Abstract
Background:
Recent advances in stem cell technologies have rekindled an interest in the use of cell therapies to treat patients with Parkinson’s disease. Although the transplantation of dopaminergic mesencephalic human fetal brain tissue has previously been reported in the treatment of patients with Parkinson’s disease, this method is limited by the availability of tissue obtained from each human embryo.
Objective:
Our study aimed to isolate, culture, proliferate, and differentiate dopaminergic neurons from human neuroepithelial stem cells obtained from embryo reduction procedures performed in multifetal pregnancies following in vitro fertilization.
Materials and Methods:
A total of 201 human embryos were dissected for isolation and culture of neuroepithelial stem cells for proliferation and differentiation into dopaminergic neurons. All embryos were obtained from embryo reduction procedures performed in multifetal pregnancies after in vitro fertilization treatments.
Results:
Human neuroepithelial stem cells were isolated and cultured from embryos from 6.0 to 8.0 weeks. Neuroepithelial stem cells were successfully isolated, proliferated, and differentiated into dopaminergic neurons. The cells adhered to the surfaces of cell culture plates after 2 days and could be proliferated and differentiated into neurons within 4 days. Cultured cells expressed the dopaminergic marker tyrosine hydroxylase after 6 days, suggesting that these cells were successfully differentiated into dopaminergic neurons.
Conclusion:
The successful isolation, culture, proliferation, and differentiation of human dopaminergic neurons from embryo reductions performed for multifetal pregnancies after in vitro fertilization suggests that this pathway may serve as a potential source of cell therapy materials for use in the treatment of Parkinson’s disease.
Keywords: Parkinson’s disease, human neuroepithelial stem cells, dopaminergic neuron, in vitro fertilization
1. BACKGROUND
Parkinson’s disease (PD) is among the most commonly occurring neurodegenerative disorders characterized by the loss of dopamine-producing neurons. PD is characterized by symptoms such as tremor; muscle stiffness; slowing of movement; impaired posture and balance; loss of automatic movement; and changes in speech and writing. To date, no cure exists for PD, although medications and surgery can help reduce symptoms (1). Over the last 20 years, several molecular, genetic, and stem cell therapeutic approaches have been developed to counteract or slow PD progression (1). Dopaminergic neuronal precursors derived from human fetal mesencephalic (FM) cells, embryonic stem cells, or induced pluripotent stem (iPS) cells and transplanted into the putamen can lead to the generation of mature dopaminergic neurons, which can ameliorate disease-induced motor impairments (2). By 1979, intracerebral grafts derived from FM tissue, which is rich in dopaminergic neuroblasts, were reported to ameliorate the symptoms of a rat model of PD (3). Clinical studies have been performed using human FM tissue since the late 1980s, providing valuable insight into the basic principles underlying cell therapy in PD (4). These studies demonstrated that human embryonic dopaminergic neurons transplanted into patients with PD could survive (5), produce dopamine, and form synapses with host neurons (6), indicating that dopaminergic grafts could become functionally integrated into the host brain. Some patients who received FM tissue transplants no longer required medication after grafting. However, 15%–57% of patients in the treatment groups from two clinical studies developed graft-induced dyskinesia (5, 7). These contrasting outcomes in response to the transplantation of FM tissue indicate that although this approach can be effective in some PD cases, this technique requires improvements with regard to the preparation of donor tissue and modulating the immune response (8). These findings also highlighted the potential for human FM tissue transplantation to treat patients with PD; however, the clinical application of transplantation therapy was hindered by two main issues. First, the collection of human fetal tissue can be difficult, requiring the accurate identification of embryonic age and the isolation of the ventral mesencephalon containing dopaminergic neurons, and can result in adverse effects when implanted into the brains of patients with PD. Second, and the most important, is the question of whether using tissue obtained from dead, aborted human fetuses for transplantation into patients with PD to ameliorate severe motor symptoms is ethically and morally justified (2).
In Vietnam, assisted reproductive technologies have been developed over the last 20 years. To date, Vietnamese law has not banned multiple embryo transfers during the process of in vitro fertilization, and the rate of multiple pregnancies remains high. In cases with 3 or more developing embryos, physicians routinely perform pregnancy reductions to avoid obstetric complications in women, such as premature births or low birth weights, in addition to non-medical problems, such as the emotional or financial difficulties of raising more than one or two infants (9, 10). Embryo reduction is typically performed at 6-8 weeks of gestational age (11). Embryonic tissues obtained from reductions have a known and accurate age, are aseptic, and are still alive.
2. OBJECTIVE
With the goal of identifying a reliable source of dopaminergic neurons for the treatment of patients with PD, we performed this study to determine whether dopaminergic neurons can be differentiated from isolated and proliferated neuroepithelial stem cells obtained from embryo reductions following in vitro fertilization.
3. MATERIAL AND METHODS
Embryo selection
A total of 201 embryos were collected 6.0–8.0 weeks after embryo transfer during the process of embryo reduction for multifetal pregnancies following in vitro fertilization from October 2014 to April 2016. Embryos were obtained with maternal consent from three centers: Reproductive Health and Tissue Engineering Center of Hanoi Medical University Hospital, Reproductive Health Center of Postal Hospital, and Reproductive Health Center of Hanoi Obstetrics and Gynecology Hospital. The accurate identification of embryo age was determined based on the date of embryo transfer, corresponding to 6.0–8.0 weeks.
Cell suspension preparation
The embryos were collected using a follicle aspiration needle. Neural tubes containing neuroepithelial stem cells were identified as the segments above the liver. The dissection of neural tube segments was performed under an inverted microscope under sterile conditions. Neural tube segments were incubated with 1.2 IU dispase at 37 °C for 3 minutes, followed by incubation in 0.05% trypsin-EDTA for 3 minutes, and washing three times with Dulbecco’s modified Eagle medium (DMEM)/F12 (1:1). The pieces of tissues were placed in a 5-ml tube containing DMEM/F12 and passed through a pipette tip 20 times to lyse the cell suspension. The suspension was filtered through a 70-μm membrane to obtain single cells and small cell aggregates. A small volume of the cell suspension was stained with trypan blue (10 μl of trypan blue combined with 10 μl cell suspension) to identify the cell survival rate. Cell numbers were counted using a Neubauer counting chamber.
Cell culture
The isolated cells were cultured on 6-well plates at a cell density of 2–2.2 × 104/cm2 in DMEM (Invitrogen, Carlsbad, CA) and F12 (DMEM/F12; 1:1) supplemented with 10% fetal bovine serum, 100 mg/ml transferrin, 25 ng/ml insulin, 20 nM progesterone, 62 mM putrescine, 30 nM selenite, 20 ng/ml epidermal growth factor, fibroblast growth factor-2 (Sigma), 100 UI/ml penicillin, 100 μg/ml streptomycin, and 0.25 μg/ml amphotericin B at 37 °C in a humidified atmosphere of 5% CO2 and 95% air. The medium was replaced every 2 days. After culturing for 7–10 days, the cells were harvested for identification by Giemsa staining, Cajal silver staining, immunocytochemistry staining, and transmission electron microscope (TEM) characterization.
Cajal silver staining
The cells were fixed in a fixative solution for 24 hours. After washing with water, the cells were transferred into 1.5% silver nitrate solution for 4 days at room temperature. The cells were washed in water for 1 minute and incubated in 1% pyrogallic acid for 1 day at 37 °C. The cells were then washed in water for 5 minutes and dehydrated through 95% and 100% alcohol before being cleared in xylene.
Transmission electron microscope preparation
Cells were fixed in 1 mm tissue blocks in 2.5% glutaraldehyde/cacodylate in 0.1 M phosphate buffer (PB, pH 7.2–7.4) overnight. After fixation, the cells were washed in 0.1 M cacodylate three times, post-fixed in 1% osmium tetroxide in 0.1 M PB for 2 hours at room temperature, and washed in 0.1 M cacodylate three times. The samples were dehydrated in a graded series of 50%, 70%, 95%, and 100% ethanol for 2 × 15 minutes for each step, followed by incubation in 100% propylene oxide for 2 × 15 minutes. Cells were embedded in epoxy overnight and in beam capsules at 60°C for 48 hours. Sections were cut into 30-40-nm-thick slices and collected onto a grid, which was stained with uranyl acetate for 10 minutes and lead citrate for 5 minutes. The sections were dried sections and observed under TEM (JEOL JEM 1010).
Immunocytochemistry staining
Cells were rehydrated in 0.1 M phosphate-buffered saline (PBS) for 30 minutes, treated with sodium citrate buffer (pH 6.0) at 95 °C for 15 minutes, and cooled to room temperature for 30 minutes. Cells were then washed in PBS and blocked with blocking solution (0.2 M PB, 0.05 M NaCl, and 0.3% Triton X-100) containing 5% normal goat serum for 2 hours at room temperature. Cells were incubated overnight at 4 °C in primary antiserum diluted in blocking solution without goat serum. Antisera included anti-tyrosine hydroxylase (1:750; catalog # ab-112; Abcam) and anti-vimentin (1:300; catalog # ab 92745; Abcam). Cells were washed with PBS for 2 hours and incubated with goat anti-rabbit Alexa Fluor 546 (1:200; Invitrogen) in blocking solution for 2 hours at room temperature. Cells were washed in 0.1 M PBS for 2 hours, counterstained with Sytox (1:10000; Invitrogen), mounted in Fluoromount G, and coverslipped for analysis using fluorescent microscopy.
Ethical issues
Embryos were considered discarded biological products and were collected with maternal agreement, and appropriate written consent was obtained. The study was approved by the Ethics Committee of Hanoi Medical University.
4. RESULTS
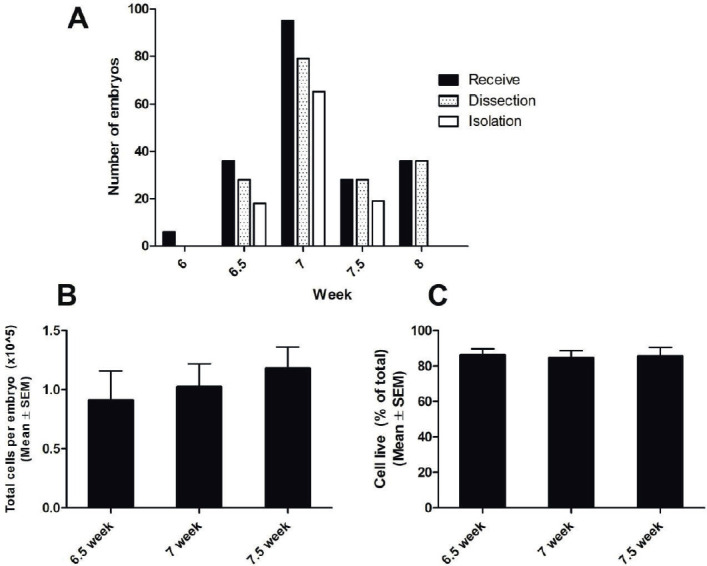
The ages of 201 human embryos were determined based on the date of embryo transfer, ranging from 6.0 to 8.0 weeks old, including 6 embryos at 6.0 weeks, 36 embryos at 6.5 weeks, 95 embryos at 7.0 weeks, 28 embryos at 7.5 weeks, and 36 embryos at 8.0 weeks (Figure 1). The embryos were removed as small pieces. Figure 1 shows the total number of embryos received from embryo reductions performed for multifetal pregnancies following in vitro fertilization, in addition to the numbers of embryos that were successfully dissected and isolated at 6.0, 6.5, 7.0, 7.5, and 8.0 weeks after embryo transfer (Figure 1A)., We were unable to successfully isolate neuroepithelial stem cells from embryos at 6.0 weeks or 8.0 weeks, due to the embryos being too small or due to the initial formation of the spine. The average cell numbers isolated from each embryo at 6.5, 7.0, and 7.5 weeks were 0.96 ± 0.14 × 105, 1.02 ± 0.17 × 105, and 1.08 ± 0.20 × 105, respectively (Figure 1B). No significant difference in the numbers of cells isolated was observed between these three stages. Using trypan blue staining, we determined that 84.7% - 86.1% of isolated cells survived (Figure 1C). Therefore, human neuroepithelial stem cells were successfully isolated from embryos 6.5–7.5 weeks after embryo transfer.
Figure 1. Isolation of neuroepithelial stem cells from human embryos. A: The number of embryos received, dissected, and from which stem cells were isolated at 6.0, 6.5, 7.0, 7.5, and 8.0 weeks. B: The average number of cells isolated from a single embryo (mean ± SEM) at 6.5, 7.0, and 7.5 weeks. C: The percentage of viable cells after isolation.
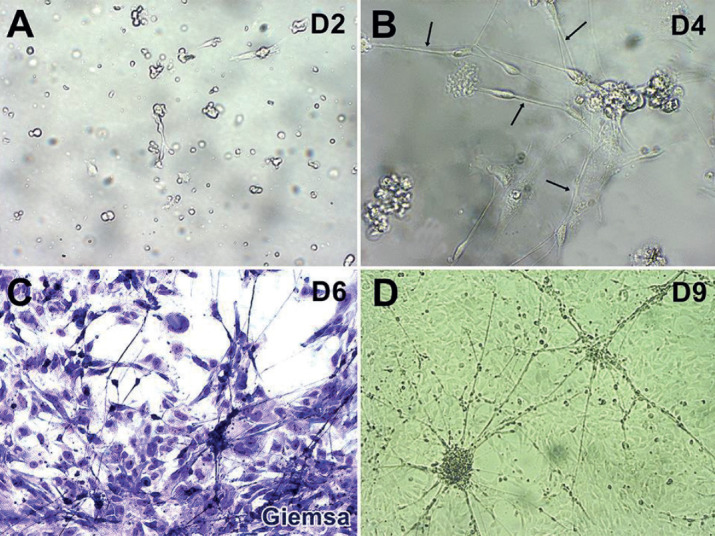
To examine whether these neuroepithelial stem cells could proliferate and differentiate, we cultured them in DMEM/F12 supplemented medium (see methods). At day 2, the stem cells adhered to the culture plate without neuronal polarization (Figure 2A). At day 4, neuronal polarization was initiated, and branches from the cell body resembling neurites were visible (12) (Figure 2B). The neurites continued to elongate on days 6 and 9 (Figure 2C and 2D).
Figure 2. Proliferation and differentiation of neuroepithelial stem cells. A: Cell adhesion on the culture plate at day 2. B: Neuronal polarization initiated at day 4. C and D: Cells proliferated and differentiated at day 6 (C) and day 9 (D). Arrows in B: branches from the cell body.
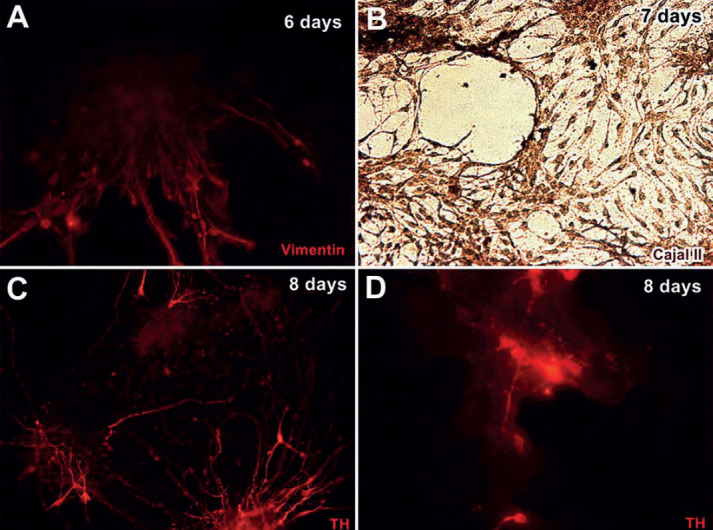
To test if cultured cells at days 6 and 7 could be characterized as neural cells, the cells were stained with vimentin antibody and Cajal silver stain. All cells were positive for vimentin antibody staining Cajal silver staining at day 7 (Figure 3A and 3B), suggesting that these cells were neural cells. Interestingly, at day 8, some cells appeared positive for tyrosine hydroxylase (TH) antibody staining, which is a marker of dopaminergic neurons (Figure 3C and 3D), indicating that some of the cultured cells differentiated into dopaminergic neurons.
Figure 3. Neuroepithelial stem cells differentiated into dopaminergic neurons. A: Cultured cells were visualized using an anti-vimentin antibody at day 6 (red). B: Cells were positive for Cajal silver staining at day 7 (brown). C and D: Some cells were positively stained with anti-tyrosine hydroxylase (TH) antibody at day 8 (red).
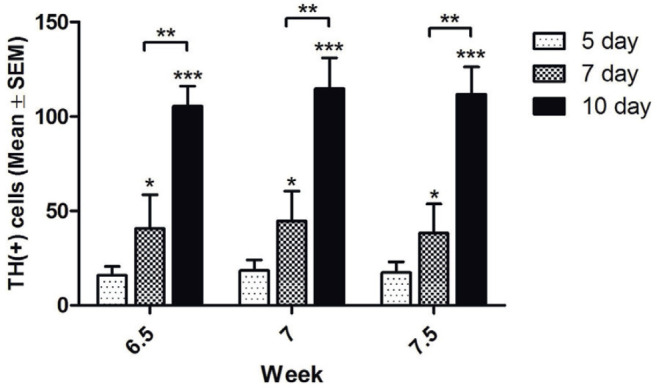
To evaluate whether the cultured cells continually differentiated into dopaminergic neurons, we calculated the average numbers of cells expressing TH in each well (2.2 cm diameter). In the 6.5-week group, the numbers of TH-positive cells were 15.9 ± 4.8, 40.7 ± 17.9, and 105.4 ± 10.7 at days 5, 7, and 10, respectively. In the 7.0-week group, these numbers were 18.4 ± 5.7, 44.7 ± 15.9, and 114.7 ± 16.4, respectively. The respective numbers of TH-positive cells in the 7.5-week group were 17.4 ± 5.6, 38.2 ± 15.5, and 111.8 ± 14.4. Significant differences in the numbers of TH-positive cells between the three groups were observed at days 7 and 10 (Figure 4).
Figure 4. Cultured cells continually differentiated into dopaminergic neurons. The numbers of tyrosine hydroxylase (TH)-positive cells at days 5, 7, and 10 in the 6.5-, 7.0-, and 7.5-week groups. *: P < 0.05; data represent the mean ± SEM.
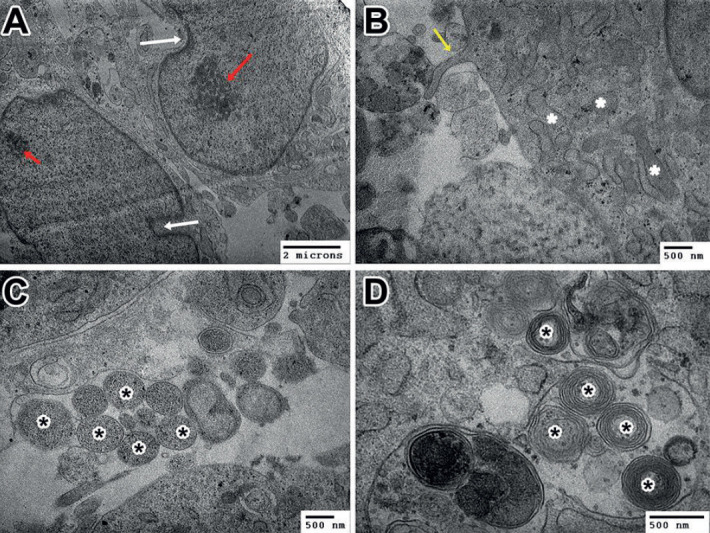
We assessed the morphological characteristics of cultured cells at day 10 by TEM. At day 10, some cells still possessed the morphological characteristics of neural stem cells, with irregularly shaped nuclei that frequently showed deep indentations in the nuclear membrane and one or two nucleoli (Figure 5A). Many cells had differentiated into neurons, with branches (Figure 5B and C) and myelinated axons (Figure 5D). These findings demonstrated that human neuroepithelial stem cells were successfully isolated from the embryos obtained from multifetal pregnancy reductions and that the isolated stem cells were able to proliferate and differentiated into dopaminergic neurons.
Figure 5. Cultured cells at day 10 expressed the morphological characteristics of neural stem cells and neurons. A: Neural stem cells; white arrows: indentations; red arrows: nucleoli. B: Neurites protruded from the cell body (yellow arrow), rough endoplasmic reticulum (*). C and D: Cultured cells have branches (* in C) and myelinated axons (* in D).
5. DISCUSSION
The brain is considered an immune-privileged site, making it relatively permissive for immune reactions, which could contribute to the success of cell therapy in the brain (13). The replacement of dopaminergic cells in patients with PD using transplantation was examined several decades ago, but this transplantation of dopaminergic cells has yet to be developed into a clinically competitive treatment. Dopaminergic cells for therapy can be derived from human FM tissue, neural stem cells, or iPS cells. However, neural stem cells used for transplantation can also differentiate into other cell types, such as astrocytes and oligodendrocytes, in addition to dopaminergic neurons (14). Moreover, the acquisition and in vitro expansion of neural stem cells to obtain a sufficient number of donor cells can be difficult (8). In cell therapy approaches using iPS cells, tumorigenesis has been reported as a major problem. Tumor formation was attributed to residual immature cells present in the graft with high proliferation activity. Residual iPS donor cells can cause teratoma formation in the brain; however, recent improvements in induction technology have minimized this problem (15, 16). However, even if the cells are appropriately differentiated into a neural linage, the risk of neural overgrowth among immature proliferative neural cells remains. Therefore, the preparation of the donor cells from iPS cells requires tight control of the cell phenotype and maturation. Hargus et al indicated that PD patient iPS cell-derived dopaminergic neurons survived at high numbers, showed arborization, and mediated functional effects in an animal model of PD, including reduced amphetamine- and apomorphine-induced rotation asymmetry; however, only a few dopaminergic neurons projected into the host striatum at 16 weeks after transplantation (15). However, both the efficient differentiation of human iPS cells into dopaminergic neurons and the behavioral functionality of dopaminergic neurons derived from human iPS cells have yet to be demonstrated in clinical trials. Cell therapy for patients with PD using donor cells obtained from the mesencephalon of aborted embryos represents the most common type of transplant performed in the clinic, first described in the late 1980s. A large number of studies have established that fetal dopaminergic neurons can survive and grow following intrastriatal transplantation into the brains of patients with PD patient. The strongest evidence that human fetal dopaminergic grafts can give rise to clinically competitive improvements comes from two patients who were subjected to the bilateral intrastriatal transplantation of human FM tissue (17, 18). The motor improvements observed in both patients were sustained for up to 18 years after grafting, including more than 10 years after the withdrawal of dopaminergic medication (17, 18). However, several challenges must be overcome when using fetal dopaminergic neurons obtained from the mesencephalon of aborted human embryos, including [1] the identification of the accurate embryo age; [2] the difficulty identifying the fetus in embryo abortions; [3] and non-living embryos obtained from drug-derived abortions.
Embryo reductions are frequently performed in multifetal pregnancies following in vitro fertilization treatments. In Vietnam, no laws limit the number of embryos that can be used in each transfer, and in vitro fertilization patients are typically implanted with 2-4 embryos. Therefore, multiple pregnancies sometimes occur. Women carrying three or more fetuses are at risk of developing various problems, including a greatly increased risk of premature delivery, which can be detrimental to the health and survival of the fetuses. In addition, non-medical challenges are associated with multiple pregnancies, such as the parents’ emotional or financial readiness to care for multiple infants. Thus, multifetal pregnancy reductions have been advocated as a method for reducing these risks (9, 10, 19). Embryonic products derived from multifetal pregnancy reductions represent an excellent source for the isolation of neural stem cells, as post-isolation cells from these procedures are associated with high survival rates, and their aseptic acquisition makes them suitable for proliferation. We collected embryos from multifetal pregnancy reductions to examine the ability to isolate and culture these neural stem cells into dopaminergic neurons. The survival rate of the stem cells obtained in this study was approximately 85%, with no specimens infected with fungal infections. Although dopaminergic neurons have been identified in FM tissue, the embryos obtained from multifetal pregnancy reductions are typically not intact, making the exact localization of the middle brain difficult. Thus, we collected multiple neural segments. The 6-week-old embryo was too small to be able to distinguish the neural tube in embryos obtained from embryo reduction procedures. By contrast, in 8-week-old embryos, the spine had begun to form (data not shown), preventing neural tube isolation. In embryos 6.5-7.5 weeks old, the neural tube could be identified. The accurate identification of the neural tube facilitated the isolation of neuroepithelial stem cells, which were successfully cultured, proliferated, and differentiated into dopaminergic neurons in our study. Consistent with our study, Wictorin et al also used dissociated ventral mesencephalons obtained from 6–8-week-old human embryos for transplantation into the brain of a PD rat model (20).
In our study, the culture time varied depending on the embryo. Most cells adhere to the culture plate after 2 days. Although many stem cells proliferate and differentiate gradually, neurites were observed protruding from the cell body after 4 days, and myelinated axons formed, demonstrating that these cells could develop a neuronal morphology, although some cells retained the morphological characteristics of neural stem cells, characterized by deep indentations in irregularly shaped nuclei (21). Immunocytochemical staining using TH antibodies revealed that some neurons were TH-positive, suggesting that the neural stem cells successfully differentiated into dopaminergic neurons. Thus, the cells expressed both the morphological and functional characteristics of dopaminergic neurons after 6–8 days in culture. Banker et al also reported that rodent brain–derived isolated neurons displayed in vitro neuronal polarity after 7 days (22, 23). However, these neurons displayed several minor neurites as early as day 1 after being plated in culture, compared with the appearance of neurites on day 2–3 in our study. This difference may be due to differences in the species or timing of cell isolation. After 7–10 days of culture, the cells began to spread out and fill the culture plate and could be used for cryopreservation or grafting.
6. CONCLUSION
In summary, this study demonstrated that neuroepithelial stem cells obtained 6.5–7.5-week-old embryos obtained through embryo reduction procedures performed in multifetal pregnancies after in vitro fertilization could be isolated, cultured, proliferated, and differentiated into human dopaminergic neurons. This is the first report describing the use of embryo reduction–derived products in cultures to develop a dopaminergic neuronal source for use in cell therapy for patients with PD, which opens a new avenue for the use of embryo reduction–derived products as a cellular source for the treatment of patients with PD. The development of human embryo reduction–derived dopaminergic neurons can be exploited in cell therapy approaches in animal models of PD.
Ethical approval:
This study was approved by the Ethics Committee of Hanoi Medical University.
Author’s contribution:
Nguyen Thi Binh and Nguyen Khang Son contributed equally to this article. Nguyen Thi Binh and Nguyen Khang Son gave a substantial contribution to the acquisition, analysis, and data interpretation. Nguyen Minh Duc and Nguyen Manh Ha had a part in preparing the article for drafting and revising it critically for important intellectual content. Each author gave final approval of the version to be published and agreed to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Conflicts of interest:
There are no conflicts of interest to declare.
Financial support and sponsorship:
Nil.
REFERENCES
- 1.Pires AO, Teixeira FG, Mendes-Pinheiro B, Serra SC, Sousa N, Salgado AJ. Old and new challenges in Parkinson’s disease therapeutics. Prog Neurobiol. 2017;156:69–89. doi: 10.1016/j.pneurobio.2017.04.006. [DOI] [PubMed] [Google Scholar]
- 2.Lindvall O. Clinical translation of stem cell transplantation in Parkinson’s disease. J Intern Med. 2016;279(1):30–40. doi: 10.1111/joim.12415. [DOI] [PubMed] [Google Scholar]
- 3.Perlow MJ, Freed WJ, Hoffer BJ, Seiger A, Olson L, Wyatt RJ. Brain grafts reduce motor abnormalities produced by destruction of nigrostriatal dopamine system. Science. 1979;204(4393):643–647. doi: 10.1126/science.571147. [DOI] [PubMed] [Google Scholar]
- 4.Barker RA, Barrett J, Mason SL, Bjorklund A. Fetal dopaminergic transplantation trials and the future of neural grafting in Parkinson’s disease. Lancet Neurol. 2013;12(1):84–91. doi: 10.1016/S1474-4422(12)70295-8. [DOI] [PubMed] [Google Scholar]
- 5.Freed CR, Greene PE, Breeze RE, et al. Transplantation of embryonic dopamine neurons for severe Parkinson’s disease. N Engl J Med. 2001;344(10):710–719. doi: 10.1056/NEJM200103083441002. [DOI] [PubMed] [Google Scholar]
- 6.Brundin P, Kordower JH. Neuropathology in transplants in Parkinson’s disease: implications for disease pathogenesis and the future of cell therapy. Prog Brain Res. 2012;200:221–241. doi: 10.1016/B978-0-444-59575-1.00010-7. [DOI] [PubMed] [Google Scholar]
- 7.Olanow CW, Goetz CG, Kordower JH, et al. A double-blind controlled trial of bilateral fetal nigral transplantation in Parkinson’s disease. Ann Neurol. 2003;54(3):403–414. doi: 10.1002/ana.10720. [DOI] [PubMed] [Google Scholar]
- 8.Morizane A, Takahashi J. Cell therapy for Parkinson’s disease. Neurol Med Chir (Tokyo) 2016;56(3):102–109. doi: 10.2176/nmc.ra.2015-0303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.ACOG educational bulletin. Special problems of multiple gestation. Number 253, November 1998 (Replaces Number 131, August 1989). American College of Obstetricians and Gynecologists. Int J Gynaecol Obstet. 1999;64(3):323–333. [PubMed] [Google Scholar]
- 10.Tabsh KM. Transabdominal multifetal pregnancy reduction: report of 40 cases. Obstet Gynecol. 1990;75(5):739–741. [PubMed] [Google Scholar]
- 11.Zipori Y, Haas J, Berger H, Barzilay E. Multifetal reduction of triplets to twins compared with non-reduced twins: a meta-analysis. Reprod Biomed Online. 2017;35(1):87–93. doi: 10.1016/j.rbmo.2017.04.001. [DOI] [PubMed] [Google Scholar]
- 12.Flynn KC. The cytoskeleton and neurite initiation. Bioarchitecture. 2013;3(4):86–109. doi: 10.4161/bioa.26259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Barker RA, Widner H. Immune problems in central nervous system cell therapy. NeuroRx. 2004;1(4):472–481. doi: 10.1602/neurorx.1.4.472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gage FH, Ray J, Fisher LJ. Isolation, characterization, and use of stem cells from the CNS. Annu Rev Neurosci. 1995;18:159–192. doi: 10.1146/annurev.ne.18.030195.001111. [DOI] [PubMed] [Google Scholar]
- 15.Hargus G, Cooper O, Deleidi M, et al. Differentiated Parkinson patient-derived induced pluripotent stem cells grow in the adult rodent brain and reduce motor asymmetry in Parkinsonian rats. Proc Natl Acad Sci U S A. 2010;107(36):15921–15926. doi: 10.1073/pnas.1010209107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Doi D, Morizane A, Kikuchi T, et al. Prolonged maturation culture favors a reduction in the tumorigenicity and the dopaminergic function of human ESC-derived neural cells in a primate model of Parkinson’s disease. Stem Cells. 2012;30(5):935–945. doi: 10.1002/stem.1060. [DOI] [PubMed] [Google Scholar]
- 17.Politis M, Wu K, Loane C, et al. Serotonergic neurons mediate dyskinesia side effects in Parkinson’s patients with neural transplants. Sci Transl Med. 2010;2(38):38ra46. doi: 10.1126/scitranslmed.3000976. [DOI] [PubMed] [Google Scholar]
- 18.Kefalopoulou Z, Politis M, Piccini P, et al. Long-term clinical outcome of fetal cell transplantation for Parkinson disease: two case reports. JAMA Neurol. 2014;71(1):83–87. doi: 10.1001/jamaneurol.2013.4749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Berkowitz RL, Lynch L, Lapinski R, Bergh P. First-trimester transabdominal multifetal pregnancy reduction: a report of two hundred completed cases. Am J Obstet Gynecol. 1993;169(1):17–21. doi: 10.1016/0002-9378(93)90124-2. [DOI] [PubMed] [Google Scholar]
- 20.Wictorin K, Brundin P, Sauer H, Lindvall O, Bjorklund A. Long distance directed axonal growth from human dopaminergic mesencephalic neuroblasts implanted along the nigrostriatal pathway in 6-hydroxydopamine lesioned adult rats. J Comp Neurol. 1992;323(4):475–494. doi: 10.1002/cne.903230403. [DOI] [PubMed] [Google Scholar]
- 21.Doetsch F, Garcia-Verdugo JM, Alvarez-Buylla A. Cellular composition and three-dimensional organization of the subventricular germinal zone in the adult mammalian brain. J Neurosci. 1997;17(13):5046–5061. doi: 10.1523/JNEUROSCI.17-13-05046.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Craig AM, Banker G. Neuronal polarity. Annu Rev Neurosci. 1994;17:267–310. doi: 10.1146/annurev.ne.17.030194.001411. [DOI] [PubMed] [Google Scholar]
- 23.Dotti CG, Sullivan CA, Banker GA. The establishment of polarity by hippocampal neurons in culture. J Neurosci. 1988;8(4):1454–1468. doi: 10.1523/JNEUROSCI.08-04-01454.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]


