Abstract
Some patients with pollen-food allergy syndrome (PFAS) develop systemic symptoms along with oral allergy syndrome upon ingesting processed foods as well as raw fruits and vegetables. This study aimed to investigate the efficacy of subcutaneous immunotherapy with birch pollen extract for patients with PFAS associated with soybean allergy. Subcutaneous immunotherapy with birch pollen extract was introduced in 6 patients with PFAS caused by alder/birch pollen and soybean allergy. An oral food challenge for ingestible amount of soy milk was performed before and 1 year after initiating subcutaneous immunotherapy with birch pollen extract. Before the treatment, the median intake of soy milk was 1.5 mL (interquartile range [IQR], 1–2 mL). One year after the treatment initiation, the median intake of soy milk increased significantly to 150 mL (IQR, 20–200 mL). Systemic reactions occurred in 4 of 6 patients in the rapid escalation phase of the treatment. The results thus suggest that subcutaneous immunotherapy with birch pollen extract could be beneficial for patients with PFAS associated with soybean allergy despite concerns regarding systemic reactions.
Keywords: Allergen immunotherapy, Birch pollen, Pollen-food allergy syndrome, Soybean allergy, Subcutaneous immunotherapy
INTRODUCTION
Pollen-food allergy syndrome (PFAS) is an allergic disease caused by a cross-reaction between pollens and vegetable foods. Patients with PFAS usually present with oral symptoms such as oral discomfort and sore throat upon ingesting raw fruits and vegetables, which is referred to as oral allergy syndrome (OAS). However, some patients with PFAS may develop systemic symptoms and require restriction of processed foods often have trouble in their dietary lives [1,2]. Some of them develop systemic symptoms upon ingesting soybean and are often placed on a restricted diet at home and school. In this study, subcutaneous immunotherapy with birch pollen extract (Birch SCIT) was introduced in patients with PFAS and soybean allergy.
CASE REPORT
We recruited 6 Japanese patients with PFAS (mean age, 9.5 years; interquartile range [IQR], 6–10 years) who had experienced nonoral symptoms such as abdominal pain, skin erythema, and respiratory symptoms upon ingesting some soy foods and required soybean restriction in the school lunch program (Table 1). All of them had specific IgE antibody titers of class 3 or higher against alder and birch, and class 2 or higher against Gly m 4, but did not necessarily develop allergic rhinitis to birch pollen. Birch Mix for injection (ALK-Abelló A/S, Hørsholm, Denmark) contained red birch and white birch and was used for Birch SCIT [3].
Table 1. Background factors (n=6).
| Background factors | Value | |
|---|---|---|
| Male sex | 2 (33.3) | |
| Age of study participation (yr) | 9.5 (6–10) | |
| Age of onset (yr) | 7.0 (3–8) | |
| Comorbidity | ||
| Bronchial asthma | 3 (50.0) | |
| Allergic rhinitis | 2 (33.3) | |
| Cedar pollinosis | 2 (33.3) | |
| Atopic dermatitis | 3 (50.0) | |
| Type1 food allergy | 2 (33.3) | |
| Ingestible amount of soy milk (mL) | 1.5 (1–2) | |
| Total IgE titer (IU/mL) | 733.0 (510.3–1,554.3) | |
| Specific IgE titer | ||
| Birch (IUA/mL) | 186.0 (154.0–295.3) | |
| Alder (IUA/mL) | 40.6 (39.5–55.2) | |
| Gly m 4 (UA/mL) | 48.7 (21.5–73.3) | |
| Soybean (IUA/mL) | 1.2 (0.7–9.4) | |
| Dermatophagoides pteronyssinus (IUA/mL) | 76.0 (0.8–166.0) | |
| Dermatophagoides farinae (IUA/mL) | 115.3 (2.2–276.3) | |
| Japanese cedar (IUA/mL) | 12.4 (11.8–25.2) | |
Values are presented as number (%) or median (interquartile range).
Background factors of patients were evaluated at the time of study participation.
Age of onset was defined as the age at which PFAS with soybean allergy was confirmed by interview.
PFAS, pollen-food allergy syndrome.
Birch SCIT was introduced by rush subcutaneous immunotherapy with the Birch Mix solution (Birch rSCIT). A threshold concentration was determined from results of an intradermal test using the Birch Mix solution. An initial dose of the Birch Mix solution was the threshold or 1/10 times the threshold concentration (1:2×106 -1:2×104 [w/v]). A target dose in the rapid escalation phase was 1:2×103 (w/v) in 0.3 mL -1:2×10 (w/v) in 0.05 mL. In the maintenance phase, the antigen dose of 1: 2×102 (w/v) in 0.05 mL was administered once a month in the outpatient clinic.
An oral food challenge (OFC) test with soy milk (protein content 3.5%) was performed before and 1 year after initiating Birch SCIT. The initial amount of soy milk OFC was 1 mL, and the amount of soy milk was gradually increased to 200 mL, intake status of processed soybean foods, sprouts, and apples were evaluated by interview before and 1 year after Birch SCIT initiation. The restriction of soybean in the school lunch program was confirmed on the school life management instruction sheet.
We obtained an approval regarding the use of the imported Birch Mix for injection from the Yao Municipal Hospital Ethics Committee (YMH-H28-17). Written informed consent was obtained from the parents regarding participation in the study and reporting of study results. This study was approved by the Yao Municipal Hospital Ethics Committee (YMH-051820-85).
Ingestible amount of soy milk, and serum allergen-specific IgE antibody titers were examined before and 1 year after initiating Birch SCIT using the Wilcoxon signed rank test. The difference was considered significant if p was less than 0.05.
A total of 11 systemic reactions (SRs), including 8 skin symptoms and 3 respiratory symptoms, were observed in 8 of 68 injections (11.8%) in the rapid escalation phase. Four of 6 patients (67%) showed the SRs in the rapid escalation phase. In patients 1, 2, 4, and 5 who showed the SRs in the rapid escalation phase, the dose was gradually increased to 1:2×102 (w/v) in 0.05 mL by the conventional method in the outpatient clinic.
The median ingestible amount of soy milk was 1.5 mL (IQR, 1–2 mL) before the treatment and significantly increased to 150 mL (IQR, 100–200 mL) 1 year after initiating Birch SCIT (p = 0.04) (Fig. 1).
Fig. 1. Ingestible amount of soy milk by OFC before and 1 year after Birch SCIT initiation.
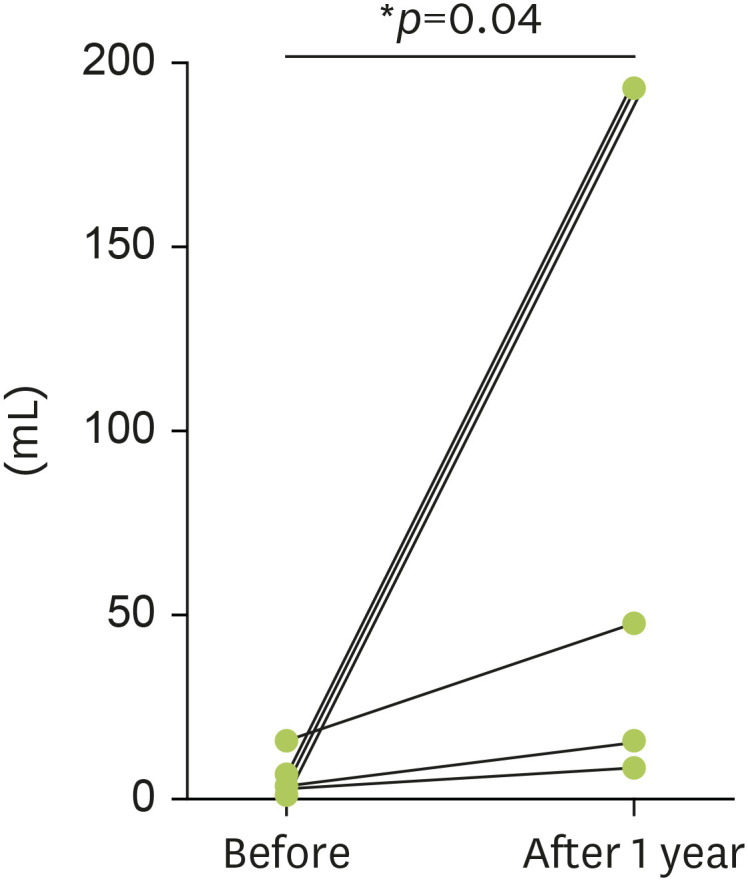
In the Birch SCIT group, the median ingestible amount of soy milk was significantly increased 1 year after initiating Birch SCIT. OFC, oral food challenge; Birch SCIT, subcutaneous immunotherapy with birch pollen extract. *p<0.05.
The specific IgE antibody titers to birch, alder, and Gly m 4 did not change significantly before and 1 year after initiating Birch SCIT.
One year after initiating the treatment, all patients were free from dietary restriction of soybean intake in their school lunch programs (Table 2).
Table 2. Dietary intake before and 1 year after Birch SCIT initiation.
| Dietary intake | Patient | ||||||
|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | ||
| Processed soy foods | |||||||
| Before | Possible | Impossible | Possible | Impossible | Impossible | Impossible | |
| After 1 year | Possible | Possible | Possible | Partial | Possible | Possible | |
| Sprouts | |||||||
| Before | Possible | Impossible | Impossible | Impossible | Impossible | Impossible | |
| After 1 year | Possible | Partial | Partial | Possible | Possible | Partial | |
| Apples | |||||||
| Before | Impossible | Impossible | Possible | Impossible | Impossible | Impossible | |
| After 1 year | Partial | Partial | Possible | Partial | Partial | Possible | |
| Restriction of soybean in the school lunch program | |||||||
| Before | Yes | Yes | Yes | Yes | Yes | Yes | |
| After 1 year | None | None | None | None | None | None | |
Birch SCIT, subcutaneous immunotherapy with birch pollen extract; possible, no restriction of target food ingesting; partial, some range restriction of target food ingesting; impossible, complete restriction of target food ingesting.
Of the patients who needed restriction of apple intake before the treatment, Patient 6 was able to ingest apples without restriction, whereas patients 1, 2, 4, and 5 were able to ingest them partially 1 year after initiating the treatment (Table 2).
DISCUSSION
Allergen-specific immunotherapy (AIT) is expected to be effective in treating PFAS, which is unlikely to heal spontaneously after its onset. However, the efficacy of AIT with pollen extract to PFAS has differed greatly in various reports, and its evaluation has not been established [4,5,6,7,8].
This is the first report specifically evaluating soy milk intake of therapeutic effect on PFAS with Birch SCIT. The median intake of soy milk was significantly increased, and all patients were able to ingest soybean without restriction in their school lunch programs 1 year after initiating Birch SCIT. Birch SCIT could be suitable for patients with PFAS associated with soybean allergy.
The efficacy of AIT with Bet v1, the major birch pollen allergen has been evaluated with apple OFC in some studies [4,5,6]. However, the method for apple OFC used in these studies differed greatly in the amount of apple intake and the definition of symptoms. In our study, all patients were able to ingest apples partially or completely. The efficacy of apple intake with Birch SCIT could be expected depending on the target setting of each patient.
The antigenic homology between the components in the Birch Mix solution and Japanese birch and alder pollen is unknown. Tsumagari et al. [3] reported that 15 of 19 Japanese children (79%) showed improved or markedly improved OAS for raw fruits with Birch SCIT after 1 year; based on their report, we used the birch pollen extract from the same manufacturer. The target antigen dose of Birch SCIT to achieve sufficient efficacy with AIT has not been established [9]. In our study, the desired therapeutic effect was obtained with the maintenance dose of 1:2×102 (w/v) in 0.05 mL, but the incidence of SRs was 67% in the rapid escalation phase, indicating that the protocol for Birch rSCIT needs further improvement.
The limitation of this study is that it is not a randomized controlled trial. In addition, this study was conducted with a small number of patients at a single facility. A multicenter collaborative study will be necessary in the future.
In conclusion, our findings indicate that Birch SCIT should be taken into account in patients with difficulty in ingesting soy foods, and having trouble in dealing with soybean in the school lunch program.
ACKNOWLEDGEMENTS
We would like to extend special thanks to Dr. Kazuyuki Kurihara, Department of Pediatrics, Kanagawa Children's Medical Center for his valuable advice regarding the introduction of Birch SCIT.
Footnotes
Conflict of Interest: The authors have no financial conflicts of interest.
- Conceptualization: Masaaki Hamada, Masakazu Kagawa.
- Formal analysis: Masaaki Hamada, Ichiro Tanaka.
- Investigation: Masaaki Hamada.
- Methodology: Masaaki Hamada, Masakazu Kagawa.
- Project administration: Masaaki Hamada, Masakazu Kagawa.
- Writing - original draft: Masaaki Hamada.
- Writing - review & editing: Ichiro Tanaka.
References
- 1.Werfel T, Asero R, Ballmer-Weber BK, Beyer K, Enrique E, Knulst AC, Mari A, Muraro A, Ollert M, Poulsen LK, Vieths S, Worm M, Hoffmann-Sommergruber K. Position paper of the EAACI: food allergy due to immunological cross-reactions with common inhalant allergens. Allergy. 2015;70:1079–1090. doi: 10.1111/all.12666. [DOI] [PubMed] [Google Scholar]
- 2.Kim MA, Kim DK, Yang HJ, Yoo Y, Ahn Y, Park HS, Lee HJ, Jeong YY, Kim BS, Bae WY, Jang AS, Park Y, Koh YI, Lee J, Lim DH, Kim JH, Lee SM, Kim YM, Jun YJ, Kim HY, Kim Y, Choi JH Work Group for Rhinitis, the Korean Academy of Asthma, Allergy and Clinical Immunology. Pollen-food allergy syndrome in Korean pollinosis patients: a nationwide survey. Allergy Asthma Immunol Res. 2018;10:648–661. doi: 10.4168/aair.2018.10.6.648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tsumagari S, Mori S, Ishizu H, Tanaka Y, Okamoto Y, Kurihara K. Evaluation of the effectiveness of subcutaneous immunotherapy using birch pollen extract for pollen-food allergy syndrome. Arerugi. 2018;67:211–218. doi: 10.15036/arerugi.67.211. [Japanese] [DOI] [PubMed] [Google Scholar]
- 4.Asero R. Effects of birch pollen-specific immunotherapy on apple allergy in birch pollen-hypersensitive patients. Clin Exp Allergy. 1998;28:1368–1373. doi: 10.1046/j.1365-2222.1998.00399.x. [DOI] [PubMed] [Google Scholar]
- 5.Hansen KS, Khinchi MS, Skov PS, Bindslev-Jensen C, Poulsen LK, Malling HJ. Food allergy to apple and specific immunotherapy with birch pollen. Mol Nutr Food Res. 2004;48:441–448. doi: 10.1002/mnfr.200400037. [DOI] [PubMed] [Google Scholar]
- 6.Bucher X, Pichler WJ, Dahinden CA, Helbling A. Effect of tree pollen specific, subcutaneous immunotherapy on the oral allergy syndrome to apple and hazelnut. Allergy. 2004;59:1272–1276. doi: 10.1111/j.1398-9995.2004.00626.x. [DOI] [PubMed] [Google Scholar]
- 7.Cox L, Nelson H, Lockey R, Calabria C, Chacko T, Finegold I, Nelson M, Weber R, Bernstein DI, Blessing-Moore J, Khan DA, Lang DM, Nicklas RA, Oppenheimer J, Portnoy JM, Randolph C, Schuller DE, Spector SL, Tilles S, Wallace D. Allergen immunotherapy: a practice parameter third update. J Allergy Clin Immunol. 2011;127:S1–55. doi: 10.1016/j.jaci.2010.09.034. [DOI] [PubMed] [Google Scholar]
- 8.Walker SM, Durham SR, Till SJ, Roberts G, Corrigan CJ, Leech SC, Krishna MT, Rajakulasingham RK, Williams A, Chantrell J, Dixon L, Frew AJ, Nasser SM British Society for Allergy and Clinical Immunology. Immunotherapy for allergic rhinitis. Clin Exp Allergy. 2011;41:1177–1200. doi: 10.1111/j.1365-2222.2011.03794.x. [DOI] [PubMed] [Google Scholar]
- 9.Pajno GB, Fernandez-Rivas M, Arasi S, Roberts G, Akdis CA, Alvaro-Lozano M, Beyer K, Bindslev-Jensen C, Burks W, Ebisawa M, Eigenmann P, Knol E, Nadeau KC, Poulsen LK, van Ree R, Santos AF, du Toit G, Dhami S, Nurmatov U, Boloh Y, Makela M, O'Mahony L, Papadopoulos N, Sackesen C, Agache I, Angier E, Halken S, Jutel M, Lau S, Pfaar O, Ryan D, Sturm G, Varga EM, van Wijk RG, Sheikh A, Muraro A EAACI Allergen Immunotherapy Guidelines Group. EAACI Guidelines on allergen immunotherapy: IgE-mediated food allergy. Allergy. 2018;73:799–815. doi: 10.1111/all.13319. [DOI] [PubMed] [Google Scholar]


