Abstract
Background
Recent trends of hepatocellular carcinoma (HCC) mortality and outcome remain unknown in the United States. We investigated the recent trends of primary liver cancer (excluding intrahepatic cholangiocarcinoma) mortality and HCC stage, treatment, and overall survival (OS) in the United States.
Methods
The National Center for Health Statistics Database was analyzed to investigate the trend of primary liver cancer mortality. We analyzed the Surveillance, Epidemiology, and End Results 18 Database to assess the temporal trend of tumor size, stage, treatment, and OS of HCC. We investigated the association between HCC diagnosis year and OS using Cox regression analysis. All statistical tests were 2-sided.
Results
During 2000-2018, liver cancer mortality rates increased until 2013, plateaued during 2013-2016 (annual percent change = 0.1%/y, 95% confidence interval [CI] = −2.1%/y to 2.4%/y, P = .92), and started to decline during 2016-2018 (annual percent change = −1.5%/y, 95% CI = −3.2%/y to 0.2%/y, P = .08). However, mortality continues to increase in American Indian and Alaska Native, individuals aged 65 years or older, and in 33 states. There was a 0.61% (95% CI = 0.53% to 0.69%, P < .001) increase in localized stage HCC and a 0.86-mm (95% CI = −1.10 to −0.62 mm, P < .001) decrease in median tumor size per year. The 1-year OS rate increased from 36.3% (95% CI = 34.3% to 38.3%) to 58.1% (95% CI = 56.9% to 59.4%) during 2000-2015, and the 5-year OS rate almost doubled from 11.7% (95% CI = 10.4% to 13.1%) to 21.3% (95% CI = 20.2% to 22.4%) during 2000-2011. Diagnosis year (per year) (adjusted hazard ratio = 0.96, 95% CI = 0.96 to 0.97) was independently associated with OS in multivariable analysis.
Conclusions
Primary liver cancer mortality rates have started to decline in the United States with demographic and state-level variation. With an increasing detection of localized HCC, the OS of HCC has improved over the past decades.
Primary liver cancer remains the fourth-leading cause of cancer-related death worldwide (1,2). Most primary liver cancers (>80%) are hepatocellular carcinoma (HCC), with intrahepatic cholangiocarcinoma (iCCA) being the second-most common and accounting for 10%-15% (2). The majority of HCCs occur in patients with cirrhosis, which can be due to several etiologies, including chronic hepatitis B virus (HBV), hepatitis C virus (HCV), heavy alcohol use, or nonalcoholic fatty liver disease. HCC incidence rates in the United States increased over several decades; however, recent data from the Surveillance, Epidemiology, and End Results (SEER) Program showed incidence rates plateaued in 2013 and then started to decline in 2016 (3,4).
Small improvement in HCC survival during the 1970s to 2011 in the United States has been attributed to increased earlier detection, refinement of staging systems and treatment allocation algorithms, and improvement in HCC therapies (5,6). With gradual improvements in HCC surveillance, it is anticipated that more patients will be diagnosed at early stages and therefore able to receive curative treatments (7). Although a previous modeling study in 2014 reported that liver cancer incidence and mortality would continue to increase by 2030 in the United States (8), it is possible that liver cancer mortality rates may instead plateau or decline sooner than previously anticipated due to a decrease in HCC incidence and improvement in overall survival (OS).
An earlier study indicated substantial demographic and regional variation in the burden of HCC due to the difference in the prevalence of risk factors, access to care, and socioeconomic disparities; however, there are fewer data evaluating disparities in HCC prognosis (2). The aim of this study is to investigate the recent temporal trends of demographic and state-level variation in primary liver cancer mortality (excluding iCCA), and tumor burden, receipt of curative treatment, and OS of HCC in the United States.
Methods
Data Sources
US Cancer Mortality data between 2000 and 2018, collected and maintained by the National Center for Health Statistics (NCHS) (9), were curated and analyzed using SEER*Stat software (10). Although this database included nationwide mortality data, mortality statistics specific for HCC are not available. To estimate the trend of HCC mortality, we reported primary liver cancer mortality excluding iCCA by International Classification for Diseases (ICD) version 10 codes for the underlying cause of death using codes C22.0–C22.9 (malignant neoplasm of liver and intrahepatic bile ducts), excluding C22.1 (iCCA) (11).
We assumed that the trend of primary liver cancer mortality excluding iCCA from the NCHS Database (9) would reflect the trend of national HCC mortality. Therefore, we performed a sensitivity analysis using the SEER 18 Database (12) to assess the accuracy of our estimates and calculate the HCC-specific incidence-based (IB) mortality rate during 2000-2017 by linking the characteristics of HCC at diagnosis to the death certificate (5,13). HCC cases were identified using ICD for Oncology, 3rd edition, codes (site: C22.0; histology: 8170–8175). HCC stage, tumor size, types of treatment, and survival data were also curated from SEER 18 Database (12).
Study Variables
The study variables included sex, age, race or ethnicity(non-Hispanic White, Black, American Indian and Alaska Native [AI and AN], Asian and Pacific Islander [API], and Hispanic), state, tumor stage, diameter of the largest tumor (available since 2004), the proportion receiving curative treatments, and OS. SEER Summary Stage 2000 was used for HCC staging: localized (confined to liver), regional (spread to regional lymph nodes), and distant (extrahepatic metastasis) stages (12).
Statistical Analyses
Mortality rates, tumor stage, largest tumor size, curative treatment, and OS data were ascertained using SEER*Stat software (version 8.3.6.1) (10). Mortality rates were age adjusted to the 2000 US standard population and expressed per 100 000 person-years (PY) (14,15). Primary liver cancer mortality rates were calculated as the number of deaths attributed to liver cancer excluding iCCA over person-time at-risk individuals in the US general population using the NCHS Database (9). HCC IB mortality rate was calculated as the number of deaths attributed to HCC over person-time at-risk individuals by linking the characteristics of HCC at diagnosis to the death certificate in the SEER-18 regions (12). Attribution to HCC was made when the cause of death on the death certificate was liver cancer ICD 10 codes C22.0–C22.9 (malignant neoplasm of liver and intrahepatic bile ducts), excluding C22.1 (intrahepatic bile duct cancer), and the deceased was listed in the registry as having been diagnosed with HCC (site: C22.0; histology: 8170–8175) at an earlier time.
The National Cancer Institute’s Joinpoint Regression Analysis program (version 4.8.0.1) was used to quantify trends in primary liver cancer mortality overall and by sex, age, race or ethnicity, and state (16). The best-fitting log-linear regression model was applied to calculate annual percentage changes (APCs) and 95% confidence intervals (CIs) and identify the calendar years (ie, the joinpoints) when APCs changed statistically significantly (P < .05). We reported average APC (AAPC), which is a summary measure of the trend over a prespecified fixed interval and was computed as a weighted average of the APCs from the joinpoint model, with the weights equal to the length of the APC interval (17).
For survival analyses, patients who had less than 1 month follow-up period after diagnosis, diagnosed on the basis of a death certificate only or newly diagnosed at autopsy, were excluded. One-, 3-, and 5-year OS were estimated separately for each HCC year of diagnosis between 2000 and 2015 according to the Kaplan-Meier method. Trends in OS were estimated using a weighted linear model with inverse-variance weighting of the standard errors obtained from the OS estimates using the Kaplan-Meier estimator (18). Cox proportional hazard models were used to investigate the association between HCC diagnosis year and OS after adjusting for sex, age, race or ethnicity, state, tumor stage, and receipt of curative treatment. Missing values for variables in the multivariable model were imputed using chained equations algorithm as proposed by Van Buuren and Groothuis-Oudshoorn (19). A total of 5 complete data sets were generated, and the regression estimates are the average coefficients among each of the complete data and variance equal to the imputation-corrected variance-covariance matrix. The proportional hazards assumption was assessed both graphically and quantitatively using the scaled Schoenfeld residuals and the goodness-of-fit test as proposed by Grambsch and Therneau (20). All survival analyses were performed using R statistical software (version 4.0.2; R Foundation, Vienna, Austria) with 2-sided tests and a statistical significance level of .05.
Results
Trends of Primary Liver Cancer Mortality in the United States
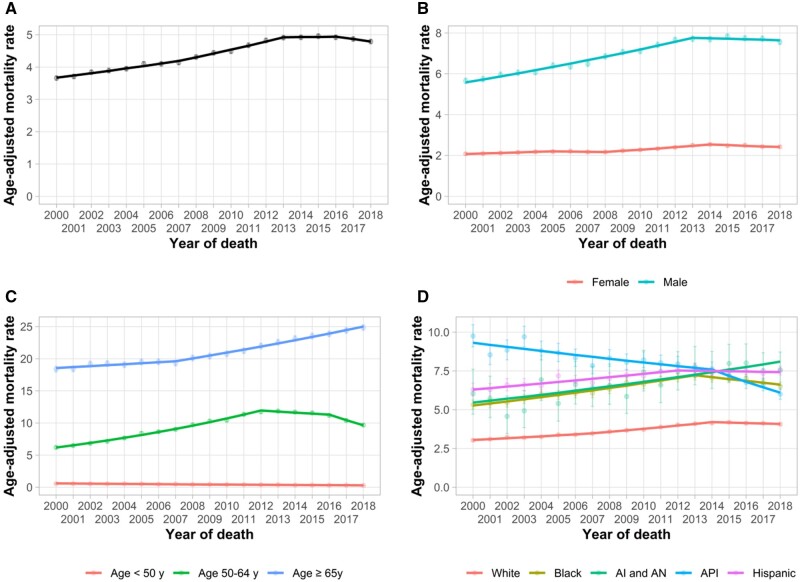
During 2000-2018, a total of 289 567 deaths were attributed to primary liver cancer, with an overall mortality rate of 4.5 per 100 000 PY (95% CI = 4.5 to 4.5). Trends in liver cancer mortality are shown in Table 1 and Figure 1. Nationally, liver cancer mortality increased with an AAPC of 1.5%/y (95% CI = 1.1%/y to 1.9%/y, P < .001); however, we noted variation over the study period. Specifically, mortality rates initially increased by 1.9%/y (95% CI = 1.5%/y to 2.3%/y, P < .001) during 2000-2007, 2.7%/y (95% CI = 2.2%/y to 3.3%/y, P < .001) during 2007-2013, plateaued during 2013-2016 (APC = 0.1%/y, 95% CI = −2.1%/y to 2.4%/y, P = .92), and then started to decline during 2016-2018 (APC = −1.5%/y, 95% CI = −3.2%/y to 0.2%/y, P = .08). In subgroup analyses, mortality rates recently declined in women, Blacks and APIs, and individuals aged younger than 65 years; plateaued in males, Whites, and Hispanics; and increased in AIs and ANs and individuals aged 65 years and older.
Table 1.
Age-adjusted primary liver cancer mortality rates and joinpoint trends, 2000-2018, by sex, age, and race or ethnicity in the NCHS Database
| Characteristic | Mortality rates per 100 000 PY |
Trend 1a |
Trend 2 |
Trend 3 |
2000-2018 AAPC |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 2000 | 2016 | 2018 | Years | APC (95% CI) | P | Years | APC (95% CI) | P | Years | APC (95% CI) | P | AAPC (95% CI) | P b | |
| Overallc | 3.7 | 4.9 | 4.8 | 2000-2007 | 1.9 (1.5 to 2.3) | <.001 | 2007-2013 | 2.7 (2.2 to 3.3) | <.001 | 2013-2016 | 0.1 (−2.1 to 2.4) | .92 | 1.5 (1.1 to 1.9) | <.001 |
| Sex | ||||||||||||||
| Male | 5.7 | 7.7 | 7.6 | 2000-2013 | 2.6 (2.3 to 2.8) | <.001 | 2013-2018 | −0.3 (−1.1 to 0.4) | .39 | — | — | — | 1.8 (1.5 to 2.0) | <.001 |
| Femalec | 2.1 | 2.5 | 2.4 | 2000-2005 | 1.2 (0.5 to 1.9) | .005 | 2005-2008 | −0.5 (−3.6 to 2.6) | .71 | 2008-2014 | 2.6 (2.0 to 3.3) | <.001 | 0.9 (0.3 to 1.4) | .001 |
| Age, y | — | — | — | — | — | — | — | — | — | — | — | — | — | — |
| <50 | 0.6 | 0.3 | 0.3 | 2000-2018 | −3.7 (−4.1 to −3.3) | <.001 | — | — | — | — | — | — | −3.7 (−4.1 to −3.3) | <.001 |
| 50-64 | 6.2 | 11.2 | 9.7 | 2000-2012 | 5.6 (5.3 to 6.0) | <.001 | 2012-2016 | −1.4 (−3.2 to 0.5) | .14 | 2016-2018 | −7.6 (−11.2 to −3.9) | .001 | 2.5 (1.9 to 3.1) | <.001 |
| ≥65 | 18.4 | 23.9 | 24.9 | 2000-2007 | 0.8 (0.4 to 1.2) | <.001 | 2007-2018 | 2.2 (2.1 to 2.4) | <.001 | — | — | — | 1.7 (1.5 to 1.8) | <.001 |
| Race or ethnicity | ||||||||||||||
| Non-Hispanic White | 3.0 | 4.1 | 4.1 | 2000-2007 | 1.9 (1.5 to 2.3) | <.001 | 2007-2014 | 2.7 (2.3 to 3.2) | <.001 | 2014-2018 | −0.7 (−1.5 to 0.1) | .07 | 1.6 (1.4 to 1.9) | <.001 |
| Non-Hispanic Black | 5.3 | 7.0 | 6.5 | 2000-2013 | 2.4 (2.1 to 2.8) | <.001 | 2013-2018 | −1.7 (−2.7 to −0.7) | .003 | — | — | — | 1.3 (0.9 to 1.6) | <.001 |
| Non-Hispanic AI and AN | 6.0 | 8.0 | 7.6 | 2000-2018 | 2.2 (1.4 to 3.0) | <.001 | — | — | — | — | — | — | 2.2 (1.4 to 3.0) | <.001 |
| Non-Hispanic API | 9.8 | 6.8 | 6.0 | 2000-2014 | −1.5 (−2.0 to −0.9) | <.001 | 2014-2018 | −5.3 (−8.4 to −2.2) | .003 | — | — | — | −2.3 (−3.1 to −1.6) | <.001 |
| Hispanic | 6.3 | 7.5 | 7.5 | 2000-2012 | 1.5 (1.0 to 2.0) | <.001 | 2012-2018 | −0.2 (−1.2 to 0.7) | .59 | — | — | — | 0.9 (0.5 to 1.3) | <.001 |
Joinpoint software selected joinpoint when annual percentage changed statistically significantly (P < .05). Thus, subgroups with steady incidence trend over the study period have only 1 trend. AAPC = average annual percent change; AI and AN = American Indian and Alaska Native; APC = annual percent change; API = Asian and Pacific Islander; CI = confidence interval; HCC = hepatocellular carcinoma; NCHS = National Center for Health Statistics; PY = person-years.
A 2-sided Wald’s test was used to calculate the P values and test for statistically significant trends.
There were more than 3 joinpoint trends. The fourth trend for overall primary liver cancer mortality was −1.5 (95% CI = −3.2 to 0.2, P = .08) during 2016-2018; the fourth trend for female primary liver cancer mortality was −1.2 (95% CI = −1.9 to −0.6, P = .003) during 2014-2018.
Figure 1.
Age-adjusted primary liver cancer (excluding intrahepatic cholangiocarcinoma) mortality trends, 2000-2018, by sex, age, and race or ethnicity in the National Center for Health Statistics Database. A) Overall primary liver cancer mortality trends. B) Primary liver cancer mortality trends by sex. C) Primary liver cancer mortality trends by age. D) Primary liver cancer mortality trends by race or ethnicity. Error bars represent the 95% confidence intervals. AI and AN = American Indian and Alaska Native; API = Asian and Pacific Islander.
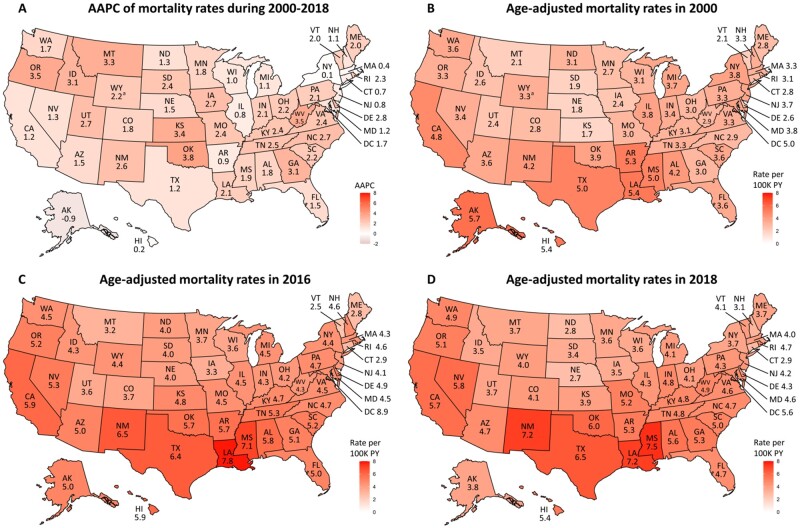
There was notable state-level variation in primary liver cancer mortality (Figure 2). In 2000, mortality rates were highest in Alaska (5.7 per 100 000 PY, 95% CI = 3.4 to 9.0 per 100 000 PY) and lowest in Kansas (1.7 per 100 000 PY, 95% CI = 1.3 to 2.3 per 100 000 PY), with a mortality rate ratio of 3.4 between the 2 states. State-level variation in mortality rates decreased in 2018, with a mortality rate ratio of 2.8 between states with the highest (Mississippi at 7.5 per 100 000 PY, 95% CI = 6.6 to 8.4 per 100 000 PY) and lowest (Nebraska at 2.7 per 100 000 PY, 95% CI = 2.1 to 3.5 per 100 000 PY) mortality. Mortality continued to increase in 33 states, plateaued in 15 states and the District of Columbia, and decreased in 2 states in recent years (Supplementary Table 1, available online). Notably, 15 states with plateaued mortality had larger populations and showed downtrending mortality that did not reach statistical significance.
Figure 2.
State-specific age-adjusted primary liver cancer mortality rates (excluding intrahepatic cholangiocarcinoma) and average annual percentage changes (AAPC) during 2000-2018 in National Center for Health Statistics Database. A) State-specific AAPC of primary liver cancer mortality rates during 2000-2018. B) State-specific mortality rates of primary liver cancer in 2000. C) State-specific mortality rates of primary liver cancer in 2016. D) State-specific mortality rates of primary liver cancer in 2018. aIndicates age-adjusted mortality rate in 2000 were not available. Earliest reported mortality rate was 2001 in WY, which was used to calculate AAPC in (A) and map 2000 mortality rates in (B). PY = person-years.
Sensitivity analyses using the SEER 18 Database revealed HCC IB mortality trends in the SEER regions are comparable with the primary liver cancer mortality trend of the entire United States. Similar to the national trend of liver cancer mortality, HCC IB mortality rates increased until 2013 and then plateaued during 2013 and 2017 (APC = −0.7%, 95% CI = −2.3% to 0.9%, P = .36). Subgroup analyses showed mortality rates recently declined in APIs (2012-2017; APC = −3.5%, 95% CI = −4.9% to −1.9%, P < .001) and individuals aged younger than 50 years (2003-2017; APC = −5.0%, 95% CI = −5.9% to −4.2%, P < .001) and 50-64 years (2013-2017; APC = −4.9%, 95% CI = −8.0% to −1.7%, P = .007); plateaued in males (2012-2017; APC = −0.1%, 95% CI = −1.4% to 1.2%, P = .84), Whites (2014-2017; APC = −1.8%, 95% CI = −4.4% to 1.0%, P = .18), Blacks (2013-2017; APC = −2.6%, 95% CI = −7.3% to 2.2%, P = .25), and Hispanics (2013-2017; APC = −0.9%, 95% CI = −4.2% to 2.6%, P = .58); and increased in females (2002-2017; APC = 2.8%, 95% CI = 2.3% to 3.3%, P < .001), AIs and ANs (2000-2017; APC = 5.4%, 95% CI = 3.9% to 6.8%, P < .001), and individuals aged older than 65 years (2002-2017; APC = 3.8%, 95% CI = 3.4% to 4.1%, P < .001).
Trends of HCC Tumor Burden and Curative Treatment in the United States
Over the entire study period, more than one-half (56.8%, 95% CI = 56.5% to 57.2%) of tumors were detected at a localized stage, and approximately one-fourth (27.0%, 95% CI = 26.7% to 27.3%) of patients received potentially curative treatment (Table 2). A higher proportion of male and Black individuals presented with more advanced stage HCC and were less likely to receive curative treatment. There was also state-level variation in tumor stage and receipt of curative treatment, with the highest proportion of curative treatment receipt among patients in Alaska (40.0%, 95% CI = 30.6% to 50.0%) and lowest in New Mexico (20.1%, 95% CI = 18.3% to 21.9%).
Table 2.
HCC stage, median tumor size, proportion of curative treatment receipt, and OS rate, by sex, age, race or ethnicity, and state in SEER 18 Database
| Characteristic | 2000-2016 (n = 74 610) |
2004-2016 (n = 64 285) | 2000-2016 (n = 74 610) | 2000-2015 (n = 68 876) |
||||
|---|---|---|---|---|---|---|---|---|
| Tumor stagea |
Median tumor size, cm (95% CI)a | Proportion of receiving curative Tx, % (95% CI)a | OS rates |
|||||
| Localized, % (95% CI) | Regional, % (95% CI) | Distant, % (95% CI) | 1-y OS, % (95% CI) | 3-y OS, % (95% CI) | 5-y OS, % (95% CI) | |||
| Overall | 56.8 (56.5 to 57.2) | 29.1 (28.7 to 29.4) | 14.1 (13.8 to 14.4) | 5.0 (5.0 to 5.0) | 27.0 (26.7 to 27.3) | 50.9 (50.6 to 51.3) | 27.6 (27.2 to 27.9) | 19.4 (19.1 to 19.8) |
| Sex | ||||||||
| Male | 55.1 (54.7 to 55.5) | 30.2 (29.8 to 30.6) | 14.7 (14.4 to 15.0) | 5.1 (5.1 to 5.1) | 26.3 (25.9 to 26.6) | 50.1 (49.7 to 50.5) | 26.8 (26.4 to 27.2) | 18.8 (18.4 to 19.1) |
| Female | 62.5 (61.7 to 63.2) | 25.5 (24.9 to 26.2) | 12.0 (11.5 to 12.5) | 4.7 (4.6 to 4.8) | 29.4 (28.8 to 30.1) | 53.5 (52.7 to 54.2) | 30.2 (29.4 to 30.9) | 21.6 (20.9 to 22.3) |
| Age, y | ||||||||
| <50 | 51.9 (50.6 to 53.1) | 30.6 (29.4 to 31.8) | 17.5 (16.6 to 18.5) | 5.8 (5.6 to 6.0) | 32.6 (31.5 to 33.8) | 52.9 (51.7 to 54.2) | 33.2 (32.0 to 34.4) | 26.7 (25.6 to 27.9) |
| 50-64 | 56.8 (56.3 to 57.3) | 29.6 (29.2 to 30.1) | 13.6 (13.2 to 13.9) | 4.5 (4.4 to 4.5) | 28.9 (28.5 to 29.4) | 53.7 (53.2 to 54.3) | 30.9 (30.4 to 31.4) | 23.0 (22.5 to 23.5) |
| ≥65 | 58.0 (57.4 to 58.6) | 28.0 (27.5 to 28.6) | 14.0 (13.6 to 14.4) | 5.6 (5.5 to 5.7) | 23.4 (23.0 to 23.9) | 46.9 (46.3 to 47.5) | 22.0 (21.5 to 22.5) | 13.0 (12.6 to 13.5) |
| Race or ethnicitya | ||||||||
| Non-Hispanic White | 57.0 (56.5 to 57.5) | 28.9 (28.4 to 29.4) | 14.1 (13.7 to 14.5) | 4.8 (4.8 to 4.9) | 28.1 (27.7 to 28.6) | 49.9 (49.4 to 50.4) | 26.6 (26.1 to 27.1) | 18.9 (18.4 to 19.3) |
| Non-Hispanic Black | 52.2 (51.2 to 53.3) | 30.8 (29.8 to 31.7) | 17.0 (16.2 to 17.8) | 5.4 (5.3 to 5.5) | 22.5 (21.7 to 23.3) | 45.7 (44.7 to 46.7) | 22.6 (21.7 to 23.5) | 14.7 (13.9 to 15.6) |
| Non-Hispanic, AI and AN | 54.9 (51.3 to 58.5) | 33.1 (29.8 to 36.6) | 12.0 (9.8 to 14.5) | 5.6 (5.5 to 5.7) | 23.7 (20.9 to 26.6) | 50.2 (46.9 to 53.8) | 27.5 (24.4 to 31.0) | 17.6 (14.8 to 21.0) |
| Non-Hispanic, API | 57.5 (56.6 to 58.4) | 29.4 (28.6 to 30.2) | 13.1 (12.5 to 13.7) | 4.8 (4.5 to 5.1) | 32.5 (31.7 to 33.3) | 56.7 (55.8 to 57.5) | 35.0 (34.1 to 35.9) | 25.7 (24.9 to 26.6) |
| Hispanic | 59.0 (58.1 to 59.9) | 27.9 (27.1 to 28.7) | 13.1 (12.5 to 13.7) | 4.8 (4.7 to 4.8) | 22.3 (21.7 to 23.0) | 51.9 (51.1 to 52.8) | 26.5 (25.7 to 27.4) | 18.0 (17.3 to 18.8) |
| State | ||||||||
| Alaska | 55.6 (45.2 to 65.5) | 30.3 (21.5 to 40.4) | 14.1 (8.0 to 22.6) | 4.6 (3.9 to 5.4) | 40.0 (30.6 to 50.0) | 59.1 (50.2 to 69.6) | 34.2 (25.6 to 45.7) | 25.6 (17.7 to 37.2) |
| California | 57.5 (57.0 to 58.0) | 29.0 (28.5 to 29.5) | 13.5 (13.2 to 13.9) | 5.0 (5.0 to 5.0) | 25.2 (24.7 to 25.6) | 52.8 (52.3 to 53.3) | 28.7 (28.2 to 29.2) | 19.9 (19.4 to 20.4) |
| Connecticut | 57.8 (55.9 to 59.7) | 28.5 (26.8 to 30.2) | 13.7 (12.4 to 15.0) | 4.6 (4.4 to 4.7) | 34.5 (32.8 to 36.3) | 53.5 (51.7 to 55.3) | 29.6 (27.9 to 31.4) | 20.1 (18.5 to 21.9) |
| Georgia | 54.1 (52.8 to 55.4) | 30.5 (29.3 to 31.7) | 15.4 (14.4 to 16.3) | 5.3 (5.1 to 5.4) | 24.0 (22.9 to 25.1) | 46.8 (45.5 to 48.1) | 24.3 (23.1 to 25.5) | 17.5 (16.4 to 18.7) |
| Hawaii | 56.0 (53.5 to 58.4) | 31.1 (28.8 to 33.5) | 12.9 (11.3 to 14.7) | 5.8 (5.5 to 6.0) | 37.9 (35.6 to 40.2) | 52.1 (49.7 to 54.5) | 29.7 (27.5 to 32.1) | 20.5 (18.4 to 22.7) |
| Iowa | 51.0 (48.5 to 53.4) | 34.9 (32.6 to 37.3) | 14.1 (12.5 to 15.9) | 5.1 (4.9 to 5.4) | 23.0 (21.0 to 25.0) | 47.8 (45.5 to 50.3) | 25.5 (23.3 to 27.8) | 18.7 (16.7 to 21.0) |
| Kentucky | 56.3 (54.4 to 58.2) | 28.7 (27.0 to 30.4) | 15.0 (13.7 to 16.4) | 5.2 (5.0 to 5.4) | 30.5 (28.8 to 32.1) | 45.6 (43.8 to 47.4) | 24.6 (23.0 to 26.3) | 17.1 (15.6 to 18.7) |
| Louisiana | 57.3 (55.7 to 58.8) | 27.5 (26.1 to 28.9) | 15.3 (14.1 to 16.4) | 5.3 (5.1 to 5.4) | 24.6 (23.3 to 26.0) | 44.5 (42.9 to 46.1) | 21.8 (20.4 to 23.3) | 16.3 (15.0 to 17.7) |
| Michigan | 54.9 (53.2 to 56.6) | 29.2 (27.7 to 30.8) | 15.9 (14.7 to 17.2) | 5.0 (4.8 to 5.2) | 30.6 (29.0 to 32.1) | 47.1 (45.4 to 48.8) | 26.1 (24.6 to 27.7) | 18.5 (17.1 to 20.0) |
| New Jersey | 57.4 (56.1 to 58.7) | 26.3 (25.2 to 27.4) | 16.3 (15.3 to 17.3) | 5.0 (4.9 to 5.1) | 31.0 (29.9 to 32.1) | 50.2 (49.1 to 51.5) | 27.7 (26.6 to 28.8) | 20.5 (19.4 to 21.6) |
| New Mexico | 62.1 (59.7 to 64.4) | 22.5 (20.5 to 24.6) | 15.5 (13.7 to 17.3) | 5.2 (5.0 to 5.4) | 20.1 (18.3 to 21.9) | 47.5 (45.3 to 49.9) | 23.9 (21.8 to 26.1) | 16.7 (14.8 to 18.8) |
| Utah | 57.8 (54.7 to 60.8) | 29.9 (27.2 to 32.8) | 12.3 (10.4 to 14.5) | 4.9 (4.6 to 5.2) | 28.3 (25.7 to 31.1) | 49.7 (46.8 to 52.8) | 28.0 (25.3 to 31.1) | 20.0 (17.4 to 23.0) |
| Washington | 55.6 (54.1 to 57.1) | 32.9 (31.5 to 34.3) | 11.5 (10.6 to 12.5) | 4.4 (4.3 to 4.6) | 31.6 (30.2 to 32.9) | 55.0 (53.5 to 56.4) | 30.7 (29.3 to 32.2) | 21.8 (20.4 to 23.2) |
Percents were calculated after excluding missing data for race or ethnicity (0.2%), tumor size (17.3%), tumor stage (8.9%), and treatment modality (0.9%). AI and AN = American Indian and Alaska Native; API = Asian and Pacific Islander; CI = confidence interval; HCC = hepatocellular carcinoma; OS = overall survival; SEER = Surveillance, Epidemiology, and End Results; Tx = treatment.
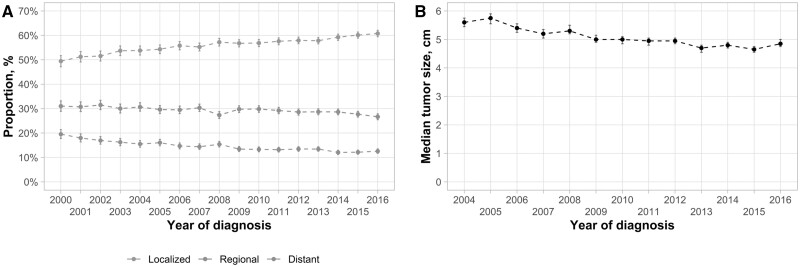
An increasing proportion of tumors was detected at a localized stage over time (Figure 3, A). There was an annual 0.61% (95% CI = 0.53% to 0.69%, P < .001) increase in localized stage detection, increasing from 49.4% (95% CI = 47.2% to 51.7%) in 2000 to 60.8% (95% CI = 59.4% to 62.1%) in 2016. This trend was seen in all demographic subgroups except AIs and ANs (Supplementary Table 2, available online). Similarly, there was a 0.86-mm (95% CI = −1.10 to −0.62 mm, P < .001) decrease in the median diameter of the largest tumor per year (Figure 3, B), with a median diameter of 56.0 mm in 2004 and 48.5 mm in 2016; this trend was also seen in most subgroups (Supplementary Table 2, available online). Despite improvements in localized tumor detection, the proportion of patients receiving potentially curative treatment did not change over time (−0.08%, 95% CI = −0.45% to 0.29%, P = .66).
Figure 3.
Trends of hepatocellular carcinoma (HCC) tumor stages (2000-2016) and median tumor sizes (2004-2016) in Surveillance, Epidemiology, and End Results 18 Database. A) Trends of HCC tumor stages during 2000-2016. B) Trends of median tumor sizes during 2004-2016. Error bars represent the 95% confidence intervals.
Trends of OS for HCC Patients in the United States
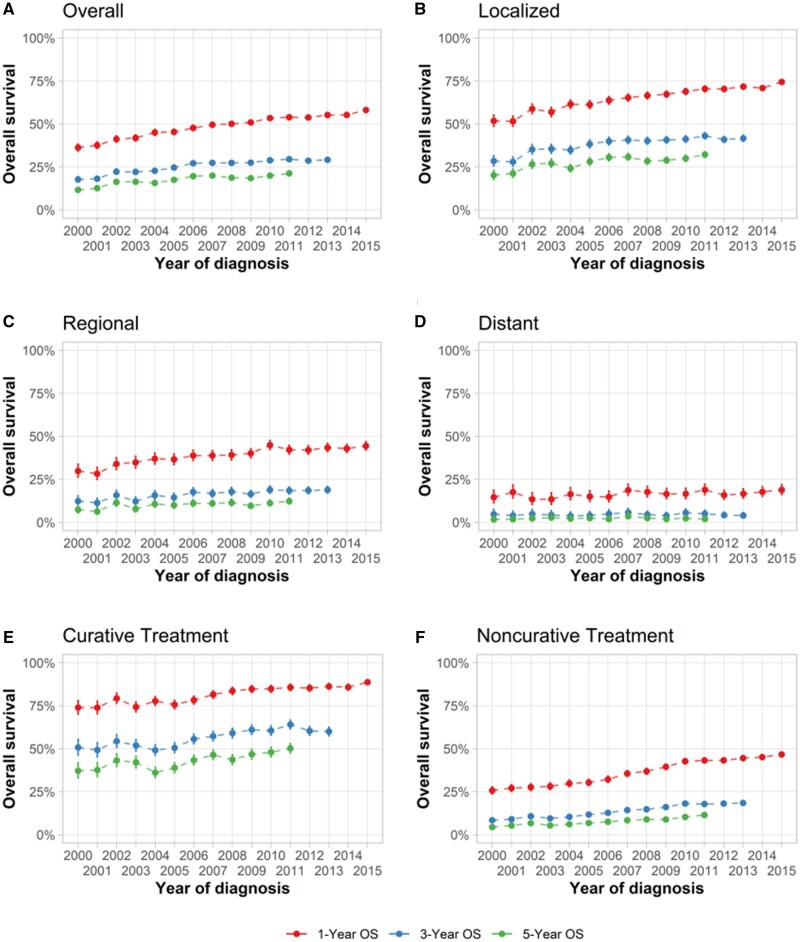
Overall 1-, 3-, and 5-year OS rates were 50.9% (95% CI = 50.6% to 51.3%), 27.6 % (95% CI = 27.2% to 27.9%), and 19.4 % (95% CI = 19.1% to 19.8%), respectively, across the study period (Table 2). OS was higher in younger patients, females, and APIs, whereas it was worse in Blacks. State-level variation was seen in OS, with 5-year OS being 57% higher in Alaska (25.6%, 95% CI = 17.7% to 37.2%) than in Louisiana (16.3%, 95% CI = 15.0% to 17.7%).
From 2000 to 2015, there were increases in 1-, 3-, and 5-year OS rates (Figure 4, A). One-year OS increased from 36.3% (95% CI = 34.3% to 38.3%) to 58.1% (95% CI = 56.9% to 59.4%) during 2000-2015, and the 5-year OS rate almost doubled from 11.7% (95% CI = 10.4% to 13.1%) to 21.3% (95% CI = 20.2% to 22.4%) during 2000-2011. Improvement in survival was more notable in individuals with localized HCC (Figure 4, B) than those with regional (Figure 4, C) or distant HCC (Figure 4, D). Similarly, substantial improvement in OS was noted among individuals receiving curative treatments (Figure 4, E) and noncurative treatments (Figure 4, F).
Figure 4.
Trends of 1-, 3-, 5-year overall survival (OS) of hepatocellular carcinoma (HCC) patients by tumor stages and receipt of curative treatments in Surveillance, Epidemiology, and End Results 18 Database. A) Trends of 1-, 3-, and 5-year OS of overall HCC patients. B) Trends of 1-, 3-, and 5-year OS of HCC patients with localized disease. C) Trends of 1-, 3-, and 5-year OS of HCC patients with regional disease. D) Trends of 1-, 3-, and 5-year OS of HCC patients with distant disease. E) Trends of 1-, 3-, and 5-year OS of HCC patients receiving curative treatment. F) Trends of 1-, 3-, and 5-year OS of HCC patients receiving noncurative treatment. Error bars represent the 95% confidence intervals.
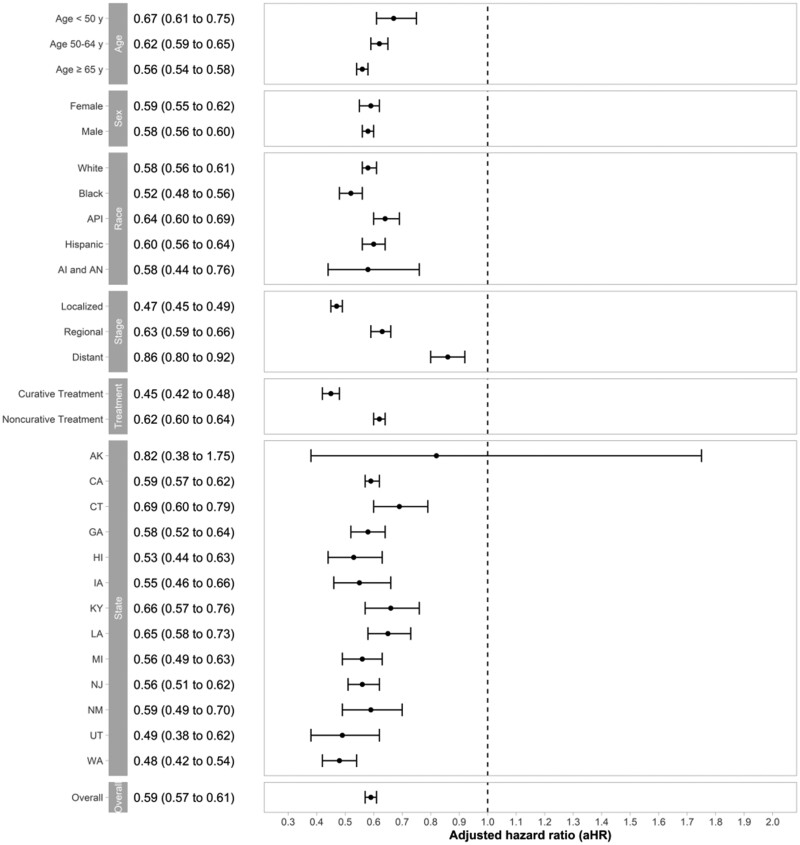
In multivariable Cox regression analysis, year of diagnosis (adjusted hazard ratio = 0.96 per year, 95% CI = 0.96 to 0.97 per year, P < .001) was independently associated with OS (Table 3). With HCC patients diagnosed in 2000 as a reference group, individuals diagnosed with HCC in 2015 had a 41% decreased risk of death (adjusted hazard ratio = 0.59, 95% CI = 0.57 to 0.61, P < .001), with similar trends observed in all the subgroups (Figure 5). In addition to the year of diagnosis, male sex, older age, Black race, regional or distant stage, lack of curative treatment, and particular states were associated with poor OS (Table 3).
Table 3.
Univariate and multivariable Cox proportional hazards model assessing factors associated with HCC overall survival in SEER 18 Database
| Characteristics | Univariate analysis |
Multivariable analysis |
||
|---|---|---|---|---|
| HR (95% CI) | P | aHR (95% CI) | P | |
| Year of diagnosis, per year | 0.97 (0.97 to 0.97) | <.001 | 0.96 (0.96 to 0.97) | <.001 |
| Sex | ||||
| Female | Ref | Ref | Ref | Ref |
| Male | 1.10 (1.08 to 1.12) | <.001 | 1.09 (1.07 to 1.11) | <.001 |
| Age, per 10 y | 1.16 (1.15 to 1.17) | <.001 | 1.15 (1.14 to 1.16) | <.001 |
| Race or ethnicitya | ||||
| Non-Hispanic White | Ref | Ref | Ref | Ref |
| Non-Hispanic Black | 1.14 (1.11 to 1.17) | <.001 | 1.10 (1.07 to 1.13) | <.001 |
| Non-Hispanic AI and AN | 1.00 (0.92 to 1.08) | 1.00 | 1.04 (0.96 to 1.13) | .35 |
| Non-Hispanic API | 0.81 (0.79 to 0.83) | <.001 | 0.82 (0.79 to 0.84) | <.001 |
| Hispanic | 0.98 (0.96 to 1.00) | .08 | 1.00 (0.97 to 1.02) | .72 |
| State | ||||
| California | Ref | Ref | Ref | Ref |
| Alaska | 0.84 (0.67 to 1.06) | .15 | 0.88 (0.68 to 1.13) | .32 |
| Connecticut | 1.00 (0.95 to 1.04) | .82 | 1.03 (0.99 to 1.08) | .18 |
| Georgia | 1.15 (1.11 to 1.18) | <.001 | 1.13 (1.09 to 1.17) | <.001 |
| Hawaii | 1.03 (0.98 to 1.09) | .24 | 1.32 (1.24 to 1.39) | <.001 |
| Iowa | 1.09 (1.03 to 1.15) | .003 | 1.06 (1.01 to 1.12) | .03 |
| Kentucky | 1.16 (1.11 to 1.21) | <.001 | 1.26 (1.21 to 1.32) | <.001 |
| Louisiana | 1.21 (1.17 to 1.26) | <.001 | 1.21 (1.17 to 1.26) | <.001 |
| Michigan | 1.10 (1.06 to 1.15) | <.001 | 1.13 (1.08 to 1.17) | <.001 |
| New Jersey | 1.03 (1.00 to 1.06) | .04 | 1.02 (0.99 to 1.06) | .16 |
| New Mexico | 1.15 (1.09 to 1.21) | <.001 | 1.11 (1.05 to 1.17) | <.001 |
| Utah | 1.04 (0.97 to 1.12) | .24 | 1.13 (1.05 to 1.21) | <.001 |
| Washington | 0.95 (0.91 to 0.98) | .002 | 1.06 (1.02 to 1.10) | .001 |
| Stagea | ||||
| Localized | Ref | Ref | Ref | Ref |
| Regional | 2.01 (1.97 to 2.05) | <.001 | 1.64 (1.60 to 1.67) | <.001 |
| Distant | 4.02 (3.92 to 4.12) | <.001 | 2.57 (2.50 to 2.64) | <.001 |
| Treatment modalitya | — | — | — | — |
| Noncurative treatment | Ref | Ref | Ref | Ref |
| Curative treatment | 0.30 (0.29 to 0.30) | <.001 | 0.35 (0.34 to 0.36) | <.001 |
Missing data for race or ethnicity (0.2%), tumor stage (8.9%), and treatment modality (0.9%) were imputed as described in the Methods section. aHR = adjusted hazard ratio; AI and AN = American Indian and Alaska Native; API = Asian and Pacific Islander; CI = confidence interval; HCC = hepatocellular carcinoma; HR = hazard ratio; SEER = Surveillance, Epidemiology, and End Results.
Figure 5.
Improvement in overall survival of hepatocellular carcinoma (HCC) diagnosed in 2015 compared with HCC diagnosed in 2000 in Surveillance, Epidemiology, and End Results 18 Database. Adjusted hazard ratio of the diagnosis year 2015 with the diagnosis year of 2000 as a reference group. Error bars represent the 95% confidence intervals.
Discussion
In this study, we investigated the temporal trends of primary liver cancer mortality (excluding iCCA) and tumor burden at presentation, receipt of curative treatment, and OS of HCC in the United States. Despite earlier projections of rising liver cancer mortality over the next decade (8), we demonstrated for the first time, to our knowledge, that liver cancer mortality rates have recently plateaued and begun to decline in the United States. Similar to recent changes in HCC incidence, we observed differences by age, race or ethnicity, and geography. Most notably, mortality rates appear to be decreasing in individuals aged younger than 65 years but continued to increase in individuals aged 65 years and older and AIs and ANs. The changes in liver cancer mortality have occurred concurrently with improvements in early tumor detection and increased OS among patients with HCC.
Compared with the recently reported 2.1% AAPC of liver cancer mortality during 1999-2016 (21), we found a lower AAPC of 1.5% with continued follow-up through 2018. Although mortality rates of liver cancer increased over the entire study period, they recently plateaued and have now started showing a downward trend. These changes likely reflect recently observed decreases in HCC incidence (4,22). For example, increased antiviral treatment programs for chronic hepatitis in the United States was reported to be associated with statistically significant reductions in HCC incidence (23-25). Studies have even demonstrated potential benefit of antiviral treatment in patients with a history of HCC given improvement in liver dysfunction (26,27). Our results showed that HCC mortality reduction is most notable in API, likely due to the effectiveness of HBV vaccination and antivirals to curve down the incidence of HBV-associated HCC and improved liver function in patients with HBV-associated HCC (2,28,29). Outside of antiviral treatment reducing HCC mortality, we also noted improved OS among HCC patients, afforded by increased early detection and improved posttreatment outcomes.
We noted increasing mortality in individuals aged 65 years and older. This could be due to increasing burden of chronic liver disease or cirrhosis associated with metabolic syndrome, which is an important risk factor for HCC especially in elderly individuals (30). One other subgroup in whom mortality rates continue to increase and are now the highest is among AIs and ANs. Our recent study showed HCC incidence rates also continue to rise only in AIs and ANs, which likely contributed to the rising HCC mortality rate in this group (22). Higher disease burden of HCV, diabetes and metabolic syndrome in AIs and ANs as well as lower rates of screening and access to HCV treatment and hepatology care due to the socioeconomic and cultural barriers are the main drivers of the increasing burden of HCC (31,32). Similarly, incidence rates of HCC remain high in Hispanics, likely attributed to the growing burden of metabolic syndrome and non-alcoholic fatty liver disease (NAFLD) in this population (22). Finally, we also found Black-White disparities, with Black patients having the worst OS (33). This survival disparity may be partly driven by differences in tumor stage and receipt of curative treatment, although many other patient-, provider-, and system-level interrelated factors, including patient barriers to medical care, issues of medical mistrust, and provider implicit bias, likely contribute to survival disparity in Blacks (34,35). Although tumor growth biology and growth patterns can differ between patients, existing data do not suggest differences in tumor biology by race or ethnicity, suggesting disparities are more related to health-care delivery factors (36). The result highlights the urgency of targeted interventions and resource allocation to prevent liver cancer mortality among racial or ethnic minorities.
Several studies have reported improving early detection and OS in HCC patients in recent years (5,37). Indeed, our study also showed considerable improvement in OS, especially in HCC patients with localized disease. The increased proportion of localized HCC and decreased median size of tumors indicate the role of surveillance and early diagnosis as a determinant of improving prognosis (38,39).
The improved survival among HCC patients receiving curative and noncurative treatments and the year of diagnosis as an independent prognostic factor suggests that refinement of HCC treatment allocation and efficacy likely contribute to improvements in survival as well. This is consistent with the fact that the treatment allocation algorithm for HCC has been revolutionized during the past 2 decades (6,40). For example, surgical technique, perioperative management, and patient selection for HCC resection have all improved (41). Liver transplant organ allocation policy and selection of appropriate candidates have improved for HCC patients, which led to an excellent long-term outcome with very low risk of posttransplant cancer recurrence (42). Technical improvement and candidate selection for transarterial chemoembolization led to a decreased risk of post-transarterial chemoembolization liver failure and local tumor progression (43). Finally, there were improvements in cirrhosis management over the same time period, especially in HCC patients with viral hepatitis-induced liver cirrhosis (26). Although the survival improvement for patients with advanced HCC is not as obvious as for those with localized or regional disease, we did observe a substantial increase in 1-year survival. This survival improvement could be related to the approval of sorafenib as the first systemic therapy for advanced HCC in 2008 (44,45). With continued advances in therapy for advanced HCC (2,46,47), it is expected the prognosis of patients with advanced HCC will also continue to improve rapidly in the next 3-5 years.
We also noted state-level variations in liver cancer mortality, tumor stage, receipt of curative treatment, and OS. Higher liver cancer mortality states are clustered in the southern US, although it is unclear if this is simply related to higher incidence rates, higher prevalence of risk factors (eg, obesity, diabetes), and/or other geographic disparities in early detection and treatment receipt. Future studies should investigate the causes of this geographic disparity to inform future intervention strategies.
We acknowledge there are some limitations to analyzing the large cancer registry data database. First of all, the database does not provide data regarding underlying liver disease etiology or other granular clinical data, including receipt of viral hepatitis treatment and liver disease severity, which is an important prognostic factor in patients with HCC. In addition, the SEER Program records the staging information using SEER Summary Stage 2000 across the study period instead of the commonly used Barcelona Clinic Liver Cancer staging system, which not only considers tumor burden but also incorporates liver function and patient performance status. We reported primary liver cancer mortality excluding iCCA to estimate HCC mortality in our main analysis because mortality statistics specific for HCC are not available in the NCHS database (9). However, our sensitivity analysis showed that liver cancer mortality excluding iCCA in the entire United States closely mirrors the HCC mortality trend in the SEER 18 database, which is representative of the general population covering approximately 28% of the United States (12). Finally, the tumor extent specific variable, treatment, and survival data were not available in the NCHS database (9); thus, we used the SEER 18 database to investigate the temporal trends of tumor extent at diagnosis, treatment, and OS. It should be noted that there could still be variation in the management and outcome of HCC in the remaining 72% of the US population that are not represented in the SEER 18 registries.
In conclusion, the mortality of primary liver cancer appears to have plateaued and started downtrending. In addition to decreasing incidence, increased early HCC detection and advances in treatment allocation and efficacy likely contributed to observed decreases in mortality. It is expected that the prognosis of patients with HCC will continue to improve with the advent of novel curative, locoregional, and systemic treatments. Future studies should further investigate the underlying causes of demographic and state-level variation in liver cancer mortality and outcome. Finally, targeted interventions and resource allocation are needed to minimize HCC disparities in the United States.
Funding
This work is supported by the American College of Gastroenterology (Junior Faculty Development Award), United States Department of Defense (CA191051), Cedars-Sinai Medical Center (Clinical Scholar award), Huiying Foundation, and National Institutes of Health (R01 MD12565 and U01 CA230694).
Notes
Role of the funders: The funders had no role in the collection of data; the design and conduct of the study; management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication.
Disclosures: Dr Yang provides a consulting service for Exact Sciences, Gilead and Eisai. Dr Singal has been on advisory boards and served as a consultant for Wako Diagnostics, Glycotest, Exact Sciences, Roche, Genentech, Bayer, Eisai, BMS, Exelixis, AstraZeneca, Merck, and TARGET PharmaSolutions.
Author contributions: Conceptualization, J.D.Y.; Methodology, Y.-T.L., M.L., and J.D.Y.; Investigation, Y.-T.L., J.J.W., and M.L.; Formal Analysis, M.L.; Writing—Original Draft, Y.-T.L., J.J.W., M.L., and J.D.Y.; Writing—Review & Editing, Y.-T.L., J.J.W., M.L., M.N., K.K., V.G.A., N.E.R., S.C.L., H.-R.T., N.N.N., A.G.S., and J.D.Y.; Funding Acquisition, A.G.S. and J.D.Y.; Resources, J.D.Y.; Supervision, J.D.Y.
Acknowledgements: This study used the NCHS and SEER 18 Databases. The authors acknowledge the efforts of the National Cancer Institute, Division of Cancer Control and Population Sciences; and the Surveillance, Epidemiology, and End Results Program tumor registries in the creation of the NCHS and SEER 18 Databases.
Supplementary Material
Data Availability
The data underlying this article are available in NCHS Database: Mortality—All COD, Aggregated With State, Total U.S. (1990-2018) <Katrina/Rita Population Adjustment> and SEER 18 Database: Incidence-Based Mortality—SEER Research Data, 18 Registries, Nov 2019 Sub (2000-2017) at https://seer.cancer.gov/data/access.html, and can be accessed with Surveillance Research Program, National Cancer Institute SEER*Stat software (seer.cancer.gov/seerstat).
References
- 1. Fitzmaurice C, Allen C, Barber RM, et al. ; Global Burden of Disease Cancer Collaboration. Global, regional, and national cancer incidence, mortality, years of life lost, years lived with disability, and disability-adjusted life-years for 32 cancer groups, 1990 to 2015: a systematic analysis for the Global Burden of Disease Study. JAMA Oncol. 2017;3(4):524–548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Yang JD, Hainaut P, Gores GJ, et al. A global view of hepatocellular carcinoma: trends, risk, prevention and management. Nat Rev Gastroenterol Hepatol. 2019;16(10):589–604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Shiels MS, O'Brien TR.. Recent decline in hepatocellular carcinoma rates in the United States. Gastroenterology. 2020;158(5):1503–1505.e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Rich NE, Yopp AC, Singal AG, et al. Hepatocellular carcinoma incidence is decreasing among younger adults in the United States. Clin Gastroenterol Hepatol. 2020;18(1):242–248.e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Njei B, Rotman Y, Ditah I, et al. Emerging trends in hepatocellular carcinoma incidence and mortality. Hepatology. 2015;61(1):191–199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Marrero JA, Kulik LM, Sirlin CB, et al. Diagnosis, staging, and management of hepatocellular carcinoma: 2018 practice guidance by the American Association for the Study of Liver Diseases. Hepatology. 2018;68(2):723–750. [DOI] [PubMed] [Google Scholar]
- 7. Yang JD, Luu M, Singal AG, et al. Factors associated with detection and survival of T1 hepatocellular carcinoma in the United States: National Cancer Database analysis. J Natl Compr Canc Netw. 2020;18(9):1210–1220. [DOI] [PubMed] [Google Scholar]
- 8. Rahib L, Smith BD, Aizenberg R, et al. Projecting cancer incidence and deaths to 2030: the unexpected burden of thyroid, liver, and pancreas cancers in the United States. Cancer Res. 2014;74(11):2913–2921. [DOI] [PubMed] [Google Scholar]
- 9.Surveillance, Epidemiology, and End Results (SEER) Program (www.seer.cancer.gov) SEERStat Database: Mortality - All COD, Aggregated With State, Total U.S. (1990-2018) <Katrina/Rita Population Adjustment>, National Cancer Institute, DCCPS, Surveillance Research Program, released May 2020. Underlying mortality data provided by NCHS (www.cdc.gov/nchs). https://seer.cancer.gov/data/access.html. Accessed Aug 09, 2020.
- 10.Surveillance Research Program, National Cancer Institute SEERStat software version <8.3.6>. https://seer.cancer.gov/seerstat/. Accessed Aug 09, 2020.
- 11. Altekruse SF, Henley SJ, Cucinelli JE, et al. Changing hepatocellular carcinoma incidence and liver cancer mortality rates in the United States. Am J Gastroenterol. 2014;109(4):542–553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Surveillance, Epidemiology, and End Results (SEER) Program (www.seer.cancer.gov) SEERStat Database: Incidence-Based Mortality - SEER Research Data, 18 Registries, Nov 2019 Sub (2000-2017) - Linked To County Attributes - Time Dependent (1990-2017) Income/Rurality, 1969-2018 Counties, National Cancer Institute, DCCPS, Surveillance Research Program, released April 2020, based on the November 2019 submission. https://seer.cancer.gov/data/access.html. Accessed Aug 09, 2020.
- 13. Chu KC, Miller BA, Feuer EJ, et al. A method for partitioning cancer mortality trends by factors associated with diagnosis: an application to female breast cancer. J Clin Epidemiol. 1994;47(12):1451–1461. [DOI] [PubMed] [Google Scholar]
- 14. Anderson RN, Rosenberg HM.. Age standardization of death rates: implementation of the year 2000 standard. Natl Vital Stat Rep. 1998;47(3):1–16. 20. [PubMed] [Google Scholar]
- 15. Klein RJ, Schoenborn CA.. Age adjustment using the 2000 projected U.S. population. Healthy People 2010. Stat Notes. 2001;(20):1–10. [PubMed] [Google Scholar]
- 16.Joinpoint Regression Program, Version 4.8.0.1 - April 2020; Statistical Methodology and Applications Branch, Surveillance Research Program, National Cancer Institute. https://surveillance.cancer.gov/joinpoint/. Accessed Aug 09, 2020.
- 17. Clegg LX, Hankey BF, Tiwari R, et al. Estimating average annual per cent change in trend analysis. Statist Med. 2009;28(29):3670–3682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Fox J. Applied Regression Analysis, Linear Models, and Related Methods. Thousand Oaks, CA: Sage Publications, Inc; 1997. [Google Scholar]
- 19. van Buuren S, Groothuis-Oudshoorn K. Mice: multivariate imputation by chained equations in R. 2011;45(3):1–67. [Google Scholar]
- 20. Grambsch PM, Therneau TM.. Proportional hazards tests and diagnostics based on weighted residuals. Biometrika. 1994;81(3):515–526. [Google Scholar]
- 21. Tapper EB, Parikh ND.. Mortality due to cirrhosis and liver cancer in the United States, 1999-2016: observational study. BMJ. 2018;362:k2817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Lee Y-T, Wang JJ, Luu M, et al. State-level hepatocellular carcinoma incidence and association with obesity and physical activity in the United States [published online ahead of print]. Hepatology. 2021. doi: 10.1002/hep.31811. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 23. Beste LA, Green P, Berry K, et al. Hepatitis C-related hepatocellular carcinoma incidence in the Veterans Health Administration after introduction of direct-acting antivirals. JAMA. 2020;324(10):1003–1005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Nahon P, Layese R, Bourcier V, et al. Incidence of hepatocellular carcinoma after direct antiviral therapy for HCV in patients with cirrhosis included in surveillance programs. Gastroenterology. 2018;155(5):1436–1450.e6. [DOI] [PubMed] [Google Scholar]
- 25. Kanwal F, Kramer J, Asch SM, et al. Risk of hepatocellular cancer in HCV patients treated with direct-acting antiviral agents. Gastroenterology. 2017;153(4):996–1005.e1. [DOI] [PubMed] [Google Scholar]
- 26. Singal AG, Rich NE, Mehta N, et al. Direct-acting antiviral therapy for hepatitis C virus infection is associated with increased survival in patients with a history of hepatocellular carcinoma. Gastroenterology. 2019;157(5):1253–1263.e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Singal AG, Rich NE, Mehta N, et al. Direct-acting antiviral therapy not associated with recurrence of hepatocellular carcinoma in a multicenter North American cohort study. Gastroenterology. 2019;156(6):1683–1692.e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Chang MH, You SL, Chen CJ, et al. Long-term effects of hepatitis B immunization of infants in preventing liver cancer. Gastroenterology. 2016;151(3):472–480.e1. [DOI] [PubMed] [Google Scholar]
- 29. Yin J, Li N, Han Y, et al. Effect of antiviral treatment with nucleotide/nucleoside analogs on postoperative prognosis of hepatitis B virus-related hepatocellular carcinoma: a two-stage longitudinal clinical study. J Clin Oncol. 2013;31(29):3647–3655. [DOI] [PubMed] [Google Scholar]
- 30. Younossi ZM, Stepanova M, Ong J, et al. Nonalcoholic steatohepatitis is the most rapidly increasing indication for liver transplantation in the United States. Clin Gastroenterol Hepatol. 2021;19(3):580–589.e5. [DOI] [PubMed] [Google Scholar]
- 31. Reilley B, Leston J, Hariri S, et al. Birth cohort testing for hepatitis C virus - Indian Health Service 2012-2015. MMWR Morb Mortal Wkly Rep. 2016;65(18):467–469. [DOI] [PubMed] [Google Scholar]
- 32. Leston J, Finkbonner J.. The need to expand access to hepatitis C virus drugs in the Indian Health Service. JAMA. 2016;316(8):817–818. [DOI] [PubMed] [Google Scholar]
- 33. Rich NE, Hester C, Odewole M, et al. Racial and ethnic differences in presentation and outcomes of hepatocellular carcinoma. Clin Gastroenterol Hepatol. 2019;17(3):551–559.e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Alvidrez J, Castille D, Laude-Sharp M, et al. The National Institute on Minority Health and Health Disparities Research Framework. Am J Public Health. 2019;109(S1):S16–S20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Rich NE, Carr C, Yopp AC, et al. Racial and ethnic disparities in survival among patients with hepatocellular carcinoma in the United States: a systematic review and meta-analysis. Clin Gastroenterol Hepatol. 2020;S1542-3565(20)31725-0. doi:10.1016/j.cgh.2020.12.029. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Rich NE, John BV, Parikh ND, et al. Hepatocellular carcinoma demonstrates heterogeneous growth patterns in a multicenter cohort of patients with cirrhosis. Hepatology. 2020;72(5):1654–1665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. De Toni EN, Schlesinger-Raab A, Fuchs M, et al. Age independent survival benefit for patients with hepatocellular carcinoma (HCC) without metastases at diagnosis: a population-based study. Gut. 2020;69(1):168–176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Simmons OL, Feng Y, Parikh ND, et al. Primary care provider practice patterns and barriers to hepatocellular carcinoma surveillance. Clin Gastroenterol Hepatol. 2019;17(4):766–773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Choi DT, Kum HC, Park S, et al. Hepatocellular carcinoma screening is associated with increased survival of patients with cirrhosis. Clin Gastroenterol Hepatol. 2019;17(5):976–987.e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Llovet JM, Bru C, Bruix J.. Prognosis of hepatocellular carcinoma: the BCLC staging classification. Semin Liver Dis. 1999;19(03):329–338. [DOI] [PubMed] [Google Scholar]
- 41. Allaire M, Goumard C, Lim C, et al. New frontiers in liver resection for hepatocellular carcinoma. JHEP Rep. 2020;2(4):100134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Rich NE, Parikh ND, Singal AG.. Hepatocellular carcinoma and liver transplantation: changing patterns and practices. Curr Treat Options Gastro. 2017;15(2):296–304. [DOI] [PubMed] [Google Scholar]
- 43. Piscaglia F, Ogasawara S.. Patient selection for transarterial chemoembolization in hepatocellular carcinoma: importance of benefit/risk assessment. Liver Cancer. 2018;7(1):104–119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Llovet JM, Ricci S, Mazzaferro V, et al. Sorafenib in advanced hepatocellular carcinoma. N Engl J Med. 2008;359(4):378–390. [DOI] [PubMed] [Google Scholar]
- 45. Cheng AL, Kang YK, Chen Z, et al. Efficacy and safety of sorafenib in patients in the Asia-Pacific region with advanced hepatocellular carcinoma: a phase III randomised, double-blind, placebo-controlled trial. Lancet Oncol. 2009;10(1):25–34. [DOI] [PubMed] [Google Scholar]
- 46. Finn RS, Qin S, Ikeda M, et al. Atezolizumab plus bevacizumab in unresectable hepatocellular carcinoma. N Engl J Med. 2020;382(20):1894–1905. [DOI] [PubMed] [Google Scholar]
- 47. Finn RS, Ryoo BY, Merle P, et al. ; KEYNOTE-240 investigators. Pembrolizumab as second-line therapy in patients with advanced hepatocellular carcinoma in KEYNOTE-240: a randomized, double-blind, phase III trial. J Clin Oncol. 2020;38(3):193–202. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data underlying this article are available in NCHS Database: Mortality—All COD, Aggregated With State, Total U.S. (1990-2018) <Katrina/Rita Population Adjustment> and SEER 18 Database: Incidence-Based Mortality—SEER Research Data, 18 Registries, Nov 2019 Sub (2000-2017) at https://seer.cancer.gov/data/access.html, and can be accessed with Surveillance Research Program, National Cancer Institute SEER*Stat software (seer.cancer.gov/seerstat).