Abstract
Background:
Stimulant (cocaine and/or methamphetamine) use has increased among people with opioid use disorder. We conducted a systematic review of medications for stimulant use disorders in this population.
Methods:
We searched for randomized controlled trials in multiple databases through April 2019, and dual-screened studies using pre-specified inclusion criteria. Primary outcomes were abstinence defined as stimulant-negative urine screens for ≥3 consecutive weeks; overall use as the proportion of stimulant-negative urine specimens; and retention as the proportion of participants who completed treatment. We rated strength of evidence using established criteria and conducted meta-analyses of comparable interventions and outcomes.
Results:
Thirty-four trials of 22 medications focused on cocaine use disorder in patients with opioid use disorder. Most studies enrolled participants stabilized on opioid maintenence therapy, generally methadone. None of the six studies that assessed abstinence found significant differences between groups. We found moderate-strength evidence that antidepressants (desipramine, bupropion, and fluoxetine) worsened retention. There was moderate-strength evidence that disulfiram worsened treatment retention (N=605, RR 0.86, 95% CI 0.77 to 0.95). We found low-strength evidence that psychostimulants (mazindol and dexamphetamine) may reduce cocaine use, though the difference was not statistically significant (standard mean difference 0.35 [95% CI −0.05 to 0.74]). There was only 1 trial for methamphetamine use disorder, which showed insufficient-strength evidence for naltrexone.
Conclusions:
Co-occurring stimulant/opioid use disorder is an important problem for targeting future research. Medication trials for methamphetamine use disorder are lacking in this population. Most of the medications studied for cocaine use were ineffective, although psychostimulants warrant further study.
Keywords: pharmacotherapy, substance use disorder, cocaine, amphetamine, stimulant, systematic review
1. INTRODUCTION
While the United States (U.S.) is in the midst of an opioid epidemic, stimulant use disorders have been increasing in people with existing opioid use disorder (OUD). Among treatment-seeking people with OUD, reports of past-month methamphetamine use nearly doubled from 18.8% to 34.2% between 2011 and 2017 (Ellis et al., 2018). Similarly, amongst people with prescription OUD in 2015, 31.5% reported cocaine use disorders in the prior year (Han et al., 2017). While there are Food and Drug Administration (FDA)-approved medications for OUD (Substance Abuse and Mental Health Services Administration (SAMHSA), 2018), untreated stimulant use disorders complicate treatment and are associated with poorer outcomes, including increases in hospitalization and overdose deaths (Centers for Disease Control and Prevention; Seth et al., 2018; Winkelman et al., 2018). In previous systematic reviews (SRs) (Chan et al., 2019a; Chan et al., 2019b) we examined various medications for stimulant use disorders; however, many of the studies reviewed excluded populations with co-occurring OUD. We sought to conduct a more in depth review the evidence for medications for stimulant use disorder treatment specifically in people with co-occurring OUD.
2. METHODS
2.1. Data sources and search strategies
This SR is part of a larger report commissioned by the U.S. Veterans Health Administration (VHA) that examined the benefits and harms of medications for cocaine and methamphetamine use disorders (Chan et al., 2018). We searched Ovid MEDLINE, OvidPsycINFO, and Ovid EBM Reviews Cochrane Database of Systematic Reviews through April 2019 (see online supplement eMethods 1 for full search strategy). We reviewed the bibliographies of relevant articles and contacted experts to identify additional studies. To identify in-progress or unpublished studies, we searched ClinicalTrials.gov, OpenTrials, and the World Health Organization (WHO) International Clinical Trials Registry Platform (ICTRP). The review protocol was registered to PROSPERO before study initiation (CRD42018085667). Our methods and reporting follow PRISMA guidelines (Moher et al.).
2.2. Study selection
We included randomized controlled trials (RCTs) that enrolled adults with cocaine or methamphetamine use disorders and co-occurring OUD and compared pharmacotherapies to one another, placebo, usual care, or psychotherapy. We excluded studies and comparisons examining participants with comorbid psychotic spectrum or bipolar disorders. We excluded studies that did not perform urine drug screening (UDS) at least once per week, and/or had less than four-weeks follow-up. The parameters and scope of the review are available in our PICOTS (population, interventions, comparators, outcomes, timing, setting, and study design) table in eMethods 2 of the online supplement, while study selection criteria are detailed in eMethods 3.
We dual reviewed and evaluated titles and abstracts for 13% of the search yield to ensure reliability. Two investigators independently reviewed the full text of all potentially relevant articles for inclusion, and discordant results were resolved through consensus.
2.3. Data abstraction and quality assessment
One investigator abstracted details related to study design; setting; population; intervention and follow-up; co-interventions; outcomes; and harms. A second investigator confirmed the abstraction. Outcomes of interest were defined based on outcomes used in prior reviews, as well as guidance from our Technical Expert Panel (TEP) prior to beginning our review, with focus on standardized measures that would allow for potential meta-analysis, including: abstinence from stimulants, defined as three or more consecutive weeks of negative UDS; overall use, analyzed as the proportion of UDS samples that were cocaine- or methamphetamine-negative; treatment retention defined as the proportion of randomized participants who completed treatment; and harms, specifically, reported adverse effects leading to treatment dropout and severe adverse events. We also noted whether participants in each study were receiving opioid maintenance treatment upon enrollment or not, and whether they were started or continued on concurrent medications for opioid use disorder during the trial.
Two reviewers independently assessed the quality of each included RCT using a tool developed by the Cochrane Collaboration (Higgins and Green, 2011) (quality criteria and ratings are in online supplement eMethods 4 [eTables 1 and 2]), and classified the risk of bias (ROB) as low, unclear, or high. For studies identified in our search that were included in prior SRs, we reviewed the primary studies to confirm enrollment of co-occurring OUD populations and abstracted results from those studies in our analysis.
2.4. Data synthesis and analysis
We qualitatively synthesized the evidence, and combined the findings of trials with comparable interventions and outcome measures in random-effects meta-analyses (DerSimonian and Laird, 1986). We used RevMan 5.3 (Review Manager, 2014) to calculate the overall relative risk (RR) and 95% confidence intervals (CI) for each outcome in the treatment group compared with placebo. We assessed statistical heterogeneity among the pooled studies using the I2 statistic (Higgins and Thompson, 2002; Higgins et al., 2003).
We assessed the overall strength of evidence (SOE) for each outcome as high, moderate, low, or insufficient using an established method (Berkman et al., 2013). Although the small number of trials for each medication precluded quantitative analysis for publication bias, we assessed publication bias qualitatively by considering whether or not it was likely that negative studies were selectively withheld from publication (Guyatt et al., 2011). We considered factors such as number of positive studies included, review of study sponsorship, and searched clinicaltrials.gov to ensure no studies that should have been reported had remained unpublished.
3. RESULTS
Our search yielded 5,862 publications, of which we selected 486 for full-text review (eFigure 1, online supplement). We included 35 RCTs of 23 different medications, including anticonvulsants, antidepressants, antipsychotics, dopamine agonists, medications for OUD, medications approved by the FDA for other substance use disorders (SUDs) (NIDA), psychostimulants, and various other pharmacotherapies (Table 1). Of the 35 RCTs we included, 24 were reviewed in seven previous SRs, and 11 were newly identified (Table 1) in our search. 34 trials were of treatments for cocaine use disorder, while only one RCT (Tiihonen et al., 2012) studied treatment of amphetamine use disorder using naltrexone implant in participants with comorbid OUD.
Table 1.
Pharmacotherapies for stimulant use disorder studied in participants with comorbid opioid use disorder
| Drug class | N trials and sources | Drug or drug combination | N trials |
|---|---|---|---|
| Interventions for cocaine use disorder | |||
| Antidepressants | 10 RCTs from 4 previous SRs (Castells et al., 2016; Castells et al., 2009; Minozzi et al., 2015a; Pani et al., 2011) | Bupropion | 2 (Margolin et al., 1995b; Poling et al., 2006) |
| Desipramine | 6 (Arndt et al., 1992; Kolar et al., 1992; Kosten et al., 2003; Kosten et al., 1992; O’Brien et al., 1988; Oliveto et al., 1999) | ||
| Fluoxetine | 2 (Grabowski et al., 1995; Winstanley et al., 2011) | ||
| Anticonvulsants | 4 RCTs from 2 previous SRs (Castells et al., 2009; Minozzi et al., 2015b) | Gabapentin | 1 (Gonzalez et al., 2007) |
| Tiagabine | 2 (Gonzalez et al., 2007; Gonzalez et al., 2003) | ||
| Topiramate | 1 (Umbricht et al., 2014) | ||
| Antipsychotics | 2 RCTs: 1 from the current search; 1 from 3 previous SRs (Castells et al., 2016; Castells et al., 2009; Indave et al., 2016) | Aripiprazole | 1 (Moran et al., 2017) |
| Risperidone | 1 (Grabowski et al., 2004) | ||
| Dopamine agonists | 4 RCTs from 3 previous SRs (Castells et al., 2009; Minozzi et al., 2015a; Pani et al., 2011) | Amantadine | 3 (Handelsman et al., 1995; Kolar et al., 1992; Kosten et al., 1992) |
| Bromocriptine | 1 (Handelsman et al., 1997) | ||
| Medications for opioid use disorder | 4 RCTs: 1 from the current search; 3 from previous SR (Castells et al., 2009; Pani et al., 2011) | Buprenorphine | 3 (Oliveto et al., 1999; Schottenfeld et al., 2005; Schottenfeld et al., 1997) |
| Buprenorphine-naloxone | 1 (Ling et al., 2016) | ||
| Methadone | 3 (Oliveto et al., 1999; Schottenfeld et al., 2005; Schottenfeld et al., 1997) | ||
| Medications for other substance use disorders | 7 RCTs: 5 from the current search; 2 from 2 previous SRs (Castells et al., 2009; Pani et al., 2010) | Disulfiram | 6 (Carroll et al., 2012; George et al., 2000; Kosten et al., 2013; Oliveto et al., 2011; Petrakis et al., 2000; Schottenfeld et al., 2014) |
| Varenicline | 1 (Poling et al., 2010) | ||
| Psychostimulants | 4 RCTs included in 3 previous SRs (Castells et al., 2016; Castells et al., 2009; Indave et al., 2016) | Mazindol | 2 (Margolin et al., 1995a; Margolin et al., 1997) |
| Dexamphetamine | 1 (Grabowski et al., 2004) | ||
| Methylphenidate | 1 (Dursteler-MacFarland et al., 2013) | ||
| Other pharmacotherapies | 3 RCTs from the current search; 1 RCT from a previous SR (Castells et al., 2009) | Carvedilol | 1 (Sofuoglu et al., 2017) |
| Magnesium L-aspartate | 1 (Margolin et al., 2003) | ||
| Progesterone | 1 (Sofuoglu et al., 2007) | ||
| Mecamylamine | 1 (Reid et al., 2005a) | ||
| Medications for amphetamine/methamphetamine use disorder | |||
| Medications for other substance use disorders | 1 RCT from the current search | Naltrexone | 1 (Tiihonen et al., 2012) |
Abbreviations: N = number of; RCT = randomized controlled trial; SR = systematic review
A majority (16) of included trials enrolled participants who were already receiving opioid maintenance treatment (OMT), though 10 trials enrolled participants who had not recently received OMT; 9 trials either enrolled a mix of treatment stabilized and treatment naïve or did not include this information. Aside from the five trials of MOUD for stimulant use (Ling et al., 2016; Oliveto et al., 1999; Schottenfeld et al., 2005; Schottenfeld et al., 1997; Tiihonen et al., 2012), the majority of studies used methadone concurrently with the study medication (26); three studies used buprenorphine, and one study used diacetylmorphine.
3.1. Medications for Cocaine Use Disorder in Participants with Opioid Use Disorder
3.1.1. Antidepressants
Ten RCTs studied antidepressants for treatment of cocaine use disorder in populations that were generally stable on methadone maintenance (Table 1).: desipramine in six trials(Arndt et al., 1992; Kolar et al., 1992; Kosten et al., 2003; Kosten et al., 1992; O’Brien et al., 1988; Oliveto et al., 1999), bupropion in two trials(Margolin et al., 1995b; Poling et al., 2006), and fluoxetine in two trials (Table 1). Few studies were high-quality, and most were underpowered.
There was moderate SOE that antidepressants worsen treatment retention (10 RCTs, combined N=1,006; RR of dropout 1.22, 95% CI 1.05 to 1.41) and withdrawals due to adverse events (five RCTs, combined N=492; RR 2.47, 95% CI 1.03 to 5.90) compared to placebo (Pani et al., 2011). Only one unclear-ROB study examined cocaine use during the trial period and found no difference between the antidepressant bupropion and placebo (Poling et al., 2006). None of the antidepressant trials reported abstinence outcomes,
3.1.2. Anticonvulsants
Three placebo-controlled trials of anticonvulsants were reviewed in two previous SRs (Castells et al., 2009; Minozzi et al., 2015b): one unclear-ROB trial of tiagabine, one unclear-ROB trial with separate arms for gabapentin and tiagabine, and one low-ROB trial of topiramate (Table 1).
Combined retention data from all three trials (N=292) show moderate-strength evidence of worse retention with anticonvulsants compared with placebo (RR 0.86, 95% CI 0.76 to 0.97;; Figure 3), and low-strength evidence for no effect on cocaine use or abstinence in cocaine users with comorbid OUD.
Figure 3. Treatment retention in randomized controlled trials of psychostimulants, anticonvulsants, dopamine agonists, disulfiram, and opiate agonists in participants with dual cocaine/opioid use disorders.
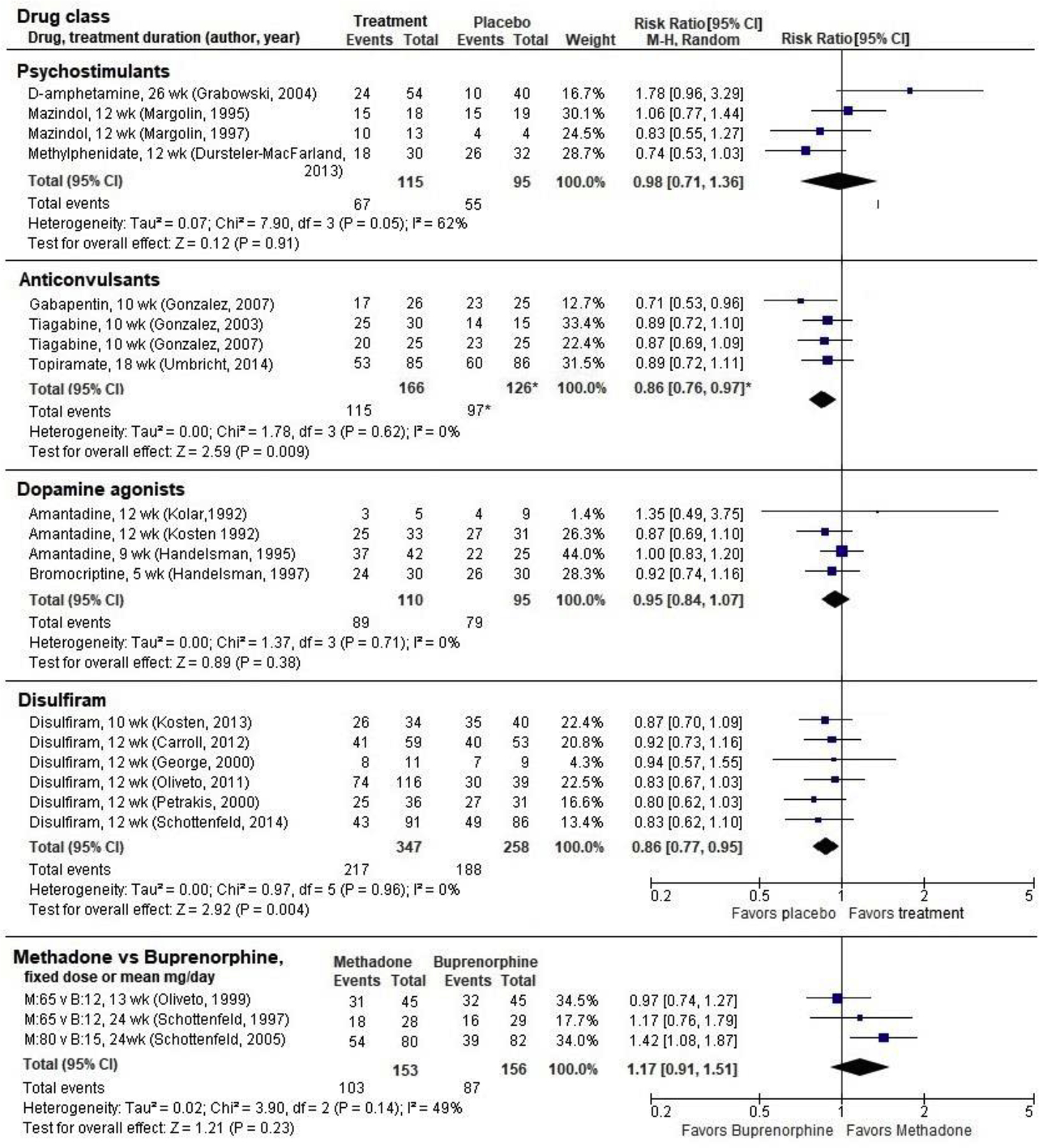
* Gonzalez. 2007 included 2 active treatment arms. The weights and combined estimate have been adjusted to represent the placebo arm only once in the analysis.
3.1.3. Antipsychotics
Two antipsychotic medications were studied as interventions of cocaine use disorder in participants with OUD: risperidone (Grabowski et al., 2004) and aripiprazole (Moran et al., 2017). These studies provide insufficient-strength evidence for treating cocaine use disorder with antipsychotics in people with comorbid OUD.
The risperidone study included a two-week medically supervised stabilization period prior to a 24-week medication trial of risperidone (two or four mg) or placebo; participants were not required to be cocaine-free at the start. This unclear-ROB study reported fewer dropouts with risperidone than placebo, although the difference was not statistically significant (N=96, RR 0.77, 95% CI 0.59 to 1.00) (Indave et al., 2016). The aripiprazole study was conducted among participants recently stabilized on methadone, receiving contingency management and counseling, who completed a 12-week medically-supervised withdrawal from cocaine prior to initiating treatment (Moran et al., 2017). Those who achieved continuous cocaine abstinence during weeks 11 and 12 (N=18) were randomized to 15 mg of aripiprazole or placebo, with contingency management continuing through the two-week induction phase. Time to both lapse (first cocaine-positive UDS; hazard ratio [HR]=0.45, 95% C I0.14 to 1.42, P=0.17) and relapse (two consecutive cocaine-positive UDSs or missed urines; HR= 0.31, 95% CI 0.07 to 1.27, P=0.10) were similar between groups, and there were no differences in abstinence, retention, or harms. The study was discontinued early due to the small number of participants able to achieve abstinence in weeks 11 and 12 (18 of 41 enrolled) and rated high ROB.
3.1.4. Dopamine agonists
Three RCTs of amantadine and one RCT of bromocriptine were conducted in methadone or buprenorphine-maintained participants with cocaine use disorder (Table 1). These studies reviewed in a previous SR (Minozzi et al., 2015a). We pooled retention outcomes from all studies (N=205) and found no difference between dopamine agonists and placebo (RR 0.95, 95% CI 0.84 to 1.07; Figure 3). There were also no differences in cocaine use, and abstinence was not reported. Three of the studies had unclear ROB and the fourth had high ROB (eTable 2, online supplement).). These studies provide low-strength evidence that dopamine agonists are not effective for reducing cocaine use nor improving treatment retention in an OUD population.
3.1.5. Medications for Opioid Use Disorder (MOUDs)
Four trials examined the effects of MOUDs for cocaine use disorder in participants with comorbid OUD. Three trials compared buprenorphine directly with methadone (Ling et al., 2016)(Table 1). One of these studies included four treatment arms with two dose levels of each drug (methadone 20 mg versus 65 mg daily, buprenorphine four mg versus 12 mg daily; (Schottenfeld et al., 1997). For meta-analysis we included only the higher drug dosages from this study, which are closer (although still lower) to the dosages used in clinical practice and in the other two studies (Oliveto et al., 1999; Schottenfeld et al., 2005). Abstinence was greater for those taking methadone than buprenorphine in our pooled analysis of 2 low-ROB RCTs (N=219; RR 1.85, 95% CI 1.25 to 2.75; Figure 1). Similarly, there was no significant difference in treatment retention when all three studies were pooled (N=309, RR 1.17, 95% CI 0.91 to 1.51; Figure 3). These studies were reviewed in previous SRs (Castells et al., 2009; Pani et al., 2011).
Figure 1.
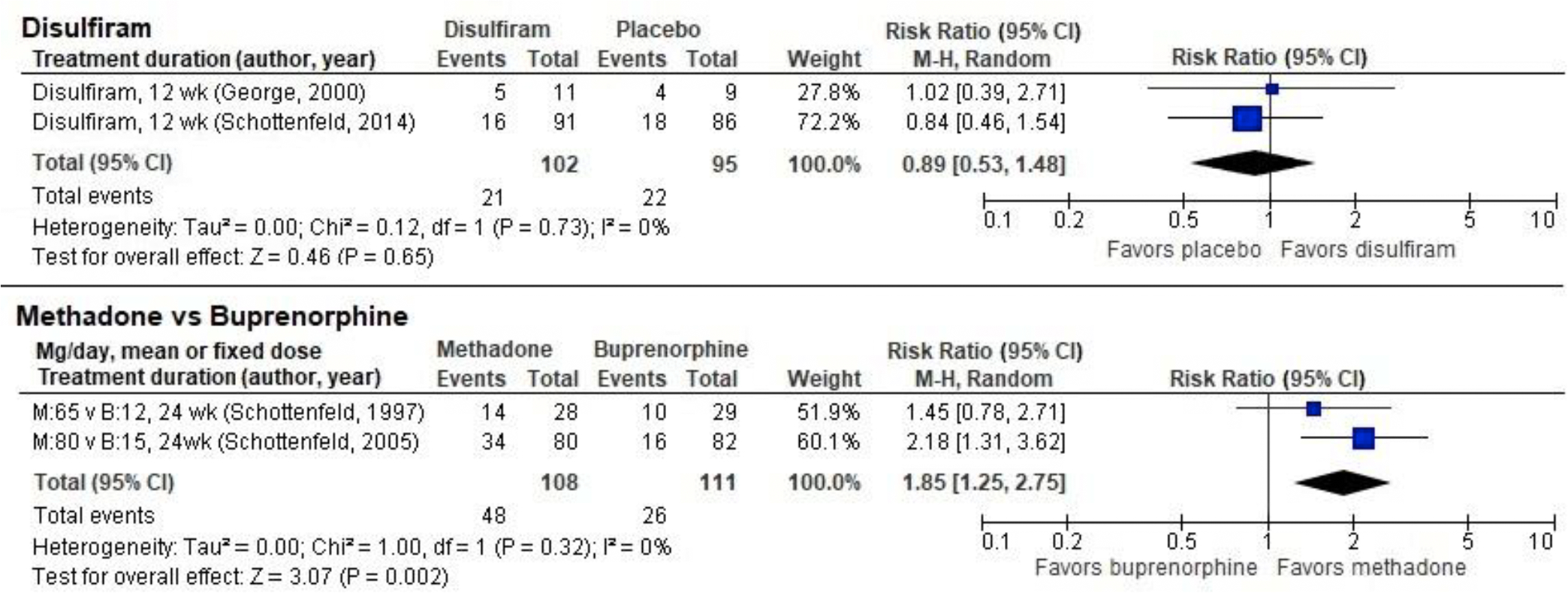
Abstinence for 3 or more consecutive weeks in randomized controlled trials of disulfiram and opiate agonists in participants with dual cocaine/opioid use disorders
The current search identified one large (N=302), low-ROB trial of buprenorphine in combination with naloxone at two dosages (16–4 mg and 4–1 mg buprenorphine-naloxone; (Ling et al., 2016). The higher dose arm experienced significantly less cocaine use compared with placebo (UDS-negative OR 1.71, P=0.02); the lower dose arm experienced no differences in use (OR 1.09, P=0.11). There were no differences in retention or abstinence at either dosage.
Taken together these studies provide insufficient-strength evidence for the effectiveness of MOUDs for treatment of cocaine use disorder in those with comorbid OUD.
3.1.6. Medications FDA-approved for other substance use disorders
We identified six studies of disulfiram, which is FDA-approved for alcohol use disorder, to treat cocaine use disorder in participants with comorbid OUD (Carroll et al., 2012; George et al., 2000; Kosten et al., 2013; Oliveto et al., 2011; Petrakis et al., 2000; Schottenfeld et al., 2014)(Table 1). Combined retention data from all six studies (N=605) provides moderate-strength evidence that disulfiram worsened treatment retention compared with placebo (RR 0.86, 95% CI 0.77 to 0.95; Figure 3). Combining data on abstinence from one low-ROB RCT (Schottenfeld et al., 2014) and one unclear-ROB RCT (George et al., 2000) revealed no significant differences (N=207, RR 0.89, 95% CI 0.53 to 1.48; Figure 1). Three trials of disulfiram reported conflicting findings on cocaine use; data were not available to conduct meta-analysis. One study (Kosten et al., 2013) found significant reduction in use (RR 1.58, 95% CI 1.39 to 1.79), another study (Oliveto et al., 2011) found significant increase in use (RR 0.61, 95% CI 0.52 to 0.71), and a third study (Carroll et al., 2012) found no difference from placebo. These provide insufficient-strength evidence for the effect of disulfiram on cocaine use in those with OUD.
Varenicline, an FDA-approved treatment for tobacco use disorder, was studied in one small (N=31), unclear-ROB trial that reported no statistically significant differences in cocaine use reduction or treatment retention (Poling et al., 2010). No adverse events occurred in the study, and abstinence was not reported.
3.1.7. Psychostimulants
Four trials of psychostimulant medications were reviewed in three previous SRs; (Castells et al., 2016; Castells et al., 2009; Indave et al., 2016). Mazindol was studied in two unclear-ROB RCTs (Margolin et al., 1995a; Margolin et al., 1997), dexamphetamine in one unclear-ROB RCT (Grabowski et al., 2004), and methylphenidate in one high-ROB RCT (Dursteler-MacFarland et al., 2013). We found no effect of psychostimulants on retention when the four studies were pooled (N=210; RR=0.98, 95% CI 0.71 to 1.36; Figure 3), although the findings were mixed across studies and statistical heterogeneity was on the margin of significance (P=0.05, I2=62%; Figure 3). Cocaine-free urinalyses occurred more frequently with psychostimulants than placebo, but the difference was not statistically significant in a pooled analysis that combined cocaine use data from the studies of mazindol and dexamphetamine (N=115, standardized mean difference [SMD]= 0.35, 95% CI −0.05 to 0.74; Figure 2) using data reported in a previous SR (Castells et al., 2009). None of the studies reported abstinence.
Figure 2.
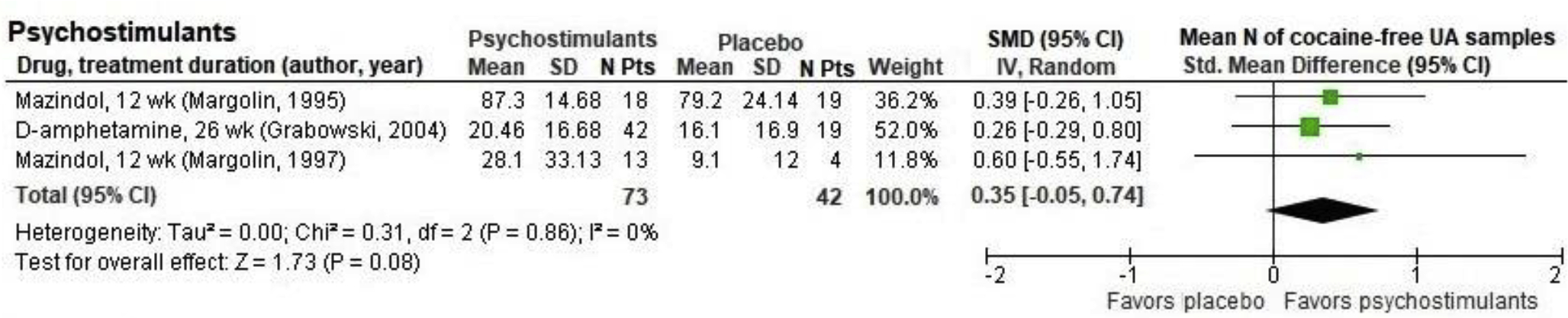
Cocaine-free urinalysis outcomes in randomized placebo-controlled trials of psychostimulants in participants with dual cocaine/opioid use disorders
3.1.8. Other medications (carvedilol, magnesium L-aspartate hydrochloride, mecamylamine, progesterone)
Four studies examined various other medications for treatment of cocaine use disorder in comorbid OUD participants (Table 1). A study of carvedilol (25 mg, 50 mg, placebo) in 106 methadone-maintained participants did not assess abstinence but found differences in cocaine use based on dosing; the lower (25 mg) dosage arm had lower rates of positive UDS compared to placebo, while the higher (50 mg) dosage arm had higher rates of positive UDS (25 mg 51% versus 50 mg 75% versus placebo 59%, P=0.03). There were no statistically significant differences in retention (76% versus 66% versus 56%, P=0.21) (Sofuoglu et al., 2017). A study of magnesium L-aspartate versus placebo found no differences in cocaine use (74.5% versus 75.5%, P=NS) or treatment retention (10.9 versus 8.9 weeks in treatment, P>0.5) (Margolin et al., 2003). A small study (N=35) of mecamylamine patches versus placebo in OUD participants on either methadone or levomethadyl acetate (LAAM) therapy found no differences in cocaine use reduction or treatment retention (13 of 17 retained versus 17 of 18, P=0.13) (Reid et al., 2005). Another study randomized 45 OUD participants on methadone maintenance therapy to progesterone (up to 600 mg) or placebo and found no differences in treatment retention (73% versus 93%, P=0.12); no data were available for abstinence or use outcomes (Sofuoglu et al., 2007). As a whole these were small trials that provide insufficient-strength evidence for use of these medications to treat cocaine use in people with OUD.
3.2. Medications for Methamphetamine Use Disorder in Participants with Opioid Use Disorder
We identified one RCT that examined a naltrexone implant (not available in the U.S.) for methamphetamine/amphetamine use disorder in participants with co-occurring OUD (Tiihonen et al., 2012). This was a multi-site trial conducted in Russia of 100 participants with co-occurring amphetamine and opioid dependence randomized to naltrexone implant (1000 mg) or placebo for 10 weeks. The treatment group had a greater percentage of negative UDS than placebo, but this difference was not statistically significant (40% versus 24%, P=0.09). Treatment participants had increased retention versus placebo (52% versus 28%, P=0.01) (Tiihonen et al., 2012). This study had high ROB due to changes to the protocol after study initiation and provides insufficient-strength evidence for naltrexone implant treatment of methamphetamine use disorder in people with existing OUD.
3.3. Publication Bias
There were too few trials for any medication to conduct quantitative estimates of publication bias. However, we felt there was low likelihood of publication bias because: 1) the body of evidence is largely negative - we did not find a disproportionate number of positive studies, 2) most of the published studies were not industry sponsored, and 3) we searched clinicaltrials.gov and did not find additional studies that should have been reported (Guyatt et al., 2011).
4. DISCUSSION
Against a backdrop of increasing co-use of opioids and stimulants and their associated health consequences (Winkelman et al., 2018), this review summarizes 35 RCTs examining multiple classes of medications used for treatment of cocaine and methamphetamine use disorders in people with OUD. Unfortunately, we found no strong evidence that any drug class was effective in increasing abstinence, reducing use, or improving retention rates for stimulant use disorders. We found antidepressants had no effect for cocaine, but there was moderate-strength evidence that they worsened study retention and increased harms (Chan et al., 2019b). While we found low-strength evidence that psychostimulants might reduce cocaine use, these were small trials that may not be replicable. We did find low-strength evidence that methadone may increase cocaine treatment retention over other MOUDs such as buprenorphine; however, it is unclear the degree to which any potential benefit from opioid agonist therapy relates to the medications themselves, the dosages used, or the effect of participation in highly structured opioid treatment programs with frequent behavioral therapy. We also found conflicting evidence for disulfiram reducing cocaine use, but evidence of worsened treatment retention. All other medication classes had insufficient evidence to draw conclusions. We found only one trial of treatment of methamphetamine use disorder in people with OUD.
This review is the first to summarize evidence for medications trialed for stimulant use disorders in patients with OUD. Our review highlights several implications. First, our review found almost no evidence regarding treatment of methamphetamine use disorder in people with OUD. Given that co-use of methamphetamine and opioids is increasing in prevalence (Al-Tayyib et al., 2017; Winkelman et al., 2018), additional research specific to methamphetamine use in people with OUD is urgently needed. While we identified additional trials of medications for methamphetamine use disorder, including a recent trial of mirtazapine that showed reduced methamphetamine use (Coffin et al., 2019), it was unclear whether participants with OUD were included. Second, while we found trials of medications for cocaine use disorder, there were few trials in each class, mainly small studies of varying quality, had high risk of bias, and lacked power to detect differences--larger multi-site trials would strengthen the evidence base.
4.1. Limitations
Through the review process we noted a wide variety of definitions of abstinence. For the purpose of this review we narrowly defined abstinence as at least three weeks of negative UDS in order to compare efficacy across trials—this meant excluding findings from studies that reported other measures of abstinence, decreasing the size of our review. Substance use disorder researchers should seek to standardize outcome definitions in future studies to enable meta-analyses of results. Similarly, the included studies had varied treatment duration, which may have affected treatment retention estimates.
5. CONCLUSIONS
We found little evidence that any drug class was effective in increasing abstinence, reducing use, or improving treatment retention for those with cocaine or methamphetamine use disorder and co-occurring OUD. Antidepressants and disulfiram may worsen treatment retention outcomes when used for treatment of cocaine use disorders in participants with comorbid OUD. Almost no evidence exists of medications to treat methamphetamine use disorder in people with OUD--additional trials would advance the field.
Supplementary Material
Table 2.
Summary of the evidence on pharmacotherapies in participants with comorbid stimulant and opioid use disorders
| Outcome | N studies per outcome | Summary of findings by outcome | Strength of Evidence* |
|---|---|---|---|
| Antidepressants (bupropion, desipramine, and fluoxetine) | |||
| Abstinence for ≥3 consecutive weeks | NA | NA | No evidence |
| Use | 1 RCT of bupropion (Poling et al., 2006) | No difference. One unclear-ROB RCT (N=106) reported no difference in use of cocaine. | Insufficient |
| Retention | 10 RCTs: 6 of desipramine (Arndt et al., 1992; Kolar et al., 1992; Kosten et al., 2003; Kosten et al., 1992; O’Brien et al., 1988; Oliveto et al., 1999); 2 of bupropion (Margolin et al., 1995b; Poling et al., 2006); 2 of fluoxetine (Grabowski et al., 1995; Winstanley et al., 2011) |
Favors placebo. A previous SR (Pani et al., 2011) found RR for dropout 1.22 (95% CI 1.05 to 1.41) combining 10 RCTs (N=1,006). | Moderate |
| Harms | 5 RCTs: 3 of desipramine (Arndt et al., 1992; Kolar et al., 1992; Kosten et al., 1992); 1 of bupropion (Margolin et al., 1995b); 1 of fluoxetine (Winstanley et al., 2011) |
Favors placebo. A previous SR (Pani et al., 2011) reported a combined RR of withdrawal due to an adverse event RR of 2.47 (95% CI 1.03 to 5.90; 5 RCTs, N=492). Severe AEs NR. |
Moderate |
| Anticonvulsants (gabapentin, tiagabine, and topiramate) | |||
| Abstinence for ≥3 consecutive weeks | 1 RCT of topiramate (Umbricht et al., 2014) | No difference. 1 low-ROB RCT (N=171) with 4 arms for topiramate vs placebo, +/− contingency management: longest duration of cocaine abstinence (mean weeks ± SE): 3.8 + 0.8 for TOP/CM, 3.7 ± 0.7 for TOP/Non-CM, 4.4 ± 0.7 for P/CM, for 3.5 ± 0.6 P/Non-CM. | Low |
| Use | 1 RCT of topiramate (Umbricht et al., 2014) | No difference. 1 low-ROB RCT (N=171) % of UA that were cocaine-negative: OR: 1.051, 95% CI: 0.6 to 1.84; p = 0.86, | Low |
| Retention | 3 RCTs of 4 medications: 2 of tiagabine (Gonzalez et al., 2007; Gonzalez et al., 2003); 1 of gabapentin (Gonzalez et al., 2007); 1 of topiramate (Umbricht et al., 2014) |
Favors placebo. Worse retention with anticonvulsants in 3 trials (N=292), pooled RR 0.86 (0.76 to 0.97). | Moderate |
| Harms | 3 RCTs (Gonzalez et al., 2007; Gonzalez et al., 2003; Umbricht et al., 2014) |
No difference. Severe AEs: None occurred in 2 RCTs. NR in 1 RCT. Dropouts due to AEs: None occurred in 2 RCTs; 6 vs 7 in 1 trial of topiramate. |
Low |
| Antipsychotics (aripiprazole and risperidone) | |||
| Abstinence for ≥3 consecutive weeks | NA | NA | No evidence |
| Use | 1 RCT of aripiprazole (Moran et al., 2017) | No difference. A high-ROB RCT of aripiprazole (N=18) found no differences in cocaine use. | Insufficient |
| Lapse and relapse | No difference. A high-ROB RCT of aripiprazole (N=18) found no differences in cocaine lapse or relapse. | Insufficient | |
| Insufficient | |||
| Retention | 1 RCT of risperidone (Grabowski et al., 2004) 1 RCT of aripiprazole (Moran et al., 2017) |
No difference. A high-ROB RCT of aripiprazole (N=18) and an unclear-ROB RCT of risperidone (N=96) found no difference in retention between groups. | Insufficient |
| Harms | 1 RCT of aripiprazole (Moran et al., 2017) |
No difference. A high-ROB RCT of aripiprazole found no difference in withdrawal due to AEs between groups. Severe AEs: NR |
Insufficient |
| Dopamine agonists | |||
| Abstinence for ≥3 consecutive weeks | NA | NA | No evidence |
| Use | 4 RCTs: 3 of amantadine (Handelsman et al., 1995; Kolar et al., 1992; Kosten et al., 1992) and 1 of bromocriptine (Handelsman et al., 1997) | No difference. There were no differences in cocaine use in 3 studies of amantadine (2 unclear-ROB and 1 high-ROB, or in 1 study of bromocriptine (unclear-ROB). | Low |
| Retention | 4 RCTs: 3 of amantadine (Handelsman et al., 1995; Kolar et al., 1992; Kosten et al., 1992) and 1 of bromocriptine (Handelsman et al., 1997) | No difference. Meta-analysis combining 3 studies of amantadine and 1 study of bromocriptine found no difference, without significant heterogeneity: RR 0.95, 95% CI 0.84 to 1.07 (N=205). | Low |
| Harms | 4 RCTs: 3 of amantadine (Handelsman et al., 1995; Kolar et al., 1992; Kosten et al., 1992) and 1 of bromocriptine (Handelsman et al., 1997) |
No difference. Withdrawal due to AEs: no difference from placebo in 1 study of bromocriptine and 2 studies of amantadine. Severe AEs: None occurred in 1 study of bromocriptine and 1 study of amantadine. |
Low |
| Medications for Opioid Use Disorder (methadone, buprenorphine, and buprenorphine combined with naloxone) | |||
| Abstinence for ≥3 consecutive weeks | 2 RCTs of methadone vs buprenorphine (Schottenfeld et al., 2005; Schottenfeld et al., 1997) | Methadone vs buprenorphine: Favors methadone. Continuous abstinence more frequent with methadone, combining 2 low-ROB RCTs (N=219): RR 1.85, 95% CI 1.25 to 2.75. | Low |
| 1 RCT of buprenorphine- naloxone vs placebo (Ling et al., 2016) |
Buprenorphine-naloxone vs placebo: No difference. 1 low-ROB RCT (N=302). No significant difference in abstinence (days 25–54). |
||
| Use | 1 RCT of buprenorphine- naloxone vs placebo (Ling et al., 2016) | Favors buprenorphine-naloxone: in a low-ROB RCT (N=302), more UDS(−) at higher dose (16–4 mg buprenorphine-naloxone) OR 1.71, P=0.022. No difference at lower dose (4–1 mg buprenorphine-naloxone) OR 1.09, P=0.105. | Insufficient |
| 1 RCT of naltrexone implant (Tiihonen et al., 2012) |
Favors naltrexone in 1 high-ROB RCT; % of drug free UDS (−): 38% vs 16%; P=0.01 Amphetamine: Week 10 UDS (−): 40% vs 24%; P=0.09 Heroin: Week 10 UDS (−): 52% vs 20%, P = 0.001 |
Insufficient | |
| Retention | 3 RCTs of methadone vs buprenorphine (Oliveto et al., 1999; Schottenfeld et al., 2005; Schottenfeld et al., 1997) |
Methadone vs buprenorphine: No difference. 2 low-ROB and 1 unclear-ROB RCTs (N=309), pooled RR 1.17 (95% CI 0.91 to 1.51). |
Low |
| 1 RCT of buprenorphine- naloxone vs placebo (Ling et al., 2016) |
Buprenorphine-naloxone vs placebo: No difference. 1 low-ROB RCT (N=302), high dose vs low dose vs placebo: 100% vs 98% vs 99%. |
Insufficient | |
| 1 RCT of naltrexone implant (Tiihonen et al., 2012) |
Favors naltrexone in 1 high-ROB RCT: 26/50 (52%) vs 14/50 (28%); RR=1.86 (1.11–3.12) |
Insufficient | |
| Harms | 2 RCTs (Oliveto et al., 1999; Schottenfeld et al., 2005) |
Methadone vs buprenorphine: No difference. Dropouts due to AE: NR Severe AEs: 1 |
Insufficient |
| 1 RCT of naltrexone implant (Tiihonen et al., 2012) | Naltrexone implant vs placebo: No difference. | Insufficient | |
| Medications FDA-approved for other substance use disorders (disulfiram, varenicline) | |||
| Abstinence for ≥3 consecutive weeks | 2 RCTs of disulfiram (George et al., 2000; Schottenfeld et al., 2014) | No difference. Pooled RR 0.89 (95% CI 0.53 to 1.48) based on 1 low-ROB RCT and 1 unclear- ROB RCT(N=207) | Low |
| Use | 1 RCT of varenicline (Poling et al., 2010) | No difference. 1 unclear-ROB RCT (N=31) found no difference in slope over time or between groups (Z = 0.20, p < 0.84). | Insufficient |
| Retention | 6 RCTs of disulfiram (Carroll et al., 2012; George et al., 2000; Kosten et al., 2013; Oliveto et al., 2011; Petrakis et al., 2000; Schottenfeld et al., 2014) | Favors placebo. Significantly worse treatment retention with disulfiram compared to placebo, 6 studies combined (N=605). RR 0.86, 95% CI 0.77 to 0.95. | Moderate |
| 1 RCT of varenicline (Poling et al., 2010) | No difference. 1 unclear-ROB RCT (N=31) found no difference (log rank χ2 = 1.3,p < 0.26) | ||
| Harms | 6 RCTs of disulfiram (Carroll et al., 2012; George et al., 2000; Kosten et al., 2013; Oliveto et al., 2011; Petrakis et al., 2000; Schottenfeld et al., 2014) 1 RCT of varenicline (Poling et al., 2010) |
No difference. | Low |
| Psychostimulants (dexamphetamine, mazindol, and methylphenidate) | |||
| Abstinence for ≥3 consecutive weeks | NA | NA | No evidence |
| Use | 3 RCTs: 2 of mazindol (Margolin et al., 1995a; Margolin et al., 1997) 1 of dexamphetamine (Grabowski et al., 2004) |
No difference. Cocaine-free UDSs occurred more frequently with psychostimulants compared with placebo in 3 Unclear/high ROB RCTs, but the difference did not reach statistical significance (standardized mean difference (SMD) 0.35, 95% CI −0.05 to 0.74; 3 RCTs, N=115). | Low |
| Retention | 4 RCTs: 2 of mazindol (Margolin et al., 1995a; Margolin et al., 1997); 1 of dexamphetamine (Grabowski et al., 2004); 1 of methylphenidate (Dursteler-MacFarland et al., 2013) |
No difference. (RR 0.98, 95% CI 0.71 to 1.36; N=210), although statistical heterogeneity was on the margin of significance (P=0.05; Figure 3). | Low |
| Harms | NA | NA | No evidence |
| Medications for amphetamine/methamphetamine use disorder | |||
| Abstinence for ≥3 consecutive weeks | NA | NA | Insufficient |
| Use | 1 RCT of naltrexone implant (Tiihonen et al., 2012) |
Favors naltrexone in 1 high-ROB RCT; % of drug free UDS (−): 38% vs 16%; P=0.01 Amphetamine: Week 10 UDS (−): 40% vs 24%; P=0.09 Heroin: Week 10 UDS (−): 52% vs 20%, P = 0.001 |
Insufficient |
| Retention | 1 RCT of naltrexone implant (Tiihonen et al., 2012) |
Favors naltrexone in 1 high-ROB RCT: 26/50 (52%) vs 14/50 (28%); RR=1.86 (1.11–3.12) |
Insufficient |
| Harms | 1 RCT of naltrexone implant (Tiihonen et al., 2012) | No difference. | Insufficient |
Abbreviations: AE = adverse event; N = number of; NA = not applicable; P = p-value; RCT = randomized controlled trial; ROB = risk of bias; UDS = urinary drug screen; RR = risk ratio; SMD = standard mean difference; SR = systematic review
Highlights.
Stimulant use has increased among people with opioid use disorder
Clinical trials have studied 21 medications for cocaine use in people with OUD
Only one medication has been studied for methamphetamine use in people with OUD
No medication had clear benefits; antidepressants and disulfiram had worse retention
Psychostimulant trials showed potential benefits for cocaine use in this population
Acknowledgments:
The findings and conclusions in this document are those of the authors who are responsible for its contents; the findings and conclusions do not necessarily represent the views of the Department of Veterans Affairs or the United States government.
Funding: This research was funded by the Department of Veterans Affairs, Veterans Health Administration, Office of Research and Development, Quality Enhancement Research Initiative. Dr. Chan’s time was supported by AHRQ PCOR K12 (K12HS022981). Dr. Korthuis’ time was supported by the National Institutes of Health, National Institute on Drug Abuse (UG1DA015815, UG3DA044831).
Role of the Funding Source: Nothing declared
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest: No conflict declared
Systematic Review Registration: PROSPERO: CRD42018085667
REFERENCES
- Review Manager (RevMan) [Computer program]. Version 5.3. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2014. [Google Scholar]
- Al-Tayyib A, Koester S, Langegger S, Raville L, 2017. Heroin and Methamphetamine Injection: An Emerging Drug Use Pattern. Subst Use Misuse 52(8), 1051–1058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arndt IO, Dorozynsky L, Woody GE, McLellan AT, O’Brien CP, 1992. Desipramine treatment of cocaine dependence in methadone-maintained patients. Archives of general psychiatry 49(11), 888–893. [DOI] [PubMed] [Google Scholar]
- Berkman N, Lohr K, Ansari M, McDonagh M, Balk E, Whitlock E, Reston J, Bass E, Butler M, Gartlehner G, Hartling L, Kane R, McPheeters M, Morgan L, Morton S, Viswanathan M, Sista P, Chang S, 2013. Grading the Strength of a Body of Evidence When Assessing Health Care Interventions for the Effective Health Care Program of the Agency for Healthcare Research and Quality: An Update. Agency for Healthcare Research and Quality; Methods Guide for Comparative Effectiveness Reviews (AHRQ Publication No. 13(14)-EHC130-EF; ), Rockville, MD, pp. http://www.effectivehealthcare.ahrq.gov/ehc/products/457/1752/methods-guidance-grading-evidence-131118.pdf. [PubMed] [Google Scholar]
- Carroll KM, Nich C, Shi JM, Eagan D, Ball SA, 2012. Efficacy of disulfiram and Twelve Step Facilitation in cocaine-dependent individuals maintained on methadone: a randomized placebo-controlled trial. Drug and alcohol dependence 126(1–2), 224–231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castells X, Cunill R, Perez-Mana C, Vidal X, Capella D, 2016. Psychostimulant drugs for cocaine dependence. The Cochrane database of systematic reviews 9, CD007380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castells X, Kosten TR, Capella D, Vidal X, Colom J, Casas M, 2009. Efficacy of opiate maintenance therapy and adjunctive interventions for opioid dependence with comorbid cocaine use disorders: A systematic review and meta-analysis of controlled clinical trials. The American journal of drug and alcohol abuse 35(5), 339–349. [DOI] [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention, 2019. Annual Surveillance Report of Drug-Related Risks and Outcomes — United States Surveillance Special Report. Centers for Disease Control and Prevention, U.S. Department of Health and Human Services. Published November 1, 2019. Accessed July 6, 2020, from https://www.cdc.gov/drugoverdose/pdf/pubs/2019-cdc-drug-surveillance-report.pdf. [Google Scholar]
- Chan B, Freeman M, Kondo K, Ayers C, Montgomery J, Paynter R, Kansagara D, 2019a. Pharmacotherapy for methamphetamine/amphetamine use disorder-a systematic review and meta-analysis. Addiction 114(12), 2122–2136. [DOI] [PubMed] [Google Scholar]
- Chan B, Kondo K, Ayers C, Freeman M, Montgomery J, Paynter R, Kansagara D, 2018. Pharmacotherapy for Stimulant Use Disorders: A Systematic Review of the Evidence. VA ESP Project #05–225. [PubMed] [Google Scholar]
- Chan B, Kondo K, Freeman M, Ayers C, Montgomery J, Kansagara D, 2019b. Pharmacotherapy for Cocaine Use Disorder-a Systematic Review and Meta-analysis. J Gen Intern Med. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coffin PO, Santos GM, Hern J, Vittinghoff E, Walker JE, Matheson T, Santos D, Colfax G, Batki SL, 2019. Effects of Mirtazapine for Methamphetamine Use Disorder Among Cisgender Men and Transgender Women Who Have Sex With Men: A Placebo-Controlled Randomized Clinical Trial. JAMA Psychiatry 77(3), 246–255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DerSimonian R, Laird N, 1986. Meta-analysis in clinical trials. Control Clin Trials 7(3), 177–188. [DOI] [PubMed] [Google Scholar]
- Dursteler-MacFarland KM, Farronato NS, Strasser J, Boss J, Kuntze MF, Petitjean SA, Burki C, Wiesbeck GA, 2013. A randomized, controlled, pilot trial of methylphenidate and cognitive-behavioral group therapy for cocaine dependence in heroin prescription. Journal of clinical psychopharmacology 33(1), 104–108. [DOI] [PubMed] [Google Scholar]
- Ellis MS, Kasper ZA, Cicero TJ, 2018. Twin epidemics: The surging rise of methamphetamine use in chronic opioid users. Drug Alcohol Depend 193, 14–20. [DOI] [PubMed] [Google Scholar]
- George TP, Chawarski MC, Pakes J, Carroll KM, Kosten TR, Schottenfeld RS, 2000. Disulfiram versus placebo for cocaine dependence in buprenorphine-maintained subjects: a preliminary trial. Biological psychiatry 47(12), 1080–1086. [DOI] [PubMed] [Google Scholar]
- Grabowski J, Rhoades H, Stotts A, Cowan K, Kopecky C, Dougherty A, Moeller FG, Hassan S, Schmitz J, 2004. Agonist-like or antagonist-like treatment for cocaine dependence with methadone for heroin dependence: two double-blind randomized clinical trials. Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology 29(5), 969–981. [DOI] [PubMed] [Google Scholar]
- Guyatt GH, Oxman AD, Montori V, Vist G, Kunz R, Brozek J, Alonso-Coello P, Djulbegovic B, Atkins D, Falck-Ytter Y, Williams JW Jr., Meerpohl J, Norris SL, Akl EA, Schunemann HJ, 2011. GRADE guidelines: 5. Rating the quality of evidence--publication bias. J Clin Epidemiol 64(12), 1277–1282. [DOI] [PubMed] [Google Scholar]
- Han B, Compton WM, Blanco C, Crane E, Lee J, Jones CM, 2017. Prescription Opioid Use, Misuse, and Use Disorders in U.S. Adults: 2015 National Survey on Drug Use and Health. Ann Intern Med 167(5), 293–301. [DOI] [PubMed] [Google Scholar]
- Higgins J, Green S, 2011. Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0. 2019-05-17. URL:http://handbook.cochrane.org/. Accessed: 2019-05-17. (Archived by WebCite® at http://www.webcitation.org/78Rgk6X9K). http://handbook.cochrane.org/. (Accessed March 24 2017).
- Higgins JP, Thompson SG, 2002. Quantifying heterogeneity in a meta-analysis. Stat Med 21(11), 1539–1558. [DOI] [PubMed] [Google Scholar]
- Higgins JP, Thompson SG, Deeks JJ, Altman DG, 2003. Measuring inconsistency in meta-analyses. Bmj 327(7414), 557–560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Indave BI, Minozzi S, Pani PP, Amato L, 2016. Antipsychotic medications for cocaine dependence. The Cochrane database of systematic reviews 3, CD006306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolar AF, Brown BS, Weddington WW, Haertzen CC, Michaelson BS, Jaffe JH, 1992. Treatment of cocaine dependence in methadone maintenance clients: a pilot study comparing the efficacy of desipramine and amantadine. The International journal of the addictions 27(7), 849–868. [DOI] [PubMed] [Google Scholar]
- Kosten T, Oliveto A, Feingold A, Poling J, Sevarino K, McCance-Katz E, Stine S, Gonzalez G, Gonsai K, 2003. Desipramine and contingency management for cocaine and opiate dependence in buprenorphine maintained patients. Drug and alcohol dependence 70(3), 315–325. [DOI] [PubMed] [Google Scholar]
- Kosten TR, Morgan CM, Falcione J, Schottenfeld RS, 1992. Pharmacotherapy for cocaine-abusing methadone-maintained patients using amantadine or desipramine. Archives of general psychiatry 49(11), 894–898. [DOI] [PubMed] [Google Scholar]
- Kosten TR, Wu G, Huang W, Harding MJ, Hamon SC, Lappalainen J, Nielsen DA, 2013. Pharmacogenetic randomized trial for cocaine abuse: disulfiram and dopamine beta-hydroxylase. Biological psychiatry 73(3), 219–224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ling W, Hillhouse MP, Saxon AJ, Mooney LJ, Thomas CM, Ang A, Matthews AG, Hasson A, Annon J, Sparenborg S, Liu DS, McCormack J, Church S, Swafford W, Drexler K, Schuman C, Ross S, Wiest K, Korthuis PT, Lawson W, Brigham GS, Knox PC, Dawes M, Rotrosen J, 2016. Buprenorphine + naloxone plus naltrexone for the treatment of cocaine dependence: the Cocaine Use Reduction with Buprenorphine (CURB) study. Addiction (Abingdon, England) 111(8), 1416–1427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Margolin A, Avants SK, Kosten TR, 1995a. Mazindol for relapse prevention to cocaine abuse in methadone-maintained patients. The American journal of drug and alcohol abuse 21(4), 469–481. [DOI] [PubMed] [Google Scholar]
- Margolin A, Avants SK, Malison RT, Kosten TR, 1997. High- and low-dose mazindol for cocaine dependence in methadone- maintained patients: A preliminary evaluation. Substance Abuse: Substance Abuse 18(3), 125. [Google Scholar]
- Margolin A, Kantak K, Copenhaver M, Avants SK, 2003. A preliminary, controlled investigation of magnesium L-aspartate hydrochloride for illicit cocaine and opiate use in methadone-maintained patients. Journal of addictive diseases 22(2), 49–61. [DOI] [PubMed] [Google Scholar]
- Margolin A, Kosten TR, Avants SK, Wilkins J, Ling W, Beckson M, Arndt IO, Cornish J, Ascher JA, Li SH, 1995b. A multicenter trial of bupropion for cocaine dependence in methadone-maintained patients. Drug and alcohol dependence 40(2), 125–131. [DOI] [PubMed] [Google Scholar]
- Minozzi S, Amato L, Pani PP, Solimini R, Vecchi S, De Crescenzo F, Zuccaro P, Davoli M, 2015a. Dopamine agonists for the treatment of cocaine dependence. The Cochrane database of systematic reviews(5), CD003352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minozzi S, Cinquini M, Amato L, Davoli M, Farrell MF, Pani PP, Vecchi S, 2015b. Anticonvulsants for cocaine dependence. The Cochrane database of systematic reviews(4), CD006754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moher D, Liberati A, Tetzlaff J, Altman DG, The PRISMA Group (2009), Preferred Reporting Items for Systematic Reviews and Meta-Analyses: The PRISMA Statement. PLoS Med 6(6): e1000097. doi: 10.1371/journal.pmed1000097. 2019-05-17. URL:http://www.prisma-statement.org. Accessed: 2019-05-17. (Archived by WebCite® at http://www.webcitation.org/78ReC3Ln8). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moran LM, Phillips KA, Kowalczyk WJ, Ghitza UE, Agage DA, Epstein DH, Preston KL, 2017. Aripiprazole for cocaine abstinence: a randomized-controlled trial with ecological momentary assessment. Behavioural pharmacology 28(1), 63–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NIDA, 2020, June 2. Addiction Medications. Retrieved from https://www.drugabuse.gov/publications/principles-adolescent-substance-use-disorder-treatment-research-based-guide/evidence-based-approaches-to-treating-adolescent-substance-use-disorders/addiction-medications on 2020, July 6.
- O’Brien CP, Childress AR, Arndt IO, McLellan AT, Woody GE, Maany I, 1988. Pharmacological and behavioral treatments of cocaine dependence: controlled studies. The Journal of clinical psychiatry 49 Suppl, 17–22. [PubMed] [Google Scholar]
- Oliveto A, Poling J, Mancino MJ, Feldman Z, Cubells JF, Pruzinsky R, Gonsai K, Cargile C, Sofuoglu M, Chopra MP, Gonzalez-Haddad G, Carroll KM, Kosten TR, 2011. Randomized, double blind, placebo-controlled trial of disulfiram for the treatment of cocaine dependence in methadone-stabilized patients. Drug and alcohol dependence 113(2–3), 184–191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oliveto AH, Feingold A, Schottenfeld R, Jatlow P, Kosten TR, 1999. Desipramine in opioid-dependent cocaine abusers maintained on buprenorphine vs methadone. Archives of general psychiatry 56(9), 812–820. [DOI] [PubMed] [Google Scholar]
- Pani PP, Trogu E, Vecchi S, Amato L, 2011. Antidepressants for cocaine dependence and problematic cocaine use. The Cochrane database of systematic reviews(12), CD002950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petrakis IL, Carroll KM, Nich C, Gordon LT, McCance-Katz EF, Frankforter T, Rounsaville BJ, 2000. Disulfiram treatment for cocaine dependence in methadone-maintained opioid addicts. Addiction (Abingdon, England) 95(2), 219–228. [DOI] [PubMed] [Google Scholar]
- Poling J, Oliveto A, Petry N, Sofuoglu M, Gonsai K, Gonzalez G, Martell B, Kosten TR, 2006. Six-month trial of bupropion with contingency management for cocaine dependence in a methadone-maintained population. Archives of general psychiatry 63(2), 219–228. [DOI] [PubMed] [Google Scholar]
- Poling J, Rounsaville B, Gonsai K, Severino K, Sofuoglu M, 2010. The safety and efficacy of varenicline in cocaine using smokers maintained on methadone: a pilot study. The American journal on addictions 19(5), 401–408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reid MS, Angrist B, Baker SA, O’Leary S, Stone J, Schwartz M, Leiderman D, Montgomery A, Elkashef A, Majewska D, Robinson J, Rotrosen J, 2005. A placebo controlled, double-blind study of mecamylamine treatment for cocaine dependence in patients enrolled in an opiate replacement program. Substance abuse 26(2), 5–14. [DOI] [PubMed] [Google Scholar]
- Schottenfeld RS, Chawarski MC, Cubells JF, George TP, Lappalainen J, Kosten TR, 2014. Randomized clinical trial of disulfiram for cocaine dependence or abuse during buprenorphine treatment. Drug and alcohol dependence 136, 36–42. [DOI] [PubMed] [Google Scholar]
- Schottenfeld RS, Chawarski MC, Pakes JR, Pantalon MV, Carroll KM, Kosten TR, 2005. Methadone versus buprenorphine with contingency management or performance feedback for cocaine and opioid dependence. The American journal of psychiatry 162(2), 340–349. [DOI] [PubMed] [Google Scholar]
- Schottenfeld RS, Pakes JR, Oliveto A, Ziedonis D, Kosten TR, 1997. Buprenorphine vs methadone maintenance treatment for concurrent opioid dependence and cocaine abuse. Archives of general psychiatry 54(8), 713–720. [DOI] [PubMed] [Google Scholar]
- Seth P, Scholl L, Rudd RA, Bacon S, 2018. Overdose Deaths Involving Opioids, Cocaine, and Psychostimulants — United States, 2015–2016. MMWR Morb Mortal Wkly Rep 67, 349–358. https://www.cdc.gov/mmwr/volumes/367/wr/mm6712a6711.htm?s_cid=mm6712a6711_w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sofuoglu M, Poling J, Babuscio T, Gonsai K, Severino K, Nich C, Carroll KM, 2017. Carvedilol does not reduce cocaine use in methadone-maintained cocaine users. Journal of substance abuse treatment 73, 63–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sofuoglu M, Poling J, Gonzalez G, Gonsai K, Oliveto A, Kosten TR, 2007. Progesterone effects on cocaine use in male cocaine users maintained on methadone: a randomized, double-blind, pilot study. Experimental and clinical psychopharmacology 15(5), 453–460. [DOI] [PubMed] [Google Scholar]
- Substance Abuse and Mental Health Services Administration (SAMHSA), 2018. Medications for Opioid Use Disorder - For Healthcare and Addiction Professionals, Policymakers, Patients, and Families. Treatment Improvement Protocol TIP 63. HHS Publication No. (SMA) 18–5063FULLDOC. US Department of Health and Human Services, Substance Abuse and Mental Health Services Administration, Center for Substance Abuse Treatment.
- Tiihonen J, Krupitsky E, Verbitskaya E, Blokhina E, Mamontova O, Fohr J, Tuomola P, Kuoppasalmi K, Kiviniemi V, Zwartau E, 2012. Naltrexone implant for the treatment of polydrug dependence: a randomized controlled trial. The American journal of psychiatry 169(5), 531–536. [DOI] [PubMed] [Google Scholar]
- Winkelman TNA, Admon LK, Jennings L, Shippee ND, Richardson CR, Bart G, 2018. Evaluation of Amphetamine-Related Hospitalizations and Associated Clinical Outcomes and Costs in the United States. JAMA network open 1(6), e183758. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


