Abstract
Background
The quantity of skeletal muscles has recently been reported to have prognostic value in patients with non‐small‐cell lung cancer (NSCLC) treated with second‐line immunotherapy. However, the prognostic role of skeletal muscle assessment in NSCLC patients undergoing first‐line immuno‐oncology (IO) combinatorial treatment (IO‐chemotherapy) has not been elucidated.
Methods
We retrospectively reviewed 36 patients with NSCLC undergoing first‐line IO‐chemotherapy between April 2018 and June 2021 in our hospital. The cross‐sectional area of the erector spinae muscle (ESMCSA) was evaluated by manual tracing on computed tomography scans at the level of the 12th thoracic vertebra before initiating IO‐chemotherapy. To minimize deviation due to physique, the ESMCSA was adjusted by body surface area (BSA) (ESMCSA to BSA ratio: ESMCSA/BSA). A survival time analysis was performed using the Kaplan–Meier method and log‐rank test. A multivariate analysis with Cox proportional hazards model was conducted to investigate the prognostic value of the ESMCSA/BSA and inflammatory and nutritional indices.
Results
The median progression‐free survival (PFS) and overall survival (OS) were 6.5 and 16.6 months, respectively. Intergroup comparison by the log‐rank test revealed that there was no significant difference in the median PFS, but the median OS was significantly long in the high ESMCSA/BSA (>19 cm2/m2) (high ESMCSA/BSA group, p = 0.0373). The multivariate analysis showed that ESMCSA/BSA was an independent prognostic factor for OS (hazard ratio 0.79, p = 0.044).
Conclusions
The results of this study indicate that the pretreatment ESMCSA/BSA may be a potential prognostic factor in NSCLC patients receiving first‐line IO‐chemotherapy.
Keywords: carcinoma, erector spinae muscles, immunotherapy, non‐small‐cell lung cancer, nutrition assessment
In non‐small‐cell lung cancer patients undergoing first‐line immuno‐oncology combinatorial treatment, pretreatment erector spinae muscle/body surface area (ESMCSA/BSA) was associated with survival outcome. The lower ESMCSA/BSA group had significantly shorter overall survival. This finding was validated by the multivariate analysis performed in this study.

INTRODUCTION
Immuno‐oncology (IO) combinatorial treatment (IO‐chemotherapy), which combines platinum doublet chemotherapy with immune checkpoint inhibitors (ICIs), such as anti‐programmed cell death‐1 (PD‐1)/programmed death ligand‐1 (PD‐L1) antibody and anti‐cytotoxic T‐lymphocyte antigen‐4 antibody, is one of the optimal first‐line treatment strategies for advanced or recurrent non‐small‐cell lung cancer (NSCLC) patients with negative or unknown status of oncogenic driver mutations. The concomitant use of ICIs with cytotoxic chemotherapy agents induces upregulation of antitumor immunity, resulting in synergistic therapeutic effects. 1 Specifically, several phase III clinical trials demonstrated that IO‐chemotherapy is associated with better survival outcomes and treatment responses compared to conventional cytotoxic chemotherapy regimens. 2 , 3 , 4 , 5 , 6
In patients with advanced NSCLC, potential nutritional status, physical activity represented by performance status (PS), and serum albumin levels are well‐known prognostic factors. 7 , 8 , 9 Exhaustion due to tumorigenic systemic inflammation and toxicity of anticancer agents could accelerate malnutrition, leading to cancer cachexia. 10 Numerous studies have demonstrated that inflammation‐ and nutrition‐associated indices, which consist of hematological and/or biochemical parameters, have prognostic potential in patients with advanced NSCLC. It has also been reported that a radiological assessment of the quantity and quality of skeletal muscles and its time‐dependent changes are also associated with prognosis in patients with advanced NSCLC. 11 , 12 , 13 , 14 , 15 Antigravity muscles, which are involved in posture maintenance, are considered to better reflect the quality and quantity of physical activity compared to other skeletal muscles. 16 Previous studies have shown that a skeletal muscle index calculated using measurements at the level of the third lumbar vertebra on computed tomography (CT) scans were significantly associated with progression‐free survival (PFS) and overall survival (OS) outcomes in NSCLC patients treated with ICI monotherapy. 12 , 13 , 14 , 15
The erector spinae muscles (ESMs) are included in the imaging level of a chest CT scan and can be quantitatively evaluated in mediastinal settings. In patients with advanced lung cancer, chest CT scans are routinely performed to evaluate tumor staging and treatment response to chemotherapy, which allows for a time series evaluation of ESMs. In recent years, the quantity of ESMs has been reported as a prognostic factor in patients with chronic obstructive pulmonary disease, 17 idiopathic pulmonary fibrosis, 18 pulmonary nontuberculous mycobacteria, 19 and community‐acquired pneumonia. 20 However, to the best of our knowledge, the prognostic value of ESMs has not been investigated in patients with advanced NSCLC who were treated with first‐line IO‐chemotherapy. Therefore, we hypothesized that in patients with advanced NSCLC treated with first‐line IO‐chemotherapy the quantity of ESMs before IO‐chemotherapy could be associated with their prognosis. The aim of this study was to examine the predictive and prognostic value of ESMs in patients with NSCLC undergoing first‐line IO‐chemotherapy.
METHODS
Patients and settings
The study was conducted retrospectively at a single institution. All methods used in this study were performed in accordance with the amended Declaration of Helsinki. The Institutional Review Board of Shinshu University School of Medicine approved the study (approval number: 4772) and waived the need for informed consent owing to its retrospective design. Instead, an opt‐out document for this study was posted on the website of Shinshu University School of Medicine. The data for analysis were collected from electrical medical records. We extracted information from patients who were histologically diagnosed with NSCLC between April 2018 and June 2021. All patients met the following criteria: having advanced or recurrent NSCLC and receiving at least one cycle of ICIs in combination with platinum‐based chemotherapy as first‐line chemotherapy.
Data collection
Data on patient characteristics included age, sex, smoking history, performance status (PS) evaluated by the Eastern Cooperative Oncology Group scale, body mass index (BMI), body surface area (BSA), histology findings, tumor stage assessed by tumor‐node‐metastasis eighth stage classification, 21 PD‐L1 status, and oncogenic driver mutations. PD‐L1 status was evaluated in archival or newly obtained tumor samples by means of immunohistochemical analysis with the use of the 22C3 antibody. The baseline data before initiating IO‐chemotherapy included white blood cell counts and its fractions, and serum albumin levels. Based on these parameters, the neutrophil‐to‐lymphocyte ratio (NLR), prognostic nutrition index (PNI), and advanced lung cancer inflammation index (ALI) were generated. The NLR was calculated as the ratio of absolute neutrophil count (ANC) to lymphocyte count (ALC), and patients were classified as having <5 or ≥5. The PNI score was calculated as 10× albumin + 0.005 × ALC, and patients were classified as having ≤40 or >40. The ALI score was calculated as (BMI × albumin)/NLR, with patients classified as having <18 or ≥18. The cutoff values for NLR, PNI, and ALI were determined according to the respective seminal reports. 22 , 23 , 24
Clinical course
Data on first‐line IO‐chemotherapy, adverse events (evaluated by the Common Terminology Criteria for Adverse Events version 5.0), 25 treatment response (evaluated by the Response Evaluation Criteria in Solid Tumors version 1.1), 26 and the survival time were collected. PFS for first‐line IO‐chemotherapy was defined as the period from the initiation of IO‐chemotherapy until death or disease progression. OS was defined as the period from the initiation of IO‐chemotherapy until either a fatal event or censored observation.
Quantitative analysis of the ESM area
All patients underwent chest CT during an inspiratory breath‐hold in the supine position with a 64‐row multidetector CT scanner (LightSpeed VCT, GE Healthcare). The CT scanner settings were 120 kV tube voltage, variable tube current, 64 × 0.625 mm collimation, and a 0.4‐s rotation time. Image reconstruction was performed with the standard algorithm for the mediastinum and a slice thickness of 1.25 mm. Following a method described in previous studies, 19 , 27 a quantitative analysis of the ESM area was performed using a commercially available workstation (EV Insite, PSP Co.) before initiating IO‐chemotherapy. In summary, the position of the analysis slice was determined at the level of the lower margin of the 12th thoracic vertebra, and the limb of the left and right ESMs area was bordered by manual tracing on a single axial image. The cross‐sectional areas (CSAs) of both ESMs (ESMCSA) were automatically calculated, and the ESMCSA was presented as the sum of the area of right and left ESMs. The ESMCSA was adjusted by the BSA, and the ratio of the ESMCSA to the BSA was employed in the analysis as ESMCSA/BSA. The chest CT images were reviewed by two expert pulmonologists (T.A. and Y.K., with 10 and 22 years of experience, respectively) by consensus. The correlation of the physique index with the ESMCSA was also evaluated.
Statistical analysis
Spearman's rank correlation coefficient was used to investigate the correlation between the ESMCSA and physique variables. A receiver operating characteristic (ROC) curve was constructed using the pretreatment ESMCSA/BSA as the test variables and death events (death or survival and censored events) as the state variables. The optimal cutoff value for the ESMCSA/BSA was assessed by calculating the area under the ROC curves for predicting death events to perform a comparative analysis. A Kaplan–Meier analysis was performed to plot the PFS and OS curves, and the log‐rank test was used for intergroup comparisons of PFS and OS. A Cox proportional hazards model was used to identify the prognostic factors for PFS and OS. Statistically significant variables were determined using the univariate model and clinically important variables were further analyzed using the multivariate analysis. All statistical analyses were performed using EZR (Saitama Medical Center, Jichi Medical University, Saitama, Japan), a graphical user interface for R (The R Foundation for Statistical Computing). Statistical significance was set at p < 0.05. 28
RESULTS
Patient characteristics
During the study period, data on 38 patients with advanced or recurrent NSCLC treated with first‐line IO‐chemotherapy were extracted. One patient was excluded due to missing CT scan data and another patient was excluded due to receipt of IO‐chemotherapy as second‐line chemotherapy. A total of 36 patients were included in the analysis. The patient characteristics are presented in Table 1. The cohort comprised 31 (86.1%) male and five (13.9%) female patients, with a median age of 68.5 years (range 49–79 years).
TABLE 1.
Patient characteristics (n = 36)
| n | % | |
|---|---|---|
| Median age, year (range) | 68.5 (49–79) | |
| Male sex | 31 | 86.1 |
| Smoking status | ||
| Yes/no | 33/3 | 91.7/8.3 |
| BMI, kg/m2 | 21.6 ± 3.4 | |
| BSA, m2 | 1.64 ± 0.16 | |
| ECOG‐PS | ||
| 0,1 | 28 | 77.8 |
| 2,3 | 8 | 22.2 |
| Histology | ||
| Adeno | 24 | 66.7 |
| Squamous | 6 | 16.7 |
| NSCLC‐NOS | 5 | 13.9 |
| Large cell | 1 | 2.8 |
| Tumor stage | ||
| III | 3 | 8.3 |
| IVA | 11 | 30.1 |
| IVB | 21 | 58.3 |
| Post. Ope | 1 | 2.8 |
| PD‐L1 status | ||
| <1% | 7 | 19.4 |
| ≥1% | 23 | 63.9 |
| Unknown | 6 | 16.7 |
| Presence of driver mutation | 4 | 11.1 |
| Clinical parameters | ||
| NLR | 4.9 ± 3.3 a | |
| <5/≥5 | 22/14 | 61.1/38.9 |
| PNI | 41.2 ± 6.0 a | |
| ≤40/>40 | 15/21 | 41.7/58.3 |
| ALI | 20.9 ± 14.5 a | |
| <18/≥18 | 17/19 | 47.2/52.8 |
| ESMCSA, cm2 | 29.6 ± 7.3 a | |
| ESMCSA/BSA, cm2/m2 | 17.9 ± 3.5 a |
Abbreviations: ALI, advanced lung cancer inflammation index; BMI, body mass index; BSA, body surface area; CSA, cross‐sectional area; ECOG‐PS, Eastern Cooperative Oncology Group performance status; ESM, erector spinae muscles; NLR, neutrophil to lymphocyte ratio; NOS, not otherwise specified; NSCLC, non‐small cell lung cancer; PD‐L1, programmed cell death ligand 1; PNI, prognostic nutrition index.
Mean ± standard deviation.
More than 80% of patients had nonsquamous histology, including 24 (66.7%) adenocarcinoma, five (13.9%) NSCLC not otherwise specified, and one (2.8%) large cell carcinoma. PD‐L1 status was available in 30 (83.3%) patients, of which 23 (63.9%) had positive expression (≥1%), and four (11.1%) carried the oncogenic driver mutation. The baseline ESMCSA was 29.6 ± 7.3 cm2. Since a significant difference was found between the ESMCSA and BSA, the ESMCSA was adjusted by the BSA (r = 0.599, p < 0.001; Supporting Information Table S1) (ESMCSA to BSA ratio: ESMCSA/BSA). The baseline ESMCSA/BSA was 17.9 ± 3.5 cm2/m2.
Adverse events
All the patients exhibited adverse events (AEs). Neutropenia was the most frequent grade 3 AE (30.6%), followed by adrenal insufficiency and bacterial pneumonia (8.3% each), excluding the hematological and gastrointestinal toxicities (Table 2).
TABLE 2.
Adverse events for IO‐chemotherapy (n = 36)
| Variables | Any grade n (%) | Grade3≤ n (%) |
|---|---|---|
| Neutropenia | 19 (52.3) | 11 (30.6) |
| Anemia | 21 (58.3) | 1 (2.8) |
| Thrombocytopenia | 14 (38.9) | 3 (8.3) |
| Nausea | 24 (66.7) | 8 (22.2) |
| Diarrhea | 5 (13.9) | 0 (0) |
| Rash | 14 (38.9) | 0 (0) |
| Liver dysfunction | 9 (25) | 1 (2.8) |
| Renal dysfunction | 8 (22.2) | 0 (0) |
| Peripheral neuropathy | 7 (19.4) | 1 (2.8) |
| Thyroid dysfunction | 6 (16.7) | 0 (0) |
| Interstitial lung disease | 4 (11.1) | 0 (0) |
| Adrenal insufficiency | 5 (13.9) | 3 (8.3) |
| Bacterial pneumonia | 3 (8.3) | 3 (8.3) |
| Bacterial infection | 3 (8.3) | 1 (2.8) |
Treatment response and survival time analysis
The treatment regimen, efficacy, and clinical course are presented in Table 3. Twenty‐five patients (69.4%) received platinum‐doublet chemotherapy with pembrolizumab, six (16.7%) received platinum‐doublet chemotherapy with atezolizumab, and five (13.9%) received platinum‐doublet chemotherapy with nivolumab and ipilimumab as first‐line IO‐chemotherapy. The objective response rate and disease control rate for first‐line IO‐chemotherapy were 58.3% (95% confidence interval [CI] 40.8–74.5) and 86.1% (95% CI 70.5–95.3), respectively. During the observational period, 23 patients (63.9%) showed disease progression and 13 (36.1%) patients died. The median PFS and OS in the entire cohort were 6.5 months (95% CI 4.8–10.5) and 16.6 months (95% CI 11.5 to not applicable [NA]), respectively (Figure 1). Table 4 presents the results of the comparative analysis of PFS and OS according to baseline patient characteristics, clinical parameters, and ESMCSA/BSA. The median OS was long in the group with a high ESMCSA/BSA (>19 cm2/m2) (≤19 vs. >19 cm2/m2, 11.6 months vs. NA, p = 0.0373) (Figure 2). The results of the univariate analysis for PFS and OS are presented in Table 5. There were no significant prognostic factors associated with PFS. The baseline ESMCSA/BSA was found to be a significant prognostic factor for OS (hazard ratio [HR] 0.78, 95% CI 0.62–0.98, p = 0.041). The multivariate analysis using the Cox proportional hazards model demonstrated that the baseline ESMCSA/BSA was an independent prognostic factor for OS (HR 0.79, 95% CI 0.62–0.99, p = 0.0442) (Table 6).
TABLE 3.
Clinical course (n = 36)
| n | % | |
|---|---|---|
| Treatment regimen | ||
| Pembrolizumab + chemo | 25 | 69.4 |
| Atezolizumab + chemo | 6 | 16.7 |
| Nivo + Ipi + chemo | 5 | 13.9 |
| Treatment response | ||
| ORR (%), 95% CI | 58.3 | 40.8–74.5 |
| DCR (%), 95% CI | 86.1 | 70.5–95.3 |
| No. of progression events | 23 | 63.9 |
| No. of mortality events | 13 | 36.1 |
Abbreviations: CI, confidence interval; DCR, disease control rate; Ipi, ipilimumab; Nivo, nivolumab; ORR, objective response rate.
FIGURE 1.
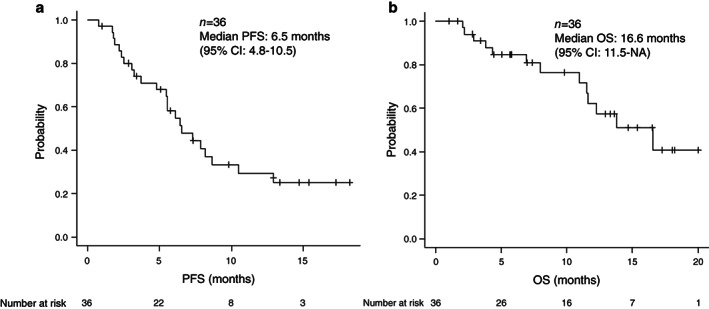
The Kaplan–Meier curve for the entire cohort (n = 36) is presented. The median progression‐free survival (PFS) was 6.5 months (a) and median overall survival was 16.6 months (b)
TABLE 4.
Survival time comparison by log‐rank test (n = 36)
| Variables | PFS | OS | ||
|---|---|---|---|---|
| Months | p value | Months | p value | |
| Age | 6.5 vs. 5.5 | 0.543 | 13.8 vs 16.6 | 0.254 |
| ≥70 (n = 19) vs. <70 | ||||
| Sex | 8.2 vs. 6.4 | 0.34 | 16.6 vs. 13.8 | 0.494 |
| Male (n = 31) vs. female | ||||
| ECOG‐PS | 5.6 vs. 6.5 | 0.558 | NA vs. 16.6 | 0.634 |
| 2,3 (n = 8) vs. 0,1 | ||||
| PD‐L1 | 7.3 vs. 8.2 | 0.997 | NA vs. 13.8 | 0.865 |
| ≥1% (n = 23) vs. <1% | ||||
| Driver mutation | 5.6 vs. 7.3 | 0.928 | NA vs. 13.8 | 0.318 |
| Yes (n = 4) vs. no | ||||
| NLR | 8.6 vs. 6.4 | 0.494 | 12.3 vs. 16.6 | 0.998 |
| ≥5 (n = 14) vs. <5 | ||||
| PNI | 5.6 vs. 7.3 | 0.982 | 16.6 vs. 13.8 | 0.97 |
| ≤40 (n = 15) vs. >40 | ||||
| ALI | 6.5 vs. 6.4 | 0.452 | 12.3 vs. 16.6 | 0.926 |
| <18 (n = 17) vs. ≥18 | ||||
| ESMCSA/BSA | 6.1 vs. 7.9 | 0.374 | 11.6 vs. NA | 0.0373 |
| ≤19 cm2/m2 (n = 23) vs. >19 cm2/m2 | ||||
Abbreviations: ALI, advanced lung cancer inflammation index; BSA, body surface area; CSA, cross‐sectional area; ECOG‐PS, Eastern Cooperative Oncology Group performance status; ESM, erector spinae muscle; NA, not applicable; NLR, neutrophil‐to‐lymphocyte ratio; OS, overall survival; PD‐L1, programmed cell death ligand 1; PFS, progression‐free survival; PNI, prognostic nutrition index.
FIGURE 2.
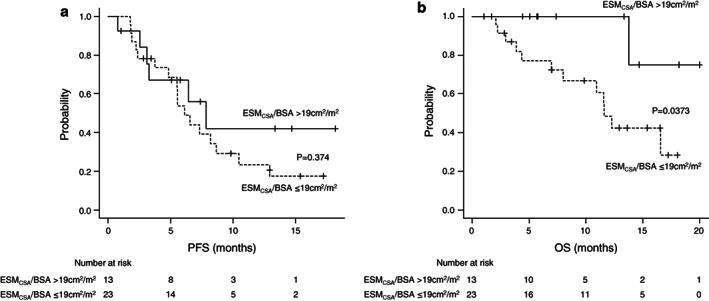
Survival time comparison according to the ESMCSA/BSA is shown. The median PFS tended to be long in the high ESMCSA/BSA group (>19 cm2/m2, solid line), with no statistical significance (a). The median OS was significantly long in the high ESMCSA/BSA group (b). BSA, body surface area; CSA, cross‐sectional area; ESM, erector spinae muscle; OS, overall survival; PFS, progression free survival
TABLE 5.
Univariate analysis for PFS and OS (n = 36)
| Variables | PFS | OS | ||
|---|---|---|---|---|
| HR (95% CI) | p value | HR (95% CI) | p value | |
| Age | 1.03 (0.97–1.1) | 0.323 | 1.01 (0.93–1.09) | 0.818 |
| Male sex | 1.64 (0.59–4.6) | 0.346 | 0.59 (0.13–2.71) | 0.499 |
| ECOG‐PS 2,3 | 0.73 (0.25–2.14) | 0.561 | 1.37 (0.37–5.03) | 0.635 |
| PD‐L1 of ≥1% or <1% | 0.99 (0.36–2.79) | 0.997 | 0.89 (0.24–3.37) | 0.865 |
| Presence of driver mutation | 1.47 (0.43–4.95) | 0.538 | NA | NA |
| NLR | 0.99 (0.85–1.16) | 0.902 | 1.07 (0.89–1.28) | 0.497 |
| PNI | 0.99 (0.93–1.06) | 0.816 | 0.97 (0.88–1.07) | 0.545 |
| ALI | 0.99 (0.97–1.03) | 0.983 | 0.97 (0.93–1.02) | 0.281 |
| ESMCSA/BSA | 0.91 (0.79–1.05) | 0.201 | 0.78 (0.62–0.98) | 0.041 |
Abbreviations: ALI, advanced lung cancer inflammation index; BSA, body surface area; CI, confidence interval; CSA, cross‐sectional area; ECOG‐PS, Eastern Cooperative Oncology Group performance status; ESM, erector spinae muscle; HR, hazard ratio; NA, not applicable; NLR, neutrophil‐to‐lymphocyte ratio; OS, overall survival; PD‐L1, programmed cell death ligand 1; PFS, progression‐free survival; PNI, prognostic nutrition index.
TABLE 6.
Cox proportional hazards model for OS (n = 36) a
| Variables | HR (95% CI) | p value |
|---|---|---|
| ECOG‐PS 2,3 | 1.43 (0.37–5.55) | 0.605 |
| ESMCSA/BSA | 0.79 (0.62–0.99) | 0.0442 |
Abbreviations: BSA, body surface area; CI, confidence interval; CSA, cross‐sectional area; ESM, erector spinae muscle; HR, hazard ratio; OS, overall survival; PS, performance status.
Including 13 mortality events.
DISCUSSION
The present study was conducted to investigate pretreatment ESMs as prognostic markers in patients with advanced NSCLC receiving first‐line IO‐chemotherapy. The survival time analysis using log‐rank test showed that the group with a high ESMCSA/BSA had significantly long OS, and the univariate analysis revealed that the ESMCSA/BSA was significantly associated with poor OS. The baseline ESMCSA/BSA was identified as an independent prognostic factor for OS in the multivariate analysis using the Cox proportional hazards model. There were no significant prognostic values in the survival time analysis for the inflammation‐ and nutrition‐related indices evaluated in this study. To our knowledge, this is the first study to demonstrate the prognostic relevance of ESMCSA/BSA in patients with NSCLC undergoing first‐line IO‐chemotherapy.
In patients with advanced cancer, it is well known that tumorigenic systemic inflammation leads to muscle proteolysis, lipolysis, and appetite suppression by involving various inflammatory cytokines. 10 In turn, this leads to further skeletal muscle loss and undernutrition, resulting in a state known as cachexia. In patients with NSCLC treated with second‐line ICI therapy, a poor PS is a well‐known adverse prognostic factor. 29 In recent years, several clinical parameters derived from hematological and/or biochemical laboratory test results have been explored to evaluate the predictive and prognostic value of second‐line ICI therapy in NSCLC patients in real‐world settings. The NLR, 30 PNI score, 31 and ALI score, 32 which were investigated in this study, have been well described as potential prognostic factors in advanced NSCLC undergoing second‐line ICI therapy. However, the prognostic significance of inflammatory and nutritional factors in NSCLC patients undergoing first‐line IO‐chemotherapy is limited owing to the novel nature of its populations. Morimoto et al. recently reported that in a multicenter retrospective study of NSCLC patients undergoing IO‐chemotherapy, patients with cachexia had significantly shorter PFS than those without cachexia. OS tended to be shorter in the cachexia group than in those without, with no statistical significance in that study. 33 This suggests that neoplastic inflammation and malnutrition have prognostic relevance even in patients with NSCLC undergoing IO‐chemotherapy regimens.
The skeletal muscle index evaluated by CT scans at the level of the third lumbar vertebra has been well investigated as an objective nutrition‐related indicator. Takada et al. reported that the skeletal muscle index was an independent prognostic factor for response rate and survival time outcomes in patients with advanced NSCLC treated with second‐line anti‐PD‐1 antibody therapy. 14 Similarly, in their multicenter observational study, Cortellini et al. also reported that a lower skeletal muscle index was significantly associated with shorter OS time in advanced NSCLC patients treated with anti PD‐1/PD‐L1 antibody therapy. 15 It was noted that these previous studies included patients who received second‐line ICI therapy and did not concurrently investigate nutritional and inflammatory indices other than muscle indices. In addition, skeletal muscle assessment was based on the level of the third lumbar vertebra, and the ESMs have not been validated. ESMs is a parameter that has shown its prognostic value in several respiratory diseases. ESMs at the level of the 12th thoracic vertebra have the advantage of providing an easy and comprehensive evaluation on chest CT scans. The quantity of ESMs at the level of the 12th thoracic vertebra has been reported to be correlated with skeletal muscles at the level of fourth lumber vertebra, 34 suggesting that it may have diagnostic value identical to that of the lumbar level muscles.
The results of the present study showed that a lower baseline ESMCSA/BSA tended to have a shorter PFS without statistical significance. Meanwhile, a lower baseline ESMCSA/BSA was considered an independent adverse prognostic factor for OS. These findings suggest that the baseline ESMCSA/BSA may be a potential prognostic marker rather than a predictive marker in NSCLC patients undergoing first‐line IO‐chemotherapy. In the investigated inflammatory and nutritional indices, none of these was associated with OS in the univariate analysis. The reason for this result is unclear, but our consideration is that clinical parameters consisting of hematological and biochemical tests, while better than single test values, might vary widely depending on the patient's background factors. In this respect, the baseline ESMCSA/BSA, which also varies with age and physique, may have a relatively good prognostic ability since it is adjusted by the physique variable.
The present study has several limitations. First, it was a retrospective study conducted at a single institution with a relatively small sample size. These factors could potentially cause a patient selection bias. Therefore, the interpretation of our results should be cautious, and a prospective study with a large sample size is desirable in the future. Second, this study lacked clinical details of second‐line chemotherapy after IO‐chemotherapy, which could affect the survival time. Third, the ESMCSA was measured by manual tracing at specific prespecified CT levels. Previous studies have employed auto‐tracing using a specific analysis software. It is uncertain which method is superior as a tracing method; however, manual tracing can be implemented using software installed in existing electronic medical records systems and might be cost‐effective.
Despite the above limitations, this is the first study to suggest the prognostic implications of ESMCSA/BSA assessment in patients with NSCLC receiving first‐line IO‐chemotherapy. In our study cohort, the ESMCSA/BSA seemed to have a superior potential prognostic ability compared to the other nutritional and inflammatory indices. Since this study was conducted in a small number of patients and the observation period was short, further follow‐up and case accumulation should be attempted in the future to verify the prognostic value of the ESMCSA/BSA.
CONFLICT OF INTEREST
The authors declare no competing interests.
AUTHOR CONTRIBUTIONS
The study was originally conceived by Y.K. and T.A., and Y.S. handled the data collection and analysis. All the authors contributed to the conception of the study design. The first draft of the manuscript was written by T.A., and all the authors referred to and agreed to the final manuscript.
ETHICS APPROVAL
The present study was approved by the Institutional Review Board of Shinshu University School of Medicine (approval no. 4772), and the study was performed in accordance with the ethical standards of the amended Declaration of Helsinki.
Supporting information
Table S1. Presenting the results of Spearman's rank correlation coefficient. Among the parameters examined, there was the strongest positive correlation between ESMCSA and BSA.
ACKNOWLEDGMENTS
This research did not receive any specific grant from funding agencies in the public, commercial, or not‐for‐profit sectors.
Araki T, Kitaguchi Y, Suzuki Y, Komatsu M, Sonehara K, Wada Y, et al. Prognostic implication of erector spinae muscles in non‐small‐cell lung cancer patients treated with immuno‐oncology combinatorial chemotherapy. Thorac Cancer. 2021;12:2857–2864. 10.1111/1759-7714.14142
REFERENCES
- 1. Langer CJ, Gadgeel SM, Borghaei H, Papadimitrakopoulou VA, Patnaik A, Powell SF, et al. Carboplatin and pemetrexed with or without pembrolizumab for advanced, non‐squamous non‐small‐cell lung cancer: a randomized, phase 2 cohort of the open‐label KEYNOTE‐021 study. Lancet Oncol. 2016;17:1497–508. 10.1016/S1470-2045(16)30498-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Garassino MC, Gadgeel S, Esteban E, Felip E, Speranza G, Domine M, et al. Patient‐reported outcomes following pembrolizumab or placebo plus pemetrexed and platinum in patients with previously untreated, metastatic, non‐squamous non‐small‐cell lung cancer (KEYNOTE‐189): a multicentre, double‐blind, randomised, placebo‐controlled, phase 3 trial. Lancet Oncol. 2020;21:387–97. 10.1016/S1470-2045(19)30801-0 [DOI] [PubMed] [Google Scholar]
- 3. Paz‐Ares L, Vicente D, Tafreshi A, Robinson A, Soto Parra H, Mazières J, et al. A randomized, placebo‐controlled trial of pembrolizumab plus chemotherapy in patients with metastatic squamous NSCLC: protocol‐specified final analysis of KEYNOTE‐407. J Thorac Oncol. 2020;15:1657–69. 10.1016/j.jtho.2020.06.015 [DOI] [PubMed] [Google Scholar]
- 4. Reck M, Mok TSK, Nishio M, Jotte RM, Cappuzzo F, Orlandi F, et al. Atezolizumab plus bevacizumab and chemotherapy in non‐small‐cell lung cancer (IMpower150): key subgroup analyses of patients with EGFR mutations or baseline liver metastases in a randomised, open‐label phase 3 trial. Lancet Respir Med. 2019;7:387–401. 10.1016/S2213-2600(19)30084-0 [DOI] [PubMed] [Google Scholar]
- 5. West H, McCleod M, Hussein M, Morabito A, Rittmeyer A, Conter HJ, et al. Atezolizumab in combination with carboplatin plus nab‐paclitaxel chemotherapy compared with chemotherapy alone as first‐line treatment for metastatic non‐squamous non‐small‐cell lung cancer (IMpower130): a multicentre, randomised, open‐label, phase 3 trial. Lancet Oncol. 2019;20:924–37. 10.1016/S1470-2045(19)30167-6 [DOI] [PubMed] [Google Scholar]
- 6. Paz‐Ares L, Ciuleanu TE, Cobo M, Schenker M, Zurawski B, Menezes J, et al. First‐line nivolumab plus ipilimumab combined with two cycles of chemotherapy in patients with non‐small‐cell lung cancer (CheckMate 9LA): an international, randomised, open‐label, phase 3 trial [published correction appears in Lancet Oncol. 2021 Mar;22(3):e92]. Lancet Oncol. 2021;22:198–211. 10.1016/S1470-2045(20)30641-0 [DOI] [PubMed] [Google Scholar]
- 7. Espinosa E, Feliu J, Zamora P, Barón MG, Sánchez JJ, Ordónez A, et al. Serum albumin and other prognostic factors related to response and survival in patients with advanced non‐small cell lung cancer. Lung Cancer. 1995;12:67–76. 10.1016/0169-5002(95)00407-r [DOI] [PubMed] [Google Scholar]
- 8. Ray P, Quantin X, Grenìer J, Pujol JL. Predictive factors of tumor response and prognostic factors of survival during lung cancer chemotherapy. Cancer Detect Prev. 1998;22:293–304. 10.1046/j.1525-1500.1998.cdoa43.x [DOI] [PubMed] [Google Scholar]
- 9. Dall'Olio FG, Maggio I, Massucci M, Mollica V, Fragomeno B, Ardizzoni A. ECOG performance status ≥2 as a prognostic factor in patients with advanced non small cell lung cancer treated with immune checkpoint inhibitors‐a systematic review and meta‐analysis of real world data. Lung Cancer. 2020;145:95–104. 10.1016/j.lungcan.2020.04.027 [DOI] [PubMed] [Google Scholar]
- 10. Fearon K, Strasser F, Anker SD, Bosaeus I, Bruera E, Fainsinger RL, et al. Definition and classification of cancer cachexia: an international consensus. Lancet Oncol. 2011;12:489–95. 10.1016/S1470-2045(10)70218-7 [DOI] [PubMed] [Google Scholar]
- 11. Cortellini A, Palumbo P, Porzio G, Verna L, Giordano AV, Masciocchi C, et al. Single‐institution study of correlations between skeletal muscle mass, its density, and clinical outcomes in non‐small cell lung cancer patients treated with first‐line chemotherapy. Thorac Cancer. 2018;9:1623–30. 10.1111/1759-7714.12870 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Nishioka N, Uchino J, Hirai S, Katayama Y, Yoshimura A, Okura N, et al. Association of sarcopenia with and efficacy of anti‐PD‐1/PD‐L1 therapy in non‐small‐cell lung cancer. J Clin Med. 2019;8:450. 10.3390/jcm8040450 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Cortellini A, Verna L, Porzio G, Bozzetti F, Palumbo P, Masciocchi C, et al. Predictive value of skeletal muscle mass for immunotherapy with nivolumab in non‐small cell lung cancer patients: a "hypothesis‐generator" preliminary report. Thorac Cancer. 2019;10:347–51. 10.1111/1759-7714.12965 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Takada K, Yoneshima Y, Tanaka K, Okamoto I, Shimokawa M, Wakasu S, et al. Clinical impact of skeletal muscle area in patients with non‐small cell lung cancer treated with anti‐PD‐1 inhibitors. J Cancer Res Clin Oncol. 2020;146:1217–25. 10.1007/s00432-020-03146-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Cortellini A, Bozzetti F, Palumbo P, Brocco D, Di Marino P, Tinari N, et al. Weighing the role of skeletal muscle mass and muscle density in cancer patients receiving PD‐1/PD‐L1 checkpoint inhibitors: a multicenter real‐life study. Sci Rep. 2020;10:1456. 10.1038/s41598-020-58498-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Ikezoe T, Mori N, Nakamura M, Ichihashi N. Effects of age and inactivity due to prolonged bed rest on atrophy of trunk muscles. Eur J Appl Physiol. 2012;112:43–8. 10.1007/s00421-011-1952-x [DOI] [PubMed] [Google Scholar]
- 17. Tanimura K, Sato S, Fuseya Y, Hasegawa K, Uemasu K, Sato A, et al. Quantitative assessment of erector spinae muscles in patients with chronic obstructive pulmonary disease. Novel chest computed tomography‐derived index for prognosis. Ann Am Thorac Soc. 2016;13:334–41. 10.1513/AnnalsATS.201507-446OC [DOI] [PubMed] [Google Scholar]
- 18. Nakano A, Ohkubo H, Taniguchi H, Kondoh Y, Matsuda T, Yagi M, et al. Early decrease in erector spinae muscle area and future risk of mortality in idiopathic pulmonary fibrosis. Sci Rep. 2020;10:2312. 10.1038/s41598-020-59100-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Asakura T, Yamada Y, Suzuki S, Namkoong H, Okamori S, Kusumoto T, et al. Quantitative assessment of erector spinae muscles in patients with Mycobacterium avium complex lung disease. Respir Med. 2018;145:66–72. 10.1016/j.rmed.2018.10.023 [DOI] [PubMed] [Google Scholar]
- 20. Yoshikawa H, Komiya K, Yamamoto T, Fujita N, Oka H, Okabe E, et al. Quantitative assessment of erector spinae muscles and prognosis in elderly patients with pneumonia. Sci Rep. 2021;11:4319. 10.1038/s41598-021-83995-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Goldstraw P, Chansky K, Crowley J, Rami‐Porta R, Asamura H, Eberhardt WEE, et al. The IASLC lung cancer staging project: proposals for revision of the TNM stage groupings in the forthcoming (eighth) edition of the TNM classification for lung cancer. J Thorac Oncol. 2016;11(1):39–51. 10.1016/j.jtho.2015.09.009 [DOI] [PubMed] [Google Scholar]
- 22. Proctor MJ, Morrison DS, Talwar D, Balmer SM, Fletcher CD, O'Reilly DS, et al. A comparison of inflammation‐based prognostic scores in patients with cancer. A Glasgow Inflammation Outcome Study. Eur J Cancer. 2011;47:2633–41. 10.1016/j.ejca.2011.03.028 [DOI] [PubMed] [Google Scholar]
- 23. Buzby GP, Mullen JL, Matthews DC, Hobbs CL, Rosato EF. Prognostic nutritional index in gastrointestinal surgery. Am J Surg. 1980;139:160–7. 10.1016/0002-9610(80)90246-9 [DOI] [PubMed] [Google Scholar]
- 24. Jafri SH, Shi R, Mills G. Advance lung cancer inflammation index (ALI) at diagnosis is a prognostic marker in patients with metastatic non‐small cell lung cancer (NSCLC): a retrospective review. BMC Cancer. 2013;13:158. 10.1186/1471-2407-13-158 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Trotti A, Colevas AD, Setser A, Rusch V, Jaques D, Budach V, et al. CTCAE v3.0: Development of a comprehensive grading system for the adverse effects of cancer treatment. Semin Radiat Oncol. 2003;13:176–81. 10.1016/S1053-4296(03)00031-6 [DOI] [PubMed] [Google Scholar]
- 26. Schwartz LH, Litière S, de Vries E, Ford R, Gwyther S, Mandrekar S, et al. RECIST 1.1 – update and clarification: from the RECIST committee. Eur J Cancer. 2016;62:132–7. 10.1016/j.ejca.2016.03.081 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Minegishi Y, Inoue S, Sato K, Abe K, Murano H, Furuyama K, et al. Smaller erector spinae muscle size is associated with inability to recover activities of daily living after pneumonia treatment. Respir Investig. 2019;57:191–7. 10.1016/j.resinv.2018.11.002 [DOI] [PubMed] [Google Scholar]
- 28. Kanda Y. Investigation of the freely available easy‐to‐use software 'EZR' for medical statistics. Bone Marrow Transplant. 2013;48:452–8. 10.1038/bmt.2012.244 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Facchinetti F, Mazzaschi G, Barbieri F, Passiglia F, Mazzoni F, Berardi R, et al. First‐line pembrolizumab in advanced non‐small cell lung cancer patients with poor performance status. Eur J Cancer. 2020;130:155–67. 10.1016/j.ejca.2020.02.023 [DOI] [PubMed] [Google Scholar]
- 30. Diem S, Schmid S, Krapf M, Flatz L, Born D, Jochum W, et al. Neutrophil‐to‐lymphocyte ratio (NLR) and platelet‐to‐lymphocyte ratio (PLR) as prognostic markers in patients with non‐small cell lung cancer (NSCLC) treated with nivolumab. Lung Cancer. 2017;111:176–81. 10.1016/j.lungcan.2017.07.024 [DOI] [PubMed] [Google Scholar]
- 31. Matsubara T, Takamori S, Haratake N, Toyozawa R, Miura N, Shimokawa M, et al. The impact of immune‐inflammation‐nutritional parameters on the prognosis of non‐small cell lung cancer patients treated with atezolizumab. J Thorac Dis. 2020;12:1520–8. 10.21037/jtd.2020.02.27 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Shiroyama T, Suzuki H, Tamiya M, Tamiya A, Tanaka A, Okamoto N, et al. Pretreatment advanced lung cancer inflammation index (ALI) for predicting early progression in nivolumab‐treated patients with advanced non‐small cell lung cancer. Cancer Med. 2018;7:13–20. 10.1002/cam4.1234 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Morimoto K, Uchino J, Yokoi T, Kijima T, Goto Y, Nakao A, et al. Impact of cancer cachexia on the therapeutic outcome of combined chemoimmunotherapy in patients with non‐small cell lung cancer: a retrospective study. Oncoimmunology. 2021;10:1950411. 10.1080/2162402X.2021.1950411 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Canvasser LD, Mazurek AA, Cron DC, Terjimanian MN, Chang ET, Lee CS, et al. Paraspinous muscle as a predictor of surgical outcome. J Surg Res. 2014;192:76–81. 10.1016/j.jss.2014.05.057 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Presenting the results of Spearman's rank correlation coefficient. Among the parameters examined, there was the strongest positive correlation between ESMCSA and BSA.


