Abstract
Background
Biological sex and gender have been reported to affect incidence and overall survival (OS) of curatively treated gastroesophageal cancer. The aim of this study was to compare palliative treatment allocation and OS between women and men with advanced gastroesophageal cancer.
Methods
Patients with an unresectable or metastatic esophageal (including cardia) adenocarcinoma (EAC) or squamous cell carcinoma (ESCC) or gastric adenocarcinoma (GAC) diagnosed in 2015-2018 were identified in the Netherlands Cancer Registry. Treatment allocation was compared using χ2 tests and multivariable logistic regression analyses, and OS using the Kaplan-Meier method with log-rank test and Cox proportional hazards analysis. All statistical tests were 2-sided.
Results
Of patients with EAC (n = 3077), ESCC (n = 794), and GAC (n = 1836), 18.0%, 39.4%, and 39.1% were women, respectively. Women less often received systemic treatment compared with men for EAC (42.7% vs 47.4%, P = .045) and GAC (33.8% vs 38.8%, P = .03) but not for ESCC (33.2% vs 39.5%, P = .07). Women had a lower probability of receiving systemic treatment for GAC in multivariable analyses (odds ratio [OR] = 0.79, 95% confidence interval [CI] = 0.62 to 1.00) but not for EAC (OR = 0.86, 95% CI = 0.69 to 1.06) and ESCC (OR = 0.81, 95% CI = 0.57 to 1.14). Median OS was lower in women with EAC (4.4 vs 5.2 months, P = .04) but did not differ after adjustment for patient and tumor characteristics and systemic treatment administration.
Conclusions
We observed statistically significant and clinically relevant gender differences in systemic treatment administration and OS in advanced gastroesophageal cancer. Causes of these disparities may be sex based (ie, related to tumor biology) as well as gender based (eg, related to differences in treatment choices).
Gastroesophageal cancer occurs more frequently in men (1-3). In the Netherlands, approximately 750 women are diagnosed with an esophageal or gastroesophageal junction or cardia carcinoma annually compared with 2200 men (1). This difference is smaller in gastric cancer, with a yearly incidence of 450 women and 700 men (1).
Although the overrepresentation of men in the incidence of gastroesophageal cancer has been described frequently (2-4), less is known about gender differences in outcomes in this patient population. Overall, men have poorer outcomes in a wide range of cancer types (1–6). However, poorer survival in women has been described in gastric cancer (7,8), whereas similar survival rates in women have been observed in esophageal cancer (4,9) and even better outcomes in women younger than 55 years with esophageal squamous cell carcinoma (ESCC) (9).
Causes of disparities in incidence and outcomes between men and women with gastroesophageal cancer can be based on either biological factors (ie, sex) or sociocultural factors (ie, gender-related factors). Biological factors include differences in the distribution of molecular subtypes or genetic causes (10). Gender-based causes may include individual exposure to risk factors such as obesity, smoking, and alcohol (9,11), but also treatment choices and factors associated with the need for and access to health care (12).
Earlier studies comparing outcomes between women and men in metastatic gastroesophageal cancer did not consider the use of palliative systemic treatment (8,9), although this may differ and influence survival. Exploration of differences in both clinical characteristics and the probability of receiving treatment in advanced gastroesophageal cancer could help to provide an understanding of possible differences in outcome. The aim of this population-based study was to compare patient and tumor characteristics as well as treatment allocation and overall survival (OS) between women and men in a nationwide cohort of patients with unresectable or metastatic gastroesophageal cancer.
Methods
Data Collection
Patients 18 years or older with a histologically confirmed esophageal (including gastroesophageal junction or cardia) adenocarcinoma (EAC) or ESCC or gastric adenocarcinoma (GAC) diagnosed with synchronous metastases (cM1) or an unresectable carcinoma at initial diagnosis between 2015 and 2018 were identified from the Netherlands Cancer Registry (NCR). The NCR is a population-based registry that covers the total Dutch population of more than 17 million people and is directly linked to the nationwide network and registry of histo- and cytopathology that comprises all histologically confirmed cancer diagnoses. The hospital in which the initial diagnostic assessment was performed was considered the hospital of diagnosis. Patient and tumor characteristics at initial diagnosis, including gender identity, and information about initial treatment and follow-up were extracted from the hospital’s medical records by specially trained data managers. Data on vital status were obtained by annual linkage to the Dutch Personal Records Database and updated until February 1, 2020. Clinical staging was performed according to the TNM seventh (2015-2016) and eighth editions (2017-2018) (13,14). Dutch guidelines recommend initial staging with gastroscopy with biopsies and CT scan in all patients, and endoscopic ultrasonography, fluorodeoxyglucose positron emission tomography/CT, and diagnostic laparoscopy on indication (15,16).
Type of treatment was subdivided in the following categories: systemic treatment, radiotherapy on the primary tumor (without systemic treatment), radiotherapy on metastases, or surgical resection. Systemic treatment was also subdivided in chemoradiotherapy (ie, systemic treatment with long scheme radiotherapy, ie, ≥23 fractions) and systemic treatment without long-term radiotherapy. If none of these treatments was applied, patients were assumed to have received best supportive care only.
Statistical Analysis
All analyses were fully stratified for primary tumor location in combination with histology: EAC, ESCC, and GAC. Patient and tumor characteristics were displayed with counts and percentages or medians and interquartile ranges (IQRs) for men and women separately. Differences were analyzed using χ2, Fisher exact, or Mann-Whitney U tests, whichever was appropriate. Unadjusted differences in the probability of receiving systemic treatment between genders were analyzed with χ2 tests. To identify possible differences in systemic treatment administration among age groups, age-stratified χ2 tests were also performed. Additionally, multivariable logistic regression analyses were used to identify the adjusted difference between genders in the probability of receiving systemic treatment. Age, performance status, number of comorbidities, Lauren classification (only for EAC and GAC subgroups), tumor stage, metastases locations, and hospital volume were included in the full model as covariates. Hospital volume is associated with the probability of receiving curative or palliative treatment for gastroesophageal cancer in the Netherlands (17–19) and was calculated using the number of patients diagnosed with gastroesophageal cancer per hospital in 2015-2018, subdivided in quartiles based on these volumes. Statistical significance of the adjusted differences between genders was determined with likelihood-ratio tests, comparing the full model with the full model without gender.
OS was calculated from day of diagnosis in survival analyses for all EAC, ESCC, and GAC patients, and log-rank tests were performed to compare OS between genders. Multivariable Cox proportional hazards analyses were used to determine the effect of gender on OS by comparing the models including gender, the interaction between gender and systemic treatment, treatment, and clinical covariates (age, performance status, number of comorbidities, tumor stage, Lauren classification [in EAC and GAC], metastases locations, and hospital volume) with a model including systemic treatment and clinical covariates only by using likelihood-ratio tests. Adequacy of the proportional hazards assumption was tested using Kaplan-Meier curves for survival functions.
All tests were 2-sided, and a P value less than .05 was considered statistically significant. Analyses were performed using SAS software (version 9.4, SAS Institute, Cary, NC).
Ethical Approval
According to the Central Committee on Research involving Human Subjects, this type of study does not require approval from an ethics committee in the Netherlands. The study was approved by the Privacy Review Board of the NCR and the scientific committee of the Dutch Upper GI Cancer Group. The reporting of this study followed the Strengthening the Reporting of Observational Studies in Epidemiology guidelines (47).
Results
Patient Selection
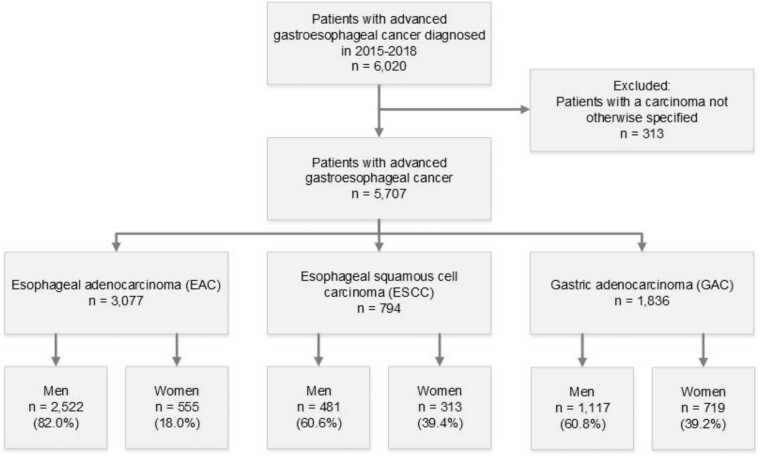
After exclusion of patients with a carcinoma not otherwise specified (n = 313), 5707 patients with an advanced gastroesophageal adenocarcinoma or squamous cell carcinoma were identified (Figure 1). Carcinoma not otherwise specified was equally distributed among men and women.
Figure 1.
Flowchart of patient selection. Patients displayed in the blue boxes were included for analyses.
Of all 5707 patients with an unresectable carcinoma and/or metastases tumor included, most patients had an EAC (n = 3077, 53.9%), followed by GAC (n = 1836, 32.1%) and ESCC (n = 794, 13.9%). Of EAC, ESCC, and GAC patients, 18.0%, 39.4%, and 39.1% were women, respectively.
Baseline Characteristics
In all subtypes, patients older than 75 years were more frequently women (Table 1). Women with EAC and GAC had fewer comorbidities than men. Women with GAC more often had a diffuse-type tumor (39.1%) compared with men (31.9%) and less often an intestinal tumor type (28.0% vs 34.3%, P = .01). The proportion of diffuse GACs declined with increasing age in both genders. In women, this proportion declined gradually from 50.9% in patients aged 55 years and younger to 30.5% in patients older than 75 years compared with 44.0% to 28.4%, respectively, in men. A signet cell histology was relatively more frequently found in women with EAC (5.8% vs 3.8%, P = .046) and GAC (17.8% vs 11.8%, P < .001). Women with EAC less often had distant metastases at 2 or more locations (43.1% vs 49.4%, P = .007). Women with ESCC and GAC less often had liver metastases (19.2% vs 25.8%, P = .03, and 24.6% vs 36.3%, P < .001, respectively), whereas peritoneal metastases of GAC were more often diagnosed in women (55.8% vs 49.4%, P = .008). There were no differences in performance and HER2 status between women and men in any of the groups.
Table 1.
Baseline characteristics of included patients (n = 5707)
| Characteristics | EAC (n = 3077) |
ESCC (n = 794) |
GAC (n = 1836) |
||||||
|---|---|---|---|---|---|---|---|---|---|
| Men | Women | P | Men | Women | P | Men | Women | P | |
| (n = 2522) | (n = 555) | (n = 481) | (n = 313) | (n = 1117) | (n = 719) | ||||
| No. (%) | No. (%) | No. (%) | No. (%) | No. (%) | No. (%) | ||||
| Age, y | |||||||||
| ≤55 | 329 (13.0) | 78 (14.1) | .01a | 42 (8.7) | 22 (7.0) | .005a | 134 (12.0) | 108 (15.0) | .009a |
| 56-65 | 695 (27.6) | 153 (27.6) | 154 (32.0) | 81 (25.9) | 201 (18.0) | 121(16.8) | |||
| 66-75 | 947 (37.5) | 173 (31.2) | 209 (43.5) | 129 (41.2) | 401 (35.9) | 211 (29.3) | |||
| >75 | 551 (21.8) | 151 (27.2) | 76 (15.8) | 81 (25.9) | 381 (34.1) | 279 (38.8) | |||
| Performance status | .09b | .72b | .07b | ||||||
| 0-1 | 1325 (52.5) | 263 (47.4) | 236 (49.1) | 146 (46.6) | 483 (43.2) | 272 (37.8) | |||
| ≥2 | 403 (16.0) | 101 (18.2) | 92 (19.1) | 59 (18.8) | 188 (16.8) | 137 (19.1) | |||
| Unknown | 794 (31.5) | 191 (34.4) | 153 (31.8) | 108 (34.5) | 446 (39.9) | 310 (43.1) | |||
| No. of comorbidities | .045b | .17b | .002b | ||||||
| 0 | 1174 (46.6) | 284 (51.2) | 203 (42.2) | 144 (46.0) | 487 (43.6) | 362 (50.3) | |||
| 1 | 756 (30.0) | 168 (30.3) | 153 (31.8) | 109 (34.8) | 355 (31.8) | 211 (29.3) | |||
| ≥2 | 467 (18.5) | 76 (13.7) | 100 (20.8) | 49 (15.7) | 218 (19.5) | 100 (13.9) | |||
| Unknown | 125 (5.0) | 27 (4.9) | 25 (5.2) | 11 (3.5) | 57 (5.1) | 46 (6.4) | |||
| Tumor stage | .06b | .62b | .41b | ||||||
| cT4bM0 | 26 (1.0) | 11 (2.0) | 88 (18.3) | 53 (16.9) | 67 (6.0) | 50 (7.0) | |||
| cM1 | 2496 (99.0) | 544 (98.0) | 393 (81.7) | 260 (83.1) | 1050 (94.0) | 669 (93.0) | |||
| Lauren classificationb | .15b | .01b | |||||||
| Intestinal | 1118 (44.3) | 241 (43.4) | — | — | 383 (34.3) | 201 (28.0) | |||
| Diffuse | 352 (14.0) | 99 (17.8) | — | — | 356 (31.9) | 281 (39.1) | |||
| Mixed | 46 (1.8) | 12 (2.2) | — | — | 39 (3.5) | 24 (3.3) | |||
| Indeterminate | 72 (2.9) | 12 (2.2) | — | — | 10 (0.9) | 10 (1.4) | |||
| Unknown | 934 (37.0) | 191 (34.4) | — | — | 329 (29.5) | 203 (28.2) | |||
| Signet ring cell carcinomab | 98 (3.9) | 32 (5.8) | .046b | — | — | 132 (11.8) | 128 (17.8) | <.001b | |
| Differentiation grade | .30b | .11b | .18b | ||||||
| Good/moderate | 637 (25.3) | 135 (24.3) | 159 (33.1) | 122 (39.0) | 158 (14.1) | 87 (12.1) | |||
| Poor | 1088 (43.1) | 259 (46.7) | 156 (32.4) | 82 (26.2) | 636 (56.9) | 440 (61.2) | |||
| Unknown | 797 (31.6) | 161 (29.0) | 166 (34.5) | 109 (34.8) | 323 (28.9) | 192 (26.7) | |||
| HER2 statusb | .36b | .12b | |||||||
| Positive | 378 (15.0) | 78 (14.1) | — | — | 98 (8.8) | 47 (6.5) | |||
| Negative | 1145 (45.4) | 239 (43.1) | — | — | 555 (49.7) | 348 (48.4) | |||
| Unknown | 999 (39.6) | 238 (42.9) | — | — | 464 (41.5) | 324 (45.1) | |||
| Metastases locations | .007b | .33b | .55b | ||||||
| 0 | 26 (1.0) | 11 (2.0) | 88 (18) | 53 (17) | 67 (6) | 50 (7) | |||
| 1 | 1249 (49.5) | 305 (55.0) | 234 (49) | 169 (54) | 660 (59) | 409 (57) | |||
| ≥2 | 1247 (49.4) | 239 (43.1) | 159 (33) | 91 (29) | 390 (35) | 260 (36) | |||
| Extraregional lymph node metastases | 1230 (48.8) | 264 (47.6) | .61b | 233 (48.4) | 154 (49.2) | .83b | 316 (28.3) | 172 (23.9) | .04b |
| Liver metastases | 1347 (53.4) | 281 (50.6) | .24b | 124 (25.8) | 60 (19.2) | .03b | 406 (36.3) | 177 (24.6) | <.001b |
| Peritoneal metastases | 251 (10.0) | 65 (11.7) | .22b | 15 (3.1) | 6 (1.9) | .30b | 552 (49.4) | 401 (55.8) | .008b |
| Lung metastases | 592 (23.5) | 112 (20.2) | .10b | 125 (26.0) | 85 (27.2) | .72b | 127 (11.4) | 78 (10.8) | .73b |
| Bone metastases | 495 (19.6) | 87 (15.7) | .03b | 68 (14.1) | 42 (13.4) | .78b | 76 (6.8) | 58 (8.1) | .31b |
| Other metastases locations | 394 (15.6) | 75 (13.5) | .21b | 52 (10.8) | 28 (8.9) | .48b | 104 (9.3) | 107 (14.9) | <.001b |
P value was calculated using a 2-sided χ2 test. EAC = esophageal adenocarcinoma; ESCC = esophageal squamous cell carcinoma; GAC = gastric adenocarcinoma; IQR = interquartile range.
Lauren classification, signet ring cell carcinoma, and HER2 status are applicable only in esophageal and gastric adenocarcinomas.
Treatment
Among women with EAC, 42.7% received systemic treatment (including chemoradiotherapy) compared with 47.4% of men with EAC (P = .045), a difference that was observed in GAC as well (33.8% vs 38.8%, P = .03; Table 2; Figure 2). The proportion of women treated with systemic therapy for ESCC was not statistically significant lower (33.2%) than in men (39.5%, P = .07). The proportion of women who received best supportive care only was larger for EAC (35.3% vs 30.5%, P = .03) and GAC (58.4% vs 51.1%, P = .003) and did not differ for ESCC (31.6% vs 29.9%, P = .61).
Table 2.
Treatment characteristics of included patients stratified for tumor location and histology
| Type of treatment | EAC (n = 3077) |
ESCC (n = 794) |
GAC (n = 1836) |
||||||
|---|---|---|---|---|---|---|---|---|---|
| Men | Women | P a | Men | Women | P a | Men | Women | P a | |
| (n = 2522) | (n = 555) | (n = 481) | (n = 313) | (n = 1117) | (n = 719) | ||||
| No. (%) | No. (%) | No. (%) | No. (%) | No. (%) | No. (%) | ||||
| Systemic treatment (including chemoradiotherapy) | 1195 (47.4) | 237 (42.7) | .045 | 190 (39.5) | 104 (33.2) | .07 | 433 (38.8) | 243 (33.8) | .03 |
| Systemic treatment (but not chemoradiotherapyb) | 1115 (44.2) | 217 (39.1) | .03 | 91 (18.9) | 53 (16.9) | .48 | 431 (38.6) | 241 (33.5) | .03 |
| Chemoradiotherapyb | 80 (3.2) | 20 (3.6) | .60 | 99 (20.6) | 51 (16.3) | .13 | 2 (0.2) | 2 (0.5) | .66 |
| Radiotherapy primary tumor (without systemic treatment) | 495 (19.6) | 113 (20.4) | .70 | 131 (27.2) | 104 (33.2) | .07 | 68 (6.1) | 26 (3.6) | .02 |
| Radiotherapy metastases | 217 (8.6) | 33 (5.9) | .04 | 36 (7.5) | 23 (7.3) | .94 | 18 (1.6) | 19 (2.6) | .13 |
| Surgical resection | 39 (1.5) | 12 (2.2) | .30 | 18 (3.7) | 10 (3.2) | .68 | 76 (6.8) | 40 (5.6) | .28 |
| Best supportive care only | 769 (30.5) | 196 (35.3) | .03 | 144 (29.9) | 99 (31.6) | .61 | 571 (51.1) | 419 (58.3) | .003 |
Two-sided P values are from χ2 tests. EAC = esophageal adenocarcinoma; ESCC = esophageal squamous cell carcinoma; GAC = gastric adenocarcinoma.
Chemoradiotherapy was defined as systemic treatment with concurrent long-term radiotherapy.
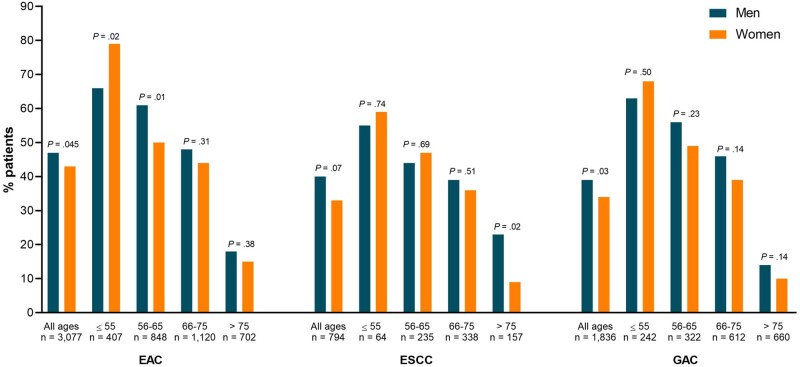
Figure 2.
Systemic treatment administration (including chemoradiotherapy) stratified for gender and age in patients with esophageal adenocarcinoma (EAC), esophageal squamous cell carcinoma (ESCC), and gastric adenocarcinoma (GAC). Statistically significant differences between men and women are marked with asterisks. The exact number of men and women per age group is listed in Table 2. P values were calculated using a 2-sided χ2 test.
When stratified for age, the proportion of women aged 55 years and younger who received systemic treatment was statistically significantly higher for EAC compared with men (79.5% vs 65.7%, P = .02; Figure 2) and did not differ for ESCC (59.1% vs 54.8%, P = .74) and GAC (67.6% vs 63.4%, P = .50). Women with EAC aged 56-65 years less often received systemic treatment compared with men (50.3% vs 61.3%, P = .01). Among women with ESCC older than 75 years, the proportion who received systemic treatment was 8.6% compared with 22.4% of men (P = .02). When we restricted our analyses to patients aged 76-80 years in the highest age subgroup (ie, >75 years), because the proportion of women older than 80 in this subgroup was larger than in men, systemic treatment administration in women compared with men did not statistically significantly differ in EAC (33.3% vs 26.2%, respectively, P = .27), ESCC (11.9% vs 27.5%, P = .06), or GAC (17.3% vs 21.8%, P = .32).
The adjusted odds ratios (ORs) for receiving systemic treatment for women with EAC, ESCC, and GAC were 0.86 (95% confidence interval [CI] = 0.69 to 1.06), 0.81 (95% CI = 0.57 to 1.14), and 0.79 (95% CI = 0.62 to 1.00; Table 3), respectively. Accordingly, the results of the likelihood-ratio tests were in line with these results: GAC (χ²1 = 4.01, P = .045), EAC (χ²1 = 1.95, P = .16), and ESCC (χ²1 = 1.47, P = .23).
Table 3.
Multivariable logistic regression analyses for the probability of receiving systemic treatment (including chemoradiotherapy) in EAC, ESCC, and GAC patients
| Characteristics | EAC (n = 3077) |
ESCC (n = 794) |
GAC (n = 1836) |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| OR (95% CI) | P | OR (95% CI) | P | OR (95% CI) | P | |||||||
| Gender | ||||||||||||
| Men | Referent | Referent | Referent | |||||||||
| Women | 0.86 (0.69 to 1.06) | .16b | 0.81 (0.57 to 1.14) | .23c | 0.79 (0.62 to 1.00) | .046d | ||||||
| Age | 0.93 (0.92 to 0.94) | <.001 | 0.94 (0.92 to 0.96) | <.001 | 0.91 (0.91 to 0.93) | <.001 | ||||||
| Performance status | ||||||||||||
| 0-1 | Referent | <.001 | Referent | <.001 | Referent | <.001 | ||||||
| ≥2 | 0.18 (0.14 to 0.23) | 0.28 (0.17 to 0.44) | 0.29 (0.21 to 0.40) | |||||||||
| Unknown | 0.29 (0.24 to 0.34) | 0.21 (0.14 to 0.32) | 0.24 (0.19 to 0.31) | |||||||||
| No. of comorbidities | ||||||||||||
| 0 | Referent | .004 | Referent | .80 | Referent | <.001 | ||||||
| 1 | 0.88 (0.74 to 1.06) | 0.95 (0.65 to 1.39) | 0.99 (0.77 to 1.28) | |||||||||
| ≥2 | 0.65 (0.53 to 0.82) | 0.79 (0.49 to 1.27) | 0.55 (0.39 to 0.78) | |||||||||
| Unknown | 1.07 (0.72 to 1.57) | 1.04 (0.45 to 2.33) | 0.57 (0.34 to 0.94) | |||||||||
| Lauren classification | ||||||||||||
| Intestinal | Referent | .01 | N/A | — | Referent | .45 | ||||||
| Diffuse | 0.73 (0.57 to 0.93) | — | 0.85 (0.64 to 1.14) | |||||||||
| Mixed | 1.24 (0.67 to 2.34) | — | 0.74 (0.39 to 1.38) | |||||||||
| Indeterminate | 0.81 (0.50 to 1.34) | — | 0.45 (0.12 to 1.65) | |||||||||
| Unknown | 0.75 (0.63 to 0.90) | — | 0.80 (0.60 to 1.07) | |||||||||
| Stage | ||||||||||||
| cT4bM0 | 0.93 (0.45 to 2.07) | 1.26 (0.70 to 2.28) | 0.73 (0.43 to 1.25) | |||||||||
| cM1 | Referent | .87 | Referent | .43 | Referent | .25 | ||||||
| Hospital volumea | ||||||||||||
| Q1 | 0.76 (0.57 to 1.00) | 0.55 (0.29 to 1.05) | 0.93 (0.63 to 1.38) | |||||||||
| Q2 | 1.12 (0.89 to 1.40) | 0.43 (0.26 to 0.72) | 1.24 (0.92 to 1.66) | |||||||||
| Q3 | 1.15 (0.93 to 1.40) | 0.75 (0.51 to 1.10) | 0.81 (0.61 to 1.07) | |||||||||
| Q4 | Referent | .03 | Referent | .007 | Referent | .07 | ||||||
| Extraregional lymph node metastases | 0.90 (0.76 to 1.07) | .22 | 1.11 (0.71 to 1.70) | .63 | 0.82 (0.62 to 1.08) | .16 | ||||||
| Liver metastases | 1.20 (1.03 to 1.43) | .046 | 1.04 (0.66 to 1.61) | .88 | 1.10 (0.83 to 1.48) | .50 | ||||||
| Peritoneal metastases | 0.74 (0.57 to 0.98) | .04 | 0.63 (0.20 to 1.97) | .43 | 0.85 (0.64 to 1.13) | .26 | ||||||
| Lung metastases | 0.91 (0.74 to 1.10) | .32 | 0.73 (0.48 to 1.10) | .14 | 0.91 (0.63 to 1.31) | .60 | ||||||
| Bone metastases | 0.81 (0.62 to 1.00) | .05 | 0.44 (0.25 to 0.78) | .004 | 0.55 (0.35 to 0.86) | .009 | ||||||
| Other metastases locations | 0.61 (0.48 to 0.77) | <.001 | 0.58 (0.31 to 1.09) | .09 | 1.10 (0.78 to 1.57) | .59 | ||||||
Volume of hospital of diagnosis. Per hospital, the volume of gastroesophageal cancer patients diagnosed with gastroesophageal cancer between 2015 and 2018 was calculated. Subsequently, hospitals were categorized into quartiles (Q1-4) according to these volumes, which resulted in hospitals in which less than 25 (Q1), 25-61 (Q2), 61-140 (Q3), and over 140 (Q4) patients were diagnosed in 2015-2018. CI = confidence interval; cM1 = metastatic; EAC = esophageal adenocarcinoma; ESCC = esophageal squamous cell carcinoma; GAC = gastric adenocarcinoma; OR = odds ratio.
Likelihood-ratio tests comparing the full model and the full model without gender: EAC: χ2 = 1.95, 2-sided P = .16.
Likelihood-ratio tests comparing the full model and the full model without gender: ESCC: χ2 = 1.47, 2-sided P = .23.
Likelihood-ratio tests comparing the full model and the full model without gender: GAC: χ2 = 4.01, 2-sided P = .045.
Increasing age and higher performance status were independently associated with a lower probability of systemic treatment administration in all groups. In EAC and ESCC, being diagnosed in a high-volume hospital was associated with a higher chance of receiving systemic treatment. If hospital volume was not added to the model, then the adjusted odds ratios for women with EAC, ESCC, and GAC were 0.86 (95% CI = 0.96 to 1.07), 0.81 (95% CI = 0.58 to 1.14), and 0.80 (95% CI = 0.63 to 1.00), respectively.
Overall Survival
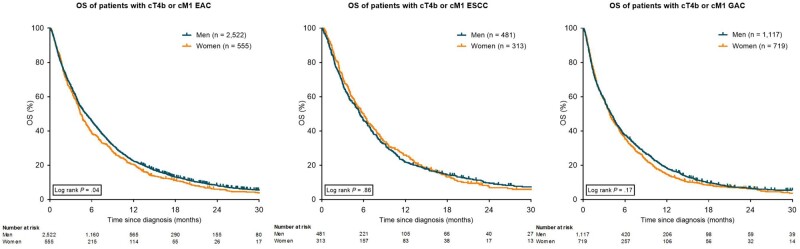
A statistically significant difference in median OS to the disadvantage of women compared with men was observed in EAC (4.4 months, IQR = 1.9-9.9 months vs 5.2 months, IQR = 2.0-11.0 months, P = .04), but not in ESCC (5.9 months, IQR = 2.5-12.5 months vs 5.4 months, IQR = 10.9-2.3 months, P = .86) and GAC (3.8 months, IQR = 1.5-8.6 months vs 4.0 months, IQR = 1.4-9.8 months, P = .17; Figure 3).
Figure 3.
Kaplan-Meier curves for overall survival (OS) in patients with esophageal adenocarcinoma (EAC), esophageal squamous cell carcinoma (ESCC), and gastric adenocarcinoma (GAC), stratified for gender. All statistical tests were 2-sided. cM1 = metastatic; cT4b = unresectable.
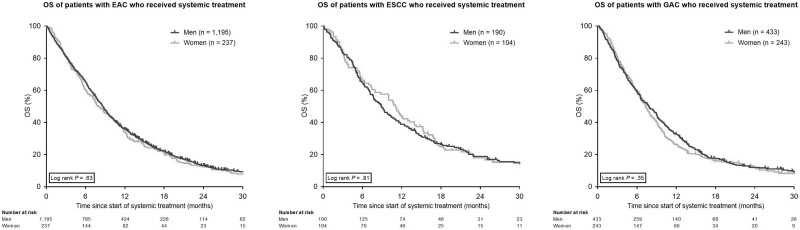
The median OS of patients who received systemic treatment did not differ between women and men with EAC (8.0 months, IQR = 3.7-15.0 months vs 8.6 months, IQR = 4.1-16.2 months, P = .63), ESCC (11.1 months, IQR = 3.8-18.4 months vs 8.9 months, IQR = 4.6-20.0 months, P = .81), or GAC (7.2 months, IQR = 3.7-12.4 months vs 7.9 months, IQR = 3.4-14.0 months, P = .35; Figure 4).
Figure 4.
Kaplan-Meier curves for overall survival (OS) in patients with esophageal adenocarcinoma (EAC), esophageal squamous cell carcinoma (ESCC), and gastric adenocarcinoma (GAC) who received systemic treatment (including chemoradiotherapy), stratified for gender. All statistical tests were 2-sided. Age is in years.
After comparison of multivariable Cox regression models, women did not have an increased risk of dying after adjustment for clinical covariates, systemic treatment, and the interaction between gender and systemic treatment (EAC: hazard ratio [HR] = 0.99, 95% CI = 0.88 to 1.12, χ²1 = 0.24, P = .89; ESCC: HR = 0.93, 95% CI = 0.78 to 1.12, χ²1 = 0.72, P = .70; GAC: HR = 0.97, 95% CI = 0.86 to 1.10, χ²1 = 0.23, P = .89; Table 4). The association between systemic treatment and OS was statistically significant in all groups, whereas no independent association between gender or the interaction between gender and systemic treatment was observed in any of the groups (Table 4).
Table 4.
Multivariable Cox proportional hazards regression analyses for overall survival in EAC, ESCC, and GAC patients
| Characteristics | EAC (n = 3077) |
ESCC (n = 794) |
GAC (n = 1836) |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| HR (95% CI) | P | HR (95% CI) | P | HR (95% CI) | P | |||||||
| Gender | ||||||||||||
| Men | Referent | .90b | Referent | .46c | Referent | .64d | ||||||
| Women | 0.99 (0.88 to 1.12) | 0.93 (0.78 to 1.12) | 0.97 (0.86 to 1.10) | |||||||||
| Systemic treatment | 0.32 (0.27 to 0.38) | <.001 | 0.41 (0.31 to 0.53) | <.001 | 0.39 (0.33 to 0.46) | <.001 | ||||||
| Gendera systemic treatment | 0.96 (0.79 to 1.16) | .66 | 0.88 (0.64 to 1.22) | .44 | 0.96 (0.79 to 1.18) | .73 | ||||||
| Age | 1.00 (0.99 to 1.00) | .03 | 0.99 (0.99 to 1.00) | .11 | 1.00 (1.00 to 1.01) | .25 | ||||||
| Performance status | ||||||||||||
| 0-1 | Referent | Referent | Referent | |||||||||
| ≥2 | 1.66 (1.49 to 1.85) | 1.79 (1.46 to 2.19) | <.001 | 1.37 (1.19 to 1.57) | <.001 | |||||||
| Unknown | 1.62 (1.49 to 1.77) | <.001 | 1.64 (1.38 to 1.96) | <.001 | 1.51 (1.35 to 1.68) | <.001 | ||||||
| No. of comorbidities | ||||||||||||
| 0 | Referent | Referent | Referent | |||||||||
| 1 | 1.11 (1.02 to 1.22) | .02 | 1.06 (0.90 to 1.26) | .49 | 1.10 (0.98 to 1.23) | .12 | ||||||
| ≥2 | 0.90 (0.81 to 1.01) | .06 | 1.07 (0.87 to 1.31) | .53 | 1.05 (0.91 to 1.21) | .52 | ||||||
| Unknown | 0.95 (0.80 to 1.13) | .56 | 1.22 (0.85 to 1.75) | .29 | 0.93 (0.75 to 1.15) | .48 | ||||||
| Lauren classification | ||||||||||||
| Intestinal | Referent | — | Referent | |||||||||
| Diffuse | 1.38 (1.24 to 1.55) | <.001 | — | — | 1.29 (1.13 to 1.46) | <.001 | ||||||
| Mixed | 1.65 (1.26 to 2.16) | <.001 | — | — | 1.05 (0.80 to 1.37) | .74 | ||||||
| Indeterminate | 1.03 (0.82 to 1.30) | .80 | — | — | 0.82 (0.51 to 1.30) | .40 | ||||||
| Unknown | 1.09 (1.00 to 1.18) | .05 | — | — | 1.28 (1.13 to 1.45) | <.001 | ||||||
| HER2 status | ||||||||||||
| Negative | Referent | — | Referent | |||||||||
| Positive | 0.75 (0.67 to 0.84) | <.001 | — | — | 0.97 (0.80 to 1.16) | .70 | ||||||
| Unknown | 1.16 (1.06 to 1.26) | <.001 | — | — | 1.13 (1.01 to 1.27) | .04 | ||||||
| Stage | ||||||||||||
| cT4bM0 | 1.05 (0.73 to 1.51) | .79 | 1.28 (0.98 to 1.66) | .07 | 0.67 (0.53 to 0.85) | .001 | ||||||
| cM1 | Referent | Referent | Referent | |||||||||
| Hospital volumea | ||||||||||||
| Q1 | 1.14 (1.00 to 1.29) | .04 | 1.05 (0.80 to 1.39) | .74 | 1.35 (1.14 to 1.59) | <.001 | ||||||
| Q2 | 1.09 (0.98 to 1.20) | .13 | 0.98 (0.79 to 1.21) | .84 | 1.45 (1.27 to 1.65) | <.001 | ||||||
| Q3 | 1.11 (1.01 to 1.21) | .03 | 0.98 (0.82 to 1.18) | .85 | 1.34 (1.19 to 1.51) | <.001 | ||||||
| Q4 | Referent | Referent | Referent | |||||||||
| Extraregional lymph node metastases | 1.27 (1.17 to 1.37) | <.001 | 1.08 (0.90 to 1.29) | .39 | 1.18 (1.05 to 1.33) | .005 | ||||||
| Liver metastases | 1.77 (1.63 to 1.92) | <.001 | 1.88 (1.56 to 2.28) | <.001 | 1.42 (1.26 to 1.60) | <.001 | ||||||
| Peritoneal metastases | 1.85 (1.64 to 2.10) | <.001 | 2.18 (1.39 to 3.42) | <.001 | 1.32 (1.17 to 1.48) | <.001 | ||||||
| Lung metastases | 1.22 (1.12 to 1.33) | <.001 | 1.25 (1.04 to 1.50) | .02 | 1.09 (0.94 to 1.27) | .27 | ||||||
| Bone metastases | 1.42 (1.29 to 1.56) | <.001 | 1.28 (1.03 to 1.59) | .03 | 2.06 (1.71 to 2.49) | <.001 | ||||||
| Other metastases locations | 1.33 (1.19 to 1.47) | <.001 | 1.41 (1.11 to 1.81) | .006 | 1.18 (1.05 to 1.33) | .005 | ||||||
Volume of hospital of diagnosis. Per hospital, the volume of gastroesophageal cancer patients that was diagnosed with gastroesophageal cancer between 2015 and 2018 was calculated. Subsequently, hospitals were categorized into quartiles (Q1-4) according to these volumes, which resulted in hospitals in which less than 25 (Q1), 25-61 (Q2), 61-140 (Q3), and greater than 140 (Q4) patients were diagnosed in 2015-2018. CI = confidence interval; cM1 = metastatic; EAC = esophageal adenocarcinoma; ESCC = esophageal squamous cell carcinoma; GAC = gastric adenocarcinoma; HR = hazard ratio.
Likelihood-ratio tests comparing the full model and the full model without gender and the interaction between gender and systemic treatment: EAC: χ2 = 0.24, 2-sided P = .89.
Likelihood-ratio tests comparing the full model and the full model without gender and the interaction between gender and systemic treatment: ESCC: χ2 = 0.72, 2-sided P = .70.
Likelihood-ratio tests comparing the full model and the full model without gender and the interaction between gender and systemic treatment: GAC: χ2 = 0.23, 2-sided P = .89.
Discussion
In addition to the well-known disparity in gastroesophageal cancer incidence between women and men, our results in a nationwide cohort of patients with unresectable or metastatic gastroesophageal cancer revealed statistically significant and clinically relevant gender differences in both patient characteristics (eg, less comorbidities in women), tumor characteristics (eg, more often a diffuse histology in women), patterns of metastasis (eg, less often liver metastasis and more often peritoneal metastasis in women), and treatment allocation (less systemic treatment administration in women). Most importantly, although women have a decreased risk of dying from many cancer types (2–4), we observed an increased risk of dying in women with EAC. Because these survival disparities were not observed in women and men who received systemic treatment and in multivariable analyses after adjustment for clinical covariates and systemic treatment, this gap could at least partly be explained by the smaller proportion of women treated with systemic therapy, which was observed especially in patients older than 55 years. Our findings support the assumption that both sex- and gender-based factors could contribute to disparities in treatment allocation and outcomes of patients with unresectable and metastatic esophageal and gastric cancer. A clear distinction between sex-based causes (eg, differences in tumor biology) and gender-based factors (ie, those related to sociocultural factors and behavior) is important to understand these differences.
Sex-based causes of the observed survival gap could include different exposure to sex hormones as well as differences in tumor biology. An explanation could be the suggested protective effect of female sex hormones (estrogens) because a more aggressive cancer biology has been observed in men and postmenopausal women compared with premenopausal women in several cancer types, including esophageal cancer (9,20). Interestingly, we observed that women were more often diagnosed with a diffuse-type GAC and signet cell ring EAC and GAC, which is in line with earlier studies (7,21) and may have contributed to their poorer survival rates (22). Moreover, women more often had peritoneal and less often liver metastasis, which is in line with colorectal cancer and likely to reflect differences in tumor biology, because peritoneal metastases are more frequently found in patients with a diffuse histology tumor (23,24). Other sex differences in tumor biology of gastroesophageal cancer are increasingly reported as well. For example, women with GAC more frequently have a microsatellite instable tumor, whereas tumors associated with the Epstein-Barr virus are more frequently found in men (21). In addition, sex differences in efficacy and toxicity of systemic treatment have been reported (5,30,31) and may have contributed to differences in survival as well. Unfortunately, data on toxicity as well as microsatellite instable tumors were not available in our study. More research on differences in biology and treatment response is necessary to understand differences in outcome and improve the balance between efficacy and toxicity for both men and women.
Female sex hormones may not only influence tumor biology or treatment response but also play a role in the development of gastroesophageal cancer. Although risk factors such as abdominal adiposity and gastroesophageal reflux disease are more common in men, they cannot fully explain the overrepresentation of men in the incidence of EAC (6,10,25). To illustrate, men have a 2.5 times greater risk to develop a Barrett esophagus but a 3-7 times greater risk to subsequently develop EAC (11). In addition, higher incidence rates of ESCC in women compared with men have been reported despite lower prevalence of the behavioral risk factors of smoking and alcohol (26). It is therefore suggested that female sex hormones decrease the risk of esophageal and gastric cancer (9,20,27–29).
Interestingly, the overall proportion of women with EAC and GAC who received systemic treatment was statistically significantly lower than the proportion of men and numerically lower in ESCC. Hospital volume was found to play a role in the probability of receiving systemic treatment in EAC and ESCC but did not influence the gender disparity in multivariable analysis. Besides performance status, age, and a diffuse histology, being a woman was independently associated with a lower chance of receiving systemic treatment for GAC (OR = 0.79), and, although not statistically significant, odds ratios were below 1 in EAC (OR = 0.86) and ESCC (OR = 0.79). Moreover, the survival difference in favor of men with EAC, which was not observed in multivariable analysis after adjustment for clinical covariates, systemic treatment, and the interaction between gender and systemic treatment, suggests that women are undertreated. These differences are worrisome, because systemic treatment not only prolongs survival (32) but also improves patients’ quality of life (33). On the other side of the equation, some men could be overtreated, because only best supportive care may be the best option in selected patients, for example, those with a short life expectancy (34). Both over- and undertreatment are examples of suboptimal care and require further examination.
To understand the gender-based causes for the statistically significant and clinically relevant difference in treatment allocation observed in our study, we propose a research agenda based on the Andersen healthcare utilization model, a framework that describes 3 domains of determinants for health services (12,35). The first domain consists of predisposing factors: beliefs and preferences of the individual. Gender has earlier been identified as the most independent predictor of patient preferences (36). Because, for example, women have appeared to be more likely to prefer palliative care (37), this may have affected treatment choices. Factors enabling or impeding health-care use are the second domain and include access to health insurance or family support. In the Dutch population aged 55 years and older, women are overrepresented and less often married than men (38,39). Being single has been associated with a higher probability of refraining from esophageal cancer treatment (40,41). We hypothesize that lack of spousal support may contribute to different treatment choices. Another factor that may also impede access in these patients is that physicians may be influenced by stereotypes and biased in treatment propositions and recommendations. For example, single patients have been offered treatment less often because of the assumption that they do not have enough support (42). Gender stereotypes are also known to exist in medical diagnosis and decisions: physicians are more likely to interpret symptoms in women as psychosocial, and illnesses in men are investigated and treated more extensively despite the same severity of symptoms (43–45). Awareness of these unconscious biases among physicians is urgently needed to narrow the treatment gap (45). The third domain includes the need factors. Differences in the need for care may exist, for example, due to differences in perception of disease symptoms between men and women (46). Future qualitative studies that explore a patient’s disease perception and preferences as well as environmental or social factors and physicians’ possible unconscious biases in proposing and recommending treatments could be valuable in identifying causes for this disparity.
In conclusion, not only patient characteristics, such as comorbidities, but also tumor characteristics, such as histology, as well as palliative systemic treatment allocation and OS differ statistically significantly between all men and women with advanced EAC. Although behavioral factors influence for example the presence of comorbidities, other differences, such as the higher frequency of women with signet cell GAC, cannot be explained by differences in behavior and support the hypothesis of a sexual dimorphism in cancer susceptibility and biology (6,20). An independent association between gender and OS was not observed after adjustment for clinical covariates, treatment, and the interaction between gender and treatment, suggesting the observed inferior survival in women with EAC might result from less frequent systemic treatment administration. Thus, more consequent systemic treatment administration in women may constitute an example for an opportunity to improve patient outcomes. The reasons for differences in treatment allocation, including potential differences in individual preferences and beliefs and the relative contributions of both physicians and patients, need further investigation.
Funding
Not applicable.
Notes
Role of the funders: Not applicable.
Disclosures: RHAV reports grants from BMS and Roche. ADW has received consulting fees from BMS, Servier Suisse, Merck, MSD, Bayer, EMD Serono, Lilly, Celgene, Shire, Pierre-Fabre, and Pfizer, non-financial support (for congress participations) from Sanofi, Astra-Zeneca, AbbVIE and Ipsen and an educational grant from Roche to EORTC. VEPPL received educational grants and unrestricted research grants from Roche. MGHvO reports grants from Amgen, BMS, Lilly, Nordic, Merck, Roche and Servier. SSG reports a research grant from Olympus and consulting fees from Medtronic. MIvBH reports research grants from Olympus and Stryker, in addition to consulting fees from Medtronic, Mylan and Johnson and Johnson. HWMvL reports a consult/advisory role for BMS, Celgene, Lilly, Merck, and Nordic, and Servier and has received unrestricted research funding from Bayer, BMS, Celgene, Lilly, Merck Serono, MSD, Nordic, Philips, Roche and Servier. The other authors declare that they have no conflicts of interest.
Author contributions: Study concepts: WD, ADW, HvL. Study design: WD, ADW, MGHvO, RHAV, HvL. Data acquisition: WD, RHAV. Quality control of data and algorithms: WD, RHAV, MGHvO. Data analysis and interpretation: WD, MCK, ADW, RHAV, MGHvO, VEPPL, HvL. Statistical analysis: WD. Manuscript preparation: WD. Manuscript editing: WD, MCK, ADW, MGHvO, HvL. Manuscript review: all authors.
Prior presentations: Part of this study was presented at ESMO Virtual Congress, 2020.
Acknowledgements: The authors thank the registration team of the Netherlands Comprehensive Cancer Organisation (IKNL) for the collection of data for the Netherlands Cancer Registry. The authors thank Marije Wolvers from the Clinical Research Unit of the University of Amsterdam for her help in the statistical methodology.
Data Availability
The data that support the findings of this study are available from the Netherlands Cancer Registry. Restrictions apply to the availability of these data, which were used under license for this study.
References
- 1.Netherlands Comprehensive Cancer Organization (IKNL). Dutch Cancer Figures.
- 2. Ferlay J, Colombet M, Soerjomataram I, et al. Cancer incidence and mortality patterns in Europe: estimates for 40 countries and 25 major cancers in 2018. Eur J Cancer. 2018;103:356–387. [DOI] [PubMed] [Google Scholar]
- 3. Siegel RL, Miller KD, Jemal A.. Cancer statistics, 2020. CA Cancer J Clin. 2020;70(1):7–30. [DOI] [PubMed] [Google Scholar]
- 4. Najari BB, Rink M, Li PS, et al. Sex disparities in cancer mortality: the risks of being a man in the United States. J Urol. 2013;189(4):1470–1474. [DOI] [PubMed] [Google Scholar]
- 5. Özdemir BC, Csajka C, Dotto GP, Wagner AD.. Sex differences in efficacy and toxicity of systemic treatments: an undervalued issue in the era of precision oncology. J Clin Oncol. 2018;36(26):2680–2683. [DOI] [PubMed] [Google Scholar]
- 6. Mauvais-Jarvis F, Bairey MN, Barnes PJ, et al. Sex and gender: modifiers of health, disease, and medicine. Lancet. 2020;396(10250):565–582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Kim HW, Kim JH, Lim BJ, et al. Sex disparity in gastric cancer: female sex is a poor prognostic factor for advanced gastric cancer. Ann Surg Oncol. 2016;23(13):4344–4351. [DOI] [PubMed] [Google Scholar]
- 8. Yang D, Hendifar A, Lenz C, et al. Survival of metastatic gastric cancer: significance of age, sex and race/ethnicity. J Gastrointest Oncol. 2011;2(2):77–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Bohanes P, Yang D, Chhibar RS, et al. Influence of sex on the survival of patients with esophageal cancer. J Clin Oncol. 2012;30(18):2265–2272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Wagner AD, Oertelt-Prigione S, Adjei A, et al. Gender medicine and oncology: report and consensus of an ESMO workshop. Cancer-Related Cogn Impair. 2019;30:1914–1924. [DOI] [PubMed] [Google Scholar]
- 11. Wong A, Fitzgerald RC.. Epidemiologic risk factors for Barrett’s esophagus and associated adenocarcinoma. Clin Gastroenterol Hepatol. 2005;3(1):1–10. [DOI] [PubMed] [Google Scholar]
- 12. Anderson RM. Revisiting the behavioral model and access to medical care: does it matter? J Health Soc Behav. 1995;36(1):1–10. [PubMed] [Google Scholar]
- 13. Edge SB, Compton CC.. The American Joint Committee on Cancer: The 7th Edition of the AJCC Cancer Staging Manual and the future of TNM. Ann Surg Oncol. 2010;17(6):1471–1474. [DOI] [PubMed] [Google Scholar]
- 14. Brierley JD, Gospodarowicz MK, Whittekind C.. TNM Classification of Malignant Tumours. John Wiley & Sons; 2017. [Google Scholar]
- 15.Dutch Clinical Practice Guidelines for Gastric Carcinoma, version 2.2. 2016. www.oncoline.nl. Accessed December 13, 2018.
- 16.Dutch Clinical Practice Guidelines for Esophageal Carcinoma, version 3.1. 2014. www.oncoline.nl. Accessed December 13, 2018.
- 17. Van Putten M, Koëter M, Van Laarhoven HWM, et al. Hospital of diagnosis influences the probability of receiving curative treatment for esophageal cancer. Ann Surg. 2018;267(2):303–310. [DOI] [PubMed] [Google Scholar]
- 18. Van Putten M, Verhoeven RHA, Van SJ, et al. Hospital of diagnosis and probability of having surgical treatment for resectable gastric cancer. Br J Surg. 2016;103(3):233–241. [DOI] [PubMed] [Google Scholar]
- 19. Dijksterhuis WPM, Verhoeven RHA, Pape M, et al. Hospital volume and beyond first-line palliative systemic treatment in metastatic oesophagogastric adenocarcinoma: a population-based study. Eur J Cancer. 2020;139:107–118. [DOI] [PubMed] [Google Scholar]
- 20. Clocchiatti A, Cora E, Zhang Y, Dotto GP.. Sexual dimorphism in cancer. Nat Rev Cancer. 2016;16(5):330–339. [DOI] [PubMed] [Google Scholar]
- 21. Bass AJ, Thorsson V, Shmulevich I, et al. Comprehensive molecular characterization of gastric adenocarcinoma. Nature. 2014;513(7517):202–209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. van der Kaaij RT, Koemans WJ, van Putten M, et al. A population-based study on intestinal and diffuse type adenocarcinoma of the oesophagus and stomach in the Netherlands between 1989 and 2015. Eur J Cancer. 2020;130:23–31. [DOI] [PubMed] [Google Scholar]
- 23. Lemmens VE, Klaver YL, Verwaal VJ, Rutten HJ, Coebergh JWW, De Hingh IH.. Predictors and survival of synchronous peritoneal carcinomatosis of colorectal origin: a population-based study. Int J Cancer. 2011;128(11):2717–2725. [DOI] [PubMed] [Google Scholar]
- 24. Koemans WJ, Luijten J, van der Kaaij RT, et al. The metastatic pattern of intestinal and diffuse type gastric carcinoma - a Dutch national cohort study. Cancer Epidemiol. 2020;69:101846. [DOI] [PubMed] [Google Scholar]
- 25. Petrick JL, Hyland PL, Caron P, et al. Associations between prediagnostic concentrations of circulating sex steroid hormones and esophageal/gastric cardia adenocarcinoma among men. J Natl Cancer Inst. 2019;111(1):34–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Nobel TB, Livschitz J, Eljalby M, et al. Unique considerations for females undergoing esophagectomy. Ann Surg. 2020;272(1):113–117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Petrick JL, Hyland PL, Caron P, et al. Associations between prediagnostic concentrations of circulating sex steroid hormones and esophageal/gastric cardia adenocarcinoma among men. J Natl Cancer Inst. 2019;111(1):34–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Yang H, Sukocheva OA, Hussey DJ, Watson DI.. Estrogen, male dominance and esophageal adenocarcinoma: is there a link? World J Gastroenterol. 2012;18(5):393–400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Chandanos E, Lagergren J.. Oestrogen and the enigmatic male predominance of gastric cancer. Eur J Cancer. 2008;44(16):2397–2403. [DOI] [PubMed] [Google Scholar]
- 30. Davidson M, Wagner AD, Kouvelakis K, et al. Influence of sex on chemotherapy efficacy and toxicity in oesophagogastric cancer: a pooled analysis of four randomised trials. Eur J Cancer. 2019;121:40–47. [DOI] [PubMed] [Google Scholar]
- 31. Cristina V, Mahachie J, Mauer M, et al. Association of patient sex with chemotherapy-related toxic effects: a retrospective analysis of the PETACC-3 trial conducted by the EORTC gastrointestinal group. JAMA Oncol. 2018;4(7):1003–1006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Veer ET, Mohammad NH, Van Valkenhoef G, et al. The efficacy and safety of first-line chemotherapy in advanced esophagogastric cancer: a network meta-analysis. J Natl Cancer Inst. 2016;108(10):1–13. [DOI] [PubMed] [Google Scholar]
- 33. Van Kleef JJ, Ter VE, Van Den Boorn HG, et al. Quality of life during palliative systemic therapy for esophagogastric cancer: systematic review and meta-analysis. J Natl Cancer Inst. 2020;112(1):12–29. [DOI] [PubMed] [Google Scholar]
- 34. Merchant SJ, Lajkosz K, Brogly SB, et al. The final 30 days of life: a study of patients with gastrointestinal cancer in Ontario, Canada. J Palliat Care. 2017;32(3-4):92–100. [DOI] [PubMed] [Google Scholar]
- 35. Babitsch B, Gohl D, Von Lengerke T.. Re-revisiting Andersen’s behavioral model of health services use: a systematic review of studies from 1998-2011. GMS Psycho-Social-Med. 2012;9(2011):1–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Wessels H, Graeff A, Wynia K, et al. Gender‐related needs and preferences in cancer care indicate the need for an individualized approach to cancer patients. Oncologist. 2010;15(6):648–655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Saeed F, Hoerger M, Norton SA, Guancial E, Epstein RM, Duberstein PR.. Preference for palliative care in cancer patients: are men and women alike? J Pain Symptom Manage. 2018;56(1):1–6.e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Centraal Bureau voor de Statistiek (CBS). Bevolking; geslacht, leeftijd en burgerlijke staat. 2019. https://opendata.cbs.nl/statline/#/CBS/nl/dataset/7461bev/table?ts=1593714454135. Accessed July 7, 2020.
- 39.Centraal Bureau voor de Statistiek (CBS). Mannen en vrouwen per leeftijdsgroep. 2019. https://www.cbs.nl/nl-nl/achtergrond/2018/35/mannen-en-vrouwen-per-leeftijdsgroep. Accessed July 7, 2020.
- 40. Paniagua CA, Haug KL, Zhao L, Reddy RM.. Association between marital status and racial disparities in esophageal cancer care. J Clin Oncol Pract. 2020;16(6):e498–e506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Du L, Kim JJ, Chen B, Zhu S, Dai N.. Marital status is associated with superior survival in patients with esophageal cancer: a surveillance, epidemiology, and end results study. Oncotarget. 2017;8(56):95965–95972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. DelFattore J. Death by stereotype? Cancer treatment in unmarried patients. N Engl J Med. 2019;381(10):982–985. [DOI] [PubMed] [Google Scholar]
- 43. Andersson J, Salander P, Hamberg K.. Using patients’ narratives to reveal gender stereotypes among medical students. Acad Med. 2013;88(7):1015–1021. [DOI] [PubMed] [Google Scholar]
- 44. Hamberg K, Risberg G, Johansson EE, Westman G.. Gender bias in physicians’ management of neck pain: a study of the answers in a Swedish national examination. J Womens Health Gend Based Med. 2002;11(7):653–666. [DOI] [PubMed] [Google Scholar]
- 45. Fitzgerald C, Hurst S.. Implicit bias in healthcare professionals: a systematic review. BMC Med Ethics. 2017;18(1):19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Cheung WY, Le LW, Gagliese L, Zimmermann C.. Age and gender differences in symptom intensity and symptom clusters among patients with metastatic cancer. Support Care Cancer. 2011;19(3):417–423. [DOI] [PubMed] [Google Scholar]
- 47. Von EE, Altman DG, Egger M, Pocock SJ, Gøtzsche PC, Vandenbroucke JP.. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. PLoS Med. 2007;4(10):1623–1627. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available from the Netherlands Cancer Registry. Restrictions apply to the availability of these data, which were used under license for this study.