Abstract
Immune checkpoint inhibitors (ICIs) have become standard pharmacological therapies in patients with non‐small‐cell lung cancer (NSCLC). Because elderly patients with NSCLC are often excluded from clinical trials as a result of lower functional capacity or comorbidities, the prognostic impact of chronological age on the efficacy of ICIs is unclear. The National Cancer Database was queried for stage IV NSCLC patients between 2014 and 2015. Associations between ICI therapy and clinical characteristics were assessed using chi‐squared tests. Kaplan–Meier curves were compared using the log‐rank test. A Cox proportional hazards model was used to identify clinical characteristics predictive of overall survival (OS). This study included 24 136 patients with stage IV NSCLC aged ≥75 years and 62 037 patients with stage IV NSCLC aged <75 years. Patients aged ≥75 years treated with ICIs had significantly longer OS than those not treated with ICIs (hazard ratio [HR] 0.61, 95% confidence interval [CI] 0.58–0.64, p < 0.0001). The corresponding HR in patients aged <75 years was 0.67 (95% CI 0.65–0.68, p < 0.0001). Cox modeling confirmed the survival benefit of ICI therapy in patients aged ≥75 years (HR for patients not receiving ICIs 1.63 [95% CI: 1.55–1.71], p < 0.0001). The corresponding HR in patients aged <75 years was 1.47 (95% CI 1.43–1.51, p < 0.0001). Chronological age does not appear to negatively impact the survival benefit of ICI therapy in patients with stage IV NSCLC according to this large real‐world database analysis.
Keywords: age, immune checkpoint inhibitor, non‐small‐cell lung cancer, programmed cell death‐1, survival
In stage IV non‐small‐cell lung cancer patients aged <75 years, the hazard ratio of patients treated with immunocheckpoint inhibitors compared with those without was 0.67. The corresponding hazard ratio in patients aged ≥75 years was 0.61. Chronological age does not appear to negatively impact the survival benefit from immunocheckpoint inhibitors in patients with stage IV non‐small‐cell lung cancer according to real‐world national cancer database analysis.
INTRODUCTION
Lung cancer has one of the highest case‐fatality rates of all malignancies, and non‐small‐cell lung cancer (NSCLC) accounts for 85% of lung cancers. 1 Immune checkpoint inhibitors (ICIs) targeting programmed cell death‐1 (PD‐1) or programmed cell death‐ligand 1 (PD‐L1) have been widely adopted for therapy of NSCLC. 2 , 3 , 4 , 5 , 6 , 7 Because of the small number of elderly patients with NSCLC included in the clinical trials of these drugs, the efficacy of ICIs among elderly patients with NSCLC remains unclear. 8 , 9 The age‐dependent loss of immune function is called immune senescence and is associated with decreased immune surveillance functions of both innate and adaptive immunity. 10 Aging is associated with decreased antigen presentation by dendric cells, decreased numbers of naïve CD8+ T cells, and reduced chemotaxis by neutrophils and macrophages. 10 , 11 Given the potential for immune senescence, the efficacy of ICIs in elderly patients remains controversial. The aim of the current study was to clarify whether chronological age was a significant prognostic factor in patients with advanced NSCLC treated with ICIs using real‐world data from the National Cancer Database (NCDB).
METHODS
NCDB
The NCDB is a joint project between the Commission on Cancer (CoC) of the American College of Surgeons and the American Cancer Society. The CoC NCDB and the participating hospitals were the sources of the de‐identified data used herein. These organizations have not verified the data and are not responsible for the statistical validity of the data analysis nor the conclusions derived by the authors.
Statistical analyses
Clinical characteristics were summarized using contingency tables. The associations between ICI (yes vs. no) and clinical demographics were compared using the chi‐squared test. Survival curves were evaluated using the Kaplan–Meier method and compared between the two groups using the log‐rank test. The hazard ratios (HR) for survival between groups with 95% confidence intervals (CI) were estimated using Cox proportional hazards models. A Cox proportional hazards model was used to identify the independent prognostic factor. All Cox proportional hazards analyses were performed using JMP 14.0 (SAS Institute Inc.). p < 0.05 was considered statistically significant.
RESULTS
Patient characteristics
A total of 86 173 patients with stage IV NSCLC were selected for the analysis. Among patients aged <75 years, 8968 of 62 037 (14.5%) received ICIs, whereas 2241 of 24 136 (9.3%) patients aged ≥75 years received ICIs. The relationships between administration of ICIs and clinical factors, stratified by chronological age (≥75 vs. <75 years), are shown in Table 1.
TABLE 1.
Clinical characteristics of patients with stage IV non‐small‐cell lung cancer aged <75 and ≥75 years (n = 86 173)
| Factors | <75 years old (n = 62 037) | ≥75 years old (n = 24 136) | ||||
|---|---|---|---|---|---|---|
| Immune checkpoint inhibitor | p value | Immune checkpoint inhibitor | p value | |||
| Yes (n = 8968) | No (n = 53 069) | Yes (n = 2241) | No (n = 21 895) | |||
| Sex | ||||||
| Male | 4645 (14%) | 28 309 (86%) | 0.0067 | 1222 (10%) | 11 211 (90%) | 0.0027 |
| Female | 4323 (15%) | 24 760 (85%) | 1019 (9%) | 10 684 (91%) | ||
| Race | ||||||
| Whites | 7424 (15%) | 42 528 (85%) | <0.0001 | 1973 (9%) | 18 910 (91%) | 0.0272 |
| Others | 1544 (13%) | 10 541 (87%) | 268 (8%) | 2985 (92%) | ||
| Institution | ||||||
| Others | 5916 (15%) | 34 715 (85%) | 0.3131 | 1560 (9%) | 15 535 (91%) | 0.1877 |
| Academic | 3052 (14%) | 18 354 (86%) | 681 (10%) | 6360 (90%) | ||
| Charlson–Deyo score | ||||||
| ≥2 | 909 (12%) | 6791 (88%) | <0.0001 | 342 (8%) | 3753 (92%) | 0.0247 |
| ≤1 | 8059 (15%) | 46 278 (85%) | 1899 (9%) | 18 142 (91%) | ||
| Year of diagnosis | ||||||
| 2014 | 3754 (12%) | 27 238 (88%) | <0.0001 | 786 (7%) | 11 155 (93%) | <0.0001 |
| 2015 | 5214 (17%) | 25 831 (83%) | 1455 (12%) | 10 740 (88%) | ||
| Histology | ||||||
| Others | 2430 (10%) | 21 293 (90%) | <0.0001 | 694 (7%) | 12 826 (93%) | <0.0001 |
| Adenocarcinoma NOS | 6538 (17%) | 31 776 (83%) | 1547 (12%) | 9069 (88%) | ||
| Nodal status | ||||||
| N0 | 2211 (13%) | 15 375 (87%) | <0.0001 | 719 (8%) | 8308 (92%) | <0.0001 |
| ≥N1 | 6757 (15%) | 37 694 (85%) | 1522 (10%) | 13 587 (90%) | ||
| Brain metastasis | ||||||
| Yes | 709 (9%) | 7295 (91%) | <0.0001 | 89 (5%) | 1682 (95%) | <0.0001 |
| No | 8259 (15%) | 45 774 (85%) | 2152 (10%) | 20 213 (90%) | ||
| Bone metastasis | ||||||
| Yes | 1403 (14%) | 8520 (86%) | 0.3343 | 303 (8%) | 3337 (92%) | 0.0301 |
| No | 7565 (15%) | 44 549 (85%) | 1938 (9%) | 18 558 (91%) | ||
| Liver metastasis | ||||||
| Yes | 464 (11%) | 3692 (89%) | <0.0001 | 97 (6%) | 1448 (94%) | <0.0001 |
| No | 8504 (15%) | 49 377 (85%) | 2144 (9%) | 20 447 (91%) | ||
| Surgery for primary lesion | ||||||
| Yes | 225 (11%) | 1773 (89%) | <0.0001 | 38 (8%) | 418 (92%) | 0.5155 |
| No | 8743 (15%) | 51 296 (85%) | 2203 (9%) | 21 477 (91%) | ||
| Radiation | ||||||
| Yes | 4479 (14%) | 28 430 (86%) | <0.0001 | 865 (9%) | 8314 (91%) | 0.5680 |
| No | 4489 (15%) | 24 639 (85%) | 1376 (9%) | 13 581 (91%) | ||
| Chemotherapy | ||||||
| Yes | 7398 (17%) | 34 928 (83%) | <0.0001 | 1444 (13%) | 9752 (87%) | <0.0001 |
| No | 1570 (8%) | 18 141 (92%) | 797 (6%) | 12 143 (94%) | ||
Abbreviation: NOS, not otherwise specified.
Univariate survival analyses of patients with stage IV NSCLC treated with or without ICIs stratified by chronological age
Kaplan–Meier curves of OS among patients with stage IV NSCLC stratified by chronological age are shown in Figure 1. Among patients aged <75 years, those who received ICIs had significantly longer OS than those who did not receive ICIs (median OS 14.5 vs. 7.8 months, HR 0.67 [95% CI 0.65–0.68], p < 0.0001; Figure 1(a)). Similarly, among patients aged ≥75 years, those who received ICIs had significantly longer OS than the those who did not receive ICIs (median OS 11.9 vs. 5.4 months, HR 0.61 [95% CI 0.58–0.64], p < 0.000; Figure 1(b)).
FIGURE 1.
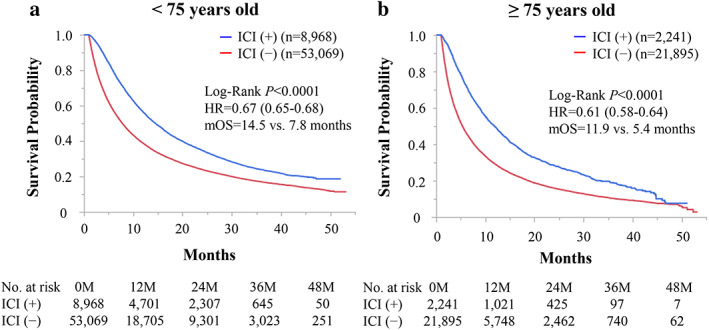
Kaplan–Meier curves of overall survival (OS) among patients with stage IV non‐small‐cell lung cancer treated with immune checkpoint inhibitors (ICIs) stratified by chronological age. (a) Among patients aged <75 years, those receiving ICIs had significantly longer OS than those who did not receive ICIs. (b) Among patients aged ≥75 years, those receiving ICIs had significantly longer OS those who did not receive ICIs. Hr, hazard ratio; mOS, median overall survival
Univariate and multivariate analyses of OS among patients with stage IV NSCLC aged <75 and ≥75 years
The results of univariate and multivariate analyses of OS among patients with stage IV NSCLC aged <75 and ≥75 years are shown in Table 2. Multivariate analysis of OS among patients aged <75 years demonstrated that male sex, white race, uninsured status, nonacademic institution, Charlson‐Deyo score ≥ 2, diagnosis in 2014, nonadenocarcinoma not otherwise specified [NOS] histology, nodal status ≥ N1, bone metastasis, liver metastasis, no surgery of the primary lesion, radiation, chemotherapy, and no ICI therapy were independent predictors of shorter OS (HR for patients not receiving ICIs 1.47 [95% CI 1.43–1.51], p < 0.0001; Table 2). Among patients aged ≥75 years, multivariate analysis of OS showed that male sex, white race, nonacademic institution, Charlson‐Deyo score ≥ 2, diagnosis in 2014, nonadenocarcinoma NOS histology, nodal status ≥ N1, brain metastasis, bone metastasis, liver metastasis, no surgery of the primary lesion, chemotherapy, and no ICI therapy were independent predictors of shorter OS (HR for patients not receiving ICIs 1.63 [95% CI 1.55–1.71], p < 0.0001; Table 2). In multivariate analyses, both no chemotherapy and no ICI therapy were independent predictors of shorter OS in patients with stage IV NSCLC aged <75 and ≥75 years. In each group, the HR for patients not receiving chemotherapy was greater than that for patients not receiving ICI.
TABLE 2.
Multivariate analyses of overall survival in patients with stage IV non‐small‐cell lung cancer aged <75 and ≥75 years
| Factors | <75 years old (n = 62 037) | ≥75 years old (n = 24 136) | ||
|---|---|---|---|---|
| Univariate | Multivariable | Univariate | Multivariable | |
| HR (95% CI) | HR (95% CI) | HR (95% CI) | HR (95% CI) | |
| p value | p value | p value | p value | |
| Sex | ||||
| Male | 1.27 (1.24–1.29) | 1.22 (1.20–1.24) | 1.17 (1.14–1.21) | 1.19 (1.16–1.23) |
| Female | <0.0001 | <0.0001 | <0.0001 | <0.0001 |
| Race | ||||
| Whites | 1.13 (1.11–1.16) | 1.14 (1.11–1.17) | 1.16 (1.11–1.21) | 1.16 (1.11–1.21) |
| Others | <0.0001 | <0.0001 | <0.0001 | <0.0001 |
| Insurance status | ||||
| Uninsured | 1.12 (1.07–1.18) | 1.06 (1.01–1.12) | 1.01 (0.80–1.25) | 1.16 (0.94–1.46) |
| Insured | <0.0001 | 0.0118 | 0.9307 | 0.1906 |
| Institution | ||||
| Others | 1.20 (1.17–1.22) | 1.19 (1.16–1.21) | 1.18 (1.14–1.21) | 1.14 (1.11–1.18) |
| Academic | <0.0001 | <0.0001 | <0.0001 | <0.0001 |
| Charlson–Deyo score | ||||
| ≥2 | 1.37 (1.33–1.40) | 1.26 (1.23–1.30) | 1.33 (1.28–1.38) | 1.22 (1.18–1.27) |
| ≤1 | <0.0001 | <0.0001 | <0.0001 | <0.0001 |
| Year of diagnosis | ||||
| 2014 | 1.05 (1.03–1.07) | 1.05 (1.02–1.07) | 1.04 (1.01–1.07) | 0.91 (0.88–0.94) |
| 2015 | <0.0001 | <0.0001 | 0.0088 | <0.0001 |
| Histology | ||||
| Others | 1.23 (1.21–1.25) | 1.13 (1.11–1.15) | 1.14 (1.11–1.17) | 1.05 (1.02–1.08) |
| Adenocarcinoma not otherwise specified | <0.0001 | <0.0001 | <0.0001 | 0.0014 |
| Nodal status | ||||
| ≥N1 | 1.22 (1.19–1.24) | 1.33 (1.30–1.36) | 1.17 (1.14–1.21) | 1.31 (1.27–1.35) |
| N0 | <0.0001 | <0.0001 | <0.0001 | <0.0001 |
| Brain metastasis | ||||
| Yes | 1.09 (1.06–1.12) | 1.02 (0.99–1.05) | 1.24 (1.18–1.31) | 1.25 (1.18–1.32) |
| No | <0.0001 | 0.2439 | <0.0001 | <0.0001 |
| Bone metastasis | ||||
| Yes | 1.24 (1.21–1.27) | 1.13 (1.11–1.15) | 1.17 (1.13–1.22) | 1.20 (1.15–1.25) |
| No | <0.0001 | <0.0001 | <0.0001 | <0.0001 |
| Liver metastasis | ||||
| Yes | 1.45 (1.40–1.50) | 1.32 (1.27–1.36) | 1.32 (1.25–1.39) | 1.26 (1.19–1.33) |
| No | <0.0001 | <0.0001 | <0.0001 | <0.0001 |
| Surgery for primary lesion | ||||
| No | 2.02 (1.90–2.14) | 2.04 (1.92–2.16) | 1.85 (1.66–2.08) | 1.83 (1.64–2.06) |
| Yes | <0.0001 | <0.0001 | <0.0001 | <0.0001 |
| Radiation | ||||
| Yes | 1.10 (1.08–1.12) | 1.12 (1.10–1.14) | 1.06 (1.03–1.09) | 1.00 (0.98–1.04) |
| No | <0.0001 | <0.0001 | <0.0001 | 0.7468 |
| Chemotherapy | ||||
| No | 2.19 (2.15–2.23) | 2.30 (2.25–2.34) | 2.11 (2.05–2.17) | 2.18 (2.12–2.24) |
| Yes | <0.0001 | <0.0001 | <0.0001 | <0.0001 |
| Immune checkpoint inhibitor | ||||
| No | 1.50 (1.46–1.54) | 1.47 (1.43–1.51) | 1.65 (1.57–1.73) | 1.63 (1.55–1.71) |
| Yes | <0.0001 | <0.0001 | <0.0001 | <0.0001 |
Abbreviations: CI, confidence interval; HR, hazard ratio; NOS, not otherwise specified.
DISCUSSION
The efficacy of ICIs among elderly patients is controversial given the potential for immune senescence. Aging may reduce the potency of the effector phase of the cancer immunity cycle because of decreased antigen presentation by dendritic cells and decreased numbers of naïve CD8+ T cells. 10 , 11 However, patients with melanoma aged ≥60 years old receiving ICIs responded more efficiently to anti‐PD‐1 inhibitors than younger patients. 12 One potential explanation of this phenomenon is that in younger patients, regulatory T cells are more abundant in the tumor microenvironment 12 and negatively impact the efficacy of ICIs. 13 In addition, frequencies of CD8+ T cells, which play significant roles in the cancer immunity cycle, were decreased in melanoma tumors from younger patients. 12 Thus, despite immune senescence, elderly patients with cancer can still potentially benefit from ICI therapy.
An Italian multicenter retrospective study investigated the efficacy and safety of anti‐PD‐1 immunotherapy among patients with advanced NSCLC aged ≥75 years and found that the efficacy and toxicity of ICIs in elderly patients were comparable with the profiles observed in younger patients. 14 A meta‐analysis showed that ICI therapy and non‐ICI therapy of NSCLC had comparable efficacy in patients aged ≥65 years and those aged <65 years. 15 Another meta‐analysis of a large clinical dataset suggested that NSCLC patients aged ≥65 years can benefit even more from ICI therapy than younger patients. 16 In the current study, we found that the HR for death among patients with stage IV NSCLC aged ≥75 years who did not receive ICIs was greater than among those aged <75 years (1.63 vs. 1.48). A survival benefit of ICI therapy was observed in both age strata. Consistently, the HR for death among patients with stage IV NSCLC aged ≥75 years who received ICIs was smaller than that among those who did not receive ICIs (1.23 vs. 1.32). These results suggest that aging does not negatively impact ICI efficacy in patients with stage IV NSCLC, in line with these previous reports. 8 , 12 , 14 , 15 , 16 , 17
In multivariate analyses, the HRs for patients not receiving chemotherapy were greater than those for patients not receiving ICI in patients with stage IV NSCLC aged <75 and ≥75 years. One of the explanations of this phenomenon is that cytotoxic chemotherapy is generally not suitable for frail patients and/or those with poor performance status due to their increased incidence of adverse events, but ICI may be a potential treatment option for such population. Therefore, patients receiving ICI may have included more frail patients compared with those treated with chemotherapy. Such bias arising from patients' selection may potentially result in the greater HR for patients not receiving chemotherapy. In addition, NCDB lacks data about names of medications, dose, and number of cycles, so detailed subset analysis cannot be conducted. These points make it difficult to compare the HRs of patients who received chemotherapy and those with ICI. Further advanced analysis is needed to determine the clinical impact of chemotherapy and that of ICI.
Our study had several limitations. First, the NCDB lacks several prognostic factors, including performance status, PD‐L1 expression level, genetic mutation status, laboratory data, line of therapy, and immune‐related adverse events. 18 Analyses including these factors may further elucidate correlates of the safety and efficacy of ICIs in elderly NSCLC patients. Second, this was a retrospective study and potential biases associated with physician decisions and/or patient status cannot be ruled out. Our findings should be validated in future well‐designed prospective studies.
In conclusion, the present study showed that chronological age does not appear to impact the survival benefit of ICIs in patients with stage IV NSCLC. These findings should be validated in future prospective studies.
CONFLICT OF INTEREST
Takefumi Komiya received travel fees from Merck. All authors declare no conflicts of interest associated with this study.
ACKNOWLEDGMENTS
We thank Edanz (https://jp.edanz.com/ac) for editing a draft of this manuscript. The publication fee was supported by the Kaibara Morikazu Medical Science Promotion Foundation.
Takamori S, Shimokawa M, Komiya T. Prognostic impact of chronological age on efficacy of immune checkpoint inhibitors in non‐small‐cell lung cancer: Real‐world data from 86 173 patients. Thorac Cancer. 2021;12:2943–2948. 10.1111/1759-7714.14178
Funding information Kaibara Morikazu Medical Science Promotion Foundation
REFERENCES
- 1. Zappa C, Mousa SA. Non‐small cell lung cancer: current treatment and future advances. Transl Lung Cancer Res. 2016;5(3):288–300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Gandhi L, Rodriguez‐Abreu D, Gadgeel S, et al. Pembrolizumab plus chemotherapy in metastatic non‐small‐cell lung cancer. N Engl J Med. 2018;378(22):2078–92. [DOI] [PubMed] [Google Scholar]
- 3. West H, McCleod M, Hussein M, Morabito A, Rittmeyer A, Conter HJ, et al. Atezolizumab in combination with carboplatin plus nab‐paclitaxel chemotherapy compared with chemotherapy alone as first‐line treatment for metastatic non‐squamous non‐small‐cell lung cancer (IMpower130): a multicentre, randomised, open‐label, phase 3 trial. Lancet Oncol. 2019;20(7):924–37. [DOI] [PubMed] [Google Scholar]
- 4. Socinski MA, Jotte RM, Cappuzzo F, Orlandi F, Stroyakovskiy D, Nogami N, et al. Atezolizumab for first‐line treatment of metastatic nonsquamous NSCLC. N Engl J Med. 2018;378(24):2288–301. [DOI] [PubMed] [Google Scholar]
- 5. Mok TSK, Wu YL, Kudaba I, Kowalski DM, Cho BC, Turna HZ, et al. Pembrolizumab versus chemotherapy for previously untreated, PD‐L1‐expressing, locally advanced or metastatic non‐small‐cell lung cancer (KEYNOTE‐042): a randomised, open‐label, controlled, phase 3 trial. Lancet. 2019;393(10183):1819–30. [DOI] [PubMed] [Google Scholar]
- 6. Paz‐Ares L, Luft A, Vicente D, Tafreshi A, Gümüş M, Mazières J, et al. Pembrolizumab plus chemotherapy for squamous non‐small‐cell lung Cancer. N Engl J Med. 2018;379(21):2040–51. [DOI] [PubMed] [Google Scholar]
- 7. Carbone DP, Reck M, Paz‐Ares L, Creelan B, Horn L, Steins M, et al. First‐line Nivolumab in stage IV or recurrent non–small‐cell lung Cancer. N Engl J Med. 2017;376(25):2415–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Nosaki K, Saka H, Hosomi Y, Baas P, de Castro G Jr, Reck M, et al. Safety and efficacy of pembrolizumab monotherapy in elderly patients with PD‐L1‐positive advanced non‐small‐cell lung cancer: pooled analysis from the KEYNOTE‐010, KEYNOTE‐024, and KEYNOTE‐042 studies. Lung Cancer. 2019;135:188–95. [DOI] [PubMed] [Google Scholar]
- 9. Yamaguchi O, Imai H, Minemura H, Suzuki K, Wasamoto S, Umeda Y, et al. Efficacy and safety of immune checkpoint inhibitor monotherapy in pretreated elderly patients with non‐small cell lung cancer. Cancer Chemother Pharmacol. 2020;85(4):761–71. [DOI] [PubMed] [Google Scholar]
- 10. Nikolich‐Žugich J. The twilight of immunity: emerging concepts in aging of the immune system. Nat Immunol. 2018;19(1):10–9. [DOI] [PubMed] [Google Scholar]
- 11. Solana R, Tarazona R, Gayoso I, Lesur O, Dupuis G, Fulop T. Innate immunosenescence: effect of aging on cells and receptors of the innate immune system in humans. Semin Immunol. 2012;24(5):331–41. [DOI] [PubMed] [Google Scholar]
- 12. Kugel CH 3rd, Douglass SM, Webster MR, et al. Age correlates with response to anti‐PD1, reflecting age‐related differences in Intratumoral effector and regulatory T‐cell populations. Clin Cancer Res. 2018;24(21):5347–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Kim HR, Park HJ, Son J, Lee JG, Chung KY, Cho NH, et al. Tumor microenvironment dictates regulatory T cell phenotype: upregulated immune checkpoints reinforce suppressive function. J Immunother Cancer. 2019;7(1):339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Luciani A, Marra A, Toschi L, Cortinovis D, Fava S, Filipazzi V, et al. Efficacy and safety of anti‐PD‐1 immunotherapy in patients aged ≥ 75years with non‐small‐cell lung cancer (NSCLC): an Italian, multicenter, retrospective study. Clin Lung Cancer. 2020;21(6):e567–71. [DOI] [PubMed] [Google Scholar]
- 15. Sun YM, Wang Y, Sun XX, Chen J, Gong ZP, Meng HY. Clinical efficacy of immune checkpoint inhibitors in older non‐small‐cell lung cancer patients: a meta‐analysis. Front Oncol. 2020;10:558454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Wu Q, Wang Q, Tang X, et al. Correlation between patients' age and cancer immunotherapy efficacy. Oncoimmunology. 2019;8(4):e1568810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Youn B, Trikalinos NA, Mor V, Wilson IB, Dahabreh IJ. Real‐world use and survival outcomes of immune checkpoint inhibitors in older adults with non‐small cell lung cancer. Cancer. 2020;126(5):978–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Takamori S, Takada K, Shimokawa M, Matsubara T, Fujishita T, Ito K, et al. Clinical utility of pretreatment Glasgow prognostic score in non‐small‐cell lung cancer patients treated with immune checkpoint inhibitors. Lung Cancer. 2021;152:27–33. [DOI] [PubMed] [Google Scholar]


