Abstract
Background
Supporting well-informed decisions about breast cancer screening requires communicating that inconsequential disease may be detected, leading to overdiagnosis and overtreatment. Having previously shown that telling women about overdetection improved informed choice, we investigated effects on screening knowledge and participation over 2 years.
Methods
We conducted a community-based, parallel-group, randomized controlled trial in Australia. Participants were women aged 48-50 years, without personal or strong family history of breast cancer, who had not undergone mammography in the past 2 years. We randomly assigned 879 women to receive the intervention decision aid (evidence-based information on overdetection, breast cancer mortality reduction, and false-positives) or control decision aid (identical but without overdetection information). We interviewed 838 women postintervention and recontacted them for follow-up at 6 months and 1 and 2 years. Main outcomes for this report are screening knowledge and participation.
Results
We interviewed 790, 746, and 712 participants at 6 months, 1, and 2 years, respectively. The intervention group demonstrated superior knowledge throughout follow-up. After 2 years, conceptual knowledge was adequate in 123 (34.4%) of 358 women in the intervention group compared with 71 (20.1%) of 354 control participants(odds ratio = 2.04, 95% confidence interval = 1.46 to 2.85). Groups were similar in total screening participation (200 [55.1%] vs 204 [56.0%]; = 0.97, 95% confidence interval = 0.73 to 1.29).
Conclusions
A brief decision aid produced lasting improvement in women’s understanding of potential consequences of screening, including overdetection, without changing participation rates. These findings support the use of decision aids for breast cancer screening.
Mammography screening can reduce a woman’s risk of dying from breast cancer but entails the risk of having a cancer diagnosed that would not have presented clinically in her lifetime—termed overdetection or overdiagnosis (1). Such a diagnosis, and the resulting overtreatment, can harm women physically and emotionally (2), for example, through treatment side effects (3-5) and psychosocial consequences of being labeled with a diagnosis of cancer (6‐8).
Growing scientific attention to overdetection has highlighted the importance of ensuring that women invited to breast screening are informed about the potential for harm in addition to benefit (9‐11). After a thorough evidence review, the Independent UK Panel on Breast Cancer Screening wrote that “information should be made available in a transparent and objective way to women invited to screening so that they can make informed decisions” (12). Momentum is shifting from persuasive approaches to screening communication (13) toward more balanced information, giving people the opportunity to make informed choices whether to screen or not based on their personal assessment of the trade-offs between potential outcomes (14‐16).
Decision aids are tools to communicate evidence-based information about benefits and harms of different health-care options to help people make informed decisions. Numerous randomized trials show decision aids improve knowledge and facilitate informed choice in various settings, including cancer screening (17). However, earlier breast screening decision aid trials (18,19) did not involve women aged 50 years (when screening is initiated in many countries), and the decision aids paid little attention to overdetection. We developed the world’s first mammography decision aid giving comprehensive explanatory and quantitative information about overdetection along with other important breast screening outcomes (20). Both intervention and control versions included information about breast cancer deaths averted and false-positive screening results, but only the intervention decision aid contained information about overdetection (21).
The aim of our study was to investigate for the first time the effects of including evidence-based consumer-friendly information about overdetection of breast cancer in a decision aid in a community sample of women around the target age for starting breast screening in Australia. Mammographic screening is offered free of charge to women in Australia, and treatment is available to all through the public health-care system. We previously published short-term findings showing that overdetection information statistically significantly improved knowledge and increased the number of women making an informed choice about breast screening (22). Here, we present the effects of the intervention on a range of additional prespecified outcomes collected from the same study cohort over 2 years of follow-up, importantly including both self-reported and objectively measured data on participation in screening.
Methods
Study Design and Participants
The study protocol has been published (20). We did a community-based parallel-group randomized controlled trial in New South Wales (NSW), Australia. NSW is Australia’s most populous state, with a predominantly urban population (75% living in major cities). The NSW Electoral Commission randomly sampled women aged 48-50 years from the electoral roll, which covers 98% of the eligible population. Trained interviewers from an independent nonprofit company telephoned potential participants to ascertain their eligibility. Exclusion criteria were mammography in the last 2 years; personal history of breast cancer; high risk of breast cancer (eg, strong family history); or inadequate language ability. Interviewers told potential participants the study involved information about breast screening (without mentioning overdetection) and recorded oral consent.
The University of Sydney Human Research Ethics Committee approved the trial (2012/1429), and the NSW Population & Health Services Research Ethics Committee additionally approved the linkage with BreastScreen NSW data (2018HRE0203). All participants provided informed consent to participate. The study was performed in accordance with the Declaration of Helsinki.
Randomization and Masking
Randomization was performed using a computer system, with allocation concealment. We assigned participants to either the intervention or control group in a 1:1 ratio. Women were told the study was about a new booklet aiming to provide clear and useful information for women about breast cancer screening. They knew they would receive 1 of 2 versions of an information booklet but did not know how these differed (ie, inclusion vs omission of overdetection content).
Procedures
The decision aid and details about development, piloting, and preliminary evaluation are published elsewhere (see Box 1 for brief overview) (21,22). Quantitative content was from a published model of mammography screening outcomes for Australian women (23). The model incorporates estimates of breast cancer mortality reduction and overdetection from meta-analysis of randomized trials, adjusted to reflect the effect of undergoing screening (not merely being invited) (24). These estimates were applied to Australian incidence and mortality data to quantify cumulative outcomes of biennial screening from age 50-69 years vs no screening over this period, and the 20-year cumulative likelihood of a false-positive was modeled from Australian data (23).
Box 1.
Intervention and control decision aids
The intervention contained evidence-based explanatory and quantitative information about benefits and harms of mammography (breast cancer mortality reduction, overdetection, and false positives). To help users consider what it might be like to experience downstream consequences of screening, we briefly described how abnormal mammograms are followed up and how breast cancer is treated. The control booklet omitted overdetection content (aligning in that regard with the standard NSW screening program leaflet used at the time) but was otherwise identical to the intervention (i.e. included quantitative information about mortality and false positives, unlike the standard program leaflet).
Content and presentation were guided by qualitative research, input from layperson collaborators and independent experts, and thorough piloting with iterative revisions. Outcome frequencies (per 1000 women screened biennially) were illustrated with icon arrays and summarized in a table. Materials were posted for women to read at home, consistent with the setup of the Australian breast screening program, which is directly accessible by women without referral. No provider consultation, training or counseling was incorporated.
The intervention decision aid (updated to reflect the expanded age group now targeted by the BreastScreen program) is freely available online: https://ses.library.usyd.edu.au/handle/2123/16658
After conducting recruitment and a baseline interview by telephone, we randomly assigned participants and sent their allocated decision aid to their nominated postal address. We gathered self-reported outcome data using standardized questions (see the Supplementary Methods, available online) in structured computer-assisted telephone interviews at approximately 3 weeks (previously reported) (22), 6 months (including a subset of outcomes as per protocol) (20), 1 year, and 2 years after randomization (by which time all participants were within the age group invited by the public screening program). Participants’ study group allocation was not displayed to interviewers. All women who completed the 3-week follow-up were approached for every subsequent follow-up, unless they withdrew from the trial.
To complement self-reported screening participation data, we obtained data on mammograms provided either within the state’s public screening program (BreastScreen NSW) or externally and reimbursed through the federal government’s Medicare system (Medicare Benefits Schedule). During the final telephone interview, participants were invited to give written consent for their screening records to be checked to verify self-reported data on mammography (further details in the Supplementary Methods, available online). Participants who agreed to consider this request were posted an information statement, consent form, and prepaid return envelope. To maximize data quality, participants received a brief phone call to remind them, without coercion, to return the consent form if they wished.
Outcomes
The published protocol details sample size calculations, outcomes, measures, and timing of their administration (20). We have published postintervention results assessed at 3 weeks for the primary outcome (informed choice about breast screening) and most secondary outcomes, as well as data on women’s utilization of the study materials and details of our knowledge marking scheme (22). Briefly, informed choice was assessed at the initial follow-up as a dichotomous outcome, whereby a woman was judged to have made an informed choice if she had adequate knowledge and her attitudes and intentions were consistent. Comparing the proportion of women in the intervention and control groups who made an informed choice revealed a statistically significant difference favoring the intervention group (22).
Additional outcomes measured for the first time after the initial follow-up were screening participation, decision regret, and quality of life. During the 6-month interview, women were asked whether they had had a mammogram since joining the study and, at each successive follow-up, whether they had had a mammogram since the previous interview. We assessed regret about screening decisions with the Decision Regret Scale (25), defining heightened regret as scores of 25 or above in line with previous literature (26). We investigated quality of life using the Consequences of Screening in Breast Cancer questionnaire (27). This multidimensional condition-specific instrument was purpose designed to capture aspects of well-being that may be related to breast screening (eg, dejection and impact on behavior and sleep); higher scores indicate poorer psychosocial health.
This study is registered with the Australian New Zealand Clinical Trials Registry, number ACTRN12613001035718.
Statistical Analysis
We used the statistical software SPSS (IBM Corporation, Armonk, NY) and SAS (SAS Institute, Cary, NC). We included all participants as randomly assigned, except those lost to follow-up and those for whom some outcome data were missing because of incomplete answers (see Figure 1). We analyzed binary outcomes using logistic regression and continuous outcomes using linear regression models, both with generalized estimating equations to account for repeated measurements over time. The analytical models were prespecified to include only a small set of independent variables, namely those reflecting any effects of study group, time, and the potential interaction between group and time. We adjusted for baseline measurement of the outcome where applicable (ie, for screening attitudes and intentions). Analyses of linked screening data are described in the Supplementary Methods (available online). Tests of statistical significance were 2-sided (α of 5%).
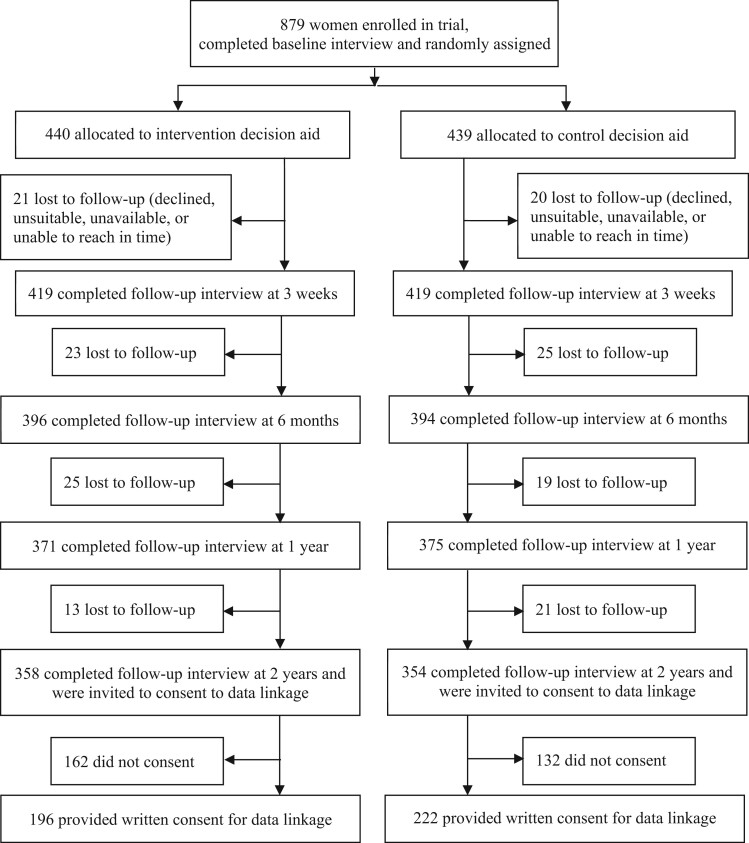
Figure 1.
Trial profile extended to include long-term follow-up. Further details about recruitment and procedures prior to randomization have been published (22).
Results
A total of 879 participants were randomly allocated in the trial; 838 completed the first follow-up (22) and were eligible to participate in subsequent follow-ups from September 2014 to August 2016. During 3 rounds of interviews, we obtained long-term follow-up data from 790 (94.3%), 746 (89.0%), and 712 (85.0%), respectively, of eligible participants, with numbers well balanced across groups (Figure 1). Sociodemographic and other baseline characteristics were similar between study groups (22) and for women who did and did not provide long-term data (Supplementary Table 1, available online). Of the 712 women participating in the final interview, 418 (58.7%) provided written consent for data linkage to verify self-reported screening mammography (Figure 1).
Table 1 presents results for knowledge, including key component subscales (Supplementary Table 2, available online, shows all subscales). Overall, knowledge (sum total of all knowledge items about 3 key screening outcomes: breast cancer mortality benefit, overdetection, and false-positives) across the 2-year follow-up period was statistically significantly higher in the intervention group than the control group. The difference between study groups diminished somewhat over time but remained statistically significant after 1 and 2 years. At 2 years, the mean total knowledge score among intervention group participants was 9.57 compared with 8.89 among control participants (difference = 0.68, 95% confidence interval [CI] = 0.30 to 1.05). This persisting difference was entirely attributable to the intervention group’s superior understanding of the concept of overdetection.
Table 1.
Total knowledge, knowledge of overdetection, and adequate knowledge determined using different thresholdsa
| Outcome | <1 month follow-up |
1 year follow-up |
2 years follow-up |
P interaction b | |||
|---|---|---|---|---|---|---|---|
| Intervention group (n = 419) | Control group (n = 419) | Intervention group (n = 371) | Control group (n = 375) | Intervention group (n = 358) | Control group (n = 354) | ||
| Total knowledge, mean, 22 marks available | 13.49c | 11.84 | 9.73c | 8.89 | 9.57c | 8.89 | .004 |
| Conceptual, 11 marks available | 8.85c | 7.33 | 8.03c | 7.31 | 7.91c | 7.31 | <.001 |
| Numerical, 11 marks available | 4.64 | 4.50 | 1.70 | 1.58 | 1.66 | 1.58 | .94 |
| Overdetection knowledge, mean, 10 marks available | 6.19c | 4.05 | 4.74c | 3.92 | 4.66c | 3.99 | <.001 |
| Conceptual, 7 marks available | 5.05c | 3.48 | 4.26c | 3.56 | 4.20c | 3.61 | <.001 |
| Numerical, 3 marks available | 1.14c | 0.57 | 0.48c | 0.37 | 0.46 | 0.38 | <.001 |
| Adequate conceptual knowledge, all subscales, No. (%) | 248 (59.2)c | 87 (20.8) | 150 (40.4)c | 76 (20.3) | 123 (34.4)c | 71 (20.1) | <.001 |
| Mortality benefit subscale | 370 (88.3) | 382 (91.2) | 324 (87.3) | 318 (84.8) | 304 (84.9) | 295 (83.3) | .12 |
| False-positives subscale | 415 (99.0) | 418 (99.8) | 370 (99.7) | 371 (98.9) | 352 (98.3) | 351 (99.2) | .10 |
| Overdetection subscale | 279 (66.6)c | 93 (22.2) | 168 (45.3)c | 90 (24.0) | 149 (41.6)c | 88 (24.9) | <.001 |
| Adequate conceptual + numerical knowledge, all subscales, No. (%) | 122 (29.1)c | 71 (16.9) | 22 (5.9) | 17 (4.5) | 22 (6.1) | 19 (5.4) | .28 |
| Mortality benefit subscale | 272 (64.9) | 256 (61.1) | 111 (29.9) | 93 (24.8) | 98 (27.4) | 87 (24.6) | .86 |
| False-positives subscale | 244 (58.2)c | 278 (66.3) | 144 (38.8) | 151 (40.3) | 148 (41.3) | 155 (43.8) | .27 |
| Overdetection subscale | 230 (54.9)c | 112 (26.7) | 96 (25.9)c | 65 (17.3) | 93 (26.0)c | 64 (18.1) | <.001 |
Knowledge questions are provided in the Supplementary Methods (available online). The marking scheme and score thresholds have been published (22). Supplementary Tables 2-4 (available online) provide additional data and analyses. Knowledge was not assessed at the 6-month follow-up, as per the protocol (20).
Two-sided P value for group x time interaction.
Indicates where group differences are statistically significant at that timepoint.
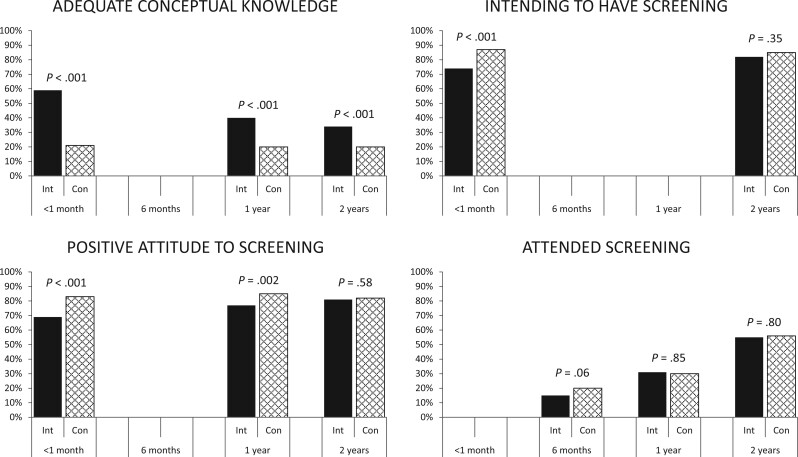
Given that no consensus exists on what level of knowledge constitutes being informed, we classified knowledge as adequate using 2 differently defined thresholds (22). The primary threshold, set a priori, required women to demonstrate some numerical knowledge (as well as moderately good conceptual knowledge) about all 3 screening outcomes; the alternative, conceptually focused threshold disregarded the numerical items but required a high level of performance on the conceptual items regarding all 3 screening outcomes. At the first comparison between study groups, shortly postintervention, statistically significantly more women in the intervention group had adequate knowledge (22). After 1 and 2 years, few women in either group were able to demonstrate the numerical knowledge required to meet the primary threshold, but the intervention group continued to show a statistically significant advantage (albeit, diminishing somewhat over time) using the conceptual knowledge threshold (Table 1, Figure 2). At 2 years, conceptual knowledge was adequate in 123 (34.4%) of 358 women in the intervention group compared with 71 (20.1%) of 354 control participants (odds ratio [OR] = 2.04, 95% CI = 1.46 to 2.85). Again, the observed effect was because of better knowledge of the overdetection content.
Figure 2.
Key self-reported outcomes. For all outcomes shown, the group x time interaction is statistically significant (P < .05). Con = control group; Int = intervention group.
Women’s attitudes toward breast screening remained generally positive over the 2-year follow-up period but were less positive in the intervention group than the control group. The group difference on total attitude scores diminished over time as intervention group scores approached those of the control group; the difference remained statistically significant at 1 year but had disappeared after 2 years (Table 2). When examining the proportions of study groups meeting our threshold for a positive attitude (22), fewer women in the intervention group had positive attitudes across the first year of follow-up, but the groups no longer differed after 2 years (287 [80.6%] vs 285 [81.9%]; OR = 0.89, 95% CI = 0.59 to 1.34) (Table 3, Figure 2).
Table 2.
Psychosocial outcomes, mean scoresa
| Outcome | Range of scores possible | <1 month follow-up |
1 year follow-up |
2 years follow-up |
P interaction b | |||
|---|---|---|---|---|---|---|---|---|
| Intervention group (n = 419) | Control group (n = 419) | Intervention group (n = 371) | Control group (n = 375) | Intervention group (n = 358) | Control group (n = 354) | |||
| Attitudes to screening | 6-30 | 24.53c | 26.05 | 25.23c | 26.20 | 25.37c | 26.01 | .008 |
| Anxiety | 20-80 | 29.71 | 29.64 | 31.74 | 32.17 | 31.76 | 32.98 | .62 |
| Breast cancer worry | 1-4 | 1.67c | 1.84 | 1.58c | 1.70 | 1.63 | 1.68 | .09 |
| Quality of lifed | 0-87 | 1.57 | 2.25 | 1.71 | 2.00 | 1.30 | 1.28 | .10 |
The Supplementary Methods (available online) and published protocol (20) give further details on outcomes and measurement. Supplementary Tables 2 and 3 (available online) provide additional data and analyses, including 6-month data for anxiety and worry.
Two-sided P value for group x time interaction.
Indicates where group differences are statistically significant at that timepoint.
The first data collected for quality of life are from 6-month follow-up, which included 396 women in the intervention group and 394 controls. Quality-of-life data are missing for maximum 108 cases within 1 round. For other variables, data are missing for maximum 21 cases in a round.
Table 3.
| Outcome | <1 month follow-up |
1 year follow-up |
2 years follow-up |
P interaction c | |||
|---|---|---|---|---|---|---|---|
| Intervention group, No. (%) | Control group, No. (%) | Intervention group, No. (%) | Control group, No. (%) | Intervention group, No. (%) | Control group, No. (%) | ||
| All women participating | 419 | 419 | 371 | 375 | 358 | 354 | |
| Positive attitude to screening (score ≥24) | 282 (68.9)d | 340 (83.3) | 280 (76.5)d | 317 (85.2) | 287 (80.6) | 285 (81.9) | .004 |
| Intending to screen (definitely/likely) | 308 (73.5)d | 363 (86.6) | — | — | 293 (81.8) | 301 (85.0) | .002 |
| Heightened decision regret (score >25)e | 59 (15.5) | 54 (14.0) | 64 (17.5) | 55 (14.9) | — | — | .81 |
| Attended screening (self-reported)e | 60 (15.2) | 80 (20.3) | 116 (31.1) | 116 (30.4) | 200 (55.1) | 204 (56.0) | .03 |
| Women participating in data linkage | 196 | 222 | 196 | 222 | 196 | 222 | |
| Attended screening (BSNSW/MBS record)e | 43 (21.9) | 54 (24.3) | 85 (43.4) | 80 (36.0) | 137 (69.9) | 154 (69.4) | .03 |
The Supplementary Methods (available online) and published protocol (20) give further details on outcomes and measurement. See Supplementary Table 4 (available online) for full analyses comparing groups over time and Supplementary Table 7 and Supplementary Figure 1 (available online) for additional analyses using linked screening data. BSNSW=Breast Screen New South Wales; MBS = Medicare Benefits Schedule.
For some variables, data are missing (max. 24 cases in a round).
Two-sided P value for group x time interaction.
Indicates where group differences are statistically significant at that timepoint.
The first data for regret and screening attendance are from 6-month follow-up including 396 women in the intervention group and 394 controls [attitude and intention were not assessed at 6 months, in line with the protocol (20)]. Decision regret was not assessed at 2-year follow-up (programming error). Self-report mammography numbers shown at each timepoint include all those responding in the current follow-up round and any women recorded as screened in an earlier round.
For women’s intentions about undergoing breast screening in the next 2-3 years, positive intentions prevailed in both groups at 2-year follow-up, as they had previously. The group by time interaction was highly statistically significant: at the first comparison postintervention, fewer women in the intervention group than the control group intended to be screened, whereas this difference had largely dissipated by 2 years (293 [81.8%] vs 301 [85.0%]; OR = 0.81, 95% CI = 0.52 to 1.23) (Table 3, Figure 2).
During the first 6 months of follow-up, slightly fewer women in the intervention group reported attending screening compared with the control group. By the 1- and 2-year follow-ups, the groups were similar in terms of the cumulative proportion of women reporting having a mammogram (Table 3, Figure 2): at 2 years, 200 women in the intervention group (55.1%) compared with 204 control participants (56.0%; OR = 0.97, 95% CI = 0.73 to 1.29). We observed a similar pattern when examining the data from screening records, and agreement between self-report and screening records was high (Supplementary Tables 6 and 7, available online).
Overall, women in the intervention group were less worried about developing breast cancer than were control participants (Table 2). Regret about screening decisions was low overall, with few women in either group expressing heightened regret (Table 3). Mean scores for anxiety and quality of life were similar between groups throughout the follow-up period (Table 2). Supplementary Table 2 (available online) includes data for additional outcomes (perceived risk of breast cancer, anticipated regret for screening and not screening) for which findings over longer-term follow-up remained similar to those previously reported (22).
Discussion
This article reports new evidence of how informing women about overdetection in breast cancer screening affects their long-term knowledge, screening participation, and psychosocial outcomes. Both study groups received a decision aid providing information about the breast cancer mortality benefit from screening and the likelihood of false-positives, whereas the intervention additionally explained the chance of overdetection. Our key findings are that the intervention durably increased women’s understanding of potential outcomes of breast screening, with overall knowledge remaining superior in the intervention group over 2 years of follow-up, and that screening participation rates during this period did not differ between study groups. We also observed a sustained reduction in worry about developing breast cancer among the intervention group, suggesting a further positive effect of the intervention.
The unique strengths of our study are its randomized design, long follow-up period of 2 years with high participant retention, and objective measurement of screening behavior. As far as we know, such a long-term effect of a decision aid on knowledge has not been shown before, with most studies focusing on short-term effects (17). To complement self-reported screening participation data, we used data linkage (with individual consent) to obtain matched records of mammograms provided through either the statewide public screening program or the national Medicare health insurer (the 2 main avenues for provision of mammography to this population). As expected, we found relatively few women in this cohort were screened outside the organized program (7%; Supplementary Table 7, available online—noting that although 50 years is the recommended starting age, women aged 40-49 years can also access mammography either within or outside the program). Agreement between self-report and screening records was high (among those willing to have their data cross-checked), suggesting that self-report may be considered a reliable way to measure participation. To further our understanding of how and why the study intervention worked, we have explored the psychological pathways involved (28) and will separately report an embedded longitudinal qualitative study.
Ours is the first randomized trial to investigate long-term effects of providing information about overdetection in the context of real-world individual breast screening decisions at the recommended starting age, addressing an important evidence gap identified by systematic reviews of breast screening decision aids (29,30) and independent reviews of mammography screening (12,31,32). Recent randomized trials in Germany, Spain, and Italy (33‐35) each compared a decision aid promoting informed choice against a standard leaflet control. Like ours, these trials showed improvement in informed choice (primary outcome) and found unchanged attendance rates at follow-up (33‐35). However, in the European studies, follow-up duration was limited to approximately 3 months, and because interventions differed substantially from control materials, findings cannot be directly attributed to overdetection information. By contrast, our trial was designed to specifically investigate the effect of explaining overdetection (which was not mentioned in the standard screening leaflet at the time). The fact that the observed knowledge gain was limited to the overdetection component suggests our experimental manipulation succeeded in targeting our particular question.
Our finding that the intervention did not affect screening participation is highly relevant and of broad interest to screening programs, public health, and clinical audiences. Screening programs tasked with monitoring uptake as a performance indicator may be reluctant to inform women about overdetection because of concern that doing so could reduce participation. Observational data from the breast screening program in England show little change in uptake (36) after overdetection was incorporated into information materials provided with screening invitations (37). However, this could be a consequence of the new information not being read and understood. We designed our trial to rigorously investigate whether our intervention could improve understanding of overdetection and how it would influence behavior over 2 years (corresponding to the recommended screening interval in Australia and many other countries) by prespecifying screening participation as an important secondary outcome (20) for which we collected both self-reported and externally verified data. Our results now provide robust evidence that women can be well informed about overdetection and retain some learning over an extended time frame, without affecting screening participation. These findings could facilitate greater adoption of an informed choice approach to screening communication.
In determining our a priori threshold for adequate knowledge, we set a high benchmark by including numerical information, whereas other studies have often considered only conceptual understanding. Although knowledge scores did decline over follow-up, the new learning demonstrated by participants was achieved and maintained after a relatively brief intervention. Women spent on average 15 minutes reading the intervention (3 minutes more than the control), which contained information going against the more positive and persuasive general public discourse about mammography and was sent to them once with no subsequent reinforcement (22). Their learning would likely be bolstered if the information was reinforced somehow, for example, by resending the booklet with each biennial screening invitation and with support from health practitioners.
We designed our trial to assess intervention effects under the best possible circumstances (ie, focusing on efficacy rather than effectiveness). Participants were highly committed to the study and may have engaged more with the materials because of the follow-up interviews, possibly limiting the extent to which findings would generalize beyond a research setting. The main limitation of our study is that it was conducted largely independently of the established screening program. In this respect, our trial was similar to one in Germany (35), whereas the Spanish and Italian trials involved participation by local public screening programs (33,34). An unanswered question therefore remains around how our intervention would influence participation and subsequent outcomes if it were implemented into routine care within population screening, and this is an important avenue for future research. However, enabling women to make better-informed choices is an ethically critical goal in its own right, regardless of any downstream impact on participation. Small reductions in screening participation might reduce both benefit and harm (eg, unnecessary biopsies, surgeries, and complications).
This study found that improving women’s understanding of overdetection produced a durable gain in knowledge and did not adversely affect well-being, including as women participated in screening during the trial. Worry about developing breast cancer was consistently lower in the intervention group than among control participants, and anxiety scores were low throughout follow-up with no group difference observed. These results confirm that providing women with information about overdetection did not cause distress, which should reassure screening programs and practitioners. Our findings show that such information will help inform women effectively and durably, without adverse psychosocial effects and without reducing screening participation. The available evidence therefore supports a policy of providing this information to women routinely (9,10,38).
Funding
This work was supported by the Australian National Health and Medical Research Council (NHMRC project grant number 1062389). JH is supported by an NHMRC Early Career Fellowship (1112509), NH is supported by an NHMRC Investigator (Leader) Grant (1194410), and KMcC is supported by an NHMRC Principal Research Fellowship (1121110).
Notes
Role of the funder: The funding source had no role in study design, data collection, analysis, interpretation, or report writing. The corresponding author had full access to all study data and final responsibility for the decision to submit for publication.
Disclosures: The authors declare no competing interests.
Author contributions: Conceptualization and methodology: All authors. Funding acquisition: KMcC, AB, JJ, NH, HD, KMcG. Project administration: JH. Supervision: KMcC, JJ, LI, AB, KMcG, NH, HD. Data curation and formal analysis: JH, KMcG. Writing—original draft: JH. Writing—review & editing: All authors.
Acknowledgements: We thank Tessa Copp and Caitlin Semsarian for administrative support; layperson contributors Hazel Thornton and Jenn Kidd for input into study design and intervention development; Hunter Research Foundation for recruitment and interviewing services; NSW Ministry of Health for NSW BreastScreen data; Services Australia for provision of the Medicare Benefits Schedule (MBS) data; and the Centre for Health Record Linkage for performing data linkage. We are particularly grateful to all women who took part.
Prior presentations: An earlier version of this work was presented at the International Shared Decision Making Conference in Lyon, France, in July 2017 and the Preventing Overdiagnosis Conference in Quebec City, Canada, in August 2017.
Data Availability
The data underlying this article will be shared on reasonable request to the corresponding author.
Supplementary Material
References
- 1. Brawley OW, Paller CJ.. Overdiagnosis in the age of digital cancer screening. J Natl Cancer Inst. 2021;113(1):1–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. de Ligt KM, Heins M, Verloop J. , et al. Patient-reported health problems and healthcare use after treatment for early-stage breast cancer. Breast. 2019;46:4–11. [DOI] [PubMed] [Google Scholar]
- 3. Smith BD, Jiang J, Shih YC. , et al. Cost and complications of local therapies for early-stage breast cancer. J Natl Cancer Inst. 2017;109(1):djw178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Taylor C, Correa C, Duane FK. , et al. ; for the Early Breast Cancer Trialists’ Collaborative Group. Estimating the risks of breast cancer radiotherapy: evidence from modern radiation doses to the lungs and heart and from previous randomized trials. J Clin Oncol. 2017;35(15):1641–1649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Mehta LS, Watson KE, Barac A. , et al. Cardiovascular disease and breast cancer: where these entities intersect. Circulation. 2018;137(8):e30-66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Thornton H. Pairing accountability with responsibility–the consequences of screening “promotion.” Med Sci Monit. 2001;7(3):531–533. [PubMed] [Google Scholar]
- 7. Ganz PA. Quality-of-life issues in patients with ductal carcinoma in situ. J Natl Cancer Inst Monogr. 2010;2010(41):218–222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Fallowfield L, Jenkins V.. Psychosocial/survivorship issues in breast cancer: Are we doing better? J Natl Cancer Inst. 2014;107(1):dju335. [DOI] [PubMed] [Google Scholar]
- 9. Stefanek ME. Uninformed compliance or informed choice? A needed shift in our approach to cancer screening. J Natl Cancer Inst. 2011;103(24):1821–1826. [DOI] [PubMed] [Google Scholar]
- 10. Klarenbach S, Sims-Jones N, Lewin G. , et al. Recommendations on screening for breast cancer in women aged 40-74 years who are not at increased risk for breast cancer. CMAJ. 2018;190(49):E1441–1451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Mathioudakis AG, Salakari M, Pylkkanen L. , et al. Systematic review on women’s values and preferences concerning breast cancer screening and diagnostic services. Psycho‐Oncology. 2019;28(5):939–947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Independent UK Panel on Breast Cancer Screening. The benefits and harms of breast cancer screening: an independent review. Lancet. 2012;380(9855):1778–1786. [DOI] [PubMed] [Google Scholar]
- 13. Woloshin S, Schwartz LM, Black WC. , et al. Cancer screening campaigns–getting past uninformative persuasion. N Engl J Med. 2012;367(18):1677–1679. [DOI] [PubMed] [Google Scholar]
- 14. Pace LE, Keating NL.. A systematic assessment of benefits and risks to guide breast cancer screening decisions. JAMA. 2014;311(13):1327–1335. [DOI] [PubMed] [Google Scholar]
- 15. Kramer BS, Elmore JG.. Projecting the benefits and harms of mammography using statistical models: Proof or proofiness? J Natl Cancer Inst. 2015;107(7):djv145. [DOI] [PubMed] [Google Scholar]
- 16. Pignone M. Presenting benefits and downsides to facilitate high-quality decision-making about cancer screening. J Natl Cancer Inst. 2016;108(6):djv433. [DOI] [PubMed] [Google Scholar]
- 17. Stacey D, Legare F, Lewis K. , et al. Decision aids for people facing health treatment or screening decisions. Cochrane Database Syst Rev. 2017;4(4):CD001431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Mathieu E, Barratt A, Davey HM. , et al. Informed choice in mammography screening: a randomized trial of a decision aid for 70-year-old women. Arch Intern Med. 2007;167(19):2039–2046. [DOI] [PubMed] [Google Scholar]
- 19. Mathieu E, Barratt AL, McGeechan K. , et al. Helping women make choices about mammography screening: an online randomized trial of a decision aid for 40-year-old women. Patient Educ Couns. 2010;81(1):63–72. [DOI] [PubMed] [Google Scholar]
- 20. Hersch J, Barratt A, Jansen J. , et al. The effect of information about overdetection of breast cancer on women’s decision-making about mammography screening: study protocol for a randomised controlled trial. BMJ Open. 2014;4(5):e004990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Hersch J, Jansen J, Barratt A. , et al. Overdetection in breast cancer screening: development and preliminary evaluation of a decision aid. BMJ Open. 2014;4(9):e006016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Hersch J, Barratt A, Jansen J. , et al. Use of a decision aid including information on overdetection to support informed choice about breast cancer screening: a randomised controlled trial. Lancet. 2015;385(9978):1642–1652. [DOI] [PubMed] [Google Scholar]
- 23. Jacklyn G, Howard K, Irwig L. , et al. Impact of extending screening mammography to older women: information to support informed choices. Int J Cancer. 2017;141(8):1540–1550. [DOI] [PubMed] [Google Scholar]
- 24. Jacklyn G, Glasziou P, Macaskill P. , et al. Meta-analysis of breast cancer mortality benefit and overdiagnosis adjusted for adherence: improving information on the effects of attending screening mammography. Br J Cancer. 2016;114(11):1269–1276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Brehaut JC, O’Connor AM, Wood TJ. , et al. Validation of a decision regret scale. Med Decis Making. 2003;23(4):281–292. [DOI] [PubMed] [Google Scholar]
- 26. Mack JW, Cronin AM, Kang TI.. Decisional regret among parents of children with cancer. J Clin Oncol. 2016;34(33):4023–4029. [DOI] [PubMed] [Google Scholar]
- 27. Brodersen J, Thorsen H.. Consequences of Screening in Breast Cancer (COS-BC): development of a questionnaire. Scand J Prim Health Care. 2008;26(4):251–256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Hersch J, McGeechan K, Barratt A. , et al. How information about overdetection changes breast cancer screening decisions: a mediation analysis within a randomised controlled trial. BMJ Open. 2017;7(10):e016246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Ivlev I, Hickman EN, McDonagh MS. , et al. Use of patient decision aids increased younger women’s reluctance to begin screening mammography: A systematic review and meta-analysis. J Gen Intern Med. 2017;32(7):803–812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Martinez-Alonso M, Carles-Lavila M, Perez-Lacasta MJ. , et al. Assessment of the effects of decision aids about breast cancer screening: a systematic review and meta-analysis. BMJ Open. 2017;7(10):e016894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Chiolero A, Rodondi N.. Lessons from the Swiss Medical Board recommendation against mammography screening programs. JAMA Intern Med. 2014;174(10):1541–1542. [DOI] [PubMed] [Google Scholar]
- 32. Barratt A, Jorgensen KJ, Autier P.. Reform of the national screening mammography program in France. JAMA Intern Med. 2018;178(2):177–178. [DOI] [PubMed] [Google Scholar]
- 33. Roberto A, Colombo C, Candiani G. , et al. A dynamic web-based decision aid to improve informed choice in organised breast cancer screening. A pragmatic randomised trial in Italy. Br J Cancer. 2020;123(5):714–721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Perez-Lacasta MJ, Martinez-Alonso M, Garcia M. , et al. ; with the InforMa Group. Effect of information about the benefits and harms of mammography on women’s decision making: the InforMa randomised controlled trial. PLoS One. 2019;14(3):e0214057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Reder M, Kolip P.. Does a decision aid improve informed choice in mammography screening? Results from a randomised controlled trial. PLoS One. 2017;12(12):e0189148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.NHS Digital. Breast screening programme England, 2018-19. https://digital.nhs.uk/data-and-information/publications/statistical/breast-screening-programme/england---2018-19. Accessed April 4, 2021.
- 37. Forbes LJ, Ramirez AJ,; the Expert group on Information about Breast Screening. Expert group on Information about Breast Screening. Offering informed choice about breast screening. J Med Screen. 2014;21(4):194–200. [DOI] [PubMed] [Google Scholar]
- 38. Schünemann HJ, Lerda D, Quinn C. , et al. ; for the European Commission Initiative on Breast Cancer (ECIBC) Contributor Group. Breast cancer screening and diagnosis: a synopsis of the European Breast Guidelines. Ann Intern Med. 2020;172(1):46–56. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data underlying this article will be shared on reasonable request to the corresponding author.