Abstract
Background
The benefit of adjuvant aromatase inhibitors (AI) vs tamoxifen has been investigated in randomized clinical trials for premenopausal and postmenopausal patients with early, estrogen receptor–positive (ER+) breast cancer. The optimal endocrine treatment for chemotherapy-treated perimenopausal women, who generally develop chemotherapy-induced amenorrhea, is uncertain.
Methods
All Dutch women who received adjuvant chemotherapy and endocrine treatment for stage I-III, ER+ (>10% positive cells), invasive breast cancer diagnosed between 2004 and 2007 were identified through the Netherlands Cancer Registry. Included women were considered perimenopausal based on an age at diagnosis of 45 to 50 years (n = 2295). For each patient, AI treatment duration relative to total endocrine treatment duration was calculated. Predominantly tamoxifen-treated patients (AI < 25%) were compared with those receiving AI and tamoxifen for a similar duration (AI 25%-75%) and those mostly using AI (AI > 75%). Adjusted hazard ratios (HRs) for recurrence-free survival (RFS) and overall survival were calculated using time-dependent Cox regression.
Results
After an average follow-up of 7.6 years, 377 RFS events occurred. Women mostly receiving AI (AI > 75%) had the best RFS (adjusted HR = 0.63, 95% confidence interval = 0.46 to 0.86) followed by those receiving AI 25% to 75% (adjusted HR = 0.85, 95% confidence interval = 0.65 to 1.12) compared with predominantly tamoxifen-treated women. Trend analyses showed that every 10% increase in AI-endocrine treatment ratio reduced RFS event risk by 5% (2-sided Ptrend = .002). In total, 236 deaths occurred; hazard ratios for overall survival showed similar trends.
Conclusions
These results suggest that the best adjuvant endocrine treatment for chemotherapy-treated, ER+ breast cancer patients diagnosed aged 45-50 years consists of mainly AI followed by a switch strategy and mainly tamoxifen.
Adjuvant endocrine treatment for premenopausal women with estrogen receptor–positive (ER+) and/or progesterone receptor (PR)–positive breast cancer has long consisted of tamoxifen. The Suppression of Ovarian Function Trial (SOFT) and the Tamoxifen and Exemestane Trial (TEXT) results have challenged this standard by showing that tamoxifen plus ovarian ablation (OA) improve disease-free survival and overall survival (OS) at 8 years compared with tamoxifen alone, and exemestane, an aromatase inhibitor (AI), plus OA led to even higher rates of freedom of recurrence (1-3).
For postmenopausal patients, 5 years of tamoxifen followed by an AI for 2 to 3 years, tamoxifen for 2 to 3 years followed by an AI for up to 5 years, or upfront AI for 5 years are common endocrine treatment regimens (4,5).
For endocrine treatment purposes, women are usually considered premenopausal until definite menopause occurs. Menopausal status at diagnosis is often used as the basis for endocrine treatment allocation. However, the menopausal transition, or perimenopause, is a gradual process caused by the continuing decline in ovarian reserve (6). Perimenopausal women experience, among other symptoms, irregular cycle lengths and an increased susceptibility for the ovarian suppressive effects of chemotherapy (7).
Chemotherapy-induced amenorrhea is a well-known side effect that on average occurs in 77% (95% confidence interval [CI] = 71% to 83%) of premenopausal women treated with chemotherapy 40 years of age and older (8). Although considered an adverse event of chemotherapy, chemotherapy-induced amenorrhea is associated with improved outcome in women younger than 40 years of age diagnosed with hormone receptor–positive breast cancer (9). In women 40 years of age and older, postchemotherapy amenorrhea often proves to be irreversible and is also called chemotherapy-induced menopause. However, estrogen depletion increases the production of gonadotropins, which stimulate the ovaries and can cause ovarian function recovery (10).
Ovarian function recovery can occur during tamoxifen as well as AI treatment and may initially remain unnoticed (11). Although tamoxifen remains an active drug in the presence of ovarian function recovery, its occurrence renders AI treatment ineffective (12). It is therefore unclear if AI treatment in perimenopausal women with chemotherapy-induced amenorrhea is safe or whether tamoxifen should be preferred instead. This study aims to determine the optimal endocrine treatment (AI or tamoxifen) for chemotherapy-treated women who were 45-50 years at breast cancer diagnosis, likely perimenopausal and thus at a high risk of developing chemotherapy–induced amenorrhea or menopause.
Methods
Patient Selection
Through the Netherlands Cancer Registry, we identified all Dutch women who were 45-50 years old when diagnosed with a T1-4NanyM0 ER+ primary breast cancer between 2004 and 2007. Eligible women had no history of prior malignancy and received adjuvant chemotherapy and endocrine treatment.
From its establishment in 1989, the prospective population-based Netherlands Cancer Registry has registered all newly diagnosed, histologically confirmed cancers. For this study, additional information on body mass index (BMI), treatments, and disease recurrences were gathered. All data are obtained directly from patient hospital records by trained registrars. Vital status is acquired from the municipal population registry. Cause of death is not registered because of Dutch privacy regulations.
Registrars from the Netherlands Cancer Registry derived ER, PR, and HER2 status from local pathology reports. According to Dutch guidelines, tumors were considered ER+ and/or PR+ when immunohistochemistry stained greater than 10% of tumor cells positive (13). HER2 positivity was demonstrated by polymerase chain reaction, in situ hybridization, or a 3+ score on immunohistochemistry (14).
Ethics Approval
This project was approved by the Medical Ethical Committee of the Netherlands Cancer Institute—Antoni van Leeuwenhoek hospital (PTC12.1262/NBCP).
Statistical Analysis
Because of frequent treatment switches between tamoxifen and AI, we used the AI–endocrine treatment ratio, as previously described, to determine the predominant endocrine treatment received (15). In short, at any event time during follow-up, we calculated the AI–endocrine treatment ratio
For women with missing treatment start dates, stop date of previous treatment or date of diagnosis was used instead. For women with missing stop dates, the respective date of subsequent treatment start, disease recurrence, end of follow-up, or death were used.
We used the AI–endocrine treatment ratio in a time-dependent manner to group patients into those mainly tamoxifen treated (AI < 25%), mostly AI treated (AI > 75%), or those with roughly similar durations of tamoxifen and AI treatment (AI 25%-75%). In addition, we also evaluated trends by analyzing the AI–endocrine treatment ratio as a continuous variable.
Study endpoints were recurrence-free survival (RFS) and OS. RFS was defined as time from cancer diagnosis to disease recurrence (ipsilateral, local, regional, or distant) or death from any cause, whichever occurred first. OS was calculated as time from cancer diagnosis to death from any cause (16). However, time was left truncated at the start of first endocrine treatment. Patients without an RFS or OS event at the end of follow-up and those lost to follow-up were censored.
The association between the AI–endocrine treatment ratio and RFS and OS was assessed by Cox regression and adjusted for age at diagnosis, trastuzumab use, grade, number of positive lymph nodes, pathologic T-stage (pT-stage), PR status, HER2 status, and OA. Due to low numbers, women for whom nodal status was not known (n = 11) were excluded from Cox regression analyses. Trastuzumab use and OA were used as time-dependent variables, with follow-up as the time scale. The proportional hazards assumption was tested using Schoenfeld residuals. If violated, an interaction between the covariate and follow-up time centered at 5 years was added to the model. Treatment group–specific survival functions were estimated by multivariable Cox models and plotted at the average covariate values. The 95% confidence intervals for 5-year RFS and OS rates were estimated with 1000 bootstrap samples.
Sensitivity analyses were performed by adjusting for BMI, number of treatment switches, total endocrine treatment duration, and type of first endocrine treatment received (tamoxifen vs AI). In addition, calculations were done excluding women with missing start and stop dates of the first endocrine treatment as well as women who did not receive OA. Furthermore, analyses were repeated using alternative categorizations of the AI–treatment ratio. Lastly, we investigated whether AI treatment benefit differed by PR and HER2 status.
Statistical analyses were performed using R version 3.6.3 and Stata SE 15.
Results
Study Population
We identified 2295 women who were 45-50 years of age, when diagnosed between 2004 and 2007, with an ER+ invasive breast cancer. We included 204 (8.9%) of these women in a previous study on the optimal endocrine treatment of ER+HER2+breast cancer patients (15).
All patients received adjuvant chemotherapy and endocrine treatment. Endocrine treatment consisted of tamoxifen and/or AI. Most women started on tamoxifen (1903 of 2295, 82.9%). The average duration of endocrine treatment was 5.5 years. For the 1504 of 2295 (65.5%) women who received endocrine treatment beyond 5 years, the average treatment duration was 6.5 years. Only 2 of these women received therapy for 10 or more years.
Supplementary Figure 1 (available online) summarizes details on missing treatment start and stop dates.
The majority of patients switched between endocrine treatment modalities (Supplementary Figure 2, A-C, available online). Only 34.7% of patients (796 of 2295) received 1 type of treatment. Of these nonswitchers, 56.3% (448 of 796) received tamoxifen and 43.7% received an AI (348 of 796) (Supplementary Table 1, available online). Baseline characteristics are shown per treatment group as defined by the AI–endocrine treatment ratio at the end of follow-up (AI < 25%, AI 25%-75%, and AI > 75%) (Table 1). At that time, 47.5% (1091 of 2295) of patients had received an AI for 25%-75% of their endocrine treatment duration, 27.2% (624 of 2295) had received an AI less than 25%, and 25.3% (580 of 2295) of patients had received an AI greater than 75%. Most women had pathologic T-stage 2 (1178 of 2239, 52.6%) and grade II-III (1801 of 2085, 86.4%) tumors. Metastases to 1 and more lymph nodes were present in 72.1% (1647 of 2284) of women. Of all the ER+ tumors, 87.3% (1908 of 2185) coexpressed PR. HER2 positivity was observed in 6.9% (39 of 567) and 3.9% (39 of 990) of women treated with an AI less than 25% and 25%-75% of their endocrine treatment duration vs 38.2% (194 of 508) in women who received an AI greater than 75% of the time, respectively. Chemotherapy regimens contained an anthracycline in 96.6% (2218 of 2295) of all women (Table 1).
Table 1.
Baseline characteristics of all 2295 ER+ breast cancer patients by AI–endocrine treatment ratio at the end of follow-upa
| Characteristic | AI < 25% | AI 25%-75% | AI > 75% |
|---|---|---|---|
| Total, No. (%) | 624 (100) | 1091 (100) | 580 (100) |
| Mean age (range), y | 47.4 (45-50) | 47.8 (45-50) | 47.9 (45-50) |
| pT-stage, No. (%) | |||
| 1 | 232 (37.1) | 451 (41.3) | 226 (39.0) |
| 2 | 333 (53.4) | 548 (50.3) | 297 (51.2) |
| 3 | 39 (6.3) | 64 (5.9) | 29 (5.0) |
| 4 | 8 (1.3) | 8 (0.7) | 4 (0.7) |
| Unknown | 12 (1.9) | 20 (1.8) | 24 (4.1) |
| Grade, No. (%) | |||
| I | 69 (11.1) | 150 (13.8) | 65 (11.2) |
| II | 282 (45.2) | 514 (47.1) | 243 (41.9) |
| III | 211 (33.8) | 334 (30.6) | 217 (37.4) |
| Unknown | 62 (9.9) | 93 (8.5) | 55 (9.5) |
| Positive lymph nodes, No. (%) | |||
| 0 | 151 (24.2) | 312 (28.6) | 174 (30.0) |
| 1-3 | 324 (51.9) | 566 (51.9) | 251 (43.2) |
| 4-9 | 93 (14.9) | 153 (14.0) | 113 (19.5) |
| >10 | 53 (8.5) | 56 (5.1) | 38 (6.6) |
| Unknown | 3 (0.5) | 4 (0.4) | 4 (0.7) |
| PR status, No. (%) | |||
| Negative | 80 (12.8) | 97 (8.9) | 100 (17.2) |
| Positive | 519 (83.2) | 938 (86.0) | 451 (77.8) |
| Unknown | 25 (4.0) | 56 (5.1) | 29 (5.0) |
| HER2 status, No. (%) | |||
| Negative | 528 (84.6) | 951 (87.1) | 314 (54.1) |
| Positive | 39 (6.3) | 39 (3.6) | 194 (33.5) |
| Unknown | 57 (9.1) | 101 (9.3) | 72 (12.4) |
|
Trastuzumab, No. (%) |
|||
| No | 603 (96.6) | 1068 (97.9) | 452 (77.9) |
| Yes | 21(3.4) | 23 (2.1) | 128 (22.1) |
| Ovarian ablation, No. (%) | |||
| Yesb | 155 (24.8) | 221 (20.3) | 122 (21.0) |
| Surgery | 54 | 129 | 69 |
| GnRH | 121 | 128 | 80 |
| No | 469 (75.2) | 870 (79.7) | 458 (79.0) |
| Chemotherapyc, No. (%) | |||
| Yes | 624 (100) | 1091 (100) | 580 (100) |
| Anthracycline based | 481 (77.1) | 861 (78.9) | 367 (63.3) |
| Anthracycline and taxane based | 118 (18.9) | 207 (19.0) | 184 (31.7) |
| Other | 25 (4.0) | 23 (2.1) | 29 (5.0) |
| No | 0 (0) | 0 (0) | 0 (0) |
The AI–endocrine treatment ratio is defined as the percentage of total endocrine treatment duration (AI+tamoxifen) that was spent on AI treatment. AI = aromatase inhibitor; ER+ = estrogen receptor positive; GnRH = gonadotropin-releasing hormone agonist; PR = progesterone receptor; pT-stage = pathologic T-stage.
Numbers may not add up because some patients received a GnRH before their surgery.
Anthracycline-based schedules: doxorubicin + cyclofosfamide (AC), 5-FU + epirubicin + cyclofosfamide (FEC/CEF), 5-FU + doxorubicin + cyclofosfamide (FAC/CAF). Anthracycline- and taxane-based schedules: docetaxel + doxorubicin + cyclofosfamide (TAC), doxorubicin + docetaxel (DA).
Recurrence-Free Survival
During an average follow-up time of 7.6 years, a total 377 RFS events were observed, most of these (71.1%, 268 of 377) involved distant metastases (Supplementary Table 2, available online). At the end of follow-up, 29.6% (185 of 624) of the women who received an AI less than 25% of their endocrine treatment duration experienced a disease recurrence compared with 10.8% (118 of 1091) and 12.8% (74 of 580) in women treated AI 25%-75% and AI greater than 75%, respectively.
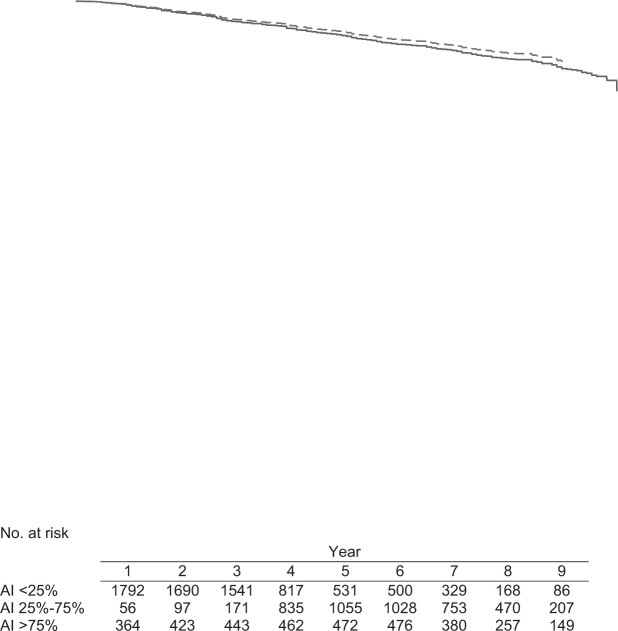
Compared with women who were treated with an AI less than 25% of their endocrine treatment duration, women who received an AI greater than 75% had a statistically significant improvement in RFS, with an adjusted hazard ratio (HR) of 0.63 (95% CI = 0.46 to 0.86) and an adjusted 5-year RFS rate of 94.5% (95% CI = 93.0% to 96.8%) vs 91.4% (95% CI = 90.2% to 94.7%) (Figure 1; Table 2). Women treated with AI 25%-75% did not have a different risk of RFS compared with women treated with AI less than 25% (adjusted HR = 0.85, 95% CI = 0.65 to 1.12; adjusted 5-year RFS rate of 92.3% [95% CI = 90.6% to 95.3%] vs 91.4% [95% CI = 90.2% to 94.7%]). When the AI–endocrine treatment ratio was used as a continuous variable, an adjusted hazard ratio of 0.95 (95% CI = 0.91 to 0.98, Ptrend = .002) was observed, indicating that the risk of an RFS event is reduced by 5% for each additional 10% increase in AI–endocrine treatment ratio.
Figure 1.
Adjusted survival function of recurrence-free survival (RFS) for 2284 estrogen receptor–positive breast cancer patients according to aromatase inhibitor (AI)–endocrinetreatment ratio. Adjusted 5-year RFS rates are 91.4% (95% CI = 90.2% to 94.7%) vs 92.3% (95% CI = 90.6% to 95.3%) vs 94.5% (95% CI = 93.0% to 96.8%) for AI less than 25%, AI 25%-75%, and AI greater than 75%, respectively. The AI–endocrine treatment ratio, included in the model as a time-dependent variable, is defined as the percentage of total endocrine treatment duration (AI+tamoxifen) spent on AI treatment. The survival functions are obtained from a Cox model at average values of age at diagnosis, trastuzumab use (included as a time-dependent variable), grade, number of positive lymph nodes, pathologic T-stage, progesterone receptor status, HER2 status, and ovarian ablation (included as a time-dependent variable).
Table 2.
Multivariable Cox regression for RFS in 2284 ER+ breast cancer patients
| Characteristic | Events | Adjusted HR (95% CI) | P a |
|---|---|---|---|
| AI–endocrine treatment ratiob | |||
| AI < 25% | 185 | 1.00 (Reference) | |
| AI 25%-75% | 118 | 0.85 (0.65 to 1.12) | .27 |
| AI>75% | 74 | 0.63 (0.46 to 0.86) | .004 |
| Age | 1.05 (0.98 to 1.11) | .11 | |
| Age*(follow-up time − 5)c | 1.03 (1.00 to 1.05) | .01 | |
| Trastuzumab | |||
| No | 375 | 1.00 | |
| Yes | 2 | 0.56 (0.13 to 2.39) | .44 |
| Grade | |||
| I | 60 | 0.45 (0.29 to 0.69) | <.001 |
| II | 26 | 0.61 (0.48 to 0.77) | <.001 |
| III | 138 | 1.00 | |
| Unknown | 153 | 1.38 (1.00 to 1.90) | .05 |
| Positive lymph nodes | |||
| 0 | 70 | 1.00 | |
| 1-3 | 156 | 1.37 (1.02 to 1.83) | .03 |
| 4-9 | 89 | 2.29 (1.66 to 3.16) | <.001 |
| >10 | 62 | 4.55 (3.17 to 6.52) | <.001 |
| pT-stage | |||
| 1 | 119 | 1.00 | |
| 2 | 201 | 1.23 (0.98 to 1.55) | .07 |
| 3 | 39 | 1.50 (1.02 to 2.19) | .04 |
| 4 | 8 | 1.68 (0.79 to 3.57) | .18 |
| Unknown | 10 | 1.11 (0.56 to 2.18) | .75 |
| PR status | |||
| Negative | 77 | 1.00 | |
| Positive | 277 | 0.50 (0.38 to 0.64) | <.001 |
| Unknown | 23 | 0.75 (0.46 to 1.20) | .23 |
| HER2 status | |||
| Negative | 289 | 1.00 | |
| Positive | 51 | 1.17 (0.83 to 1.66) | .36 |
| Unknown | 37 | 0.76 (0.53 to 1.09) | .14 |
| Ovarian ablation | |||
| No | 308 | 1.00 | |
| Yes | 69 | 1.25 (0.95 to 1.64) | .10 |
P values are based on a 2-sided Wald test. AI = aromatase inhibitor; CI = confidence interval; ER+ = estrogen receptor positive; HR = hazard ratio; PR = progesterone receptor; pT-stage = pathologic T-stage; RFS = recurrence-free survival.
The AI–endocrine treatment ratio, included in the model as a time-dependent variable, is defined as the percentage of total endocrine treatment duration (AI+tamoxifen) spent on AI treatment.
Interaction between age at diagnosis and follow-up time centered at 5 years was included to accommodate nonproportional hazards. At 5 years of follow-up, 2 patients who differ 1 year in age have an adjusted hazard ratio of 1.05, meaning that the older patient has a 5% higher risk of a RFS event compared with the younger patient. The hazard ratio increases by 3% for each additional year of follow-up, so, for example, at 6 years of follow-up, the adjusted hazard ratio equals exp{ln(1.05)+(follow-up time-5)*ln(1.03)}= 1.08.
Overall Survival
During an average follow-up time of 7.7 years, 236 deaths were observed. Average follow-up times differed slightly by AI–endocrine treatment ratio and were 7.1 years, 7.8 years, and 8.0 years for women who were treated with an AI less than 25%, AI 25%-75%, and AI greater than 75%, respectively.
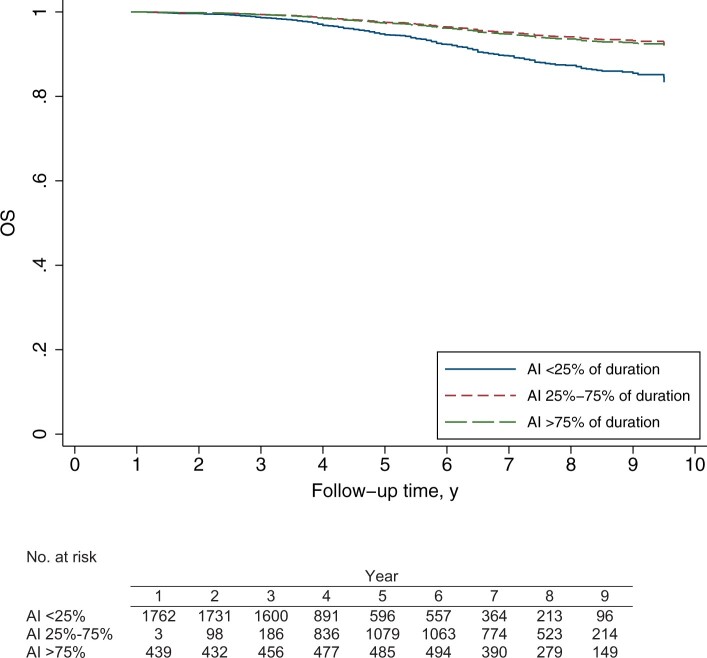
Compared with women with an AI–endocrine treatment ratio less than 25%, women who received an AI greater than 75% experienced better OS (adjusted HR = 0.50, 95% CI = 0.34 to 0.74; adjusted 5-year OS rate = 97.3% [95% CI = 96.4% to 98.4%] vs 94.6% [95% CI = 93.6% to 96.1%], respectively) (Figure 2; Table 3). OS was also better during the first few years of follow-up for women who were treated with AI 25%-75% compared with women who received AI less than 25% (adjusted 5-year OS rate of 97.6% [95% CI = 97.0% to 98.4%] vs 94.6% [95% CI = 93.6% to 96.1%], respectively) (Figure 2). The hazard ratios between women who were treated with an AI 25%-75% vs AI less than 25% vary with time because the proportional hazards assumption was violated. At 5 years, the risk of dying was reduced by approximately 70% when receiving AI 25%-75% compared with AI less than 25% (adjusted HR = 0.32, 95% CI = 0.21 to 0.49, at 5 years). After year 5, the relative risk of dying increased for each additional 1-year increment in follow-up time. This can be calculated as follows:
Figure 2.
Adjusted survival function of overall survival (OS) for 2284 estrogen receptor–positive breast cancer patients according to aromatase inhibitor (AI)–endocrine treatment ratio. Adjusted 5-year OS rates were 94.6% (95% CI = 93.6% to 96.1%) vs 97.6% (95% CI = 97.0% to 98.4%) vs 97.3% (95% CI = 96.4% to 98.4%) for AI less than 25%, 25%-75%, and AI greater than 75%, respectively. The AI–endocrine treatment ratio, included in the model as a time-dependent variable, is defined as the percentage of total endocrine treatment duration (AI+tamoxifen) spent on AI treatment. The survival functions are obtained from a Cox model at average values of age at diagnosis, trastuzumab use (included as a time-dependent variable), grade, number of positive lymph nodes, pathologic T-stage, progesterone receptor status, HER2 status, and ovarian ablation (included as a time-dependent variable).
Table 3.
Multivariable Cox regression for OS in 2284 ER+ breast cancer patients
| Characteristic | Events | Adjusted HR (95% CI) | P a |
|---|---|---|---|
| AI–endocrine treatment ratiob | |||
| AI < 25% | 127 | 1.00 | |
| AI 25%-75% | 62 | 0.32 (0.21 to 0.49) | <.001 |
| AI 25%-75% * (follow-up time − 5)c | 1.42 (1.12 to 1.80) | .003 | |
| AI > 75% | 47 | 0.50 (0.34 to 0.74) | <.001 |
| Age | 1.05 (0.97 to 1.13) | .19 | |
| Trastuzumab | |||
| No | 233 | 1.00 | |
| Yes | 3 | 2.46 (0.72 to 8.40) | .15 |
| Grade | |||
| I | 43 | 0.33 (0.17 to 0.61) | <.001 |
| II | 12 | 0.55 (0.40 to 0.75) | <.001 |
| II * (follow-up time − 5)c | 1.22 (1.05 to 1.43) | .009 | |
| III | 82 | 1.00 | |
| Unknown | 99 | 1.41 (0.97 to 2.07) | .07 |
| Positive lymph nodes | |||
| 0 | 43 | 1.00 | |
| 1-3 | 93 | 1.34 (0.93 to 1.94) | .11 |
| 4-9 | 56 | 2.31 (1.54 to 3.47) | <.001 |
| >10 | 44 | 4.76 (3.06 to 7.38) | <.001 |
| pT stage | |||
| 1 | 78 | 1.00 | |
| 2 | 120 | 1.08 (0.81 to 1.45) | .57 |
| 3 | 25 | 1.27 (0.79 to 2.04) | .31 |
| 4 | 6 | 1.76 (0.73 to 4.20) | .20 |
| Unknown | 7 | 1.07 (0.48 to 2.41) | .85 |
| PR status | |||
| Negative | 54 | 1.00 | |
| Positive | 170 | 0.46 (0.34 to 0.64) | <.001 |
| Unknown | 12 | 0.59 (0.31 to 1.12) | .11 |
| HER2 status | |||
| Negative | 180 | 1.00 | |
| Positive | 33 | 0.99 (0.63 to 1.54) | .98 |
| Unknown | 23 | 0.95 (0.61 to 1.49) | .85 |
| Ovarian ablation | |||
| No | 196 | 1.00 | |
| Yes | 40 | 1.12 (0.79 to 1.59) | .50 |
P values are based on a 2-sided Wald test. AI = aromatase inhibitor; CI = confidence interval; ER+ = estrogen receptor positive; HR = hazard ratio; OS = overall survival; PR = progesterone receptor.
The AI–endocrine treatment ratio, included in the model as a time-dependent variable, is defined as the percentage of total endocrine treatment duration (AI+tamoxifen) spent on AI treatment.
Interaction between the covariates and follow-up time centered at 5 years was included to accommodate nonproportional hazards. At 5 years of follow-up, patients with an AI 25%-75% ratio had a smaller chance of an OS event then patients with a AI less than 25% ratio (adjusted HR = 0.32). The hazard ratio increases by 42% for each additional year of follow-up, so at 6 years of follow-up the adjusted hazard ratio for AI 25%-75% ratio vs AI less than 25% ratio = exp{ln(0.32) + (follow-up time-5) * ln(1.42)} = 0.45. At 5 years of follow-up, patients with grade II tumors had a smaller chance of an OS event then patients with a grade III tumor (adjusted HR = 0.55). The hazard ratio increases by 22% for each additional year of follow-up, so at 6 years of follow-up the adjusted hazard ratio for grade II tumors vs grade III tumors = exp{ln(0.55) + (follow-up time − 5) * ln(1.22)} = 0.67.
For example, at 6 years of follow-up, women who received an AI 25%-75% vs AI less than 25% had an adjusted hazard ratio of 0.45 (95% CI = 0.33 to 0.66) (Figure 2; Table 3).
When the AI–endocrine treatment ratio was used as a continuous variable, an adjusted hazard ratio of 0.90 (95% CI = 0.86 to 0.93, Ptrend < .001) was observed, indicating that the risk of dying is reduced by 10% for each additional 10% increase in the AI–endocrine treatment ratio.
Sensitivity Analyses
The effects of AI treatment on RFS and OS, adjusted for BMI, total endocrine treatment duration, number of treatment switches, and type of first endocrine treatment received and including only women whose start or stop date of first endocrine treatment was known, were comparable with the AI treatment effect from the main models (Supplementary Table 3, available online). Separate analyses for the 498 women who ever received OA at any stage during endocrine treatment were again very similar to the overall AI treatment effect (Supplementary Table 4, available online). Analyses using alternative AI–endocrine treatment ratio cutoffs (AI 0%–30%-70%–100%, AI 0%–40%-60%–100%, AI 0%–50%–100%, AI 0%-100%) also showed similar patterns (Supplementary Table 5, available online).
Lastly, results for OS and RFS did not differ by PR (Supplementary Table 6, available online) or HER2 status (Supplementary Table 7, available online) (all Pinteraction>.05).
Discussion
Our study represents a rigorous, well-annotated, prospective, population-based cohort study in a well-defined patient population. The data are derived from the Netherlands Cancer Registry, which is known to provide highly accurate and complete cancer incidence.
Our study is the first, to our knowledge, to systematically address the relative efficacy of adjuvant AI over tamoxifen in breast cancer patients 45-50 years at diagnosis who are likely perimenopausal and commonly excluded from randomized-controlled trials, including the SOFT and TEXT trials where only 31.9% of all patients were 45-49 years of age (3). Therefore, all current recommendations on adjuvant endocrine therapy for perimenopausal women are based on extrapolations of data from premenopausal or postmenopausal women. Our results emphasize the clinically relevant beneficial effect of AI treatment after chemotherapy in this particular age group and support the abovementioned extrapolation, reassuring both breast cancer oncologists and patients worldwide.
The aim of this study was to assess whether chemotherapy-treated women 45-50 years at breast cancer diagnosis, who are likely perimenopausal and thus at a high risk of developing amenorrhea or menopause, derive more benefit from AI treatment compared with tamoxifen. We found that RFS and OS improved considerably with an increasing AI–endocrine treatment ratio (ie, the longer a woman is treated with an AI compared with tamoxifen).
The findings of our study are in line with posthoc analyses conducted within the monotherapy arms of the Breast International Group 1–98 trial (17). In this study, the authors investigated the effect of tamoxifen and letrozole on disease-free survival in women with either chemotherapy-induced menopause or recent natural menopause (aged ≤55 years). After correction for prognostic factors, a statistically significant disease-free survival benefit of AI vs tamoxifen was observed in the women with chemotherapy-induced menopause (adjusted HR = 0.21, 95% CI = 0.05 to 0.94) (17).
A recent meta-analysis on the incidence and risk factors of chemotherapy-induced amenorrhea found that 77% (95% CI = 71% to 83%) of premenopausal, chemotherapy-treated women 40 years of age and older experience amenorrhea, irrespective of the type of chemotherapy administered (8). Overall, risk factors for chemotherapy-induced amenorrhea were age at diagnosis older than 40 years and tamoxifen treatment following chemotherapy (8,18).
Although chemotherapy-induced amenorrhea is considered a chemotherapeutic side effect, its occurrence has an independent positive effect on the outcome of women diagnosed with hormone-sensitive breast cancer younger than 40 years of age (9,19,20).
The cessation of menses in women with chemotherapy-induced amenorrhea 40 years of age and older often proves permanent (21). In some cases, however, amenorrhea is temporary and ovarian function recovery, either clinical (menstruation or pregnancies) or subclinical (follicle stimulating hormone (FSH), luteinizing hormone(LH), estradiol (E2)blood levels in the premenopausal range), occurs. Ovarian function recovery after chemotherapy-induced amenorrhea typically takes place within the first 2 years following chemotherapy (22). Yet cases of ovarian function recovery have been described many years after finishing chemotherapeutic treatment (10).
Consistent with the Breast International Group 1–98 trial, we found that AI treatment improves the outcome of women who are at increased risk for chemotherapy-induced amenorrhea, even though AI are partly responsible for the ovarian function recovery that diminishes their anticancer effect (17). Indeed, 1 study found a 2-year disease free survival of 82% in women with ovarian function recovery compared with 100% for women without recovery of ovarian function after AI treatment (HR = 9.3, 95% CI = 3.3 to 48, P = .04) (23). Researchers of other studies, however, were unable to observe a difference in outcome (9). Unfortunately, at this time it is not possible to predict which women will experience ovarian function recovery after chemotherapy-induced amenorrhea (22,24,25).
The weight of the evidence suggests that a younger age at chemotherapy initiation predicts for future occurrence of ovarian function recovery. Biochemical ovarian function monitoring is therefore highly recommended in young patients on AI treatment. Caution is needed when selecting the appropriate methodology because some assays may not be sensitive enough or interact with the metabolites of steroidal AI (26).
Our study has some limitations. First, we lack information about the women’s actual menopausal status, both preceding and following chemotherapy. However, based on the age restriction of our study population (45-50 years), it is very likely that a large proportion of the women in our cohort were perimenopausal before chemotherapy initiation (27). Hence, our assumption that these women are at an increased risk for chemotherapy-induced amenorrhea is probably justified because age at diagnosis is a reliable predictive factor (8). In addition, because of their age at diagnosis, they are highly likely to experience permanent amenorrhea (21,22,24,25).
Our assumption of increased risk for chemotherapy-induced amenorrhea is further supported by the high percentage of women in our cohort who received anthracycline- or cyclophosphamide-containing chemotherapy regimens. Besides age at diagnosis, the abovementioned chemotherapy regimens are known to increase the risk of developing chemotherapy-induced amenorrhea (8).
Furthermore, some of the women in our cohort may have been postmenopausal before chemotherapy initiation. Because it is known from the literature that postmenopausal women derive a clinically significant benefit from AI treatment, inclusion of these women in our cohort may have enhanced the AI treatment effect. However, we believe the putative enhancement to be very small, because the number of postmenopausal women at diagnosis is expected to be less than 10% (27). In addition, women could have become postmenopausal due to bilateral oophorectomy performed for benign causes. However, the possible effect on our results is expected to be negligible because, based on information derived from Statistics Netherlands and the nationwide network and registry of histo- and cytopathology in the Netherlands, less than 1% of women aged 45-50 years had undergone bilateral oophorectomy between 2004 and 2007 in the Netherlands (28,29).
Unfortunately, we also lack information on side effects and patient adherence. Side effects are very common among women who receive endocrine treatment and may cause treatment discontinuation (3). Women with hormone receptor–positive breast cancer should be encouraged to adhere to their assigned endocrine treatment due to its observed effectiveness. In SOFT and TEXT, the proportion of women who discontinued endocrine treatment was somewhat larger in the exemestane plus ovarian function suppression group compared with the tamoxifen plus ovarian function suppression group (23.7% vs 19.3%) (3). Here, we propose that an age at diagnosis of 45-50 years and having received adjuvant chemotherapy are clinical variables that may be a great motivation for patients to adhere to an AI because of its superior efficacy compared with tamoxifen. Development and application of additional biomarkers and/or clinical profiles that can accurately predict side effects and therapy resistance would be a tremendous aid to oncologists in selecting the most appropriate treatment for each breast cancer patient.
Furthermore, we found that 194 of 508 (38.2%) women in our predominantly AI-treated (AI > 75%) patient subset were HER2+ compared with 6.9% and 3.9% in the predominantly tamoxifen-treated (AI < 25%) and AI-intermediate groups (AI 25%-75%). Women whose tumors coexpress ER and HER2 are considered high risk and have an unfavorable prognosis. However, the majority of women with ER+HER2+ tumors in our cohort received both chemotherapy and trastuzumab (Table 1). Due to the effectiveness of trastuzumab in HER2+ breast cancers, this formerly poor prognostic group now has a favorable prognosis. Historically, AI are favored over tamoxifen in high-risk perimenopausal and postmenopausal women with ER+ tumors. An enrichment of women with ER+HER2+ breast cancers who received trastuzumab treatment may have enhanced the relative effectiveness of AI in our cohort. However, treatment effect did not differ by HER2 status (Pinteraction > .05), with similar trends as observed in the main analysis, indicating that the effect of HER2 status on our results is at best small (Supplementary Table 5, available online).
Our analyses may have suffered from confounding by indication, because women with amenorrhea postchemotherapy are more likely to receive an AI compared with women without amenorrhea, and development of chemotherapy-induced amenorrhea itself is a favorable prognostic factor. To address this issue, we analyzed the AI–endocrine treatment ratio in the 498 patients who had undergone OA, based on the assumption that predominantly patients without chemotherapy-induced amenorrhea received OA. Again, this showed the same trends as in the main analysis (Supplementary Table 3, available online).
Although comparable with the updated SOFT and TEXT trials, follow-up times in our cohort are still relatively short, with an average follow-up of 7.6 years for RFS and 7.7 years for OS, respectively. Nevertheless, we observed better RFS and OS for women who mainly received an AI compared with those who mostly received tamoxifen, irrespective of HER2 status. Analysis after longer follow-up is part of our future plan to fully appreciate the effect of AI on OS in the treatment of perimenopausal breast cancer patients.
It should also be noted that the Dutch population is predominantly White. Between 2004 and 2007, when the women in our cohort were diagnosed, only 7.7%-8.5% of all 45- to 50-year-old women living in the Netherlands were of non-Dutch, non-Western decent (30). Therefore, caution is advised, because our results may not directly translate to a predominantly non-White/non-Caucasian population.
Lastly, using the AI–endocrine treatment ratio metric was not meant to identify an optimal combination of AI and tamoxifen, but to optimally exploit the information provided by the large group of treatment switchers.
In conclusion, ER+ breast cancer patients diagnosed between 45 and 50 years of age derive statistically significant RFS and OS benefit from treatment with predominantly AI after chemotherapy. AI can therefore be considered for all women in this age group with chemotherapy-induced amenorrhea under strict monitoring of ovarian function to detect early signs of ovarian function recovery. Patients should be instructed to contact their physician when clinical signs of ovarian function recovery occur, including—among others—vaginal bleeding and the cessation of menopausal symptoms. At this point, biochemical assessment of menopausal status should be performed. In case of ovarian function recovery, ovarian suppression or ablation should be added to AI treatment. When deemed inappropriate, tamoxifen remains a suitable alternative for these women.
Funding
This work was supported by grants from The Netherlands Organization for Health Research and Development (Project number 836021019), A Sister’s Hope, and De Vrienden van UMC Utrecht.
Notes
Role of the funder: None of the funders had any influence on study design; data collection; and/or project management; data analysis, interpretation; or manuscript preparation, review or approval.
Disclosures: GSS has received institutional research support funding from AstraZeneca, Merck, Novartis, and Roche outside the submitted work. SCL reports grants from ZonMw and A Sister's Hope during the conduct of the study. SCL is an advisory board member for AstraZeneca, Cergentis, IBM, Pfizer and Roche and received grants from AstraZeneca, Eurocept-pharmaceuticals, Genentech, Novartis, Pfizer, Roche, Tesaro and Immunomedics, in addition, SCL received nonfinancial support from Genentech, Novartis, Roche, Tesaro and Immunomedics and other from AstraZeneca, Pfizer, Cergentis, IBM and Bayer outside of this study. All remaining authors have declared no conflicts of interest.
Author contributions: Conceptualization: SCL, MH, SS, GSS, PJD, EvdW. Data curation: GMHED, SS. Formal Analysis: GMHED, KJ, MH. Funding acquisition: SCL. Investigation: SCL, GMHED, KJ, MH, SS, GSS, PJD, EvdW. Methodology: GMHED, KJ, MH. Project administration: GMHED. Resources: SCL, SS, MH. Software: GMHED, KJ. Supervision: SCL. Validation: GMHED, KJ. Visualization: GMHED, KJ. Writing—original draft: GMHED, KJ, MH, SCL. Writing—review & editing: SCL, GMHED, KJ, MH, SS, GSS, PJD, EvdW.
Prior presentation: This work was previously presented at the ASCO annual meeting, Chicago, Illinois, June 5th 2016. Dackus G, Józwiak K, Sonke GS, et al. Optimal endocrine therapy for breast cancer patients 45–50 years of age at diagnosis. Journal of Clinical Oncology. 2016; 34(15_suppl):551–551. The abstract was granted a Merit award.
Acknowledgements: The authors would like to thank the Netherlands Comprehensive Cancer Organization for maintaining and collecting information in the Netherlands Cancer Registry and in particular all registrars for the collection and completion of additional variables for the Netherlands Breast Cancer Project, a project that this study is part of. In addition, we would like to thank The Netherlands Organization for Health Research and Development (ZonMW), A Sisters Hope and De Vrienden van UMC Utrecht for their financial support in conducting this study.
Data Availability
The data that support the findings of this study are available from the Netherlands Cancer Registry, hosted by the Netherlands Comprehensive Cancer Centre (IKNL) but restrictions apply to the availability of these data, which were used under license for this study, and so are not publicly available. Data are however available from the authors upon reasonable request and with permission of The Netherlands Comprehensive Cancer Centre (IKNL).
Supplementary Material
References
- 1. Francis PA, Regan MM, Fleming GF, et al. Adjuvant ovarian suppression in premenopausal breast cancer. N Engl J Med. 2015;372(5):436–446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Pagani O, Regan MM, Walley BA, et al. Adjuvant exemestane with ovarian suppression in premenopausal breast cancer. N Engl J Med. 2014;371(2):107–118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Francis PA, Pagani O, Fleming GF, et al. Tailoring adjuvant endocrine therapy for premenopausal breast cancer. N Engl J Med. 2018;379(2):122–137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Burstein HJ, Lacchetti C, Anderson H, et al. Adjuvant endocrine therapy for women with hormone receptor-positive breast cancer: ASCO clinical practice guideline focused update. J Clin Oncol. 2019;37(5):423–438. [DOI] [PubMed] [Google Scholar]
- 5. Gradishar WJ, Anderson BO, Abraham J, et al. Breast cancer, version 3.2020, NCCN clinical practice guidelines in oncology. J Natl Compr Canc Netw. 2020;18(4):452–478. [DOI] [PubMed] [Google Scholar]
- 6. De Vos FY, van Laarhoven HW, Laven JS, et al. Menopausal status and adjuvant hormonal therapy for breast cancer patients: a practical guideline. Crit Rev Oncol Hematol. 2012;84(2):252–260. [DOI] [PubMed] [Google Scholar]
- 7. Prior JC. Clearing confusion about perimenopause. BCMJ. 2005;47(10):538–542. [Google Scholar]
- 8. Zavos A, Valachis A.. Risk of chemotherapy-induced amenorrhea in patients with breast cancer: a systematic review and meta-analysis. Acta Oncol. 2016;55(6):664–670. [DOI] [PubMed] [Google Scholar]
- 9. Pagani O, O’Neill A, Castiglione M, et al. Prognostic impact of amenorrhoea after adjuvant chemotherapy in premenopausal breast cancer patients with axillary node involvement: results of the International Breast Cancer Study Group (IBCSG) Trial VI. Eur J Cancer. 1998;34(5):632–640. [DOI] [PubMed] [Google Scholar]
- 10. Smith IE, Dowsett M, Yap YS, et al. Adjuvant aromatase inhibitors for early breast cancer after chemotherapy-induced amenorrhoea: caution and suggested guidelines. J Clin Oncol. 2006;24(16):2444–2447. [DOI] [PubMed] [Google Scholar]
- 11. Mourits MJ, de Vries EG, ten Hoor KA, van der Zee AG, Willemse PH.. Beware of amenorrhea during tamoxifen: it may be a wolf in sheep's clothing. J Clin Oncol. 2007;25(24):3787–3788; author reply 3788–3789. [DOI] [PubMed] [Google Scholar]
- 12.Early Breast Cancer Trialists' Collaborative G. Effects of chemotherapy and hormonal therapy for early breast cancer on recurrence and 15-year survival: an overview of the randomised trials. Lancet. 2005;365(9472):1687–1717. [DOI] [PubMed] [Google Scholar]
- 13.The Dutch Guideline Database. https://www.oncoline.nl/richtlijn/item/index.php?pagina=/richtlijn/item/pagina.php&richtlijn_id=1097. Accessed February 25, 2021.
- 14. Wolff AC, Hammond ME, Hicks DG, et al. Recommendations for human epidermal growth factor receptor 2 testing in breast cancer: American Society of Clinical Oncology/College of American Pathologists clinical practice guideline update. J Clin Oncol. 2013;31(31):3997–4013. [DOI] [PubMed] [Google Scholar]
- 15. Dackus GMHE, Jóźwiak K, Sonke GS, et al. Optimal adjuvant endocrine treatment of ER+/HER2+ breast cancer patients by age at diagnosis: a population-based cohort study. Eur J Cancer. 2018;90:92–101. [DOI] [PubMed] [Google Scholar]
- 16. Hudis CA, Barlow WE, Costantino JP, et al. Proposal for standardized definitions for efficacy end points in adjuvant breast cancer trials: The STEEP system. J Clin Oncol. 2007;25(15):2127–2132. [DOI] [PubMed] [Google Scholar]
- 17. Chirgwin J, Sun Z, Smith I, et al. The advantage of letrozole over tamoxifen in the BIG 1-98 trial is consistent in younger postmenopausal women and in those with chemotherapy-induced menopause. Breast Cancer Res Treat. 2012;131(1):295–306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Walshe JM, Denduluri N, Swain SM.. Amenorrhea in premenopausal women after adjuvant chemotherapy for breast cancer. J Clin Oncol. 2006;24(36):5769–5779. [DOI] [PubMed] [Google Scholar]
- 19. Poikonen P, Saarto T, Elomaa I, Joensuu H, Blomqvist C.. Prognostic effect of amenorrhoea and elevated serum gonadotropin levels induced by adjuvant chemotherapy in premenopausal node-positive breast cancer patients. Eur J Cancer. 2000;36(1):43–48. [DOI] [PubMed] [Google Scholar]
- 20. Swain SM, Jeong JH, Geyer CE Jr, et al. Longer therapy, iatrogenic amenorrhea, and survival in early breast cancer. N Engl J Med. 2010;362(22):2053–2065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Vanhuyse M, Fournier C, Bonneterre J.. Chemotherapy-induced amenorrhea: Influence on disease-free survival and overall survival in receptor-positive premenopausal early breast cancer patients. Ann Oncol. 2005;16(8):1283–1288. [DOI] [PubMed] [Google Scholar]
- 22. Vriens IJ, De Bie AJ, Aarts MJ, et al. The correlation of age with chemotherapy-induced ovarian function failure in breast cancer patients. Oncotarget. 2017;8(7):11372–11379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Guerrero A, Gavila J, Folkerd E, et al. Incidence and predictors of ovarian function recovery (OFR) in breast cancer (BC) patients with chemotherapy-induced amenorrhea (CIA) who switched from tamoxifen to exemestane. Ann Oncol. 2013;24(3):674–679. [DOI] [PubMed] [Google Scholar]
- 24. Henry NL, Xia R, Banerjee M, et al. Predictors of recovery of ovarian function during aromatase inhibitor therapy. Ann Oncol. 2013;24(8):2011–2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Krekow LK, Hellerstedt BA, Collea RP, et al. Incidence and predictive factors for recovery of ovarian function in amenorrheic women in their 40s treated with letrozole. J Clin Oncol. 2016;34(14):1594–1600. [DOI] [PubMed] [Google Scholar]
- 26. Folkerd EJ, Lonning PE, Dowsett M.. Interpreting plasma estrogen levels in breast cancer: caution needed. J Clin Oncol. 2014;32(14):1396–1400. [DOI] [PubMed] [Google Scholar]
- 27. Mishra GD, Pandeya N, Dobson AJ, et al. Early menarche, nulliparity and the risk for premature and early natural menopause. Hum Reprod. 2017;32(3):679–686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Casparie M, Tiebosch AT, Burger G, et al. Pathology databanking and biobanking in The Netherlands, a central role for PALGA, the nationwide histopathology and cytopathology data network and archive. Cell Oncol. 2007;29(1):19–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Statistics Netherlands Database. 2019. https://statline.cbs.nl/Statweb/publication/?DM=SLNL&PA=37325&D1=0&D2=l&D3=46-52&D4=0&D5=0&D6=8-12&HDR=T,G3,G4,G5&STB=G1,G2&CHARTTYPE=1&VW=T Accessed March 10, 2019.
- 30.Statistics Netherlands Database 2021. https://opendata.cbs.nl/statline/#/CBS/en/dataset/37325eng/table?ts=1611664539697. Accessed January 26, 2021.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data that support the findings of this study are available from the Netherlands Cancer Registry, hosted by the Netherlands Comprehensive Cancer Centre (IKNL) but restrictions apply to the availability of these data, which were used under license for this study, and so are not publicly available. Data are however available from the authors upon reasonable request and with permission of The Netherlands Comprehensive Cancer Centre (IKNL).