Abstract
Background
Obesity at breast cancer (BC) diagnosis has been associated with poor outcome, although the magnitude of effect in different BC subtypes is uncertain. We report on the association of obesity or overweight at diagnosis of nonmetastatic BC with disease-free (DFS) and overall survival (OS) in the following defined subtypes: hormone receptor positive/HER2 negative (HR+HER2−), HER2 positive (HER2+), and triple negative (TNBC).
Methods
We searched MEDLINE, EMBASE, and COCHRANE databases up to January 1, 2019. Study eligibility was performed independently by 2 authors. Studies reporting hazard ratios (HRs) of OS and/or DFS for obesity or overweight in BC subtypes were included. The pooled hazard ratio was computed and weighted using generic inverse variance and random effects models.
Results
Twenty-seven studies were included. Obese compared with nonobese women had worse DFS in all subtypes: the hazard ratios were 1.26 (95% confidence interval [CI] = 1.13 to 1.41, P < .001) for HR+HER2− BC, 1.16 (95% CI = 1.06 to 1.26, P < .001) for HER2+ BC, and 1.17 (95% CI = 1.06 to 1.29, P = .001) for TNBC. OS was also worse in obese vs nonobese women (HR+HER2− BC HR = 1.39, 95% CI = 1.20 to 1.62, P < .001; HER2+ BC HR = 1.18, 95% CI = 1.05 to 1.33, P = .006; and TNBC HR = 1.32, 95% CI = 1.13 to 1.53, P < .001). As opposed to obesity, overweight was not associated with either DFS or OS in HER2+ BC (HR = 1.02, 95% CI = 0.81 to 1.28, P = .85; and HR = 0.96, 95% CI = 0.76 to 1.21, P = .99, respectively) or TNBC (HR = 1.04, 95% CI = 0.93 to 1.18, P = .49; and HR = 1.08, 95% CI = 0.81 to 1.44, P = .17), respectively. In HR+HER2− BC, being overweight was associated with worse OS (HR = 1.14, 95% CI = 1.07 to 1.22, P < .001).
Conclusions
Obesity was associated with modestly worse DFS and OS in all BC subtypes.
Obesity is recognized as being associated with poor prognosis in several cancers, including breast cancer (BC) (1,2). Key prior meta-analyses of obesity and localized BC outcome have focused on all BCs (1) (hazard ratio [HR] for mortality in obese vs nonobese = 1.41, 95% confidence interval [CI] = 1.29 to 1.53) disease-free survival (DFS) or BC subdivided by hormone receptor status only (2). For example, in a literature-based meta-analysis, the hazard ratio for overall mortality in obese vs nonobese was 1.31 (95% CI = 1.17 to 1.46) for hormone receptor positive (HR+) and 1.18 (95% CI = 1.06 to 1.31) for hormone receptor negative (HR−) BC (Pdifference = .31) (2). In a more recent meta-analysis that focused on triple-negative BC (TNBC), obesity was not associated with DFS or overall survival (OS) (HR = 0.93 and 1.07, respectively); however, inclusion of studies was incomplete and numbers of patients in the included studies were small (3).
Differences in obesity associations among studies may reflect patient selection, a factor that is particularly important in the comparison of observational and interventional studies. Even when body mass index (BMI) is similar, metabolically healthier patients (ie, those without diabetes or cardiovascular disease) may be more likely to be enrolled into intervention trials, particularly those that include cardiotoxic treatments. These metabolically healthy patients are less likely than metabolically unhealthy patients to have obesity-associated attributes such as hyperinsulinemia, dysglycemia, dyslipidemia, and inflammation that may mediate associations of obesity with poor BC outcomes, even when BMIs are similar, and they have lower rates of non-BC deaths, leading to different associations of obesity with outcomes.
Previous comprehensive meta-analyses have not comprehensively examined obesity associations across BC subtypes, nor have they focused on BCs diagnosed since the introduction of routine HER2 testing. Although there is a growing consensus that obesity is associated with poor outcomes in HR+ BC, there is less evidence in those HR+ BCs that are also shown to be HER2−. There has also been limited and inconsistent evidence regarding the association of obesity with BC outcome in more aggressive BC subtypes such as TNBC and HER2 positive (HER2+) (4-6). For example, despite the suboptimal meta-analysis in TNBC discussed above, Turkoz et al. (4) reported worse DFS for obese vs nonobese patients in the HER2+ and TNBC subgroups (HR = 1.51, 95% CI = 1.1 to 2.1; and HR = 1.41, 95% CI = 1.0 to 2.0, respectively), whereas Sparano et al. (5) did not find statistically significant associations of obesity with DFS in these 2 populations (HR = 1.06, 95% CI = 0.82 to 1.38; and HR = 1.03, 95% CI = 0.81 to 1.30, respectively). Some of the differences across studies may reflect patient selection as discussed above; they may also reflect advances in adjuvant treatment in HER2+ and TNBC that may result in different associations of BMI with outcomes than was seen in earlier cohorts receiving less intensive therapy.
Given the limitations of prior meta-analyses and the continuing appearance of studies examining the association of obesity with BC outcomes, we conducted a literature-based meta-analysis with the goal of clarifying the association of body size with outcomes in nonmetastatic BC across the spectrum of immunohistochemically defined BC subtypes (HR+HER2−, HER2+, and TNBC) in women receiving modern adjuvant therapies.
Methods
Protocol and Literature Search
The study protocol was published by Prospero (registration number CRD42020130723) (7) and followed the PRISMA guidelines for meta-analyses of observational studies (8).
Our main analysis compared DFS and OS in obese and nonobese groups, accepting obesity as defined in each study. A sensitivity analysis was performed restricted to studies with obesity defined as BMI ≥ 30 kg/m2. The outcome of BC–specific survival (BCSS) was deemed secondary because fewer studies reported this outcome. In general, BCSS was defined as survival until death from BC. We investigated potential sources of heterogeneity, notably study design (observational vs interventional studies). In addition to obesity, some studies also reported results for overweight (BMI = 25-30 kg/m2) vs lower BMI; these were analyzed in a prespecified subgroup analysis.
Search Criteria
A comprehensive search of MEDLINE, EMBASE, and COCHRANE databases from inception to January 1, 2019, was performed. Abstracts presented at the American Society of Clinical Oncology Annual Meeting, the American Society of Clinical Oncology Breast Cancer Symposium, the San Antonio Breast Cancer Symposium, and the European Society for Medical Oncology Annual Meeting between 2014 and 2018 were also searched. Authors were contacted to obtain further data if abstracts without corresponding articles were identified. Manual searches of the reference lists of all pertinent reviews were also undertaken. The first 200 results of a Google Scholar search of subject headings “obesity” and “breast cancer” and “prognosis” or “outcome” were also reviewed for additional studies.
An electronic search was conducted by a professional librarian on the OvidSP search platform in the MEDLINE, EMBASE, and COCHRANE databases. Both subject headings and text word terms for BC, obesity, and prognosis terms were used. The results were limited to nonmetastatic BC study terms. The complete search strategy is provided in Box 1.
Box 1.
Search strategy
exp Breast Neoplasms/or Carcinoma, Ductal, Breast/or (breast adj2 (cancer* or neoplasm* or carcinoma* or tumo? r* or adenocarcinoma*)).ti, ab, kw. or ((neoplasms/or carcinoma/or adenocarcinoma/) AND (breast/or mammary glands, human/or nipples/or breast diseases/))
AND
body mass index/or body size/or body weight/or overweight/or obesity/or obesity, abdominal/ or obesity, metabolically benign/or obesity, morbid/or waist circumference/or waist-height ratio/ or waist-hip ratio/or body fat distribution/or adiposity/or ((body adj (mass or size or weight or fat)) or overweight or obes* or adiposity or (waist* adj3 (circumference or ratio))).ti, ab, kw.
AND
prognosis/or disease-free survival/or medical futility/or treatment outcome/or treatment failure/
or
disease progression/or remission, spontaneous/or morbidity/or incidence/or prevalence/or mortality/or “cause of death”/or fatal outcome/or mortality, premature/or survival rate/ survival analysis/or disease-free survival/or kaplan-meier estimate/or proportional hazards models/or
(prognosis or surviv* or outcome* or disease progres* or remission* or morbid* or mortality or “cause of death” or recover* or recur* or relaps*).ti, ab, kw. exclude animals
Identification of Studies
Studies that reported outcomes only in relation to hormone receptor status without stratifying by HER2 status were excluded. Only studies that reported hormone receptor and HER2 identified by immunohistochemistry or fluorescence in situ hybridization were included. Hormone receptor positivity included estrogen receptor and/or progesterone receptor–positive BCs as defined by the authors. Reports of observational or interventional studies in any language involving newly diagnosed nonmetastatic invasive BC populations that compared DFS, OS, and/or BCSS in obese vs nonobese patients were included if they contained the following information: 1) OS, DFS, and/or BCSS reported by BMI category according to hormone receptor and HER2 status; 2) measurement of body size around the time of diagnosis, reported as BMI, to allow classification as obese (World Health Organization definition of obesity of BMI ≥ 30 kg/m2) vs the reference category normal weight (BMI = 18.5-24.9 kg/m2), or nonobese category (BMI < 25 kg/m2). If similar, authors’ definition of body size categories was accepted; and 3) explicit reporting of hazard ratios associating body size with DFS, OS, and or BCSS by BC subtype. Of note, individual studies’ definitions of these outcomes were accepted.
Data Extraction
Data were extracted independently by 2 authors (A.E.L. and S.V.S.) using standardized data collection forms. Any discrepancies were resolved by consensus. Reasons for exclusion of studies were recorded. When necessary, additional information was requested from study authors.
A.E.L. and S.V.S. used the Newcastle-Ottawa Scale independently to assess risk of bias (9). We applied this assessment to both observational and interventional studies because in the clinical trials, authors performed post hoc analyses using a population that was randomly assigned to a different treatment; obesity was not relatedto the randomization in these trials.
Statistical Analysis
Meta-analysis was performed using Review Manager 5.3 software (The Cochrane Collaboration, 2014). Outcomes were included if at least 3 studies that met the criteria were available. Pooled estimates of HR outcomes were computed using random-effects modeling (10) and generic inverse variance (11), and forest plots were used to display the results. Random effects modeling was chosen because it was possible effects would differ across studies due to differences in population and treatment, irrespective of the magnitude of statistical heterogeneity. Clinical heterogeneity of included studies was assessed using Cochran Q test, the I2 statistic (11). I2 greater than 50% was classified as having substantial heterogeneity, and this was discussed accordingly. Publication bias was assessed using visual inspection of a funnel plot, which was most useful with more than 10 studies. Statistical tests were 2-sided, and statistical significance was defined as P less than .05.
Results
Included Studies
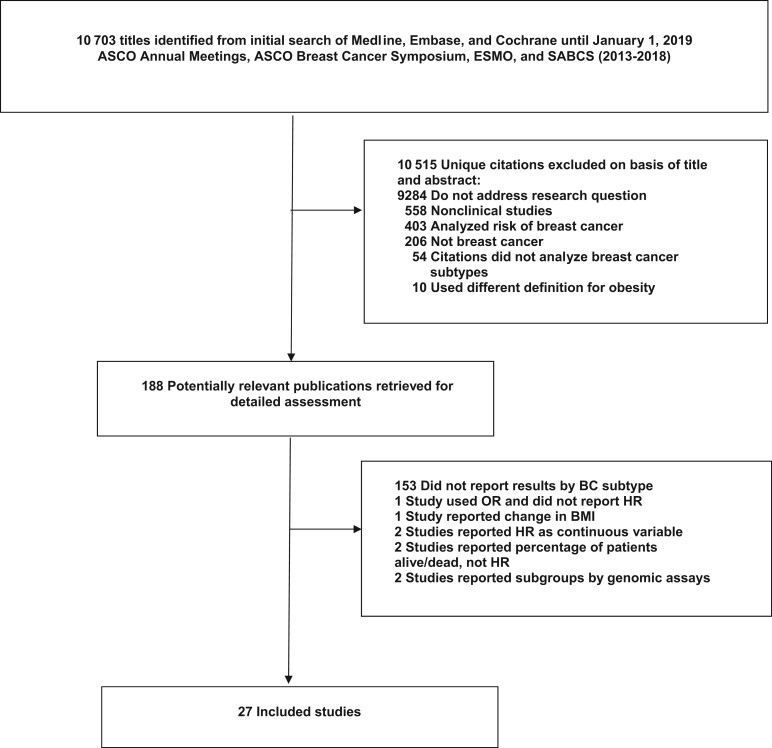
Reviewed publications are summarized in Figure 1. A total of 10 703 studies were identified, with most excluded on the basis of titles and abstracts. There were 188 reports retrieved for detailed assessment, of which 161 were excluded: 153 studies did not report results by BC subtype, 1 study reported only odds ratios, 1 investigated change in BMI rather than BMI at diagnosis, 2 used BMI as a continuous variable, 2 reported percentage of patients alive or dead, and 2 classified BC by genomic assays. The remaining 27 studies, 21 observational and 6 interventional, met the eligibility criteria.
Figure 1.
Flow diagram. ASCO = American Society of Clinical Oncology; BC = breast cancer; BMI = body mass index; ESMO = European Society for Medical Oncology; HR = hazard ratio; OR = odds ratio; SABCS = San Antonio Breast Cancer Symposium.
The characteristics of included publications are summarized in Table 1, and baseline patient and tumor characteristics are provided in Supplementary Table 1 (available online). All studies used BMI to characterize body size; the majority (19 studies; Table 1, subset A1) defined obesity as BMI ≥ 30 kg/m2, and 8 reports used lower cut-points (subset A2), for example, BMI greater than or equal to 28, 25, or 24 kg/m2. For the main analysis, we included the 27 studies in subsets A1 and A2 (plots shown in Figures 2 and 3), and for the sensitivity analysis, only the 19 in subset A1 (Supplementary Figure 2, available online). Eleven studies included information on associations of overweight vs lower BMI with BC outcomes (subset B). All 6 interventional studies (5,13,19–22) had longer than 5 years of follow-up and larger sample sizes (from 1250 to 8381). After request, De la Cruz et al. (27) provided additional information on all BC subtypes. Some studies reported information on tumor stage, histologic subtype, and menopausal status (see Supplementary Table 1, available online); however, associations of body size with BC outcomes in relation to these variables in combination with BC subtype were infrequently reported. As a result, it was not possible to conduct meta-analyses of BMI prognostic associations by BC subtype within these subsets apart from TNBC in pre- or postmenopausal patients.
Table 1.
Characteristics of included studies
| Study | No. of patients | No. of HR+HER2, HER2+, and TNBC | Type of study | Median follow-up, mo | Comparison groups by BMI, kg/m2 | HR+HER2− |
HER2+ |
TNBC |
||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| DFS | OS | BCSS | DFS | OS | BCSS | DFS | OS | BCSS | ||||||
| Subset A1: studies comparing obese with nonobese, No. of studiesa | — | — | — | — | — | 8 | 7 | 6 | 11 | 9 | 5 | 12 | 11 | 6 |
| Jeon et al., 2015 (12) | 41 021 | 21 094, 8005, 7436 | Obs | 92 | ≥30 vs <18.5-24.9 | No | Yes | Yes | No | Yesc | Yesc | No | Yes | Yes |
| Pajares et al., 2013 (13) GEICAM/BCIR trial | 5683 | — | Int | 93.4 | 35 vs <25 | Yesd | Yes | Yes | Yesd | Yes | Yes | Yesd | Yes | Yes |
| Sparano et al., 2012 (5) ECOG1199 trial | 4770 | — | Int | 94.8 | ≥30 vs <25 | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes |
| Sun et al., 2015 (14) | 1109 | 714, 72, 197 | Obs | 162 | ≥30 vs <25 | No | Yes | Yes | No | No | No | No | Yes | Yes |
| Ademuyiwa et al., 2011 (15) | 418 | 0, 0, 418 | Obs | 37.1 | ≥30 vs <25 | No | No | No | No | No | No | Yes | Yes | No |
| Dawood et al., 2012 (16) | 2311 | 0, 0, 2311 | Obs | 39 | ≥30 vs <25 | No | No | No | No | No | No | Yes | Yes | No |
| Paul et al., 2016 (17) | 74 | 0, 0, 74 | Obs | 68 | ≥30 vs <25 | No | No | No | No | No | No | Yes | Yes | No |
| Tait et al., 2014 (18) | 448 | 0, 0, 448 | Obs | 40.1 | ≥30 vs <25 | No | No | No | No | No | No | Yes | No | No |
| Widschwendter et al., 2015 (19) | 3754 | 2045, 883, 742 | Int | 65 | ≥30 vs <25 | Yes | Yes | No | Yes | Yes | No | Yes | Yes | No |
| Cecchini et al., 2016 (20) NSABB-31 trial | 2102 | 0, 2102, 0 | Int | 99.6e | ≥30 vs <25 | No | No | No | Yesc,g | Yesc | No | No | No | No |
| Crozier et al., 2013 (21) N9831 trial | 3017 | 0, 3017, 0 | Int | 63.6 | ≥30 vs <25 | No | No | No | Yes | No | No | No | No | No |
| Martel et al., 2018 (22) ALTTO trial | 8381 | 0, 8381, 0 | Int | na | ≥30 vs <25 | No | No | No | Yes | Yes | No | No | No | No |
| Mazzarella et al., 2013 (23) | 1250 | 0, 1250, 0 | Obs | 98.4 | ≥30 vs <25 | No | No | No | Yesc | Yesc | No | No | No | No |
| Turkoz et al., 2013 (4) | 733 | 561, 65, 107 | Obs | 29 | ≥30 vs 18.5-24.9 | Yes | No | Yes | Yes | No | Yes | Yes | No | Yes |
| Robinson et al., 2014 (24) | 1155 | 1155, 0, 0 | Obs | 67.2 | 30-40 vs ≥18.5-24.9 | Yes | No | No | No | No | No | No | No | No |
| Kawai et al., 2016 (25) | 20 090 | 13 838, 1485, 2993 | Obs | 80.4 | ≥30 vs <18.5-21.8 | Yesd | No | Yes | Yesd,f | No | Yesf | Yesd | No | Yes |
| Lara-Medina et al., 2011 (26) | 1048 | 1167, 421, 477 | Obs | 17 | ≥30 vs <18.5-21.8 | Yes | Yes | No | Yesf | Yes§ | No | Yes | Yes | No |
| De La Cruz et al., 2017 (27) | 1415 | 0, 0, 1495 | Obs | 61.2 | ≥30 vs <30 | No | No | No | No | No | No | Yes | Yes | No |
| Liu et al., 2018 (28) | 273 | 135, 94, 44 | Obs | 32.6 | ≥30 vs <30 | Yes | Yes | No | Yes | Yes | No | Yes | Yes | No |
| Subset A2: studies comparing obese with nonobese, No. of studiesb | — | — | — | — | — | 3 | 2 | 0 | 2 | 2 | 0 | 6 | 7 | 1 |
| Bao et al., 2016 (29) | 518 | 0, 0, 518 | Obs | 109.2 | ≥28 vs 18.5-23.9 | No | No | No | No | No | No | Yes | Yes | No |
| Chen et al., 2016 (30) | 206 | 0, 0, 206 | Obs | 59 | ≥25 vs <25 | No | No | No | No | No | No | Yes | Yes | No |
| Cho et al., 2018 (6) | 5668 | 3352, 1151, 793 | Obs | na | ≥25 vs <25 | Yes | Yes | No | Yesc | Yesc | No | Yes | Yes | No |
| Sato et al., 2017 (31) | 1924 | 1371, 258, 295 | Obs | 73 | ≥25 vs <25 | Yes | Yes | No | Yesc | Yesc | No | Yes | Yes | No |
| Ohara et al., 2015 (32) | 184 | 184, 0, 0 | Obs | 46.1 | ≥25 vs <25 | Yesd | No | No | No | No | No | No | No | No |
| Al Jarroudi et al., 2017 (33) | 115 | 0, 0, 115 | Obs | na | ≥ 25 vs <25 | No | No | No | No | No | No | Yes | Yes | No |
| Hao et al, 2015 (34) | 1106 | 0, 0, 1106 | Obs | 44.8 | >24 vs ≤24 | No | No | No | No | No | No | No | Yes | Yes |
| Asaga et al., 2013 (35) | 135 | 0, 0, 135 | Obs | 49.2 | >25 vs <18.5 | No | No | No | No | No | No | Yes | Yes | No |
| Subset B: studies comparing overweight with nonoverweight, No. of studies | — | — | — | — | — | 2 | 3 | 3 | 5 | 4 | 2 | 6 | 6 | 3 |
| Sun et al., 2015 (14) | 1109 | 714, 72, 197 | Obs | 162 | 25-29.9 vs <25 | No | Yes | Yes | No | No | No | No | Yes | Yes |
| Ademuyiwa et al., 2011 (15) | 418 | 0, 0, 418 | Obs | 37.1 | 25-29.9 vs <25 | No | No | No | No | No | No | Yes | Yes | No |
| Dawood et al., 2012 (16) | 2311 | 0, 0, 2311 | Obs | 39 | 25-29.9 vs <25 | No | No | No | No | No | No | Yes | Yes | No |
| Paul et al., 2016 (17) | 74 | 0, 0, 74 | Obs | 68 | 25-29.9 vs <25 | No | No | No | No | No | No | Yes | Yes | No |
| Cecchini et al., 2016 (20) NSABP-31 trial | 2102 | 0, 2102, 0 | Int | 99.6e | 25-29.9 vs <25 | No | No | No | Yesc,g | Yesc | No | No | No | No |
| Crozier et al., 2013 (21) N9831 trial | 3017 | 0, 3017, 0 | Int | 63.6 | 25-29.9 vs <25 | No | No | No | Yes | No | No | No | No | No |
| Mazzarella et al., 2013 (23) | 1250 | 0, 1250, 0 | Obs | 98.4 | 25-29.9 vs <25 | No | No | No | Yes | Yes | No | No | No | No |
| Jeon et al., 2015 (12) | 41 021 | 21 094, 8005, 7436 | Obs | 92 | 25-29.9 vs 18.5-24.9 | No | Yes | Yes | No | Yesc | Yesc | No | Yes | Yes |
| Kawai et al., 2016 (25) | 20 090 | 13 838, 1485, 2993 | Obs | 80.4 | 25-29.9 vs <18.5-21.8 | Yesd | No | Yes | Yesd,f | No | Yesf | Yesd | No | Yes |
| Tait et al., 2014 (18) | 448 | 0, 0, 448 | Obs | 40.1 | 25-29.9 vs <25 | No | No | No | No | No | No | Yes | No | No |
| Widschwendter et al., 2015 (19) | 3754 | 2045, 883, 742 | Int | 65 | 25-29.9 vs <25 | Yes | Yes | No | Yes | Yes | No | Yes | Yes | No |
Obese defined as BMI ≥30 kg/m2. ALTTO = Adjuvant Lapatinib And/Or Trastuzumab Treatment Optimisation; BCR = breast cancer recurrence; BCSS = breast cancer–specific survival; BMI = body mass index; DFS = disease-free survival; ECOG = Eastern Cooperative Oncology Group; HR = hormone receptor; Int = interventional; NA = not available; NSABP = National Surgical Adjuvant Breast and Bowel Project; Obs = observational; OS = overall survival; RFS = recurrence-free survival; TNBC = triple-negative breast cancer; Yes = available outcomes according to each tumor subtype.
Obese defined as BMI ≥28, 25, or 24 kg/m2.
HER2+ further divided into HR+ and HR−.
RFS.
Mean follow-up.
HER2+ HR− only.
BCR.
Figure 2.
Association of obesity with disease-free survival (DFS) and overall survival (OS) in relation to breast cancer (BC) subtypes: hormone receptor positive and HER2 negative (HR+HER2−), HER2 positive (HER2+), and triple negative. A) Association of obesity at breast cancer diagnosis with DFS in HR+HER2− BC is shown. B) Association of obesity at BC diagnosis with OS in HR+HER2− BC is shown. C) Association of obesity at BC diagnosis with DFS in HER2+ BC is shown. D) Association of obesity at BC diagnosis with OS in HER2+ BC is shown. E) Association of obesity at BC diagnosis with DFS in triple-negative (TN) BC is shown. F) Association of obesity at BC diagnosis with OS in TNBC is shown. BMI = body mass index; CI = confidence interval; IV = inverse variance; SE = standard error.
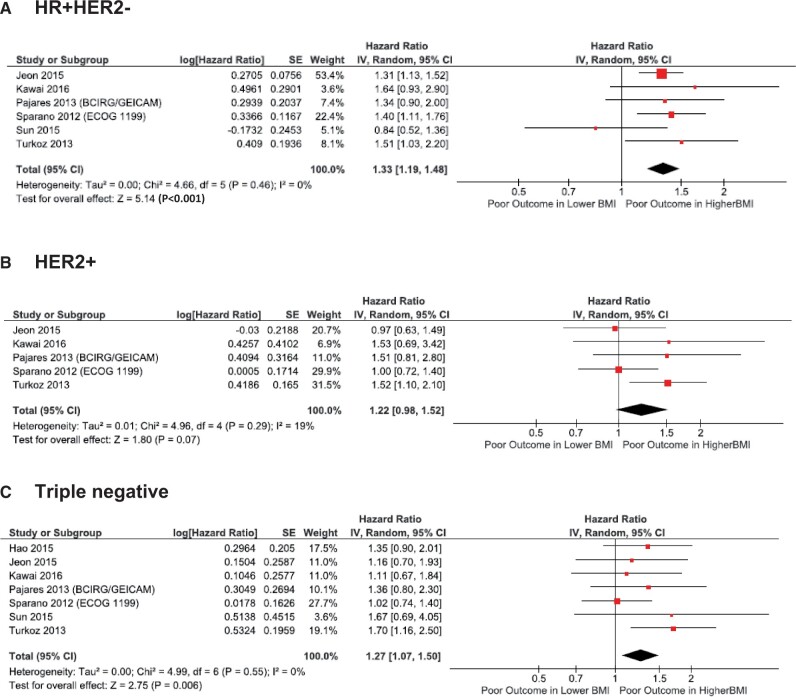
Figure 3.
Association of obesity with breast cancer (BC)–specific survival in relation to BC subtypes. Results for (A) hormone receptor-positive and HER2 negative (HR+HER2−), (B) HER2 positive (HER2+), and (C) triple-negative BC subtypes are shown. BMI = body mass index; CI = confidence interval; IV = inverse variance; SE = standard error.
The Newcastle-Ottawa quality rating of these studies is provided in Table 2. The overall quality of studies was good (mean overall score = 6) with moderate risk of bias. As expected, studies reported as abstracts only received lower scores.
Table 2.
Newcastle-Ottawa Quality Assessment Scale for cohort studiesa
| Study | Selection |
Comparability | Outcome |
Overall score | |||||
|---|---|---|---|---|---|---|---|---|---|
| Exposed representationb | Nonexposed selectionb | Ascertainment of obesityc | Outcome absent at study startd | Adjustment by age and nodal status or stagee | Outcome assessmentb | Follow-up lengthf | Adequacy of follow-upg | ||
| Ademuyiwa et al., 2011 (15) | Y | Y | Y | Y | Y | Y | — | — | 6 |
| Al Jarroudi et al., 2017 (33) | Y | Y | Y | Y | Y | Y | — | — | 6 |
| Asaga et al., 2013 (35) | Y | Y | Y | Y | — | Y | — | Y | 6 |
| Bao et al., 2016 (29) | Y | Y | Y | Y | Y | Y | Y | Y | 8 |
| Cecchini et al., 2016 (20) NSABB-3 trial | Y | Y | Y | Y | Y | Y | Y | — | 7 |
| Chen et al., 2016 (30) | Y | Y | Y | Y | — | Y | — | Y | 6 |
| Cho et al., 2018 (6) | Y | Y | Y | Y | Y | Y | — | — | 6 |
| Crozier et al., 2013 (21) N9831 trial | Y | Y | Y | Y | Y | Y | Y | Y | 8 |
| Dawoodet al., 2012 (16) | Y | Y | Y | Y | Y | Y | — | — | 6 |
| De La Cruz et al., 2017 (27) | Y | Y | — | Y | — | Y | Y | — | 5 |
| Hao et al., 2015 (34) | Y | Y | Y | Y | Y | Y | — | — | 6 |
| Jeon et al., 2015 (12) | Y | Y | Y | Y | Y | Y | Y | — | 7 |
| Kawai et al., 2016 (25) | Y | Y | Y | Y | Y | Y | Y | — | 7 |
| Lara-Medina et al., 2011 (26) | Y | Y | Y | Y | — | Y | — | — | 5 |
| Liu et al., 2018 (28) | Y | Y | Y | Y | — | Y | — | — | 5 |
| Martel et al., 2018 (22) ALTTO trial | Y | Y | Y | Y | Y | Y | — | — | 6 |
| Mazzarella et al., 2013 (23) | Y | Y | Y | Y | Y | Y | Y | Y | 8 |
| Ohara et al., 2015 (32) | Y | Y | Y | Y | — | Y | — | Y | 6 |
| Pajares et al., 2013 (13) Geicam/BCIRG trial | Y | Y | Y | Y | Y | Y | Y | — | 7 |
| Paul et al, 2016 (17) | — | — | Y | Y | — | Y | Y | — | 4 |
| Robinson et al., 2014 (24) | Y | Y | — | Y | Y | Y | Y | — | 6 |
| Sato et al., 2017 (31) | Y | Y | — | Y | — | Y | Y | — | 5 |
| Sparano et al., 2017 (5) | Y | Y | Y | Y | Y | Y | Y | — | 7 |
| Sun et al., 2015 (14) | Y | Y | Y | Y | Y | Y | Y | — | 7 |
| Tait et al., 2014 (18) | Y | Y | Y | Y | Y | Y | — | — | 6 |
| Turkoz et al., 2013 (4) | Y | Y | Y | Y | Y | Y | — | — | 6 |
| Widschwendter et al., 2015 (19) | Y | Y | Y | Y | Y | Y | Y | — | 7 |
Studies with a score greater than 7 were considered as having a low risk of bias, a score of 5-7 having a moderate risk of bias, and a score of less than 5 having a high risk of bias. ALTTO = Adjuvant Lapatinib And/Or Trastuzumab Treatment Optimisation; Geicam/BCIRG = Spanish Breast cancer Research Group/Breast Cancer International Research Group; NSABP = National Surgical Adjuvant Breast and Bowel Project.
Y = no major bias.
Y = investigator measured.
Y = yes.
Y = adjusted.
Y = median 5 or more years.
Y = adequate.
Outcomes
Prognostic Association of Obesity and Overweight in HR+HER2− BC
In the HR+HER2− subgroup, obesity was associated with worse DFS (HR = 1.26, 95% CI = 1.13 to 1.41, P < .001; Figure 2, A) (4–6,13,19,24–26,28,31,32) and OS (HR = 1.39, 95% CI = 1.20 to 1.62, P < .001; Figure 2, B) (5,6,12,14,19,24,26,28,31). The sensitivity analysis of studies that defined obesity as BMI ≥ 30 kg/m2 showed similar results for both DFS (HR = 1.26, 95% CI = 1.12 to 1.41, P < .001; Supplementary Figure 1, A, available online) and OS (HR = 1.31, 95% CI = 1.16 to 1.49, P < .001; Supplementary Figure 1, B, available online). The associations of obesity with DFS and OS were similar in observational vs interventional studies (DFS HR = 1.30, 95% CI = 1.08 to 1.56 vs HR = 1.24, 95% CI = 1.08 to 1.41, subgroup difference P = .66; and OS HR = 1.39, 95% CI = 1.06 to 1.83 vs HR = 1.36, 95% CI = 1.16 to 1.58, subgroup difference P = .88).
An analysis of the impact of overweight status on DFS was not performed because only 2 studies (19,25) reported on this association in the HR+HER2− BC subtype. For OS, overweight was associated with worse OS (HR = 1.14, 95% CI = 1.07 to 1.22, P = .001) in a meta-analysis of 3 studies (12,14,19). An adverse association of obesity with BCSS was also observed (HR = 1.33, 95% CI = 1.19 to 1.48, P < .001) in a meta-analysis of 6 studies (4,5,12,14,24,25).
Prognostic Associations of Obesity and Overweight in HER2+ BC
In the HER2+ subgroup, obesity was associated with worse DFS (HR = 1.16, 95% CI = 1.06 to 1.26, P < .001; Figure 2, C) (4–6,13,20–23,25,26,28,31) and OS (HR = 1.18, 95% CI = 1.05 to 1.33, P = .006; Figure 2, D) (5,6,13,19,20,22,23,26,28,31). The sensitivity analysis of studies that defined obesity as BMI of 30 kg/m2 or greater showed similar results for both DFS (HR = 1.17, 95% CI = 1.07 to 1.27, P < .001; Supplementary Figure 1, C, available online) and OS (HR = 1.19, 1.04 to 1.36, P < .009; Supplementary Figure 1, D, available online).
When comparing observational vs interventional studies, obesity associations with DFS (HR = 1.20, 95% CI = 0.98 to 1.46 vs HR = 1.14, 95% CI = 1.04 to 1.26, respectively, subgroup difference P = .68) and for OS did not differ statistically significantly in observational vs in interventional studies (HR = 1.34, 95% CI = 1.13 to 1.57 vs HR = 1.10, 95% CI = 0.95 to 1.27, respectively, subgroup difference P = .08).
Few studies further classified the HER2+ population according to hormone receptor status [HER2+/HR+ (12,23,31) and HER2+/HR− (12,23,25,31,35)]. No statistically significant prognostic association was observed in either subgroup (DFS HER2+/HR+ HR = 0.98, 95% CI = 0.77 to 1.25, P = .88; HER2+/HR− HR = 1.12, 95% CI = 0.95 to 1.31, P = .24; OS HER2+/HR+ HR = 0.96, 95% CI = 0.76 to 1.21, P = .74; and HER2+/HR− HR = 1.24, 95% CI = 0.94 to 1.64, P = .14).
Four studies (13,20,21,23) presented data in women who did not receive adjuvant trastuzumab; in this subgroup, higher vs lower BMI was not associated with DFS (HR = 1.03, 95% CI = 0.88 to 1.22, P = .70). Because only 2 studies (20,21) provided information on outcome in women who received adjuvant trastuzumab, a meta-analysis was not possible. Given only 2 studies reported prognostic associations of BMI with OS in the HER2+ in populations [Pajares et al. (13) HR = 1.41, 95% CI = 0.80 to 2.50; and Mazzarella et al. (23) HR = 1.46, 95% CI 0.95 to 2.24] that did not receive adjuvant trastuzumab, a meta-analysis was not feasible. No studies reported OS in the HER2+ group that received adjuvant trastuzumab.
Obesity was not statistically significantly associated with BCSS in the HER2+ subtype (HR = 1.22, 95% CI = 0.98 to 1.52, P = .07); however, the hazard ratio was similar to that observed in meta-analyses of BCSS in HR+HER2− and TNBC, and it is possible the small number of studies reporting this outcome (4,5,12,24,25) contributed to this lack of statistical significance. No studies provided BCSS according to patients who received adjuvant anti-HER2 treatment such as trastuzumab.
Overweight was not associated with DFS (HR = 1.02, 95% CI = 0.81 to 1.28, P = .85) or OS (HR = 0.96, 95% CI = 0.76 to 1.21, P = .99) in HER2+ BC.
Prognostic Associations of Obesity and Overweight in TNBC
In the TNBC subgroup, obesity was associated with poor DFS (HR = 1.17, 95% CI = 1.06 to 1.29, P = .001; Figure 2, E) (4–6,13,19,25–27) and OS (HR = 1.32, 95% CI = 1.13 to 1.53, P < .001; Figure 2, F) (5,6,13–17,19,26–31,33,35). The sensitivity analysis of studies that defined obesity as BMI 30 kg/m2 or greater showed similar results for both DFS (HR = 1.12, 95% CI = 1.01 to 1.25, P = .03; Supplementary Figure 1, E, available online) and OS (HR = 1.17, 95% CI = 1.00 to 1.37, P = .05; Supplementary Figure 1, F, available online).
Pooled HRs for obesity for both DFS and OS did not differ statistically significantly in observational vs interventional studies (DFS HR = 1.16, 95% CI = 1.04 to 1.30 vs HR = 1.25, 95% CI = 0.94 to 1.65, P = .64 for subgroup difference; OS HR = 1.34 95% CI = 1.16 to 1.60 vs HR = 1.27, 95% CI = 0.93 to 1.75, P = .80 for subgroup difference).
Seven studies (4,5,14,22,24,25,34) reported associations of obesity with BCSS in the TNBC subgroup, and a meta-analysis of these findings also demonstrated an adverse prognostic association (HR = 1.27, 95% CI = 1.07 to 1.50, P = .006).
Limited meta-analyses of obesity associations in TNBC by menopausal status were possible (see Supplementary Table 2, available online). In 3 meta-analyses (which each included 3 studies) (31,33,34), obesity was associated with worse OS but not DFS in premenopausal women (4,31,33) (HR = 2.40, 95% CI = 1.50 to 3.86, P < .001; and HR = 1.44, 95% CI = 0.68 to 3.03, P = .34, respectively). Obesity was not associated with OS in postmenopausal women (HR = 1.03, 95% CI = 0.70 to 1.50, P = .89) (31,33,34). Due to the small number of included reports, these results should be interpreted cautiously. Overweight was not associated with worse DFS (HR = 1.04, 95% CI = 0.93 to 1.18, P = .49) or OS (HR = 1.08, 95% CI = 0.81 to 1.44, P = .17) in TNBC.
Heterogeneity and Publication Bias
Low heterogeneity was observed for DFS, OS, and BCSS meta-analyses in the HR+HER2− (I2 = 35.0%, P = .13; I2 = 39.0%, P = .11; and I2= 0.0%, P = .46, respectively) and HER2+ subgroups (I2 = 0.0%, P = .57; I2 = 11.0%, P = .33; and I2 = 19.0%, P = .29, respectively). However, in TNBC, whereas low heterogeneity was identified in the DFS (I2 = 22.0%, P = .19) and BCCS meta-analyses (I2 = 0.0%, P = .55), high heterogeneity (I2 = 56.0%, P = .002) was observed in the OS analysis. No notable asymmetry or evidence of publication bias was observed in relation to studies included in any of the BC subtype primary analyses (Supplementary Figure 1, A-E, available online).
Discussion
Our results suggest that obesity is modestly, but statistically significantly, associated with worse DFS and OS in all BC subtypes. Obesity was also statistically significantly associated with worse BCSS in the HR+HER2− and TNBC populations.
Hazard ratios for OS and DFS were modestly higher in the HR+HER2− BC meta-analyses than the other 2 subtypes, but 95% confidence intervals overlapped, and it cannot be concluded that statistically significant differences in prognostic associations of obesity exist across BC subtypes. Although a statistically significant association of obesity with BCSS was not identified in HER2+ BC, only 5 studies were included in that meta-analysis and the hazard ratio of 1.22 was similar to the hazard ratios seen in the HR+HER2− and TNBC meta-analyses (HRs = 1.33 in 6 studies and 1.22 in 7 studies, respectively), with overlapping 95% confidence intervals across the BC subtypes. As a result, it cannot be concluded that the association of obesity with BCSS differs among BC subtypes. As opposed to obesity, overweight status was not associated with worse outcomes in HER2+ or TNBC. Because only 2 studies reported associations of overweight with DFS in the HR+HER2− subgroup, a meta-analysis could not be performed. A modest but statistically significant association of overweight (BMI = 25-30 kg/m2) with OS in the HR+HER2− was observed in a meta-analysis of 3 studies (HR = 1.14, 95% CI = 1.07 to 1.22). Additional research examining associations of overweight in all BC subtypes is needed. Evaluation of HER2 status in the large adjuvant aromatase inhibitor trials would be of particular interest because that would allow investigation of obesity associations in large groups of women with HR+HER2− as well as HR+HER2+ BC.
Previous meta-analyses have not examined prognostic associations of BMI in BC subtypes that included assessment of HER2 status. Similar to previous meta-analyses in HR+ BC that did not consider HER2 status, obesity was associated with poor outcomes in HR+HER2− BC in our study. There is little overlap between the studies included in the prior meta-analysis and our current analysis, reflecting our requirement that HER2 status be used to define BC subtypes. Obesity was also associated with worse outcomes in TNBC, similar to a prior meta-analysis in HR− BC that did not consider HER2 status (with little overlap in included studies), but it is inconsistent with a small meta-analysis (3) that included fewer studies.
In our analysis of the HER2+ subgroup, although there was an overall adverse association of BMI with DFS and OS, associations were not consistently seen when this subgroup was further classified according to hormone receptor status. We were not able to analyze the particular subgroup that received adjuvant HER2-targeted treatment such as trastuzumab because fewer (<2) studies reported this subanalysis. It is possible prognostic associations are different in the presence or absence of HER2 targeted treatment, affecting the results of our meta-analyses in this BC subtype. Furthermore, although obesity was not statistically significantly associated with BCSS in HER2+ BC, only 5 studies were included in this BCSS meta-analysis, and the observed HR was numerically similar to the HRs observed for DFS and OS in HER2+ BC (HR = 1.16, 95% CI = 1.06 to 1.26; and HR = 1.18, 95% CI = 1.05 to 1.33, respectively). As a result, it should not be concluded that obesity has different associations with DFS, OS, and BCSS in HER2+ BC. Any observed inconsistencies in HER2+ meta-analyses may reflect these treatment differences; they may also reflect low power or heterogeneity or nonrepresentativeness of studies that reported results in HER2+ BC by HR subgroups. Additional research in HER2+ BC, particularly in those receiving targeted adjuvant therapy, is urgently needed.
We were unable to comprehensively examine associations of obesity with outcome according to menopausal status because few of the included studies reported these associations. Meta-analyses were possible only in TNBC where a small subgroup of studies provided the required information. Results were inconsistent with no evidence of an association of obesity with OS in postmenopausal women but some evidence of poorer OS (and possibly DFS) in premenopausal women. These limited data do not allow conclusions to be drawn regarding differential associations of obesity with BC outcomes in pre- vs postmenopausal women.
A number of biologic mechanisms linking obesity to BC outcomes have been proposed; the contributions of these mechanisms may differ across BC subtypes. Excess estradiol production in adipose tissue of obese participants may lead to higher estrogen exposure, particularly in postmenopausal women, contributing to the association of obesity with outcome in HR+ BC; it is possible these associations may differ in Luminal A (less aggressive) and Luminal B (more aggressive) BCs. Only 1 of the included studies examined obesity associations in luminal A vs luminal B HR+ BCs and did not find an association of obesity with DFS or BCSS in either group (25). Observations (36) that obesity is associated with risk of developing less aggressive (HR per 5 kg/m2 = 1.44, 95% CI = 1.10 to 1.90, P = .009) but not more aggressive postmenopausal HR+HER2− BC and that postmenopausal hormone therapy users have a lower risk of developing less aggressive HR+HER2− BC (HR per 5 kg/m2 = 0.68, 95% CI = 0.50 to 0.94, P = .018) suggest that these associations are complex and underscore the importance of additional research to examine these associations. Other biologic factors that appear to contribute to the association of obesity with BC prognosis include insulin resistance with associated hyperinsulinemia and dysglycemia, altered adipokines (notably higher leptin and lower adiponectin), and localized or systemic inflammation. These biologic effects are present in central obesity (37), and they are likely to be highly relevant to differing degrees across BC subtypes and to be highly relevant in HR− BCs, regardless of HER2 status, which are unlikely to be affected by endogenous estrogen levels. Research is needed to explore the potential contributions of these and other biologic mediators to outcomes across BC subtypes.
These obesity-associated alterations are also associated with an increased risk of type 2 diabetes, cardiovascular disease, and other conditions that may lead to worse OS independent of BC recurrence or death. This may have affected our analyses of DFS and OS outcomes (both of which include deaths from non-BC causes) but would be unlikely to affect BCSS. The persistence of adverse prognostic associations of obesity in meta-analyses of BCSS discussed above provides evidence that the associations we have identified reflect, at least in part, potential direct impacts of obesity on BC.
Other possible explanations for the worse BC outcomes in obese individuals include underdosing of administered treatments with the widespread practice of dose capping in obese patients rather than prescribing full weight-based cytotoxic chemotherapy. This practice is less common in recent years as appreciation of the tolerance and/or benefits of full-dose chemotherapy in obese individuals has gained greater appreciation (38). Our observations that prognostic associations of obesity were more similar in observational studies (where underdosing may have been more common) than in intervention studies (where standard dosing would be required) suggests this is not the major mechanism underlying these prognostic associations. Obesity has also been associated with multiple potentially confounding factors such as increased age and delayed diagnosis with associated higher stage, which could contribute in part to worse outcomes. However, the impact of these potential confounders was minimized in our meta-analyses because most included studies controlled for these factors in their prognostic analyses as shown in Table 2. The distribution of molecular subtypes differs by race or ethnicity, and this may partially account for racial or ethnic disparities in BC outcomes, particularly among younger women (20,21). The extent to which obesity associations with BC outcomes may differ by race and BC subtype deserves further study (39).
In this meta-analysis, higher heterogeneity was observed in studies of TNBC. This likely reflects, at least in part, greater biologic heterogeneity in this BC subtype; associations of obesity with BC outcomes may vary across different TNBC subtypes. Recent research using genomic analysis identified at least 6 TNBC subtypes (40,41); future research is needed to investigate the role of obesity in biologically different subtypes of TNBC.
Our study has limitations. Few studies reported obesity associations according to menopausal status, and a meta-analysis of obesity associations across BC subtypes by menopausal status was not feasible except for 3 studies in the TNBC case. Some caution is needed in interpreting results of BCSS analyses, particularly in the HER2+ subtype because fewer studies reported this outcome. As discussed above, this is an important outcome because it excludes obesity-related deaths that are not due to BC; future studies should prioritize this outcome. The use of a literature-based vs individual patient approach did not permit detailed adjustment for key covariates (including stage and treatment) in our meta-analyses.
Strengths of our research include the broad literature search process, the evaluation of study quality, and inclusion of modern cohorts that included HER2 data. Major confounding factors such as age and stage were adjusted for in many studies. The ascertainment of outcome was through record linkage or direct inquiry in all the studies. Included studies were carried out in diverse locations around the world, with a variety of population of BC contributing to generalizability of the results.
We found evidence that obesity was associated with poorer BC outcomes in all BC subgroups, with the potential for larger prognostic associations in HR+HER2−. Overweight was not associated with DFS in any BC subtype and with OS only in the HR+HER2−, although data were limited. Priorities for future research have been discussed above.
Funding
This work was supported by The Breast Cancer Research Foundation (United States) and Hold’em For Life Charities (Canada).
Notes
Role of the funder: Not applicable.
Disclosures: Dr Eitan has provided expert testimony for Genentech/Roche and consulting for Apobiologix, Sandoz, Agendia, and Novartis. Dr Lohmann has contracted Research with Roche/Genentech, and has research funding in kind from Epic Sciences. Dr Goodwin has received research funding in kind from Epic Sciences. The other authors have no disclosures.
Author contributions: Conceptualization: AEL, SVS, ME, PG. Methodology: AEL, SVS, ME, EA, PJG. Software: AEL, EA, ME. Data curation: AEL, ME. Writing- Original draft preparation: AEL, SVS, IP, DR, ME, EA, PJG. Visualization, Investigation: AEL, SVS, IP, DR, ME, PJG. Supervision: PJG. Software, Validation: AEL, EA, ME. Writing- Reviewing and Editing: AEL, SVS, EA, PJG.
Data Availability
The data underlying this article will be shared on reasonable request to the corresponding author.
Supplementary Material
References
- 1. Chan DS, Vieira AR, Aune D, et al. Body mass index and survival in women with breast cancer-systematic literature review and meta-analysis of 82 follow-up studies. Ann Oncol. 2014;25(10):1901–1914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Niraula S, Ocana A, Ennis M, et al. Body size and breast cancer prognosis in relation to hormone receptor and menopausal status: a meta-analysis. Breast Cancer Res Treat. 2012;134(2):769–781. [DOI] [PubMed] [Google Scholar]
- 3. Mei L, He L, Song Y, et al. Association between obesity with disease-free survival and overall survival in triple-negative breast cancer: a meta-analysis. Medicine (Baltimore). 2018;97(19):e0719–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Turkoz FP, Solak M, PetekkayaI, et al. The prognostic impact of obesity on molecular subtypes of breast cancer in premenopausal women. J BUON. 2013;18(2):335–341. [PubMed] [Google Scholar]
- 5. Sparano JA, Wang M, Zhao F, et al. Obesity at diagnosis is associated with inferior outcomes in hormone receptor‐positive operable breast cancer. Cancer. 2012;118(23):5937–5946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Cho WK, Choi DH, Park YH, et al. Effect of body mass index on survival in breast cancer patients according to subtype, metabolic syndrome, and treatment. Clin Breast Cancer. 2018;18(5):e1141–e1147. [DOI] [PubMed] [Google Scholar]
- 7. Lohmann AE, Soldera S, Pimentel I, Ribnikar D, Ennis M, Amir E, Goodwin PJ. Association of obesity with breast cancer outcome in relation to cancer subtypes. PROSPERO 2020. CRD42020130723. https://www.crd.york.ac.uk/prospero/display_record.php?ID=CRD42020130723. Accessed January 3, 2021. [DOI] [PMC free article] [PubMed]
- 8. Moher D, Liberati A, Tetzlaff J, et al. The PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. 2009;6(7):e1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Stang A. Critical evaluation of the Newcastle Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur J Epidemiol. 2010;25(9):603–605. [DOI] [PubMed] [Google Scholar]
- 10. Borenstein M, Hedges LV, Higgins JP, et al. A basic introduction to fixed-effect and random-effects models for meta-analysis. Res Synth Method. 2010;1(2):97–111. [DOI] [PubMed] [Google Scholar]
- 11. Higgins JPT, Green S (eds). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration; 2011. www.handbook.cochrane.org. Accessed January 3, 2021.
- 12. Jeon Y, Kang S, Park MH, et al. Relationship between body mass index and the expression of hormone receptors or human epidermal growth factor receptor 2 with respect to breast cancer survival. BMC Cancer. 2015;15(1):865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Pajares B, Pollán M, Martín M, et al. Obesity and survival in operable breast cancer patients treated with adjuvant anthracyclines and taxanes according to pathological subtypes: a pooled analysis. Breast Cancer Res. 2013;15(6):R105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Sun X, Nichols H, Robinson W, et al. Post-diagnosis adiposity and survival among breast cancer patients: influence of breast cancer subtype. Cancer Causes Control. 2015;26(12):1803–1811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Ademuyiwa FO, Groman A, O'Connor T, et al. Impact of body mass index on clinical outcomes in triple-negative breast cancer. Cancer. 2011;117(18):4132–4140. [DOI] [PubMed] [Google Scholar]
- 16. Dawood S, Lei X, Litton J, et al. Impact of body mass index on survival outcome among women with early stage triple-negative breast cancer. Clinical Breast Cancer. 2012;12(5):364–372. [DOI] [PubMed] [Google Scholar]
- 17. Paul S, Yendala R, Raja NP, et al. Influence of obesity on survival in non-metastatic triple negative breast cancer; a retrospective analysis. J Clin Oncol. 2016;34(15_suppl):e12546. [Google Scholar]
- 18. Tait S, Pacheco J, Gao F, et al. Body mass index, diabetes, and triple-negative breast cancer prognosis. Breast Cancer Res Treat. 2014;146(1):189–197. [DOI] [PubMed] [Google Scholar]
- 19. Widschwendter P, Friedl TW, Schwentner L, et al. The influence of obesity on survival in early, high-risk breast cancer: results from the randomized SUCCESS A trial. Breast Cancer Res. 2015;17(1):129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Cecchini RS, Swain SM, Costantino JP, et al. Body mass index at diagnosis and breast cancer survival prognosis in clinical trial populations from NRG oncology/NSABP B-30, B-31, B-34, and B-38. Cancer Epidemiol Biomarkers Prev. 2016;25(1):51–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Crozier JA, Moreno‐Aspitia A, Ballman KV, et al. Effect of body mass index on tumor characteristics and disease‐free survival in patients from the HER2‐positive adjuvant trastuzumab trial N9831. Cancer. 2013;119(13):2447–2454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Martel S, Lambertini M, Agbor-Tarh D, et al. Impact of body mass index (BMI) and weight change after treatment in patients (pts) with HER2-positive (HER2+) early breast cancer (EBC): secondary analysis of the ALTTO BIG 2-06 trial. J Clin Oncol. 2018;36(15_suppl):10067. [Google Scholar]
- 23. Mazzarella L, Disalvatore D, Bagnardi V, et al. Obesity increases the incidence of distant metastases in oestrogen receptor-negative human epidermal growth factor receptor 2-positive breast cancer patients. Eur J Cancer. 2013;49(17):3588–3597. [DOI] [PubMed] [Google Scholar]
- 24. Robinson PJ, Bell RJ, Davis SR.. Obesity is associated with a poorer prognosis in women with hormone receptor positive breast cancer. Maturitas. 2014;79(3):279–286. [DOI] [PubMed] [Google Scholar]
- 25. Kawai M, Tomotaki A, Miyata H, et al. Body mass index and survival after diagnosis of invasive breast cancer: a study based on the Japanese National Clinical Database—Breast Cancer Registry. Cancer Med. 2016;5(6):1328–1340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Lara-Medina F, Pérez-Sánchez V, Saavedra-Pérez D, et al. Triple-negative breast cancer in Hispanic patients: high prevalence, poor prognosis, and association with menopausal status, body mass index, and parity. Cancer. 2011;117(16):3658–3669. [DOI] [PubMed] [Google Scholar]
- 27. De la Cruz K, Antonio G, Enriquez D, Morante Z, et al. Survival prognostic value of obesity in localized triple negative breast cancer in Peruvian women. J Clin Oncol. 2017;35(15_suppl):e12100. [Google Scholar]
- 28. Liu Y, Saraf A, Catanese B, et al. Obesity and survival in the neoadjuvant breast cancer setting: role of tumor subtype in an ethnically diverse population. Breast Cancer Res Treat. 2018;167(1):277–288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Bao PP, Cai H, Peng P, et al. Body mass index and weight change in relation to triple-negative breast cancer survival. Cancer Causes Control. 2016;27(2):229–236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Chen H, Ding A, Wang M.. Impact of central obesity on prognostic outcome of triple negative breast cancer in Chinese women. Springer Plus. 2016;5:1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Sato M, Terai S, Tachikawa H, Maeda H, Yamammoto M, Tomioka N, Watanabe K, Takahashi M. Obesity is associated with poor prognosis of Japanese breast cancer, especially in ER positive/HER2 negative subtype, which tendency is prominent. Paper presented at San Antonio Breast Cancer Symposium; December 2017; San Antonio, TX. Abstract P1-07-13.
- 32. Ohara M, Akimoto E, Noma M, et al. Prognostic impact of progesterone receptor status combined with body mass index in breast cancer patients treated with adjuvant aromatase inhibitor. Oncol Lett. 2015;10(5):3286–3292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Al Jarroudi O, Abda N, Seddik Y, et al. Overweight: is it a prognostic factor in women with triple-negative breast cancer? Asian Pac J Cancer Prev. 2017;18(6):1519–1523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Hao S, Liu Y, Yu K, et al. Overweight as a prognostic factor for triple-negative breast cancers in Chinese women. PLoS One. 2015;10(6):e0129741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Asaga S, Kinoshita T, Hojo T, et al. Prognostic factors for triple-negative breast cancer patients receiving preoperative systemic chemotherapy. Clin Breast Cancer. 2013;13(1):40–46. [DOI] [PubMed] [Google Scholar]
- 36. Nattenmüller CJ, Kriegsmann M, Sookthai D, et al. Obesity as risk factor for subtypes of breast cancer: results from a prospective cohort study. BMC Cancer. 2018;18(1):616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Lohmann AE, Goodwin PJ, Chlebowski RT, et al. Association of obesity-related metabolic disruptions with cancer risk and outcome. J Clin Oncol. 2016;34(35):4249–4255. [DOI] [PubMed] [Google Scholar]
- 38. Griggs JJ, Sorbero ME, Lyman GH.. Undertreatment of obese women receiving breast cancer chemotherapy. Arch Intern Med. 2005;165(11):1267–1273. [DOI] [PubMed] [Google Scholar]
- 39. Acheampong T, Kehm RD, Terry MB, Argov EL, Tehranifar P.. Incidence trends of breast cancer molecular subtypes by age and race/ethnicity in the US From 2010 to 2016. JAMA Netw Open. 2020;3(8):e2013226–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Burstein MD, Tsimelzon A, Poage GM, et al. Comprehensive genomic analysis identifies novel subtypes and targets of triple-negative breast cancer. Clin Cancer Res. 2015;21(7):1688–1698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Perou CM. Molecular stratification of triple‐negative breast cancers. Oncologist. 2010;15(S5):39–48. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data underlying this article will be shared on reasonable request to the corresponding author.