Abstract
Breast irradiation has long been utilized in the adjuvant or metastatic setting to eliminate microscopic disease or to palliate existing disease, respectively. However, preclinical data have demonstrated that radiation can also alter the tumor microenvironment and induce antitumor immune responses. As a result, multiple clinical studies have been undertaken and have reported synergy between radiation and immune checkpoint blockade across various cancer types. Given recent clinical successes with immune checkpoint blockade in both early-stage and metastatic breast cancer, there has been substantial interest in combining radiation and immunotherapy to enhance local and systemic immune responses. Herein, we review the preclinical rationale for combining radiotherapy and immunotherapy, the early clinical trials that have adopted this strategy in breast cancer, and the landscape of ongoing relevant clinical trials. Finally, we propose future directions based on promising preclinical studies that integrate radiation, checkpoint blockade, and novel agents for the treatment of breast cancer.
Keywords: Cancer immunotherapy, Metastatic breast cancer, Triple-negative breast cancer, Tumor microenvironment, Radiation Oncology
Introduction
Radiotherapy (RT) is a cornerstone of breast cancer treatment with multiple trials demonstrating the efficacy of RT in preventing local recurrence and improving survival.1 This efficacy was predicated on the cell intrinsic cytotoxic activity of RT according to historical studies in radiobiology.2 Prior studies examining the mechanistic underpinning of RT largely focused on the DNA-damaging properties and the resultant cell death; however, preclinical and clinical evidence generated over the last decade suggest that RT-induced cell death can also alter the tumor microenvironment (TME) and trigger an anti-tumor inflammatory response.3-6 In light of recent clinical successes with immune checkpoint blockade (ICB)-mediated immune modulation in breast cancer together with a growing body of preclinical and clinical data demonstrating synergy between ICB and RT,7-9 the clinical application of ICB with RT is an active area of investigation. Herein, we review the preclinical and clinical rationale for combining RT and ICB in breast cancer, early data from recent clinical trials, and relevant future directions.
Preclinical Rationale for Combining RT and ICB
The immune system has long been recognized for its role in RT-mediated tumor responses. In a seminal 1979 paper, Stone and colleagues demonstrated in a murine model of fibrosarcoma that the response to RT is T cell-dependent.10 Since that time, others have further elucidated the immune mechanisms that support the response to RT showing that the immune response elicited by RT is dependent on CD8+ cytotoxic T cells producing interferon gamma.11-13 RT generates this response through its ability to induce an immunogenic cell death in which inflammatory molecules such as high-mobility group box protein 1 (HMGB1),14 calreticulin,15 and cytosolic DNA16 are released from irradiated cells resulting in a type I interferon response and subsequent anti-tumor CD8+ T cell responses.17 Thus, strategies that augment specific T cell responses may synergize to produce more robust RT-mediated tumor responses as demonstrated in multiple in preclinical models.18 Moreover, it has been hypothesized that anti-tumor immune responses generated in the irradiated tumor may lead to systemic anti-tumor immunity also known as the abscopal effect. In 1953, R.H. Mole first described the abscopal effect (from “ab scopus” meaning away from target) as the phenomenon by which RT induces the spontaneous regression of a distant, unirradiated lesion likely through immune-mediated mechanisms.19 The abscopal effect of RT has since been reported in a variety of solid cancers, including papillary adenocarcinoma,20 melanoma,21 renal cell carcinoma,22 and hepatocellular carcinoma;23 however, cases are exceedingly rare.24
Several preclinical studies have demonstrated that targeting different aspects of the immune system can produce more robust anti-tumor immunity following RT. Several investigators have shown preclinical synergy with RT and immunoadjuvants such as FMS-like tyrosine kinase receptor 3 ligand (Flt3-L), a growth factor for dendritic cells.25-27 The combination of RT with an immunoadjuvant was tested in a proof-of-principle trial in which RT was combined with granulocyte-macrophage colony-stimulating factor (GM-CSF) and produced an abscopal response in nearly 30% of (11/41) patients.28 Of note, 36% (5/14) of women with metastatic breast cancer participating in the trial demonstrated a partial response in an unirradiated lesion.
The identification of immune checkpoints such as cytotoxic T lymphocyte-associated antigen 4 (CTLA-4) and programmed cell death-(ligand) 1 [PD-(L)1] and the subsequent successful development of drugs that target those ligands, created a novel opportunity to potentially augment the abscopal responses to RT. Early preclinical studies combining RT with ICB were performed in murine models of breast cancer and demonstrated synergy. For example, in 2005, Demaria and colleagues demonstrated that combining RT with an anti-CTLA-4 antibody significantly delayed metastases and improved survival in a breast cancer model.29 Moreover, this synergy was augmented by administering short courses of fractionated RT and by administering anti-CTLA-4 prior to RT.30, 31
Additional preclinical studies have demonstrated that RT further supports anti-tumor immunity by altering the TME. For example, in a murine model of breast cancer, PD-L1 was upregulated on tumor cells following irradiation.18 Accordingly, PD-L1 blockade amplified the anti-tumor response to RT in a CD8+ T cell-dependent manner. As such, upregulation of PD-L1 in the TME following RT could potentially be overcome by ICB. Similar observations were made by in a murine model of melanoma, in which tumors that were resistant to anti-CTLA-4 and RT were shown to upregulate PD-L1 in a non-redundant immune pathway of evasion.32 In this study, PD-L1 blockade was shown to reinvigorate exhausted T cells and enhance response to RT and anti-CTLA-4.
In addition to the regulation of immune checkpoints, RT can also influence the diversity of T cell clones by increasing the number and diversity of tumor antigens. For example, RT was shown to increase the diversity of the T cell receptor (TCR) repertoire of tumor-associated lymphocytes.32 These findings were recapitulated on functional analysis of a phase II trial combining anti-CTLA-4 and RT in metastatic non-small cell lung cancer.33 Patients who responded to treatment had a significant T cell clonal expansion compared to non-responders. In one patient with a complete response, two new T cell clones were identified that suggested that RT enhanced the expression of new immunogenic mutations. Recent preclinical studies have further shown that neoantigens upregulated by RT are recognized by specific T cells whose activity can be augmented by immunization with RT-elicited epitopes.34
In total, these data indicate that the full efficacy of RT depends on eliciting an anti-tumor immune response. RT does this in part through upregulating the expression of immunogenic mutations that can be used for antigen cross-presentation and releasing pro-inflammatory molecules. Further, this process can be enhanced by adding immune checkpoint inhibitors.
Clinical Rationale for ICB in Breast Cancer
Several trials have shown limited activity with anti-PD-1/PD-L1 monotherapy in metastatic breast cancer with objective response rates (ORRs) from 5% to 24% reported. The most robust responses have been observed in triple negative breast cancer (TNBC), in PD-L1-positive tumors, and in the first-line palliative setting.35-40 In fact, two large phase III trials (IMpassion130 and KEYNOTE-355) have demonstrated clinical benefit with concurrent ICB and chemotherapy combinations in patients with PD-L1-positive advanced TNBC.41-43 Moreover, grade 3 or higher toxicities were observed in 48% to 68% of all patients. Ongoing clinical trials are focused on combining ICB with cytotoxic or molecularly-targeted agents across all subtypes of breast cancer. In a recent overview of the immune-oncology landscape, ICB was most commonly co-administered with chemotherapy or HER2-directed therapy with these combinations comprising 35% (64 of 185) of all active ICB trials in breast cancer.44
Thus, in the metastatic setting, there are several challenges to overcome if ICB regimens are to be more broadly relevant. First, there are limited ICB options for patients with PD-L1-negative tumors or combined positive score (CPS) < 10 by the 22C3 assay. Second, novel ICB combinations are needed to minimize toxicity - in IMpassion130, approximately 16% of patients discontinued at least one agent due to adverse events. Finally, it is unclear if patients should remain on therapy or transition to new ICB agents after progression on ICB.
In early stage breast cancer, two clinical trials (I-SPY2 and KEYNOTE-522) have demonstrated promising activity with neoadjuvant ICB and chemotherapy. In the phase II I-SPY2 trial, estimated pathologic complete response (pCR) rates more than doubled with the addition of ICB to standard neoadjuvant chemotherapy (NAC) among patients with early TNBC.45 The estimated pCR rate also improved in the estrogen receptor positive cohort of I-SPY2 from 13% to 34%. Similarly, the phase III KEYNOTE-522 trial demonstrated a significant improvement in pCR rates from 51.2% with NAC alone to 64.8% with the addition of ICB in patients with early TNBC.46 Of note, the rate of grade 3 or higher adverse events was 78% in the ICB-chemotherapy arm. Although exploratory, a subgroup analysis demonstrated a pronounced benefit among node-positive patients, which suggests possible T cell priming in involved lymph nodes. Strategies exploring ICB combinations to optimize cure rates while minimizing treatment toxicity are both in development and underway.
Clinical Rationale for RT with ICB in Breast Cancer
Among current ICB trials in breast cancer, only 7% (13 of 185) of active trials combine RT and ICB.44 In an early single-arm trial, 73 patients with metastatic cancer, including six women with breast cancer, were treated with concurrent SBRT and pembrolizumab.47 In this study, the ORR of the entire cohort was 13.2%. Given that the response rate was not stratified by tumor type, it is unclear what is the true clinical benefit for metastatic breast cancer. Nonetheless, the trial demonstrated that the combination of SBRT and ICB was generally well tolerated and safe without significant side effects.
Despite the modest number of breast cancer dedicated RT-ICB trials, several key trials have been reported (Table 1). In the first study of RT with ICB for the treatment of breast cancer, the safety and efficacy of brain RT and concurrent CTLA4-mediated immune modulation with tremelimumab, +/− trastuzumab, a HER2-directed antibody, were explored in patients with HER2-negative or HER2-positive brain metastases.48 The primary endpoint was 12-week non-central nervous system (CNS) disease control rate (DCR). The 12-week non-CNS DCR was 10% (2/20 patients) in the HER2-negative cohort and 33% (2/6 patients) in the HER2-positive cohort. One patient with heavily pretreated, trastuzumab resistant, HER2-positive disease experienced a durable partial response with evidence of peripheral T-cell activation. Given the encouraging responses observed in the HER2-positive safety cohort, a clinical trial to determine efficacy of ICB with trastuzumab for HER2+ breast cancer brain metastases is planned.
Table 1. Published trials combining radiotherapy and immune checkpoint blockade in metastatic breast cancer.
AE, adverse event; AST, aspartate aminotransferase; CI, confidence interval; CR, complete response; HR+/HER2−, hormone receptor-positive/human epidermal growth factor receptor 2-negative; mBC, metastatic breast cancer; mTNBC, metastatic triple negative breast cancer; ORR, objective response rate; PR, partial response; RT, radiation therapy; WBRT, whole brain radiotherapy.
| Trial | Phase | N | Tumor Type | Intervention | ORR | Toxicity |
|---|---|---|---|---|---|---|
| McArthur et. al.48 | - | 20 | HER2+ or HER2− mBC with brain metastases | Concurrent WBRT and Tremelimumab | 12-week non-CNS DCR 10% in HER2− mBC 33% in HER2+ mBC |
15 grade 3 AE (fatigue, diarrhea, colitis), 0 grade 4 or 5 AE |
| Voorwerk et. al. (TONIC)49 | 2 | 12 | mTNBC | Sequential RT (24 Gy in 3 fractions) and Atezolizumab | 12% (1 PR) 95% CI, 0.2-38.5% |
3 grade 3 AE, 0 grade 4 AE, 1 grade 5 AE (nivolumab-related) |
| Ho et. al.54 | 2 | 17 | mTNBC | Concurrent RT (24 Gy in 3 fractions) and Pembrolizumab | 17.6% (3 CR) 95% CI, 4.7-44.2% |
3 grade 3 AE (fatigue, infection, lymphopenia), 1 grade 4 AE (lymphopenia), 0 grade 5 AE |
| Barroso-Sousa et. al.55 | 2 | 8 | HR+/HER2− mBC | Pembrolizumab prior (2-7 days) to RT (20 Gy in 5 fractions) | 0% | 1 grade 3 AE (AST elevation), 0 grade 4 or 5 AE |
The phase II TONIC trial tested whether priming with RT or chemotherapy prior to ICB can alter the tumor microenvironment and increase tumor sensitivity to PD-1/PD-L1 blockade.49 In this non-comparative study, 67 women with metastatic TNBC were randomized to nivolumab, a human monoclonal antibody to PD-1, without induction or with 2-week low-dose induction with RT (3 x 8Gy), cyclophosphamide, cisplatin or doxorubicin. Although the ORR of the entire cohort was 20%, the ORR for the RT arm was only 8% (1/12 patients) while the ORR for the “no induction” cohort was 17% (2/12 patients). Based on the preset criteria by Simon two-stage design, the RT arm was discontinued for further study.
There are several possible explanations for the poor response rate to sequential RT and ICB on TONIC, which was notably lower than the response rate in the “no induction” control arm. For example, due to the small patient cohorts, several patient characteristics were unbalanced, including decreased stromal tumor-infiltrating lymphocytes (TILs) at baseline in the RT cohort.50 TILs have been showed to be important predictors for response to immunotherapy in breast cancer.51 Moreover, the trial included a two-week waiting period between RT and ICB to test whether induction could turn “cold” into “hot” tumors. Preclinical evidence suggests that concurrent RT or ICB induction prior to RT may be more effective in generating an immunologic response.31, 52, 53 Thus, sequential administration of RT prior to ICB may have hindered any potential synergy. Finally, irradiation of only a single index lesion was allowed to detect an abscopal response in any untreated lesion. However, treatment of multiple metastatic lesions may potentially increase systemic response rates to RT and ICB by both debulking metastatic disease and exposing additional neoantigens that may enhance T cell priming.
In a multi-institutional phase II trial of concurrent standard-of-care palliative RT with ICB in 17 women with heavily pretreated, metastatic TNBC, patients received SBRT (30 Gy in 5 fractions) with pembrolizumab, a humanized, monoclonal antibody to PD-1.54 In contrast to the TONIC study, pembrolizumab was administered within three days after the first fraction of RT. The ORR of the entire cohort was 17.6% (3/17 patients), which compared favorably to the response rate of 5% with pembrolizumab monotherapy in a similar PD-L1 unselected population in cohort A of KEYNOTE-086.39 Of the women who were radiographically evaluable at week 13, 33% (3/9 patients) demonstrated a complete response (CR) with 100% reduction of tumor volume outside the irradiated field. The anti-tumor response was durable, ranging from 18 weeks to >108 weeks, consistent with a systemic immunologic response to the combination.
One exceptional responder treated on the pembrolizumab/RT metastatic TNBC trial, who previously received treatment with a colony-stimulating factor 1 receptor antibody, had no evaluable disease at time of last follow-up at 108 weeks. Although the 3 patients achieving a CR were PD-L1 positive at baseline, PD-L1 expression was not correlated with ORR and progression free survival overall. Limitations of the trial include a modestly-sized patient cohort and the single arm, non-randomized design. Nonetheless, the durability of some of the results are encouraging for this heavily pretreated population with a historically poor prognosis. Further clinical investigations are warranted to further delineate the interplay between RT and ICB in metastatic TNBC.
Finally, the efficacy of RT and ICB was also tested in a phase II trial of women with heavily pretreated, hormone receptor-positive (HR+)/HER2-negative breast metastatic breast cancer.55 Patients received pembrolizumab followed by palliative RT (20 Gy in 5 fractions) within 2 to 7 days. There were no objective responses observed in the first eight patients that were enrolled and the trial was consequently closed to accrual per the Simon two-stage design. The lack of clinical activity in this study may reflect the innate resistance of metastatic HR+ disease to ICB as demonstrated in other studies,36, 37 the allowance for bone RT on study which may not represent the optimal RT target to generate synergy, the low prevalence of PD-L1+ tumors, and the fact that the RT dose was lower than in previously reported trials. Moreover, given that anti-PD-1 acts on both new and exhausted T-cells,56-58 RT after ICB may eradicate any newly infiltrated or activated T-cells.
Insights for Future RT and ICB Trials
These early experiences with RT plus ICB have provided important insights that should directly inform future trials. First, the identification of predictive biomarkers is needed to optimize response to combination RT and ICB.59 Several candidate pretreatment biomarkers include PD-L1 expression, tumor infiltrating lymphocytes, and tumor mutational burden. In several trials, PD-1/PD-L1 status has served as a critical biomarker for response to ICB monotherapy or in combination with chemotherapy. For example, response rates to pembrolizumab monotherapy varied drastically between the unselected cohort A and PD-L1-positive cohort B of KEYNOTE-086.38, 39 Similarly, in IMpassion130, the overall survival benefit to ICB-chemotherapy for metastatic TNBC was limited to patients with PD-L1+ tumors.41, 42
Unfortunately, there is limited prospective validation of PD-L1 expression as a predictive biomarker for response to RT and ICB. In a phase II trial of pembrolizumab and RT in metastatic TNBC, it was noted that all three patients with complete responses were PD-L1 positive; however, PD-L1 was not statistically correlated with response or survival due to the limited sample size. Moreover, in the metastatic non-small cell lung cancer (NSCLC), patients with PD-L1-negative tumors experienced the greatest clinical benefit with concurrent RT and ICB.8 By contrast to ICB-chemotherapy trials, RT may induce the expression of PD-L1,18, 32 and so pretreatment PD-L1 or PD-1 status may fall short as a prognostic factor.
Another key question of combining RT and ICB is the question of which lesion is the optimal target for RT to induce an abscopal response. To date, it is unclear whether this would be visceral, lymph node, or bone metastases. In the phase II trial of pembrolizumab and RT in HR+/HER2-negative breast cancer, all eight patients received palliative RT to the bone and there were no objective responses observed. While the palliative RT doses may have contributed to this futility, this also raises the possibility that bone lesions may not be an optimal target for RT-ICB combinations. Although bone is a highly vascular organ that contains high levels of multiple immune cells, a large retrospective trial of patients with metastatic NSCLC demonstrated that patients with bone metastases had decreased immunotherapy efficiency.60 Given that these patients experienced early progression and death compared to patients without bone metastases, it is hypothesized that immunotherapy cannot overcome the negative prognostic factor of bone dissemination. Bone metastases may also promote the differentiation of myeloid-derived suppressor cells that may limit the efficacy of RT-ICB.
Similarly, the presence of liver metastases is a negative prognostic factor for survival and response to immunotherapy.61 This is mediated in part through the apoptosis of CD8+ T cells upon interaction with monocyte-derived macrophages within the liver. However, in preclinical models, liver-directed RT promotes T cell survival by eliminating the population of suppressive macrophages. Therefore, liver-directed RT may improve systemic responses to ICB with the caveat that these patients are at increased mortality risk at baseline. Lastly, while lymph node recurrences or metastases may be common, preclinical studies have demonstrated reduced synergy between RT and ICB with irradiation of the draining lymph nodes in breast cancer.62 Given that PD-1 inhibition promotes T cell activation or reinvigoration, nodal RT may negate any benefit from ICB activation.
Finally, the question of whether RT should be delivered to multiple metastatic sites or a single lesion remains. The TONIC trial allowed RT to a single index lesion, which may have limited its synergy with ICB. However, the benefit of irradiating multiple metastatic lesions would be two-fold. First, the immune response generated by RT-ICB may be insufficient to handle the tumor burden associated with multiple metastases; therefore, multisite irradiation would serve to debulk oligometastatic disease. Indeed, SBRT for oligometastatic disease has been shown to improve survival with minimal toxicity in both phase II trial and meta-analyses.63, 64 Second, multisite RT may help expose neoantigens that can prime a T cell response. Indeed, multisite SBRT and pembrolizumab was associated with improved survival, increased expression of innate and adaptive immune genes, and decreased expression of DNA repair genes.65 Lastly, preclinical models demonstrated a role for “high-dose” RT to the primary tumor site and “low-dose” RT to secondary metastatic lesions to reprogram the inhibitory TME of secondary sites.66 In total, multisite irradiation may improve response rates to RT-ICB and promote anti-tumor immunity.
Ongoing and Future Trials of RT and ICB in Metastatic Breast Cancer
Given the observed synergy between RT and ICB in breast cancer, there are several ongoing trials exploring the combination in the metastatic, preoperative, and adjuvant settings. A recent search on clinicatrials.gov of breast cancer clinical trials with the terms “radiation” and “pembrolizumab or nivolumab or ipilimumab or atezolizumab or durvalumab or tremelimumab,” yielded 46 clinical trials as of January 11, 2021. In the metastatic setting, we identified 18 clinical trials with 5 actively recruiting, 3 not yet recruiting, and 8 active but not recruiting (Table 2). For example, TROG 17.05 AZTEC (NCT03464942) is an ongoing phase II trial in women with metastatic TNBC without brain metastases who have received one or fewer prior lines of chemotherapy.67 Patients are randomized to one of two SBRT dose fractionations (20 Gy in 1 fraction or 24 Gy in 3 fractions) followed by up to 24 months of atezolizumab, a humanized, PD-L1-directed monoclonal antibody. It is hoped that this study will elucidate whether single fraction or multi-fraction SBRT is more efficacious in combination with ICB.
Table 2. Combination radiotherapy and immune checkpoint blockade clinical trials in metastatic breast cancer.
HR+/HER2−, hormone receptor-positive/human epidermal growth factor receptor 2-negative; mBC, metastatic breast cancer; mNSCLC, metastatic non-small cell lung cancer; mTNBC, metastatic triple negative breast cancer; NCT, national clinical trial; RT, radiation therapy; SBRT, stereotactic body radiotherapy; SRS, stereotactic radiosurgery.
| NCT Number | Phase | N | Status | Tumor Type | Intervention | Sponsor |
|---|---|---|---|---|---|---|
| NCT03004183 | 2 | 57 | Recruiting | mTNBC or mNSCLC | ADV/HSV-tk + Valacyclovir + Pembrolizumab + SBRT (30 Gy in 5 fractions) | Houston Methodist Cancer Center |
| NCT03449238 | 1/2 | 41 | Recruiting | mBC with brain metastases | Pembrolizumab + SRS | Weill Cornell College of Cornell University |
| NCT03464942 | 2 | 52 | Recruiting | mTNBC | Atezolizumab + SBRT (20 Gy in 1 fraction or 24 Gy in 3 fractions) | Peter MacCallum Cancer Centre, Australia; Trans Tasman Radiation Oncology Group, TROG |
| NCT03524170 | 1 | 20 | Recruiting | HR+/HER2− mBC | M7824 (Anti-PDL1/TGFβ Trap) + RT | MD Anderson Cancer Center |
| NCT03789097 | 1/2 | 56 | Recruiting | Multiple tumor types, including mBC | Pembrolizumab + CDX-301 (Flt3 ligand) + RT + Poly ICLC (TLR3 agonist) | Icahn School of Medicine at Mount Sinai |
| NCT03915678 | 2 | 247 | Not yet recruiting | Multiple tumor types, including mTNBC | BDB001 (TLR7/8 agonist) + Pembrolizumab + SBRT (27-60 Gy in 3-5 fractions) | Institut Bergonié |
| NCT04683679 | 2 | 56 | Not yet recruiting | mTNBC | Olaparib + Pembrolizumab + SBRT (24-27 Gy in 3 fractions) | Memorial Sloan Kettering Cancer Center |
| NCT04690855 | 2 | 23 | Not yet recruiting | mTNBC | Talazoparib + Atezolizumab + SBRT (24 Gy in 3 fractions) | Emory Winship Cancer Institute |
Given the promising response rates to RT-ICB in the metastatic TNBC setting to date, several ongoing and planned trials seek to combine additional therapeutic agents in order to further augment synergy. For example, the AGADIR trial (NCT03915678) is an upcoming trial from the Institut Bergonié that will combine atezolizumab, RT, and BDB001, a Toll-like receptor (Tlr) 7/8 agonist.68 Using a Simon two-stage design, this trial will enroll 247 patients over six independent cohorts, including an arm with women with anti-PD-1/PD-L1 refractory metastatic TNBC.
Three additional trials will examine the addition of a poly ADP ribose polymerase (PARP) inhibitor to RT and ICB. The phase II TARA trial (NCT04690855) will enroll 23 women who are germline BRCA1/2 (gBRCA1/2) pathogenic variant negative (e.g. gBRCA1/2 wild-type or gBRCA1/2 variants of uncertain significance) with PD-L1 positive metastatic TNBC.69 Patients will receive hypofractionated RT (24 Gy in 3 fractions) within 72 hours of receiving atezolizumab, plus concurrent talazoparib, which is an orally bioavailable PARP inhibitor. Another study (NCT04683679) will randomize women with metastatic TNBC to RT (8-9 Gy x 3 fractions), pembrolizumab, with or without olaparib, another orally bioavailable PARP inhibitor.70 Lastly, an upcoming phase II trial will test the combination of dostarlimab, a humanized anti-PD-1 monoclonal antibody, SBRT (24 Gy in 3 fractions), and niraparib, a PARP inhibitor, in metastatic TNBC (either PD-L1-negative or PD-L1-positive with progression on ICB). Thus, numerous studies of promising combinations of ICB/RT combinations are planned or ongoing for the treatment of metastatic TNBC.
Ongoing and Future Trials of RT and ICB in Early Stage Breast Cancer
In the preoperative setting, we identified 7 clinical trials of RT and ICB in breast cancer with 3 actively recruiting, 3 not yet recruiting, and 1 active but not recruiting (Table 3). An active phase I/II trial of the preoperative combination of pembrolizumab and RT in 50 women with operable, early stage TNBC recently completed enrollment (NCT03366844). Patients received one cycle of lead-in pembrolizumab, followed by concurrent pembrolizumab and RT (24 Gy in 3 fractions) and then standard of care NAC. In an interim analysis of the first 20 enrolled patients presented at the 2020 San Antonio Breast Cancer Symposium, the pCR rate with this combination was 60% (12 of 20; residual cancer burden, RCB, 0) and 15% (3 of 20) had a near pCR (RCB 1).71 Notably, there were no observed grade 3 or 4 toxicities during RT plus ICB. Although we caution against cross-trial comparisons due to different treatment arms, the grade 3 or higher toxicity rate favorably compared to a 78% incidence of grade 3 or higher toxicities with concurrent pembrolizumab and NAC in KEYNOTE-522.46 This preoperative RT/ICB trial was recently expanded to include patients with high risk, hormone receptor-positive, HER2-negative breast cancer.
Table 3. Preoperative and adjuvant radiotherapy and immune checkpoint blockade clinical trials in breast cancer.
cT1, clinical T1 tumor (tumor size ≤2 cm); HR+/HER2−, hormone receptor-positive/human epidermal growth factor receptor 2-negative breast cancer; HT, neoadjuvant hormone therapy; IORT, intraoperative radiotherapy; LN+, lymph node-positive; NAC, neoadjuvant chemotherapy; NCT, national clinical trial; RT, radiation therapy; TNBC, triple negative breast cancer
| NCT Number | Phase | N | Status | Tumor Type | Intervention | Sponsor |
|---|---|---|---|---|---|---|
| Preoperative Trials | ||||||
| NCT02977468 | 1 | 15 | Recruiting | TNBC | Pembrolizumab + IORT | Columbia University |
| NCT03366844 | 1/2 | 60 | Recruiting | TNBC or HR+/HER2− | Pembrolizumab + RT (24 Gy in 3 fractions) | Cedars-Sinai Medical Center |
| NCT03804944 | 2 | 100 | Recruiting | HR+/HER2− | HT + Pembrolizumab + CDX-301 (Flt3 ligand) + RT (24 Gy in 3 fractions) | Weill Medical College of Cornell University |
| NCT03872505 | 2 | 140 | Not yet recruiting | TNBC | Durvalumab ± RT (24 Gy in 3 fractions) + Chemotherapy | Cedars-Sinai Medical Center |
| NCT04443348 | 2 | 120 | Not yet recruiting | TNBC or high-risk HR+/HER2− (grade 2-3 or high genomic assay score) | No RT or 9 Gy in 3 fractions or 24 Gy in 3 fractions + Chemotherapy + Pembrolizumab with exploratory proton cohort | Massachusetts General Hospital (TBCRC) |
| NCT04454528 | 1/2 | 36 | Not yet recruiting | TNBC or HR+/HER2− or HER2+ cT1 | Surgery ± Pembrolizumab ± RT | University of Pennsylvania |
| Adjuvant Trials | ||||||
| NCT02954874 | 3 | 1,000 | Recruiting | TNBC with residual disease after NAC | RT ± Pembrolizumab | National Cancer Institute |
| NCT03818685 | 2 | 114 | Recruiting | TNBC with residual disease after NAC | RT + Ipilimumab and Nivolumab OR Capecitabine | Centre Léon Bérard |
In a phase I preoperative “window of opportunity study” of pembrolizumab with intraoperative radiotherapy (IORT) in women with newly-diagnosed early stage TNBC, patients will receive 1-2 cycles of preoperative pembrolizumab followed by IORT at time of surgery, with change in tumor infiltrating lymphocytes as the primary endpoint. (NCT02977468). Lastly, the “Converting HR+ Breast Cancer into an Individualized Vaccine (CBCV)” study is a multi-institutional phase II trial in women with operable hormone receptor-positive, HER2-negative breast cancer (NCT03804944). Patients will receive 4 months of neoadjuvant hormone therapy followed by randomization to RT alone (8 Gy x 3 fractions) or RT with various immunotherapy combinations (pembrolizumab alone, CDX-301 [Flt-3 ligand] alone, or both).
To address the critical question of RT dose in the preoperative space, the Translational Breast Cancer Research Consortium (TBCRC) is currently undertaking the P-RAD trial (NCT04443348) in women with node-positive TNBC or high-risk hormone receptor-positive, HER2-negative early stage breast cancer (either histologic grade II-III or high-risk genomic assay score [Oncotype RS>25, high risk MammaPrint, PAM50, EndoPredict, or Prosigna score]).72 This phase II trial will randomize patients to no RT, conventional RT boost (9 Gy in 3 fractions), or high-dose RT (24 Gy in 3 fractions), concurrently with pembrolizumab, and followed by chemotherapy. Response will be assessed in a biopsy-proven metastatic lymph node as a measure of abscopal response outside of the field of RT. A third, unrandomized cohort will receive high-dose proton-based RT with an exploratory endpoint of cosmesis.
Ongoing Trials of RT and ICB in the Adjuvant Setting
Lastly, several trials are addressing the question of whether there is a role for RT plus ICB in the adjuvant setting for breast cancer. Two large trials are currently exploring this combination in patients with TNBC with residual disease following NAC (Table 2). SWOG S1418/NRG BR006 (NCT02954874) is a phase III randomized trial that is randomizing women with ≥ 1 cm residual tumor or positive lymph nodes after NAC to either observation or pembrolizumab before or concurrent with RT.73 The accrual goal is 1,000 patients with two planned interim analyses. Similarly, given the poor outcomes associated with residual disease in TNBC,74 the BreastImmune03 trial (NCT03818685) is a phase II trial randomized women with residual disease to either adjuvant capecitabine per CREATE-X75 or nivolumab and ipilimumab. RT is administered one week prior to C1D1 of immunotherapy/capecitabine.
Future Directions
Given the success of the anti-PD-1/PD-L1 therapies, future trials will likely all include one of these PD-1/PD-L1 directed therapies. Combinations with RT will likely combine RT and an anti-PD-1/PD-L1 with other agents that can augment the anti-tumor immune response elicited by the combination. Preclinical studies looking at augmenting RT-induced anti-tumor immunity have focused on two strategies: increasing the initial inflammatory response or preventing suppression of the immune response. Aside from the addition of further chemotherapy or radiosensitizers as detailed above, preclinical studies demonstrate that potential immunostimulatory triple combinations targeting the Type I interferon pathway such as STING (stimulator of interferon genes) agonists17, 76, 77, TLR ligands, or T-cell cytokines such as IL-1578 may hold the key to increasing anti-tumor immunity following RT.
Preclinical studies have demonstrated that the immune-mediate effects of RT are dependent on the STING-cGAS pathway.17, 77 Specifically, RT generates cytosolic DNA, which is sensed by cGAS and leads to the activation of STING in tumor cells and dendritic cells in the TME. This results in the induction of type I interferons (interferon-β) and promotes antigen uptake by the innate immune system and cross-priming of T cells. In preclinical models, intratumor STING agonists were shown to potentiate tumor response to RT. Given that type I interferon signaling can induce PD-L1 expression, trials combining anti-PD-L1, STING agonists, and RT are currently underway. Potential toxicities of STING agonists include cytokine release storm and inflammatory- and immune-related toxicities. Similarly, TLR ligands, which are immunomodulatory and commonly used as vaccine adjuvants, may be used in combination with RT and ICB to potentiate an anti-tumor immune response.79
Additional targeted therapies include PARP inhibitors, which are currently being proposed in combination with RT and ICB. In tumors that are deficient in homologous recombination, PARP inhibitors can induce synthetic lethality by preventing DNA repair and replication.80 As such, PARP inhibition can act as a radiosensitizer by delaying single strand break repair and causing double stranded breaks. The rationale for combining RT and PARP inhibition would be to potentiate RT-induced cell death, which can be recognized by reinvigorated T cells following ICB.
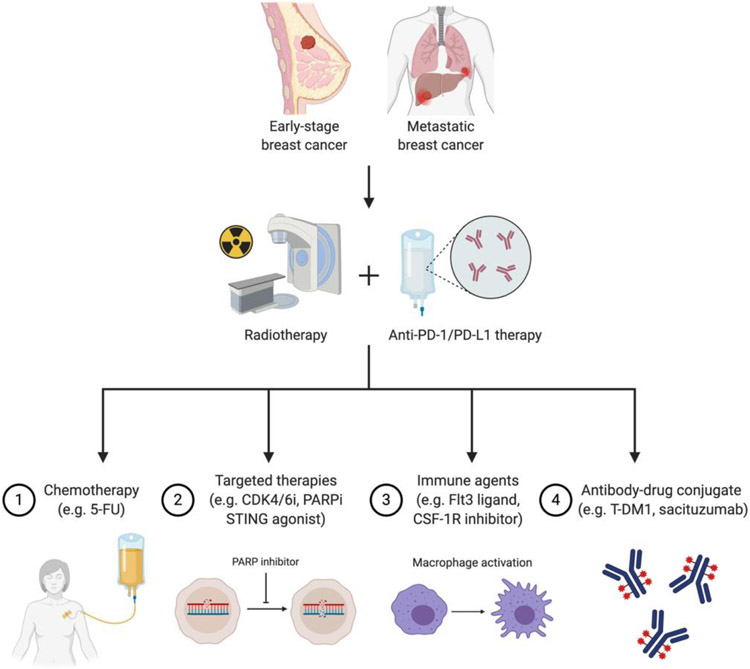
Other groups have also found that preventing RT-induced immune suppression by targeting inhibitory pathways such as TGF-β81 or suppressive immune cells such as macrophages82 or myeloid-derived suppressor cells (MDSC)83 can enhance the anti-tumor immune response elicited by RT. The next generation of trials will hopefully test some of these preclinical findings to help fully realize the immunomodulatory potential of RT (Figure 1).
Figure 1. Future strategies for augmenting synergism between radiation and immunotherapy in breast cancer.
Abbreviations: 5-FU, fluorouracil; CDK4/6i, cyclin-dependent kinase 4/6 inhibitor; CSF-1R, colony-stimulating factor receptor 1; Flt3, fms like tyrosine kinase 3; PARPi, poly ADP ribose polymerase inhibitor; STING, stimulator of interferon genes; T-DM1, ado-trastuzumab emtansine. Created with BioRender.com.
Conclusion
There is increasing preclinical and clinical evidence of potential synergy between RT and ICB in breast cancer. Early trials that have adopted this strategy have provided critical insight into the design of future clinical trials combining RT and ICB. These lessons are reflected in the diverse landscape of ongoing relevant trials in the metastatic and curative intent setting. Moving forward, critical questions remain including how to augment the immunogenic response to RT plus ICB through immunomodulatory agents or DNA damage repair mechanisms. Moreover, given increasing use of ICB in the neoadjuvant setting across solid tumors, ongoing trials will elucidate the role of ICB and RT in the preoperative setting for early stage breast cancer. Finally, trials of adjuvant ICB with standard-of-care RT will attempt to improve oncologic outcomes in patients with residual disease following neoadjuvant treatment. To this end, there are several promising avenues for the integration of RT and ICB into the breast cancer treatment paradigm.
Clinical Practice Points.
Several preclinical studies have demonstrated synergistic activity between radiotherapy and immune checkpoint blockade.
Although recent trials have demonstrated promising activity with immune checkpoint blockade in both early stage and metastatic breast cancer, there are several challenges to overcome if these regimens are to be more broadly relevant.
Early trials combining radiotherapy and immunotherapy in breast cancer have demonstrated safety and potential clinical activity in triple negative breast cancer.
These early trials have offered critical insight into patient selection, biomarkers, sequencing, and radiotherapy targets and doses.
Ongoing and planned trials integrating radiotherapy and immunotherapy in breast cancer cover a broad landscape in the curative intent and metastatic setting.
Acknowledgements:
The review described was supported in part by Cedars-Sinai Cancer, which had no role in the preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication.
Footnotes
Conflicts of Interest: HLM reports grants and personal fees from Bristol-Myers Squibb, Merck, AstraZeneca, and MedImmune; personal fees from Daiichi-Sankyo, Eli Lilly and Company, Pfizer, Genentech, Immunomedics, Puma Biotech, Amgen, Seattle Genetics, Genomic Health, and Spectrum Pharmaceuticals outside the submitted work.
References
- 1.Early Breast Cancer Trialists' Collaborative G, Darby S, McGale P, et al. Effect of radiotherapy after breast-conserving surgery on 10-year recurrence and 15-year breast cancer death: meta-analysis of individual patient data for 10,801 women in 17 randomised trials. Lancet. 2011;378:1707–1716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mavragani IV, Nikitaki Z, Kalospyros SA, Georgakilas AG. Ionizing Radiation and Complex DNA Damage: From Prediction to Detection Challenges and Biological Significance. Cancers (Basel). 2019;11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Apetoh L, Ghiringhelli F, Tesniere A, et al. The interaction between HMGB1 and TLR4 dictates the outcome of anticancer chemotherapy and radiotherapy. Immunological reviews. 2007;220:47–59. [DOI] [PubMed] [Google Scholar]
- 4.Fucikova J, Kepp O, Kasikova L, et al. Detection of immunogenic cell death and its relevance for cancer therapy. Cell Death Dis. 2020;11:1013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Burnette BC, Liang H, Lee Y, et al. The efficacy of radiotherapy relies upon induction of type i interferon-dependent innate and adaptive immunity. Cancer Res. 2011;71:2488–2496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Monjazeb AM, Schalper KA, Villarroel-Espindola F, Nguyen A, Shiao SL, Young K. Effects of Radiation on the Tumor Microenvironment. Semin Radiat Oncol. 2020;30:145–157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Postow MA, Callahan MK, Barker CA, et al. Immunologic correlates of the abscopal effect in a patient with melanoma. N Engl J Med. 2012;366:925–931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Theelen W, Peulen HMU, Lalezari F, et al. Effect of Pembrolizumab After Stereotactic Body Radiotherapy vs Pembrolizumab Alone on Tumor Response in Patients With Advanced Non-Small Cell Lung Cancer: Results of the PEMBRO-RT Phase 2 Randomized Clinical Trial. JAMA Oncol. 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Theelen W, Chen D, Verma V, et al. Pembrolizumab with or without radiotherapy for metastatic non-small-cell lung cancer: a pooled analysis of two randomised trials. Lancet Respir Med. 2020. [DOI] [PubMed] [Google Scholar]
- 10.Stone HB, Peters LJ, Milas L. Effect of host immune capability on radiocurability and subsequent transplantability of a murine fibrosarcoma. J Natl Cancer Inst. 1979;63:1229–1235. [PubMed] [Google Scholar]
- 11.Gerber SA, Sedlacek AL, Cron KR, Murphy SP, Frelinger JG, Lord EM. IFN-gamma mediates the antitumor effects of radiation therapy in a murine colon tumor. The American journal of pathology. 2013;182:2345–2354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lim JY, Gerber SA, Murphy SP, Lord EM. Type I interferons induced by radiation therapy mediate recruitment and effector function of CD8(+) T cells. Cancer Immunol Immunother. 2014;63:259–271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lugade AA, Moran JP, Gerber SA, Rose RC, Frelinger JG, Lord EM. Local radiation therapy of B16 melanoma tumors increases the generation of tumor antigen-specific effector cells that traffic to the tumor. Journal of immunology. 2005;174:7516–7523. [DOI] [PubMed] [Google Scholar]
- 14.Apetoh L, Ghiringhelli F, Tesniere A, et al. Toll-like receptor 4–dependent contribution of the immune system to anticancer chemotherapy and radiotherapy. Nature medicine. 2007;13:1050–1059. [DOI] [PubMed] [Google Scholar]
- 15.Obeid M, Panaretakis T, Joza N, et al. Calreticulin exposure is required for the immunogenicity of γ-irradiation and UVC light-induced apoptosis. Cell Death & Differentiation. 2007;14:1848–1850. [DOI] [PubMed] [Google Scholar]
- 16.Vanpouille-Box C, Alard A, Aryankalayil MJ, et al. DNA exonuclease Trex1 regulates radiotherapy-induced tumour immunogenicity. Nat Commun. 2017;8:ncomms15618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Deng L, Liang H, Xu M, et al. STING-Dependent Cytosolic DNA Sensing Promotes Radiation-Induced Type I Interferon-Dependent Antitumor Immunity in Immunogenic Tumors. Immunity. 2014;41:843–852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Deng L, Liang H, Burnette B, et al. Irradiation and anti-PD-L1 treatment synergistically promote antitumor immunity in mice. J Clin Invest. 2014;124:687–695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mole RH. Whole body irradiation; radiobiology or medicine? Br J Radiol. 1953;26:234–241. [DOI] [PubMed] [Google Scholar]
- 20.Ehlers G, Fridman M. Abscopal effect of radiation in papillary adenocarcinoma. Br J Radiol. 1973;46:220–222. [DOI] [PubMed] [Google Scholar]
- 21.Kingsley DP. An interesting case of possible abscopal effect in malignant melanoma. Br J Radiol. 1975;48:863–866. [DOI] [PubMed] [Google Scholar]
- 22.Wersall PJ, Blomgren H, Pisa P, Lax I, Kalkner KM, Svedman C. Regression of non-irradiated metastases after extracranial stereotactic radiotherapy in metastatic renal cell carcinoma. Acta Oncol. 2006;45:493–497. [DOI] [PubMed] [Google Scholar]
- 23.Ohba K, Omagari K, Nakamura T, et al. Abscopal regression of hepatocellular carcinoma after radiotherapy for bone metastasis. Gut. 1998;43:575–577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Abuodeh Y, Venkat P, Kim S. Systematic review of case reports on the abscopal effect. Curr Probl Cancer. 2016;40:25–37. [DOI] [PubMed] [Google Scholar]
- 25.Demaria S, Ng B, Devitt ML, et al. Ionizing radiation inhibition of distant untreated tumors (abscopal effect) is immune mediated. Int J Radiat Oncol Biol Phys. 2004;58:862–870. [DOI] [PubMed] [Google Scholar]
- 26.Chakravarty PK, Alfieri A, Thomas EK, et al. Flt3-ligand administration after radiation therapy prolongs survival in a murine model of metastatic lung cancer. Cancer Res. 1999;59:6028–6032. [PubMed] [Google Scholar]
- 27.Camphausen K, Moses MA, Menard C, et al. Radiation abscopal antitumor effect is mediated through p53. Cancer Res. 2003;63:1990–1993. [PubMed] [Google Scholar]
- 28.Golden EB, Chhabra A, Chachoua A, et al. Local radiotherapy and granulocyte-macrophage colony-stimulating factor to generate abscopal responses in patients with metastatic solid tumours: a proof-of-principle trial. Lancet Oncol. 2015;16:795–803. [DOI] [PubMed] [Google Scholar]
- 29.Demaria S, Kawashima N, Yang AM, et al. Immune-mediated inhibition of metastases after treatment with local radiation and CTLA-4 blockade in a mouse model of breast cancer. Clin Cancer Res. 2005;11:728–734. [PubMed] [Google Scholar]
- 30.Dewan MZ, Galloway AE, Kawashima N, et al. Fractionated but not single-dose radiotherapy induces an immune-mediated abscopal effect when combined with anti-CTLA-4 antibody. Clin Cancer Res. 2009;15:5379–5388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Young KH, Baird JR, Savage T, et al. Optimizing Timing of Immunotherapy Improves Control of Tumors by Hypofractionated Radiation Therapy. PLoS One. 2016;11:e0157164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Twyman-Saint Victor C, Rech AJ, Maity A, et al. Radiation and dual checkpoint blockade activate non-redundant immune mechanisms in cancer. Nature. 2015;520:373–377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Formenti SC, Rudqvist NP, Golden E, et al. Radiotherapy induces responses of lung cancer to CTLA-4 blockade. Nat Med. 2018;24:1845–1851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lhuillier C, Rudqvist NP, Yamazaki T, et al. Radiotherapy-exposed CD8+ and CD4+ neoantigens enhance tumor control. J Clin Invest. 2021;131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Nanda R, Chow LQ, Dees EC, et al. Pembrolizumab in Patients With Advanced Triple-Negative Breast Cancer: Phase Ib KEYNOTE-012 Study. J Clin Oncol. 2016;34:2460–2467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rugo HS, Delord JP, Im SA, et al. Safety and Antitumor Activity of Pembrolizumab in Patients with Estrogen Receptor-Positive/Human Epidermal Growth Factor Receptor 2-Negative Advanced Breast Cancer. Clin Cancer Res. 2018;24:2804–2811. [DOI] [PubMed] [Google Scholar]
- 37.Dirix LY, Takacs I, Jerusalem G, et al. Avelumab, an anti-PD-L1 antibody, in patients with locally advanced or metastatic breast cancer: a phase 1b JAVELIN Solid Tumor study. Breast Cancer Res Treat. 2018;167:671–686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Adams S, Loi S, Toppmeyer D, et al. Pembrolizumab monotherapy for previously untreated, PD-L1-positive, metastatic triple-negative breast cancer: cohort B of the phase II KEYNOTE-086 study. Ann Oncol. 2019;30:405–411. [DOI] [PubMed] [Google Scholar]
- 39.Adams S, Schmid P, Rugo HS, et al. Pembrolizumab monotherapy for previously treated metastatic triple-negative breast cancer: cohort A of the phase II KEYNOTE-086 study. Ann Oncol. 2019;30:397–404. [DOI] [PubMed] [Google Scholar]
- 40.Emens LA, Cruz C, Eder JP, et al. Long-term Clinical Outcomes and Biomarker Analyses of Atezolizumab Therapy for Patients With Metastatic Triple-Negative Breast Cancer: A Phase 1 Study. JAMA Oncol. 2019;5:74–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Schmid P, Adams S, Rugo HS, et al. Atezolizumab and Nab-Paclitaxel in Advanced Triple-Negative Breast Cancer. N Engl J Med. 2018;379:2108–2121. [DOI] [PubMed] [Google Scholar]
- 42.Schmid P, Rugo HS, Adams S, et al. Atezolizumab plus nab-paclitaxel as first-line treatment for unresectable, locally advanced or metastatic triple-negative breast cancer (IMpassion130): updated efficacy results from a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Oncol. 2020;21:44–59. [DOI] [PubMed] [Google Scholar]
- 43.Cortes J, Cescon DW, Rugo HS, et al. Pembrolizumab plus chemotherapy versus placebo plus chemotherapy for previously untreated locally recurrent inoperable or metastatic triple-negative breast cancer (KEYNOTE-355): a randomised, placebo-controlled, double-blind, phase 3 clinical trial. Lancet. 2020;396:1817–1828. [DOI] [PubMed] [Google Scholar]
- 44.Esteva FJ, Hubbard-Lucey VM, Tang J, Pusztai L. Immunotherapy and targeted therapy combinations in metastatic breast cancer. Lancet Oncol. 2019;20:e175–e186. [DOI] [PubMed] [Google Scholar]
- 45.Nanda R, Liu MC, Yau C, et al. Effect of Pembrolizumab Plus Neoadjuvant Chemotherapy on Pathologic Complete Response in Women With Early-Stage Breast Cancer: An Analysis of the Ongoing Phase 2 Adaptively Randomized I-SPY2 Trial. JAMA Oncol. 2020;6:676–684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Schmid P, Cortes J, Pusztai L, et al. Pembrolizumab for Early Triple-Negative Breast Cancer. N Engl J Med. 2020;382:810–821. [DOI] [PubMed] [Google Scholar]
- 47.Luke JJ, Lemons JM, Karrison TG, et al. Safety and Clinical Activity of Pembrolizumab and Multisite Stereotactic Body Radiotherapy in Patients With Advanced Solid Tumors. J Clin Oncol. 2018;36:1611–1618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.McArthur H, Beal K, Halpenny D, et al. Abstract 4705: CTLA4 blockade with HER2-directed therapy (H) yields clinical benefit in women undergoing radiation therapy (RT) for HER2-positive (HER2+) breast cancer brain metastases (BCBM). Cancer Research. 2017;77:4705–4705. [Google Scholar]
- 49.Voorwerk L, Slagter M, Horlings HM, et al. Immune induction strategies in metastatic triple-negative breast cancer to enhance the sensitivity to PD-1 blockade: the TONIC trial. Nat Med. 2019;25:920–928. [DOI] [PubMed] [Google Scholar]
- 50.Demaria S, Romano E, Brackstone M, Formenti SC. Immune induction strategies to enhance responses to PD-1 blockade: lessons from the TONIC trial. J Immunother Cancer. 2019;7:318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Savas P, Salgado R, Denkert C, et al. Clinical relevance of host immunity in breast cancer: from TILs to the clinic. Nature reviews. Clinical oncology 2016;13:228–241. [DOI] [PubMed] [Google Scholar]
- 52.Dovedi SJ, Adlard AL, Lipowska-Bhalla G, et al. Acquired resistance to fractionated radiotherapy can be overcome by concurrent PD-L1 blockade. Cancer Res. 2014;74:5458–5468. [DOI] [PubMed] [Google Scholar]
- 53.Buchwald ZS, Wynne J, Nasti TH, et al. Radiation, Immune Checkpoint Blockade and the Abscopal Effect: A Critical Review on Timing, Dose and Fractionation. Front Oncol. 2018;8:612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ho AY, Barker CA, Arnold BB, et al. A phase 2 clinical trialassessing theefficacy and safety of pembrolizumab and radiotherapy in patients with metastatic triple-negative breast cancer. Cancer. 2020;126:850–860. [DOI] [PubMed] [Google Scholar]
- 55.Barroso-Sousa R, Krop IE, Trippa L, et al. A Phase II Study of Pembrolizumab in Combination With Palliative Radiotherapy for Hormone Receptor-positive Metastatic Breast Cancer. Clin Breast Cancer. 2020;20:238–245. [DOI] [PubMed] [Google Scholar]
- 56.Barber DL, Wherry EJ, Masopust D, et al. Restoring function in exhausted CD8 T cells during chronic viral infection. Nature. 2006;439:682–687. [DOI] [PubMed] [Google Scholar]
- 57.Iwai Y, Ishida M, Tanaka Y, Okazaki T, Honjo T, Minato N. Involvement of PD-L1 on tumor cells in the escape from host immune system and tumor immunotherapy by PD-L1 blockade. Proc Natl Acad Sci U S A. 2002;99:12293–12297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Ahn E, Araki K, Hashimoto M, et al. Role of PD-1 during effector CD8 T cell differentiation. Proc Natl Acad Sci U S A. 2018;115:4749–4754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Gibney GT, Weiner LM, Atkins MB. Predictive biomarkers for checkpoint inhibitor-based immunotherapy. Lancet Oncol. 2016;17:e542–e551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Landi L, D'Inca F, Gelibter A, et al. Bone metastases and immunotherapy in patients with advanced non-small-cell lung cancer. J Immunother Cancer. 2019;7:316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Yu J, Green MD, Li S, et al. Liver metastasis restrains immunotherapy efficacy via macrophage-mediated T cell elimination. Nat Med. 2021;27:152–164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Marciscano AE, Ghasemzadeh A, Nirschl TR, et al. Elective Nodal Irradiation Attenuates the Combinatorial Efficacy of Stereotactic Radiation Therapy and Immunotherapy. Clin Cancer Res. 2018;24:5058–5071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Palma DA, Olson R, Harrow S, et al. Stereotactic Ablative Radiotherapy for the Comprehensive Treatment of Oligometastatic Cancers: Long-Term Results of the SABR-COMET Phase II Randomized Trial. J Clin Oncol. 2020;38:2830–2838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Lehrer EJ, Singh R, Wang M, et al. Safety and Survival Rates Associated With Ablative Stereotactic Radiotherapy for Patients With Oligometastatic Cancer: A Systematic Review and Meta-analysis. JAMA Oncol. 2021;7:92–106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Luke JJ, Onderdonk BE, Bhave SR, et al. Improved Survival Associated with Local Tumor Response Following Multisite Radiotherapy and Pembrolizumab: Secondary Analysis of a Phase I Trial. Clin Cancer Res. 2020;26:6437–6444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Barsoumian HB, Ramapriyan R, Younes AI, et al. Low-dose radiation treatment enhances systemic antitumor immune responses by overcoming the inhibitory stroma. J Immunother Cancer. 2020;8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.US National Library of Medicine. Stereotactic Radiation and Immunotherapy in Patients With Advanced Triple Negative Breast Cancer.
- 68.US National Library of Medicine. Atezolizumab Combined With BDB001 AnD Immunogenic Radiotherapy in Patients With Advanced Solid Tumors.
- 69.US National Library of Medicine. A Study to Evaluate TAlazoparib, Radiotherapy and Atezolizumab in gBRCA 1/2 Negative Patients With PD-L1+ Metastatic Triple Negative Breast Cancer (TARA).
- 70.US National Library of Medicine. A Study of Radiation Therapy With Pembrolizumab and Olaparib in Women Who Have Triple-Negative Breast Cancer.
- 71.McArthur H, Shiao S, Karlan S, et al. Abstract P3-09-09: Pre-operative pembrolizumab (pembro) with radiation therapy (RT) in patients with operable triple-negative breast cancer (TNBC). Cancer Research. 2020;80:P3-09-09-P03-09-09. [Google Scholar]
- 72.Ho AY, Wright JL, Blitzblau RC, et al. Optimizing Radiation Therapy to Boost Systemic Immune Responses in Breast Cancer: A Critical Review for Breast Radiation Oncologists. Int J Radiat Oncol Biol Phys. 2020;108:227–241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Pusztai L, Barlow W, Ganz P, et al. Abstract OT1-02-04: SWOG S1418/NRG -BR006: A randomized, phase III trial to evaluate the efficacy and safety of MK-3475 as adjuvant therapy for triple receptor-negative breast cancer with > 1 cm residual invasive cancer or positive lymph nodes (>pN1mic) after neoadjuvant chemotherapy. Cancer Research. 2018;78:OT1-02-04-OT01-02-04. [Google Scholar]
- 74.Mougalian SS, Hernandez M, Lei X, et al. Ten-Year Outcomes of Patients With Breast Cancer With Cytologically Confirmed Axillary Lymph Node Metastases and Pathologic Complete Response After Primary Systemic Chemotherapy. JAMA Oncol. 2016;2:508–516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Masuda N, Lee SJ, Ohtani S, et al. Adjuvant Capecitabine for Breast Cancer after Preoperative Chemotherapy. N Engl J Med. 2017;376:2147–2159. [DOI] [PubMed] [Google Scholar]
- 76.Baird JR, Friedman D, Cottam B, et al. Radiotherapy Combined with Novel STING-Targeting Oligonucleotides Results in Regression of Established Tumors. Cancer Res. 2016;76:50–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Vanpouille-Box C, Alard A, Aryankalayil MJ, et al. DNA exonuclease Trex1 regulates radiotherapy-induced tumour immunogenicity. Nat Commun. 2017;8:15618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Pilones KA, Charpentier M, Garcia-Martinez E, et al. Radiotherapy Cooperates with IL15 to Induce Antitumor Immune Responses. Cancer immunology research. 2020;8:1054–1063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Urban-Wojciuk Z, Khan MM, Oyler BL, et al. The Role of TLRs in Anti-cancer Immunity and Tumor Rejection. Front Immunol. 2019;10:2388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Jannetti SA, Zeglis BM, Zalutsky MR, Reiner T. Poly(ADP-Ribose)Polymerase (PARP) Inhibitors and Radiation Therapy. Front Pharmacol. 2020;11:170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Vanpouille-Box C, Diamond JM, Pilones KA, et al. TGFbeta Is a Master Regulator of Radiation Therapy-Induced Antitumor Immunity. Cancer Res. 2015;75:2232–2242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Shiao SL, Ruffell B, DeNardo DG, Faddegon BA, Park CC, Coussens LM. TH2-Polarized CD4+ T Cells and Macrophages Limit Efficacy of Radiotherapy. Cancer immunology research. 2015;3:518–525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Liang H, Deng L, Hou Y, et al. Host STING-dependent MDSC mobilization drives extrinsic radiation resistance. Nat Commun. 2017;8:1736. [DOI] [PMC free article] [PubMed] [Google Scholar]