Abstract
Background
Egg allergy is one of the most common food allergies in childhood with increasing prevalence in Hong Kong. While ample studies were published on its optimal diagnosis, there was limited data on predictors for the natural history of egg allergy in Asian populations.
Objective
This study aimed to characterize the clinical course and outcome of children with egg allergy and identify its prognostic factors.
Methods
All Chinese children with immediate-type egg allergy being followed since ≥3 years old in allergy clinic of our university-affiliated teaching hospital were reviewed to determine if they outgrew egg allergy at the latest follow-up. The predictive values of clinical and atopic factors for resolution of egg allergy were analyzed on Kaplan-Meier curves, and factors independently associated with persistent egg allergy was analyzed by logistic regression.
Results
Seventy-six patients with median (interquartile range) age 8.9 years (6.3–13.0 years) were recruited. They initially presented with egg-allergic reactions at 1.0 years (0.7–1.7 years). Fifty-four children (71%) were able to tolerate egg at a median of 36 months from initial reaction. Patients with concomitant peanut allergy and those with initial reaction at ≥1 year old were more likely to have persistent egg allergy (p = 0.015 and p = 0.027 respectively). Skin prick test wheal ≥6 mm to egg yolk and egg white individually as well as to both egg yolk and egg white were predictors for egg allergy persistence (respective, p < 0.001, p = 0.001, and p = 0.001 by log-rank tests). Logistic regression showed that initial SPT ≥ 6 mm to egg yolk was the only independent predictor for persistent egg allergy (B = 2.59 ± 0.98, p = 0.008).
Conclusion
Most Chinese children with immediate-type egg allergy can tolerate egg in long run. SPT wheal size to egg, concomitant peanut allergy and initial presentation after infancy may predict egg allergy persistence.
Keywords: Egg allergy, Natural history, Peanut allergy, Persistence, Prediction
INTRODUCTION
The global prevalence of food allergy in children is increasing [1]. Egg allergy is one of the most common food allergies in young children, which affected up to one-tenth of Australian infants [2]. Among Chinese preschoolers, shellfish (15.8%), egg (9.1%), and peanut (8.1%) were the leading causes of adverse food reactions [3]. The estimated prevalence of adverse reactions to egg was 7.3 per 1,000 children aged 2–7 years in Hong Kong. In a comparative study funded by EuroPrevall, sensitization to egg was amongst the top 3 foods in Chinese children living in Hong Kong and Guangzhou while it was uncommonly seen in Chinese from Shaoguan as well as Russian and Indian schoolchildren [4]. Egg is an important source of protein in weaning diet for infants and is commonly found in processed foods and baked goods. Accidental ingestion of egg or egg-containing foods is common [5], and such imposes anxiety and stress on egg-allergic children and their families. Egg allergy may also coexist with other food allergies or allergic diseases [6], and parents with these egg-allergic children had impaired quality of life [7]. A number of studies from Western countries and Korea reported that egg allergy resolved over time [8,9,10,11]. An early report that followed the natural course of challenge-proven egg allergy showed that half of children regularly tolerated egg by 4–12 years of age [8]. Another study on Korean children with atopic dermatitis and egg allergy found that 41% and 60% of them developed tolerance to egg by 3 and 5 years old respectively [11]. These observations suggested that some egg-allergic children had persistent egg allergy in adolescence. There is limited data on the natural history and epidemiology of egg allergy as well as prognostic indicators for its persistence in Chinese children. Previous studies reported specific-IgE levels, age of first intake of egg and skin prick test (SPT) results to predict outcomes of egg allergy [11,12,13]. It is important to know if these prognostic factors are also applicable to Chinese egg-allergic children. This study aimed to describe the natural history of egg allergy in Chinese children and identify any factor that might predict the persistence of egg allergy.
MATERIALS AND METHODS
This study recruited children younger than 18 years old with egg allergy who were referred to the allergy clinic of our university-affiliated teaching hospital between January 2003 and December 2017. The inclusion criteria for subjects were as follows: (1) history of allergic reactions (e.g., hives, facial, eyelid or lip swelling, cough, dyspnea, wheeze, vomiting, diarrhea, abdominal pain, low blood pressure, drowsiness, loss of consciousness) within 2 hours following ingestion or skin contact with hen's egg; (2) wheal size to egg yolk and/or egg white ≥ 3 mm larger than negative control by SPT; and (3) aged 3 years or older at first clinic consultation and followed for ≥3 years. Patients who experienced delayed adverse reactions (>2 hours after egg ingestion or contact) were excluded. The Joint Chinese University of Hong Kong-New Territories East Cluster Clinical Research Ethics Committee approved this study (reference no. 2016.637). Subjects' parents gave informed verbal consent to participate.
Demographics and personal and family history of allergic diseases of eligible subjects were retrieved from the Hospital Authority Electronic Patient Record and/or medical record folders. Egg allergy was considered to be resolved when subjects acquired oral tolerance to egg with the absence of adverse reactions after reintroduction of regular egg ingestion at home for 3 months or longer, or tolerated a graded dose oral food challenge to hen's egg. In both categories, subjects must tolerate hen's egg at an amount appropriate to their age and weight.
The following information was reviewed from medical records: demographics, possible prognostic factors (e.g., age at first presentation, anaphylaxis at first presentation, SPT details, personal history of eczema and food allergy, family history of allergic diseases, and food allergy). The diagnosis of anaphylaxis was made according to the National Institute of Allergy and Infectious Diseases (NIAID)/FAAN criteria [14]. SPT to egg yolk and egg white is a useful test to determine the presence of specific IgE (sIgE) to egg [15]. Histamine (10 mg/mL) and normal saline were included as positive and negative controls respectively, and SPT also included food allergen extracts cow's milk, soybean, wheat and peanut as well as Dermatophagoides pteronyssinus (ALK-Abelló A/S, Hørsholm, Denmark). Egg allergy diagnosis was made by the presence of suggestive clinical history after egg ingestion and ≥3 mm wheal by SPT. Peanut allergy was diagnosed by suggestive adverse reactions after peanut ingestion and positive SPT or peanut-sIgE level [16].
The natural course of egg allergy from the first allergic reaction was estimated using Kaplan-Meier curves. The relationship between different demographic, clinical and disease-related factors and the cumulative probability of egg allergy persistence was analyzed using log-rank test. Multivariate logistic regression was also performed to identify independent factor(s) for persistent egg allergy among the significant ones from univariate analyses. Statistical analyses were performed on IBM SPSS Statistics ver. 22.0 (IBM Co., Armonk, NY, USA). A p value < 0.05 was considered to be statistically significant.
RESULTS
Subjects
Seventy-six patients with median (interquartile range [IQR]) age of 8.9 years (6.3–13.0 years) met the recruitment criteria, with 52 (68.4%) being male. Table 1 summarizes their baseline characteristics. The median (IQR) age at first egg-allergic reaction was 1.0 years (0.7–1.7 years), with 44 patients (57.9%) presented initially before 2 years of age. The main presenting features included urticaria (90.8%) and angioedema (53.9%), while 13 patients (17.1%) had anaphylaxis. Ninety percent of patients had family history of allergic diseases. Seventy-two patients (94.7%) suffered from eczema. Regarding SPT results at baseline, 33 patients had ≥6 mm wheal to egg yolk while 37 patients had ≥ 6 mm wheal to egg white. Twenty-five patients (32.9%) had ≥ 6 mm wheal to both egg yolk and egg white.
Table 1. Baseline clinical and atopic characteristics of our 76 subjects.
| Characteristic | Value | |
|---|---|---|
| Age at recruitment (yr) | 8.9 (6.3–13.0) | |
| Male sex | 52 (68.4) | |
| Age at initial presentation (yr) | 1.0 (0.7–1.7) | |
| Younger than 2 years old at initial presentation | 58 (76.3) | |
| Presenting clinical features for egg allergy | ||
| Urticaria | 69 (90.8) | |
| Eczematous rash | 10 (13.2) | |
| Angioedema | 41 (53.9) | |
| Vomiting, diarrhea and/or abdominal cramp | 14 (18.4) | |
| Shortness of breath, cyanosis and/or wheezing | 13 (17.1) | |
| Anaphylaxis* | 13 (17.1) | |
| Coexisting allergic diseases at the time of recruitment | ||
| Asthma | 25 (32.9) | |
| Allergic rhinitis | 42 (55.3) | |
| Eczema | 72 (94.7) | |
| Family history of allergic diseases† in first-degree relatives | 68 (89.5) | |
| Wheal size for skin prick tests with allergen extracts (mm) | ||
| Egg white | 5.0 (4.0–7.6) | |
| Egg yolk | 4.0 (3.5–7.0) | |
| Cow's milk | 1.0 (0–3.1) | |
| Peanut | 3.0 (0–6.6) | |
| Soybean | 0 (0–2.1) | |
| Wheat | 0 (0–2) | |
| Dermatophagoides pteronyssinus | 5.0 (0.8–8.3) | |
Values are presented as median (interquartile range) or number (%).
*Cardiovascular, respiratory, and/or neurological involvement. †One or more for asthma, allergic rhinitis, eczema and food allergy.
Natural history and predictors for egg allergy persistence
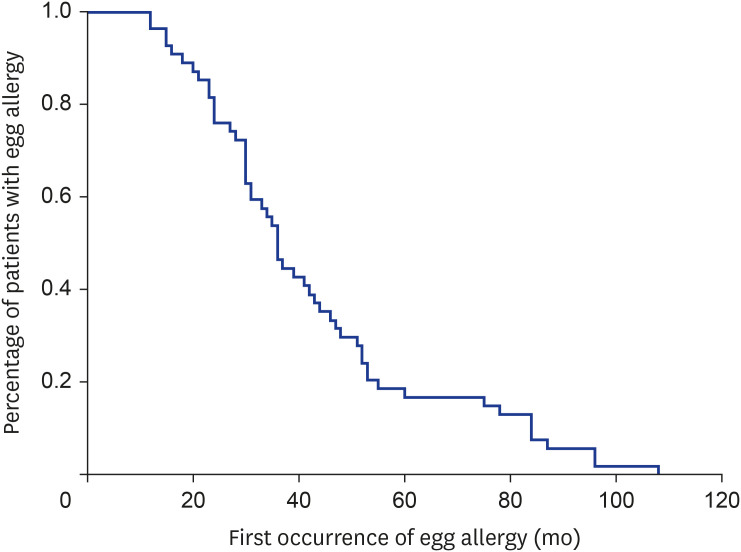
Amongst 76 recruited patients, 54 (71.1%) developed tolerance to egg at a median (IQR) duration of 36.0 months (27.3–52.0 months) while 22 patients (28.9%) had persistent egg allergy. Fig. 1 shows the Kaplan-Meier curve demonstrating the cumulative persistence of egg allergy. Patients who outgrew egg allergy achieved this at a median 36 months (range, 2–96 months) from their first allergic reactions. Table 2 summarizes possible predicting factors for the development of egg tolerance. Patients with concomitant peanut allergy and those experienced the first allergic reaction at ≥1 year old were more likely to have persistent egg allergy (p = 0.015 and p = 0.027 respectively).
Fig. 1. Kaplan-Meier curve for development of egg tolerance from initial presentation.
Table 2. Predictors for the development of egg tolerance.
| Factor | Egg tolerance | p value* | ||
|---|---|---|---|---|
| No. (%) | Median time (mo) (95% CI) | |||
| Sex | 1.000 | |||
| Male | 38/52 (73.1) | 39 (20–57) | ||
| Female | 16/24 (66.7) | 52 (41–62) | ||
| Age at first allergic reaction | 0.027 | |||
| <1 yr | 34/43 (79.1) | 41 (28–53) | ||
| ≥1 yr | 20/33 (60.1) | 78 (37–118) | ||
| <2 yr | 44/62 (71.0) | 48 (0–97) | ||
| ≥2 yr | 10/14 (71.4) | 47 (37–57) | ||
| Wheal size of first SPT to egg white | 0.001 | |||
| <6 mm | 33/40 (82.5) | 42 (31–52) | ||
| ≥6 mm | 21/36 (58.3) | 55 (7–102) | ||
| Wheal size of first SPT to egg yolk | <0.001 | |||
| <6 mm | 39/43 (90.7) | 36 (30–41) | ||
| ≥6 mm | 15/33 (45.5) | 48 (38–57) | ||
| Wheal size of first SPT to both egg white and egg yolk | 0.001 | |||
| <6 mm | 43/51 (84.3) | 42 (31–52) | ||
| ≥6 mm | 11/25 (44.0) | 55 (7–102) | ||
| Coexisting peanut allergy | 0.015 | |||
| No | 38/48 (79.2) | 36 (28–43) | ||
| Yes | 16/28 (57.1) | 84 (34–133) | ||
| Anaphylaxis as first presentation | 0.091 | |||
| No | 49/67 (73.1) | 44 (34–54) | ||
| Yes | 5/9 (55.6) | 108 (73–143) | ||
| Physician-diagnosed eczema | 0.480 | |||
| No | 14/17 (82.4) | 46 (24–68) | ||
| Yes | 40/59 (67.8) | 51 (42–60) | ||
CI, confidence interval; SPT, skin prick test.
*Analyzed by log-rank test.
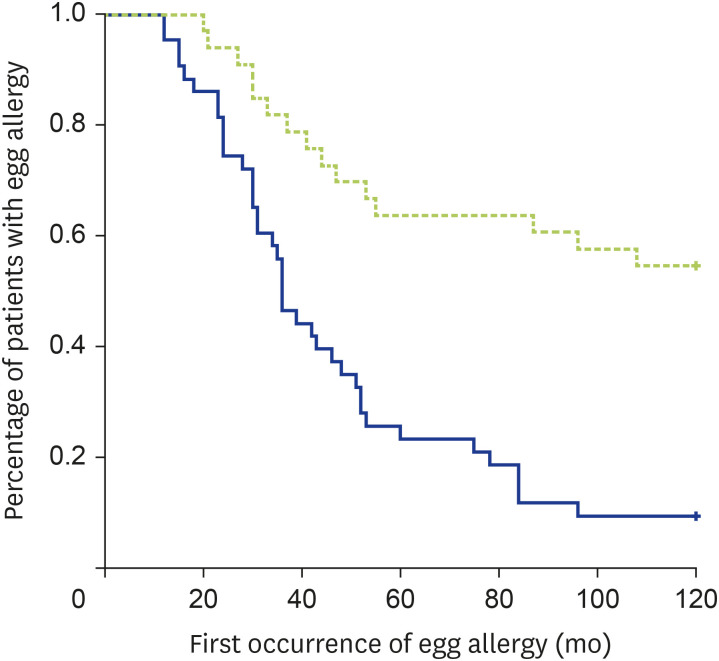
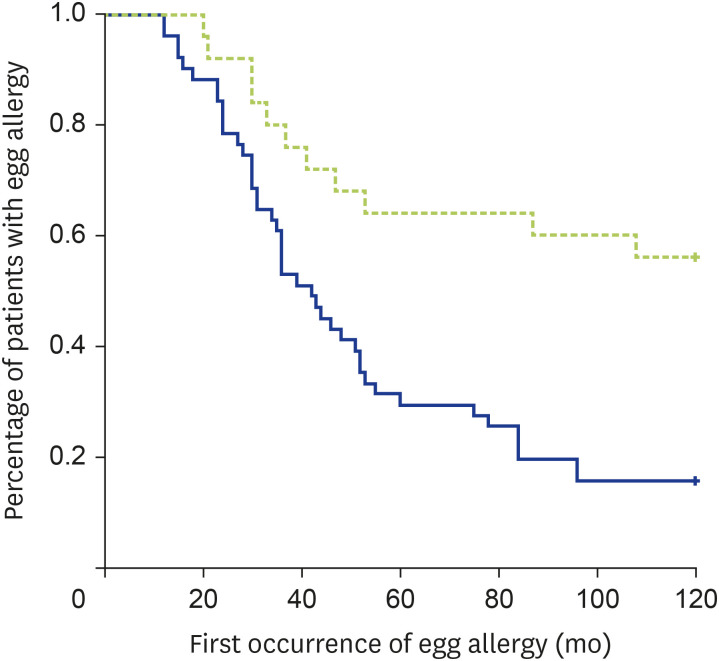
Univariate analysis using log-rank test showed that the development of egg tolerance was lower in patients with initial SPT wheal ≥ 6 mm to egg yolk (p < 0.001, Fig. 2), initial SPT wheal ≥ 6 mm to combined egg yolk and egg white (P = 0.001, Fig. 3) and initial SPT wheal ≥ 6 mm to egg white (p = 0.001). Only 15 of 33 patients (45.5%) with initial SPT ≥ 6 mm to egg yolk, 11 of 25 patients (44.0%) with initial SPT ≥ 6 mm to both egg white and egg yolk, and 21 of 36 patients (58.3%) with initial SPT ≥ 6 mm to egg white developed egg tolerance (respective p < 0.0001, p < 0.001, and p = 0.038). All other factors enlisted in Table 1 were not significantly associated with egg allergy persistence in our Chinese children. Logistic regression revealed initial SPT ≥ 6 mm to egg yolk to be the only independent predictor for persistent egg allergy (B = 2.59 ± 0.98, p = 0.008).
Fig. 2. Kaplan-Meier curve on the relationship between egg allergy resolution and initial SPT ≥ 6 mm for egg yolk (p < 0.001 by log-rank test). Green and blue lines indicate initial SPT wheal ≥ 6 mm and <6 mm respectively.
Fig. 3. Kaplan-Meier curve on the relationship between egg allergy resolution and initial SPT ≥ 6 mm for both egg white and egg yolk (p = 0.001 by log-rank test). Green and blue lines indicate initial SPT wheal ≥ 6 mm and <6 mm respectively.
DISCUSSION
The present study reviewed the natural history of 76 children with immediate-type egg allergy who attended our pediatric allergy clinic since 3 years or older and spanned over a 15-year period. We found that most children (71%) were able to tolerate egg at a median follow-up of 36 months, which was consistent with earlier reports in Western countries and Korea [8,10,11]. Importantly, SPT wheal size to egg, concomitant peanut allergy and initial presentation after infancy predicted persistence of egg allergy in our Chinese children.
Across Europe, 9,336 newborns enrolled in the EuroPrevall birth cohort study were followed up to 2 years of age [17]. Eighty-six infants had challenge-confirmed egg allergy by the end of follow-up, and half of them could tolerate egg within 1 year after the initial diagnosis. In a multicenter cohort, 213 infants aged 3–15 months with a clinical history of immediate-type egg allergy were followed longitudinally to a median age of 74 months [18]. Egg allergy resolution was established based on successful ingestion, which was found in 105 of them (49.3%). This figure of egg allergy resolution was similar to that found in the above multi-national European study. The reported independent predictors for resolution to be features of initial allergic reactions and baseline egg sIgE level.
Egg white was believed to be more allergenic than egg yolk, and several studies reported a significant association between persistence of egg allergy and positive SPT or sIgE against egg white [12,13,15]. Nonetheless, Horino et al. [19] found that only 15% of patients with challenge-proven allergy to boiled egg yolk could tolerate egg yolk after 3 years. This study was the first to report SPT wheal size to egg yolk as a significant prognostic factor for persistent egg allergy in children. Allergy to egg yolk might be more important than previously thought. Regarding the testing for natural history of egg allergy, 2 studies reported SPT to egg white to have high sensitivity but low specificity for predicting the persistence of egg allergy [15,20]. Our results from SPT positivity to egg yolk (Fig. 2) supported such possible benefit of testing for egg yolk sensitization.
Recent studies reported the usefulness of measuring sIgE against allergens to improve egg allergy diagnosis. Five major proteins with allergenic properties in hen's eggs have been identified, which are known as Gal d 1 to Gal d 5 [15]. Ovomucoid (Gal d 1) and ovalbumin (Gal d 2) were the proteins being linked to IgE-mediated allergy to egg, and both proteins are found in egg white. Alpha-livetin (Gal d 5) was reported to be the major allergen in egg yolk that caused allergic reactions. There is a tendency for allergy centers to move from conducting SPT alone to taking blood for serum sIgE to egg and more recently egg white components (ovomucoid, ovalbumin and conalbumin) [21]. Nonetheless, it remains unclear whether the levels of these sIgE were able to identify patients with persistent egg allergy. Marriage et al. showed that sIgE against ovomucoid was one of the most useful tests to identify persistent egg allergy, while this test could predict persistent allergy to heated and uncooked egg at different cutoff values [21,22]. In another study, 101 of 124 Korean children (81.5%) tolerated egg [23]. Similar to our results, peanut allergy was more common among those with persistent egg allergy. Presence of atopic dermatitis and wheat allergy were other predictors for persistent egg allergy, while ≥30% reduction of egg white sIgE level after 12 months from diagnosis significantly predicted the development of egg tolerance. Such information would be helpful in the counselling of our patients whether they might be able to tolerate extensively heated egg in the near future.
This study had several limitations. First, we did not ascertain egg tolerance by double-blind, placebo-controlled food challenges. In the Australian HealthNuts study, 140 infants with challenge-confirmed raw egg allergy were followed until 2 years of age [24]. Egg allergy resolved in 66 infants (47%) in this follow-up period, and those with baked egg allergy at 1 year old was more likely to have persistent egg allergy. Such oral food challenge may add predictive value for egg allergy persistence. The optimal cutoff for predicting resolution of food allergy might be age-dependent. In the HealthNuts study, the 95% positive predictive values of peanut sIgE for its resolution varied from 34 kUA/L in children younger than 2 years to 2.1 kUA/L at 4 years of age [25]. As this study recruited children 3 years or older at baseline, our results on SPT cutoff to egg white and yolk might not be extrapolated to infants with egg allergy. Secondly, this retrospective study was liable to recall and selection biases especially regarding the timing and presentation of patients' initial allergic reactions. Besides, this study only recruited patients with immediate (IgE)-type egg allergy whereas egg allergy in real-life is caused by IgE, non-IgE and mixed IgE and non-IgE reactions (e.g., eczema, eosinophilic esophagitis). Our findings were not applicable to the latter groups of egg-allergic patients. The apparent inclusion of ‘eczematous rash’ in Table 1 as presenting features of egg allergy was due to the observation that some patients presented with more than one allergic manifestation after egg ingestion, including urticaria and angioedema (immediate-type allergy) and eczematous rash (more related to delayed hypersensitivity). As this study was conducted in our tertiary allergy clinic, the recruited patients might also suffer from more severe egg-allergic reactions who would less likely outgrow their food allergy. Lastly, we did not evaluate the roles of newer biomarkers of IgE-mediated food allergy such as basophil activation test, IgG4 to IgE ratio and mast cell activation test [26].
In conclusion, this study showed that the majority of Chinese children with immediate-type egg allergy were able to tolerate egg upon long term follow-up. Wheal size of SPT to egg yolk as well as both egg yolk and white, concomitant peanut allergy and occurrence of first allergic reaction after infancy were possible predictors for egg allergy persistence. Further studies should focus on the roles of sIgE to egg and its individual allergens and their longitudinal changes as prognostic indicators for the development of egg tolerance.
ACKNOWLEDGEMENTS
This study was partly supported by Research Fellowship Scheme (reference no. 0418004) of Health and Medical Research Fund, Hong Kong Special Administrative Region Government.
Footnotes
Conflict of Interest: The authors have no financial conflicts of interest.
- Conceptualization: Ting Fan Leung.
- Formal analysis: Noelle Anne Ngai, Ting Fan Leung.
- Investigation: Noelle Anne Ngai, Agnes Sze Yin Leung, Ting Fan Leung.
- Methodology: Noelle Anne Ngai, Agnes Sze Yin Leung, Jonathan Chi Ho Leung, Oi Man Chan, Ting Fan Leung.
- Project administration: Ting Fan Leung.
- Writing - original draft: Noelle Anne Ngai.
- Writing - review & editing: Noelle Anne Ngai, Agnes Sze Yin Leung, Jonathan Chi Ho Leung, Oi Man Chan, Ting Fan Leung.
References
- 1.Prescott SL, Pawankar R, Allen KJ, Campbell DE, Sinn JKh, Fiocchi A, Ebisawa M, Sampson HA, Beyer K, Lee BW. A global survey of changing patterns of food allergy burden in children. World Allergy Organ J. 2013;6:21. doi: 10.1186/1939-4551-6-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Osborne NJ, Koplin JJ, Martin PE, Gurrin LC, Lowe AJ, Matheson MC, Ponsonby AL, Wake M, Tang ML, Dharmage SC, Allen KJ HealthNuts Investigators. Prevalence of challenge-proven IgE-mediated food allergy using population-based sampling and predetermined challenge criteria in infants. J Allergy Clin Immunol. 2011;127:668–76.e1-2. doi: 10.1016/j.jaci.2011.01.039. [DOI] [PubMed] [Google Scholar]
- 3.Leung TF, Yung E, Wong YS, Lam CW, Wong GW. Parent-reported adverse food reactions in Hong Kong Chinese pre-schoolers: epidemiology, clinical spectrum and risk factors. Pediatr Allergy Immunol. 2009;20:339–346. doi: 10.1111/j.1399-3038.2008.00801.x. [DOI] [PubMed] [Google Scholar]
- 4.Li J, Ogorodova LM, Mahesh PA, Wang MH, Fedorova OS, Leung TF, Fernandez-Rivas M, Mills ENC, Potts J, Kummeling I, Versteeg SA, van Ree R, Yazdanbakhsh M, Burney PGJ, Wong GWK. Comparative Study of Food Allergies in Children from China, India, and Russia: The EuroPrevall-INCO Surveys. J Allergy Clin Immunol Pract. 2020;8:1349–58.e16. doi: 10.1016/j.jaip.2019.11.042. [DOI] [PubMed] [Google Scholar]
- 5.Boyano-Martínez T, Pedrosa M, Quirce S, García-Ara C. Accidental allergic reactions in children allergic to hen's egg. J Investig Allergol Clin Immunol. 2012;22:109–115. [PubMed] [Google Scholar]
- 6.Tariq SM, Matthews SM, Hakim EA, Arshad SH. Egg allergy in infancy predicts respiratory allergic disease by 4 years of age. Pediatr Allergy Immunol. 2000;11:162–167. doi: 10.1034/j.1399-3038.2000.00077.x. [DOI] [PubMed] [Google Scholar]
- 7.Leung TF, Yung E, Wong YS, Li CY, Wong GW. Quality-of-life assessment in Chinese families with food-allergic children. Clin Exp Allergy. 2009;39:890–896. doi: 10.1111/j.1365-2222.2008.03192.x. [DOI] [PubMed] [Google Scholar]
- 8.Dannaeus A, Inganäs M. A follow-up study of children with food allergy. Clinical course in relation to serum IgE- and IgG-antibody levels to milk, egg and fish. Clin Allergy. 1981;11:533–539. doi: 10.1111/j.1365-2222.1981.tb02171.x. [DOI] [PubMed] [Google Scholar]
- 9.Savage JH, Matsui EC, Skripak JM, Wood RA. The natural history of egg allergy. J Allergy Clin Immunol. 2007;120:1413–1417. doi: 10.1016/j.jaci.2007.09.040. [DOI] [PubMed] [Google Scholar]
- 10.Ford RP, Taylor B. Natural history of egg hypersensitivity. Arch Dis Child. 1982;57:649–652. doi: 10.1136/adc.57.9.649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kim J, Chung Y, Kwon J, Ahn K, Lee S. Natural history of egg allergy and prognostic factors of the development of tolerance for egg allergy in children with atopic dermatitis (AD) J Allergy Clin Immunol. 2008;121:S239. [Google Scholar]
- 12.Boyano-Martínez T, García-Ara C, Díaz-Pena JM, Martín-Esteban M. Prediction of tolerance on the basis of quantification of egg white-specific IgE antibodies in children with egg allergy. J Allergy Clin Immunol. 2002;110:304–309. doi: 10.1067/mai.2002.126081. [DOI] [PubMed] [Google Scholar]
- 13.Shek LP, Soderstrom L, Ahlstedt S, Beyer K, Sampson HA. Determination of food specific IgE levels over time can predict the development of tolerance in cow's milk and hen's egg allergy. J Allergy Clin Immunol. 2004;114:387–391. doi: 10.1016/j.jaci.2004.04.032. [DOI] [PubMed] [Google Scholar]
- 14.Sampson HA, Muñoz-Furlong A, Campbell RL, Adkinson NF, Jr, Bock SA, Branum A, Brown SG, Camargo CA, Jr, Cydulka R, Galli SJ, Gidudu J, Gruchalla RS, Harlor AD, Jr, Hepner DL, Lewis LM, Lieberman PL, Metcalfe DD, O'Connor R, Muraro A, Rudman A, Schmitt C, Scherrer D, Simons FE, Thomas S, Wood JP, Decker WW. Second symposium on the definition and management of anaphylaxis: summary report--Second National Institute of Allergy and Infectious Disease/Food Allergy and Anaphylaxis Network symposium. J Allergy Clin Immunol. 2006;117:391–397. doi: 10.1016/j.jaci.2005.12.1303. [DOI] [PubMed] [Google Scholar]
- 15.Caubet JC, Wang J. Current understanding of egg allergy. Pediatr Clin North Am. 2011;58:427–443. doi: 10.1016/j.pcl.2011.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Manivannan V, Decker WW, Stead LG, Li JT, Campbell RL. Visual representation of National Institute of Allergy and Infectious Disease and Food Allergy and Anaphylaxis Network criteria for anaphylaxis. Int J Emerg Med. 2009;2:3–5. doi: 10.1007/s12245-009-0093-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Xepapadaki P, Fiocchi A, Grabenhenrich L, Roberts G, Grimshaw KE, Fiandor A, Larco JI, Sigurdardottir S, Clausen M, Papadopoulos NG, Dahdah L, Mackie A, Sprikkelman AB, Schoemaker AA, Dubakiene R, Butiene I, Kowalski ML, Zeman K, Gavrili S, Keil T, Beyer K. Incidence and natural history of hen's egg allergy in the first 2 years of life-the EuroPrevall birth cohort study. Allergy. 2016;71:350–357. doi: 10.1111/all.12801. [DOI] [PubMed] [Google Scholar]
- 18.Sicherer SH, Wood RA, Vickery BP, Jones SM, Liu AH, Fleischer DM, Dawson P, Mayer L, Burks AW, Grishin A, Stablein D, Sampson HA. The natural history of egg allergy in an observational cohort. J Allergy Clin Immunol. 2014;133:492–499. doi: 10.1016/j.jaci.2013.12.1041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Horino S, Kitazawa H, Satou T, Miura K. Hyperresponsiveness to boiled egg yolk in early life leads to prolonged egg allergy. Allergy Asthma Immunol Res. 2019;11:433–437. doi: 10.4168/aair.2019.11.3.433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hill DJ, Heine RG, Hosking CS. The diagnostic value of skin prick testing in children with food allergy. Pediatr Allergy Immunol. 2004;15:435–441. doi: 10.1111/j.1399-3038.2004.00188.x. [DOI] [PubMed] [Google Scholar]
- 21.Celik-Bilgili S, Mehl A, Verstege A, Staden U, Nocon M, Beyer K, Niggemann B. The predictive value of specific immunoglobulin E levels in serum for the outcome of oral food challenges. Clin Exp Allergy. 2005;35:268–273. doi: 10.1111/j.1365-2222.2005.02150.x. [DOI] [PubMed] [Google Scholar]
- 22.Marriage DE, Erlewyn-Lajeunesse M, Unsworth DJ, Henderson AJ. Unscrambling egg allergy: the diagnostic value of specific IgE concentrations and skin prick tests for ovomucoid and egg white in the management of children with hen's egg allergy. ISRN Allergy. 2012;2012:627545. doi: 10.5402/2012/627545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kim JD, Kim SY, Kwak EJ, Sol IS, Kim MJ, Kim YH, Kim KW, Sohn MH. Reduction rate of specific IgE level as a predictor of persistent egg allergy in children. Allergy Asthma Immunol Res. 2019;11:498–507. doi: 10.4168/aair.2019.11.4.498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Peters RL, Dharmage SC, Gurrin LC, Koplin JJ, Ponsonby AL, Lowe AJ, Tang ML, Tey D, Robinson M, Hill D, Czech H, Thiele L, Osborne NJ, Allen KJ HealthNuts study. The natural history and clinical predictors of egg allergy in the first 2 years of life: a prospective, population-based cohort study. J Allergy Clin Immunol. 2014;133:485–491. doi: 10.1016/j.jaci.2013.11.032. [DOI] [PubMed] [Google Scholar]
- 25.Peters RL, Allen KJ, Dharmage SC, Tang ML, Koplin JJ, Ponsonby AL, Lowe AJ, Hill D, Gurrin LC HealthNuts Study. Skin prick test responses and allergen-specific IgE levels as predictors of peanut, egg, and sesame allergy in infants. J Allergy Clin Immunol. 2013;132:874–880. doi: 10.1016/j.jaci.2013.05.038. [DOI] [PubMed] [Google Scholar]
- 26.Foong RX, Santos AF. Biomarkers of diagnosis and resolution of food allergy. Pediatr Allergy Immunol. 2021;32:223–233. doi: 10.1111/pai.13389. [DOI] [PubMed] [Google Scholar]