an IV, there may be residual confounding by unobserved characteristics. Still, given the difficulties of past RCTs—the gold-standard for addressing unobserved confounding—, IV regression may be the best alternative to mitigate bias. Although limitations of the CMS-2728 Form may contribute to residual confounding,42 the IV model mitigates these unobserved biases. Second, we cannot prove the assumptions of the IV model. However, we provide evidence that the dialysis start day is randomly distributed and also show that dialysis start day does not affect long-term Medicare enrollment, the most likely alternative mechanism through which dialysis start day could affect outcomes. Third, we could not assess differences in short-term costs between months 1–6 given data limitations. However, our sensitivity analyses demonstrate no differences in extended long-term costs, from month 7 through 12 (in our primary analysis) and month 24 (in our secondary analysis), which is likely the more relevant policy metric. Fourth, our findings may not generalize beyond the uninsured. Because IV estimates are of local average treatment effects, they apply only to patients who switched dialysis modality due to retroactive Medicare coverage. However, as previously discussed, the uninsured population is younger and more likely to use PD and thus may be more representative of patients willing to consider PD.
CONCLUSIONS
In summary, we find no evidence that increasing PD use for patients with incident ESKD would offer reductions in mortality, hospitalizations, or cost. Policy makers eager to promote home dialysis should temper expectations of improved outcomes and reduced spending.
Supplementary Material
HIGHLIGHTS.
While observational studies suggest that peritoneal dialysis offers improved outcomes and lower costs than hemodialysis, these studies are fraught with selection bias. Researchers have attempted randomized controlled trials to study the initial choice of dialysis modality (peritoneal dialysis or in-center hemodialysis) but have failed to enroll enough patients.
We conducted an instrumental variable analysis, exploiting an idiosyncratic policy encouraging PD use in 58,539 uninsured adults with end-stage kidney disease. The initial choice of dialysis modality (peritoneal dialysis or in-center hemodialysis) did not result in statistically significant differences in mortality, hospitalizations, or costs.
Policy makers have already implemented policies financially incentivizing peritoneal dialysis over in-center hemodialysis. Even if these policies increase peritoneal dialysis use, they should temper expectations of improved outcomes or reduced spending.
Acknowledgments:
The data reported here have been supplied by the United States Renal Data System (USRDS). The interpretation and reporting of these data are the responsibility of the authors and should not be seen as an official policy or interpretation of the U.S. government.
Funding/Support:
This work was supported by the National Institutes of Health (NIH) through the National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK) and the National Institute of Aging (NIA): EL receives support from NIDDK K08DK118213, GMC receives support from NIDDK K24 DK085446, JB receives support from NIA P30 AG17253, and DL receives support from NIA NIA R01 AG062277-01. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH.
EL is also supported by the University Kidney Research Organization.
Funding/Support:
This work was supported by grants K08 DK118213 and K24 DK085446 from the National Institute of Diabetes and Digestive and Kidney Diseases and grants P30 AG17253 and R01 AG062277-01 from the National Institute on Aging.
Role of the Funder/Sponsor:
The funder had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication.
Financial Disclosures:
Dr. Lin reports receiving a grant from the National Institute of Diabetes and Digestive and Kidney Diseases during the conduct of the study; personal fees from Acumen, LLC, outside the submitted work; and sits on the American Society of Nephrology - Quality Committee and National Kidney Foundation - Scientific Advisory Board. Mrs. Lung reports no conflict of interest. Dr. Chertow reports receiving a grant from the National Institute of Diabetes and Digestive and Kidney Diseases during the conduct of the study; personal fees from Satellite Healthcare, Akebia, AstraZeneca, Baxter, Cricket, Gilead, Reata, Sanifit, Vertex, Angion, Bayer, and ReCor outside the submitted work; grants from Amgen outside the submitted work; other support from CloudCath, Durect, DxNow, Eliaz Therapeutics, Outset outside the submitted work; and personal fees and other support from Ardelyx, DiaMedica, Miromatrix, and Unicycive outside the submitted work. Dr. Bhattacharya reports receiving a grant from National Institute on Aging during the conduct of the study and personal fees from Acumen, LLC, outside the submitted work. Dr. Lakdawalla reports receiving a grant from National Institute on Aging during the conduct of the study and other support from Precision Medicine Group outside the submitted work. No other disclosures were reported.
Conflict of Interest Disclosures:
Dr Lin reported receiving a grant from the National Institute of Diabetes and Digestive and Kidney Diseases during the conduct of the study; personal fees from Acumen, LLC, outside the submitted work; and sits on the American Society of Nephrology - Quality Committee and National Kidney Foundation - Scientific Advisory Board. Dr Chertow reported receiving a grant from the National Institute of Diabetes and Digestive and Kidney Diseases during the conduct of the study; personal fees from, and being a member of the Board of Directors of, Satellite Healthcare, being on the Trial Steering Committee for Akebia, AstraZeneca, Gilead, outside the submitted work, grants to institution to support staff from Amgen outside the submitted work, being a Scientific Advisor to Baxter, Cricket, DiaMedica, Reata, and being a Scientific Advisor with stock options to Ardelyx, CloudCath, Durect, DxNow, Eliaz Therapeutics, Miromatrix, Unicycive, and Outset outside the submitted work; and being a DSMB member with Angion, a DSMB co-chair with Bayer and DSMB chair for ReCor outside the submitted work. Dr Bhattacharya reported receiving a grant from National Institute on Aging during the conduct of the study and personal fees from Acumen, LLC, outside the submitted work. Dr Lakdawalla reported receiving grant R01 AG062277-01 from National Institute on Aging during the conduct of the study; support from Precision Medicine Group outside the submitted work; personal fees from Amgen, Biogen, Genentech, GRAIL, Edwards Lifesciences, Novartis, Otsuka, Perrigo, and Pfizer outside the submitted work. No other disclosures were reported. No other disclosures were reported.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.United States Renal Data System. 2019 USRDS Annual Data Report: Epidemiology of Kidney Disease in the United States. National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases; 2019. Accessed May 7, 2020. https://usrds.org/2019/view/Default.aspx [Google Scholar]
- 2.Hartman M, Martin AB, Benson J, Catlin A. National Health Care Spending In 2018: Growth Driven By Accelerations In Medicare And Private Insurance Spending. Health Aff (Millwood). 2019;39(1):8–17. doi: 10.1377/hlthaff.2019.01451 [DOI] [PubMed] [Google Scholar]
- 3.Liu FX, Treharne C, Culleton B, Crowe L, Arici M. The financial impact of increasing home-based high dose haemodialysis and peritoneal dialysis. BMC Nephrol. 2014;15:161. doi: 10.1186/1471-2369-15-161 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Shih Y-CT, Guo A, Just PM, Mujais S. Impact of initial dialysis modality and modality switches on Medicare expenditures of end-stage renal disease patients. Kidney Int. 2005;68(1):319–329. doi: 10.1111/j.1523-1755.2005.00413.x [DOI] [PubMed] [Google Scholar]
- 5.Krahn MD, Bremner KE, de Oliveira C, et al. Home Dialysis Is Associated with Lower Costs and Better Survival than Other Modalities: A Population-Based Study in Ontario, Canada. Perit Dial Int. 2019;39(6):553–561. doi: 10.3747/pdi.2018.00268 [DOI] [PubMed] [Google Scholar]
- 6.Copley C, Humer C. U.S. seeks to cut dialysis costs with more home care versus clinics. Published March 3, 2019. Accessed May 7, 2020. https://www.reuters.com/article/us-usa-healthcare-dialysis/u-s-seeks-to-cut-dialysis-costs-with-more-home-care-versus-clinics-idUSKCN1QL0G6
- 7.The White House. Executive Order on Advancing American Kidney Health Published July 10, 2019. Accessed September 18, 2019. https://www.whitehouse.gov/presidential-actions/executive-order-advancing-american-kidney-health/
- 8.Chan CT, Collins K, Ditschman EP, et al. Overcoming Barriers for Uptake and Continued Use of Home Dialysis: An NKF-KDOQI Conference Report. Am J Kidney Dis. Published online February 4, 2020. doi: 10.1053/j.ajkd.2019.11.007 [DOI] [PubMed] [Google Scholar]
- 9.Centers for Medicare and Medicaid Services (CMS), HHS. Medicare Program; Specialty Care Models To Improve Quality of Care and Reduce Expenditures. CMS-5527-F. Federal Register 2020;85(189):61114–61381. [Google Scholar]
- 10.Mehrotra R, Chiu Y-W, Kalantar-Zadeh K, Bargman J, Vonesh E. Similar Outcomes With Hemodialysis and Peritoneal Dialysis in Patients With End-Stage Renal Disease. Arch Intern Med. 2011;171(2):110–118. doi: 10.1001/archinternmed.2010.352 [DOI] [PubMed] [Google Scholar]
- 11.Lukowsky LR, Mehrotra R, Kheifets L, Arah OA, Nissenson AR, Kalantar-Zadeh K. Comparing mortality of peritoneal and hemodialysis patients in the first 2 years of dialysis therapy: a marginal structural model analysis. Clin J Am Soc Nephrol. 2013;8(4):619–628. doi: 10.2215/CJN.04810512 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Weinhandl ED, Foley RN, Gilbertson DT, Arneson TJ, Snyder JJ, Collins AJ. Propensity-matched mortality comparison of incident hemodialysis and peritoneal dialysis patients. J Am Soc Nephrol. 2010;21(3):499–506. doi: 10.1681/ASN.2009060635 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wong B, Ravani P, Oliver MJ, et al. Comparison of Patient Survival Between Hemodialysis and Peritoneal Dialysis Among Patients Eligible for Both Modalities. Am J Kidney Dis. 2018;71(3):344–351. doi: 10.1053/j.ajkd.2017.08.028 [DOI] [PubMed] [Google Scholar]
- 14.Liu FX, Walton SM, Leipold R, Isbell D, Golper TA. Financial Implications to Medicare from Changing the Dialysis Modality Mix under the Bundled Prospective Payment System. Perit Dial Int. 2014;34(7):749–757. doi: 10.3747/pdi.2013.00305 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Korevaar JC, Feith GW, Dekker FW, et al. Effect of starting with hemodialysis compared with peritoneal dialysis in patients new on dialysis treatment: a randomized controlled trial. Kidney Int. 2003;64(6):2222–2228. doi: 10.1046/j.1523-1755.2003.00321.x [DOI] [PubMed] [Google Scholar]
- 16.Centers for Medicare & Medicaid Services. Medicare Coverage of Kidney Dialysis and Kidney Transplant Services. Published July 2017. Accessed October 9, 2017. https://www.medicare.gov/Pubs/pdf/10128-Medicare-Coverage-ESRD.pdf
- 17.Ranganathan D, John GT, Yeoh E, et al. A Randomized Controlled Trial to Determine the Appropriate Time to Initiate Peritoneal Dialysis after Insertion of Catheter (Timely PD Study). Perit Dial Int. 2017;37(4):420–428. doi: 10.3747/pdi.2016.00066 [DOI] [PubMed] [Google Scholar]
- 18.Lin E, Chertow GM, Bhattacharya J, Lakdawalla D. Early Delays in Insurance Coverage and Long-term Use of Home-based Peritoneal Dialysis. Med Care 2020;58(7):632–642. doi: 10.1097/MLR.0000000000001350 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Angrist JD, Imbens GW. Two-Stage Least Squares Estimation of Average Causal Effects in Models with Variable Treatment Intensity. J Am Stat Assoc. 1995;90(430):431–442. doi: 10.1080/01621459.1995.10476535 [DOI] [Google Scholar]
- 20.Angrist JD, Imbens GW, Rubin DB. Identification of Causal Effects Using Instrumental Variables. J Am Stat Assoc. 1996;91(434):444–455. doi: 10.1080/01621459.1996.10476902 [DOI] [Google Scholar]
- 21.McClellan M, McNeil BJ, Newhouse JP. Does more intensive treatment of acute myocardial infarction in the elderly reduce mortality? Analysis using instrumental variables. JAMA. 1994;272(11):859–866. [PubMed] [Google Scholar]
- 22.Schneeweiss S, Seeger JD, Landon J, Walker AM. Aprotinin during Coronary-Artery Bypass Grafting and Risk of Death. N Engl J Med. 2008;358(8):771–783. doi: 10.1056/NEJMoa0707571 [DOI] [PubMed] [Google Scholar]
- 23.Wang PS, Schneeweiss S, Avorn J, et al. Risk of Death in Elderly Users of Conventional vs. Atypical Antipsychotic Medications. N Engl J Med. 2005;353(22):2335–2341. doi: 10.1056/NEJMoa052827 [DOI] [PubMed] [Google Scholar]
- 24.Lin E, Cheng XS, Chin K-K, et al. Home Dialysis in the Prospective Payment System Era. J Am Soc Nephrol. Published online May 10, 2017. doi: 10.1681/ASN.2017010041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lin E, Kurella Tamura M, Montez-Rath ME, Chertow GM. Re-evaluation of re-hospitalization and rehabilitation in renal research. Hemodial Int. 2017;21(3):422–429. doi: 10.1111/hdi.12497 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Stock JH, Yogo M. Chapter 5: Testing for Weak Instruments in Linear IV Regression. In: Identification and Inference for Econometric Models: Essays in Honor of Thomas Rothenberg. Cambridge University Press; 2005. Accessed May 7, 2020. https://www.cambridge.org/core/services/aop-cambridge-core/content/view/8AD94FF2EFD214D05D75EE35015021E4/9780511614491c5_p80-108_CBO.pdf/testing_for_weak_instruments_in_linear_iv_regression.pdf [Google Scholar]
- 27.Terza JV, Basu A, Rathouz PJ. Two-Stage Residual Inclusion Estimation: Addressing Endogeneity in Health Econometric Modeling. J Health Econ. 2008;27(3):531–543. doi: 10.1016/j.jhealeco.2007.09.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Imbens GW, Wooldridge JM. Recent Developments in the Econometrics of Program Evaluation. J Econ Lit. 2009;47(1):5–86. doi: 10.1257/jel.47.1.5 [DOI] [Google Scholar]
- 29.Imbens GW, Wooldridge JM. What’s New in Econometrics. Lecture 6: Control Function and Related Methods. Presented at the: National Bureau of Economic Research, Summer Institute; July 31, 2007; Cambridge, MA. Accessed May 7, 2020. https://www.nber.org/WNE/WNEnotes.pdf [Google Scholar]
- 30.Schomaker M, Heumann C. Bootstrap Inference When Using Multiple Imputation. Stat Med. 2018;37(14):2252–2266. doi: 10.1002/sim.7654 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Brand J, van Buuren S, le Cessie S, van den Hout W. Combining multiple imputation and bootstrap in the analysis of cost-effectiveness trial data. Stat Med. 2019;38(2):210–220. doi: 10.1002/sim.7956 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Massey FJJ. The Kolmogorov-Smirnov Test for Goodness of Fit. J Am Stat Assoc. 1951;46(253):68–78. doi: 10.1080/01621459.1951.10500769 [DOI] [Google Scholar]
- 33.Kutner NG, Zhang R, Huang Y, Wasse H. Patient Awareness and Initiation of Peritoneal Dialysis. Arch Intern Med. 2011;171(2):119–124. doi: 10.1001/archinternmed.2010.361 [DOI] [PubMed] [Google Scholar]
- 34.United States Government Accountability Office. End-Stage Renal Disease: Medicare Payment Refinements Could Promote Increased Use of Home Dialysis. Report to the Subcommittee on Health, Committee on Ways and Means, House of Representatives. GAO-16–125. Published October 2015. Accessed June 19, 2019. https://www.gao.gov/assets/680/673140.pdf [Google Scholar]
- 35.Jain AK, Blake P, Cordy P, Garg AX. Global Trends in Rates of Peritoneal Dialysis. J Am Soc Nephrol. 2012;23(3):533–544. doi: 10.1681/ASN.2011060607 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Centers for Medicare & Medicaid Services. Medicare Program; End-Stage Renal Disease Prospective Payment System, Payment for Renal Dialysis Services Furnished to Individuals With Acute Kidney Injury, and End-Stage Renal Disease Quality Incentive Program. CMS-1732-P. 42 CFR Part 413. Federal Register. 2020;85(134):42132–42208. [PubMed] [Google Scholar]
- 37.Shukla AM, Hinkamp C, Segal E, et al. What do the US advanced kidney disease patients want? Comprehensive pre-ESRD Patient Education (CPE) and choice of dialysis modality. PLoS One. 2019;14(4). doi: 10.1371/journal.pone.0215091 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Oliver MJ, Salenger P. Making Assisted Peritoneal Dialysis a Reality in the United States: A Canadian and American Viewpoint. Clin J Am Soc Nephrol. 2020;15(4):566–568. doi: 10.2215/CJN.11800919 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Iyasere OU, Brown EA, Johansson L, et al. Quality of Life and Physical Function in Older Patients on Dialysis: A Comparison of Assisted Peritoneal Dialysis with Hemodialysis. Clin J Am Soc Nephrol. 2016;11(3):423–430. doi: 10.2215/CJN.01050115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wyld M, Morton RL, Hayen A, Howard K, Webster AC. A Systematic Review and Meta-Analysis of Utility-Based Quality of Life in Chronic Kidney Disease Treatments. PLoS Med 2012;9(9):e1001307. doi: 10.1371/journal.pmed.1001307 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Juergensen E, Wuerth D, Finkelstein SH, Juergensen PH, Bekui A, Finkelstein FO. Hemodialysis and peritoneal dialysis: patients’ assessment of their satisfaction with therapy and the impact of the therapy on their lives. Clin J Am Soc Nephrol. 2006;1(6):1191–1196. doi: 10.2215/CJN.01220406 [DOI] [PubMed] [Google Scholar]
- 42.Longenecker JC, Coresh J, Klag MJ, et al. Validation of comorbid conditions on the end-stage renal disease medical evidence report: the CHOICE study. Choices for Healthy Outcomes in Caring for ESRD. J Am Soc Nephrol. 2000;11(3):520–529. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


