Abstract
Background
Plasma chemokines are biomarkers of greater disease severity, higher bacterial burden, and delayed sputum culture conversion in pulmonary tuberculosis (PTB). Whether plasma chemokines could also serve as biomarkers of unfavorable treatment outcomes in PTB is not known.
Methods
A cohort of newly diagnosed, sputum smear- and culture-positive adults with drug-sensitive PTB were recruited under the Effect of Diabetes on Tuberculosis Severity study in Chennai, India. Plasma chemokine levels measured before treatment initiation were compared between 68 cases with unfavorable outcomes (treatment failure, death, or recurrence) and 136 control individuals who had recurrence-free cure. A second validation cohort comprising newly diagnosed, culture-positive adults with drug-sensitive TB was used to measure plasma chemokine levels in 20 cases and 40 controls.
Results
Six chemokines (CCL2, CCL3, CCL4, CXCL8, CXCL10, and CX3CL1) were associated with increased risk, while CXCL1 was associated with decreased risk of unfavorable outcomes in unadjusted and adjusted analyses in the test cohort. Similarly, CCL3, CXCL8, and CXCL10 were associated with increased risk of unfavorable treatment outcomes in the validation cohort. Receiver operating characteristic analysis revealed that combinations of CCL3, CXCL8, and CXCL10 exhibited very high sensitivity and specificity in differentiating cases vs controls.
Conclusions
Our study reveals a plasma chemokine signature that can be used as a novel biomarker for predicting adverse treatment outcomes in PTB.
Keywords: tuberculosis, treatment outcomes, chemokines
A plasma chemokine signature of CCL3, CXCL8, and CXCL10 was found to be associated with unfavorable tuberculosis treatment outcomes in a test cohort and validated in an independent cohort. Thus, plasma chemokines are baseline predictors of tuberculosis treatment outcomes.
Nonsputum-based biomarkers that predict tuberculosis (TB) treatment response have the potential to target shortened vs intensified regimens for individuals at lower vs higher risk for adverse outcomes, respectively [1–4]. An advantage of blood-based biomarkers is the potential for translation into point-of-care tests that are suitable for use in low-resource settings. Several studies have assessed the role of host immune biomarkers to examine treatment outcomes, mostly in cohorts with small sample sizes that produce results with only moderate translational promise [5, 6]. Sivro et al reported that plasma levels of interleukin 6 (IL-6), CXCL9, and CXCL10 were positively associated with increased rates of recurrence in individuals with pulmonary tuberculosis (PTB) living with human immunodeficiency virus (HIV) [7]. Another study showed that a composite signature based on time to positivity in liquid culture, body mass index (BMI), and plasma levels of tumor necrosis factor–α, sIL-6R, IL-12p40, and CXCL10 predicted relapse with a sensitivity of 75%–83% and specificity of 61%–85% [8]. Both of these studies were conducted at sites in Africa, and it is presently unknown if the results are extensible to non-African populations.
Chemokines play an important role in the orchestration of the immune response during early infection and mediate protective immunity [9]. However, chemokines can also act as a double-edged sword in PTB by driving inflammation that increases lung tissue injury and can promote a pathogen-permissive host environment [10, 11]. We have previously shown that plasma chemokines are biomarkers of greater disease severity, higher bacterial burden, and delayed sputum culture conversion in South Indian PTB individuals [12]. To determine whether chemokines could also serve as predictive biomarkers for unfavorable or adverse PTB treatment outcomes in this population, we leveraged participant samples and clinical data from 2 studies conducted at different time points in Chennai, India.
METHODS
Study Population
Participants for the test cohort were enrolled from the Effect of Diabetes on Tuberculosis Severity study, a prospective cohort study (2014–2019) conducted in Chennai, India [13]. Participants from the validation cohort were enrolled from the study on Immune Responses in Pulmonary Tuberculosis (2008–2012), also conducted in Chennai, India [14]. New smear-positive adults (aged 20–75 years) suspected of having PTB disease were screened. Exclusion criteria were treatment for any prior episode of TB disease, more than 7 days treatment for incident TB, more than 7 doses of a fluoroquinolone within 30 days of screening, drug-resistant Mycobacterium tuberculosis isolate from the enrollment sputum culture, pregnant or nursing, and HIV-seropositive or taking immunosuppressive drugs. Diabetes and low BMI (<18.5 mg/kg2) were exclusion criteria for the validation cohort. The diagnosis of PTB was established by positive sputum culture on solid media (Lowenstein-Jensen media) with compatible chest X ray. TB treatment was managed by government clinics in Chennai according to the Revised National Tuberculosis Control Program standards. Participants were followed monthly through the course of the 6-month treatment and at 3-month intervals thereafter until 1 year after completion of treatment. We conducted a nested case-control study with cases defined as unfavorable treatment outcomes and matched in a 1:2 ratio to controls who were defined as having a recurrence-free cure until the end of study. Cure was defined as negative sputum cultures at months 5 and 6 of treatment without recurrent disease during follow-up. Treatment failure was defined as positive sputum culture at month 5 or 6. Death comprised all-cause mortality during TB treatment. Recurrence was defined as initial cure at month 6 but culture-proven occurrence of disease before the end of the study. There were 18 treatment failures, 16 deaths, and 34 recurrences in the test cohort and 8 failures and 12 recurrences in the validation cohort. Case-control matching was carried out on the basis of age, gender, BMI, and diabetic status. Peripheral blood was collected in heparin tubes. Following centrifugation, plasma was collected and stored at −80ºC until further analysis. Sample collection was performed at baseline (before treatment initiation).
Enzyme-linked Immunosorbent Assay
Plasma levels of chemokines were measured using the Luminex Magpix Multiplex Assay system (Bio-Rad, Hercules, CA). The Luminex Human Magnetic Assay kit 10 Plex (R & D Systems) and Luminex Human Magnetic Assay kit 1 Plex (R & D Systems) were used to measure the chemokine levels. The lowest detection limits were as follows: CCL2, 5.9 pg/mL; CCL3, 5.1 pg/mL; CCL4, 100 pg/mL; CCL5, 297 pg/mL; CCL11, 21.6 pg/mL; CCL19, 4.3 pg/mL; CCL20, 2.4 pg/mL; CXCL1, 21.1 pg/mL; CXCL8, 1.5 pg/mL; CXCL10, 2.6 pg/mL, and CX3CL1, 188 pg/mL.
Statistical Analyses
Geometric means (GMs) were used for measurements of central tendency. Statistically significant differences between cases and control groups were analyzed using the Mann-Whitney test. Receiver operator characteristic (ROC) curves were designed to test the power of each candidate chemokine in order to distinguish cases from controls. Analyses were performed using Graph-Pad PRISM, version 8.0. Univariate and multivariate analyses were performed using Stata v.15.1 (StataCorp, College Station, TX).
Ethics Statement
The test cohort was from a study that was approved by the Prof. M. Viswanathan Diabetes Research Center Ethics Committees and National Institute for Research in Tuberculosis (NIRT). The validation cohort was from a study approved by the NIRT Internal Ethical Committee. Informed written consent was obtained from all participants. All the methods were performed in accordance with the relevant institutional ethical committee guidelines.
RESULTS
Study Population
The original study design of the test cohort included 68 cases and 136 controls. However, the main analysis included 68 cases and 133 controls; 3 controls were missing due to sample unavailability. The median age was 45 years (interquartile range [IQR], 23–65) for cases and 45 years (IQR, 25–73) for controls (P = .268). There were no significant differences in gender, BMI, diabetic status, dyslipidemia, alcohol use, education level, or occupation (Table 1). There were no differences in smear or culture grades or in the presence of cavities at baseline. The control group had a higher number of smokers (either current or former; P = .0345). The validation cohort included 20 cases and 40 controls. The median age was 45 years (IQR, 20–48) for cases and 40 years (IQR, 19–61) for controls (P = .129). There were no significant differences in gender, smear, or culture grades or in the presence of cavities at baseline between the cases and controls (Table 2).
Table 1.
Baseline Demographic and Clinical Characteristics of the Study Population—Test Cohort
| Characteristic | All (n = 201) | Cases (n = 68 [34%]) | Controls (n = 133 [66%]) | P Value |
|---|---|---|---|---|
| Age, median (IQR), y | 45 (23–73) | 45 (23–65) | 45 (25–73) | .2687 |
| Gender | ||||
| Male | 170 (85%) | 60 (88%) | 110 (82%) | .2133 |
| Female | 31 (15%) | 8 (12%) | 23 (18%) | |
| Body mass index, median (IQR), kg/m2 | 17.5 (12.7–30.1) | 16.9 (12.8–25.1) | 17.6 (12.7–30.1) | .1503 |
| Diabetes mellitus status | ||||
| Yes | 116 (58%) | 42 (61%) | 74 (56%) | .4056 |
| No | 85 (42%) | 26 (39%) | 59 (44%) | |
| Chest X-ray score, median (IQR) | 38 (2–130) | 38 (5–130) | 37 (2–125) | .1943 |
| Cavity | ||||
| Yes | 54 (26%) | 18 (26%) | 36 (26%) | .942 |
| No | 118 (59%) | 40 (59%) | 78 (59%) | |
| Unknown | 29 (15%) | 10 (15%) | 19 (15%) | |
| Smear grade | ||||
| 1+ | 126 (63%) | 36 (53%) | 90 (67%) | .0565 |
| 2+ | 67 (33%) | 27 (40%) | 40 (30%) | |
| 3+ | 8 (4%) | 5 (7%) | 3 (3%) | |
| Culture grade | ||||
| 1+ | 86 (43%) | 25 (37%) | 61 (47%) | .1584 |
| 2+ | 36 (18%) | 10 (15%) | 26 (19%) | |
| 3+ | 79 (39%) | 33 (48%) | 46 (34%) | |
| Dyslipidemia | ||||
| Yes | 73 (36%) | 25 (36%) | 48 (36%) | .925 |
| No | 128 (64%) | 43 (64%) | 85 (64%) | |
| Smoking | ||||
| Yes, current smoker | 61 (30%) | 28 (41%) | 33 (25%) | .0345 |
| Yes, former smoker | 40 (20%) | 14 (21%) | 26 (20%) | |
| No, never | 100 (50%) | 26 (38%) | 74 (55%) | |
| Alcohol use | ||||
| Yes, current alcohol use | 105 (52%) | 39 (58%) | 66 (50%) | .466 |
| Yes, former alcohol use | 38 (19%) | 13 (19%) | 25 (18%) | |
| No, never | 58 (29%) | 16 (23%) | 42 (32%) | |
| Education | ||||
| Educated | 160 (80%) | 53 (78%) | 107 (80%) | .6761 |
| Uneducated | 41 (20%) | 15 (22%) | 26 (20%) | |
| Occupation | ||||
| Unemployed | 14 (7%) | 4 (6%) | 10 (7%) | .5642 |
| Unskilled worker | 102 (51%) | 40 (59%) | 62 (47%) | |
| Skilled worker | 53 (26%) | 16 (24%) | 37 (28%) | |
| Business/professional | 11 (6%) | 3 (4%) | 8 (6%) | |
| Retired/housewife | 21 (10%) | 5 (7%) | 16 (12%) |
Abbreviation: IQR, interquartile range.
Table 2.
Baseline Demographic and Clinical Characteristics of the Study Population—Validation Cohort
| Characteristic | All (n = 60) | Cases (n = 20 [34%]) | Controls (n = 40 [66%]) | P Value |
|---|---|---|---|---|
| Age, median (interquartile range), y | 45 (19–61) | 45 (20–48) | 40 (19–61) | .1297 |
| Gender | ||||
| Male | 51 (85%) | 19 (95%) | 32 (80%) | .1250 |
| Female | 9 (15%) | 1 (5%) | 8 (20%) | |
| Cavity | ||||
| Yes | 27 (45%) | 10 (50%) | 17 (43%) | .5820 |
| No | 33 (55%) | 10 (50%) | 23 (57%) | |
| Smear grade | ||||
| 1+ | 22 (36%) | 7 (35%) | 15 (38%) | .9743 |
| 2+ | 21 (35%) | 7 (35%) | 14 (35%) | |
| 3+ | 17 (29%) | 6 (30%) | 11 (27%) | |
| Culture grade | ||||
| 1+ | 20 (33%) | 7 (35%) | 13 (33%) | .9277 |
| 2+ | 20 (33%) | 7 (35%) | 13 (33%) | |
| 3+ | 20 (33%) | 6 (30%) | 14 (34%) |
Baseline Plasma Chemokine Predictors of Unfavorable Treatment Outcomes in the Test Cohort
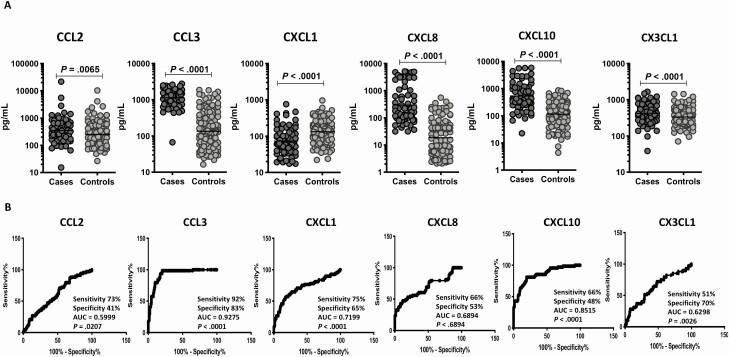
To elucidate the baseline levels of plasma chemokines in cases and controls in the test cohort, we measured the production of CC and CXC chemokines at pretreatment (Figure 1 and Supplementary Figure 1). As shown in Figure 1A, plasma levels of CCL2 (GM of 348.8 pg/mL in cases vs 251.5 pg/mL in controls), CCL3 (GM of 1122 pg/mL in cases vs 135.1 pg/mL in controls), CXCL8 (GM of 294.4 pg/mL in cases vs 18.6 pg/mL in controls), and CXCL10 (GM of 534.6 pg/mL in cases vs 115.7 pg/mL in controls) were significantly higher in cases compared with controls, while the plasma levels of CXCL1 (GM of 73.3 pg/mL in cases vs 133 pg/mL in controls) were significantly lower in cases. There were no significant differences in the plasma levels of CCL4, CCL5, CCL11, CCL19, and CCL20. To define the plasma chemokines that correlate with unfavorable treatment outcomes, we performed univariate and multivariate conditional regression analyses, with the latter correcting for age, gender, BMI, diabetic status, smoking, alcohol use, presence of cavity, smear and culture status, and occupation (Table 3). Univariate analysis showed that CCL2 (odds ratio [OR], 1.26; 95% confidence interval [CI], 1.03–1.54; P = .025), CCL3 (OR, 4.41; 95% CI, 2.84–6.83; P < .001), CCL4 (OR, 1.30; 95% CI, 1.11–1.52; P = .001), CXCL8 (OR, 2.71; 95% CI, 1.99–3.68; P < .001), and CXCL10 (OR, 2.91; 95% CI, 2.10–4.03; P < .001) were associated with increased rates of unfavorable treatment outcomes, while CXCL1 (OR, 0.50; 95% CI, .37–.67; P < .001) was associated with decreased rates of unfavorable treatment outcomes. Multivariate analysis showed that CCL2 (adjusted OR [aOR], 1.26; 95% CI, 1.02–1.56; P = .035), CCL3 (aOR, 4.89; 95% CI, 2.99–7.99; P < .001), CCL4 (aOR, 1.36; 95% CI, 1.15–1.61; P < .001), CXCL8 (aOR, 2.84; 95% CI, 2.05–3.92; P < .001), CXCL10 (aOR, 2.95; 95% CI, 2.10–4.15; P < .001), and CX3CL1 (aOR, 1.70; 95% CI, 1.15–2.53; P = .008) were still associated with significantly increased risk of unfavorable treatment outcomes, while CXCL1 (aOR, 0.45; 95% CI, .33–.63; P < .001) was still associated with significantly decreased risk of unfavorable outcomes. Finally, we performed an ROC analysis to determine the specificity and sensitivity of plasma chemokines in distinguishing cases and controls (Figure 1B). As shown, CCL3 (area under the curve [AUC] = 0.9275; sensitivity, 92%; specificity, 83%), CXCL1 (AUC = 0.7199; sensitivity, 75%; specificity, 65%), and CXCL10 (AUC = 0.8515; sensitivity, 66%; specificity, 48%) all exhibited AUC higher than 0.7. Thus, these 3 markers afforded fair to high accuracy in predicting unfavorable treatment outcomes in TB in the test cohort.
Figure 1.
Elevated baseline plasma levels of chemokines in cases in the test cohort. A, The baseline plasma levels of chemokines were measured in cases (n = 68) and controls (n = 133). The data are represented as scatter plots, with each circle representing a single individual. P values were calculated using the Mann-Whitney test with Holm’s correction for multiple comparisons. Only chemokines that showed significant P values are shown. B, Receiver operator characteristic analysis to estimate the sensitivity, specificity, and AUC was performed using chemokines to estimate the capacity of these factors to distinguish cases vs controls. Abbreviation: AUC, area under the curve.
Table 3.
Association of the Biomarker With the Treatment Outcomes at Baseline
| Univariate Model | Multivariate Model | |||
|---|---|---|---|---|
| Marker | OR (95% CI) | P Value | Adjusted ORa (95% CI) | P Value |
| CCL2 | 1.26 (1.03–1.54) | .025 | 1.26 (1.02–1.56) | .035 |
| CCL3 | 4.41 (2.84–6.83) | <.001 | 4.89 (2.99–7.99) | <.001 |
| CCL4 | 1.30 (1.11–1.52) | .001 | 1.36 (1.15–1.61) | <.001 |
| CCL5 | 1.17 (.84–1.62) | .351 | 1.18 (.83–1.66) | .359 |
| CCL11 | 0.89 (.69–1.14) | .357 | 0.89 (.69–1.17) | .407 |
| CCL19 | 1.18 (.96–1.45) | .109 | 1.18 (.95–1.46) | .142 |
| CCL20 | 0.97 (.77–1.21) | .775 | 0.97 (.77–1.23) | .830 |
| CXCL1 | 0.50 (.37–.67) | <.001 | 0.45 (.33–.63) | <.001 |
| CXCL8 | 2.71 (1.99–3.68) | <.001 | 2.84 (2.05–3.92) | <.001 |
| CXCL10 | 2.91 (2.10–4.03) | <.001 | 2.95 (2.10–4.15) | <.001 |
| CX3CL1 | 1.65 (1.14–2.38) | .008 | 1.70 (1.15–2.53) | .008 |
Abbreviations: CI, confidence interval; OR, odds ratio.
aMultivariate logistic regression models study the association of biomarker with treatment outcomes (unfavorable) and are adjusted for age in years, gender, body mass index, diabetes status, smoking status, alcohol status, and smear grading.
Baseline Plasma Chemokine Predictors of Unfavorable Treatment Outcomes in the Validation Cohort
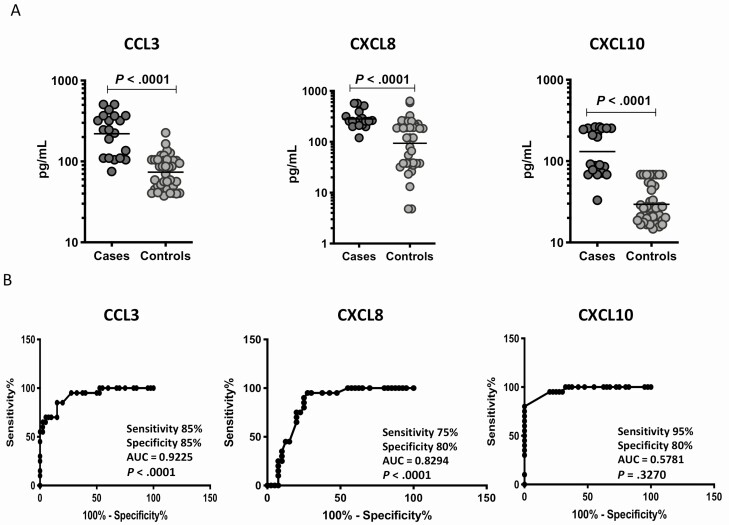
To elucidate the baseline levels of plasma chemokines in cases and controls in the validation cohort, we measured the production of CC and CXC chemokines at pretreatment (Figure 2 and Supplementary Figure 2). As shown in Figure 2A, plasma levels of CCL3 (GM of 219.7 pg/mL in cases vs 73.7 pg/mL in controls), CXCL8 (GM of 281.3 pg/mL in cases vs 93.5 pg/mL in controls), and CXCL10 (GM of 131.1 pg/mL in cases vs 29.4 pg/mL in controls) were significantly higher in cases compared with controls. There were no significant differences in the plasma levels of CCL2, CCL4, CCL5, CCL11, CCL19, CCL20, CXCL1, and CX3CL1. Next, we performed an ROC analysis to determine the specificity and sensitivity of plasma chemokines in distinguishing cases and controls (Figure 2B). As shown, only CXCL10 (AUC = 0.9694; sensitivity, 95%; specificity, 80%) exhibited AUC higher than 0.9. Thus, CXCL10 afforded fair to high accuracy in predicting unfavorable treatment outcomes in TB in the validation cohort.
Figure 2.
Elevated baseline plasma levels of chemokines in cases in the validation cohort. A, The baseline plasma levels of chemokines were measured in cases (n = 20) and controls (n = 40). The data are represented as scatter plots, with each circle representing a single individual. P values were calculated using the Mann-Whitney test with Holm’s correction for multiple comparisons. Only chemokines that showed significant P values are shown. B, Receiver operator characteristic analysis to estimate the sensitivity, specificity, and AUC was performed using chemokines to estimate the capacity of these factors to distinguish cases vs controls. Abbreviation: AUC, area under the curve.
Baseline Plasma Chemokines Are Biomarkers for Unfavorable Treatment Outcomes in PTB
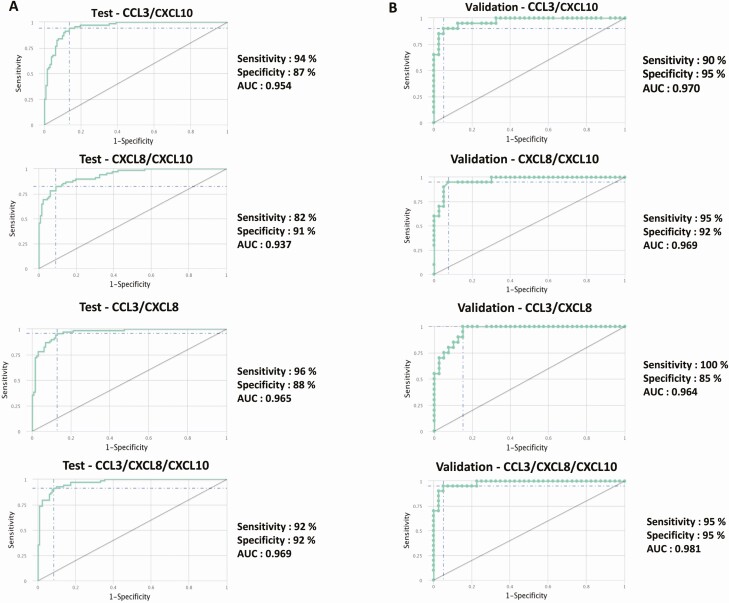
To determine if we could derive a signature of plasma chemokines that could be used as a biomarker for cases vs controls, we performed a combined ROC analysis of chemokines in both the test and validation cohorts. As shown in Figure 3A, ROC analysis of different combinations of CCL3, CXCL8, and CXCL10 exhibited a high AUC (0.937–0.969) with high sensitivity (82%–96%) and specificity (87%–92%) in differentiating cases and controls in the test cohort. Similarly, as shown in Figure 3B, the same combinations of chemokines also exhibited a high AUC (0.964–0.981) with high sensitivity (90%–100%) and specificity (85%–95%) in differentiating cases and controls in the validation cohort. Thus, plasma chemokine signatures comprising CCL3, CXCL8, and CXCL10 are highly predictive biomarkers of unfavorable treatment outcomes in PTB.
Figure 3.
Identification of biomarkers showing the strongest association using a combination of chemokines in active tuberculosis disease. The combination of receiver operator characteristic (ROC) model analysis shows the chemokines that exhibited the highest accuracy in discriminating cases and controls. ROC curves for comparing multiple markers and their combinations between cases vs controls in the test cohort (A) and validation cohort (B) are shown. Abbreviation: AUC, area under the curve.
Baseline Plasma Chemokines Are Also Biomarkers for Individual Treatment Outcomes in PTB
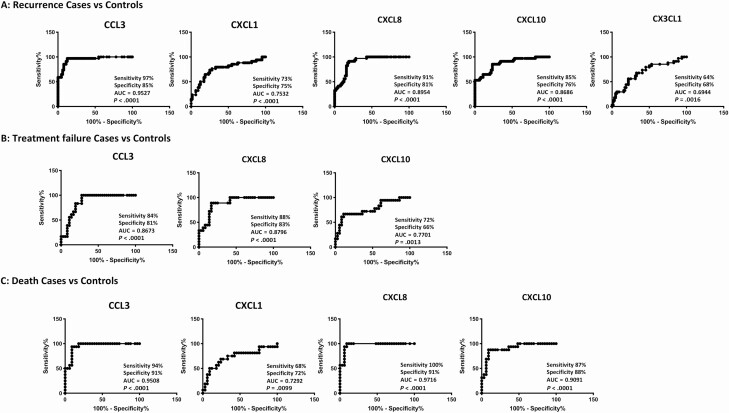
To determine if we could derive a signature of plasma chemokines that could be used as a biomarker for individual treatment outcomes (TB recurrence, TB relapse, and mortality), we performed ROC analysis of chemokines. As shown in Figure 4A, ROC analysis of CCL3 (AUC = 0.8673), CXCL1 (AUC = 0.7532), CXCL8 (AUC = 0.8954), CXCL10 (AUC = 0.8686), and CXCL11 (AUC = 0.6944) exhibited increased sensitivity and specificity in differentiating TB recurrence vs recurrence-free cure. Similarly, as shown in Figure 4B, ROC analysis of CCL3 (AUC = 0.9527), CXCL8 (AUC = 0.8796), and CXCL10 (AUC = 0.7701) exhibited increased sensitivity and specificity in differentiating TB treatment failure vs cure. Finally, as shown in Figure 4C, ROC analysis of CCL3 (AUC = 0.9508), CXCL1 (AUC = 0.7292), CXCL8 (AUC = 0.9716), and CXCL10 (AUC = 0.9091) exhibited increased sensitivity and specificity in differentiating mortality during TB treatment vs survival. Thus, different plasma chemokines serve as biomarkers of individual treatment outcomes in PTB.
Figure 4.
Plasma chemokines are biomarkers of individual treatment outcomes in active tuberculosis (TB) disease. A, Receiver operator characteristic (ROC) analysis to estimate the sensitivity, specificity, and AUC was performed using chemokines to estimate the capacity of chemokines to distinguish TB recurrence vs recurrence-free cure. Only the chemokines that showed significant P values in discrimination are shown. B, ROC analysis to estimate the sensitivity, specificity, and AUC was performed using chemokines to estimate the capacity of chemokines to distinguish treatment failure vs cure. Only the chemokines that showed significant P values in discrimination are shown. C, ROC analysis to estimate the sensitivity, specificity, and AUC was performed using chemokines to estimate the capacity of chemokines to distinguish mortality during TB treatment vs survival. Only the chemokines that showed significant P values in discrimination are shown. Abbreviation: AUC, area under the curve.
DISCUSSION
Unfavorable treatment outcomes comprising bacteriological failure, death, and TB recurrence are a major obstacle to global TB elimination [15]. Shortening the duration of chemotherapy would greatly strengthen TB control programs, but the lack of biomarkers that predict treatment response remains a roadblock [5, 6]. For example, the rate of recurrence is highly variable and has been estimated to range from 4.9% to 47% [16]. This variability is related to differences in regional epidemiology of recurrence and differences in the definitions used by the TB control programs. Therefore, there is a great need for biomarkers measured at treatment initiation that discriminate those at highest risk for adverse outcomes from those who will be cured using the standard 6-month regimen and possibly by shortened regimens. Identifying individuals at high risk of treatment failure at the time of diagnosis would allow the efficient application of intensified monitoring during and after standard treatment and define a population most likely to benefit from novel intensified or extended regimens, including using higher doses of standard antimicrobials, adding additional antimicrobials, or using adjunctive host-directed therapies [5, 6]. Finally, mortality during TB treatment is a major concern that might be lessened if at-risk individuals could be identified at baseline and offered more intensive treatment and follow-up.
Our current study takes advantage of one of the largest collections of samples from unfavorable treatment outcomes to identify baseline predictors of such outcomes. We provide evidence that several plasma chemokines appear to present with important differences in cases and controls such that they provide additional value to clinical and bacteriological parameters in identifying individuals at risk of failure, recurrence, or death. Previous studies have mainly relied on clinical and bacteriological parameters (including baseline time to positivity in culture and month 2 culture status) to predict unfavorable treatment outcomes [17–19]. However, these parameters do not exhibit high specificity and sensitivity in prediction outcomes [20]. In our study, we examined a variety of CC and CXC family of chemokines in order to determine the differences in the kinetics of expression in cases vs controls. We demonstrate that 6 chemokines (CCL2, CCL3, CCL4, CXCL8, CXCL10, and CX3CL1) is associated with increased risk of unfavorable treatment outcomes, while CXCL1 was associated with a decreased risk in the test cohort and that 3 of the same chemokines (CCL3, CXCL8, and CXCL10) are associated with a similar risk in the validation cohort. Our study also shows that 3 chemokines (CCL3, CXCL1, and CXCL10) exhibit a high degree of sensitivity and specificity in predicting unfavorable treatment outcomes at baseline in both the test and validation cohorts. Moreover, we also explored the utility of chemokines as biomarkers for individual treatment outcomes, namely, TB recurrence, treatment failure, or mortality during TB treatment. Our data clearly show the potential utility of plasma chemokine signatures as biomarkers for individual treatment outcomes as well.
Unfavorable treatment outcomes can be influenced by a variety of factors, including adherence and the presence of comorbidities such as diabetes, smoking, alcohol use, malnutrition, occupation status, and HIV status [5, 6]. In our study, we matched the cases and controls for age, gender, malnutrition, and diabetes and performed multivariate conditional regression analysis to account for the other factors. In addition, we also performed analysis to demonstrate that smear/culture grade, presence of cavity, or severity of lung pathology (as determined by chest X-ray scores) were not significantly different between the 2 groups and did not influence the outcomes of our results. Our study innovates by using a pure population of culture-positive, newly diagnosed, drug-sensitive, HIV-negative TB patients and therefore provides biomarkers that could be widely applied to different populations. Also, we only relied on culture confirmation of treatment failure and recurrence; therefore, our results are based on the gold standard of microbiological confirmation and not mere clinical or radiological confirmation as observed in other studies. Plasma chemokines have been previously described to be promising biomarkers for disease severity, bacterial burden, and delayed sputum culture conversion in PTB [12]. Moreover, plasma chemokines play a pivotal part in orchestrating the innate and adaptive immune response to TB, including the establishing and maintaining the granulomas, dictating the progression of disease, and potentially influencing transmission [21]. Thus, chemokines have both positive and negative effects on the pathogenesis of TB [10]. We are the first (to our knowledge) to reveal the importance of chemokines as predictors of unfavorable treatment outcomes.
Our study does suffer from the limitation of being performed in a validation cohort of moderate sample size, of both the testing and validating the cohort in the same geographical region, of not including individuals living with HIV, and of including all unfavorable outcomes under 1 umbrella. Also, more studies in a broader population are needed before we can conclude on the utility of these biomarkers for future use. Nevertheless, our study provides a promising first step in the process of unraveling the effect of novel nonsputum-based prognostic disease biomarkers in TB. Future validation of these findings in an adequately sized, prospective, cross-sectional, diagnostic trial would then provide an incentive to translate the results to a simple point-of-care rapid diagnostic test for treatment, shortening trials and other studies.
Supplementary Data
Supplementary materials are available at Clinical Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Notes
Acknowledgments. The authors thank the staff of the Department of Clinical Research, Department of Socio-Behavioral Research, and Department of Bacteriology, National Institute for Research in Tuberculosis, for valuable assistance with patient recruitment, bacterial cultures, and radiology. Also, they thank the staff of Prof. M. Viswanathan Diabetes Research Center, Revised National Tuberculosis Control Programme, and Chennai Corporation for valuable assistance in recruiting patients for this study. Data for this study were collected as part of the Regional Prospective Observational Research for Tuberculosis (RePORT) India Consortium.
Disclaimer. The contents of this publication are solely the responsibility of the authors and do not represent the official views of the DBT, ICMR, NIH, or CRDF Global.
Financial support. This project was funded, in whole or in part, with federal funds from the government of India’s Department of Biotechnology (DBT), the Indian Council of Medical Research (ICMR), the US National Institutes of Health (NIH), National Institute of Allergy and Infectious Diseases (NIAID), Office of AIDS Research, and distributed in part by CRDF Global (grant number USB1-31149-XX-13). This work is also funded by CRDF Global RePORT India Consortium supplemental funding (grant number OISE-17-62911-1). This work was also funded, in part, by the Division of Intramural Research, NIAID, NIH.
Potential conflicts of interest. The authors: No reported conflicts of interest. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest.
References
- 1. Walzl G, McNerney R, du Plessis N, et al. Tuberculosis: advances and challenges in development of new diagnostics and biomarkers. Lancet Infect Dis 2018; 18:e199–210. [DOI] [PubMed] [Google Scholar]
- 2. Walzl G, Ronacher K, Hanekom W, Scriba TJ, Zumla A. Immunological biomarkers of tuberculosis. Nat Rev Immunol 2011; 11:343–54. [DOI] [PubMed] [Google Scholar]
- 3. Wallis RS, Kim P, Cole S, et al. Tuberculosis biomarkers discovery: developments, needs, and challenges. Lancet Infect Dis 2013; 13:362–72. [DOI] [PubMed] [Google Scholar]
- 4. Wallis RS, Maeurer M, Mwaba P, et al. Tuberculosis—advances in development of new drugs, treatment regimens, host-directed therapies, and biomarkers. Lancet Infect Dis 2016; 16:e34–46. [DOI] [PubMed] [Google Scholar]
- 5. Goletti D, Lindestam ArlehamnCS, Scriba TJ, et al. Can we predict tuberculosis cure? What tools are available? Eur Respir J 2018;52:1801089. [DOI] [PubMed] [Google Scholar]
- 6. Rockwood N, du Bruyn E, Morris T, Wilkinson RJ. Assessment of treatment response in tuberculosis. Expert Rev Respir Med 2016; 10:643–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Sivro A, McKinnon LR, Yende-Zuma N, et al. Plasma cytokine predictors of tuberculosis recurrence in antiretroviral-treated human immunodeficiency virus-infected individuals from Durban, South Africa. Clin Infect Dis 2017; 65:819–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Ronacher K, Chegou NN, Kleynhans L, et al. Distinct serum biosignatures are associated with different tuberculosis treatment outcomes. Tuberculosis (Edinb) 2019; 118:101859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Kang DD, Lin Y, Moreno JR, Randall TD, Khader SA. Profiling early lung immune responses in the mouse model of tuberculosis. PLoS One 2011; 6:e16161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Monin L, Khader SA. Chemokines in tuberculosis: the good, the bad and the ugly. Semin Immunol 2014; 26:552–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Dorhoi A, Kaufmann SH. Pathology and immune reactivity: understanding multidimensionality in pulmonary tuberculosis. Semin Immunopathol 2016; 38:153–66. [DOI] [PubMed] [Google Scholar]
- 12. Kumar NP, Moideen K, Nancy A, et al. Plasma chemokines are biomarkers of disease severity, higher bacterial burden and delayed sputum culture conversion in pulmonary tuberculosis. Sci Rep 2019; 9:18217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Kornfeld H, West K, Kane K, et al. High prevalence and heterogeneity of diabetes in patients with TB in South India: a report from the effects of diabetes on tuberculosis severity (EDOTS) study. Chest 2016; 149:1501–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Kumar NP, Gopinath V, Sridhar R, et al. IL-10 dependent suppression of type 1, type 2 and type 17 cytokines in active pulmonary tuberculosis. PLoS One 2013; 8:e59572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Pai M, Behr MA, Dowdy D, et al. Tuberculosis. Nat Rev Dis Primers 2016; 2:16076. [DOI] [PubMed] [Google Scholar]
- 16. Mirsaeidi M, Sadikot RT. Patients at high risk of tuberculosis recurrence. Int J Mycobacteriol 2018; 7:1–6. [DOI] [PubMed] [Google Scholar]
- 17. Wallis RS, Wang C, Meyer D, Thomas N. Month 2 culture status and treatment duration as predictors of tuberculosis relapse risk in a meta-regression model. PLoS One 2013; 8:e71116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Hesseling AC, Walzl G, Enarson DA, et al. Baseline sputum time to detection predicts month two culture conversion and relapse in non-HIV-infected patients. Int J Tuberc Lung Dis 2010; 14:560–70. [PubMed] [Google Scholar]
- 19. Bark CM, Thiel BA, Johnson JL. Pretreatment time to detection of Mycobacterium tuberculosis in liquid culture is associated with relapse after therapy. J Clin Microbiol 2012; 50:538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Horne DJ, Royce SE, Gooze L, et al. Sputum monitoring during tuberculosis treatment for predicting outcome: systematic review and meta-analysis. Lancet Infect Dis 2010; 10:387–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Slight SR, Khader SA. Chemokines shape the immune responses to tuberculosis. Cytokine Growth Factor Rev 2013; 24:105–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.