Abstract
Background:
Hypertension is an established risk factor for subsequent cardiovascular and renal disease in children as well as adults. Sickle Cell Disease (SCD) is a genetic disorder associated with chronic hemolytic anemia with the major manifestation of vaso-occlusive crises. While this disease entity involves most organ systems causing vascular and pulmonary injury, little is known about blood pressure (BP) levels or prevalence of hypertension in children with SCD.
Methods:
A cross-sectional study was conducted on 56 children with SCD (54 with Hemoglobin SS disease; 2 with Hemoglobin Sβ0 thalassemia; 29 females). Study participants underwent 24-hour ambulatory BP monitoring (ABPM). Serum creatinine and cystatin C were obtained to assess estimated glomerular filtration rate (eGFR) with age-based formulas. A random urine sample was obtained to estimate urine osmolality and urine albumin to creatinine ratio.
Results:
Mean age range was 11.9(±4.5) years. Seventeen participants (30%) met criteria for hypertension based on ABPM. Of the 17 participants classified with hypertension, three had office hypertension with ambulatory hypertension; and 14 had masked hypertension detected on ABPM. Another 28 participants (50%) had some abnormal ABPM parameters in the form of either pre-hypertension and/ or lack of normal nocturnal dipping status.
Conclusion:
The prevalence of confirmed hypertension, largely manifest by masked hypertension, is high in children, as young as 6 years of age with SCD. Early identification of hypertension in SCD children can confer benefit as it is an important modifiable risk factor for progression of cardiovascular and renal disease.
Table of Contents Summary:
Early identification of hypertension, commonly seen in pediatric sickle cell disease, is important as a modifiable risk factor for progression of cardiovascular and renal disease.
Introduction:
Sickle cell disease is a chronic, debilitating, hereditary blood disorder with an occurrence in 1 in 500 African Americans. SCD results from a point mutation in the β-globin gene, resulting in abnormal expression of hemoglobin S (Hb S).1 In the presence of deoxygenation Hb S polymerizes within red blood cells, leading to erythrocyte rigidity, and membrane damage.2 Homozygous Hb SS (α2βS2), with two copies of abnormal β-globin chain, is associated with anemia, repeated ischemia-reperfusion injury, and inflammation affecting ultimately all organ systems leading to acute chest syndrome, stroke, widespread vasculopathy and kidney disease.3,4 Overall, SCD is a chronic condition that is vulnerable to vascular injury.
Reports on the prevalence of hypertension in SCD have been conflicting. Despite intermittent episodes of pulmonary hypertension, early reports described low systemic BP and a low prevalence of systemic hypertension in individuals with SCD compared to healthy controls.5–8 These reports were largely based on BP recordings obtained during hospitalization or clinic visits in adults with SCD. A more recent report described a quite different observation on BP levels in adult SCD patients. Gordeuk et. al. examined BP levels as well as renal and cardiac status in 163 SCD adults, with a second evaluation two years following the baseline measurements in 86 patients. In the entire SCD cohort, 44% met criteria for prehypertension and 10% met criteria for hypertension.9 Moreover, SCD patients with prehypertension and hypertension were at greater risk for pulmonary hypertension and renal dysfunction. Children and adults with SCD and elevated BP are at increased risk for silent cerebral infracts, and stroke,10,11 thus highlighting the importance of early recognition and management of BP abnormalities in youth with SCD. ABPM is the most appropriate BP measurement method that eliminates white-coat effect and allows assessment of nocturnal BP.12 There are very limited studies that used 24-hour ABPM to examine BP levels and patterns in children with SCD. The purpose of this study is to describe the prevalence of abnormal BP patterns in a cohort of children with SCD. All SCD participants had Hb SS or Hb Sβ0 thalassemia, the most severe genotypes of SCD.
Patients and Methods:
Study setting and study design:
We used a cross-sectional study design to investigate early markers of nephropathy including BP assessment in pediatric SCD. At enrollment, participants were clinically stable with regular attendance at the Sickle Cell Center at Nemours/ A. I. duPont Hospital for Children, Wilmington, DE, Nemours Children’s Hospital, Orlando, FL and Nemours Children’s Clinics, Pensacola, FL.
Participants:
Participants were enrolled if they had hemoglobin electrophoresis-confirmed diagnosis of SCD (Hb SS or Hb S β0 thalassemia), and were between the ages of 5 and 20 years at the time of enrollment. History of bone marrow transplant was an exclusion criterion. The protocol was approved by the Institutional Review Board at Nemours for protection of subjects, and adhered to all standards set forth by the Declaration of Helsinki. The parents of each participant younger than 18 years of age provided written informed consent, and each child above the age of seven provided assent, prior to enrollment in the study. Written informed consents were obtained by participants 18 years of age or older.
Study procedures:
Information about prior history of SCD-related complications was collected– including episodes of vaso-occlusive pain crises, acute chest syndrome, stroke, splenic sequestration, and number of hospitalizations and emergency room visits. Current need for chronic blood transfusion, and hydroxyurea therapy were recorded by completing interviews with patients and families and by reviewing the patient’s electronic medical records. All evaluations were conducted in an outpatient setting in coordination with participant’s routine hematology clinic visits. Blood samples for this study were obtained at the same time as participant’s routine clinic blood investigations, in order to prevent additional phlebotomy.
Baseline characteristics obtained on each participant included age, gender, weight, height, BMI %tile, BMI-Z score and clinic BP. Clinic BP on each participant was obtained in the seated position using a BP cuff that was appropriate for arm size. BP measurements were obtained with an oscillometric device (GE Carescape V100). A random blood sample was used for hemoglobin and hematocrit. Serum creatinine and cystatin C were obtained to assess eGFR with age-based formulas. A spot urine sample was used to estimate urine sodium, potassium, osmolality, albumin, and creatinine.
24-hour ABPM:
To obtain in-depth BP data we obtained 24-hour ABPM studies in our SCD participants utilizing the Spacelabs 90217 or 90227 ambulatory monitors (Spacelabs Medical, Issaquah, WA, USA), monitors validated in pediatric patients. Participants underwent ABPM only if they were pain-free during the BP monitoring period, and were at least four weeks from their last pain crisis or acute chest syndrome or any other recent emergency room visit or hospitalization.
ABPM was performed by a trained nurse practitioner. An appropriate sized cuff was attached to the non-dominant arm, and the device was programmed to record BP every 30 minutes during sleep and every twenty minutes during awake hours. Participants were instructed to resume their normal activities while wearing the monitor, but requested to avoid strenuous activities including sports. Each participant (or parent) recorded medication administration, activity, sleep and awake times during the study. ABPM parameters included mean SBP and DBP during the entire 24-hour, awake, and sleep periods; BP load (percentage of readings above the ambulatory 95th %tile) for both SBP and DBP during the entire 24-hour, awake, and sleep periods; and dipping which refers to the physiologic decline in SBP and DBP during sleep.12 Normal dipping is defined as a >10% decline in mean ambulatory SBP and DBP levels from awake to sleep period. ABPM measures were compared with height-specific normative pediatric ABPM data. Ambulatory SBP and DBP index (mean ambulatory blood pressure/95th %tile value based on pediatric ambulatory BP norms) was calculated. BP classification according to pediatric ABPM criteria are as follows – normal BP, white coat hypertension, prehypertension, masked hypertension, and ambulatory hypertension.12 Normal BP is defined as <90th%tile office BP, mean ambulatory BP <95th%tile and BP load <25%; White coat hypertension is defined as office BP ≥95th%tile, mean ambulatory BP <95th%tile and BP load <25%; Prehypertension is office BP ≥ 90th%tile or > 120/80 mm Hg, mean ambulatory BP <95th%tile and BP load ≥25%; Masked hypertension is defined as office BP <95th%tile, mean ambulatory BP>95th%tile, and BP load ≥25%. Ambulatory hypertension is defined as both clinic BP and ambulatory BP with mean ambulatory BP >95th %tile and BP load >50%.12, 13
Statistical Methods:
The distributions of continuous study variables in these SCD patients were summarized with means and standard deviations or, if substantially skewed, with medians and the first and third quartiles. Categorical variables were summarized with frequency counts and percentages. These data were stratified into three categories of BP status: normal, abnormal, and hypertension. Kruskal-Wallis nonparametric tests and Fisher’s exact tests were used to evaluate differences in the distributions of the continuous and categorical study variables, respectively, across these three BP groups. The significance level of each test was set a priori to 0.05. Boxplots were used to illustrate the distributions of SBP and DBP indices measured during awake and asleep periods across BP groups. The boxplots (Fig. 1) show boxes, representing the median and first and third quartile data in a group, whisker plots, extending to the most extreme data point which is no more than 1.5 times the interquartile range from the box, and data points which may be outliers. All statistical analyses were conducted using SAS v9.4 (SAS Institute, Cary, NC, USA).
Fig 1.
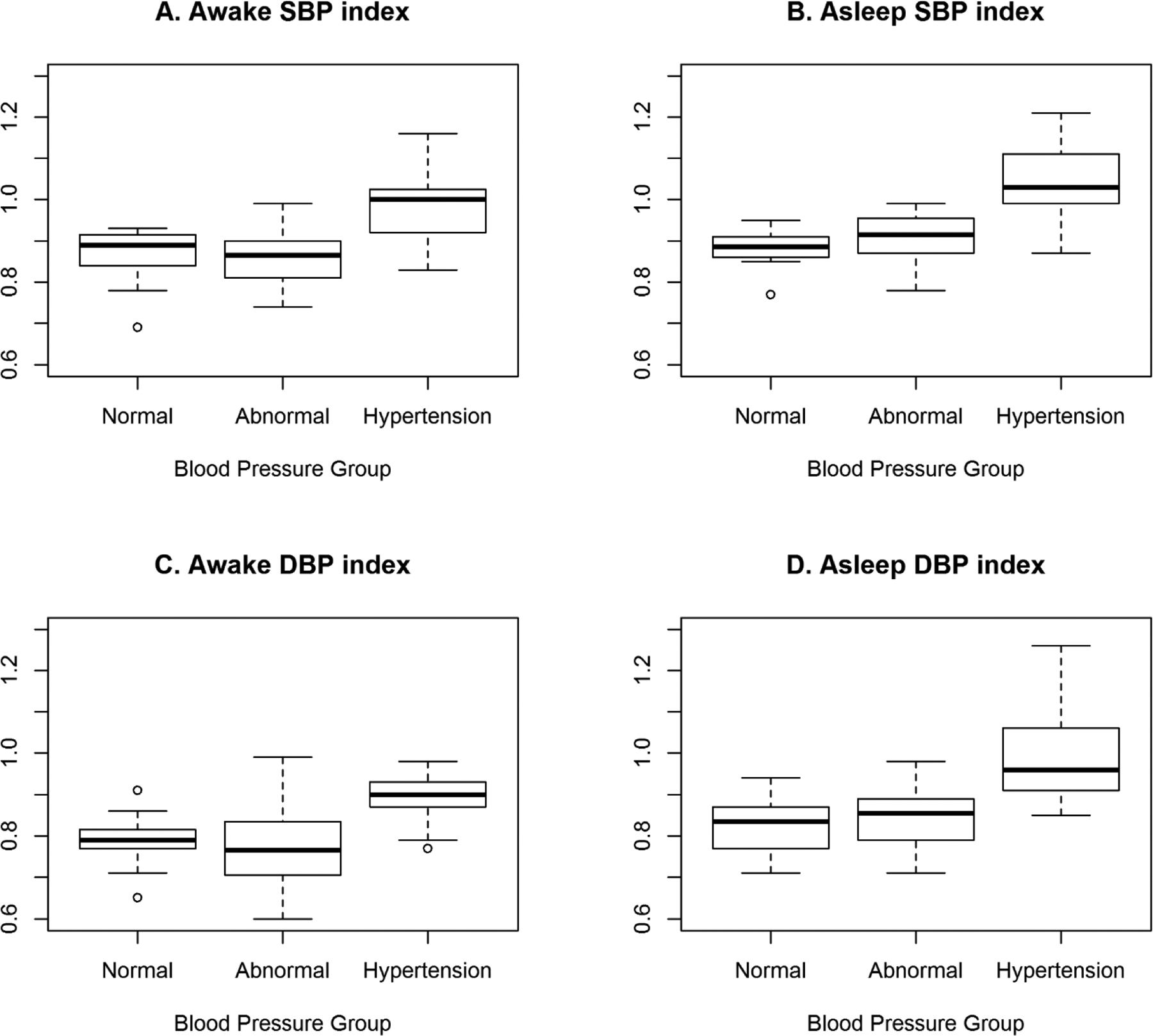
ABPM Awake and Asleep Systolic and Diastolic BP Indices Depicted as Box Plots
Results:
Between 1st of June 2015 and 1st of August 2017, we enrolled African American children with SCD. ABPM data was obtained on 56 participants; 54 with HbSS, and 2 with HbSβ0 thalassemia. ABPM recordings were obtained for the full 24 hours in 53 out of 56 participants. One participant wore the monitor for a total of 12 awake hours with 20 BP measurements recorded, a second participant had 12 BP measurements recorded over nine awake hours; and a third participant had 16 BP measurements recorded over six awake hours. There were no adverse effects reported from the ABPM study. Based on ABPM study results participants were categorized as normal ABPM, or hypertension ABPM (ambulatory hypertension, masked hypertension). The term abnormal ABPM designated participants who did not have normal ABPM or hypertension, but had other abnormal parameters on ABPM (presence of pre-hypertension and/or lack of normal nocturnal dipping).
Demographic and clinical data are presented in Table 1. Participants in the study group had ages ranging from 5 to 20 years with a mean age of 11.9 years. Mean age tended to be lower in group with ABPM hypertension (10.0 (±4.18) years) when compared to ABPM normal (12.09 (±4.32) years) and ABPM abnormal (13.04 (±4.45) years) groups (p=0.08 comparing ABPM hypertension to others). Participants in the ABPM hypertension group were smaller based on their height and weight. However, there was no significant difference in BMI and BMI z-scores between the three groups. At the time of completion of ABPM study, 62% (35/56) of the participants were on chronic hydroxyurea therapy, and 57% (32/56) of the participants were on chronic blood transfusion protocol; with no differences between the groups. Renal function described, as mean eGFR values based on serum creatinine and serum Cystatin C values were comparable between the three groups (Table 2.). There were no significant differences between the three groups in hemoglobin, and hematocrit, or urine osmolality. There were also no significant differences between the groups in median urine albumin to creatinine ratio. However, it is important to note that, 10/56 (18%) participants had microalbuminuria (urine albumin: creatinine ratio 30–300 mg/g creatinine), measured on random spot urine sample. Of those with microalbuminuria, one participant (9%) was in ABPM normal group, five (18%) were in APBM abnormal and four (23%) were in ABPM hypertension group (p=0.66). None of the participants had overt proteinuria (urine albumin: creatinine ratio >300mg/g creatinine). The concentration of sodium and potassium from spot urine samples were analyzed and expressed as urine sodium: creatinine ratios and urine potassium: creatinine ratios. Median urine sodium: creatinine ratios were relatively high and no different between the BP groups. Urine potassium: creatinine ratios were highest in the hypertensive group compared to the other groups (P = 0.02). The urine sodium: potassium ratio was also computed and found to be lowest in the hypertensive group, although not statistically significant (P = 0.17). Information on dietary intake of sodium in these patients was not available.
Table 1.
Summary of Baseline Characteristics Based on Ambulatory Blood Pressure Monitoring (ABPM)
| Variables | All | ABPM Normal | ABPM Abnormal* | ABPM Hypertension | p-value |
|---|---|---|---|---|---|
| Total, n (%) | 56 (100) | 11 (19.60) | 28 (50.00) | 17 (30.40) | |
| Gender | 0.53 | ||||
| Male, n (%) | 27 (48.20) | 7 (63.60) | 13 (46.40) | 7 (41.20) | |
| Female, n (%) | 29 (51.80) | 4 (36.40) | 15 (53.60) | 10 (58.80) | |
| Age, mean (SD) | 11.93 (±4.47) | 12.09 (±4.32) | 13.04 (±4.45) | 10.00 (±4.18) | 0.08 |
| Height (cm), mean (SD) | 144.84 (±22.04) | 141.73 (±22.99) | 152.00 (±22.17) | 135.06 (±17.65) | 0.04 |
| Weight (kg), mean (SD) | 42.06 (±19.95) | 37.59 (±16.30) | 47.24 (±18.57) | 36.41 (±22.92) | 0.15 |
| BMI, mean (SD) | 19.09 (±4.93) | 17.79 (±3.26) | 19.98 (±4.37) | 18.46 (±6.47) | 0.39 |
| BMI Z-score, mean (SD) | 0.86 (±7.42) | −0.39 (±1.30) | 2.10 (±10.47) | −0.29 (±1.36) | 0.49 |
| Hydroxyurea | 0.93 | ||||
| No, n (%) | 35 (62.50) | 7 (63.60) | 18 (64.30) | 10 (58.80) | |
| Yes, n (%) | 21 (37.50) | 4 (36.40) | 10 (35.70) | 7 (41.20) | |
| Blood Transfusions | 0.16 | ||||
| No, n (%) | 32 (57.10) | 5 (45.50) | 14 (50.00) | 13 (76.50) | |
| Yes, n (%) | 24 (42.90) | 6 (54.50) | 14 (50.00) | 4 (23.50) |
Abbreviations: ABPM – Ambulatory blood pressure monitor; BMI – Body mass index; DBP – Diastolic blood pressure; SBP – Systolic blood pressure
Presence of pre-hypertension or abnormal nocturnal dip on ABPM
Table 2.
Comparison of Clinical Characteristics Based on Ambulatory Blood Pressure Monitoring
| Variables | All (n=56) | ABPM Normal (n=11) | ABPM Abnormal (n=28) | ABPM Hypertension (n=17) | p-value |
|---|---|---|---|---|---|
| Serum Creatinine (mg/dL), mean (SD) | 0.45 (±0.15) | 0.47 (±0.17) | 0.48 (±0.15) | 0.40 (±0.15) | 0.26 |
| Serum Creatinine Based eGFR (ml/min/1.73m2), mean (SD) | 144.42 (±38.82) | 137.36 (±39.26) | 141.85 (±45.38) | 153.06 (±25.54) | 0.52 |
| Serum Cystatin C Based eGFR (ml/min/1.73m2), mean (SD) | 100.84 (±15.88) | 104.36 (±14.83) | 96.68 (±15.24) | 105.50 (±16.91) | 0.18 |
| Hemoglobin (g/dL), mean (SD) | 9.09 (±1.47) | 9.20 (±1.85) | 8.91 (±1.42) | 9.30 (±1.35) | 0.67 |
| Hematocrit (%), mean (SD) | 26.51 (±4.68) | 26.94 (±6.07) | 25.78 (±4.40) | 27.42 (±4.22) | 0.50 |
| Urine Osmolarity (mOsm/KgH2O), median [Q1, Q3] | 457.50[411, 516] | 441.50 [411, 512] | 466 [425, 518] | 446 [379, 540] | 0.82 |
| Urine Albumin : Creatinine Ratio (mg/g), median [Q1, Q3] | 10.10 [8, 24] | 10.00 [5, 24] | 10.70[8, 24] | 10.50 [8.50, 44.50] | 0.69 |
| Urine Na/Cr ratio *, median [Q1, Q3] | 25.96 [16.60, 38.03] | 37.04 [27.06, 42.43] | 18.80 [14.92, 26.87] | 28.29 [20.95, 40.23] | 0.10 |
| Urine K/Cr ratio *, median [Q1, Q3] | 5.51 [3.85, 9.75] | 7.38 [4.63, 9.14] | 4.49 [3.47, 6.17] | 8.19 [5.22, 17.09] | 0.02 |
| Urine Na : K ratio, median [Q1,Q3] | 4.40 [2.15, 5.44] | 4.68 [3.09, 5.93] | 4.56 [2.58, 5.37] | 3.45 [1.37, 5.00] | 0.17 |
| Presence of microalbuminuria, n (%) | 10 (17.90%) | 1 (9.10%) | 5 (17.90%) | 4 (23.50%) | 0.66 |
Abbreviations: eGFR - Estimated glomerular filtration rate; Q1 - First quartile; Q3 - Third quartile;
mmol/L/mg/dl
24-hour ABPM parameters:
Table 3 describes ABPM study results in our SCD study cohort. Of the 17 hypertensive SCD participants (30%), three participants had both office and ambulatory hypertension; and 14 participants had masked hypertension. Of the entire cohort, 11 participants (20%) had normal clinic BP and were normotensive on ABPM. Another 28 participants (50%) did not meet criteria for hypertension but had abnormal values in some ABPM parameters. This group, designated Abnormal ABPM, met ABPM criteria for prehypertension and/ or had absence of normal nocturnal dipping. In this group, abnormal ABPM parameters were predominantly in the sleep period with elevated systolic or diastolic BP load and/ or absence of normal nocturnal dipping. One participant in the Abnormal ABPM group had reverse systolic and diastolic nocturnal dipping (higher sleep BP than awake BP). Of the three participants who did not complete the 24-hour study and had only awake measurements, one participant was found to have all normal BP measurements while the other two participants met the criteria for masked hypertension. ABPM hypertension group had significantly higher ABPM Awake and Asleep Systolic and Diastolic BP Indices as depicted as Box Plots in Figure 1.
Table 3.
Summary of Blood Pressure Variables including Clinic and 24 hour ABPM Parameters
| Variables | All (n=56) | ABPM Normal (n=11) | ABPM Abnormal (n=28) | ABPM Hypertension (n=17) | p-value |
|---|---|---|---|---|---|
| Single Clinic BP | |||||
| SBP (mm Hg), mean (SD) | 111.13 (±14.24) | 109.27 (±13.45) | 110.75 (±13.02) | 112.94 (±17.08) | 0.79 |
| DBP (mm Hg), mean (SD) | 62.52 (±7.85) | 62.91 (±7.08) | 62.61 (±7.00) | 62.12 (±9.86) | 0.96 |
| SBP Z-score, mean (SD) | 0.64 (±1.25) | 0.46 (±1.20) | 0.38 (±1.04) | 1.14(±1.48) | 0.13 |
| DBP Z-score, m±ean (SD) | 0.11 (±0.72) | 0.09 (±0.49) | 0.01 (±0.69) | 0.27 (±0.88) | 0.52 |
| 24hr Ambulatory BP | |||||
| SBP (mm Hg), mean (SD) | 112.43 (±11.87) | 105.73 (±9.54) | 108.96 (±8.60) | 122.47 (±11.99) | <0.01 |
| DBP (mm Hg), mean (SD) | 64.16 (±8.02) | 61.09 (±6.11) | 61.11 (±6.41) | 71.18 (±7.32) | <0.01 |
| SBPi, mean (SD) | 0.91 (±0.15) | 0.87 (±0.08) | 0.85 (±0.16) | 1.01 (±0.09) | <0.01 |
| DBPi, mean (SD) | 0.82 (±0.16) | 0.80 (±0.07) | 0.77 (±0.19) | 0.92 (±0.07) | <0.01 |
| SBP Load (%), median [Q1, Q3] | 10.8 [2.00, 40.50] | 8.0 [0, 13.00] | 5.0 [0, 18.00] | 51.0 [43.00, 70.00] | <0.01 |
| DBP Load (%), median [Q1, Q3] | 7.3 [2.00, 19.00] | 4.0 [0, 9.00] | 4.5 [1.70, 13.50] | 22.0 [14.00, 26.00] | <0.01 |
Abbreviations: DBP – Diastolic blood pressure; DBPi – Diastolic blood pressure index; SBP – Systolic blood pressure; SBPi – Systolic blood pressure index
Discussion:
In our sample of 56 children with SCD, 17 participants (30%) met criteria for hypertension based on ABPM. Of the 17 hypertensive participants, three had both office and ambulatory hypertension; and 14 had masked hypertension detected on ABPM. Overall, the distribution of confirmed hypertension, largely manifested by masked hypertension, is high in pediatric participants with SCD. Another 28 participants (50%) had some abnormal ABPM parameters that were largely present in the sleep period of ABPM.
Limited data is available on hypertension in children with SCD. Reports from recent studies indicate that high BP is not uncommon in SCD children. Bodas et al.,14 in data from a retrospective chart review of 48 children with SCD, reported 17% prevalence of elevated BP readings (pre-hypertension or hypertension) based on clinic BP measurements alone. Further insight on the prevalence of abnormal BP in SCD children was reported in studies that included ABPM measurements. Shatat et.al.15 obtained ABPM data on a sample of 38 children (11–16 years of age) with SCD (25 with HbSS and 13 with HbSC). They reported ambulatory hypertension in 43.6% (17/38) of the study sample. In their study only 10% (4/38) of the children with ambulatory hypertension also had clinic hypertension, indicating that those with ambulatory hypertension were largely represented by masked hypertension. In another study, Becker et. al. obtained ABPM data on 52 adolescents with Hb SS. Of note, these participants were older in comparison to our study participants (between 12 and 18 years of age), with a higher mean age of 15 years. These investigators reported previously unrecognized hypertension in 35% of patients and pre-hypertension in 17% of patients.16 They also described absence of normal nocturnal dipping in 56% of their patients. The results of our study are comparable to these two other studies that completed ABPM in children with SCD. As with the other reports, ABPM in our cohort detected masked hypertension as the predominant pattern of hypertension. In addition, 50% of our study participants had some abnormal ABPM findings in the form of prehypertension and/or absence of normal nocturnal dipping of BP while asleep. It is also important to note that 9/10 (90%) of our participants with microalbuminuria had either an abnormal ABPM study (prehypertension and/or lack of normal nocturnal dipping status) or hypertension on ABPM study. Our study sample was somewhat different than in the previous two reports because we included younger children and our sample included only HbSS or Hb Sβ0 thalassemia; the two most severe SCD genotypes. We also reported eGFR measurements based on serum creatinine and cystatin C levels. Although eGFR levels were similar between the three groups, eGFR tended to be very high consistent with the observation of glomerular hyperfiltration commonly seen in SCD patients.
Masked hypertension is a recently recognized condition, in which BP is normal in the office setting but elevated outside of a medical setting. This condition is confirmed only on ABPM, but can be suspected in the presence of sporadic reports of high office BP from other providers or elevated BP noted at other times such as school or home measurements. Reports on masked hypertension in adults indicate that the risks for cardiovascular events among patients with masked hypertension are comparable to established hypertension.17 In children, outcome data on masked hypertension are very limited. Distribution of masked hypertension differs based on the age and population of interest. A meta-analysis by Verbek et al. reported an overall population prevalence of masked hypertension of 19% for adults and 7% for children.17 In a cohort of 592 unselected children (6 to 18 years of age), Lurbe et al. identified masked hypertension in 8%.18 Among children with diabetes, Sulakova et al. performed ABPM on 84 children (median age 14.9 years) and identified masked hypertension in 9% diabetic children.19 Mitsnefes et al. reported ABPM findings in the observational Chronic Kidney Disease in Children (CKiD) cohort study (n=198). The investigators reported confirmed hypertension (both elevated office and ambulatory BP) in 18% of children and masked systolic or diastolic hypertension in 38% of children.20 Observation studies on children with masked hypertension, without known chronic disease, describe progression to clinical hypertension, reversal to normotension, or persistence of masked hypertension.18
Abnormalities in cardiac geometry, including left ventricular hypertrophy, were also described in children and adolescents with masked hypertension.21 In the CKiD cohort, left ventricular hypertrophy was more common in children with either confirmed hypertension (34%) or with masked hypertension (20%), when compared to normotensive children (8%) with CKD. There are no standard recommendations on treating masked hypertension in children. Masked hypertension does require further monitoring, and if persistent may require anti-hypertensive therapy. In our cohort, three participants already had a diagnosis of hypertension and were being treated with anti-hypertensive therapy at the time of study enrollment. A six-year-old participant, who was diagnosed with masked hypertension after completion of ABPM study, was started on low dose angiotensin-converting enzyme inhibitor therapy, which was later discontinued due to symptoms of low BP. Theoretically, it is possible to adapt non-pharmacologic measures in these patients such as dietary sodium restriction, and increased hydration. Patients with masked hypertension may also be evaluated for evidence of target organ damage in the form of elevated left ventricular mass index, left ventricular hypertrophy, or early evidence of nephropathy in the form of microalbuminuria or overt proteinuria.
A normal (10% or greater) dip in BP level during sleep is critical for maintenance of cardiovascular health in adults22, 23. In our cohort of pediatric patients with SCD, 50% had prehypertension and/or absent normal nocturnal BP dip (both systolic and diastolic). Absent nocturnal dipping has been associated with increased risk for cardiovascular disease and stroke in adults. Abnormalities of sleep BP are cited as significant predictors of cardiovascular outcomes in patients with CKD. Also in Type 1 diabetes mellitus loss of normal nocturnal dip is associated with development of microalbuminuria; an early marker of deterioration of renal function.24 These ABPM abnormalities appear to be more prevalent in patients with chronic disease. Our findings, along with two other recent reports in children with SCD, confirm a high frequency of abnormal nocturnal BP dip.
The underlying cause for the relatively high prevalence of masked hypertension and abnormal ABPM parameters including absence of nocturnal BP dip in children with SCD is unclear. None of the participants in the abnormal ABPM or hypertension groups were obese or overweight. Hypertension is commonly associated with CKD; and patients with SCD are known to develop sickle nephropathy in young adulthood.25, 26 We detected no evidence of incipient renal disease in our SCD children with abnormal ABPM or hypertension, based on renal function and absence of overt proteinuria. Although 90% of the participants with microalbuminura had either an abnormal ABPM or hypertension, this was not statistically significant and was likely reflective of the imbalance in group sample size. Becker et. al. reported adolescents with Hb SS with microalbuminuria had significantly less nocturnal BP dipping than those without microalbuminuria.16 Clearly, in children with severe forms of SCD such as Hb SS or Hb Sβ0 thalassemia, it would be important to do routine assessments for microalbuminuria, as presence of microalbuminuria may serve as a surrogate marker for increased risk for BP abnormalities or early sickle nephropathy. However, longitudinal studies with a larger sample size will be needed to determine if there is a relationship between BP abnormalities and microalbuminuria in SCD.
Sleep-disordered breathing (SDB) is commonly associated with hypertension in adults.27 Studies on sleep quality and SDB in children and adolescents describe an association of SDB and elevated blood pressure that is commonly related to obesity.28, 29 A study on adolescents with documented SDB report a prevalence of hypertension from 4 to 14%.30 SDB, notably obstructive sleep apnea, is commonly seen in children with SCD and occurs with a much higher frequency compared to general pediatric population. In SCD patients, adenotonsillar hypertrophy with upper airway obstruction, chronic anemia and hypoxemia, hypoventilation related to chronic lung disease and pulmonary hypertension are some of the possible mechanisms cited for SDB.31, 32 With SCD, chronic anemia and worsening tissue oxygenation during sleep could increase sympathetic output and contribute to abnormal BP patterns. Although sleep screening or an overnight sleep study were not part of our study, we reviewed the medical records of all patients with masked hypertension on ABPM. We identified referrals for sleep studies in seven of the 17 patients. Of these seven, two patients had a completed sleep study that was consistent with obstructive sleep apnea, one patient had a completed sleep study that was normal, and in four patients sleep study has not been performed to date. Thus, we do not have sufficient information to implicate or dismiss a relationship of abnormal BP with SDB. Further studies will be needed to determine if sleep disorders in SCD children are associated with abnormal BP findings detected with ABPM.
Hypertension in our cohort of patients with SCD is probably secondary, as these patients lack the clinical phenotype for primary hypertension. Children with SCD have chronic disease with persistent anemia, and histories of repeated hospitalizations due to SCD-related complications and hence are under chronic stress. Theoretically, it is possible that chronic stress could contribute to hypertension in these patients. Su et. al. reported an association between severe adverse experiences in childhood with markers of endothelial dysfunction in late adolescence33. The relatively high urine potassium and lower sodium: potassium ratio in the hypertensive group could be a clue to a secondary cause of hypertension such as hyperaldosteronism, abnormalities of intra-renal endothelin-134, or other renal tubular defect in sodium transport35. However, due to the limitations of sodium and potassium measurements on a spot urine sample, further research will be needed to determine possible underlying causes of hypertension in SCD patients.
A limitation of our study is the relatively small sample size. Although our study was limited to 56 SCD children, the sample size is larger when compared to two other studies on abnormal blood pressure assessed by ABPM in children with SCD. Unlike previous reports, our study detected ambulatory hypertension in SCD children as young as 6 years of age. Findings from our study indicate that BP abnormalities are not uncommon in SCD children. Based on our findings, more attention should be given to routine BP monitoring in the management of children with SCD.
Table 4.
Summary of Blood Pressure Variables including Awake and Asleep ABPM Measurements
| Variables | All (n=56) | ABPM Normal (n=11) | ABPM Abnormal (n=28) | ABPM Hypertension (n=17) | p-value |
|---|---|---|---|---|---|
| Ambulatory Awake | |||||
| SBP (mm Hg), mean (SD) | 115.23 (±12.15) | 110.18 (±9.91) | 111.46 (±10.04) | 124.71 (±11.75) | <0.01 |
| DBP (mm Hg), mean (SD) | 66.71 (±8.34) | 64.82 (±6.16) | 63.61 (±8.20) | 73.06 (±6.32) | <0.01 |
| SBPi, mean (SD) | 0.87 (±0.19) | 0.87 (±0.07) | 0.83 (±0.19) | 0.94 (±0.23) | <0.01 |
| DBPi, mean (SD) | 0.79 (±0.18) | 0.79 (±0.07) | 0.74 (±0.23) | 0.89 (±0.06) | 0.02 |
| SBP Load (%), median [Q1,Q3] | 7.50 [0, 42.50] | 9 [0, 14.00] | 3 [0, 8.50] | 49 [37, 58] | <0.01 |
| DBP Load (%), median [Q1,Q3] | 4 [0, 14.00] | 5 [0, 10.00] | 2 [0, 5.50] | 17 [6.00, 22.00] | <0.01 |
| Ambulatory Asleep | |||||
| SBP (mm Hg), mean (SD) | 105.98 (±9.57) | 98.00 (±6.41) | 104.50 (±6.49) | 114.07 (±10.62) | <0.01 |
| DBP (mm Hg), mean (SD) | 58.17 (±6.67) | 54.60 (±4.12) | 56.46 (±4.85) | 63.73 (±7.80) | <0.01 |
| SBPi, mean (SD) | 0.93 (±0.12) | 0.88 (±0.05) | 0.89 (±0.11) | 1.03 (±0.11) | <0.01 |
| DBPi, mean (SD) | 0.87 (±0.14) | 0.83 (±0.07) | 0.82 (±0.15) | 0.98 (±0.13) | <0.01 |
| SBP Load (%), median [Q1, Q3] | 15[6, 42] | 3[0, 12] | 14 [3.00, 24.50] | 50 [46, 89] | <0.01 |
| DBP Load (%), median [Q1, Q3] | 10 [0, 24] | 6.50 [0, 8] | 9.50[0.00, 20.00] | 24 [16, 63] | 0.01 |
| Nocturnal Dip | |||||
| SBP Dip (%), median [Q1, Q3] | 8 [4, 12] | 12 [11, 13] | 6.50 [3, 9] | 10 [3, 13] | <.01 |
| DBP Dip (%), median [Q1, Q3] | 13[8, 19] | 17 [16, 19] | 10 [5.50, 16.00] | 14 [6, 20] | 0.04 |
| Normal Nocturnal BP Dip (≥10%) | |||||
| SBP | <0.01 | ||||
| No, n (%) | 30 (56.60) | 0 (0.0) | 23 (82.10) | 7 (46.70) | |
| Yes, n (%) | 23 (43.40) | 10 (100) | 5 (17.90) | 8 (53.30) | |
| DBP | 0.04 | ||||
| No, n (%) | 17 (32.10) | 0 (0.00) | 12 (42.90) | 5 (33.30) | |
| Yes, n (%) | 36 (67.90) | 10 (100) | 16 (57.10) | 10 (66.70) |
Abbreviations: DBP – Diastolic blood pressure; DBPi – Diastolic blood pressure index; SBP – Systolic blood pressure; SBPi – Systolic blood pressure index
Highlights:
SCD is a genetic disorder associated with chronic anemia and vaso occlusive crises.
Children with SCD have evidence of masked hypertension on ambulatory BP monitoring.
Masked hypertension contributes to kidney disease and cardiovascular complications.
What’s Known on This Subject:
Limited data is currently available on blood pressure disorders when assessed by 24-hour ambulatory blood pressure studies performed in children with sickle cell disease.
What This Study Adds:
In children with sickle cell disease we noted a higher rate of masked hypertension and decreased nocturnal dipping of blood pressure, and ambulatory hypertension in an individual as young as 6 years of age.
Acknowledgements:
Kelly Hussong RN and Jean Wadman RN. Ms. Hussong was responsible for patient recruitment, data collection and management. Ms. Wadman assisted with patient recruitment.
Funding source:
Research reported in this publication was supported by the National Institute of General Medical Sciences of the National Institutes of Health under Award Number P20GM109021. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. Young Investigator Grant of the National Kidney Foundation and Grant number U54-GM104941 (PI: Binder-Macleod) funded the project, in part.
Abbreviations:
- ABPM
Ambulatory blood pressure monitoring
- BP
Blood pressure
- CKD
Chronic kidney disease
- HTN
Hypertension
- SCD
Sickle cell disease
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Financial Disclosure Statement: The authors have no financial relationships relevant to this article to disclose.
Conflict of Interest Statement: The authors have no conflicts of interest relevant to this article to disclose.
References
- 1.Hassell KL. Population estimates of sickle cell disease in the U.S. Am J Prev Med. 2010;38(4 Suppl):S512–521. [DOI] [PubMed] [Google Scholar]
- 2.Bunn HF. Pathogenesis and treatment of sickle cell disease. N Engl J Med. 1997;337(11):762–769. [DOI] [PubMed] [Google Scholar]
- 3.Kaul DK, Hebbel RP. Hypoxia/reoxygenation causes inflammatory response in transgenic sickle mice but not in normal mice. J Clin Invest. 2000;106(3):411–420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Osarogiagbon UR, Choong S, Belcher JD, Vercellotti GM, Paller MS, Hebbel RP. Reperfusion injury pathophysiology in sickle transgenic mice. Blood. 2000;96(1):314–320. [PubMed] [Google Scholar]
- 5.Johnson CS, Giorgio AJ. Arterial blood pressure in adults with sickle cell disease. Arch Intern Med. 1981;141(7):891–893. [PubMed] [Google Scholar]
- 6.Rodgers GP, Walker EC, Podgor MJ. Is “relative” hypertension a risk factor for vaso-occlusive complications in sickle cell disease? Am J Med Sci. 1993;305(3):150–156. [DOI] [PubMed] [Google Scholar]
- 7.Ernst AA, Weiss SJ, Johnson WD, Takakuwa KM. Blood pressure in acute vaso-occlusive crises of sickle cell disease. South Med J. 2000;93(6):590–592. [PubMed] [Google Scholar]
- 8.Guasch A, Navarrete J, Nass K, Zayas CF. Glomerular involvement in adults with sickle cell hemoglobinopathies: Prevalence and clinical correlates of progressive renal failure. J Am Soc Nephrol. 2006;17(8):2228–2235. [DOI] [PubMed] [Google Scholar]
- 9.Gordeuk VR, Sachdev V, Taylor JG, Gladwin MT, Kato G, Castro OL. Relative systemic hypertension in patients with sickle cell disease is associated with risk of pulmonary hypertension and renal insufficiency. Am J Hematol. 2008;83(1):15–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pegelow CH, Colangelo L, Steinberg M, et al. Natural history of blood pressure in sickle cell disease: risks for stroke and death associated with relative hypertension in sickle cell anemia. Am J Med. 1997;102(2):171–177. [DOI] [PubMed] [Google Scholar]
- 11.DeBaun MR, Sarnaik SA, Rodeghier MJ, et al. Associated risk factors for silent cerebral infarcts in sickle cell anemia: low baseline hemoglobin, sex, and relative high systolic blood pressure. Blood. 2012;119(16):3684–3690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Flynn JT, Daniels SR, Hayman LL, et al. Update: ambulatory blood pressure monitoring in children and adolescents: a scientific statement from the American Heart Association. Hypertension. 2014;63(5):1116–1135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Urbina E, Alpert B, Flynn J, et al. Ambulatory blood pressure monitoring in children and adolescents: recommendations for standard assessment: a scientific statement from the American Heart Association Atherosclerosis, Hypertension, and Obesity in Youth Committee of the council on cardiovascular disease in the young and the council for high blood pressure research. Hypertension. 2008;52(3):433–451. [DOI] [PubMed] [Google Scholar]
- 14.Bodas P, Huang A, O’Riordan MA, Sedor JR, Dell KM. The prevalence of hypertension and abnormal kidney function in children with sickle cell disease -a cross sectional review. BMC Nephrol. 2013;14:237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Shatat IF, Jakson SM, Blue AE, Johnson MA, Orak JK, Kalpatthi R. Masked hypertension is prevalent in children with sickle cell disease: a Midwest Pediatric Nephrology Consortium study. Pediatr Nephrol. 2013;28(1):115–120. [DOI] [PubMed] [Google Scholar]
- 16.Becker AM, Goldberg JH, Henson M, et al. Blood pressure abnormalities in children with sickle cell anemia. Pediatr Blood Cancer. 2014;61(3):518–522. [DOI] [PubMed] [Google Scholar]
- 17.Verberk WJ, Kessels AG, de Leeuw PW. Prevalence, causes, and consequences of masked hypertension: a meta-analysis. Am J Hypertens. 2008;21(9):969–975. [DOI] [PubMed] [Google Scholar]
- 18.Lurbe E, Torro I, Alvarez V, et al. Prevalence, persistence, and clinical significance of masked hypertension in youth. Hypertension. 2005;45(4):493–498. [DOI] [PubMed] [Google Scholar]
- 19.Sulakova T, Janda J, Cerna J, et al. Arterial HTN in children with T1DM--frequent and not easy to diagnose. Pediatr Diabetes. 2009;10(7):441–448. [DOI] [PubMed] [Google Scholar]
- 20.Mitsnefes M, Flynn J, Cohn S, et al. Masked hypertension associates with left ventricular hypertrophy in children with CKD. J Am Soc Nephrol. 2010;21(1):137–144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Stabouli S, Kotsis V, Toumanidis S, Papamichael C, Constantopoulos A, Zakopoulos N. White-coat and masked hypertension in children: association with target-organ damage. Pediatr Nephrol. 2005;20(8):1151–1155. [DOI] [PubMed] [Google Scholar]
- 22.Weir MR, Blantz RC. Blood pressure and cardiovascular risks: implications of the presence or absence of a nocturnal dip in blood pressure. Curr Opin Nephrol Hypertens. 2003;12(1):57–60. [DOI] [PubMed] [Google Scholar]
- 23.Sayk F, Becker C, Teckentrup C, et al. To dip or not to dip: on the physiology of blood pressure decrease during nocturnal sleep in healthy humans. Hypertension. 2007;49(5):1070–1076. [DOI] [PubMed] [Google Scholar]
- 24.Ettinger LM, Freeman K, DiMartino-Nardi JR, Flynn JT. Microalbuminuria and abnormal ambulatory blood pressure in adolescents with type 2 diabetes mellitus. J Pediatr. 2005;147(1):67–73. [DOI] [PubMed] [Google Scholar]
- 25.Falk RJ, Jennette JC. Sickle cell nephropathy. Adv Nephrol Necker Hosp. 1994;23:133–147. [PubMed] [Google Scholar]
- 26.Wigfall DR, Ware RE, Burchinal MR, Kinney TR, Foreman JW. Prevalence and clinical correlates of glomerulopathy in children with sickle cell disease. J Pediatr. 2000;136(6):749–753. [PubMed] [Google Scholar]
- 27.Martynowicz H, Porebska I, Poreba R, Mazur G, Brzecka A. Nocturnal Blood Pressure Variability in Patients with Obstructive Sleep Apnea Syndrome. Adv Exp Med Biol. 2016;952:9–15. [DOI] [PubMed] [Google Scholar]
- 28.Archbold KH, Vasquez MM, Goodwin JL, Quan SF. Effects of sleep patterns and obesity on increases in blood pressure in a 5-year period: report from the Tucson Children’s Assessment of Sleep Apnea Study. J Pediatr. 2012;161(1):26–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Li AM, Au CT, Ng C, Lam HS, Ho CK, Wing YK. A 4-year prospective follow-up study of childhood OSA and its association with BP. Chest. 2014;145(6):1255–1263. [DOI] [PubMed] [Google Scholar]
- 30.Javaheri S, Storfer-Isser A, Rosen CL, Redline S. Sleep quality and elevated blood pressure in adolescents. Circulation. 2008;118(10):1034–1040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Strauss T, Sin S, Marcus CL, et al. Upper airway lymphoid tissue size in children with sickle cell disease. Chest. 2012;142(1):94–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Grady AJ, Hankins JS, Haberman B, Schoumacher R, Stocks RM. Hydroxyurea treatment effect on children with sickle cell disease and obstructive sleep apnea. Sleep Breath. 2017. [DOI] [PubMed] [Google Scholar]
- 33.Su S, Wang X, Kapuku G, et al. Adverse Childhood Experiences Are Associated With Detrimental Hemodynamics and Elevated Circulating Endothelin-1 in Adolescents and Young Adults. Hypertension. 2014;64:201–207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mathur S, Pollock J, Mathur S, et al. Relation of urinary endothelin-1 to stress-induced pressure natriuresis in healthy adolescents. Journal of the American Society of Hypertension. 2018;12(1): 34–41. [DOI] [PubMed] [Google Scholar]
- 35.Gamba G Role of WNK kinases in regulating tubular salt and potassium transport and in the development of hypertension. Am J Physiol Renal Physiol. 2005;288(2):F245–52. [DOI] [PubMed] [Google Scholar]


