Abstract
Background:
Poorly controlled chronic pain can lead to non-prescription use of opiates, which is a growing crisis in our communities. Transcranial magnetic stimulation (TMS) is a non-invasive therapeutic tool which has emerged as a potential treatment option for these patients. It is still unclear, however, if the dorsolateral prefrontal cortex (DLPFC) or the motor cortex (MC) is a more effective treatment location. The purpose of this study was to directly compare the effects of DLPFC versus MC TMS on pain severity and the urge to use opiates among chronic pain patients.
Methods:
Twenty-two individuals with chronic pain currently using prescription opiates were randomized to receive 10, 3000 pulse sessions of 10 Hz repetitive TMS (rTMS) to the left DLPFC (110% resting motor threshold) or left MC (90% resting motor threshold). Multivariate linear models were used to evaluate the effect of TMS on pain and opiate use, including items from the Brief Pain Inventory (BPI) as well as subjective ratings of pain, distress, and the urge for opiates.
Results:
Twenty participants (91%) completed all 10 treatment sessions and follow up visits. There was a main effect of stimulation site (F7,210=3.742, p=0.001), wherein MC stimulation decreased pain interference significantly more than DLPFC stimulation (F1,216=8.447, p=0.004). While both sites had comparable effect sizes on stress, pain, and discomfort, MC stimulation had larger effects on pain interference (Cohen’s d: 0.7) and urge to use opiates (Cohen’s d: 0.5) than DLPFC stimulation.
Conclusion:
These data suggest that the MC may be a promising target for decreasing opiate dependence and pain interference among chronic pain patients.
1.0. Introduction
Effective control of chronic pain is a top priority in the United States, as approximately 10% of adults have severe chronic pain. Existing pharmacological interventions have demonstrated limited success in chronic pain management, wherein only 30–40% of chronic pain patients, however, state they receive satisfactory (>50%) relief from their pain through pharmacological treatment (Attal et al. 2006). Many of these patients will resort to non-prescribed usage of opiates. Of individuals who misuse opioids, 80 to 90% initiated after having a legitimate prescription (Barth et al. 2013; Shei et al. 2015) and 81% endorse pain as their reason for non-medical prescription opioid use (NMPOU) (Barth et al. 2013). Low efficacy of existing medications for chronic pain may be because these treatments modulate the brain in a global fashion, rather than a neural circuit specific manner. There is now a maturing body of research suggesting that non-invasive modulation of neural circuits involved in pain processing may be a fruitful alterative treatment strategy in decreasing chronic pain.
The goal of this experiment was to evaluate two brain stimulation strategies as a tool to mitigate pain in patients currently taking chronic opiates. Transcranial magnetic stimulation (TMS) is a non-invasive neuromodulation tool which can change activity in specific cortical targets (and their related neural networks) through electromagnetic induction. The majority of TMS-based interventions for psychiatric disorders target the dorsolateral prefrontal cortex (DLPFC). In addition to its status as the Food and Drug Administration approved target for treatment resistant major depressive disorder, the DLPFC has also been investigated as a target for pain (Galhardoni et al. 2015; Lefaucheur et al. 2008; Moisset, de Andrade, and Bouhassira 2016) and for addiction (see reviews: (Diana et al. 2017; Ekhtiari et al. 2019; Gorelick, Zangen, and George 2014; Barr et al. 2011; Bellamoli et al. 2014; Grall-Bronnec and Sauvaget 2014)). A single session of TMS to the DLPFC can decrease perceived pain and corresponding brain reactivity (as measured by blood oxygen level dependent signal) in the cingulate, thalamus, midbrain and medulla (Taylor et al. 2013). Furthermore, the analgesic effects of DLPFC TMS can be blocked by naloxone, suggesting that the alleviation of pain through DLPFC TMS is related to the mobilization of endogenous opiates. Additionally, DLPFC TMS delivered postoperatively leads to less patient administered morphine use in the hospital and less opiate use in the outpatient setting (Borckardt, Weinstein, et al. 2006) and it can also decrease self-reported pain and distress among individuals with fibromyalgia (Short et al. 2011).
That said, the majority of the TMS literature in pain has focused on the primary motor cortex (DosSantos et al. 2016; Lefaucheur et al. 2008; Lefaucheur et al. 2014). In fact, the analgesic effects of TMS to the motor cortex (MC) have now been evaluated in over 128 published articles (Yang and Chang 2020). The majority of the successful MC TMS studies for pain have been in patients with acute, focused pain (e.g. phantom limb pain), rather than diffuse chronic pain (e.g. fibromyalgia, chronic lower back). These chronic pain patients are at a particularly high risk for non-medical usage of prescription opiate use due to lack of effective treatments.
A preliminary investigation into the feasibility and efficacy of these two candidate TMS stimulation sites may enable the field of brain stimulation to make a more informed choice regarding which site to pursue in a future large-scale clinical trial. This Phase 0/1 study was designed to evaluate the preliminary efficacy and therapeutic equivalence of TMS over the DLPFC versus the MC as a tool to 1) change quantitative sensory evaluation of pain and 2) qualitative self-reported ratings of pain and desire to use opiates. This was achieved through a parallel, randomized, single-blind, controlled design in a cohort of 22 individuals with chronic pain using chronic opiates.
2.0. Material and Methods
All elements of this study were reviewed and approved by the Institutional Review Board of the Medical University of South Carolina and all members of the study team received certification in Good Clinical Practice guidelines. 28 individuals with current prescription opioid use and current chronic pain were recruited from the local community. Exclusion criteria included: seizure disorder, medications known to decrease seizure threshold, pregnancy, ferromagnetic material above the shoulders, and a history of head/brain injury. Eligible participants were randomized to receive repetitive TMS (rTMS) applied over the MC or DLPFC, hereafter, referred as MC stimulation and DLPFC stimulation, respectively. Following randomization, participants received 10 sessions of real rTMS (daily, Monday-Friday at a consistent time) and 2 follow up visits (1 day, 1 week) (Table 1).
Table 1. Experimental Design.
22 individuals with chronic pain currently taking prescription opiates were randomized to receive rTMS to the left dorsolateral prefrontal cortex (DLPFC) or the left motor cortex (MC) for 10 daily sessions (10Hz, 3000 pulses/session, 5 seconds on/15 seconds off; 110% resting motor threshold @ DLPFC or 90% resting motor threshold at MC. The amplitude was higher at the DLPFC to adjust for greater scalp to cortex distance. Quantitative sensory testing (Medoc, Inc) was collected at visits 1, 6, 10 and the follow up visits 11 (1 day) and 12 (1 week). Subjective pain and opiate craving assessments and opiate use inventories were collected daily. All assessments were administered prior to rTMS treatments.
| rTMS Treatment: LDLPFC or MC [10Hz, 3000 pulses/session] | Follow Up | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Visit | * | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 |
| Participants enrolled | 28 | 22 | 21 | 21 | 21 | 21 | 21 | 20 | 20 | 20 | 20 | 20 | 20 |
| Consent & Screening | x | ||||||||||||
| rTMS | x | x | x | x | x | x | x | x | x | x | |||
| Sensory testing | x | x | x | x | x | ||||||||
| Pain & Opiate assessment | x | x | x | x | x | x | x | x | x | x | x | x | |
In-person Consent & Screening
2.1. rTMS Procedures:
Resting motor threshold (rMT) was acquired using standard procedures (PEST, (Borckardt, Weinstein, et al. 2006) and taken daily (see Supplementary Figure 4). rTMS (10Hz, 5 sec on/15 sec off, 50 pulses/train, 60 trains, 3000 pulses/session) was delivered over the left DLPFC (standardized EEG 10–20: F3, 110% rMT) or MC (20% of distance from the apex to the left tragus, covering the representation of the arm and trunk, 90% rMT) (MagVenture MagPro, Cool B65 A/P). The intensity of stimulation at these sites was based on prior research. Specifically, for MC, 90% was chosen as this was previously used in studies for pain over the MC (See Lefaucheur et al 2014 - Table 1 (bottom) for a summary of the 11 studies that have applied high frequency TMS at 90% rTMS to the motor cortex). For the left DLPFC, 110% was chosen based on previous studies applying rTMS to the DLPFC for pain (Short et al. 2011; Borckardt, Nahas, et al. 2006; Taylor, Borckardt, and George 2012). This higher intensity over the DLPFC is common in many clinical applications of rTMS, as a greater intensity is needed in order to account for the decay in electromagnetic field associated with the larger scalp-to-cortex distance in this area relative to the motor cortex location.
The location and coil orientation were marked on a nylon cap (See Figure 1 top, SIMNIBS 3.1.0 simulation; Saturnino et al 2018). The nylon cap had additional markings for the ears, center of nose, and the center of the head. In addition, specific measurements (i.e., space from inion to the cap) were taken on the cap and measured at each visit to ensure correct placement. During stimulation, participants were asked to remain awake and specifically think about their pain areas. A standardized script was read to each participant at each session asking them to begin to think about their pain over the past 24 hours, where it was located, and how it made them feel.
Figure 1. Coil Placement and Primary Outcomes.
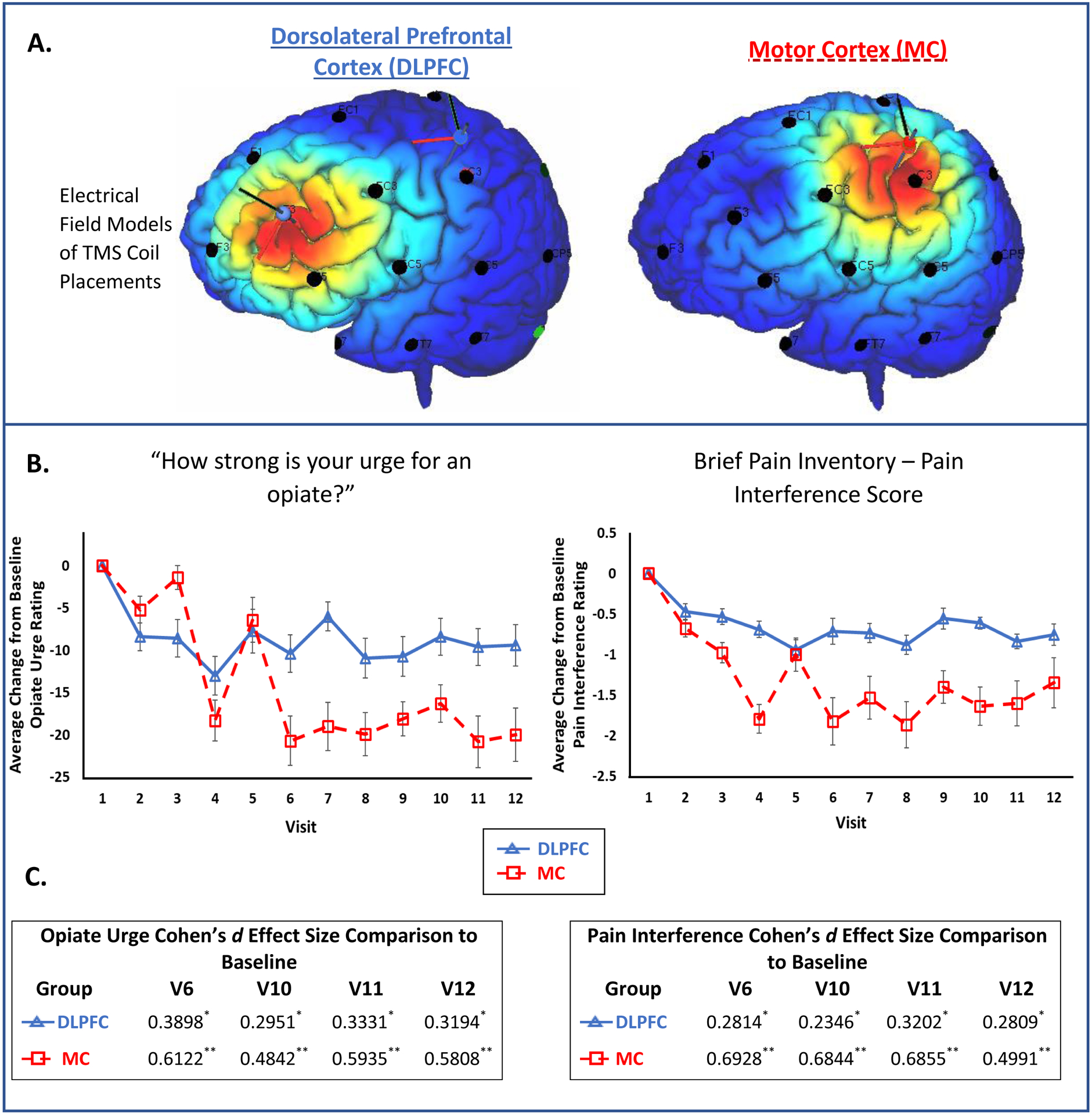
A) These chronic back pain participants currently taking prescription opiates were randomized to receive 10Hz rTMS to the left DLPFC (top left, F3 placement based on prior work in the depression and addiction field) or the left MC (top right, 20% distance from apex-tragus a territory that overlapped the somatotopic representation of the arm and trunk; SimNIBS 3.1). The e-field modeling is based on a standardized MNI template to provide a graphical illustration of the targets used. B) Self-reported assessment of pain and opiate craving was collected (see Supplementary figure 2 for full details). The data represent the average change [± standard error] from visit 1, specifically, for opiate urge (F1,216=3.248, p=0.073) and pain interference score (F1,216=8.447, p=0.004). C) The effect of rTMS on opiate urge and pain interference as defined by effect sizes for visits 6, 10, 11, and 12. Categories of the effect size are indicated (Cohen’s d): * small effect (0.2–0.5), ** moderate effect (0.5–0.8), *** large effect (>0.8).
2.2. Quantitative Pain Assessment:
Quantitative Sensory Testing (QST) was used to measure change in sensory, pain, and tolerance thresholds for heat (Medoc Ltd Advanced Medical Systems, Ramat Yishai, Israel) via the method of limits (Shy et al. 2003). A thermode was attached to the right forearm. Participants then indicated first detection of the temperature change (sensory), when the stimuli became painful (pain), and when they could no longer tolerate the heat (tolerance). This was repeated 5 times per visit and administered prior to rTMS treatments on rTMS visits 1, 6, 10, and at the 1 day (visit 11) and 1-week (visit 12) follow-up visits.
2.3. Pain and Opiate Use Assessments:
Severity of Pain and Interference of Pain with daily living was also measured via the standardized Brief Pain Inventory (BPI) questionnaire [Severity = Q3-Q6; Interference = Q9]. For each of these metrics, participants recorded their values by circling a number on a numerical rating scale (Scale 0 – 10). The pain and opiate craving inventory included five questions: ‘Q1) How STRESSED out do you feel right now? Q2) How much PAIN are you experiencing right now? Q3) How much do you WANT TO USE opiates right now? Q4) How HARD would it be for you to resist using opiates right now if it were offered to you? Q5) How much PHYSICAL DISCOMFORT do you feel right now?’. Timeline follow-back (TLFB) for prescription medications was recorded and converted to morphine equivalents. The pain and opiate use assessments were administered at all visits (visits 1–12) prior to rTMS to avoid any acute effects of TMS on the behavioral assessments (See Table 1).
2.4. Data Analysis:
The primary outcome measures were divided into 2 domains: change in quantitative sensory testing metrics and change in pain/opiate urge metrics, wherein Visit 1 was used as the baseline. A multivariate linear regression was constructed for each of these domains with site (DLPFC, MC) and time as fixed factors (SPSS v.23; IBM), including within group univariate tests for all measures. To verify that the assumptions of a regression were maintained, linearity was addressed through scatter plots, multivariate normality was addressed (Q-Q residual plot), multicollinearity was assessed with the variance inflation factor (VIF), wherein a VIF >10 suggests multicollinearity, and homoscedasticity was assessed (Goldfeld-Quandt Test of residuals). Estimated effect sizes were compiled for each dependent measure and compared across sites (Cohen’s d; small effect: 0.2–0.5; moderate effect: 0.5–0.8; large effect: >0.8).
3.0. Results
3.1. Feasibility and Tolerability:
Of the 22 individuals randomized, 20 individuals completed all 10 rTMS visits and both follow up visits. Two individuals were lost to contact (1 randomized to DLPFC, 1 to MC; 1 male, 1 female; 1 received 1 dose of rTMS, 1 received 6 doses of rTMS). Neither were at the extreme ends of the distribution for any of the demographic or health assessments performed, suggesting that this (10%) is likely a generalizable dropout rate. In addition, there was no significant difference between groups at visit 1 (p>0.05, 2 sample 2-tailed t-test) for demographic and behavioral assessments (F1,18=1.131, p=0.641) (see Supplementary Table 1).
There were no serious adverse events or unexpected adverse events. The most common adverse event was a headache, which was listed in the protocol as an anticipated risk. This was reported by 3 people, 2 of which reported it happening only 1 of the 12 study visits (both receiving MC stimulation). The other participant reported a headache on 3 of the 12 study visits (received DLPFC stimulation). Study personnel monitored these anticipated adverse events and followed up with the 3 participants. All minor headaches resolved within an hour of TMS administration, and all 3 individuals completed the study with no further issues. In addition, all but two participants completed the required TMS treatments, which further emphasizes the feasibility of these protocols.
3.2. Quantitative Pain Assessment (Supplementary Figure 1):
The multivariate linear model revealed a significant effect of site (F3,88=4.948, p=0.003), but no significant effect of time (F12,270 =0.645, p=0.803), nor a site X time interaction (F12,270=0.614, p=0.830). The main effect of site was driven by the sensory threshold assessment (F1,90=13.531, p=0.000), wherein DLPFC stimulation led to a greater increase in sensory threshold than MC stimulation. In addition, DLPFC stimulation produced a moderate effect size (Cohen’s d: −0.5) whereas MC stimulation had no effect (Cohen’s d: 0.1) (Supplementary Table 2).
3.3. Self-reported Pain and Opiate Assessments (Supplementary Figure 2):
The complete multivariate linear model (7 dependent variables) also revealed a significant effect of site (F7,210=3.742, p=0.001). This was driven by changes in pain interference, wherein MC stimulation led to a significantly greater decrease in pain interference than DLPFC stimulation (F1,216=8.447, p=0.004). MC stimulation was also associated with a greater decrease in opiate urge than DLPFC stimulation (F1,216=3.248, p=0.073), though this failed to meet significance in this pilot study.
The effect of rTMS on these variables was measured using effect sizes. Both sites produced moderate to large decreases in stress, pain, and discomfort immediately after 10 days of rTMS (visit 10) and 1 week following rTMS (visit 12), although they did not meet significance for these measures. MC stimulation also produced moderate decreases in the urge to use opiates and decreases in pain interference immediately after 10 days of rTMS and at the 1 week follow up (Figure 1). Overall, MC stimulation tended to have larger effects than DLPFC stimulation on the assessments (Supplementary Table 2).
3.4. Opiate usage (Supplementary Figure 3).
Participants were not actively encouraged to reduce their opiate usage during the course of the study, but daily usage of their prescription opiates was monitored through TLFB metrics. A univariate linear model revealed no significant effect of site (F1,90=0.802, p=0.373), visit (F4,90=0.878, p=0.480) nor a site X visit interaction (F4,90=0.067, p=0.992).
4.0. Discussion
There is an urgent need to develop novel analgesic approaches for chronic pain as these patients are underserved by currently approved analgesics and are at a high risk for misuse of prescription opiates. TMS has emerged as a non-invasive tool to modulate pain in individuals with acute, localized neuropathic pain, but until now have not been directly evaluated as a tool to decrease pain and opiate use among chronic pain patients. Here we present data from the first study directly comparing the effects of rTMS over the DLPFC versus MC as a tool to decrease pain in chronic pain patients using opioids. While both sites produced moderate to large effects on stress, pain and discomfort, MC stimulation led to a significantly greater decrease in pain than DLPFC TMS. The effect on opiate urges did not differ significantly between the 2 sites. These initial proof of principle data provide a foundation for rTMS as a novel strategy to reduce pain interference and opiate urges- an approach which should be further evaluated in Phase II randomized, double-blind sham controlled studies.
These data support and extend a foundation of research on the efficacy of MC stimulation as a pain management strategy. The surgical observation that direct electrical stimulation led to a 64–70% reduction in neuropathic or thalamic pain sparked considerable enthusiasm surrounding the MC as a TMS target (Lin et al. 2018). Typically, TMS delivered at high frequency (5–20Hz) to this area (Lefaucheur et al. 2014) can reduce pain in a somatotopic manner. Although the mechanism remains unclear, short-term analgesic effects of TMS can be blocked by naloxone, a μ opioid receptor antagonist. This has been demonstrated in MC stimulation (de Andrade et al. 2011) as well as DLPFC stimulation (Taylor et al. 2013; Taylor, Borckardt, and George 2012). This suggests that TMS-associated pain relief is related to endogenous opioid modulation.
Limitations.
This was a basic target-selection study with a relatively small sample, single blinding, and no sham control condition. Every individual received real rTMS over 1 of 2 sites (DLPFC or MC stimulation). The lack of a sham control condition precludes a definitive statement of the true effect size these rTMS protocols have on analgesia. Additionally, the approach in this experiment could be improved by the use of neuronavigation which would increase the precision of coil placement for each visit.
As an interim step along the treatment development pipeline, these data suggest that the MC and DLPFC stimulation sites may be used as a therapeutic intervention to reduce opiate urges among chronic pain patients using opioids, with the MC having additional benefit as a tool to attenuate pain interference. The outcomes of this manuscript also offer a reference set of effect sizes for future studies. They demonstrate, for the first time, that a 10-day regimen of rTMS (3–5 days/week) can be safely and effectively delivered to these patients in a manner that has high patient compliance. Finally, this study was the first to demonstrate that opiate urges could be reduced by application of rTMS, regardless of stimulation site, which is a critical finding for the advancement of new innovative strategies for both pain relief and opiate reliance in society today.
Supplementary Material
5.0 References
- Attal N, Cruccu G, Haanpaa M, Hansson P, Jensen TS, Nurmikko T, Sampaio C, Sindrup S, Wiffen P, and Efns Task Force. 2006. ‘EFNS guidelines on pharmacological treatment of neuropathic pain’, Eur J Neurol, 13: 1153–69. [DOI] [PubMed] [Google Scholar]
- Barr MS, Farzan F, Wing VC, George TP, Fitzgerald PB, and Daskalakis ZJ. 2011. ‘Repetitive transcranial magnetic stimulation and drug addiction’, Int Rev Psychiatry, 23: 454–66. [DOI] [PubMed] [Google Scholar]
- Barth KS, Maria MM, Lawson K, Shaftman S, Brady KT, and Back SE. 2013. ‘Pain and motives for use among non-treatment seeking individuals with prescription opioid dependence’, Am J Addict, 22: 486–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bellamoli E, Manganotti P, Schwartz RP, Rimondo C, Gomma M, and Serpelloni G. 2014. ‘rTMS in the treatment of drug addiction: an update about human studies’, Behav Neurol, 2014: 815215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borckardt JJ, Nahas Z, Koola J, and George MS. 2006. ‘Estimating resting motor thresholds in transcranial magnetic stimulation research and practice: a computer simulation evaluation of best methods’, J ect, 22: 169–75. [DOI] [PubMed] [Google Scholar]
- Borckardt JJ, Weinstein M, Reeves ST, Kozel FA, Nahas Z, Smith AR, Byrne TK, Morgan K, and George MS. 2006. ‘Postoperative left prefrontal repetitive transcranial magnetic stimulation reduces patient-controlled analgesia use’, Anesthesiology, 105: 557–62. [DOI] [PubMed] [Google Scholar]
- de Andrade DC, Mhalla A, Adam F, Texeira MJ, and Bouhassira D. 2011. ‘Neuropharmacological basis of rTMS-induced analgesia: the role of endogenous opioids’, Pain, 152: 320–6. [DOI] [PubMed] [Google Scholar]
- Diana Marco, Raij Tommi, Melis Miriam, Nummenmaa Aapo, Leggio Lorenzo, and Bonci Antonello. 2017. ‘Rehabilitating the addicted brain with transcranial magnetic stimulation’, Nature Reviews Neuroscience, 18: 685–93. [DOI] [PubMed] [Google Scholar]
- DosSantos MF, Ferreira N, Toback RL, Carvalho AC, and DaSilva AF. 2016. ‘Potential Mechanisms Supporting the Value of Motor Cortex Stimulation to Treat Chronic Pain Syndromes’, Front Neurosci, 10: 18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ekhtiari Hamed, Tavakoli Hosna, Addolorato Giovanni, Baeken Chris, Bonci Antonello, Campanella Salvatore, Luis Castelo-Branco Gaëlle Bouju, Clark Vincent, Claus Eric, Dannon Pinhas, Felice Alessandra, Tess den Uyl Marco Diana, Massimo di Giannantonio John Fedota, Fitzgerald Paul, Gallimberti Luigi, Grall-Bronnec Marie, and Hanlon Colleen. 2019. ‘Transcranial Electrical and Magnetic Stimulation (tES and TMS) for Addiction Medicine: A consensus paper on the present state of the science and the road ahead’, Neuroscience & Biobehavioral Reviews, 104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galhardoni R, Correia GS, Araujo H, Yeng LT, Fernandes DT, Kaziyama HH, Marcolin MA, Bouhassira D, Teixeira MJ, and de Andrade DC. 2015. ‘Repetitive transcranial magnetic stimulation in chronic pain: a review of the literature’, Arch Phys Med Rehabil, 96: S156–72. [DOI] [PubMed] [Google Scholar]
- Gorelick DA, Zangen A, and George MS. 2014. ‘Transcranial magnetic stimulation in the treatment of substance addiction’, Ann N Y Acad Sci, 1327: 79–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grall-Bronnec M, and Sauvaget A. 2014. ‘The use of repetitive transcranial magnetic stimulation for modulating craving and addictive behaviours: a critical literature review of efficacy, technical and methodological considerations’, Neurosci Biobehav Rev, 47: 592–613. [DOI] [PubMed] [Google Scholar]
- Lefaucheur JP, Andre-Obadia N, Antal A, Ayache SS, Baeken C, Benninger DH, Cantello RM, Cincotta M, de Carvalho M, De Ridder D, Devanne H, Di Lazzaro V, Filipovic SR, Hummel FC, Jaaskelainen SK, Kimiskidis VK, Koch G, Langguth B, Nyffeler T, Oliviero A, Padberg F, Poulet E, Rossi S, Rossini PM, Rothwell JC, Schonfeldt-Lecuona C, Siebner HR, Slotema CW, Stagg CJ, Valls-Sole J, Ziemann U, Paulus W, and Garcia-Larrea L. 2014. ‘Evidence-based guidelines on the therapeutic use of repetitive transcranial magnetic stimulation (rTMS)’, Clin Neurophysiol, 125: 2150–206. [DOI] [PubMed] [Google Scholar]
- Lefaucheur JP, Antal A, Ahdab R, Ciampi de Andrade D, Fregni F, Khedr EM, Nitsche M, and Paulus W. 2008. ‘The use of repetitive transcranial magnetic stimulation (rTMS) and transcranial direct current stimulation (tDCS) to relieve pain’, Brain Stimul, 1: 337–44. [DOI] [PubMed] [Google Scholar]
- Lin H, Li W, Ni J, and Wang Y. 2018. ‘Clinical study of repetitive transcranial magnetic stimulation of the motor cortex for thalamic pain’, Medicine (Baltimore), 97: e11235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moisset X, de Andrade DC, and Bouhassira D. 2016. ‘From pulses to pain relief: an update on the mechanisms of rTMS-induced analgesic effects’, Eur J Pain, 20: 689–700. [DOI] [PubMed] [Google Scholar]
- Shei A, Rice JB, Kirson NY, Bodnar K, Birnbaum HG, Holly P, and Ben-Joseph R. 2015. ‘Sources of prescription opioids among diagnosed opioid abusers’, Curr Med Res Opin, 31: 779–84. [DOI] [PubMed] [Google Scholar]
- Short EB, Borckardt JJ, Anderson BS, Frohman H, Beam W, Reeves ST, and George MS. 2011. ‘Ten sessions of adjunctive left prefrontal rTMS significantly reduces fibromyalgia pain: a randomized, controlled pilot study’, Pain, 152: 2477–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shy ME, Frohman EM, So YT, Arezzo JC, Cornblath DR, Giuliani MJ, Kincaid JC, Ochoa JL, Parry GJ, and Weimer LH. 2003. ‘Quantitative sensory testing: report of the Therapeutics and Technology Assessment Subcommittee of the American Academy of Neurology’, Neurology, 60: 898–904. [DOI] [PubMed] [Google Scholar]
- Taylor JJ, Borckardt JJ, Canterberry M, Li X, Hanlon CA, Brown TR, and George MS. 2013. ‘Naloxone-reversible modulation of pain circuitry by left prefrontal rTMS’, Neuropsychopharmacology, 38: 1189–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor JJ, Borckardt JJ, and George MS. 2012. ‘Endogenous opioids mediate left dorsolateral prefrontal cortex rTMS-induced analgesia’, Pain, 153: 1219–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Seoyon, and Chang Min Cheol. 2020. ‘Effect of Repetitive Transcranial Magnetic Stimulation on Pain Management: A Systematic Narrative Review’, Frontiers in Neurology, 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


