Abstract
Background
Most tuberculosis (TB) disease in the United States (US) is attributed to reactivation of remotely acquired latent TB infection (LTBI) in non-US-born persons who were likely infected with Mycobacterium tuberculosis in their countries of birth. Information on LTBI prevalence by country of birth could help guide local providers and health departments to scale up the LTBI screening and preventive treatment needed to advance progress toward TB elimination.
Methods
A total of 13 805 non-US-born persons at high risk of TB infection or progression to TB disease were screened for LTBI at 16 clinical sites located across the United States with a tuberculin skin test, QuantiFERON Gold In-Tube test, and T-SPOT.TB test. Bayesian latent class analysis was applied to test results to estimate LTBI prevalence and associated credible intervals (CrIs) for each country or world region of birth.
Results
Among the study population, the estimated LTBI prevalence was 31% (95% CrI, 26%–35%). Country-of-birth-level LTBI prevalence estimates were highest for persons born in Haiti, Peru, Somalia, Ethiopia, Vietnam, and Bhutan, ranging from 42% to 55%. LTBI prevalence estimates were lowest for persons born in Colombia, Malaysia, and Thailand, ranging from 8% to 13%.
Conclusions
LTBI prevalence in persons born outside the US varies widely by country. These estimates can help target community outreach efforts to the highest-risk groups.
Keywords: latent tuberculosis infection, prevalence, non-US, born persons, latent class analysis
We estimated latent tuberculosis infection (LTBI) prevalence by birth country/region for 13 805 non-US-born persons in the United States. LTBI varies widely by nativity; providers and public health programs can use this information to prioritize populations for testing.
The pace of decline in tuberculosis (TB) incidence in the United States (US) has slowed over the past 5 years [1, 2]. The 2019 provisional rate of 2.7 cases per 100 000 population—the lowest ever recorded—was achieved primarily through rapid identification and treatment of cases and infected contacts to break the chain of transmission [2]. However, since at least 2014, >80% of US TB cases were likely due to reactivation of latent TB infection (LTBI), not recent transmission [3]. Thus, progress toward TB elimination will require substantial increases in proportions of people with LTBI who are screened and complete antibiotic therapy to prevent TB disease [4–6].
More than 70% of US TB cases are now among non-US-born persons, most of whom likely acquire infection in their birth countries and progress to disease after US arrival [7, 8]. Although national guidelines recommend that persons from countries with high TB burdens be screened and treated for LTBI [9, 10], only a small fraction is screened, primarily by local health departments in the context of contact tracing [11–13]. While adult immigrants and refugees (≥15 years of age) are screened overseas for TB disease, LTBI testing is not required [14, 15]. Students, tourists, employees, and other nonpermanent residents are not routinely tested for TB or LTBI as a condition of US entry.
To efficiently design, scale up, and evaluate expanded LTBI testing and treatment programs, healthcare providers and public health authorities need detailed estimates of LTBI prevalence among non-US-born populations. Such estimates are scarce. While the National Health and Nutrition Examination Survey (NHANES) has occasionally provided data on LTBI test positivity among survey participants, it does not publicly disclose non-US-born participants’ birth countries; the information is generally available only in a US-born/non-US-born format [16]. Because non-US-born persons come from countries with wide variations in TB incidence [17], LTBI prevalence is likely to vary by birth country. Information on LTBI prevalence by birth country is therefore one way to determine which non-US-born populations in the US are in greatest need of expanded LTBI testing and treatment programs.
We used data from a prospective study that included nearly 14 000 non-US-born persons living in the US to estimate LTBI prevalence by country or world region of birth. The primary aim of the prospective study was to estimate the accuracy of tests for LTBI in persons at high risk for LTBI or progression to TB disease. Our secondary analysis aims to characterize how LTBI prevalence differs among high-risk non-US-born populations so local TB programs and healthcare providers can develop screening initiatives that prioritize those at greatest risk for LTBI.
METHODS
Setting, Population, and Dataset
The Tuberculosis Epidemiologic Studies Consortium (TBESC), funded by the Centers for Disease Control and Prevention (CDC), collaborates with TB control programs and academic institutions in 11 US states to increase diagnosis and treatment of LTBI in high-risk populations [18]. Data for this LTBI prevalence study were from a larger prospective study of the comparative abilities of TB infection tests to predict progression to TB disease. The main study recruited US-born and non-US-born persons who were (1) likely to have positive tests for TB infection (eg, immigrants, close contacts to persons with TB disease), or (2) likely to progress to TB disease if infected (eg, persons with human immunodeficiency virus [HIV] infection). Participant records included in the LTBI prevalence substudy represented (1) persons born in countries whose US populations had high (≥100 per 100 000) TB rates [19]; (2) recent arrivals (≤5 years) from countries whose US populations had moderate (10–99 per 100 000) TB rates [19]; and (3) members of local populations with documented LTBI prevalence ≥25%—persons born in Mexico (2 sites) and El Salvador (1 site), regardless of time since arrival. Country of birth was assessed by asking each participant “What country were you born in?” Sites with access to clinics that evaluate refugees used administrative information to identify potential participants. Health departments also had access to the national Electronic Disease Notification System, which provides information about arriving immigrants and refugees whose laboratory tests were negative for TB disease but whose predeparture chest radiographs were abnormal (Class B1 entrants).
Sixteen TBESC-affiliated clinics enrolled participants from July 2012 to April 2017, collected demographic and LTBI-related risk information, and drew blood simultaneously for 2 interferon-γ release assays (IGRAs)—QuantiFERON Gold In-Tube (QFT) and T-SPOT.TB (TSPOT)—followed immediately by placement of a tuberculin skin test (TST) using the Mantoux method [10]. Exclusion criteria included (1) a history of anaphylactic reaction to tuberculin; (2) current treatment for TB disease or LTBI; (3) foster children; or (4) plans to leave the United States in <2 years (the larger study followed patients for 2 years to identify progression to TB disease). For this analysis, records were also excluded if participants were diagnosed with TB disease during enrollment, were contacts of US TB cases (≥8 hours in a week in a shared airspace), did not have valid results for all 3 tests, or had been referred to the clinic for a positive TST (referrals for positive IGRAs were not enrolled). Finally, because LTBI test characteristics differ for persons with HIV and for children <5 years old [20], this analysis was restricted to HIV-negative persons ≥5 years old.
All participants provided written informed consent, assent, and/or parental permission. The study was approved by CDC’s institutional review board (IRB) and local IRBs that did not defer to CDC and was registered at ClinicalTrials.gov (identifier NCT01622140).
LTBI Prevalence Estimation
Bayesian latent class analysis (LCA) estimates the prevalence of a condition from observed test results when no gold-standard test exists [20–22]. We created an LCA model with test results from the study population to estimate LTBI prevalence as previously described [20]. In brief, we used R version 3.4.1 [23] and JAGS version 4.2.0 (open-source software) through the runjags package (version 2.0.4-2) to implement these models, with Markov chain Monte Carlo sampling to estimate parameter distributions [24]. The initial 1000 and subsequent 1000 samples were used for model adaptation and burn-in, respectively, with subsequent sampling of either (1) a minimum of 20 000 iterations or (2) enough iterations to obtain Gelman-Rubin statistics <1.05 for all sensitivity, specificity, and prevalence parameters, whichever was greater [25]. All models included a random effect to account for the conditional dependence of TB infection tests.
Estimates of LTBI Test Characteristics
Previously published LCA-based estimates of TB infection test characteristics used a subset of the HIV-negative, non-US-born TBESC study population (n = 7931), and the international cutoff (≥6 spots) for TSPOT positivity [20]. We applied the same LCA methodology to the complete TBESC cohort of non-US-born, HIV-negative persons ≥5 years old using the US Food and Drug Administration–approved cutoff (≥8 spots) for TSPOT positivity.
LTBI Prevalence Estimates by Birth Country/Region
Output from the LCA model was used to calculate the positive predictive value (PPV) of each LTBI test combination. The mean LTBI prevalence for participants from each country/region of birth c was calculated as the sum across all 8 test combinations (T) of the PPV for each combination i multiplied by the proportion of observations from country/region c with test combination i:
(see example in Supplementary Table 1). Credible intervals (CrIs) were calculated by sampling 1000 times with replacement from the LCA model posterior PPVs and multiplying by 1000 samples from multinomially distributed random numbers corresponding to the proportion for each test combination. The 95% CrI was formed from the lower 2.5th and upper 97.5th percentiles of the aggregate of these samples. We compared this approach to running separate LCA models for participants from each of the 15 countries with the most observations and obtained similar LTBI prevalence estimates (Supplementary Figure 1). Estimates could not be calculated for participants from 4 of the 15 countries with the largest sample sizes because of low counts of 1 test combination. Therefore, we chose our initial approach, since it can be applied to all countries/regions.
LTBI prevalence was estimated for each birth country with >65 observations. Prevalence estimates for countries with ≤65 observations yielded wide CrIs, indicating low precision (Supplementary Figure 2). These were either (1) grouped into United Nations world regions [26] and LTBI estimates calculated for each region, or (2) combined with neighboring regions with similar estimated LTBI prevalence (Supplementary Table 3).
Because the study did not recruit persons from countries with low TB incidence, LTBI prevalence was not estimated for North America or Western, Northern, or Southern Europe. The low-incidence Caribbean region includes 2 high-incidence countries: Haiti and the Dominican Republic. Haiti had sufficient observations to calculate LTBI prevalence; the Dominican Republic was grouped with the Central and South America region. Australia and New Zealand, which have low TB incidence, were excluded from LTBI prevalence estimates for the Oceania region.
LTBI Prevalence and US Incidence of TB Disease
Because >90% of TB cases in non-US-born persons in the United States are attributed to reactivation of remotely acquired LTBI [3], TB incidence in these populations should be a function of LTBI prevalence and risk of progression to TB disease. We assessed the correlation between birth country LTBI prevalence estimates and US TB incidence in persons from those countries [27]. We also compared number of years in the United States in our non-US-born sample to the number of years reported in the American Community Survey 2018 one-year estimate [28].
RESULTS
Participant Characteristics
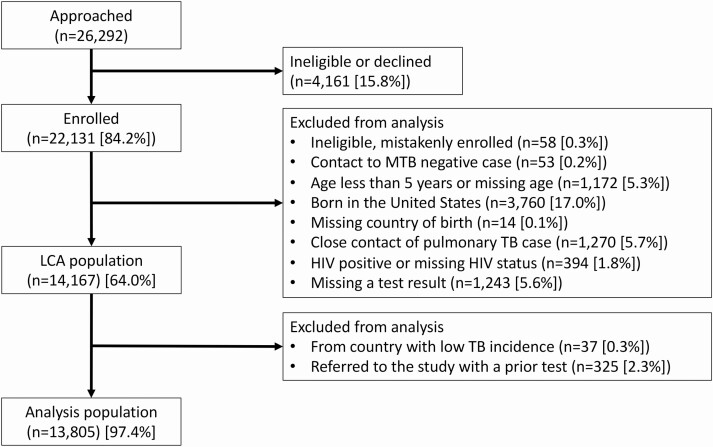
Of 22 132 persons enrolled, 13 805 had valid TST, QFT, and TSPOT test results and were HIV-negative, ≥5 years old, and born in countries whose US populations had a TB incidence of ≥10 per 100 000 (Figure 1). The most common age group was 25–44 years (Table 1); median age varied by birth country (Supplementary Figure 3). Participants born in each world region ranged from 114 in Eastern Europe to 5127 in Southeastern Asia. Refugees accounted for 60% of the study population, ranging from 1% from Central and South America to 89% from sub-Saharan Africa (Supplementary Figure 4). Of note, among participants born in countries that host significant numbers of refugees (Kenya, Malaysia, Nepal, Rwanda, and Thailand), half were children (<18 years of age) and most (>82%) were refugees whose parents fled nearby countries. Less than 30% of participants from Central and South America were recent arrivals (<1 year), compared to 85%–96% of participants from other regions.
Figure 1.
Analysis population. Persons from the original study population were excluded from this analysis if they were <5 years old, were born in the United States, were diagnosed with tuberculosis (TB) during enrollment, or were human immunodeficiency virus positive. Participants who were household contacts of infectious TB cases or were referred to a study clinic for a positive tuberculin skin test (referrals for positive interferon-γ release assays were not enrolled) were thought to have an elevated a priori risk for latent TB infection and were also excluded from the analysis. Abbreviations: HIV, human immunodeficiency virus; LCA, latent class analysis; MTB, Mycobacterium tuberculosis.
Table 1.
Characteristics of Non-US-Born Persons Screened for Latent Tuberculosis Infection in the United States (N = 13 805)
| Characteristic | All Observations (N = 13 805) |
Central and South America (n = 2179) |
Sub-Saharan Africa (n = 2468) |
North Africa and West Asia (n = 1212) |
Eastern Europe (n = 114) |
Eastern Asia (n = 5127) |
South Central Asia (n = 2439) |
Oceania (n = 266) |
|---|---|---|---|---|---|---|---|---|
| Age group | ||||||||
| 5–14 y | 2369 (17) | 155 (7) | 700 (28) | 265 (22) | 18 (16) | 763 (15) | 428 (18) | 40 (15) |
| 15–24 y | 2870 (21) | 361 (17) | 588 (24) | 236 (19) | 14 (12) | 964 (19) | 631 (26) | 76 (29) |
| 25–44 y | 5848 (42) | 1241 (57) | 922 (37) | 523 (43) | 35 (31) | 2031 (40) | 975 (40) | 121 (45) |
| 45–64 y | 2221 (16) | 357 (16) | 229 (9) | 158 (13) | 30 (26) | 1092 (21) | 330 (14) | 25 (9) |
| ≥65 y | 497 (4) | 65 (3) | 29 (1) | 30 (2) | 17 (15) | 277 (5) | 75 (3) | 4 (2) |
| Sex | ||||||||
| Female | 7119 (52) | 1470 (67) | 1185 (48) | 537 (44) | 58 (51) | 2575 (50) | 1152 (47) | 142 (53) |
| Male | 6684 (48) | 709 (33) | 1283 (52) | 675 (56) | 56 (49) | 2550 (50) | 1287 (53) | 124 (47) |
| Race/ethnicity | ||||||||
| Hispanic | 1446 (10) | 1440 (66) | 1 (0) | 0 (0) | 0 (0) | 5 (0) | 0 (0) | 0 (0) |
| Asian | 4973 (36) | 4 (0) | 7 (0) | 62 (5) | 0 (0) | 3590 (70) | 1310 (54) | 0 (0) |
| Pacific Islander | 255 (2) | 5 (0) | 0 (0) | 0 (0) | 0 (0) | 10 (0) | 2 (0) | 238 (89) |
| Black | 1982 (14) | 147 (7) | 1807 (73) | 26 (2) | 0 (0) | 2 (0) | 0 (0) | 0 (0) |
| White | 701 (5) | 44 (2) | 10 (0) | 418 (34) | 101 (89) | 4 (0) | 124 (5) | 0 (0) |
| Other | 3634 (26) | 151 (7) | 584 (24) | 586 (48) | 10 (9) | 1427 (28) | 856 (35) | 20 (8) |
| Unknown | 889 (6) | 389 (18) | 82 (3) | 128 (11) | 3 (3) | 117 (2) | 160 (7) | 10 (4) |
| Self-reported BCG vaccination | ||||||||
| No | 3364 (24) | 359 (16) | 801 (32) | 298 (25) | 9 (8) | 1283 (25) | 456 (19) | 158 (59) |
| Yes | 8881 (64) | 1554 (71) | 1335 (54) | 795 (66) | 94 (82) | 3320 (65) | 1713 (70) | 70 (26) |
| Unknown | 1560 (11) | 266 (12) | 332 (13) | 119 (10) | 11 (10) | 524 (10) | 270 (11) | 38 (14) |
| Years in United States | ||||||||
| 0–0.5 | 10 765 (78) | 446 (20) | 2243 (91) | 1158 (96) | 97 (85) | 4475 (87) | 2309 (95) | 37 (14) |
| >0.5–1 | 301 (2) | 132 (6) | 55 (2) | 8 (1) | 5 (4) | 66 (1) | 20 (1) | 15 (6) |
| >1–5 | 839 (6) | 407 (19) | 82 (3) | 38 (3) | 12 (11) | 176 (3) | 51 (2) | 73 (27) |
| >5–10 | 716 (5) | 479 (22) | 31 (1) | 1 (0) | 0 (0) | 107 (2) | 28 (1) | 70 (26) |
| >10–20 | 808 (6) | 565 (26) | 30 (1) | 3 (0) | 0 (0) | 130 (3) | 23 (1) | 57 (21) |
| >20–30 | 218 (2) | 111 (5) | 9 (0) | 1 (0) | 0 (0) | 81 (2) | 2 (0) | 14 (5) |
| >30 | 119 (1) | 37 (2) | 3 (0) | 0 (0) | 0 (0) | 78 (2) | 1 (0) | 0 (0) |
| Missing | 39 (0) | 2 (0) | 15 (1) | 3 (0) | 0 (0) | 14 (0) | 5 (0) | 0 (0) |
| Refugee | 8306 (60) | 22 (1) | 2194 (89) | 1106 (91) | 63 (55) | 2813 (55) | 2108 (86) | 0 (0) |
| Class B1a | 1502 (11) | 113 (5) | 130 (5) | 27 (2) | 46 (40) | 914 (18) | 270 (11) | 2 (1) |
Data are presented as No. (%).
aImmigrants whose overseas chest radiographs are suggestive of tuberculosis disease, but who have negative sputum microscopy smears and cultures and are cleared to travel to the United States. They are directed to report to their local health departments after arrival for further evaluation.
Estimates of LTBI Test Characteristics
LCA-based estimates of test performance yielded sensitivities of 82%, 83%, and 72% for TST, QFT, and TSPOT, respectively, and specificities of 71%, 98%, and 100% (Table 2). Among test result combinations, the greatest PPV was a positive result on all 3 tests. Negative results for all 3 tests or a lone positive TST had the lowest PPVs. All parameter estimates were similar to a previously published LCA analysis of a subset of the study population and were within published CrIs [20].
Table 2.
Estimates of Performance Characteristics of Tests for Latent Tuberculosis Infection in 13 805 Non-US-Born Persons Who Received All 3 Tests
| Parametera | Mean | (95% Credible Interval) |
|---|---|---|
| LTBI prevalence | 0.31 | (.26–.35) |
| TST sensitivity | 0.82 | (.76–.91) |
| TST specificity | 0.71 | (.70–.72) |
| QFT sensitivity | 0.83 | (.76–.93) |
| QFT specificity | 0.98 | (.96–1.00) |
| TSPOT sensitivity | 0.72 | (.64–.84) |
| TSPOT specificity | 1.00 | (.99–1.00) |
| PPV (+/+/+) | 1.00 | (1.00–1.00) |
| PPV (–/+/+) | 1.00 | (.98–1.00) |
| PPV (+/+/–) | 0.91 | (.79–1.00) |
| PPV (+/–/+) | 0.93 | (.82–1.00) |
| PPV (+/–/–) | 0.07 | (.01–.10) |
| PPV (–/+/–) | 0.66 | (.17–.96) |
| PPV (–/–/+) | 0.74 | (.21–.98) |
| PPV (–/–/–) | 0.03 | (.00–.07) |
Abbreviations: LTBI, latent tuberculosis infection; PPV, positive predictive value; QFT, QuantiFERON Gold In-Tube; TSPOT, T-SPOT.TB; TST, tuberculin skin test.
aThe + or – indicates positive or negative results in the following order: TST, QFT, TSPOT.
LTBI Prevalence Estimates by Birth Country/Region
Participants were from 127 countries, of which 28 had sufficient sample size to estimate LTBI prevalence; the rest were grouped into 7 world regions and LTBI prevalence estimated for each. Overall LTBI prevalence was 31% (95% CrI, 26%–35%), with substantial variation by birth country/region (Figure 2A and Table 3). Participants from sub-Saharan Africa and Eastern Asia had the highest regional LTBI prevalence; most country prevalences were >25%, with the highest among participants from Somalia (51.0% [95% CrI, 46.4%–55.1%]) and Ethiopia (47.4% [95% CrI, 41.4%–53.4%). In Eastern Asia, participants from Vietnam (53.0% [95% CrI, 46.7%–59.4%]) and China (37.5% [95% CrI, 31.1%–43.3%]) had the highest prevalence. Other birth countries with estimated LTBI prevalence >40% included Bhutan, Peru, and Haiti (Figure 2B). Participants from Colombia, Malaysia, and Thailand had estimated LTBI prevalence <15%.
Figure 2.
Latent tuberculosis infection (LTBI) prevalence estimates among study participants by country or world region of birth. A, Point estimates with 95% credible intervals of LTBI prevalence among high-risk non-US-born persons living in the United States by country and world region of birth. B, Countries and world regions in which LTBI prevalence was measured. With increasing LTBI prevalence, the color scale goes from yellow (<22% LTBI prevalence) to red (>32% LTBI prevalence). Abbreviations: AFG, Afghanistan; BTN, Bhutan; CHN, China; COD, Democratic Republic of the Congo; COL, Colombia; ERI, Eritrea; ETH, Ethiopia; GTM, Guatemala; HND, Honduras; HTI, Haiti; IND, India; IRN, Iran; IRQ, Iraq; KEN, Kenya; MEX, Mexico; MMR, Myanmar; MYS, Malaysia; NPL, Nepal; PER, Peru; PHL, Philippines; RWA, Rwanda; SDN, Sudan; SLV, El Salvador; SOM, Somalia; SYR, Syria; THA, Thailand; VNM, Vietnam.
Table 3.
Estimates of Latent Tuberculosis Infection Prevalence in Non-US-Born Persons by Country or World Region of Birth (N = 13 805)
| Region/Country | No. of Subjects | LTBI Prevalence, % (95% CrI) |
|---|---|---|
| Caribbean and Central and South America | ||
| Haiti | 175 | 54.8 (47.7–61.8) |
| Peru | 68 | 42.0 (31.1–52.9) |
| Mexico | 617 | 32.2 (28.1–36.6) |
| Honduras | 441 | 18.1 (13.9–22.2) |
| Guatemala | 200 | 17.3 (12.2–22.6) |
| El Salvador | 408 | 16.4 (12.3–20.7) |
| Colombia | 79 | 13.0 (6.5–20.1) |
| Other countries in region | 191 | 21.5 (15.4–27.7) |
| Eastern Europe | ||
| All countries in region | 114 | 27.0 (18.7–35.8) |
| Western Asia and Northern Africa | ||
| Syria | 135 | 24.2 (17.6–31.6) |
| Iraq | 968 | 16.1 (12.3–20.0) |
| Other countries in region | 109 | 17.5 (11.5–25.2) |
| Sub-Saharan Africa | ||
| Somalia | 610 | 51.0 (46.4–55.1) |
| Ethiopia | 260 | 47.4 (41.4–53.4) |
| Republic of the Congo | 257 | 37.5 (31.6–43.4) |
| Democratic Republic of the Congo | 424 | 35.6 (30.8–40.9) |
| Sudan | 131 | 34.8 (27.0–42.3) |
| Eritrea | 121 | 32.6 (24.2–41.1) |
| Kenya | 155 | 17.5 (11.6–23.9) |
| Rwanda | 103 | 15.6 (8.6–22.8) |
| Other countries in region | 407 | 26.1 (21.8–31.2) |
| Eastern Asia | ||
| Vietnam | 253 | 53.0 (46.7–59.4) |
| China | 270 | 37.5 (31.1–43.3) |
| Philippines | 1641 | 35.5 (31.6–38.9) |
| Myanmar | 2459 | 32.5 (29.3–35.4) |
| Malaysia | 96 | 10.9 (5.4–16.9) |
| Thailand | 281 | 8.4 (4.5–12.9) |
| Other countries in region | 127 | 27.2 (20.2–34.8) |
| South Central Asia | ||
| Bhutan | 1047 | 42.9 (39.2–46.5) |
| India | 148 | 33.8 (26.2–41.3) |
| Nepal | 688 | 22.2 (18.1–26.2) |
| Afghanistan | 374 | 21.2 (16.7–25.9) |
| Iran | 83 | 15.5 (8.2–23.0) |
| Other countries in region | 99 | 21.8 (14.2–29.3) |
| Oceania | ||
| All countries in region | 266 | 26.6 (20.1–32.9) |
Abbreviations: CrI, credible interval; LTBI, latent tuberculosis infection.
LTBI Prevalence Correlated With US Incidence of TB Disease
LTBI prevalence estimates were positively correlated with estimates of US TB incidence rates by birth country (Figure 3). Countries with notable discordances included Haiti and Vietnam, whose country LTBI prevalence estimates were high, but whose US population TB rates were moderate (26 and 36 per 100 000, respectively) [27]. Conversely, study participants from Malaysia and Thailand had very low country estimates of LTBI prevalence but moderate US rates of TB disease (17 and 16 per 100 000, respectively) [27]. Such discordance may reflect changing TB epidemiology, since study participants were more likely to have arrived in the US recently compared to the overall non-US-born population (Supplementary Table 4).
Figure 3.
Correlation between latent tuberculosis (TB) infection prevalence estimates among non-US-born study participants and TB incidence rates in the United States by country of birth. The prevalence of latent TB infection in the study population on the y-axis plotted against the average US TB disease incidence rate per 100 000 population from 2012 to 2017 on the x-axis [27] for each country or world region of birth. Abbreviations: AFG, Afghanistan; BTN, Bhutan; CHN, China; COD, Democratic Republic of the Congo; COL, Colombia; CS Am, Central and South America; ERI, Eritrea; ETH, Ethiopia; GTM, Guatemala; HND, Honduras; HTI, Haiti; IND, India; IRN, Iran; IRQ, Iraq; KEN, Kenya; LTBI, latent tuberculosis infection; MEX, Mexico; MMR, Myanmar; MYS, Malaysia; NPL, Nepal; PER, Peru; PHL, Philippines; RWA, Rwanda; SC Asia, South Central Asia; SDN, Sudan; SLV, El Salvador; SOM, Somalia; SS Af, sub-Saharan Africa; SYR, Syria; THA, Thailand; U.S., United States; VNM, Vietnam.
DISCUSSION
This large study of LTBI prevalence among non-US-born persons by country of birth provides previously unavailable information on the distribution of LTBI among non-US-born populations in the US. The study applied an LCA model to results of all 3 commercially available LTBI tests from almost 14 000 persons at high risk of TB infection. It yielded prevalence estimates for US populations born in 7 world regions and 28 countries, including the top 10 birth countries for TB cases among non-US-born persons in the US [8]. TB programs can use this information to guide practitioners and communities in the design and evaluation of programs to target LTBI screening and treatment to the highest-risk local populations.
Estimates of LTBI prevalence in non-US-born persons have largely relied on occasional surveys of TST and QFT test positivity from NHANES [29–31]. However, the NHANES sample is insufficient to provide prevalence estimates for most birth countries. This creates a challenge for local providers and health departments whose non-US-born populations have substantially different risk levels for LTBI. To plan and evaluate targeted outreach programs, health departments need to estimate the number of people likely to have LTBI and the numbers needed to test and treat to prevent a case of TB disease.
The population included in this substudy is not representative of the overall non-US-born population, because the main study sought to enroll persons likely to have a positive test for infection. Therefore, estimates of LTBI prevalence by birth country may be artificially high. Nevertheless, our study does provide useful information to help local health departments prioritize populations for LTBI screening and treatment. Since most study participants had lived in the US for <1 year, this information could be particularly useful for programs and providers who work with refugees and other recent arrivals. Jurisdictions could generate rough estimates of local LTBI prevalence by combining estimates presented here with US Census estimates of specific non-US-born populations [28].
LTBI prevalence in our study was highest among persons from sub-Saharan Africa and Eastern Asia. Persons from these regions also have the highest US incidence of TB disease [27]. The lower LTBI prevalence estimates for Northern Africa and Western Asia are reflected in lower incidence of US TB disease in persons born in these regions [27]. Birth-country-level LTBI prevalence estimates were correlated with US TB incidence in persons from the same countries. This suggests that observed differences in LTBI prevalence by birth country explain some of the variability in TB incidence after US arrival and could be used to prioritize non-US-born populations for LTBI screening and treatment.
Participants from some countries had LTBI prevalences notably discordant with their US incidence of TB disease. For example, persons from Haiti and Vietnam had the highest LTBI prevalence in our sample, whereas persons from >20 other countries had a higher US TB incidence rate [27]. Persons from Mexico have a US TB incidence of 10.6 per 100 000 [24] but an LTBI prevalence of 32%. Possible explanations include that persons from some countries enrolled in our study were particularly nonrepresentative in terms of their risk of LTBI or TB disease, or that LTBI prevalence was higher among recent arrivals from certain countries compared with entrants in years past. Of note, >80% of the population in the present study had lived in the US <1 year, which is different from the overall non-US-born population [28].
Our findings can also help clinicians and public health practitioners understand how the test-positive prevalence of TST, QFT, and TSPOT relates to LTBI prevalence among non-US-born populations in the US. The LCA model estimated QFT sensitivity and specificity at 83% and 98%, respectively, compared to 72% and 100% for TSPOT, similar to prior studies [32]. This indicates that estimates of LTBI prevalence in the non-US-born population based on a single IGRA result are likely to underestimate true LTBI prevalence by 20%–30%. The TST had similar sensitivity to the IGRAs (82%), but poorer specificity (71%), likely related to high prevalence of BCG vaccination among non-US-born persons. Thus, when practitioners need to select a test for LTBI in a non-US-born person, these data support the use of an IGRA over a TST.
Our study had several limitations. We used LCA to estimate LTBI prevalence, but as there is no gold standard for LTBI, the accuracy of the estimates cannot be directly verified (although the model outputs correspond well to data obtained from other independent sources). The study population was not a random sample of non-US-born persons, and the proportion of total persons from each country of birth living in the US captured by this study varied widely. Study participants were younger than the overall non-US-born population and were much more likely to be refugees than other recent arrivals. Therefore, LTBI estimates for countries such as Kenya, Malaysia, Nepal, Rwanda, and Thailand likely reflect the LTBI prevalence among the relatively young refugees resettled from these regions, rather than the general population. Because of significant variation in TB incidence within world regions [17], regional LTBI estimates may be inaccurate for persons born in countries with TB incidence rates discordant with the surrounding regions. Finally, 80% of the study population had lived in the US for a year or less. These estimates may not be generalizable to populations who arrived less recently given that TB incidence in many birth countries has decreased over time.
In conclusion, this study fills a gap in our understanding of LTBI prevalence among high-risk non-US-born populations in the United States. This information can inform targeted community outreach efforts to the highest-risk groups.
Supplementary Data
Supplementary materials are available at Clinical Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Notes
Author contributions. J. M. C., the primary author, was involved in design of the study, performed data analysis and interpretation, and drafted the manuscript. J. E. S. was involved with study conception, study design, and data analysis and interpretation and provided critical edits to the manuscript. T. A. was involved with study design and data analysis and interpretation and provided critical edits to the manuscript. A. N. H. was involved with data analysis and interpretation and provided critical edits to the manuscript. D. J. K. was involved with study conception and design and interpretation of the data and provided critical edits to the manuscript. C. S. H. and H. M. B. were involved with study conception and design and interpretation of the data and provided critical edits to the manuscript. K. W. was involved with study oversight and data analysis and interpretation and provided critical edits to the manuscript.
Acknowledgments. The authors thank Sarita Mohanty for her assistance with data retrieval.
Disclaimer. The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the Centers for Disease Control and Prevention (CDC)/Agency for Toxic Substances and Disease Registry or the National Institutes of Health (NIH).
Financial support. This work was supported in part by the CDC Tuberculosis Epidemiologic Studies Consortium and the NIH (grant numbers T32 AI074492 and K23 AI144040 J. M. C.) and the Georgia Clinical and Translational Science Alliance (grant number UL1 TR002378 to H. M. B. and J. M. C.).
Potential conflicts of interest. All authors: No reported conflicts of interest. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest.
References
- 1. Salinas JL, Mindra G, Haddad MB, Pratt R, Price SF, Langer AJ. Leveling of tuberculosis incidence—United States, 2013–2015. MMWR Morb Mortal Wkly Rep 2016; 65:273–8. [DOI] [PubMed] [Google Scholar]
- 2. Schwartz NG, Price SF, Pratt RH, Langer AJ. Tuberculosis—United States, 2019. MMWR Morb Mortal Wkly Rep 2020; 69:286–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Yuen CM, Kammerer JS, Marks K, Navin TR, France AM. Recent transmission of tuberculosis—United States, 2011–2014. PLoS One 2016; 11: e0153728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. LoBue PA, Mermin JH. Latent tuberculosis infection: the final frontier of tuberculosis elimination in the USA. Lancet Infect Dis 2017; 17:e327–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Menzies NA, Parriott A, Shrestha S, et al. Comparative modeling of tuberculosis epidemiology and policy outcomes in California. Am J Respir Crit Care Med 2020; 201:356–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Menzies NA, Wolf E, Connors D, et al. Progression from latent infection to active disease in dynamic tuberculosis transmission models: a systematic review of the validity of modelling assumptions. Lancet Infect Dis 2018; 18:e228–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Tsang CA, Langer AJ, Navin TR, Armstrong LR. Tuberculosis among foreign-born persons diagnosed >/=10 years after arrival in the United States, 2010–2015. MMWR Morb Mortal Wkly Rep 2017; 66:295–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Centers for Disease Control and Prevention. Reported tuberculosis in the United States, 2018. Available at: https://www.cdc.gov/tb/statistics/reports/2018/default.htm. Accessed 29 July 2020. [Google Scholar]
- 9. Bibbins-Domingo K, Grossman DC, Curry SJ, et al. Screening for latent tuberculosis infection in adults: US Preventive Services Task Force recommendation statement. JAMA 2016; 316: 962–9. [DOI] [PubMed] [Google Scholar]
- 10. Lewinsohn DM, Leonard MK, LoBue PA, et al. Official American Thoracic Society/Infectious Diseases Society of America/Centers for Disease Control and Prevention clinical practice guidelines: diagnosis of tuberculosis in adults and children. Clin Infect Dis 2017; 64:111–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Malekinejad M, Parriott A, Viitanen AP, Horvath H, Marks SM, Kahn JG. Yield of community-based tuberculosis targeted testing and treatment in foreign-born populations in the United States: a systematic review. PLoS One 2017; 12:e0180707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Collins JM, Reves RR, Belknap RW. High rates of tuberculosis and opportunities for prevention among international students in the United States. Ann Am Thorac Soc 2016; 13:522–8. [DOI] [PubMed] [Google Scholar]
- 13. Sterling TR, Bethel J, Goldberg S, Weinfurter P, Yun L, Horsburgh CR; Tuberculosis Epidemiologic Studies Consortium . The scope and impact of treatment of latent tuberculosis infection in the United States and Canada. Am J Respir Crit Care Med 2006; 173:927–31. [DOI] [PubMed] [Google Scholar]
- 14. Liu Y, Posey DL, Cetron MS, Painter JA. Effect of a culture-based screening algorithm on tuberculosis incidence in immigrants and refugees bound for the United States: a population-based cross-sectional study. Ann Intern Med 2015; 162:420–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Centers for Disease Control and Prevention. Tuberculosis technical instructions for panel physicians. Available at: https://www.cdc.gov/immigrantrefugeehealth/exams/ti/panel/tuberculosis-panel-technical-instructions.html. Accessed 29 July 2020.
- 16. Centers for Disease Control and Prevention, National Center for Health Statistics. Disclosure manual—preventing disclosure: rules for researchers. 2012. Available at: https://www.cdc.gov/rdc/data/b4/disclosuremanual.pdf. Accessed 29 July 2020.
- 17. World Health Organization. Global tuberculosis report 2019. Available at: https://www.who.int/tb/publications/global_report/en/. Accessed 29 July 2020.
- 18. Centers for Disease Control and Prevention. TBESC research projects. Available at: https://www.cdc.gov/tb/topic/research/tbesc/. Accessed 1 April 2020.
- 19. Cain KP, Benoit SR, Winston CA, Mac Kenzie WR. Tuberculosis among foreign-born persons in the United States. JAMA 2008; 300:405–12. [DOI] [PubMed] [Google Scholar]
- 20. Stout JE, Wu Y, Ho CS, et al. ; Tuberculosis Epidemiologic Studies Consortium . Evaluating latent tuberculosis infection diagnostics using latent class analysis. Thorax 2018; 73:1062–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Limmathurotsakul D, Turner EL, Wuthiekanun V, et al. Fool’s gold: why imperfect reference tests are undermining the evaluation of novel diagnostics: a reevaluation of 5 diagnostic tests for leptospirosis. Clin Infect Dis 2012; 55:322–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Pepe MS, Janes H. Insights into latent class analysis of diagnostic test performance. Biostatistics 2007; 8:474–84. [DOI] [PubMed] [Google Scholar]
- 23. R Core Team. R: a language and environment for statistical computing. 3.4.1 ed. Vienna, Austria: R Foundation for Statistical Computing,2017. [Google Scholar]
- 24. Denwood MJ. runjags: an R package providing interface utilities, model templates, parallel computing methods and additional distributions for MCMC models in JAGS. J Stat Softw 2016; 71:1–25. [Google Scholar]
- 25. Gelman A, Rubin DB. Inference from iterative simulation using multiple sequences. Stat Sci 1992; 7:457–72. [Google Scholar]
- 26..United Nations Statistics Division. Methodology: standard country of area codes for statistical use (M49). Available at: https://unstats.un.org/unsd/methodology/m49/. Accessed 1 April 2020.
- 27. Tsang CA, Langer AJ, Kammerer JS, Navin TR. US tuberculosis rates among persons born outside the United States compared with rates in their countries of birth, 2012–2016. Emerg Infect Dis 2020; 26:533–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. US Census Bureau. American community survey 1-year estimates subject tables. 2018. Available at: https://data.census.gov/cedsci/. Accessed 1 April 2020.
- 29. Miramontes R, Hill AN, Yelk Woodruff RS, et al. Tuberculosis infection in the United States: prevalence estimates from the National Health and Nutrition Examination Survey, 2011–2012. PLoS One 2015; 10:e0140881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Mancuso JD, Diffenderfer JM, Ghassemieh BJ, Horne DJ, Kao TC. The prevalence of latent tuberculosis infection in the United States. Am J Respir Crit Care Med 2016; 194:501–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Bennett DE, Courval JM, Onorato I, et al. Prevalence of tuberculosis infection in the United States population: the National Health and Nutrition Examination Survey, 1999–2000. Am J Resp Crit Care Med 2008; 177:348–55. [DOI] [PubMed] [Google Scholar]
- 32. Pai M, Zwerling A, Menzies D. Systematic review: T-cell-based assays for the diagnosis of latent tuberculosis infection: an update. Ann Intern Med 2008; 149:177–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.