Abstract
Background
Most US children with perinatal hepatitis C virus (HCV) exposure fail to receive the recommended anti-HCV antibody test at age ≥18 months. Earlier testing for viral RNA might facilitate increased screening, but sensitivity of this approach has not been established. We hypothesized that modern HCV-RNA RT-PCR platforms would adequately detect infected infants.
Methods
Nationwide Children’s Hospital electronic health records from 1/1/2008 to 30/6/2018 were reviewed to identify perinatally exposed infants tested by HCV-RNA RT-PCR at age 2–6 months. Diagnostic performance was determined using a composite case definition: (1) infected children had positive repeat HCV-RNA testing or positive anti-HCV at age ≥24 months; (2) uninfected children lacked these criteria and had negative anti-HCV at age ≥18 months.
Results
770 perinatally exposed infants underwent HCV-RNA testing at age 2–6 months. Of these, 28 (3.6%) tested positive; viremia was confirmed in all who underwent repeat testing (n = 27). Among 742 infants with negative HCV-RNA results, 226 received follow-up anti-HCV testing at age ≥18 months, of whom 223 tested negative. Three children had low-positive anti-HCV results at age 18–24 months that were negative upon retesting after age 24 months, possibly indicating waning maternal antibodies. Using the composite case definitions, early HCV-RNA screening demonstrated sensitivity of 100% (87.5–100%, Wilson-Brown 95% CI) and specificity of 100% (98.3–100%).
Conclusions
Modern HCV-RNA RT-PCR assays have excellent sensitivity for early diagnosis of perinatally acquired infection and could aid HCV surveillance given the substantial loss to follow-up at ≥18 months of age.
Keywords: hepatitis C virus, vertical transmission, perinatal infection, nucleic acid amplification test
Hepatitis C virus (HCV)-RNA testing during infancy could improve screening rates for children born to infected mothers, but diagnostic sensitivity is not established. We found excellent sensitivity of HCV-RNA reverse transcription–polymerase chain reaction performed at age 2–6 months for detection of perinatally acquired infection.
(See the Editorial Commentary by Feld and Matthews on pages e3347–8.)
Perinatal exposure to hepatitis C virus (HCV) is increasingly common in the United States due to the ongoing opioid epidemic [1–7]. Children born to viremic mothers face an approximate 6% risk of acquiring HCV infection [8], and 60–75% of infected children go on to establish chronic infection [9–11]. Although liver disease due to HCV progresses more slowly in children than adults [12], perinatally infected children face substantial risk for late complications, with up to one-third developing cirrhosis by their mid-30s [13].
Diagnosis of persistent HCV infection allows access to curative therapies that can avert long-term complications of HCV and prevent future transmission events. The American Academy of Pediatrics and others advisory bodies recommend testing all perinatally exposed infants for anti-HCV immunoglobulin G (IgG) antibody at age 18 months or older, after loss of transplacentally acquired maternal antibodies [14, 15]. However, numerous population-based studies have shown that only 10–30% of exposed infants receive this testing [16–20]. Even at centers with dedicated screening programs for exposed infants, rates of anti-HCV testing at age 18 months fail to surpass 50% [21–23].
Serum or plasma HCV-RNA testing can identify infected children before age 18 months and has been incorporated in some protocols to augment screening efforts [23]. However, the sensitivity of HCV-RNA testing is not well established in infants. A prior European study found that reverse transcription–polymerase chain reaction (RT-PCR) testing of HCV RNA had a sensitivity of 22% at birth, 79% at 1 month of age, 75% at 3 months, and 85% at 6 months [24]. Extremely poor sensitivity at birth suggested that some cases were acquired near delivery [25], while suboptimal sensitivity at later time points was thought to reflect intermittent low-level viremia [26]. The median lower limit of detection (LLOD) for PCR assays used in the European study was 150 copies/mL [24]. Newer real-time RT-PCR assays have improved analytical sensitivity [27], with LLOD down to 15 copies/mL, and may thus have better diagnostic sensitivity during early infancy. Given improved RT-PCR assays and our perception that HCV-exposed children were not being adequately tested, starting in 2015 we issued guidance to local providers advising HCV-RNA RT-PCR testing at age 2–6 months in addition to routine anti-HCV testing at 18 months or older. Here we assessed the diagnostic performance of these RT-PCR assays, hypothesizing that they would accurately detect HCV infection at age 2–6 months, thus providing a practical means to improve identification and follow-up of children with perinatally acquired HCV infection.
METHODS
Subjects and Data Sources
Nationwide Children’s Hospital (NCH) laboratory data were queried to identify all infants who underwent HCV-RNA RT-PCR testing at age 2–6 months from 1 January 2008 to 30 June 2018. Infants were included regardless of specialty or location of the ordering provider. Follow-up HCV-RNA and anti-HCV antibody results were collected through 30 June 2019 for case classification.
Indications for infant HCV-RNA testing, mother–infant demographic characteristics, and maternal HCV status were assessed by review of infant and maternal electronic health records and Ohio Department of Health (ODH) surveillance data. Infants were considered HCV exposed if (1) “perinatal HCV exposure” or “maternal HCV infection” was listed in the infant NCH medical record, (2) maternal HCV infection was recorded on the infant ODH birth certificate, or (3) a positive HCV test result (antibody or RNA) was documented for the mother prior to delivery or through 90 days postpartum in the ODH Ohio Disease Reporting System [6] or The Ohio State University Wexner Medical Center (OSUWMC) electronic health record. Infants tested for HCV-RNA due to perinatal HCV exposure were included in the study, whereas those tested for other or unknown reasons were excluded. This study was conducted with the approval of the institutional review boards of NCH, OSUWMC, and ODH.
HCV–RNA Assays
Clinical HCV-RNA RT-PCR assays conducted during the study period (1 January 2008 to 30 June 2018) used Cobas Taqman RT-PCR reagents (Roche) and included 2 types of tests: (1) a qualitative assay with LLOD of 50 IU/mL offered at NCH from 2008 to 2014 and (2) quantitative assays with LLOD of 15–75 IU/mL offered through the Associated Regional and University Pathologists reference laboratory (ARUP, Salt Lake City, UT) from 2008 to 2018. ARUP transitioned to a transcription-mediated amplification (TMA) HCV-RNA assay with an LLOD of 10 IU/mL in October 2018. The TMA results from October 2018 through June 2019 were included for follow-up case classification.
Anti-HCV IgG Antibody Assay
Anti-HCV testing conducted at NCH used the Abbott AxSYM Anti-HCV microparticle enzyme immunoassay from January 2008 to February 2012 and the Abbott Architect Anti-HCV chemilluminescent assay after February 2012. The NCH electronic health records were manually reviewed for anti-HCV results imported from outside facilities to maximize assessment of follow-up anti-HCV testing.
Case Definitions
Case definitions for perinatal HCV infection were derived from composite endpoints incorporating HCV-RNA and anti-HCV testing. Perinatal HCV infection was present if children had a (1) positive repeat HCV-RNA test after the initial positive screen or (2) positive anti-HCV test after age 24 months. Perinatal HCV infection was absent if children (1) had no documented viremia after the initial screen and (2) had a documented negative anti-HCV test after age 18 months. Children with repeatedly negative HCV-RNA testing but no follow-up antibody testing to confirm lack of perinatal infection were defined as having “presumed absence” of perinatal HCV infection. Cases with discrepant anti-HCV antibody test results over time were resolved by consideration of antibody signal-to-cutoff ratios and repeat HCV-RNA test results.
Statistical Analyses
Diagnostic characteristics of HCV-RNA RT-PCR screening were examined in comparison to the case definitions. Sensitivity was calculated as the proportion of infected children whose first HCV-RNA RT-PCR at age 2–6 months was positive. Specificity was calculated as the proportion of children without perinatal HCV infection whose first HCV-RNA RT-PCR at age 2–6 months was negative. The 95% confidence intervals (CIs) for sensitivity and specificity were determined using the Wilson-Brown method [28]. Point estimates and 95% CIs for positive and negative likelihood ratios (LR+ and LR−) were estimated by an empirical bootstrap approach developed by Marill et al [29] for use when sample sensitivities or specificities approach 100%. Calculations were derived using the mean of 10 runs of the “bootLR” program (R package version 1.0.2 accessed at https://abfriedman.shinyapps.io/bootLRshiny/), each of which represented the mean of 5 bootstrapping procedures of at least 10 000 random samplings. Incomplete study follow-up precluded determination of the true prevalence of perinatal HCV infection in our cohort. Estimates and 95% CIs for positive-predictive values (PPVs) and negative-predictive values (NPVs) were calculated by applying the LR+ and LR− 95% CI bounds to 2 different pre-test odds: (1) the prevalence reported in the literature by Benova et al [8] for children born to viremic mothers and (2) an estimated prevalence in our study population using HCV-RNA RT-PCR screening results (positive screens/total infants tested). Features of infants with and without follow-up antibody testing were compared using chi-square tests for categorical variables and Mann-Whitney U tests for continuous variables (SPSS version26; IBM Corporation). Finally, temporal trends in HCV-RNA testing and detection were explored in secondary analyses.
RESULTS
Perinatally Exposed Cohort
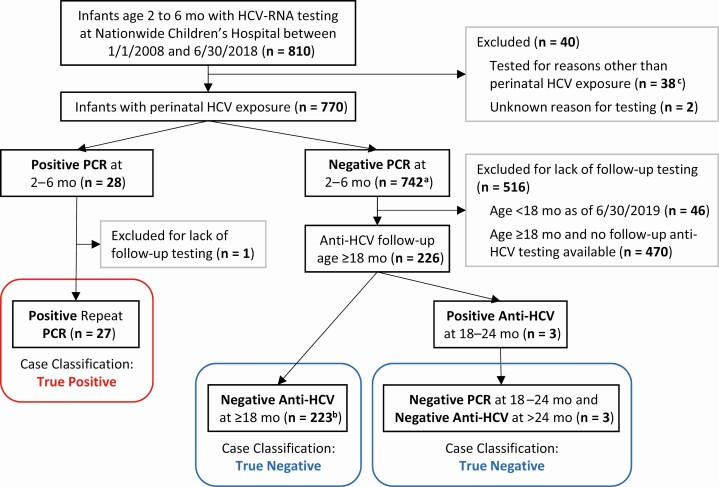
From January 2008 through June 2018, 810 infants ages 2 to 6 months underwent HCV testing by RT-PCR at the NCH laboratory (Figure 1). Of these, 770 (95.1%) were classified as perinatally exposed and included in the study. In total, 3.6% (28/770) of the HCV-exposed infants tested positive by PCR at 2–6 months of age, while the remaining 742 infants had negative PCR test results. This includes 2 infants with initial “indeterminate” PCR results who had negative PCR results on subsequent testing of a separate sample. Among the subset of children born to mothers with documented HCV viremia reported to the ODH in the year prior to delivery or up to 90 days postpartum, 4.7% (16/341) tested positive for HCV-RNA at age 2–6 months.
Figure 1.
Follow-up and case classification of infants tested for HCV-RNA at age 2–6 mo. aIncludes 2 children with indeterminate HCV-RNA RT-PCR results that were negative upon repeat testing. bIncludes 2 children with indeterminate anti-HCV antibody results that were negative upon repeat testing. cReasons for testing included: unexplained liver disease in infant (n = 22), other infant medical condition (n = 1), high-risk pregnancy with unknown maternal HCV status (n = 14), potential postnatal HCV exposure (n = 1). Abbreviations: HCV, hepatitis C virus; mo, month; RT-PCR, reverse transcription–polymerase chain reaction.
The median age at initial HCV-RNA RT-PCR screening for all 770 infants was 3.3 months (interquartile range [IQR]: 2.9–4.1 months; age distribution shown in Supplementary Figure 1). Over half (51%) of the infants resided in Franklin County, but the cohort included infants from 59 Ohio counties, 2 Kentucky counties, and 4 West Virginia counties. HCV-RNA testing was ordered by NCH infectious disease providers in two-thirds of cases, with the remainder ordered by a variety of NCH and non-NCH primary care and specialty providers (Supplementary Figure 2).
Follow-up After a Positive HCV-RNA RT-PCR Screening
Repeat HCV-RNA testing was performed within a median of 48 days (IQR, 33–113 days) following a positive PCR screen for 27 of the 28 infants, all of whom had confirmed HCV viremia. The median HCV-RNA level at screening was 5.7 × 106 IU/mL (IQR, 2.2 × 106–2.4 × 107 IU/mL) (Supplementary Figure 3). Anti-HCV testing was positive in all viremic children who underwent antibody testing after age 18 months (n = 12).
Follow-up After Negative HCV-RNA RT-PCR Screening
Of 742 perinatally exposed infants with a negative HCV-RNA RT-PCR screen at age 2–6 months, 696 had reached age 18 months by 30 June 2019. Despite this, only 226 (32%) children had documentation in NCH health records of the recommended anti-HCV antibody testing at age 18 months or older. Anti-HCV tests were negative in 223 (30%) of these children, confirming the absence of perinatal HCV infection.
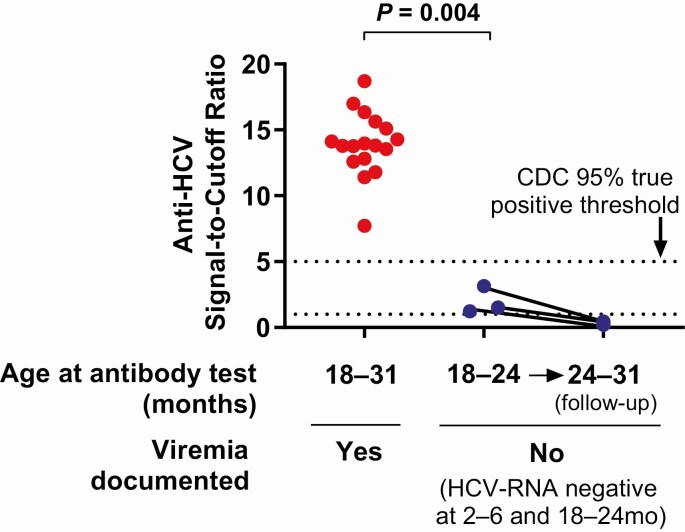
Three children tested positive for anti-HCV at age 18–24 months after a negative HCV-RNA screen at age 2–6 months. In each case, repeat HCV-RNA RT-PCR testing was negative at 18–24 months and anti-HCV was negative when retested after age 24 months. These cases were ultimately classified as uninfected based on lack of viremia and loss of anti-HCV antibody after age 24 months (Figure 1). Given the discrepant test results, the initial positive anti-HCV tests of these 3 children were further reviewed. The positive anti-HCV tests in these 3 children had signal-to-cutoff (s/co) ratios ranging from 1.24 to 3.15, below the threshold of 5.0 or less established by the Centers for Disease Control and Prevention (CDC) for the Abbott Architect anti-HCV CMIA assay to predict a probability of 95% or higher of a true-positive result [30]. These anti-HCV s/co ratios were also well below those of 17 children, ages 18–31 months, with history of HCV viremia (s/co ratio range, 7.72–18.7; P = .004) (Figure 2), including 2 who had resolved viremia prior to antibody testing (s/co ratios of 11.4 and 12.6).
Figure 2.
Comparison of anti-HCV signal-to-cutoff ratios for “positive” anti-HCV tests in HCV-exposed children with (n = 17) and without (n = 3) documented HCV viremia (Mann-Whitney U test). Abbreviations: CDC, Centers for Disease Control and Prevention; HCV, hepatitis C virus.
Approximately two-thirds (470/696) of HCV-RNA–negative children who were aged 18 months or older lacked follow-up antibody testing through the NCH (Figure 1). Repeat HCV-RNA tests available for 58 of these children were uniformly negative. Additionally, review of pediatric HCV cases reported to the ODH from 2007 to 2018 indicated that none of the 470 children had been found to have HCV infection by testing performed elsewhere in Ohio. Lack of follow-up antibody testing among HCV-RNA–negative infants was associated with residence in counties farther away from NCH (P < .001) and HCV-RNA testing outside the infectious diseases program (P < .01), but not maternal viremia or other maternal/infant demographic features examined (Supplementary Table 1).
Performance of HCV-RNA RT-PCR for Detecting Perinatal HCV Infection
Using the composite case definitions, there were 27 true-positive, 0 false-positive, 226 true-negative, and 0 false-negative HCV-RNA RT-PCR results at 2–6 months (Figure 1). The sensitivity and specificity of HCV-RNA RT-PCR testing, with 95% CIs (Wilson-Brown), were calculated to be 100% (87.5–100%) and 100% (98.3–100%), respectively (Table 1). Likelihood ratios for positive and negative screening tests, with 95% CIs calculated by bootstrapping [29], were ∞ (78.0–∞) and 0 (0–.103), respectively (Table 1). Estimates for PPVs and NPVs based on these LR+ and LR− values are given in Table 1 for 2 different assumed rates of vertical transmission: (1) 5.8% risk documented by Benova et al [8] for infants born to viremic mothers and (2) 3.6% (28/770) risk estimated in our study (which did not require documentation of maternal viremia during pregnancy).
Table 1.
Diagnostic Performance of HCV-RNA RT-PCR in Perinatally Exposed Infants Age 2–6 Months
| Point Estimate | 95% CI | |
|---|---|---|
| Sensitivity, (%)a | 100 | 87.5–100 |
| Specificity, (%)a | 100 | 98.3–100 |
| Positive likelihood ratiob | ∞ | 78.0–∞ |
| Negative likelihood ratiob | 0 | 0–.103 |
| Assume 5.8% prevalencec | ||
| Positive-predictive value, (%)d | 100 | 82.8–100 |
| Negative-predictive value, (%)d | 100 | 99.4–100 |
| Assume 3.6% prevalencee | ||
| Positive-predictive value, (%)d | 100 | 74.5–100 |
| Negative-predictive value, (%)d | 100 | 99.6–100 |
Data are presented as percentages unless otherwise indicated.
Abbreviations: CI, confidence interval; HCV, hepatitis C virus; RT-PCR, reverse transcription–polymerase chain reaction.
aCIs determined by Wilson-Brown method.
bCIs determined by bootstrapping method of Marill et al [29].
cRisk of perinatal HCV transmission from viremic mothers (Benova et al [8]).
dPredictive values and CIs derived by application of likelihood ratios to the pre-test odds associated with the given prevalence.
eEstimated risk of perinatal HCV transmission in this study cohort of infants tested due to history of maternal HCV infection (without requirement for documented maternal viremia during pregnancy).
Temporal Trends in HCV Screening
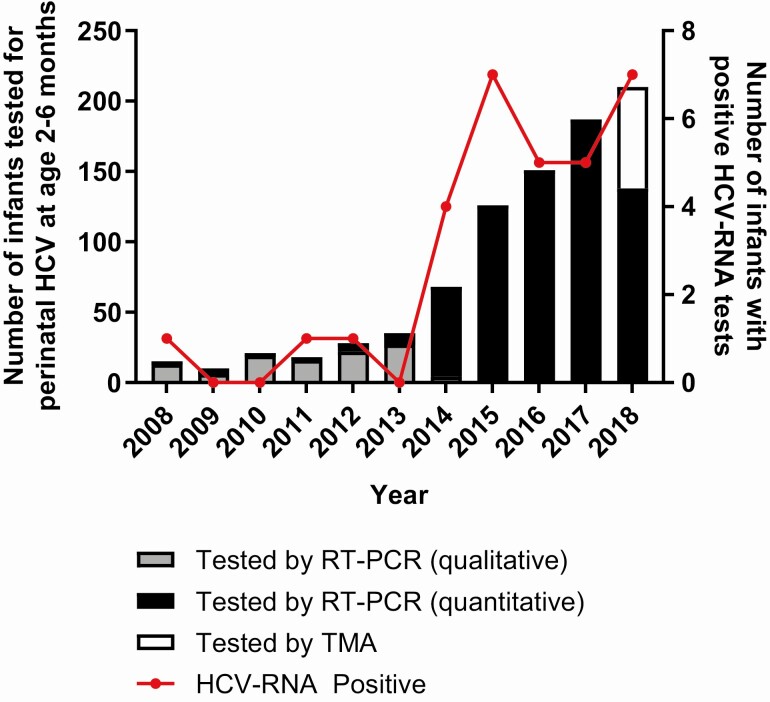
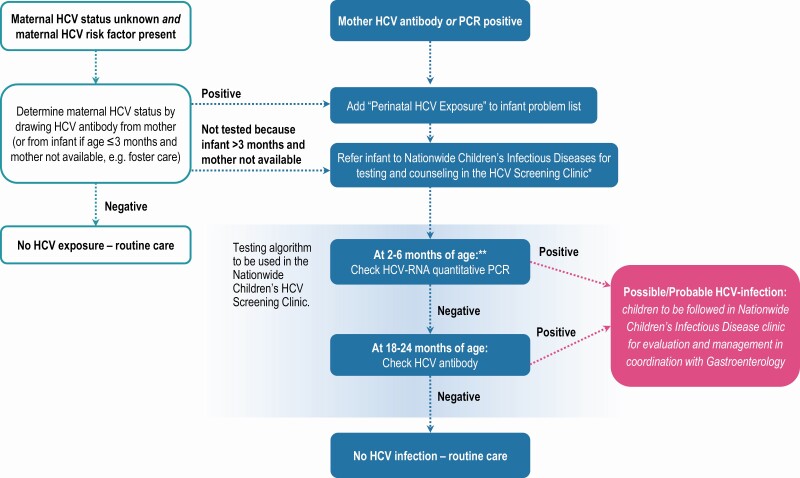
Screening for and identification of perinatal HCV infection by HCV-RNA testing in young infants increased 10-fold during the study period, from an average of 16 infants screened and 0.5 infections identified annually in 2008–2011 to over 200 infants screened and 7 infections identified in 2018 (Figure 3). This increase in infant HCV-RNA testing coincided with a period of increasing maternal HCV prevalence in Ohio [6], which, along with concerns for inadequate screening in exposed children, prompted issuance of local guidance in 2015 that exposed infants be tested for HCV-RNA at 2–6 months and anti-HCV at 18–24 months (Figure 4).
Figure 3.
Annual number of perinatally exposed infants tested (bars) and positive (red line) for HCV-RNA at age 2–6 months, 2008–2018. HCV-RNA–positive infants (red line) plotted here had either documentation of positive repeat HCV-RNA testing or HCV-RNA level ≥100 IU/m: in cases where follow-up HCV-RNA testing was not available. Abbreviations: HCV, hepatitis C virus; RT-PCR, reverse transcription– polymerase chain reaction; TMA, transcription-mediated amplification.
Figure 4.
Guidance issued to community providers in 2015 for testing infants with perinatal HCV exposure. This study includes infants tested for HCV-RNA by their primary care providers or by the Nationwide Children’s Hospital HCV Screening Clinic. *If referral for screening is not feasible, please test infant per algorithm and refer any infant who tests positive. **HCV-RNA quantitative PCR preferred at 2–6 months of age but can be done at >6 to <18 months of age if infant presents after age 6 months. Abbreviations: HCV, hepatitis C virus; PCR, polymerase chain reaction.
DISCUSSION
In this sample of over 750 perinatally HCV-exposed infants, HCV-RNA RT-PCR screening at age 2–6 months demonstrated excellent sensitivity and specificity for the diagnosis of HCV infection. There were no apparent false-positive or false-negative RT-PCR results at 2–6 months among 253 infants who had sufficient follow-up for case classification (27 confirmed true-positive and 226 true-negative), yielding a test sensitivity of 100% (95% CI, 87.5–100%) and specificity of 100% (95% CI, 98.3–100%; Wilson-Brown). Assuming a transmission rate of 5.8% for children born to HCV-viremic mothers [8], and using the calculated LR− and LR+ 95% CI bounds (Table 1), a negative HCV-RNA RT-PCR test at age 2–6 months confers a probability of 99.4% or higher of not being infected, and a positive test confers a probability of 82.8% or higher of infection. Taken together, these data indicate that a single HCV-RNA RT-PCR test at this stage of infancy may reliably rule out perinatal HCV infection.
HCV-RNA testing during early infancy had better diagnostic sensitivity in our study than reported in several prior studies. Thomas et al [31] reported a sensitivity of 89% (90% CI, 80–95%) by age 3 months, and Polywka et al [24] reported a sensitivity of 75% (95% CI, 63–85%) at age 3 months and 85% (95% CI, 75–93%) at age 6 months. We had hypothesized that modern HCV-RNA RT-PCR assays would better identify infected young infants due to advancements in analytical sensitivity that now permit detection of very low level viremia [27]. However, all infected infants in our cohort had high viral loads at 2–6 months, ranging from 3.7 × 104 to 6.3 × 107 IU/mL (Supplementary Figure 3), well above the detection threshold of older PCR assays [24]. Thus, the better diagnostic performance in our study may relate to other test characteristics, such as improved reliability and reproducibility of modern real-time RT-PCR techniques that are less susceptible to human error than prior gel-based RT-PCR assays [24, 31].
Differing case classification methodologies may also account for heterogeneous results in studies of HCV-RNA diagnostic performance. Detection of anti-HCV IgG at age 18 months or older is a commonly referenced standard for diagnosis of perinatal HCV infection. However, transplacentally acquired maternal antibodies remain detectable by enzyme immunoassay beyond 18 months of age in a small proportion of exposed-uninfected infants (eg, 1.5% in Mast et al [32]) and could contribute to misclassification of true disease states. To minimize this possibility, we used a composite endpoint requiring either the presence of anti-HCV antibodies beyond age 24 months or confirmed viremia to define infection. Three children with negative HCV-RNA screens at age 2–6 months tested positive for anti-HCV at 18–24 months and then negative for anti-HCV after age 24 months. These children were classified as HCV-uninfected by our definition but might have been classified as infected in earlier studies. We suspect that their positive anti-HCV tests at 18–24 months reflected either residual maternal antibody or simply false-positive anti-HCV results [33]. In support of this interpretation, the s/co ratios of the “positive” anti-HCV tests for these 3 children at 18–24 months were below the CDC’s 95% true-positive threshold [30] and below the ratios of children with proven infection (Figure 2). These findings suggest that s/co ratios should be considered when interpreting anti-HCV results in perinatally exposed children, as in adults [30].
The ability to assess for perinatal HCV infection earlier than the recommended 18-month antibody screen could have important clinical and public health benefits. Our study and others have documented dismally low rates of anti-HCV testing for exposed children [16, 17, 19–22, 34]. It is conceivable that the long delay from birth to 18 months, coupled with socioeconomic factors that lead to fragmented guardianship and healthcare, contribute to the low rates of anti-HCV testing. Screening as early as 2 months of age may substantially mitigate these barriers. Importantly, follow-up rates were high for infants in our study with a positive HCV-RNA screen at 2–6 months (96.4%), likely due to greater concern and attention among healthcare providers and caregivers. Thus, early identification of HCV in infancy may be associated with high probability of linkage to care for follow-up and eventual treatment with direct-acting antiviral regimens now available for chronically infected children aged 3 years and older [35]. Finally, the excellent predictive value of negative HCV-RNA testing at 2–6 months could aid health department efforts to conduct perinatal HCV surveillance, allowing focus of limited resources on the 3–5% of infants with positive screens and infants with no testing.
This study does have several limitations. The low rate of follow-up anti-HCV testing among infants with negative 2–6-month HCV-RNA tests (32%, 226/696), although illustrative of real-world challenges, substantially reduced the sample size for analysis. Receipt of follow-up antibody testing was linked to household proximity to the NCH and initial HCV-RNA testing by infectious diseases providers, but not factors expected to skew perinatal transmission risk or assessment of HCV-RNA assay performance. Second, given the low perinatal transmission rate, there were only 27 children with confirmed infection, resulting in relatively wide 95% CIs for test sensitivity (87.5–100%) despite no observed missed cases. Third, our study assessed the performance of Cobas Taqman HCV-RNA RT-PCR testing of infants ages 2–6 months at a single center. Future studies are warranted to evaluate the diagnostic performance of other modern HCV-RNA or antigen assays at time points across infancy and at additional sites.
In conclusion, we demonstrated that HCV-RNA RT-PCR testing performed well as a test for perinatal HCV infection in young infants aged 2–6 months. Further studies are needed to validate this approach in other populations of HCV-exposed infants and assess the potential impact and cost-effectiveness of diagnostic strategies incorporating infant HCV-RNA testing versus those employing 18-month antibody testing alone. As the possibility of curative therapy for HCV infection in early childhood is now upon us, the need for a practical and highly reliable strategy to identify perinatally infected children cannot be more emphasized.
Supplementary Data
Supplementary materials are available at Clinical Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Notes
Acknowledgments . The authors thank Sarah Koster for assistance querying NCH data. We also thank ODH staff for providing Vital Statistics and Ohio Disease Reporting System data. Use of these data should not be considered an endorsement of this study or its conclusions by ODH.
Financial support. This work was supported by the National Institutes of Health (grant number R01-AI096882 to J. R. H. and grant number UL1TR002733 from the National Center for Advancing Translational Sciences to The Ohio State University), and the Abigail Wexner Research Institute at Nationwide Children’s Hospital.
Potential conflicts of interest. P. J. S. has received grants from Merck, outside the submitted work; J. R. H. has received grants and nonfinancial support from Gilead, outside the submitted work. All other authors report no potential conflicts. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1. Koneru A, Nelson N, Hariri S, et al. Increased hepatitis C virus (HCV) detection in women of childbearing age and potential risk for vertical transmission—United States and Kentucky, 2011–2014. MMWR Morb Mortal Wkly Rep 2016; 65:705–10. [DOI] [PubMed] [Google Scholar]
- 2. Patrick SW, Bauer AM, Warren MD, Jones TF, Wester C. Hepatitis C virus infection among women giving birth—Tennessee and United States, 2009–2014. MMWR Morb Mortal Wkly Rep 2017; 66:470–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Ko JY, Haight SC, Schillie SF, Bohm MK, Dietz PM. National trends in hepatitis C infection by opioid use disorder status among pregnant women at delivery hospitalization–United States, 2000–2015. MMWR Morb Mortal Wkly Rep 2019; 68:833–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Zibbell JE, Iqbal K, Patel RC, et al. Increases in hepatitis C virus infection related to injection drug use among persons aged ≤30 years—Kentucky, Tennessee, Virginia, and West Virginia, 2006–2012. MMWR Morb Mortal Wkly Rep 2015; 64:453–8. [PMC free article] [PubMed] [Google Scholar]
- 5. Suryaprasad AG, White JZ, Xu F, et al. Emerging epidemic of hepatitis C virus infections among young nonurban persons who inject drugs in the United States, 2006–2012. Clin Infect Dis 2014; 59:1411–9. [DOI] [PubMed] [Google Scholar]
- 6. Gowda C, Kennedy S, Glover C, Prasad MR, Wang L, Honegger JR. Enhanced identification of maternal hepatitis C virus infection using existing public health surveillance systems. Paediatr Perinat Epidemiol 2018; 32:401–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Ly KN, Jiles RB, Teshale EH, Foster MA, Pesano RL, Holmberg SD. Hepatitis C virus infection among reproductive-aged women and children in the United States, 2006 to 2014. Ann Intern Med 2017; 166:775–82. [DOI] [PubMed] [Google Scholar]
- 8. Benova L, Mohamoud YA, Calvert C, Abu-Raddad LJ. Vertical transmission of hepatitis C virus: systematic review and meta-analysis. Clin Infect Dis 2014; 59:765–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. European Paediatric Hepatitis C Virus Network. Three broad modalities in the natural history of vertically acquired hepatitis C virus infection. Clin Infect Dis 2005; 41:45–51. [DOI] [PubMed] [Google Scholar]
- 10. Garazzino S, Calitri C, Versace A, et al. Natural history of vertically acquired HCV infection and associated autoimmune phenomena. Eur J Pediatr 2014; 173:1025–31. [DOI] [PubMed] [Google Scholar]
- 11. Indolfi G, Easterbrook P, Dusheiko G, et al. Hepatitis C virus infection in children and adolescents. Lancet Gastroenterol Hepatol 2019; 4:477–87. [DOI] [PubMed] [Google Scholar]
- 12. Mohan P, Colvin C, Glymph C, et al. Clinical spectrum and histopathologic features of chronic hepatitis C infection in children. J Pediatr 2007; 150:168–74, 74 e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Modin L, Arshad A, Wilkes B, et al. Epidemiology and natural history of hepatitis C virus infection among children and young people. J Hepatol 2019; 70:371–8. [DOI] [PubMed] [Google Scholar]
- 14. Kimberlin DW, Brady MT, Jackson MA, Long SS. Red book 2018 report of the Committe on Infections Diseases [hepatitis C]. 31st ed. Elk Grove Village, Illinois, USA: American Academy of Pediatrics, 2018. [Google Scholar]
- 15. Mack CL, Gonzalez-Peralta RP, Gupta N, et al. ; North American Society for Pediatric Gastroenterology, Hepatology, and Nutrition . NASPGHAN practice guidelines: diagnosis and management of hepatitis C infection in infants, children, and adolescents. J Pediatr Gastroenterol Nutr 2012; 54:838–55. [DOI] [PubMed] [Google Scholar]
- 16. Kuncio DE, Newbern EC, Johnson CC, Viner KM. Failure to test and identify perinatally infected children born to hepatitis C virus-infected women. Clin Infect Dis 2016; 62:980–5. [DOI] [PubMed] [Google Scholar]
- 17. Watts T, Stockman L, Martin J, Guilfoyle S, Vergeront JM. Increased risk for mother-to-infant transmission of hepatitis C virus among Medicaid recipients—Wisconsin, 2011–2015. MMWR Morb Mortal Wkly Rep 2017; 66:1136–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Delgado-Borrego A, Smith L, Jonas MM, et al. Expected and actual case ascertainment and treatment rates for children infected with hepatitis C in Florida and the United States: epidemiologic evidence from statewide and nationwide surveys. J Pediatr 2012; 161:915–21. [DOI] [PubMed] [Google Scholar]
- 19. Chappell CA, Hillier SL, Crowe D, Meyn LA, Bogen DL, Krans EE. Hepatitis C virus screening among children exposed during pregnancy. Pediatrics 2018; 141:e20173273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Lopata SM, McNeer E, Dudley JA, et al. Hepatitis C testing among perinatally exposed infants. Pediatrics 2020; 145:e20192482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Towers CV, Fortner KB. Infant follow-up postdelivery from a hepatitis C viral load positive mother. J Matern Fetal Neonatal Med 2019; 32:3303–5. [DOI] [PubMed] [Google Scholar]
- 22. Epstein RL, Sabharwal V, Wachman EM, et al. Perinatal transmission of hepatitis C virus: defining the cascade of care. J Pediatr 2018; 203:34–40 e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Abughali N, Maxwell JR, Kamath AS, Nwankwo U, Mhanna MJ. Interventions using electronic medical records improve follow up of infants born to hepatitis C virus infected mothers. Pediatr Infect Dis J 2014; 33:376–80. [DOI] [PubMed] [Google Scholar]
- 24. Polywka S, Pembrey L, Tovo PA, Newell ML. Accuracy of HCV-RNA PCR tests for diagnosis or exclusion of vertically acquired HCV infection. J Med Virol 2006; 78:305–10. [DOI] [PubMed] [Google Scholar]
- 25. Mok J, Pembrey L, Tovo PA, Newell ML; European Paediatric Hepatitis C Virus Network . When does mother to child transmission of hepatitis C virus occur? Arch Dis Child Fetal Neonatal Ed 2005; 90:F156–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Tovo PA, Pembrey LJ, Newell ML. Persistence rate and progression of vertically acquired hepatitis C infection. European Paediatric Hepatitis C Virus Infection. J Infect Dis 2000; 181:419–24. [DOI] [PubMed] [Google Scholar]
- 27. Jhaveri R, Hashem M, El-Kamary SS, et al. Hepatitis C virus (HCV) vertical transmission in 12-month-old infants born to HCV-infected women and assessment of maternal risk factors. Open Forum Infect Dis 2015; 2:ofv089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Brown LD, Cai TT, DasGupta A, et al. Interval estimation for a binomial proportion—comment–rejoinder. Stat Sci 2001; 16:101–33. [Google Scholar]
- 29. Marill KA, Chang Y, Wong KF, Friedman AB. Estimating negative likelihood ratio confidence when test sensitivity is 100%: a bootstrapping approach. Stat Methods Med Res 2017; 26:1936–48. [DOI] [PubMed] [Google Scholar]
- 30. Kamili S, Drobeniuc J, Araujo AC, Hayden TM. Laboratory diagnostics for hepatitis C virus infection. Clin Infect Dis 2012; 55:S43–8. [DOI] [PubMed] [Google Scholar]
- 31. Thomas SL, Newell ML, Peckham CS, Ades AE, Hall AJ. Use of polymerase chain reaction and antibody tests in the diagnosis of vertically transmitted hepatitis C virus infection. Eur J Clin Microbiol Infect Dis 1997; 16:711–9. [DOI] [PubMed] [Google Scholar]
- 32. Mast EE, Hwang LY, Seto DS, et al. Risk factors for perinatal transmission of hepatitis C virus (HCV) and the natural history of HCV infection acquired in infancy. J Infect Dis 2005; 192:1880–9. [DOI] [PubMed] [Google Scholar]
- 33. Moorman AC, Drobenuic J, Kamili S. Prevalence of false-positive hepatitis C antibody results, National Health and Nutrition Examination Study (NHANES) 2007–2012. J Clin Virol 2017; 89:1–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Jhaveri R. We need a new national strategy for hepatitis C virus screening. Pediatrics 2020; 145:e20193060. [DOI] [PubMed] [Google Scholar]
- 35. Schwarz KB, Rosenthal P, Murray KF, et al. Ledipasvir-sofosbuvir for 12 weeks in children 3 to <6 years old with chronic hepatitis C. Hepatology 2020; 71:422–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.