Background:
Breast implant insertion funnels have become popular adjuncts to breast implant surgery to reduce access incision length and contact of the implant with the skin of the breast. Although labeled as single-use devices, due to cost considerations, many surgeons use a new breast implant insertion funnel with each patient rather than each breast. The purpose of this study was to evaluate the prevalence of capsular contracture of the first augmentation side and compare it to the second side utilizing one insertion funnel per patient.
Methods:
Patients undergoing silicone breast augmentation or silicone augmentation mastopexy with smooth surface silicone implant and utilizing a breast implant insertion funnel were studied. Six hundred consecutive patients (1200 breasts) meeting the study criteria were evaluated. Memory Gel silicone breast implants were utilized for each patient and only patients undergoing augmentation with the same implant size were studied. Patients underwent augmentation with either inframammary or periareolar incisions.
Results:
A total of 27 capsular contractures were noted, a rate of 2.25%. The rate of capsular contracture was significantly higher with the second-side use of insertion funnels (P = 0.0179). Of the capsular contractures noted, 25.9% occurred on the first side, whereas 74.1% occurred on the second side. Capsular contracture rates were higher on the second side for both access incision locations. Capsular contracture prevalence increased with reuse of the same insertion funnel for the same patient.
Conclusion:
Based on these findings, surgeons should consider utilizing implant insertion devices as single-use, to minimize the capsular contracture risk.
INTRODUCTION
Recently, breast implant insertion devices, such as insertion funnels, have become popular to assist with inserting breast implants for both aesthetic and reconstructive surgery. A number of studies support their use and suggest that access incision length and implant insertion time may be reduced.1,2 Other studies suggest that implant funnels may reduce implant contact with the skin and capsular contracture.3,4 These devices are sold as “single-use” and single-patient devices,5 and are costly compared to other disposable breast augmentation supplies. Other devices or cost-efficient techniques made from commonly available medical supplies, such as sterile IV bags,6 or sterile gloves7 have not been widely adopted.
There is a wide variation in how implant insertion devices are used by plastic surgeons. Published articles reporting the use of implant funnels have described using a new funnel per case,1,3,4 and many surgeons performing aesthetic surgery utilize these devices in this way. Other surgeons, especially those performing reconstructive procedures in which a third-party payor covers surgical and implant supplies, may utilize more than one insertion funnel per case placing each new implant with a new insertion funnel. Finally, in a cost-saving effort and outside of product recommendations,5 a minority of surgeons may reuse implant funnels after washing and resterilizing them.
The official product insert for the insertion funnel indicates that it is intended for single use, one patient only and it not to be reused or resterilized. The insert further indicates that “reuse or resterilization may lead to diminished product performance, including loss of lubricity, potentially causing breast implant damage and implant rupture.”5 Inserting a second breast implant on the same patient with the same insertion funnel is a reuse, and it is not known whether or not this practice has an effect on the prevalence or rate of capsular contracture. We were interested in evaluating if there was a difference in the capsular contracture prevalence between the first and second side when a new, single funnel was utilized per patient. Due to the low incidence of capsular contracture among the patients studied, prevalence analysis as well as statistical testing was performed.
METHOD
Surgical Procedure
A retrospective within-subjects study was performed on 600 consecutive patients, meeting study criteria, who underwent primary smooth silicone breast augmentation or primary augmentation mastopexy by one surgeon, between 2015 and 2018. All patients included in the study were between the ages of 22 and 60 years, utilizing Mentor Memory Gel silicone implants of the same size on each side (Mentor Worldwide, Irvine, Calif.) and the off-label use of triple-antibiotic irrigation containing povidone-iodine (Betadine; Purdue Frederick Co., Norwalk, Conn.). Patients underwent a dual-plane silicone breast augmentation through inframammary or periareolar incisions, alternating the right and left sides as the first side. For patients undergoing primary augmentation mastopexy, breast implants were placed and breast tissue was closed before any mastopexy dissection. All patients received preoperative intravenous antibiotics, either a 1-g dose of cephazolin or 600 mg of clindamycin, selected based on allergy profiles. Before insertion of implants, triple-antibiotic irrigation [50,000 U of bacitracin, 1 g of Ancef (GlaxoSmithKline, Middlesex, United Kingdom), and 80 mg of gentamicin] with the addition of 50 ml of povidone-iodine in 500 ml of normal saline was used. Access incision location was selected based on the patient and surgeon preference. New, sterile retractors were used for each implant insertion. Tegaderm dressings were used as nipple shields and skin barriers for implant insertion, and a new, sterile insertion funnel, Keller Funnel II (Allergan, Dublin, Ireland) was used for each patient for insertion of the breast implants.
Analysis
All patients were evaluated at frequent follow-up appointments by both the author and a plastic surgery nurse specialist, including early postoperative visits, 1 month, 3 months, 6 months, and 1 year postoperatively. Capsular contracture was evaluated by the Baker scale. Patients with grade III or IV capsular contractures at the 1-year postoperative visit were considered as having clinical capsular contracture.
Statistical Analysis
A within-subjects retrospective analysis was performed. Prevalence rates for capsular contractures were evaluated. Chi-square analysis was used to compare the incision locations on their respective rates of capsular contracture when all four cells of the 2×2 table had more than five observations. When any cell of the 2×2 table had less than five observations in it, the Fisher exact test was used to compare the incision locations of categorical outcomes. All analyses were performed using SPSS Version 26 (IBM Corp., Armonk, N.Y.) and statistical significance was assumed at a two-tailed alpha value of 0.05.
RESULTS
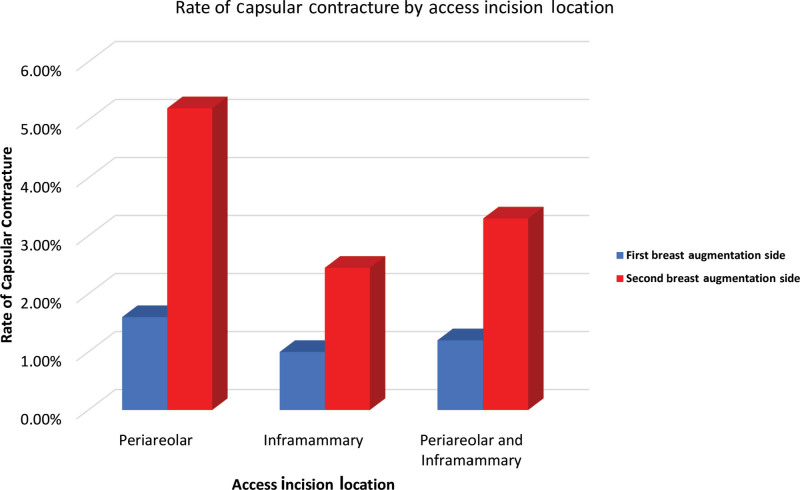
A total of 600 patients underwent 1200 breast augmentation procedures. Characteristics of the study population are shown in Table 1. Twenty-seven capsular contractures (n = 27) were noted, with an overall capsular contracture rate in the series of 2.25%. The majority of capsular contractures occurred on the second side (74.1%). The number of breast augmentation procedures (by number of breasts) undergoing silicone breast augmentation by incision location, frequency and proportion of capsular contractures, and the frequency and proportions of capsular contractures based on the order of insertion are shown in Table 2. A statistically significant difference in capsular contracture rates was detected between the first and second sides for the total number of breasts studied (P = 0.0179), with the rate being significantly higher for the periareolar incision location. The prevalence of capsular contracture for both periareolar and inframammary incisions was much higher on the second side (5.18% and 2.45%, respectively) compared to the first side (1.55% and 1.00%, respectively). The overall prevalence of second-side capsular contracture (3.3%) was also higher than that of the first-side capsular contracture (1.17%) (Fig. 1).
Table 1.
Characteristics of Patients and Implant Volume with SD for Patients Undergoing Breast Augmentation with the Aid of Insertion Funnels
| No. Patients | Mean Age (years) | Mean BMI (kg/m2) | Implant Volume (cc) |
|---|---|---|---|
| 600 | 31.7 ± 8.9 | 23.2 ± 3.1 | 338 ± 51.2 |
Table 2.
Frequency and Percentage Statistics for Capsular Contractures
| Incision Location | Total Breasts (n) | Capsular Contracture (n, %) | First Side (n, %) | Second Side (n, %) |
|---|---|---|---|---|
| Periareolar | 386 | 13 (3.36%) | 3 (1.55%) | 10 (5.18%) |
| Inframammary | 814 | 14 (1.72%) | 4 (1.00%) | 10 (2.45%) |
| Total | 1200 | 27 (2.25%) | 7 (1.17%) | 20 (3.33%) |
Fig. 1.
The rate of capsular contracture for patients undergoing specific access incision locations (periareolar and inframammary) and the combined rate for all patients evaluated in the study.
DISCUSSION
The data demonstrate that the rate of capsular contracture on the second side when using the same insertion funnel has a statistically higher capsular contracture rate compared to the first side. This is the first report demonstrating that the reuse of an implant insertion device, on the same patient and in the same case, carries with it a higher rate of capsular contracture on the second side. The implications for surgeons cannot be overemphasized. If a surgeon decides to utilize an insertion device for the placement of breast implants, they should know that there is a distinct possibility that this practice may increase the risk of capsular contracture for the patient. Although the capsular contracture rates in the present study were low and within the range typically reported in the literature,8–14 an approximate three-fold increase in the prevalence of capsular contracture on the second side was noted for all patients studied. This suggests that there may be contamination of the insertion funnel when delivering a sterile breast implant to the second breast despite limiting handling of the funnel and a “no-touch” technique.
The concept of a “no-touch” technique is a misnomer and more of a marketing term than an accurate description of how insertion funnels are currently used in practice. Surgeons experienced in the use of insertion funnels often need to open the funnel with a gloved hand as well as trim funnel length. Many surgeons place the sterile breast implant in the funnel with a gloved hand and adjust the implant within the funnel. Large implants are difficult to place within the funnel without manual placement due to the limited opening size of the proximal funnel end and the risk of the implant falling out of the funnel opening without manual contact. Often surgeons verify implant position and orientation in the pocket with a gloved finger after delivering the implant into the pocket, thereby potentially contaminating the implant. This may be necessary as implants can rotate within the funnel during insertion. For these and other reasons, a truly “no-touch” procedure is rarely accomplished with insertion funnels or other insertion devices because the surgeon either touches the interior of the funnel, touches the implant either before or after insertion, or the funnel tip contacts skin or potentially colonized breast tissue. A very disciplined approach or a truly single-use device is required to minimize potential contamination of the funnel and implant, but even in these cases, rarely is a “no-touch” accomplished.
A statistically significant difference in capsular contracture rates was detected between the first and second side when all patients were aggregated (capsular contracture rate of 1.17% for the first side and 3.33% for the second side), P = 0.0179. The prevalence of capsular contracture was much greater on the second side for both incision locations with the rate of capsular contracture at least 2.5 times the rate of the first side for either incision location. However, due to the low prevalence of capsular contracture noted in this study, it was underpowered to detect differences in rates of capsular contracture when comparing the incision locations independently. Larger sample sizes of each incision type would be required to further analyze each incision location independently.
Additional findings demonstrated higher capsular contracture rates for periareolar incisions compared to inframammary for both the first- and second-side insertions. Although periareolar access incisions are known to show higher capsular contracture rates than inframammary access incisions in previous studies,8–10 it was not previously known that this trend becomes magnified with second-side use. The rate of capsular contracture periareolar second-side insertion was 3.3 times greater than the first-side insertion, whereas inframammary second-side insertion was 2.45 times greater than the first-side insertion.
We believe that contamination of the funnel tip best explains the higher rate of capsular contracture with use of the insertion funnel on the second side. It is well-known that bacteria are associated with the skin around breast incisions and that access incisions for the placement of breast implants may transect mammary ducts containing bacteria.4,12,14 During use, an insertion funnel is placed within the skin incision such that the funnel tip comes in contact with the skin and breast tissue up to a depth of 1 cm.5 Reuse of a microscopically contaminated insertion funnel can lead to bacteria coming in contact with the second-side implant which is propelled through the insertion funnel into the second breast pocket. Implant contamination with bacteria and resulting biofilm are a known cause of capsular contracture,15–20 and this pathogenesis would explain the results of this study in which an approximate three-fold increase in capsular contracture incidence was noted on the second-side breast implant insertion.
The insertion funnel is designed as a single-use and single-patient insertion device, yet the majority of surgeons do not use insertion funnels in this way. Most aesthetic plastic surgeons utilize one insertion funnel per patient, using the device at least twice. Other doctors use one funnel to insert implant sizers before placing permanent implants, potentially contaminating the insertion funnel and permanent implants in the process. A minority of surgeons have been reported to resterilize funnels and utilize them for several cases before discarding them. Despite resterilization, multiple patient use has not been shown to be safe and effective and the infection and capsular contracture risks associated with this practice are unknown. The results of this study demonstrate that utilizing the insertion funnel on the same patient for permanent implant placement on the second side, likely increases capsular contracture risk. Therefore, it is obvious that making multiple insertions of sizers or reusing insertion funnels on multiple patients is a poor choice for optimizing patient outcomes.
The cost of insertion devices remains a barrier for some plastic surgeons to embrace this technology. The most popular device, the Keller Funnel, is costly enough to make the use of two insertion devices per case, or one device per breast, cost prohibitive for the majority of aesthetic surgeons. To encourage plastic surgeons to utilize single-use devices, there should be more cost-effective options available for inserting implants with the benefit of reduced handling and tissue contact. With a reasonable production cost and a design optimized for single, sterile use, implant manufacturers should consider providing insertion devices with implants so that all surgeons are able to utilize this technology with resulting patient benefit. This practice is likely to improve outcomes and reduce capsular contracture associated with insertion device reuse.
Newman and Davison4 reported reduced incidence of capsular contracture utilizing an implant insertion funnel with periareolar breast augmentation, however, did not evaluate which side capsular contractures occurred. Their findings suggested that implant contact with breast tissue-containing transected mammary ducts was reduced with funnel usage and that the funnel provided a protective benefit to reduce biofilm exposure. Our study has shown that the protective effect is reduced when a funnel is reused on the second side. The most plausible explanation for our findings is the likelihood of subclinical contamination of the funnel tip associated with the skin and tissue contact in the highly bacterial-colonized periareolar region. Numerous studies have shown that the nipple/areolar complex region is highly populated with bacteria,21,22 and it is likely that the funnel tip becomes contaminated with bacteria with the initial funnel use. Additional studies can evaluate funnel sterility after initial use to quantitate this potential problem.
Multivariate analysis can be an important statistical test for evaluating capsular contracture occurrence; however, there are several important weaknesses which may make other testing modalities useful. For multivariate techniques to give meaningful results, they need a large sample of data; otherwise, the results may not be meaningful due to high standard errors. In addition, since the operating surgeon is an important variable in capsular contracture occurrence, any test which does not factor the operating surgeon into the analysis is unlikely to be accurate. For example, Calobrace et al23 used multivariate analysis using backward elimination to assess potential risk factors associated with capsular contracture. However, with 34 different surgeons not being accounted for as a factor in the adjusted findings, important variations in surgeon-specific parameters likely detracted from the precision of the significant effects. The current study was designed in a within-subjects fashion to increase statistical power by using each participant as their own control and to reduce surgeon-specific variation, with only one surgeon being accounted for in the results. The within-subjects design reduced random variability amongst the study participants and amongst surgeons. Also, the within-subjects design was employed because the prevalence of the primary outcome, capsular contracture, was very low, which would not allow for multivariate analysis that could control for pertinent confounding variables. Several thousand more patients would have to be enrolled in the observational study to be able to test for confounding effects. The researcher also took measures when defining the inclusion and exclusion criteria of the population of interest to reduce potential confounders by defining a homogeneous population. The combination of the within-subjects design in a homogeneous population for a rare outcome with only one surgeon performing procedures provided a much more precise measure of association with capsular contracture.
This study demonstrates the clinically important finding that the rate of capsular contracture is higher on the second side when utilizing the same insertion device. Surgeons may want to consider utilizing a new, sterile insertion device for each breast to reduce the risk of second-side capsular contracture. More options for cost-effective, single-use insertion devices should be available. Prospective, randomized clinical trials would be helpful in determining optimal use of these devices to reduce contamination and the risk of second-side capsular contracture.
Footnotes
Published online 2 November 2021.
Disclosure: Dr. Bresnick holds a US patent, US 10,874,430 B2, for a Biofilm Protection Shield.
REFERENCES
- 1.Montemurro P, Fischer S, Schyllander S, et al. Implant insertion time and incision length in breast augmentation surgery with the Keller funnel: results from a comparative study. Aesthetic Plast Surg. 2019;43:881–889. [DOI] [PubMed] [Google Scholar]
- 2.Moyer HR, Ghazi B, Saunders N, et al. Contamination in smooth gel breast implant placement: testing a funnel versus digital insertion technique in a cadaver model. Aesthet Surg J. 2012;32:194–199. [DOI] [PubMed] [Google Scholar]
- 3.Flugstad NA, Pozner JN, Baxter RA, et al. Does implant insertion with a Funnel decrease capsular contracture? A preliminary report. Aesthet Surg J. 2016;36:550–556. [DOI] [PubMed] [Google Scholar]
- 4.Newman AN, Davison SP. Effect of Keller funnel on the rate of capsular contracture in periareolar breast augmentation. Plast Reconstr Surg Glob Open. 2018;6:e1834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Keller Funnel 2, Product insert and instructions. Allergan: 2019. [Google Scholar]
- 6.Barker AS, Law J, Nicholson M, et al. The reversed glove sleeve: A readily available and cost-effective way to achieve “No Touch” breast implant insertion. Plast Reconstr Surg Glob Open. 2020;8:e2650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Panczel G, Munhoz AM. A simple and low-cost method of sleeve to insert silicone Gel breast implants. Plast Reconstr Surg Glob Open. 2019;7:e2389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wiener TC. Relationship of incision choice to capsular contracture. Aesthetic Plast Surg. 2008;32:303–306. [DOI] [PubMed] [Google Scholar]
- 9.Jacobson JM, Gatti ME, Schaffner AD, et al. Effect of incision choice on outcomes in primary breast augmentation. Aesthet Surg J. 2012;32:456–462. [DOI] [PubMed] [Google Scholar]
- 10.Bresnick SD. Prophylactic leukotriene inhibitor therapy for the reduction of capsular contracture in primary silicone breast augmentation: experience with 1100 cases. Plast Reconst Surg. 2017;139:379e–385e. [Google Scholar]
- 11.Codner MA, Mejia JD, Locke MB, et al. A 15-year experience with primary breast augmentation. Plast Reconstr Surg. 2011;127:1300–1310. [DOI] [PubMed] [Google Scholar]
- 12.Li S, Chen L, Liu W, et al. Capsular contracture rate after breast augmentation with periareolar versus other two (inframammary and transaxillary) incisions: a meta-analysis. Aesthetic Plast Surg. 2018;42:32–37. [DOI] [PubMed] [Google Scholar]
- 13.Somogyi RB, Brown MH. Outcomes in primary breast augmentation: A single surgeon’s review of 1539 consecutive cases. Plast Reconstr Surg. 2015;135:87–97. [DOI] [PubMed] [Google Scholar]
- 14.Namnoum JD, Largent J, Kaplan HM, et al. Primary breast augmentation clinical trial outcomes stratified by surgical incision, anatomical placement and implant device type. J Plast Reconstr Aesthet Surg. 2013;66:1165–1172. [DOI] [PubMed] [Google Scholar]
- 15.Chong SJ, Deva AK. Understanding the etiology and prevention of capsular contracture: Translating science into practice. Clin Plast Surg. 2015;42:427–436. [DOI] [PubMed] [Google Scholar]
- 16.Deva AK, Adams WP, Jr, Vickery K. The role of bacterial biofilms in device-associated infection. Plast Reconstr Surg. 2013;132:1319–1328. [DOI] [PubMed] [Google Scholar]
- 17.Wolfram D, Rabensteiner E, Grundtman C, et al. T regulatory cells and TH17 cells in peri-silicone implant capsular fibrosis. Plast Reconstr Surg. 2012;129:327e–337e. [DOI] [PubMed] [Google Scholar]
- 18.Tamboto H, Vickery K, Deva AK. Subclinical (biofilm) infection causes capsular contracture in a porcine model following augmentation mammaplasty. Plast Reconstr Surg. 2010;126:835–842. [DOI] [PubMed] [Google Scholar]
- 19.Ajdic D, Zoghbi Y, Gerth D, et al. The relationship of bacterial biofilms and capsular contracture in breast implants. Aesthet Surg J. 2016;36:297–309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Araco A, Caruso R, Araco F, et al. Capsular contractures: a systematic review. Plast Reconstr Surg. 2009;124:1808–1819. [DOI] [PubMed] [Google Scholar]
- 21.Rusby JE, Brachtel EF, Michaelson JS, et al. Breast duct anatomy in the human nipple: three-dimensional patterns and clinical implications. Breast Cancer Res Treat. 2007;106:171–179. [DOI] [PubMed] [Google Scholar]
- 22.Going JJ, Moffat DF. Escaping from Flatland: clinical and biological aspects of human mammary duct anatomy in three dimensions. J Pathol. 2004;203:538–544. [DOI] [PubMed] [Google Scholar]
- 23.Calobrace MB, Stevens WG, Capizzi PJ, et al. Risk factor analysis for capsular contracture: a 10-year sientra study using round, smooth, and textured implants for breast augmentation. Plast Reconstr Surg. 2018;141(4S Sientra Shaped and Round Cohesive Gel Implants):20S–28S. [DOI] [PubMed] [Google Scholar]