Abstract
Macrolide antibiotics are well known for their antibacterial properties, but extensive research in the context of inflammatory lung disease has revealed that they also have powerful immunomodulatory properties. It has been demonstrated that these drugs are therapeutically beneficial in various lung diseases, with evidence they significantly reduce exacerbations in patients with COPD, asthma, bronchiectasis and cystic fibrosis. The efficacy demonstrated in patients infected with macrolide tolerant organisms such as Pseudomonas aeruginosa supports the concept that their efficacy is at least partly related to immunomodulatory rather than antibacterial effects. Inconsistent data and an incomplete understanding of their mechanisms of action hampers the use of macrolide antibiotics as immunomodulatory therapies. Macrolides recently demonstrated no clinically relevant immunomodulatory effects in the context of COVID-19 infection. This review provides an overview of macrolide antibiotics and discusses their immunomodulatory effects and mechanisms of action in the context of inflammatory lung disease.
Keywords: COPD, Bronchiectasis, Cystic fibrosis, Azithromycin, Neutrophils
Abbreviation list
- AMR
Antimicrobial Resistance
- AZM
Azithromycin
- BE
Bronchiectasis
- CAM
Clarithromycin
- CF
Cystic Fibrosis
- COPD
Chronic Obstructive Pulmonary Disease
- COVID-19
Coronavirus Disease 2019
- DC
Dendritic Cells
- DIR
Dirithromycin
- DPB
Diffuse panbronchiolitis
- ERY
Erythromycin
- FEV1
Forced Expiratory Volume in 1 s
- FKBP12
FK506 binding protein 12
- HCQ
Hydroxychloroquine
- hMDCs
Human monocyte-derived DCs
- ICAM-1
Intracellular Adhesion Molecule 1
- IFNγ
Interferon γ
- IkB
Inhibitor of nuclear factor kappa B
- IKK
IkB Kinase
- JM
Josamycin
- LPS
Lipopolysaccharide
- M1-like
Pro-inflammatory Macrophages
- M2-like
Anti-inflammatory Macrophages
- mBMDCs
Murine bone marrow-derived DCs
- mTOR
mammalian Target of Rapamycin
- NE
Neutrophil Elastase
- NETs
Neutrophil Extracellular Traps
- NF-κB
Nuclear Factor kappa-light-chain-enhancer of activated B cells
- PDK1
Phosphoinositide-dependent kinase 1
- PI3K
Phosphoinositide 3-Kinase
- PMA
Phorbol 12-myristate 13-acetate
- QoL
Quality of Life
- RA
Rheumatoid Arthritis
- ROS
Reactive Oxygen Species
- ROX
Roxithromycin
- S6RP
S6 Ribosomal Protein
- SARS-CoV-2
Severe Acute Respiratory Syndrome Coronavirus 2
- SLE
Systemic Lupus Erythematosus
- SPM
Spiramycin
- TNF
Tumour Necrosis Factor
- TUL
Tulathromycin
1. Introduction
1.1. Macrolide antibiotics
Macrolide antibiotics are a group of natural products produced by the genus Streptomyces [1]. They contain a macrocyclic lactone ring and are classified as 14-, 15- or 16-membered based on the number of carbon atoms within this structure [1] (Table 1 ). Macrolides are bacteriostatic, predominantly against Gram-positive bacteria, as they competitively bind the bacterial 50S ribosomal subunit thus reducing protein synthesis and preventing replication [2].
Table 1.
Classification and structure of commonly used macrolides. Information and reference images from cited sources [1,[3], [4], [5]] with chemical structures drawn using ChemSpider (Royal Society of Chemistry, 2021) [6].
| Macrolide | Classification | Structure |
|---|---|---|
| Erythromycin (ERY) | 14-membered | 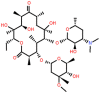 |
| Clarithromycin (CAM) | 14-membered | 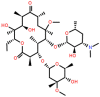 |
| Roxithromycin (ROX) | 14-membered | 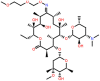 |
| Dirithromycin (DIR) | 14-membered | 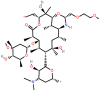 |
| Oleandomycin (OLE) (Veterinary use) |
14-membered | 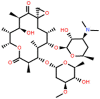 |
| Azithromycin (AZM) | 15-membered | 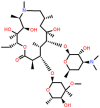 |
| Tulathromycin (TUL) (Veterinary use) |
15-membered | 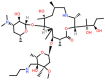 |
| Josamycin (JM) | 16-membered | 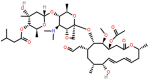 |
| Spiramycin (SPM) | 16-membered | 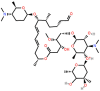 |
The immunomodulatory properties of macrolides were first described in 1987 by Kudoh and colleagues in a study of diffuse panbronchiolitis (DPB) [7], a rare, severe and progressive inflammatory lung disease driving irreversible lung damage [8] (Table 2 ). They found that long-term daily treatment with 400–600 mg of ERY suppressed DPB symptoms and increased patient life expectancy. Indeed, it is estimated that the 10-year mortality rate of DPB reduced from 90% to 10% after ERY became standard therapy. It was initially believed this was simply due to the antibiotic activity of the macrolides, but this was challenged by the observation that DPB patients are typically infected with the macrolide-resistant Gram-negative pathogen Pseudomonas aeruginosa, and that patients experienced clinical benefits despite serum levels of ERY being substantially below the antibacterial threshold [9]. This unanticipated discovery led to the theory that low-dose macrolide therapy might have immunomodulatory properties beyond its antibacterial actions. Since then, clinical and experimental research has aimed to decipher these effects and reveal the potential mechanisms of action. However, there is much controversy regarding these effects and the underlying mechanisms have yet to be completely defined.
Table 2.
A summary of key clinical studies of macrolides in respiratory disease. The listed clinical trials were conducted to establish the clinical, non-antibiotic effects seen following low-dose macrolide therapy in various respiratory diseases. Abbreviations: BE, Bronchiectasis; CF, Cystic Fibrosis; COPD, Chronic Obstructive Pulmonary Disease; QoL, Quality of Life.
| Author (Date) | Respiratory Disease | Type of Study | Macrolide | Dosage/Duration of Treatment | Clinical Outcomes |
|---|---|---|---|---|---|
| Kudoh et al. (1987) [7] | DPB | Observational cohort study | ERY | 400 mg–600mg daily; NK | Decrease in DPB symptoms, improved survival rate and QoL |
| Gibson et al. (2017) [10] | Asthma | Randomised, Placebo controlled, Double-blind Trial | AZM | 500 mg thrice weekly; 48 weeks | Reduced exacerbation number, improved QoL |
| Wong et al. (2012) [11] | BE | Randomised, Placebo-controlled, Double-blind Trial | AZM | 500 mg thrice weekly; 26 weeks | Reduced exacerbation number, no effect on lung function, no improved QoL |
| Altenburg et al. (2013) [12] | BE | Randomised, Placebo controlled, Double-blind Trial | AZM | 250 mg daily; 52 weeks | Reduced exacerbation number, improved lung function, improved symptoms and QoL |
| Serisier et al. (2013) [13] | BE | Randomised, Placebo controlled, Double-blind Trial | ERY | 400 mg twice daily; 52 weeks |
Reduced exacerbation number, improved lung function, no improved QoL, increase in macrolide-resistant bacteria, no effect on symptoms |
| Seemungal et al. (2008) [14] | COPD | Randomised, Placebo controlled, Double-blind Trial | ERY | 250 mg twice daily; 52 weeks |
Reduced number, duration and severity of exacerbations, no change in lung function |
| Albert et al. (2011) [15] | COPD | Randomised, Placebo controlled Trial | AZM | 250 mg daily; 52 weeks | Reduced exacerbation number, prolonged time until first exacerbation, improved QoL, |
| Uzun et al. (2014) [16] | COPD | Randomised, Placebo controlled, Double-blind Trial | AZM | 500 mg thrice weekly; 52 weeks | Reduced exacerbation frequency |
| Wolter et al. (2002) [17] | CF | Randomised, Placebo controlled, Double-blind Trial | AZM | 250 mg daily; 12 weeks | Maintained lung function, improved QoL, reduced exacerbation number and inflammation |
| Saiman et al. (2003) [18] | CF | Randomised, Placebo controlled, Double-blind Trial | AZM | 250 mg (<40 kg) or 500 mg (>40 kg) thrice weekly; 24 weeks |
Improved lung function, less risk of exacerbation, increased weight gain, reduced hospitalisations and antibiotic courses, improvement in physical functioning but not overall QoL |
| Saiman et al. (2010) [19] | CF | Multicenter Randomised, Placebo controlled, Double-blind Trial |
AZM | 250 mg (18–35.9 kg) or 500 mg (>or = 36 kg) thrice weekly; 24 weeks | No improvement in lung function, reduction in exacerbations, increased body weight, improvement of symptoms i.e. less cough and less productive cough |
1.2. Clinical efficacy of macrolides in lung disease
Given the success of macrolides in DPB and their subsequent use in other lung diseases (Table 2), the immunomodulatory properties of macrolides have been most widely explored in lung conditions. Chronic airway diseases, including chronic obstructive pulmonary disease (COPD), asthma, cystic fibrosis (CF) and bronchiectasis (BE), are conditions where airway inflammation gives rise to symptoms including cough, dyspnoea and excessive mucus production. Such diseases are characterised by frequent exacerbations, a reduced quality of life (QoL) and premature mortality [20]. Inhaled corticosteroids are the mainstay of anti-inflammatory treatment for many of these diseases and effectively reduce the frequency of exacerbations in a subset of patients with eosinophil-dominant inflammation [21]. However, the use of corticosteroids in neutrophil-dominant disease may exacerbate disease by delaying neutrophil apoptosis [22] and promoting the overgrowth of pathogenic bacteria, particularly Streptococcus, through suppression of cathelicidin antimicrobial peptides including LL-37 [23]. To date, there is no effective neutrophil-targeted anti-inflammatory therapy. Attempts to reduce neutrophilic inflammation, by, for example, reducing neutrophil numbers in the lung, have been unsuccessful due to increased incidence of infections. For example, blockade of CXCR2, a key receptor on neutrophils with high affinity for CXCL8 and other chemokines, resulted in increased rates of pneumonia due to bacterial overgrowth [24]. This is because CXCR2 mediates neutrophil chemotaxis and therefore its blockade likely reduces neutrophil numbers in the airway to impair bacterial clearance. Similar to CXCR2 ligands, leukotriene B4 is a key chemoattractant that aids neutrophil recruitment to the lung. Potent blockade of the leukotriene B4 receptor was associated with worse lung function and increased exacerbations of lung disease in adults and children with CF [25]. This illustrates the need to identify immunomodulatory treatments targeting neutrophilic lung diseases that are non-steroidal and do not reduce neutrophil recruitment or impair the antimicrobial functions of these cells. Macrolides have been suggested as a potential treatment particularly for neutrophilic lung diseases.
1.3. Clinical trials in chronic lung diseases
It is widely reported that long-term treatment with macrolide antibiotics, particularly 14- and 15-membered macrolides, improve clinical outcomes in several respiratory diseases (Table 2) including COPD, BE and CF which are primarily regarded as neutrophil-driven disorders. There are some apparently contradictory results between clinical trials with some trials reporting improvements in symptoms, lung function and QoL, with others reporting no clinical benefit in these areas (Table 2). However, differences in dosing and/or duration of treatment, as well as the use of different macrolides between studies, could explain these discrepancies.
These trials, however, all claim to have used doses below the reported antibacterial threshold (Table 2). For context, the commonly used daily doses of AZM and ERY for the treatment of acute respiratory infections are 500 mg and 2000–4000 mg, respectively [3]. Therefore, as macrolides show a positive effect in diseases where macrolide-resistant organisms are prominent, and at dosages below the antibacterial threshold, this suggests that clinical outcomes are not likely to be the result of antibacterial activity. This is further highlighted by reports that airway bacterial load remains unchanged following long-term low-dose therapy in chronic lung disease [26]. However, it should be noted that there is potential for antibacterial effects to occur, especially since macrolides have great capacity to accumulate within body tissues and cells, particularly phagocytes [2,27], with tissue concentrations reportedly reaching 10–100 times that of those in serum [27]. For this reason, macrolides are often taken thrice weekly instead of daily dosing in chronic diseases. The concept of macrolide accumulation causes great uncertainty when deciphering physiologically relevant drug concentrations to be used for in vitro studies and whether antimicrobial effects are in fact a major player in the mechanism behind the clinical benefit of macrolides (discussed later).
Regardless, macrolides have been shown in large scale randomized trials to reduce exacerbations in COPD, asthma, CF and BE [15,28,29] (Table 2). The magnitude of effect in these studies is remarkably similar (30–60% reduction in exacerbation frequency) despite diversity in pathophysiology between these conditions, suggesting a degree of shared biology. The results of randomized trials in these diseases have been extensively reviewed elsewhere and so are not discussed here in detail [9,30]. Nevertheless, several aspects of these trials support the view that macrolides have effects beyond traditional antimicrobial effects. In bronchiectasis, an individual patient data meta-analysis of 3 randomized trials showed an overall reduction in exacerbations of 51% along with improvements in symptoms. In responder analysis, the group of patients with the greatest response were patients chronically infected with Pseudomonas aeruginosa (rate ratio 0·36 (0·18–0·72)), an organism that is not traditionally considered susceptible to macrolide antibiotics [28]. Similarly, in CF, randomized trials of macrolides in patients chronically infected with P. aeruginosa were clearly positive with improvements in FEV1 and prolonged time to first exacerbation (0.65; 95% CI, 0.44–0.95; P = 0.03) [18] while no improvements in lung function in patients without P. aeruginosa were observed, although exacerbations were still reduced [19].
In asthma, a disease not typically associated with chronic bacterial infection, the efficacy of AZM in reducing asthma exacerbations further supports an immunomodulatory effect. In the AMAZES trial of 420 patients with asthma [10], exacerbations were reduced by 41% (0·59 [95% CI 0·47–0·74]) overall with similar results between eosinophilic and non-eosinophilic asthma (eosinophilic asthma rate ratio (0·66 (0·47–0·93)) vs non-eosinophilic (0·52 (0·29–0·94))). Together, these data support the idea that macrolides are having effects greater than would be expected from bacterial clearance alone, and their efficacy in patients without clear evidence of bacterial infection suggests an immunomodulatory mechanism. Against this, a Post-hoc analyses of the AMAZES trial did suggest a greater effect in patients with increasing H. influenzae load, using a qPCR assay with greater sensitivity than culture (incidence rate ratio 0.40, 95% CI 0.23, 0.69; p = 0.001) [31].
1.4. Macrolides in COVID-19
Given the success of macrolides in various respiratory conditions, their potent immunomodulatory effects and their possible anti-viral effects [32,33], macrolides were investigated for their efficacy in treating the Coronavirus Disease 2019 (COVID-19) pandemic which was deemed a public health emergency in March 2020.
COVID-19 is a respiratory disease caused by the Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) virus and causes symptoms of fever, shortness of breath, a chronic continuous cough and, in severe cases, pneumonia. With the number of SARS-CoV-2 infections and COVID-19 deaths increasing daily and at a time where no viable vaccine or pharmacological treatments were available, treatment options were in high demand. Indeed, various clinical trials investigating the efficacy of macrolides in treating COVID-19 were conducted. In particular, AZM, in conjunction with hydroxychloroquine (HCQ), an anti-malarial drug also used as an effective immune modifying treatment for autoimmune diseases Rheumatoid Arthritis (RA) and Systemic Lupus Erythematosus (SLE) [34], were the main focus of said trials. While predominantly observational studies reported combination therapy with AZM and HCQ promoted recovery, reduced disease symptoms, viral load and risk of hospitalisation [[35], [36], [37], [38], [39]], a number of studies reported no clinical benefit or improvement in mortality of those prescribed AZM/HCQ combination therapy compared with controls on standard therapy [[40], [41], [42], [43], [44], [45]], with some reporting the occurrence of adverse events and safety risks [41,42]. The large RECOVERY trial found no benefit of AZM on outcomes in hospitalised COVID-19 patients [44]. 7763 patients were randomised to AZM or standard care providing definitive evidence of this lack of benefit in this group.
1.5. Safety concerns and limitations
Clinical studies investigating the long-term effects of macrolides in chronic lung diseases have reported adverse events including hearing impairment and gastrointestinal complications. Furthermore, while rare, cardiotoxicity has been associated with macrolide therapy [9]. Macrolides can prolong the cardiac QT interval and inhibit metabolism of proarrhythmogenic drugs, leading to syncope and sudden death. Prolongation of the QT interval was reported in a number of COVID-19 trials of AZM and led to participants being withdrawn from the study [41,42]. Extra care must therefore be taken when prescribing macrolides, and a baseline electrocardiogram and strict surveillance for drug-drug interactions has been recommended to prevent severe cardiac events [46]. Another major concern is the rising prevalence of antimicrobial resistance (AMR). A recent meta-analysis shows significant association between long-term macrolide therapy and AMR [47]. Similarly, a retrospective study reported AMR in pneumococcal species in CF patients 4 years after macrolide treatment [48]. Therefore, a major question should be whether long-term low-dose macrolide therapy risks increasing antibiotic resistance long term.
In summary from a clinical perspective, it is perceived that macrolides have therapeutic activity extending beyond their role as antibiotics. By understanding the basis for this unexpected finding, it might be possible to improve or enhance the non-antibiotic functions of macrolides, apply them to other inflammatory diseases, avoid side effects, and limit AMR.
2. The effect of macrolides on leukocyte migration into the airspace
Clinical studies report that macrolides reduce immune cell infiltration into the lungs of asthmatics and BE patients [49,50]. Reduced lung leukocyte counts have also been reported in murine models of P. aeruginosa endobronchial infection and pulmonary fibrosis following administration of AZM and 14-membered macrolides, respectively, compared with untreated controls [51,52]. It is suggested that macrolides attenuate leukocyte migration into the lung by reducing chemokine and adhesion molecule production by airway epithelial cells. It is unclear why this does not result in similar harmful effects as CXCR2 or potent leukotriene B4 antagonism, but may be because macrolides do not entirely inhibit neutrophil migration into the lung, or that macrolides have less of a direct impact on neutrophils and alter migration of other leukocytes also.
Neutrophil chemotaxis and adhesion to bronchial epithelial cells is reported to be impaired in vitro by ERY treatment, likely because of reduced adhesion molecule expression and chemokine secretion by cultured epithelial cells [53]. ERY blocked the release of key neutrophil chemoattractants, CXCL8 and IL-6 [53]. Similarly, CAM decreased CXCL8 and IL-6 secretion by a human epithelial cell line in vitro [54]. Clinical trials report decreased levels of CXCL8 in human airways in response to macrolide treatment [49,50], and murine models of sepsis treated with macrolides show decreased inflammatory cytokine levels, including IL-6, in the airways and blood [55]. Additionally, AZM, CAM and ROX were reported to inhibit in vitro spontaneous production of soluble mediators including CXCL8 and IL-6 in COPD sputum samples [56]. In this study, ERY was described as “very weakly active” and no statistically significant changes in inflammatory molecule secretion were observed. It has also been reported that AZM does not reduce expression of CXCL1 and CXCL2, the murine chemokines involved in neutrophil chemotaxis [57], although it is important to note that this study used cultured epithelial cells from wildtype and CF murine models. Thus, although there are some inconsistencies between studies and variation between macrolides, it appears that macrolides can, in some circumstances, reduce chemoattractant production.
Macrolides are also reported to influence integrin expression. ERY inhibited the release of soluble ICAM-1, an integrin adhesion molecule, from human bronchial epithelial cells in vitro [53], and 14-membered macrolides inhibit ICAM-1 and vascular cell adhesion molecule 1 expression at the mRNA level in lung tissue and A549 cells, a human alveolar epithelial cell line [52,54]. Given the inhibitory effect of macrolides on ICAM-1 expression and clinical data showing reduced immune cell infiltration into the lung of macrolide-treated patients, this may confirm a mechanism for observed clinical findings as well as confirm discrepancies within the literature regarding ICAM-1's role as a key protein in the initial stage of transepithelial migration into the lung [58,59].
One study found that macrolides did not alter the induction of other adhesion molecule genes, such as selectins, in mice [52], despite these genes also being regulated by Nuclear Factor kappa-light-chain-enhancer of activated B cells (NF-κB) similar to integrins [60]. The reasons for these apparent discrepancies are not yet clear but may be due to these molecules not being key to transepithelial migration [61].
3. The effect of macrolides on innate immunity
3.1. Macrophages
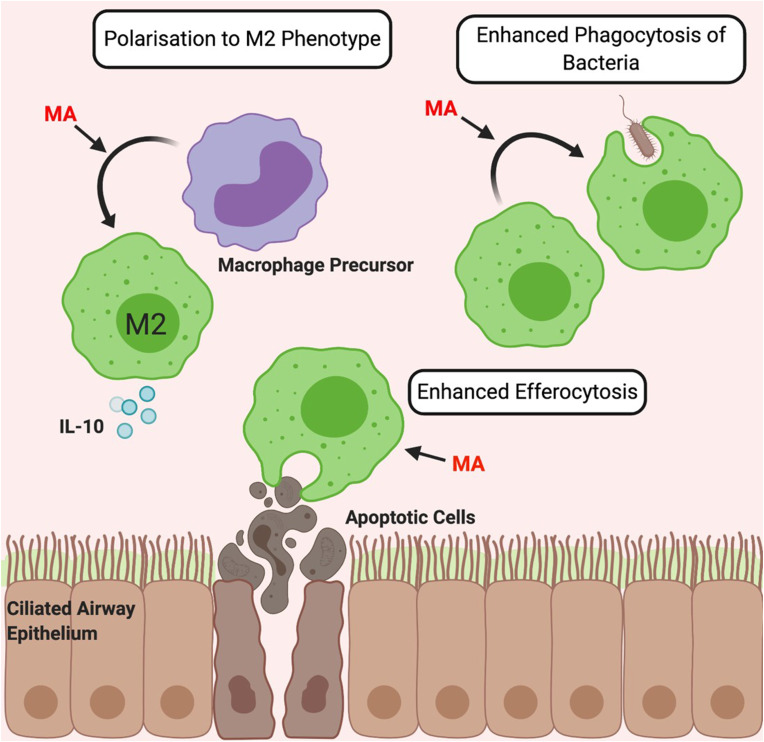
Multiple potential effects of macrolide antibiotics on macrophage function have been described (summarised in Fig. 1 ).
Fig. 1.
The Immunomodulatory Effects of Macrolides on Macrophages. Macrolide antibiotics (MA) polarise macrophage precursors towards an anti-inflammatory M2 phenotype characterised by increased levels of M2-associated molecules such as collagen, arginase and anti-inflammatory cytokine expression such as IL-10 [[62], [63], [64]]. Macrolides also enhance the phagocytic capacity of macrophages [[65], [66], [67]] and enhance efferocytosis of apoptotic cells [68].
3.1.1. Polarisation
Macrophage phenotype depends largely on the cytokine environment and is simplified to pro-inflammatory (M1-like) and anti-inflammatory (M2-like). Macrolides are consistently reported to direct macrophage precursors and existing M1 cells towards an M2 phenotype in vitro and subsequently alter macrophage cytokine production [[62], [63], [64]]. Thus, macrolides increase expression of M2-associated molecules including arginase and anti-inflammatory cytokine IL-10 in vitro, while decreasing levels of IL-12 and other pro-inflammatory molecules [[62], [63], [64]].
3.1.2. Phagocytic capacity
Macrolides also enhance the phagocytic capacity of macrophages. In vitro studies using a murine macrophage cell line and alveolar macrophages from pigs and cattle, show significantly enhanced phagocytosis of beads and Salmonella, respectively, following macrolide treatment [65,66]. In vitro human studies similarly report that macrolides enhance phagocytosis of bacteria [67]. Moreover, macrolides enhance efferocytosis, the phagocytic clearance of dead cells, as AZM increased the ability of alveolar macrophages from healthy individuals and COPD patients to phagocytose apoptotic bronchial epithelial cells and neutrophils [68]. This may be partially responsible for macrolide efficacy in COPD, where impaired clearance of apoptotic bronchial epithelial cells is considered a key part of the pathophysiology [68].
3.2. Neutrophils
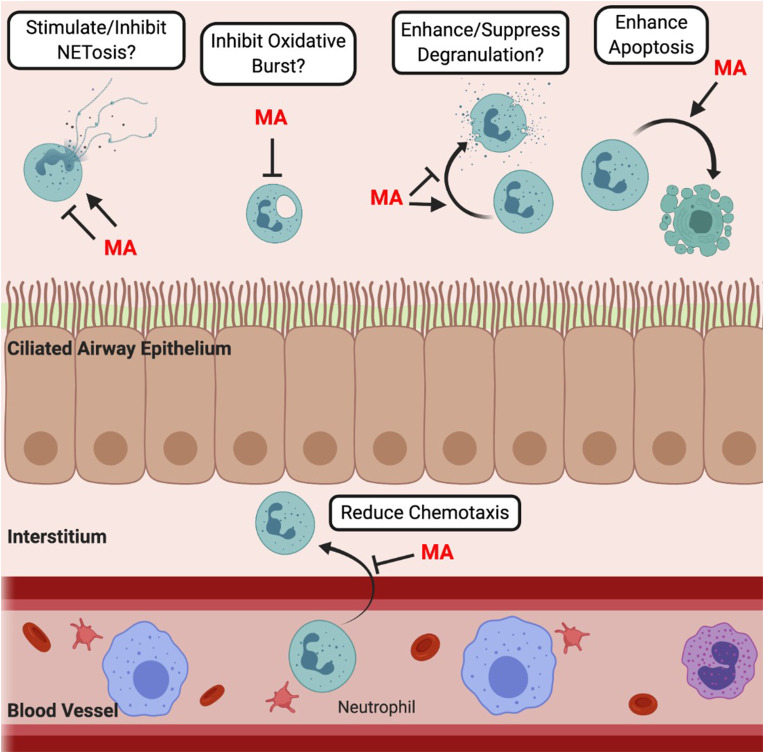
Neutrophils eliminate infection in a variety of ways including phagocytosis, degranulation and the formation of neutrophil extracellular traps (NETs). It is thought that a fundamental mechanism behind the positive clinical outcomes of macrolide treatment is the attenuation of neutrophil responses [69] (Fig. 2 ).
Fig. 2.
The Proposed Immunomodulatory Effects of Macrolide Antibiotics on Neutrophil Function. Macrolides antibiotics (MA) might affect various neutrophil functions. They have been reported to enhance apoptosis and reduce chemotaxis. Conflicting findings regarding the effect of macrolides on neutrophil degranulation [[70], [71], [72], [73], [74]], NETosis [[74], [75], [76], [77]] and the oxidative burst [74,78] highlight the lack of understanding of the impact these drugs have on neutrophil processes. Question marks indicate proposed effects of macrolides that require further experimental support.
3.2.1. Apoptosis
Prolonged neutrophil lifespan caused by delayed apoptosis is thought to be prominent in many chronic diseases [79,80]. It is thought that macrolides shorten neutrophil lifespan by inducing apoptosis, which may be one possible explanation for the reduced neutrophil numbers seen in clinical trials of macrolides [49,50] and the observed therapeutic benefits.
Initial in vitro data showed that 14- and 15-membered macrolides decrease neutrophil survival and increase apoptosis [69]. A variety of different techniques were used in this study to confirm these results, including Western blot, transmission electron microscopy and cell viability assays, highlighting a compelling case for macrolide-induced neutrophil apoptosis. Moreover, others report increased neutrophil apoptosis in healthy human volunteers following three-day treatment with AZM [73]. This study used microscopy to identify apoptosis-associated morphological changes in neutrophils and revealed prolonged effects of AZM, with increased levels of apoptotic cells being detected 28 days after the last administration of the drug. In addition, increased apoptosis of isolated blood neutrophils has been reported in calves, pigs and mice following macrolide treatment [[81], [82], [83]]. These studies strongly support the idea that macrolides induce neutrophil apoptosis.
Importantly, the benefits of neutrophil apoptosis may go deeper than simply reducing neutrophil lifespan. Promoting neutrophil apoptosis reduces the likelihood of cells undergoing necrosis and releasing inflammatory mediators into the local lung environment. In turn, enhanced neutrophil apoptosis alongside the macrolide-induced enhanced efferocytic capacity of macrophages (Section 3.1.2) may contribute to the beneficial effects of macrolides, given that efferocytosis promotes an anti-inflammatory environment [84].
3.2.2. NETosis
Macrolides are reported to modulate NETosis, the process where web-like structures composed of chromatin, histones and granule proteins are released to entrap bacteria. NET release can cause significant tissue damage in lung disease and is therefore being investigated as a potential therapeutic target [85].
Bystrzycka and colleagues (2017) demonstrated that AZM can suppress human neutrophil production of NETs induced by phorbol 12-myristate 13-acetate (PMA) [74]. Moreover, ERY suppressed NET release from human and murine neutrophils exposed in vitro to cigarette smoke, a known trigger of NETosis and an inflammatory stimulus in COPD, and reduced the number of NETs in the bronchoalveolar fluid of cigarette smoke-exposed mice [77]. In addition, a recent observational, multicohort study investigating the role of NETs in BE disease severity, and the potential of macrolide antibiotics to reduce NETs, showed that long-term low-dose AZM therapy significantly reduced NETs in sputum of both bronchiectasis and asthmatic patients, highlighting macrolides as a potential therapy for these diseases [86]. Over the course of one year, UK-based BE patients with active Pseudomonas infection were treated with 250 mg of AZM thrice weekly, and asthmatic patients enrolled in the AMAZES study [10] were treated with 500 mg AZM thrice weekly. Analysis of sputum samples obtained at baseline and following therapy showed that AZM significantly reduced NET concentration compared with either a matched cohort not receiving macrolide therapy or those receiving placebo. Of note, in comparison to asthmatics with neutrophil dominant inflammation, those with eosinophil-dominant inflammation (characterised by >3% sputum eosinophils) saw no significant reduction in NET concentration following macrolide therapy, highlighting the overall effect seen was driven by a marked reduction in NET concentration in neutrophilic disease patients. Some studies, however, report conflicting findings regarding the effect of macrolides on NETs [75,76]. AZM and CAM alone were reported to induce NET formation in vitro [75]. This study also found that neutrophils from patients with Helicobacter pylori infection undergoing combination therapy with CAM, amoxicillin and the anti-acid medication omeprazole, experienced increased levels of ex vivo NET formation compared to (i) neutrophils of healthy individuals, (ii) neutrophils before CAM treatment or (iii) neutrophils of patients undergoing alternative therapy excluding CAM. In addition, CAM, AZM and JM caused a dose-dependent increase in NET formation in cells from chronic rhinosinusitis patients [76]. Differences in study design, including neutrophil isolation methods and NETosis detection, in addition to differences in disease plus the concentrations/antibiotics used could all account for differences in results. Furthermore, NETosis is a heterogeneous process and macrolides may differentially affect these distinct NETosis pathways. Therefore, understanding the exact role macrolides play is difficult, and more research is needed to decipher exactly how macrolides influence NETosis.
3.2.3. Oxidative burst
The oxidative burst defines intracellular ROS production and is vital for efficient killing of ingested pathogens. Early in vitro evidence suggested that macrolides might impair the oxidative burst [78]. However, out of the macrolides tested, including ROX, AZM, ERY, SPM, JM and OLE, only ROX had this activity. Recent data using human neutrophils reported macrolides alone did not affect ROS production in vitro, but cells treated with the highest concentration of AZM did significantly inhibit the production of ROS [74]. Other studies report macrolides having no effect on neutrophil ROS [87] or having differential effects between different stimuli ex vivo [73]. Given the limited research on how macrolides affect oxidative burst, and that existing literature reports variable conclusions, the results should be taken with caution.
3.2.4. Degranulation
While debated, macrolides have been reported to enhance neutrophil degranulation and, if true, this could benefit patients via enhanced bacterial killing but may simultaneously increase tissue injury.
Abdelghaffar and colleagues provided evidence that macrolides directly induce neutrophil degranulation in vitro by showing that cultured primary human neutrophils released lysozyme, lactoferrin and beta-glucuronidase in a time and concentration-dependent manner after treatment with DIR, ERY and Erythromycylamine (a macrolide and metabolite of DIR) [70]. They later compared the effect of various macrolides, including ERY, ROX, AZM and CAM, on neutrophil degranulation and confirmed that macrolides could promote neutrophil degranulation [71]. Other in vitro studies demonstrate that human neutrophils experience enhanced degranulation after treatment with 14- and 15-membered macrolides [72,73]. However, one recent paper contradicts the preceding studies and proposes that macrolides have an inhibitory effect on human neutrophil degranulation [74]. This was based on the observation that AZM alone did not cause release of granular proteins, and even protected neutrophils from PMA-induced degranulation, in vitro [74]. As previously discussed, differences in study design and neutrophil preparation/handling may be behind the conflicting evidence. For example, the anticoagulant used during the isolation of blood neutrophils can activate cells, altering morphology and function [88].
In summary, the effects of macrolides on macrophages are well understood, but their precise impact on neutrophils remains to be fully defined. Macrolides are reported to favour the generation of anti-inflammatory M2 macrophages, enhance macrophage phagocytic capacity and induce neutrophil apoptosis. However, there are conflicting data on their effects on neutrophil NETosis, degranulation and the oxidative burst and further studies are required.
4. The effect of macrolides on adaptive immunity
4.1. Dendritic cells
Dendritic cell (DC) phenotypic plasticity allows for regulation of immune responses and is often perturbed in inflammatory disease where DCs perpetuate chronic inflammation and tissue damage. Macrolides seemingly polarise DCs to a tolerogenic phenotype but there are conflicting reports.
4.1.1. Surface marker expression
Three papers have reported that macrolides shift human and murine DCs towards a tolerogenic phenotype in vitro [[89], [90], [91]], evidenced by AZM treatment downregulating expression of MHC and costimulatory molecules CD40, CD86 and CD83. However, a 2007 study reported that AZM and CAM significantly enhanced expression of CD80, but not CD86 and CD40 [92]. Notably, some studies used murine bone marrow-derived DCs (mBMDCs) [88,92], which are generated in vitro in the presence of granulocyte-macrophage colony-stimulating factor and are not an ideal substitute for primary DCs. Other studies used human monocyte-derived DCs (hMDCs) [90,91], which have similar limitations, so it should be noted that results may not accurately reflect in vivo effects.
Confusingly, Polancec and colleagues showed that immature hMDCs generated in the presence of AZM have high CD86 and MHC expression compared to immature hMDCs differentiated without AZM [90]. On maturation with lipopolysaccharide (LPS), CD86 expression remained unchanged in AZM-treated hMDCs, yet CD40 and CD83 expression decreased, although decreased levels of CD86 in macrolide-treated, LPS-stimulated hMDCs are reported [[89], [90], [91]]. Thus, despite some variation between studies, it appears macrolides likely downregulate co-stimulatory molecules, at least on hMDCs, although further studies are required to determine the impact on DC phenotype in vivo.
4.1.2. Cytokine production
Macrolide-treated mBMDCs and hMDCs are reported to have enhanced IL-10 and decreased inflammatory cytokine expression including IL-6 and IL-12, providing further evidence that macrolides drive a tolerogenic DC phenotype [[89], [90], [91]]. In one study, AZM and CAM both significantly increased IL-10 production from mBMDCs but only CAM significantly reduced inflammatory IL-6 production [92]. Another study reported that AZM treatment decreased levels of both pro-inflammatory and anti-inflammatory cytokines [91]. Differences in experimental design could again account for these discrepancies but collectively data suggest macrolides increase anti-inflammatory cytokine production in vitro, suggesting they induce a tolerogenic DC phenotype. However, their impact on primary DCs in vivo has yet to be investigated.
4.2. Lymphocytes
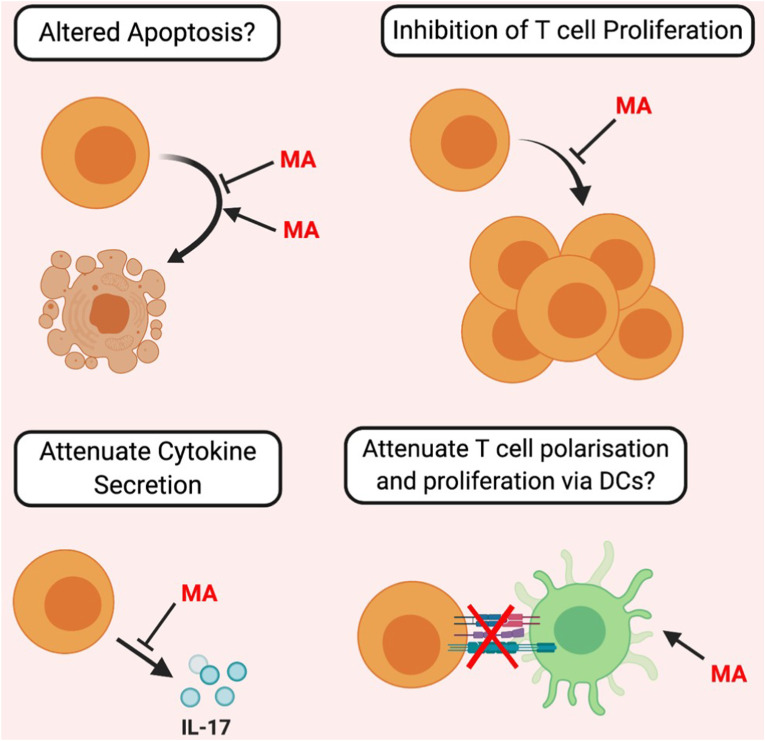
Macrolides reportedly modulate T-cell function both directly and indirectly (Fig. 3 ). Research relating to macrolides and B-cell function is lacking, with the exception of a preliminary human study indicating that in vivo antibody production was unaffected by macrolides [93].
Fig. 3.
The Proposed Immunomodulatory Effects of Macrolides on T-Cell function. Macrolide antibiotics (MA) directly influence T-cell function by attenuating cytokine secretion. Contradictory evidence (indicated by question marks) exists regarding the effect of macrolides on T-cell apoptosis, proliferation, and if, via DC attenuation, Macrolides can indirectly affect T-cell function.
4.2.1. Impact of macrolide modulation of DC function on T-cells
Macrolide induction of a tolerogenic-like DC phenotype is likely to suppress T-cell activation/proliferation or induce formation of anti-inflammatory Treg cells. AZM-treated mBMDCs have decreased T-cell stimulatory capacity compared to untreated DCs in vitro and AZM decreased IFNγ and increased IL-10 expression during a mixed lymphocyte reaction (MLR) [89], suggesting AZM-treated DCs may promote Treg differentiation. In addition, murine T-cells cultured with AZM-treated mBMDCs showed increased IL-10 production but no alteration in inflammatory cytokine production [92]. In contrast, an in vitro human study reports that AZM decreases IL-10, TNFα, and IFNγ production by T-cells during a MLR [91]. Besides differences in cell origin and experimental design, it is also important to note that in studies where cytokine levels were measured while cells were co-cultured, the source of the cytokine was not determined. When macrolides were added to both T-cells and DCs compared to DCs alone, a previously large drop in inflammatory cytokines was not observed [94]. Therefore, macrolides possibly influence T-cell cytokine secretion directly rather than indirectly via DCs. The other factors discussed below are also likely to impinge on the outcome of experiments where macrolides are added to mixed cultures containing T-cells.
4.2.2. Apoptosis
Since it was noted that macrolides reduce lymphocyte numbers in the lung of DPB patients in vivo [95], much research has examined if macrolides increase T-cell apoptosis. Macrolides increase apoptosis of activated T-cells and a Jurkat T-cell line in vitro [96,97], but this only occurred at high macrolide concentrations, i.e. ≥100μg/ml. It is possible, given that macrolides accumulate within cells over time, that these immunomodulatory effects may only be seen at high concentrations. However, it is reported that these concentrations are well above those found in human tissues [98]. Other in vitro studies similarly found lower doses of AZM and CAM did not induce T-cell apoptosis [91,98]. Also, while some studies looked at lymphocytes as a single population (and showed macrolides increased apoptosis) [96], others looked specifically at CD4+ T cells and found no effect of macrolides on apoptosis [91,97,98]. Therefore, low-dose macrolide therapy might increase apoptosis of specific T-cell subsets, highlighted by decreased CD8+ T-cells numbers and unchanged CD4+ T-cell numbers in macrolide-treated patients [95].
4.2.3. Proliferation
In vitro studies found human CD4+ T-cell proliferation was significantly inhibited by low-dose AZM therapy [91,94]. However, some report that macrolide-induced inhibition of T-cell proliferative responses was only apparent at high macrolide concentrations [91,97,98], similar to effects seen for apoptosis. This suggests the decrease in T-cell numbers seen in macrolide-treated patients may be a consequence of the reduction in pro-inflammatory cytokines involved in recruitment and proliferation, as previously discussed, rather than a direct effect of macrolides on T-cell proliferation and/or apoptosis.
4.2.4. Cytokine secretion
Lastly, research suggests macrolides directly suppress T-cell cytokine production. AZM decreased IL-17 production by human and murine Th17 cells in a dose-dependent manner highlighting a direct inhibitory effect of macrolides on T-cell cytokine production [[97], [98], [99]]. This is interesting given that Th17 responses are particularly important in providing protection from bacterial infections, a common feature of many inflammatory lung diseases, but also contributes to clinical exacerbations. Therefore, reduced IL-17 may, on the one hand, contribute to chronic bacterial infections by hampering vital immune responses but on the other, and in line with clinical data (Table 2), may benefit the patient by reducing exacerbations.
Therefore, while macrolides likely affect aspects of adaptive immunity, open questions remain. While evidence for macrolides inducing tolerogenic DCs seems largely consistent, inconclusive and conflicting studies on T-cell function highlight a need for further research.
5. Molecular mechanisms of action
5.1. PI3K/Akt/mTOR pathway
An in vitro study using a murine macrophage-like cell line showed that AZM treatment polarised M2 macrophages by stimulating the phosphorylation and activation of the Akt molecule, a serine/threonine kinase downstream of Phosphoinositide 3-Kinase (PI3K) [63]. When Akt phosphorylation was inhibited, no M2 polarisation occurred suggesting AZM acts on this pathway to polarise macrophage phenotype and possibly mediate other immunomodulatory effects. The precise mechanisms are unclear, but macrolides might upregulate the Phosphoinositide-dependent kinase 1 (PDK1) molecule, thus enhancing phosphorylation/activation of Akt, or directly influence the upstream molecule PI3K, leading to Akt recruitment and activation.
Rapamycin, a non-antibiotic macrolide, indirectly inhibits the serine/threonine kinase mammalian Target of Rapamycin (mTOR) by forming a complex with FK506 Binding Protein 12 (FKBP12) [98]. Due to structural similarity and similar immunomodulatory effects of macrolides and rapamycin, research was conducted into if and how macrolides affect mTOR. Two studies show that macrolides directly modulate mTOR and attenuate T-cell responses [97,98]. Both studies found decreased levels of phosphorylated S6 Ribosomal Protein (S6RP), a protein downstream of mTOR. Interestingly, mTOR inhibition was independent of FKBP12 [98]. Therefore, macrolides likely have a different mechanism of mTOR modulation than Rapamycin, possibly by directly binding mTOR without the need for a co-factor.
Phosphorylation by Akt can stimulate or inhibit different target proteins. Therefore, macrolide-induced immunomodulatory effects may be the result of differential effects on different proteins in the pathway i.e. Akt activation may cause some proteins to be upregulated and others to be downregulated to ultimately give rise to the effects seen.
5.2. NF-κB and AP-1
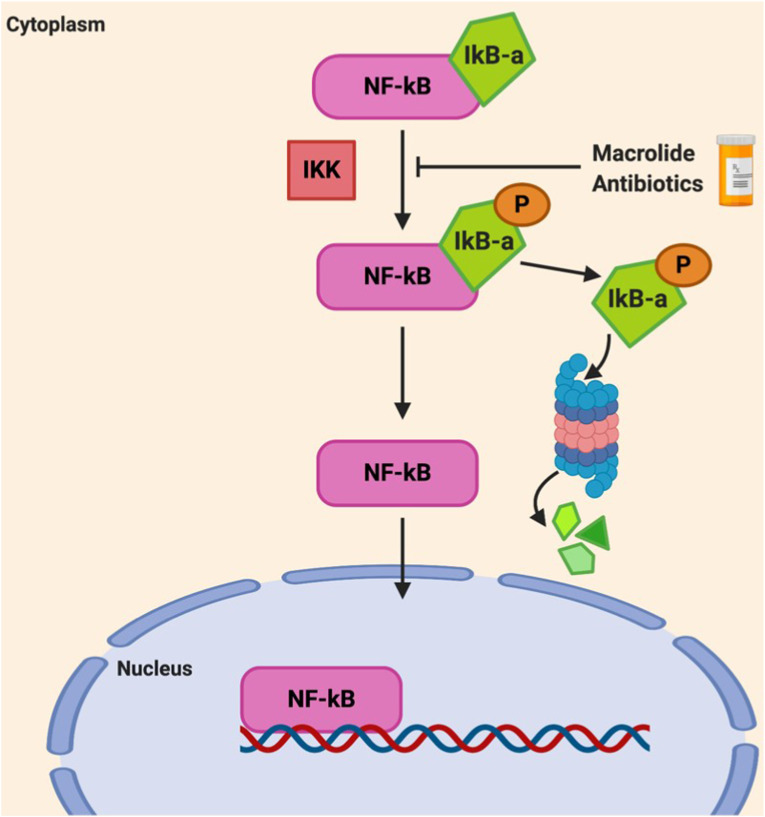
Initial research found that various 14-membered macrolides inhibit NF-κB activation in bronchial epithelial cells and peripheral blood mononuclear cells [[100], [101], [102]]. As NF-κB governs chemokine and cytokine expression, it was the proposed mechanism for macrolide-induced cytokine attenuation and further hinted that inhibition of these transcription factors may cause other anti-inflammatory effects. Macrolide treatment was found to specifically affect inhibitor of nuclear factor kappa B (IκB) proteins [81,103] (Fig. 4 ).
Fig. 4.
The Effect of Macrolides on NF-κB. In resting state, NF-κB is inactive in the cytoplasm due to inhibition by the IκB-α inhibitory protein. During inflammation, IκB-α becomes phosphorylated by IκB Kinase (IKK), which mediates degradation of IκB-α by the proteosome. The removal of IκB-α allows NF-κB to translocate into the nucleus to regulate inflammatory gene expression. Macrolides decreased the phosphorylation, and therefore degradation, of IκB-α thereby keeping NF-κB in an inactive state. (Adapted from Beinke and Ley., 2004) [104].
Using human tracheal cells, AZM treatment inhibited IκB-α degradation [103]. Likewise, TUL significantly decreased phosphorylated IκB-α levels in LPS-stimulated bovine neutrophils, therefore decreasing IκB-α degradation [81]. This suggests macrolides may inhibit IKK, the enzyme responsible for the phosphorylation and breakdown of IκB-α (Fig. 4). Therefore, it is likely macrolides have inhibitory effects on NF-κB.
6. Discussion
In summary, macrolides possess immunomodulatory properties that likely contribute to their observed efficacy in the treatment of inflammatory respiratory diseases. Their efficacy in clinical scenarios where antibiotic effects are less likely, such as eosinophilic asthma, BE and CF with Pseudomonas, further hint that immunomodulatory effects are a key mechanism behind their therapeutic action. However, much controversy exists surrounding a definitive mechanism of action for these drugs and whether antimicrobial effects are possibly a significant driver of the clinical benefits of macrolide therapy. Including the ability of macrolides to accumulate within tissues to concentrations above the antibacterial threshold, the intimate relationship between infection and host immune response alludes to the idea that targeting infection through antibacterial mechanisms may inadvertently reduce the inflammatory processes implicated in disease, and those supposedly dampened by macrolides. Multiple other findings further hint at the potential importance of antimicrobial effects. One of which is the discovery that macrolide-resistant pathogens, namely Pseudomonas, may be macrolide-sensitive in the context of the lung. This was highlighted by evidence showing that P. aeruginosa harbours increased susceptibility to macrolides when cultured in bronchoalveolar lavage fluid compared to standard cell culture media [105], findings potentially more representative of the in vivo effects occurring in the macrolide-treated lung. Also, evidence highlights Pseudomonas isolates from CF patients acquire resistance to macrolides [106], a process that would not occur in naturally macrolide-resistant organisms. This data, alongside observations that chronically infected Pseudomonas patients often show greater clinical benefit when treated with macrolides [18] (data previously overviewed in Section 1.3), highlights a compelling case for additional antibiotic effects. While outwith the scope of this review, macrolides reportedly possess additional antiviral effects [107] and these antiviral effects/improvements in host viral defense may similarly contribute to the therapeutic effects of macrolides given that a common clinical outcome of long-term macrolide therapy is reduced exacerbations, which are often of viral origin. Therefore, it may be more accurate to look at the immune modulation provided by macrolides as an interplay between improved host response to infection, dampening of dysregulated inflammation and the targeted elimination of several relevant and susceptible airway pathogens.
Regardless, the evidence throughout this review shows macrolides possess more than just antimicrobial properties. As such, macrolides may have potential in diseases where immune response dysregulation contributes to disease pathogenesis, such as in cancer and RA. This is supported by clinical data showing cancer patients, including lung cancer patients, respond better to cancer therapy when given in combination with CAM [108], and an improvement in RA symptoms after macrolide therapy [109,110]. However, as macrolide therapy comes with disadvantages (Section 1.5), it must be carefully evaluated whether the advantages of macrolides outweigh the safety concerns and future research is needed to answer questions regarding long-term safety of macrolides. Finally, by further understanding the immunological pathways targeted by macrolides and the magnitude to which their immunomodulatory effects drive clinical outcomes opposed to their antimicrobial effects, the development of non-antibiotic macrolide-like drugs could see the benefits of macrolide therapy optimised and the drawbacks addressed.
7. Conclusions
In conclusion, macrolide antibiotics have shown potent immunomodulatory properties in aspects of innate and adaptive immunity and these effects likely contribute to their therapeutic benefit in the context of inflammatory respiratory diseases alongside their well-established antimicrobial properties.
Funding
JDC is supported by the GSK/British Lung Foundation Chair of Respiratory Research and a Scottish Senior Fellowship from the Chief Scientist Office.
Acknowledgements
Figures were created using BioRender.com (2020). Macrolide structures (Table 1) were created using ChemSpider, Royal Society of Chemistry (2021).
References
- 1.Dinos G. The macrolide antibiotic renaissance. Br. J. Pharmacol. 2017;174(8):2967–2983. doi: 10.1111/bph.13936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zimmerman P., et al. The immunomodulatory effects of macrolides - a systematic review of the underlying mechanisms. Front. Immunol. 2018;9:302. doi: 10.3389/fimmu.2018.00302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.National Institute for Health and Care Excellence (NICE) British national formulary. 2019. https://bnf.nice.org.uk/ Available at:
- 4.MedChemExpress Tulathromycin A/Josamycin/Spiramycin. 2020. https://www.medchemexpress.com/Tulathromycin-A.html [online] Available at:
- 5.National Center for Advancing Technological Sciences (NCATS) Inxight: drugs. 2020. https://drugs.ncats.io/drug/P8ZQ646136 Available at: Accessed 14/4/2020.
- 6.ChemSpider Royal society of Chemistry. 2021. https://www.chemspider.com/Search.aspx?rid=ae86f8cd-5dd6-4277-949a-f70902739426 [online] Available at:
- 7.Kudoh S., et al. Clinical effects of low-dose long-term erythromycin chemotherapy on diffuse panbronchiolitis. Nihon Kyobu Shikkan Gakkai Zasshi. 1987;25:632–642. [PubMed] [Google Scholar]
- 8.Poletti V., et al. Diffuse panbronchiolitis. Eur. Respir. J. 2006;28(4):862–871. doi: 10.1183/09031936.06.00131805. [DOI] [PubMed] [Google Scholar]
- 9.Altenburg J., et al. Immunomodulatory effects of macrolide antibiotics - Part 2: advantages and disadvantages of long-term, low-dose macrolide therapy. Respir. 2011;81:75–87. doi: 10.1159/000320320. [DOI] [PubMed] [Google Scholar]
- 10.Gibson P., et al. Effect of azithromycin on asthma exacerbations and quality of life in adults with persistent uncontrolled asthma (AMAZES): a randomised, double-blind, placebo-controlled trial. Lancet. 2017;390(10095):659–668. doi: 10.1016/S0140-6736(17)31281-3. [DOI] [PubMed] [Google Scholar]
- 11.Wong C., et al. Azithromycin for prevention of exacerbations in non-cystic fibrosis bronchiectasis (EMBRACE): a randomised, double-blind, placebo-controlled trial. Lancet. 2012;380(9842):660–667. doi: 10.1016/S0140-6736(12)60953-2. [DOI] [PubMed] [Google Scholar]
- 12.Altenburg J., et al. Effect of azithromycin maintenance treatment on infectious exacerbations among patients with non-cystic fibrosis bronchiectasis: the BAT randomized controlled trial. J. Am. Med. Assoc. 2013;309(12):1251–1259. doi: 10.1001/jama.2013.1937. [DOI] [PubMed] [Google Scholar]
- 13.Serisier D., et al. Effect of long-term, low-dose erythromycin on pulmonary exacerbations among patients with non-cystic fibrosis bronchiectasis: the BLESS randomized controlled trial. J. Am. Med. Assoc. 2013;309(12):1260–1267. doi: 10.1001/jama.2013.2290. [DOI] [PubMed] [Google Scholar]
- 14.Seemungal T., et al. Long-term erythromycin therapy is associated with decreased chronic obstructive pulmonary disease exacerbations. Am. J. Respir. Crit. Care Med. 2008;178(11):1139–1147. doi: 10.1164/rccm.200801-145OC. [DOI] [PubMed] [Google Scholar]
- 15.Albert R., et al. Azithromycin for prevention of exacerbations of COPD. N. Engl. J. Med. 2011;365(8):689–698. doi: 10.1056/NEJMoa1104623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Uzun S., et al. Azithromycin maintenance treatment in patients with frequent exacerbations of chronic obstructive pulmonary disease (COLUMBUS): a randomised, double-blind, placebo-controlled trial. Lancet. Respir. Med. 2014;2(5):361–368. doi: 10.1016/S2213-2600(14)70019-0. [DOI] [PubMed] [Google Scholar]
- 17.Wolter J., et al. Effect of long-term treatment with azithromycin on disease parameters in cystic fibrosis: a randomised trial. Thorax. 2002;57(3):212–216. doi: 10.1136/thorax.57.3.212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Saiman L., et al. Azithromycin in patients with cystic fibrosis chronically infected with Pseudomonas aeruginosa: a randomized controlled trial. J. Am. Med. Assoc. 2003;290(13):1749–1756. doi: 10.1001/jama.290.13.1749. [DOI] [PubMed] [Google Scholar]
- 19.Saiman L., et al. Effect of azithromycin on pulmonary function in patients with cystic fibrosis uninfected with Pseudomonas aeruginosa: a randomised controlled trial. J. Am. Med. Assoc. 2010;303(17):1707–1715. doi: 10.1001/jama.2010.563. [DOI] [PubMed] [Google Scholar]
- 20.Forum of International Respiratory Societies (FIRS) second ed. European Respiratory Society. e-; Sheffield: 2017. The Global Impact of Respiratory Disease. 9781849840880. [Google Scholar]
- 21.Pascoe S., et al. Blood eosinophil counts, exacerbations, and response to the addition of inhaled fluticasone furoate to vilanterol in patients with chronic obstructive pulmonary disease: a secondary analysis of data from two parallel randomised controlled trials. Lancet. Respir. Med. 2015;3(6):435–442. doi: 10.1016/S2213-2600(15)00106-X. [DOI] [PubMed] [Google Scholar]
- 22.Ronchetti S., et al. How glucocorticoids affect the neutrophil life. Int. J. Mol. Sci. 2018;19(12):4090. doi: 10.3390/ijms19124090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Singanayagam A., et al. Inhaled corticosteroid suppression of cathelicidin drives dysbiosis and bacterial infection in chronic obstructive pulmonary disease. Sci. Transl. Med. 2019;11(507) doi: 10.1126/scitranslmed.aav3879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Herbold W., et al. Importance of CXC chemokine receptor 2 in alveolar neutrophil and exudate macrophage recruitment in response to pneumococcal lung infection. Infect. Immun. 2010;78(6):2620–2630. doi: 10.1128/IAI.01169-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Konstan M.W., et al. A randomised double blind, placebo controlled phase 2 trial of BIIL 284 BS (an LTB4 receptor antagonist) for the treatment of lung disease in children and adults with cystic fibrosis. J. Cyst. Fibros. 2014;13(2):148–155. doi: 10.1016/j.jcf.2013.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nakamura H., et al. Clinical and immunoregulatory effects of roxithromycin therapy for chronic respiratory infection. Eur. Respir. J. 1999;13:1371–1379. doi: 10.1183/09031936.99.13613809. [DOI] [PubMed] [Google Scholar]
- 27.Foulds G., Shepard R.M., Johnson R.B. The pharmacokinetics of azithromycin in human serum and tissues. J. Antimicrob. Chemother. 1990;25(Issue suppl_A):73–82. doi: 10.1093/jac/25.suppl_a.73. [DOI] [PubMed] [Google Scholar]
- 28.Kelly C., et al. Macrolide antibiotics for bronchiectasis. Cochrane Database Syst. Rev. 2018;3(3):CD012406. doi: 10.1002/14651858.CD012406.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chalmers J.D., et al. Long-term macrolide antibiotics for the treatment of bronchiectasis in adults: an individual participant data meta-analysis. Lancet Respir Med. 2019;7(10):845–854. doi: 10.1016/S2213-2600(19)30191-2. [DOI] [PubMed] [Google Scholar]
- 30.Cramer C.L., et al. Immunomodulatory indications of azithromycin in respiratory disease: a concise review for the clinician. Postgrad. Med. 2017;129(5):493–499. doi: 10.1080/00325481.2017.1285677. [DOI] [PubMed] [Google Scholar]
- 31.Taylor S.L., et al. Airway abundance of Haemophilus influenzae predicts response to azithromycin in adults with persistent uncontrolled asthma. ERJ. 2020;56(4) doi: 10.1183/13993003.00194-2020. [DOI] [PubMed] [Google Scholar]
- 32.Gielen V., Johnston S.L., Edwards M.R. Azithromycin induces anti-viral response in bronchial epithelial cells. Eur. Respir. J. 2010;36:646–654. doi: 10.1183/09031936.00095809. [DOI] [PubMed] [Google Scholar]
- 33.Schögler A., et al. Novel antiviral properties of azithromycin in cystic fibrosis airway epithelial cells. Eur. Respir. J. 2015;45:428–439. doi: 10.1183/09031936.00102014. [DOI] [PubMed] [Google Scholar]
- 34.Chandler L., et al. Immunomodulatory effects of hydroxychloroquine and chloroquine in viral infections and their potential application in retinal gene therapy. Int. J. Mol. Sci. 2020;21(14):4972. doi: 10.3390/ijms21144972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Abbas H.M., et al. Assessment of COVID‐19 Treatment containing both Hydroxychloroquine and Azithromycin: a natural clinical trial. Int. J. Clin. Pract. 2020;75(4) doi: 10.1111/ijcp.13856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gautret P., et al. Hydroxychloroquine and azithromycin as a treatment of COVID-19: results of an open-label non-randomized clinical trial. Int. J. Antimicrob. Agents. 2020;56(1) doi: 10.1016/j.ijantimicag.2020.105949. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 37.Sekhavati E., et al. Safety and effectiveness of azithromycin in patients with COVID-19: an open-label randomised trial. Int. J. Antimicrob. Agents. 2020;56(4) doi: 10.1016/j.ijantimicag.2020.106143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gautret P., et al. Effect of hydroxychloroquine and azithromycin as a treatment of COVID-19: results of an open-label non-randomized clinical trial, an update with an intention-to-treat analysis and clinical outcomes. Int. J. Antimicrob. Agents. 2021;57(1) doi: 10.1016/j.ijantimicag.2020.106239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Rashad A., et al. 2021. Therapeutic Efficacy of Macrolides in Management of Patients with Mild COVID-19. PREPRINT (Version 1), Research Square. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Furtado R.H.M., et al. Azithromycin in addition to standard of care versus standard of care alone in the treatment of patients admitted to the hospital with severe COVID-19 in Brazil (COALITION II): a randomised clinical trial. Lancet. 2020;396(10256):959–967. doi: 10.1016/S0140-6736(20)31862-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Cavalcanti A.B., et al. Hydroxychloroquine with or without azithromycin in mild-to-moderate covid-19. NEJM. 2020;383(21):2041–2052. doi: 10.1056/NEJMoa2019014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Molina J.M., et al. No evidence of rapid antiviral clearance or clinical benefit with the combination of hydroxychloroquine and azithromycin in patients with severe COVID-19 infection. Med. Maladies Infect. 2020;50(4):348. doi: 10.1016/j.medmal.2020.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Rosenberg E.S., et al. Association of treatment with hydroxychloroquine or azithromycin with in-hospital mortality in patients with COVID-19 in New York state. JAMACROLIDES. 2020;323(24):2493–2502. doi: 10.1001/jama.2020.8630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.RECOVERY Collaborative Group Azithromycin in patients admitted to hospital with COVID-19 (RECOVERY): a randomised, controlled, open-label, platform trial. Lancet. 2021;397(10274):605–612. doi: 10.1016/S0140-6736(21)00149-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Butler C.C., et al. Azithromycin for community treatment of suspected COVID-19 in people at increased risk of an adverse clinical course in the UK (PRINCIPLE): a randomised, controlled, open-label, adaptive platform trial. Lancet. 2021;397(10279):1063–1074. doi: 10.1016/S0140-6736(21)00461-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Smith D., et al. British Thoracic Society guideline for the use of long-term macrolides in adults with respiratory disease. Thorax. 2020;75:370–404. doi: 10.1136/thoraxjnl-2019-213929. [DOI] [PubMed] [Google Scholar]
- 47.Laska I.F., et al. The efficacy and safety of inhaled antibiotics for the treatment of bronchiectasis in adults: a systematic review and meta-analysis. Eur. Respir. J. 2019;54(63):PA2165. doi: 10.1016/S2213-2600(19)30185-7. [DOI] [PubMed] [Google Scholar]
- 48.Kasahara K., et al. Macrolide resistance of Streptococcus pneumoniae isolated during long-term macrolide therapy: difference between erythromycin and clarithromycin. J. Infect. Chemother. 2005;11(2):112–114. doi: 10.1007/s10156-005-0375-1. [DOI] [PubMed] [Google Scholar]
- 49.Simpson J., et al. Clarithromycin targets neutrophilic airway inflammation in refractory asthma. Am. J. Respir. Crit. Care Med. 2008;177(2):148–155. doi: 10.1164/rccm.200707-1134OC. [DOI] [PubMed] [Google Scholar]
- 50.Liu J., et al. Effect of low-dose, long-term roxithromycin on airway inflammation and remodelling in noncystic fibrosis bronchiectasis. Mediat. Inflamm. 2014;2014:0–14. doi: 10.1155/2014/708608. 708608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Tsai W., et al. Azithromycin blocks neutrophil recruitment in Pseudomonas endobronchial infection. Am. J. Respir. Crit. Care Med. 2004;170(12):1331–1339. doi: 10.1164/rccm.200402-200OC. [DOI] [PubMed] [Google Scholar]
- 52.Li Y., et al. Fourteen-membered ring macrolides inhibit vascular cell adhesion molecule 1 messenger RNA induction and leukocyte migration: role in preventing lung injury and fibrosis in bleomycin-challenged mice. Chest. 2002;122(6):2137–2145. doi: 10.1378/chest.122.6.2137. [DOI] [PubMed] [Google Scholar]
- 53.Khair O.A., et al. Effect of erythromycin on Haemophilus influenzae endotoxin-induced release of IL-6, IL-8 and sICAM-1 by cultured human bronchial epithelial cells. Eur. Respir. J. 1995;8(9):1451–1457. [PubMed] [Google Scholar]
- 54.Jang Y., Kwon H.-J., Lee B.-J. Effect of clarithromycin on rhinovirus-16 infection in A549 cells. Eur. Respir. J. 2006;27(1):12–19. doi: 10.1183/09031936.06.00008005. [DOI] [PubMed] [Google Scholar]
- 55.Patel A., et al. Azithromycin in Combination with Ceftriaxone reduces systemic inflammation and provides survival benefit to murine model of polymicrobial sepsis. Antimicrob. Agents Chemother. 2018;62(9) doi: 10.1128/AAC.00752-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Marjanovic N., et al. Macrolide antibiotics broadly and distinctively inhibit cytokine and chemokine production by COPD sputum cells in vitro. Pharmacol. Res. 2011;63(5):389–397. doi: 10.1016/j.phrs.2011.02.001. [DOI] [PubMed] [Google Scholar]
- 57.Gavilanes X., et al. Azithromycin fails to reduce increased expression of neutrophil-related cytokines in primary-cultured epithelial cells from cystic fibrosis mice. J. Cyst. Fibros. 2009;8(3):203–210. doi: 10.1016/j.jcf.2009.03.003. [DOI] [PubMed] [Google Scholar]
- 58.Liu L., et al. Neutrophil transmigration across monolayers of endothelial cells and airway epithelial cells is regulated by different mechanisms. Ann. N. Y. Acad. Sci. 1996;796:21–29. doi: 10.1111/j.1749-6632.1996.tb32563.x. [DOI] [PubMed] [Google Scholar]
- 59.Kidney J.C., Proud D. Neutrophil transmigration across human airway epithelial monolayers: mechanisms and dependence on electrical resistance. Am. J. Respir. Cell Mol. Biol. 2000;23(3):389–395. doi: 10.1165/ajrcmb.23.3.4068. [DOI] [PubMed] [Google Scholar]
- 60.Sehnert B., et al. NF-kB inhibitor targeted to activated endothelium demonstrates a critical role of endothelial NF-kB in immune-mediated diseases. Proc. Natl. Acad. Sci. U.S.A. 2013;110(41):16556–16561. doi: 10.1073/pnas.1218219110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Zemans R.L., Colgan S.P., Downey G.P. Transepithelial migration of neutrophils: mechanisms and implications for acute lung injury. Am. J. Respir. Cell Mol. Biol. 2009;40(9):519–535. doi: 10.1165/rcmb.2008-0348TR. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Murphy B., et al. Azithromycin alters macrophage phenotype. J. Antimicrob. Chemother. 2008;61(3):554–560. doi: 10.1093/jac/dkn007. [DOI] [PubMed] [Google Scholar]
- 63.Wang J., et al. Azithromycin promotes alternatively activated macrophage phenotype in systemic lupus erythematosus via PI3K/Akt signaling pathway. Cell Death Dis. 2018;9(11) doi: 10.1038/s41419-018-1097-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Zhang B., et al. Macrolide derivatives reduce proinflammatory macrophage activation and macrophage-mediated neurotoxicity. CNS Neurosci. Ther. 2019;25(5):591–600. doi: 10.1111/cns.13092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Xu G., et al. Effect of macrolide antibiotics on macrophage functions. Microbiol. Immunol. 1996;40(7):473–479. doi: 10.1111/j.1348-0421.1996.tb01097.x. [DOI] [PubMed] [Google Scholar]
- 66.Brumbaugh G.W., et al. Effect of tilmicosin on chemotactic, phagocytic, and bactericidal activities of bovine and porcine alveolar macrophages. Am. J. Vet. Res. 2002;63(1):36–41. doi: 10.2460/ajvr.2002.63.36. [DOI] [PubMed] [Google Scholar]
- 67.Hodge S., Reynolds P.N. Low-dose azithromycin improves phagocytosis of bacteria by both alveolar and monocyte-derived macrophages in chronic obstructive pulmonary disease subjects. Respirology. 2012;17:802–807. doi: 10.1111/j.1440-1843.2012.02135.x. [DOI] [PubMed] [Google Scholar]
- 68.Hodge S., et al. Azithromycin increases phagocytosis of apoptotic bronchial epithelial cells by alveolar macrophages. Eur. Respir. J. 2006;28:486–495. doi: 10.1183/09031936.06.00001506. [DOI] [PubMed] [Google Scholar]
- 69.Aoshiba A., Nagai A., Konno K. Erythromycin shortens neutrophil survival by accelerating apoptosis. Antimicrob. Agents Chemother. 1995;39(4):872–877. doi: 10.1128/aac.39.4.872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Abdelghaffar H., Mtairag E.M., Labro M.T. Effects of dirithromycin and erythromycylamine on human neutrophil degranulation. Antimicrob. Agents Chemother. 1994;38(7):1548–1554. doi: 10.1128/aac.38.7.1548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Abdelghaffar H., Vazifeh D., Labro M.T. Comparison of various macrolides on stimulation of human neutrophil degranulation in vitro. J. Antimicrob. Chemother. 1996;38:81–93. doi: 10.1093/jac/38.1.81. [DOI] [PubMed] [Google Scholar]
- 72.Schultz M.J., et al. Intravenous infusion of erythromycin inhibits CXC chemokine production, but augments neutrophil degranulation in whole blood stimulated with. Streptococcus pneumoniae. J. Antimicrob. Chemother. 2000;46(2):235–240. doi: 10.1093/jac/46.2.235. [DOI] [PubMed] [Google Scholar]
- 73.Culic O., et al. Azithromycin modulates neutrophil function and circulating inflammatory mediators in healthy human subjects. Eur. J. Pharmacol. 2002;450(3):277–289. doi: 10.1016/s0014-2999(02)02042-3. [DOI] [PubMed] [Google Scholar]
- 74.Bystrzycka W., et al. Azithromycin and chloramphenicol diminish neutrophil extracellular traps (NETs) release. Int. J. Mol. Sci. 2017;18(2):2666. doi: 10.3390/ijms18122666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Konstantinidis T., et al. Immunomodulatory role of clarithromycin in acinetobacter baumannii infection via formation of neutrophil extracellular traps. Antimicrob. Agents Chemother. 2016;60(2):1040–1048. doi: 10.1128/AAC.02063-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Hwang J.W., et al. Neutrophil extracellular traps in nasal secretions of patients with stable and exacerbated chronic rhinosinusitis and their contribution to induce chemokine secretion and strengthen the epithelial barrier. Clin. Exp. Allergy. 2019;49:1306–1320. doi: 10.1111/cea.13448. [DOI] [PubMed] [Google Scholar]
- 77.Zhang H., et al. Erythromycin suppresses neutrophil extracellular traps in smoking-related chronic pulmonary inflammation. Cell Death Dis. 2019;10:678. doi: 10.1038/s41419-019-1909-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Labro M.T., El Benna J., Babin-Chevaye C. Comparison of the in-vitro effect of several macrolides on the oxidative burst of human neutrophils. J. Antimicrob. Chemother. 1989;24:561–572. doi: 10.1093/jac/24.4.561. [DOI] [PubMed] [Google Scholar]
- 79.Haslett C. Granulocyte apoptosis and its role in the resolution and control of lung inflammation. Am. J. Crit. Care. Med. 1999;160(5 Pt 2) doi: 10.1164/ajrccm.160.supplement_1.4. [DOI] [PubMed] [Google Scholar]
- 80.Zhang J., et al. Delayed apoptosis by neutrophils from COPD patients is associated with altered bak, bcl-xl, and mcl-1 mRNA expression. Diagn. Pathol. 2012;7:65. doi: 10.1186/1746-1596-7-65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Fischer C., et al. Anti-inflammatory benefits of antibiotic-induced neutrophil apoptosis: tulathromycin induces caspase-3-dependent neutrophil programmed cell death and inhibits NF-κB signaling and CXCL8 transcription. Antimicrob. Agents Chemother. 2011;55(1):338–348. doi: 10.1128/AAC.01052-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Mogues R., et al. Anti-inflammatory benefits of antibiotics: tylvalosin induces apoptosis of porcine neutrophils and macrophages, promotes efferocytosis, and inhibits pro-inflammatory CXCL-8, IL1α, and LTB4 production, while inducing the release of pro-resolving Lipoxin A4 and resolvin D1. Front. Vet. Sci. 2018;5:57. doi: 10.3389/fvets.2018.00057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Al-Darraji A., et al. Azithromycin therapy reduces cardiac inflammation and mitigates adverse cardiac remodeling after myocardial infarction: potential therapeutic targets in ischemic heart disease. PLoS One. 2018;13(7) doi: 10.1371/journal.pone.0200474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Aderem A., Underhill D.M. Mechanisms of phagocytosis in macrophages. Annu. Rev. Immunol. 1999;17:593–623. doi: 10.1146/annurev.immunol.17.1.593. [DOI] [PubMed] [Google Scholar]
- 85.Porto B., Stein R. Neutrophil extracellular traps in pulmonary diseases: too much of a good thing? Front. Immunol. 2016;7:311. doi: 10.3389/fimmu.2016.00311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Keir H.R., et al. Neutrophil extracellular traps, disease severity, and antibiotic response in bronchiectasis: an international, observational, multicohort study. The Lancet Respiratory Medicine. 2021;9(8):873–884. doi: 10.1016/S2213-2600(20)30504-X. [DOI] [PubMed] [Google Scholar]
- 87.Koch C., et al. Apoptosis, oxidative metabolism and interleukin-8 production in human neutrophils exposed to azithromycin: effects of. Streptococcus pneumoniae. J. Antimicrob. Chemother. 2000;46(1):19–26. doi: 10.1093/jac/46.1.19. [DOI] [PubMed] [Google Scholar]
- 88.Haslett C., et al. Modulation of multiple neutrophil functions by preparative methods or trace concentrations of bacterial lipopolysaccharide. Am. J. Pathol. 1985;119(1):101–110. [PMC free article] [PubMed] [Google Scholar]
- 89.Iwamoto S., et al. The effect of azithromycin on the maturation and function of murine bone marrow-derived dendritic cells. Clin. Exp. Immunol. 2011;166(3):385–392. doi: 10.1111/j.1365-2249.2011.04480.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Polancec D.S., et al. Azithromycin drives in vitro GM‐CSF/IL‐4‐induced differentiation of human blood monocytes toward dendritic‐like cells with regulatory properties. J. Leukoc. Biol. 2011;91 doi: 10.1189/jlb.1210655. [DOI] [PubMed] [Google Scholar]
- 91.Lin S.J., et al. Azithromycin modulates immune response of human monocyte-derived dendritic cells and CD4+ T cells. Int. Immunopharm. 2016;40:318–326. doi: 10.1016/j.intimp.2016.09.012. [DOI] [PubMed] [Google Scholar]
- 92.Sugiyama K., et al. Differing effects of clarithromycin and azithromycin on cytokine production by murine dendritic cells. Clin. Exp. Immunol. 2007;147(3):540–546. doi: 10.1111/j.1365-2249.2007.03299.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Tomazic J., Kotnik V., Wraber B. In vivo administration of azithromycin affects lymphocyte activity in vitro. Antimicrob. Agents Chemother. 1993;37(9):1786–1789. doi: 10.1128/aac.37.9.1786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Ishida Y., Abe Y., Harabuchi Y. Effects of macrolides on antigen presentation and cytokine production by dendritic cells and T lymphocytes. Int. J. Paediatr. Otorhinolaryngol. 2007;71(2):297–305. doi: 10.1016/j.ijporl.2006.10.016. [DOI] [PubMed] [Google Scholar]
- 95.Kawakami K., et al. Phenotypic characterization of T cells in bronchoalveolar lavage fluid (BALF) and peripheral blood of patients with diffuse panbronchiolitis; the importance of cytotoxic T cells. Clin. Exp. Immunol. 1997;107:410–416. doi: 10.1111/j.1365-2249.1997.259-ce1139.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Kadota J., et al. Antibiotic-induced apoptosis in human activated peripheral lymphocytes. Int. J. Antimicrob. 2005;25(3):216–220. doi: 10.1016/j.ijantimicag.2004.10.009. [DOI] [PubMed] [Google Scholar]
- 97.Weng D., et al. Azithromycin treats diffuse panbronchiolitis by targeting T cells via inhibition of mTOR pathway. Biomed. Pharmacother. 2019;110:440–448. doi: 10.1016/j.biopha.2018.11.090. [DOI] [PubMed] [Google Scholar]
- 98.Ratzinger F., et al. Azithromycin suppresses CD$+ T-cell activation by direct modulation of mTOR activity. Sci. Rep. 2014;4:7438. doi: 10.1038/srep07438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Vandermeulen E., et al. A mechanistic study on the effect of azithromycin on T helper-17 cells. Eur. Respir. J. 2015;46(59) [Google Scholar]
- 100.Desaki M., et al. Erythromycin suppresses nuclear factor-kB and activator protein-1 activation in human bronchial epithelial cells. Biochem. Biophys. Res. 2000;267(1):124–128. doi: 10.1006/bbrc.1999.1917. [DOI] [PubMed] [Google Scholar]
- 101.Ichiyama T., et al. Clarithromycin inhibits NF-κB activation in human peripheral blood mononuclear cells and pulmonary epithelial cells. Antimicrob. Agents Chemother. 2001;45(1):44–47. doi: 10.1128/AAC.45.1.44-47.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Kikuchi T., et al. Clarithromycin suppresses lipopolysaccharide-induced interleukin-8 production by human monocytes through AP-1 and NF-κB transcription factors. J. Antimicrob. Chemother. 2002;49(5):745–755. doi: 10.1093/jac/dkf008. [DOI] [PubMed] [Google Scholar]
- 103.Aghai Z., et al. Azithromycin suppresses activation of nuclear factor-kappa B and synthesis of pro-inflammatory cytokines in tracheal aspirate cells from premature infants. Pediatr. Res. 2007;62:483–488. doi: 10.1203/PDR.0b013e318142582d. [DOI] [PubMed] [Google Scholar]
- 104.Beinke S., Ley S.C. Functions of NF-kB1 and NF-kB2 in immune cell biology. Biochem. J. 2004;382(2):393–409. doi: 10.1042/BJ20040544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Buyck J.M., et al. Increased susceptibility of P. aeruginosa to macrolides and Ketolides in eukaryotic cell culture media and biological fluids due to decreased expression of oprM and increased outer-membrane permeability. Clin. Infect. Dis. 2012;55(4):534–542. doi: 10.1093/cid/cis473. [DOI] [PubMed] [Google Scholar]
- 106.Mustafa M.H., et al. Acquired resistance to macrolides in Pseudomonas aeruginosa from cystic fibrosis patients. ERJ. 2017;49 doi: 10.1183/13993003.01847-2016. [DOI] [PubMed] [Google Scholar]
- 107.Oliver M.E., Hinks T.S.C. Azithromycin in viral infections. Rev. Med. Virol. 2021;31:e2163. doi: 10.1002/rmv.2163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Van Nuffel M.T. Repurposing drugs in oncology (ReDO) – clarithromycin as an anti-cancer agent. ecancermedicalscience. 2015;9:513. doi: 10.3332/ecancer.2015.513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Ogrendik M. Effects of clarithromycin in patients with active rheumatoid arthritis. Curr. Med. Res. Opin. 2007;23(3):515–522. doi: 10.1185/030079906X167642. [DOI] [PubMed] [Google Scholar]
- 110.Ogrendik M. Efficacy of roxithromycin in adult patients with rheumatoid arthritis who had not received disease-modifying antirheumatic drugs: a 3-month, randomised, double-blind, placebo-controlled trial. Clin. Therapeut. 2009;31(8):1754–1764. doi: 10.1016/j.clinthera.2009.08.014. [DOI] [PubMed] [Google Scholar]