Abstract
Background
There are large knowledge gaps on the transmission dynamics of Mycobacterium tuberculosis in settings where both tuberculosis and human immunodeficiency virus (HIV) are endemic. We aimed to assess the infectiousness of tuberculosis patients coinfected with HIV.
Methods
We systematically searched for studies of contacts of both HIV-positive and HIV-negative tuberculosis index cases. Our primary outcome was Mycobacterium tuberculosis infection in contacts. Data on sputum smear and lung cavitation status of index cases were extracted from each study to assess effect modification. Secondary outcomes included prevalent tuberculosis and HIV in contacts of HIV-positive and HIV-negative index cases.
Results
Of 5255 original citations identified, 32 studies met inclusion criteria, including 25 studies investigating M. tuberculosis infection (Nparticipants = 36 893), 13 on tuberculosis (Nparticipants = 18 853), and 12 on HIV positivity (Nparticipants = 18 424). Risk of M. tuberculosis infection was lower in contacts of HIV-positive index cases (odds ratio [OR], 0.67, 95% confidence interval [CI], .58–.77) but was heterogeneous (I2 = 75.1%). Two factors modified this relationship: the lung cavitary status of the index case and immunosuppression (measured through CD4 counts or HIV or acquired immunodeficiency syndrome diagnoses) among index people living with HIV. Rates of HIV were consistently higher in contacts of coinfected index cases (OR, 4.9; 95% CI, 3.0–8.0). This was modified by whether the study was in sub-Saharan Africa (OR, 2.8; 95% CI, 1.6–4.9) or in another global region (OR, 9.8; 95% CI, 5.9–16.3).
Conclusions
Tuberculosis patients coinfected with HIV are less infectious than HIV-uninfected cases when they have severe immunosuppression or paucibacillary disease. Contacts of coinfected index cases are almost 5 times more likely to also have HIV.
Keywords: human immunodeficiency virus, tuberculosis, infectiousness, transmission dynamics
Tuberculosis patients coinfected with human immunodeficiency virus (HIV) are less infectious than HIV-uninfected people when they have severe immunosuppression or paucibacillary disease. Contacts of coinfected index cases are almost 5 times more likely to also have HIV.
(See the Editorial Commentary by Kendall on pages e3456–8.)
The coepidemic of tuberculosis and human immunodeficiency virus (HIV) began more than 3 decades ago and has led to millions of deaths, the vast majority of which occur in impoverished settings with few health resources [1–3]. Individuals living with HIV are at high risk for both primary progressive disease and reactivation disease [1, 4]; moreover, people with HIV who develop tuberculosis experience accelerated HIV disease progression and severity [5, 6]. Because of this, morbidity and mortality of both diseases are substantial. The heavy toll of tuberculosis and HIV continues because of ongoing Mycobacterium tuberculosis transmission, from both HIV-seropositive and HIV-seronegative tuberculosis cases. But our understanding about transmission in the context of HIV infection is incomplete.
There are large gaps of knowledge regarding how transmission of M. tuberculosis occurs in settings where both HIV and tuberculosis are endemic [1]. The infectiousness of people with tuberculosis coinfected with HIV is unclear. A previous meta-analysis on the infectiousness of HIV-positive people with tuberculosis concluded that coinfected patients were as infectious as people who are HIV-negative and have tuberculosis after pooling 4 studies with tuberculin skin test results from HIV-negative contacts [7]. This meta-analysis displayed substantial between-study heterogeneity and was unable to explain this variability because of few available studies [7–9]. A reassessment of the infectiousness of people with tuberculosis and HIV is needed to improve our understanding of M. tuberculosis transmission dynamics where both HIV and tuberculosis are burdensome and to inform health policy decision-making.
To fill this knowledge gap, we conducted a systematic review and meta-analysis of studies evaluating M. tuberculosis infection rates in contacts of HIV-positive and HIV-negative index cases. We attempted to explain heterogeneity by stratification when possible. We also evaluated the risk of coprevalent tuberculosis and HIV-positivity in contacts exposed to HIV-positive and HIV-negative index cases.
METHODS
Search Strategy and Study Selection
This review followed the Preferred Reporting Items for Systematic Reviews and Meta-Analyses statement (Supplementary Appendix). The study protocol is registered with the International Prospective Register of Systematic Reviews (protocol #CRD42019120273). Briefly, our search strategy aimed to identify all studies that assessed the number of M. tuberculosis infections, tuberculosis, or HIV found when contact investigation was done and stratified by the HIV status of the index case. We searched journal articles of any study design in Medline, Web of Science, Biosis, and Embase electronic databases. The search was conducted with the help of a librarian database consultant and was conducted until October 2014. An identical search was then conducted for the time period of October 2014 through August 2018. We used the following search terms, adapted for each database when appropriate: “Mycobacterium tuberculosis,” tuberculosis, TB, “human immunodeficiency virus,” HIV, tuberculin, transmission, contact*. We did not restrict articles by publication date or language. Bibliographies of reviews, both systematic and descriptive, were also searched and evaluated for eligibility [1, 7, 9–15].
After the search and exclusion of duplicate articles, 2 authors (L.M. and H.W.) independently screened articles by title, abstract, and full text. If reviewers disagreed about inclusion, a consensus of authors determined manuscript eligibility. Corresponding authors were contacted for additional data if a study met eligibility criteria but exact counts were unavailable. From each article, information was collected on the study, index cases, and from contacts. We were unable to extract study-level data on outcomes from 1 eligible study [16] because only adjusted relative risks were presented in the manuscript. We therefore used an online database of this cohort from another published study [17] to re-create measures of association for the differing outcomes.
Study Definitions
Exposure
Exposure was defined as close contact, either in the same household or through the community, to a tuberculosis case. This was defined by each set of authors. We stratified by level of tuberculosis exposure (household vs community). We did not include studies with exposed healthcare workers as a previous meta-analysis [7] did because these individuals have highly distinct populations compared with other types of contacts and are at high-risk for repeated exposures and reinfections. Index cases were considered source cases and were eligible if diagnosis was confirmed either bacteriologically (sputum smear or culture positive) or radiographically.
Outcome
The primary outcome was M. tuberculosis infection in contacts of HIV-positive and HIV-negative index cases. Studies using the tuberculin skin test or an interferon gamma assay to diagnose M. tuberculosis infection were included. Various tuberculin induration cutoffs were used to define a positive tuberculin skin test. We used each study’s specified definition for a positive tuberculin skin test. A cutoff of 5- and 10-mm induration reactions to define a positive tuberculin skin test were used in 9 and 10 studies. Three studies used an induration of 10 mm or greater for HIV-negative contacts and 5 mm or greater for HIV-positive contacts. One study used QuantiFERON Gold In-Tube tests [18] and a cutoff of ≥0.35 IU/mL was used to define a positive test.
Secondary outcomes included tuberculosis and HIV in contacts of HIV-positive and HIV-negative index cases. Tuberculosis was defined as the presence of tuberculosis regardless of tuberculin skin test results. Most studies used microbiological confirmation of diseased contacts, whereas some studies used a combination of symptom screening, radiographical examinations, and tuberculin skin test results. Two studies did not specify the diagnostic method used [19, 20]. The discretion of each study’s authors to define tuberculosis was used.
HIV in contacts was defined as a positive laboratory test for HIV. The study was ineligible if HIV status was obtained through participant self-report alone. Several HIV tests (enzyme-linked immunosorbent assay [ELISA], Western blot, Determine, and Unigold) were used and we used each study’s definition of a positive HIV test.
Assessment of Study Quality
The quality of each eligible study was assessed. We used a modified version of the Newcastle-Ottawa scale for assessment of observational studies. Studies were evaluated based on 3 characteristics: adequate selection of participants (4 points), comparability of studies based on the design and analysis (1 point), and adequate ascertainment of study outcomes (3 points). This scale awards a maximum of 8 points to each study. We defined studies of high quality as those that scored ≥66.6%, moderate for 33.3%–66.6%, and low for those <33.3%.
Statistical Analysis
We estimated the odds ratio (OR) for M. tuberculosis infection and disease in contacts of HIV-positive and HIV-negative tuberculosis people for each study. We then pooled these ORs using a random effects model with DerSimonian and Laird weights. We used a random effects model, equalizing the weight of the studies to the pooled estimate, a priori because we included only observational studies [21]. We stratified the analysis by prespecified characteristics of eligible studies when available. These included contact characteristics (age, HIV status, bacillus Calmette–Guérin [BCG] vaccination status), index case characteristics (lung cavitary disease, sputum smear status, extrapulmonary disease, HIV immunosuppression, cough duration), and study characteristics (year of implementation, region, closeness of tuberculosis contact [ie, household or community]).
95% confidence intervals (CI) were used to assess statistical significance in all models. We used the I2 statistic to evaluate heterogeneity between studies [22, 23]. The I2 statistic represents the proportion of variability in included studies resulting from heterogeneity alone rather than random error. A threshold of I2 > 50% was used as indicating statistically significant heterogeneity. We assessed publication bias through inspecting funnel plot symmetry and by using the Harbord test for publication bias [24, 25]. All statistical analyses were performed with Stata, version 14.0 (StataCorp LP, College Station, Texas, USA) and R statistical software (R Foundation for Statistical Computing).
RESULTS
Study Selection and Characteristics
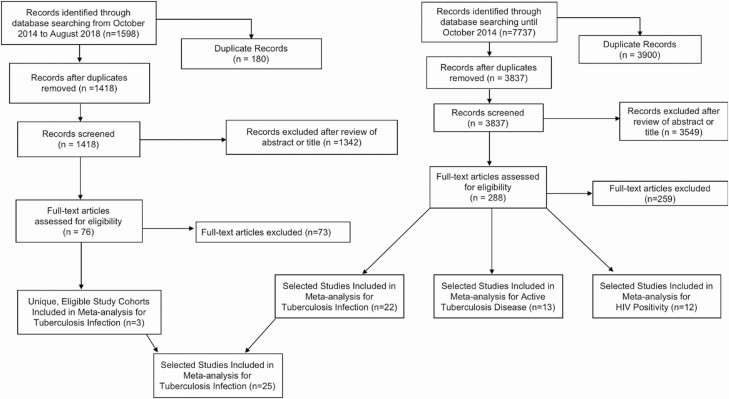
From our database searches, we found a total of 5255 original titles, of which 32 studies [2, 16–19, 26–52] met eligibility requirements and were included in the meta-analysis (Figure 1). Of these studies, 25 investigated prevalent M. tuberculosis infection [2, 16–18, 26–46], 13 on tuberculosis [2, 17–19, 26, 30, 31, 33, 34, 39, 47, 48, 52], and 12 on HIV infection among contacts [2, 17, 26, 27, 33, 37, 46, 49–52] (Table 1). Of these 3 outcome measures, 19 studies evaluated only 1 outcome, 9 studies evaluated 2, and 4 studies evaluated 3. Two studies [30, 49] had the same study population; the most recent publication [25] reported prevalent M. tuberculosis infection and disease, whereas the oldest [45] reported these 2 outcomes plus HIV infection in contacts. We took data from Klausner and colleagues [30] on M. tuberculosis infection and disease because data were most recent; data on HIV infection of contacts were taken from Baende and colleagues [49]. Among the 32 studies, 22 recruited only household contacts of tuberculosis cases, 9 studies had both household and community contacts, and 1 study did not specify the type of contact [19].
Figure 1.
Flow chart detailing literature search results for studies on the association between the HIV status of tuberculosis cases and clinical tuberculosis-related outcomes in case-contacts. *Final numbers of studies may not add to previous totals and exclusions because multiple studies investigated more than 1 outcome variable. Abbreviation: HIV, human immunodeficiency virus.
Table 1.
Summary of the 32 Observational Studies Included in the Meta-analysis on the Association Between HIV Status of Tuberculosis Cases and Clinical Tuberculosis Outcomes in their Case Contacts
| Contacts of HIV-positive TB Cases | Contacts of HIV-negative TB Cases | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| First Author, Year of Publication | Yeara | Contactb | Country | Diagnosis | N Index | N Contacts | % Yield Outcome | N Contacts | % Yield Outcome |
| Mycobacterium tuberculosis infection | |||||||||
| Martinez, 2016 | 1995–2006 | HHC | Uganda | TST, ≥10 mm | 499 | 878 | 65.7 | 974 | 73.6 |
| Alseda, 2003 | 1991–1997 | All | Spain | TST, ≥5 mmc | 437 | 199 | 31.7 | 1962 | 36.5 |
| Aibana, 2016d | 2009–2012 | HHC | Peru | TST, ≥10 mme | 4500 | 405 | 42.5 | 11 590 | 43.4 |
| Kenyon, 2002 | 1997 | HHC | Botswana | TST, ≥10 mm | 51 | 174 | 13.2 | 29 | 17.2 |
| Kifai, 2009 | 2007 | HHC | Tanzania | TST, ≥10 mme | 57 | 125 | 61.7 | 112 | 62.5 |
| Suggaravetsiri, 2003 | 2000–2002 | HHC | Thailand | TST, ≥10 mm | 499 | 487 | 46.2 | 705 | 62.1 |
| Lienhardt, 2002 | 1999–2001 | HHC | Gambia | TST, ≥10 mm | 315 | 83 | 71.1 | 1397 | 78.0 |
| Gustafson, 2008 | 1999–2000 | HHC | Guinea B. | TST, ≥10 mm | 220 | 285 | 39.2 | 738 | 42.5 |
| Naing, 2005 | 2000–2004 | HHC | Malaysia | TST, ≥10 mm | 215 | 84 | 33.3 | 320 | 51.9 |
| Cailleaux-Cezar, 2009 | 2000–2002 | All | Brazil | TST, ≥10 mmc | 276 | 110 | 51.4 | 480 | 46.5 |
| Kasambira, 2011 | 2006–2009 | HHC | SA | TST, ≥5 mm | 167 | 233 | 26.2 | 7 | 57.1 |
| Godoy, 2013 | 2005–2006 | All | Spain | TST, ≥5 mm | 1079 | 198 | 33.3 | 5173 | 28.4 |
| Biraro, 2014 | 2011–2012 | HHC | Uganda | QFT, 0.35 IU/mL | 101 | 56 | 51.8 | 207 | 67.6 |
| Fatima, 2004 | 1997–1999 | HHC | Brazil | TST, ≥10 mm | 297 | 177 | 38.4 | 22 | 45.5 |
| Reichler, 2003 | 1996 | All | USA | TST, ≥5 mm | 349 | 29 | 37.9 | 146 | 60.3 |
| Pitchenik, 1987 | 1985–1986 | HHC | USA | TST, NS | 71 | 54 | 35.2 | 108 | 43.5 |
| Cauthen, 1996 | 1985–1989 | All | USA | TST, ≥5 mm | 956 | 1095 | 31.2 | 2158 | 43.4 |
| Espinal, 2000 | 1994–1995 | HHC | Brazil | TST, ≥5 mm | 174 | 252 | 60.7 | 551 | 75.9 |
| Klausner, 1993 | 1989–1990 | HHC | Zaire | TST, ≥10 mme | 169 | 521 | 60.1 | 692 | 63.0 |
| Elliott, 1993 | 1989 | HHC | Zambia | TST, ≥5 mm | 71 | 207 | 52.2 | 141 | 70.9 |
| Nunn, 1994 | 1989–1990 | HHC | Kenya | TST, ≥5 mm | 82 | 80 | 61.3 | 223 | 58.3 |
| Garcia Ordonez, 1999 | 1995–1997 | HHC | Spain | TST, ≥5 mmc | 249 | 152 | 20.4 | 516 | 48.8 |
| Manoff, 1988 | 1988 | All | USA | TST, NS | 491 | 392 | 21.2 | 1703 | 30.4 |
| Carvalho, 2001 | 1995–1997 | All | Brazil | TST, ≥10 mm | 86 | 104 | 26.9 | 256 | 35.2 |
| Khan, 2017 | 2013–2015 | HHC | Malawi | TST, ≥10 mm | 150 | 132 | 22.0 | 170 | 44.1 |
| Tuberculosis | |||||||||
| Martinez, 2016 | 1995–2006 | HHC | Uganda | Micro | 499 | 878 | 4.0 | 974 | 4.3 |
| Aibana, 2016d | 2009–2012 | HHC | Peru | Micro, Symp | 4500 | 424 | 0.9 | 12 094 | 1.7 |
| Suggaravetsiri, 2003 | 2000–2002 | HHC | Thailand | Micro, CX | 499 | 490 | 2.9 | 710 | 4.4 |
| Cailleaux-Cezar, 2009 | 2000–2002 | All | Brazil | Micro, CX | 276 | 110 | 4.5 | 480 | 2.5 |
| Rodrigo, 1997 | 1990–1993 | All | Spain | Micro | 1079 | 163 | 4.9 | 916 | 2.4 |
| Pitchenik, 1987 | 1985–1986 | HHC | USA | NS | 71 | 54 | 1.9 | 108 | 0.9 |
| Standaert, 1989 | 1985–1986 | HHC | Burundi | Micro | NS | 48 | 12.5 | 28 | 0.0 |
| Klausner, 1993 | 1989–1990 | HHC | Zaire | Micro, Sympf | 174 | 521 | 3.1 | 692 | 4.0 |
| Elliott, 1993 | 1989 | HHC | Zambia | Micro, Symp | 71 | 207 | 3.9 | 141 | 2.8 |
| Nunn, 1994 | 1989–1990 | HHC | Kenya | Micro, Symp | 82 | 87 | 6.9 | 248 | 4.8 |
| Garcia Ordonez, 1999 | 1995–1997 | HHC | Spain | Micro, CX | 249 | 152 | 1.3 | 516 | 5.0 |
| Topley, 1996 | 1993–1994 | HHC | Malawi | Sym, CX, TST | 206 | 105 | 31.4 | 37 | 35.1 |
| Cayla, 1993 | 1990–1991 | NS | Spain | NS | 136 | 225 | 8.0 | 216 | 3.2 |
| HIV infection | |||||||||
| Martinez, 2016 | 1995–2006 | HHC | Uganda | ELISA | 499 | 915 | 16.8 | 1018 | 4.6 |
| Suggaravetsiri, 2003 | 2000–2002 | HHC | Thailand | ELISA | 499 | 376 | 13.8 | 514 | 2.5 |
| Aibana, 2016d | 2009–2016 | HHC | Peru | NS | 4500 | 419 | 2.4 | 11 959 | 0.3 |
| Nunn, 1994 | 1989–1990 | HHC | Kenya | NS | 82 | 101 | 13.9 | 250 | 0.8 |
| Elliott, 1993 | 1989 | HHC | Zambia | ELISA | 71 | 133 | 13.5 | 69 | 7.2 |
| Carvalho, 2001 | 1995–1997 | All | Brazil | ELISA and WB | 86 | 75 | 10.7 | 179 | 1.7 |
| Kifai, 2009 | 2007 | HHC | Tanzania | ELISA | 57 | 115 | 8.7 | 103 | 8.7 |
| Baende, 1990 | 1989 | HHC | Zaire | ELISA and WB | 100 | 323 | 5.9 | 410 | 2.4 |
| Hirsch-Moverman, 2015 | 2002–2006 | All | USA, Can. | NS | 651 | 184 | 23.4 | 806 | 2.7 |
| Reichler, 2003 | 1996 | All | USA | NS | 29 | 30 | 53.0 | 147 | 2.1 |
| Standaert, 1989 | 1985–1986 | HHC | Burundi | ELISA | NS | 48 | 8.3 | 28 | 7.1 |
| Mutsvangwa, 2010 | 2002–2004 | HHC | Zimbabwe | Det., Unigold | 129 | 172 | 28.5 | 50 | 12.0 |
Abbreviations: Can., Canada; CC, community contact; CX, chest radiographical exam; Det., determine; DRC, Democratic Republic of Congo; Guinea B., Guinea–Bissau; HHC, household contact; HIV, human immunodeficiency virus; Micro, microbiological testing; NS, not specified; QFT, QuantiFERON Gold In-Tube Test; SA, South Africa; Sym, symptom screening; TST, tuberculin skin test; WB, Western blot.
a Year of implementation of the study. If dates are not given for the study implementation the study publication year is given.
b All refers to studies that collected data on both household contacts and community contacts and grouped their results together. Only 1 study presented stratified results based on differing types of contacts.
c In Alseda and colleagues (2010), for all contacts unvaccinated with the BCG the definition of a positive tuberculin skin test was ≥5 mm. For all BCG-vaccinated contacts, a positive test was defined as ≥15 mm. In Cailleaux-Cezar and colleagues (2009), for all contacts unvaccinated with the BCG, the definition of a positive tuberculin skin test was ≥10 mm. For all BCG-vaccinated contacts, a positive test was defined as ≥15 mm.
d Aibana and colleagues (2016) is presented here rather than Huang and colleagues (2014), which is part of the same cohort. We present here the Aibana and colleagues study because study-level data on outcomes from contacts of HIV-positive and HIV-negative contacts were not extractable from the Huang and colleagues study. We used an online database of this Peruvian cohort and analyzed the specific outcomes (Mycobacterium tuberculosis infection, active tuberculosis, HIV infection) among contacts of HIV-positive and HIV-negative index cases for the subsequent analysis.
e For all HIV-negative participants the definition of a positive tuberculin skin test was ≥10 mm. For all HIV-positive contacts, a positive test was defined as ≥5 mm.
f A portion of the contacts diagnosed with tuberculosis were confirmed with microbiological testing.
Risk of M. tuberculosis Infection in Contacts
From the 25 studies investigating M. tuberculosis infection, the total number of household contacts of HIV-positive and HIV-negative index cases from all studies was 6513 (median, 177 [interquartile range [IQR], 104–285]) and 30 380 (median, 480 [IQR, 146–974]), respectively. One study used QuantiFERON-TB Gold in Tube [16]. Two studies did not specify the criteria for a positive tuberculin skin test but were included in the analysis because they stipulated whether subjects had either positive or negative skin test.
Risk of M. tuberculosis infection was lower in contacts of HIV-positive index cases (pooled OR, 0.67 [95% CI, .58–.77]; Table 2) but was heterogeneous (I2 = 75.1%). To evaluate the observed heterogeneity, we stratified from studies with available information (Table 2). When the tuberculosis index case had lung cavitary disease, the risk of M. tuberculosis infection was elevated in contacts of HIV-positive index cases (OR, 1.37 [95% CI, 1.05–1.78]; I2 = 0%) compared with HIV-negative index cases, whereas when the index case did not have cavitary lung lesions and contacts of HIV-positive cases had less M. tuberculosis infection (OR, 0.69 [95% CI, .47–1.04]; I2 = 83.9%). When the tuberculosis index case was stratified by sputum smear status, the relationship between risk of M. tuberculosis infection and the HIV status of the index case diverged (sputum smear positive: OR, 0.69 (95% CI, .59–.80), I2 = 63.5%; Sputum smear negative: OR, 0.41 [95% CI, .13–1.28], I2 = 93.5%). Three studies (Figure 2) also showed that people with HIV and either a low CD4 count or with acquired immunodeficiency syndrome (AIDS) status modified this relationship.
Table 2.
Pooled Random Effects Logistic Regression for the Influence of the Tuberculosis Case’s HIV Status on Mycobacterium tuberculosis Infection in Contacts, Stratified by Secondary Risk Factors
| Characteristic | No. Studies | Pooled OR (95% CI), P Value for Effecta | I 2 |
|---|---|---|---|
| Crude | |||
| All studies | 25 | 0.67 (.58–.77), <.001 | 75.1 |
| Age of contact, y | |||
| 0–4 | 3 | 0.69 (.51–.94), .018 | 0.0 |
| 5–14 | 10 | 0.61 (.45–.82), .001 | 74.9 |
| ≥15 | 4 | 0.74 (.59–.92), .008 | 0.0 |
| BCG vaccination status of contact | |||
| Vaccinated | 3 | 0.87 (.58–1.31), .504 | 82.0 |
| Unvaccinated | 3 | 0.78 (.58–1.06), .116 | 0.0 |
| HIV-negative contacts | |||
| HIV-negative contacts onlyb | 6 | 0.60 (.39–.93), <.001 | 0.86.1 |
| Form of contact with tuberculosis case | |||
| Household | 19 | 0.63 (.53–.75), <.001 | 67.5 |
| Community | 1 | 0.46 (.30–.71), <.001 | – |
| Both | 5 | 0.86 (.61–1.22), .040 | 85.0 |
| Definition of Mycobacterium tuberculosis infection c | |||
| ≥10-mm induration for all contacts | 9 | 0.58 (.42–.79), .001 | 55.0 |
| ≥5-mm induration for all contacts | 10 | 0.71 (.58–.86), <.001 | 85.8 |
| ≥10 mm for HIV-neg.; ≥5 mm for HIV-pos. | 3 | 0.93 (.81–1.08), .342 | 0 |
| Region | |||
| Asia | 2 | 0.51 (.41–.63), <.001 | 0.0 |
| Africa | 11 | 0.69 (.57–.85), <.001 | 57.0 |
| Europe | 3 | 0.66 (.29–1.48), .309 | 94.0 |
| Americas | 9 | 0.69 (.55–086), .001 | 72.9 |
| Year d | |||
| 1990 and before | 2 | 0.63 (.49–.80), <.001 | 0 |
| Post-1990 | 23 | 0.67 (.57–.78), <.001 | 77.0 |
| 2000 and before | 8 | 0.59 (.46–.75), <.001 | 78.8 |
| Post-2000 | 17 | 0.72 (.60–.86), <.001 | 69.6 |
| Sputum smear status of tuberculosis case | |||
| Positive | 13 | 0.69 (.59–.80), <.001 | 63.5 |
| Negative | 4 | 0.41 (.13–1.28), .124 | 93.5 |
| Both | 11 | 0.75 (.58–.97), .031 | 73.9 |
| Cavitary lung disease of tuberculosis case | |||
| Lung cavitary disease | 3 | 1.37 (1.05–1.78), <.018 | 0 |
| Noncavitary disease | 3 | 0.69 (.47–1.04), .074 | 83.9 |
| Both | 19 | 0.69 (.58–.83), <.001 | 76.1 |
| Extrapulmonary tuberculosis | |||
| Pulmonary cases only | 16 | 0.68 (.58–.79), <.001 | 64.2 |
| Pulmonary and extrapulmonary casese | 6 | 0.62 (.42–.91), .015 | 87.5 |
| Not specified | 1 | 0.73 (.25–2.11), .562 | - |
a In all analyses, the reference category for the measure of association are contacts of HIV-negative tuberculosis cases.
b Cauthen (2004) assumed that children under 14 in their study population were likely not HIV-infected contacts; however, this was not confirmed with HIV testing.
c Two studies did not specify their definition for a positive tuberculin skin test.
d Year of publication.
e No studies stratified both HIV status and the presence of extrapulmonary tuberculosis in the index case.
Figure 2.
Individual studies demonstrating evidence for effect modification on Mycobacterium tuberculosis infection in case contacts by clinical characteristics of the HIV-tuberculosis coinfected index case. We present here results from individual studies that show modification of the infectiousness of HIV-positive and HIV-negative index cases. These studies are stratified by the severity of tuberculosis in the index case (as measured through smear positivity and cavitary status) and the severity of the HIV status of the index case (as measured through CD4 count and AIDS status). Huang and colleagues (2014) is presented here rather than Aibana and colleagues (2016) which is part of the same cohort. We present here Huang and colleagues’ study because they include CD4 count. The study by Aibana and colleagues is presented and analyzed in the rest of the paper because study-level data on outcomes from contacts of HIV-positive and HIV-negative contacts were not extractable from the Huang and colleagues study. Abbreviations: HIV, human immunodeficiency virus; RR, relative risk; TST, tuberculin skin test. * All index cases have tuberculosis. This column stratifies tuberculosis index cases by their HIV status and other secondary modifying variables related to the severity of HIV or tuberculosis. • Adjusted for age, sex, smoking status, alcohol intake, nutritional status, number of BCG scars, household smoke exposure, relation to the tuberculosis case from household contacts; age, sex, cavitary lung disease, smear status, and treatment delay from tuberculosis cases. †Adjusted for age, education level, and alcohol status of the household contact; sputum smear and cavitary status of the tuberculosis case; and the number of individuals in the household. ¥Adjusted for female sex and sputum smear grade of the tuberculosis case, and household clustering. ¶Smear negative, culture negative index cases were grouped together with smear negative, culture positive cases in this group. The prevalence of positive skin tests was roughly equivalent in both their sets of contacts. ‡New-onset AIDS was diagnosed before contact testing while previously diagnosed AIDS was diagnosed before contact testing.
When we pooled rates from other subgroups, including different age groups, BCG vaccination, household versus community contacts, region, and study year of implementation, we found little mediation of the relationship between M. tuberculosis infection and the HIV status of the index case. For example, among contacts 0–4, 5–14, and ≥15 years old, the OR of M. tuberculosis infection among contacts of index cases living and not living with HIV did not appreciably differ and were 0.69 (95% CI, .51–.94), 0.61 (95% CI, .45–.82), and 0.74 (95% CI, .59–.92), respectively. Similarly, when stratifying by the type of contact, there was little difference in the relationship between the HIV status of the index case and M. tuberculosis infection among contacts. Among studies with household and community contacts, the odds of M. tuberculosis infection among contacts of index cases living and not living with HIV was 0.63 (95% CI, .53–.75), 0.46 (95% CI, .30–.71), and 0.86 (95% CI, .61–1.22), respectively.
Two studies investigated effect modification by the index case’s cough duration [2, 17]; both found that cough duration did not influence the relationship between M. tuberculosis infection and the differing HIV status of index cases.
Risk of Active Tuberculosis in Contacts
Of 13 studies on tuberculosis, the total number of contacts of HIV-positive and HIV-negative index cases from all studies was 2623 (median, 152 [IQR, 87–225]) and 16 230 (median, 248 [IQR, 108–692]), respectively. Nine studies confirmed tuberculosis with a microbiological examination (sputum smear or culture); 1 study used a combination of symptom screening, chest radiographical examinations, and tuberculin skin tests; and 2 studies did not specify their method of diagnosis (Table 1). Only 1 [19] of the 13 studies demonstrated a statistically significant difference in rates of tuberculosis among contacts of HIV-positive and HIV-negative tuberculosis cases. There was no statistical difference between the groups (OR, 1.07 [95% CI, .74–1.56]) and a low level of between-study heterogeneity (I2 = 39.0%) (Supplementary Appendix).
HIV Infection in Contacts
Of 12 studies on HIV infection among contacts, the total number of contacts of HIV-positive and HIV-negative index cases from all studies was 2891 (median, 153 [IQR, 88–350]) and 15 533 (median, 215 [IQR, 86–660]), respectively (Table 1). Seven of these studies were implemented in Africa, 4 from the Americas, and 1 in Asia. To diagnose HIV in contacts, 4 studies used an ELISA test, 2 used a combination of the ELISA and Western blot tests, and 3 studies did not specify.
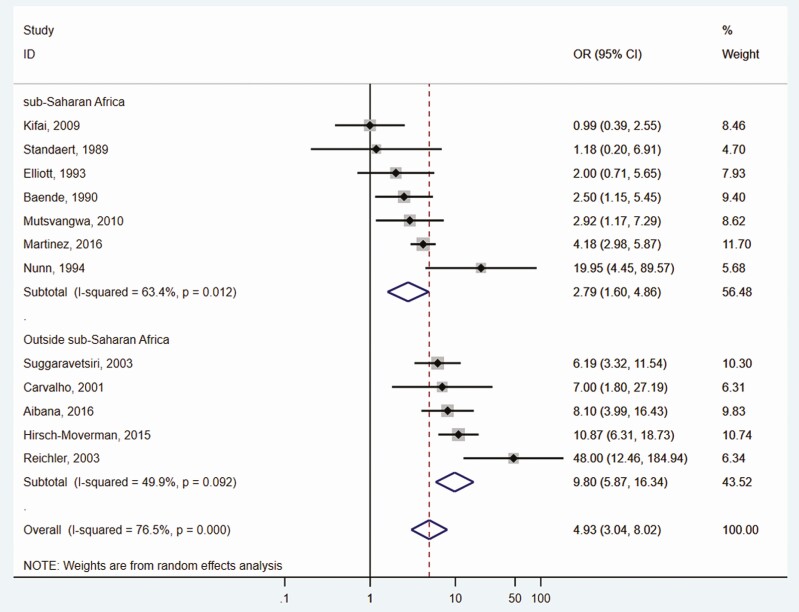
The odds ratio among contacts of HIV-positive and HIV-negative index cases ranged from 1.0 in Kifai and colleagues [27] to 48.0 in Reichler and colleagues [46]. The pooled random effects OR was 4.9 (95% CI, 3.0–8.0; I2 = 77.0%), indicating that the HIV serostatus of the index case is a marker of HIV infection among contacts. All studies had more HIV-positive contacts among HIV-positive index cases compared with HIV-negative index cases except for 1 study [27], which had an equal amount. Nine of the 12 studies had statistically significantly more HIV-positive contacts among HIV-positive index cases compared with HIV-negative index cases (Figure 3). The pooled increased odds of HIV infection among HIV-positive index cases was substantially higher in studies from countries outside of Africa (Nstudies = 5; OR, 9.80; 5.88–16.34) compared with African countries (Nstudies = 7; OR, 2.79; 1.60–4.86) (Figure 3).
Figure 3.
Risk of HIV infection among contacts of HIV-positive and HIV-negative tuberculosis cases, stratified by the study location†. Abbreviation: HIV, human immunodeficiency virus. †In all analyses, the reference category for the measure of association are case contacts of HIV-negative tuberculosis cases.
Study Quality and Publication Bias Assessment
Generally, studies were either of moderate or high study quality (Supplementary Appendix). For studies investigating M. tuberculosis infection, 13 studies were of high quality, 9 were of moderate quality, and 3 were of low quality. For studies investigating tuberculosis, 6, 5, and 2 studies were of high, moderate, and low quality. For studies investigating HIV in contacts, 6 studies of high and moderate quality, respectively.
When we assessed publication bias through inspection of funnel plots for each outcome, we found no evidence of publication bias. Harbord’s test gave nonsignificant P values of 0.56, 0.17, and 0.29 for the outcomes of M. tuberculosis infection, tuberculosis, and HIV.
CONCLUSION
Our results suggest that differential M. tuberculosis infection rates in contacts of HIV-infected and uninfected tuberculosis cases is driven predominantly by the level of immunosuppression among persons living with HIV and the lung cavitation of tuberculosis of that case. Upon stratifying our results on index case characteristics such as lung cavitary status, CD4 count, or AIDS status, we found that heterogeneity among studies reduced considerably. The rate of tuberculosis among contacts of HIV-positive index cases did not significantly differ from contacts of tuberculosis cases without HIV; however, HIV infection was 5 times more common in contacts of HIV-positive tuberculosis cases. This was modified by whether the study was in or outside of sub-Saharan Africa, likely because of the background HIV rate.
A previous systematic review on this topic was performed in 2001 [7]. After applying a random effects model to 4 contact studies, this review concluded that tuberculosis cases were not more infectious than HIV-negative tuberculosis cases. In this updated meta-analysis including 25 studies investigating M. tuberculosis infection, our results suggest that tuberculosis patients with HIV are less infectious than HIV-uninfected tuberculosis cases and that this was modified by the severity of the tuberculosis case and/or the immunosuppression of index cases with HIV. This may partially explain heterogeneity in past studies in which some studies restrict index cases to smear-positive disease, whereas others also allow paucibacillary index cases. Our results also suggest that contacts of well-controlled HIV among people with tuberculosis may also have a lower risk of M. tuberculosis infection. In Kenyon et al and Huang et al, contacts of HIV-positive people with tuberculosis and high CD4 counts had a similar risk as HIV-negative people with tuberculosis. However, Cauthen et al found that HIV-positive people with tuberculosis had similar infection risk compared with new and previously diagnosed people with AIDS (see Figure 2). Further clarification is needed to confirm these findings on well-controlled HIV and its relation to M. tuberculosis infection risk in contacts. Smear, HIV, and lung cavitation among index cases are likely to be correlated in many of these studies. Many of the paucibacillary cases in these studies may represent HIV-seropositive patients with a low CD4 counts and therefore may be investigating similar patients. Cough duration of tuberculosis cases may be an important factor because people who are HIV-positive and have tuberculosis usually present to care earlier than people who are HIV-negative [53, 54]. Only 2 studies investigated effect modification by the index case’s cough duration [3, 17]. Both of these individual studies found that it did not influence rates of M. tuberculosis infection rates in contacts of tuberculosis cases with differing HIV status, however.
An important finding of this meta-analysis is the paucity of data found on the impact of antiretroviral therapy (ART) on the infectiousness of tuberculosis patients. ART has become increasingly accessible over the past 20 years and is associated with increasing CD4 counts in individuals living with HIV [55]. Two studies [17, 29] showing decreased transmission from HIV-positive tuberculosis patients with low CD4 counts may indirectly suggest higher infectiousness in coinfected patients on ART. A recent report [45] did not show ART as an influential variable; however, this research question remains unclear and requires further studies with larger sample sizes [56].
Our results also have important implications for policy on active case finding and HIV testing among contacts of tuberculosis cases. Current evidence graded by the World Health Organization for HIV testing in contacts of tuberculosis cases is considered of “low quality” [57]. We found that contacts of HIV-positive index cases were almost 5 times more likely to also have HIV infection. Although these studies were heterogeneous, the range of estimates consistently demonstrated a marked increased risk in HIV among contacts exposed to HIV-positive index cases. This association was modified by whether the study was set in sub-Saharan Africa, where HIV prevalence is high. Contacts from sub-Saharan African countries had a lower measure of association because the background HIV prevalence is much higher and therefore contacts of HIV-negative index cases are also at high-risk to acquire HIV. These results suggest that HIV testing of all contacts of tuberculosis cases in sub-Saharan Africa regardless of the HIV status of the index should be considered. This would support current global recommendations [57]. In areas with a lower background HIV prevalence and minimal resources, HIV testing only contacts of HIV-positive index cases is likely to be a resource efficient and effective method of finding new cases of HIV.
The results presented in this meta-analysis should be taken in context with several limitations. First, because of the epidemiology of tuberculosis and the inability of the tuberculin skin test to measure recent infection, M. tuberculosis infection may not be the ideal measure of tuberculosis infectiousness. M. tuberculosis infection in a population increases with age [58–60], and nondifferential misclassification may be present if transmission occurred before the exposure event investigated. We searched for molecular clustering studies and studies using tuberculin skin test conversion in contacts; however, few have been performed with data on this topic [17, 28, 61, 62]. Second, although several studies measured effect modification between infectiousness of HIV-positive tuberculosis cases and M. tuberculosis infection in contacts, few studies used similar measurements, which limited our ability to group studies. Third, very few studies stratified their disease results by initial M. tuberculosis infection status; therefore, we could not see if our tuberculosis results were modified by infection status. Fourth, although we adjust our results by several important secondary characteristics relevant to M. tuberculosis transmission, we acknowledge that other factors may be relevant and not widely measured among studies. Last, in the prevalent tuberculosis outcome analysis, we are not able to confirm the direction of transmission between index case and contacts. Potentially, an individual with HIV may progress more quickly to symptoms and diagnosis, and thus may be misclassified as the index case.
In conclusion, our results suggest people with tuberculosis coinfected with HIV are less infectious than HIV-uninfected cases when they have severe immunosuppression or paucibacillary disease. Contacts of coinfected index cases are almost 5 times more likely to also have HIV infection strongly indicating an urgent need for integrated HIV and tuberculosis policy and intervention.
Supplementary Data
Supplementary materials are available at Clinical Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Supplementary Material
Notes
Author contributions. L. M. conceived the study, analyzed the data, wrote the first draft of the manuscript, and was the main investigator responsible for interpretation of the results. L. M., H. W., C. C., B. H., and M. E. C. contributed to study and data management. H. W., C. C., B. D. H., M. E. C., Q. L., C. C. W., and L. Z. assisted L. M. with the analytical plan and data analysis. All authors were involved in interpretation of the study results, reviewing and editing of the manuscript, and approval of the final version of the manuscript.
Acknowledgments. The authors thank the University of Georgia Interlibrary Loan office for their tireless efforts at retrieving hard-to-reach manuscripts, abstracts, and books on this topic. The authors also thank the research group at Jiangsu Province Tuberculosis Control Division at China’s Centers for Disease Control and Prevention for their useful and instructive comments on presentations of this project. Last, L. M. thanks Fernando Martinez and Maria Ines Pantoja for their constant support.
Financial support. C. C. W. and L. M. were supported in part by an investigator-initiated grant (AI 093856) and a diversity supplement grant (3R01AI093856–05W1) from the National Institutes of Allergy and Infectious Diseases. L. M. was supported by the Ruth L Kirschstein National Research Service Award. M. E. C. was supported by the Schlumberger Foundation Faculty for the Future Fellowship.
Potential conflicts of interest. The authors: No reported conflicts of interest. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest.
References
- 1. Peters JS, Andrews JR, Hatherill M, et al. Advances in the understanding of Mycobacterium tuberculosis transmission in HIV-endemic settings. Lancet Infect Dis 2019; 19:e65–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Corbett EL, Marston B, Churchyard GJ, De Cock KM. Tuberculosis in sub-Saharan Africa: opportunities, challenges, and change in the era of antiretroviral treatment. Lancet 2006; 367:926–37. [DOI] [PubMed] [Google Scholar]
- 3. Nunn P, Reid A, De Cock KM. Tuberculosis and HIV infection: the global setting. J Infect Dis 2007; 196 Suppl 1:S5–14. [DOI] [PubMed] [Google Scholar]
- 4. Martinez L, Sekandi JN, Castellanos ME, Zalwango S, Whalen CC. Infectiousness of HIV-seropositive patients with tuberculosis in a high-burden African setting. Am J Respir Crit Care Med 2016; 194:1152–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Guelar A, Gatell JM, Verdejo J, et al. A prospective study of the risk of tuberculosis among HIV-infected patients. AIDS 1993; 7:1345–9. [DOI] [PubMed] [Google Scholar]
- 6. Whalen C, Horsburgh CR, Hom D, Lahart C, Simberkoff M, Ellner J. Accelerated course of human immunodeficiency virus infection after tuberculosis. Am J Respir Crit Care Med 1995; 151:129–35. [DOI] [PubMed] [Google Scholar]
- 7. Cruciani M, Malena M, Bosco O, Gatti G, Serpelloni G. The impact of human immunodeficiency virus type 1 on infectiousness of tuberculosis: a meta-analysis. Clin Infect Dis 2001; 33:1922–30. [DOI] [PubMed] [Google Scholar]
- 8. Pai M, McCulloch M, Colford JM Jr. Meta-analysis of the impact of HIV on the infectiousness of tuberculosis: methodological concerns. Clin Infect Dis 2002; 34:1285–7. [DOI] [PubMed] [Google Scholar]
- 9. Sepkowitz KA. How contagious is tuberculosis? Clin Infect Dis 1996; 23:954–62. [DOI] [PubMed] [Google Scholar]
- 10. Fox GJ, Barry SE, Britton WJ, Marks GB. Contact investigation for tuberculosis: a systematic review and meta-analysis. Eur Respir J 2013; 41:140–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Morrison J, Pai M, Hopewell PC. Tuberculosis and latent tuberculosis infection in close contacts of people with pulmonary tuberculosis in low-income and middle-income countries: a systematic review and meta-analysis. Lancet Infect Dis 2008; 8:359–68. [DOI] [PubMed] [Google Scholar]
- 12. Shah NS, Yuen CM, Heo M, Tolman AW, Becerra MC. Yield of contact investigations in households of patients with drug-resistant tuberculosis: systematic review and meta-analysis. Clin Infect Dis 2014; 58:381–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Blok L, Sahu S, Creswell J, Alba S, Stevens R, Bakker MI. Comparative meta-analysis of tuberculosis contact investigation interventions in eleven high burden countries. PLoS One 2015; 10:e0119822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Rieder HL. Contacts of tuberculosis patients in high-incidence countries. Int J Tuberc Lung Dis 2003; 7:S333–6. [PubMed] [Google Scholar]
- 15. Martinez L, Shen Y, Mupere E, Kizza A, Hill PC, Whalen CC. Transmission of Mycobacterium tuberculosis in households and the community: a systematic review and meta-analysis. Am J Epidemiol 2017; 185:1327–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Huang CC, Tchetgen ET, Becerra MC, et al. The effect of HIV-related immunosuppression on the risk of tuberculosis transmission to household contacts. Clin Infect Dis 2014; 58:765–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Aibana O, Acharya X, Huang CC, et al. Nutritional status and tuberculosis risk in adult and pediatric household contacts. PLoS One 2016; 11:e0166333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Biraro IA, Egesa M, Toulza F, et al. Impact of co-infections and BCG immunisation on immune responses among household contacts of tuberculosis patients in a Ugandan cohort. PLoS One 2014; 9:e111517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Pitchenik AE, Burr J, Suarez M, Fertel D, Gonzalez G, Moas C. Human T-cell lymphotropic virus-III (HTLV-III) seropositivity and related disease among 71 consecutive patients in whom tuberculosis was diagnosed: a prospective study. Am Rev Respir Dis 1987;135:875–9. [DOI] [PubMed] [Google Scholar]
- 20. Cayla J, Jansa J, Iglesias B. Tuberculosis transmission from index cases HIV-1 (+) or HIV-1 (–): a case-control study. Paper presented at: IX International Conference on AIDS, Berlin, Germany, 1993. [Google Scholar]
- 21. DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials 1986; 7:177–88. [DOI] [PubMed] [Google Scholar]
- 22. Higgins JP, Thompson SG. Quantifying heterogeneity in a meta-analysis. Stat Med 2002; 21:1539–58. [DOI] [PubMed] [Google Scholar]
- 23. Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ 2003; 327:557–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ 1997; 315:629–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Harbord RM, Egger M, Sterne JA. A modified test for small-study effects in meta-analyses of controlled trials with binary endpoints. Stat Med 2006; 25:3443–57. [DOI] [PubMed] [Google Scholar]
- 26. Elliott AM, Hayes RJ, Halwiindi B, et al. The impact of HIV on infectiousness of pulmonary tuberculosis: a community study in Zambia. AIDS 1993; 7:981–7. [DOI] [PubMed] [Google Scholar]
- 27. Kifai EJ, Bakari M. Mantoux skin test reactivity among household contacts of HIV-infected and HIV un-infected patients with sputum smear positive TB in Dar es Salaam, Tanzania. East Afr J Public Health 2009; 6:211–8. [DOI] [PubMed] [Google Scholar]
- 28. Espinal MA, Peréz EN, Baéz J, et al. Infectiousness of Mycobacterium tuberculosis in HIV-1-infected patients with tuberculosis: a prospective study. Lancet 2000; 355:275–80. [DOI] [PubMed] [Google Scholar]
- 29. Kenyon TA, Creek T, Laserson K, et al. Risk factors for transmission of Mycobacterium tuberculosis from HIV-infected tuberculosis patients, Botswana. Int J Tuberc Lung Dis 2002; 6:843–50. [PubMed] [Google Scholar]
- 30. Klausner JD, Ryder RW, Baende E, et al. Mycobacterium tuberculosis in household contacts of human immunodeficiency virus type 1-seropositive patients with active pulmonary tuberculosis in Kinshasa, Zaire. J Infect Dis 1993; 168:106–11. [DOI] [PubMed] [Google Scholar]
- 31. García Ordóñez MA, Colmenero Castillo JD, Sánchez Simonet MV, García Delange MT, Causse Prados M, Juárez Fernández C. [The cost effectiveness of the study of the familial contacts of tuberculosis patients coinfected with the human immunodeficiency virus]. Rev Clin Esp 1999; 199:275–9. [PubMed] [Google Scholar]
- 32. Lienhardt C, Fielding K, Sillah J, et al. Risk factors for tuberculosis infection in sub-Saharan Africa: a contact study in The Gambia. Am J Respir Crit Care Med 2003; 168:448–55. [DOI] [PubMed] [Google Scholar]
- 33. Suggaravetsiri P, Yanai H, Chongsuvivatwong V, Naimpasan O, Akarasewi P. Integrated counseling and screening for tuberculosis and HIV among household contacts of tuberculosis patients in an endemic area of HIV infection: Chiang Rai, Thailand. Int J Tuberc Lung Dis 2003; 7:S424–31. [PubMed] [Google Scholar]
- 34. Cailleaux-Cezar M, de A Melo D, Xavier GM, et al. Tuberculosis incidence among contacts of active pulmonary tuberculosis. Int J Tuberc Lung Dis 2009; 13:190–5. [PMC free article] [PubMed] [Google Scholar]
- 35. Kasambira TS, Shah M, Adrian PV, et al. QuantiFERON-TB gold in-tube for the detection of mycobacterium tuberculosis infection in children with household tuberculosis contact. Int J Tuberc Lung Dis 2011; 15:628–34. [DOI] [PubMed] [Google Scholar]
- 36. Cauthen GM, Dooley SW, Onorato IM, et al. Transmission of Mycobacterium tuberculosis from tuberculosis patients with HIV infection or AIDS. Am J Epidemiol 1996; 144:69–77. [DOI] [PubMed] [Google Scholar]
- 37. Carvalho AC, DeRiemer K, Nunes ZB, et al. Transmission of Mycobacterium tuberculosis to contacts of HIV-infected tuberculosis patients. Am J Respir Crit Care Med 2001; 164:2166–71. [DOI] [PubMed] [Google Scholar]
- 38. Gustafson P, Lisse I, Gomes V, et al. Risk factors for positive tuberculin skin test in Guinea-Bissau. Epidemiology 2007; 18:340–7. [DOI] [PubMed] [Google Scholar]
- 39. Nunn P, Mungai M, Nyamwaya J, et al. The effect of human immunodeficiency virus type-1 on the infectiousness of tuberculosis. Tuber Lung Dis 1994; 75:25–32. [DOI] [PubMed] [Google Scholar]
- 40. Godoy P, Caylà JA, Carmona G, et al. ; Grupo de Trabajo de Estudios de Contactos de Tuberculosis de Cataluña . Smoking in tuberculosis patients increases the risk of infection in their contacts. Int J Tuberc Lung Dis 2013; 17:771–6. [DOI] [PubMed] [Google Scholar]
- 41. Alsedà M, Godoy P. [Factors associated with latent tuberculosis infection in the contacts of tuberculosis patients]. Gac Sanit 2004; 18:101–7. [DOI] [PubMed] [Google Scholar]
- 42. Manoff S, Cauthen G, Stoneburner R, Bloch A, Schultz S, Snider D. TB patients with AIDS: are they more likely to spread TB. Paper presented at: Fourth International Conference on AIDS, Stockholm, Sweden, 1988.
- 43. Naing NN, Mohammad Z, Bakar A, et al. A multi-centered study of the influence of HIV infection on the transmission of tuberculosis to household contacts three states of Malaysia. Int Med J 2007; 14:273–9. [Google Scholar]
- 44. Maria de Fatima P, Ricardo AdA, Campelo ARL, Sarinho E, Cruz M, Maia Filho V. Neonatal BCG vaccine and response to the tuberculin test in BCG vaccinated children in contact with tuberculosis patients in Recife, Brazil. J Trop Pediatr 2004;50:32–6. [DOI] [PubMed] [Google Scholar]
- 45. Khan PY, Crampin AC, Mzembe T, et al. Does antiretroviral treatment increase the infectiousness of smear-positive pulmonary tuberculosis? Int J Tuberc Lung Dis 2017; 21:1147–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Reichler MR, Bur S, Reves R, et al. Results of testing for human immunodeficiency virus infection among recent contacts of infectious tuberculosis cases in the United States. Int J Tuberc Lung Dis 2003; 7:S471–8. [PubMed] [Google Scholar]
- 47. Rodrigo T, Caylà JA, García de Olalla P, et al. Characteristics of tuberculosis patients who generate secondary cases. Int J Tuberc Lung Dis 1997; 1:352–7. [PubMed] [Google Scholar]
- 48. Topley JM, Maher D, Mbewe LN. Transmission of tuberculosis to contacts of sputum positive adults in Malawi. Arch Dis Child 1996; 74:140–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Baende E, Lelo U, Kaboto M, Williame J, Ngamboli K, Ryder R. Household contacts (HC) of HIV(+) patients in Zaire with active pulmonary tuberculosis disease (PTB) are not at increased risk of secondary M. tuberculosis (M.tb) infection. Paper presented at: 6th International Conference on AIDS, San Francisco, California, 1990.
- 50. Mutsvangwa J, Millington KA, Chaka K, et al. Identifying recent Mycobacterium tuberculosis transmission in the setting of high HIV and TB burden. Thorax 2010; 65:315–20. [DOI] [PubMed] [Google Scholar]
- 51. Hirsch-Moverman Y, Cronin WA, Chen B, Moran JA, Munk E, Reichler MR; Tuberculosis Epidemiological Studies Consortium Task Order 2 Team . HIV counseling and testing in tuberculosis contact investigations in the United States and Canada. Int J Tuberc Lung Dis 2015; 19:943–53. [DOI] [PubMed] [Google Scholar]
- 52. Standaert B, Niragira F, Kadende P, Piot P. The association of tuberculosis and HIV infection in Burundi. AIDS Res Hum Retroviruses 1989; 5:247–51. [DOI] [PubMed] [Google Scholar]
- 53. Corbett EL, Charalambous S, Moloi VM, et al. Human immunodeficiency virus and the prevalence of undiagnosed tuberculosis in African gold miners. Am J Respir Crit Care Med 2004; 170:673–9. [DOI] [PubMed] [Google Scholar]
- 54. Sekandi JN, Zalwango S, Martinez L, et al. Four degrees of separation: social contacts and health providers influence the steps to final diagnosis of active tuberculosis patients in urban Uganda. BMC Infect Dis 2015; 15:361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Lok JJ, Bosch RJ, Benson CA, et al. ; ALLRT team . Long-term increase in CD4+ T-cell counts during combination antiretroviral therapy for HIV-1 infection. AIDS 2010; 24:1867–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Borgdorff MW, De Cock KM. Provision of ART to individuals infected with HIV: impact on the epidemiology and control of tuberculosis. Int J Tuberc Lung Dis 2017; 21:1091–2. [DOI] [PubMed] [Google Scholar]
- 57. World Health Organization. Recommendations for investigating contacts of persons with infectious tuberculosis in low-and middle-income countries. No. WHO/HTM/TB/2012.9. London, United Kingdom: World Health Organization, 2012. [PubMed] [Google Scholar]
- 58. Martinez L, Arman A, Haveman N, et al. Changes in tuberculin skin test positivity over 20 years in periurban shantytowns in Lima, Peru. Am J Trop Med Hyg 2013; 89:507–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Wood R, Liang H, Wu H, et al. Changing prevalence of tuberculosis infection with increasing age in high-burden townships in South Africa. Int J Tuberc Lung Dis 2010; 14:406–12. [PMC free article] [PubMed] [Google Scholar]
- 60. Martinez L, Cords O, Horsburgh CR, Andrews JR; Pediatric TB Contact Studies Consortium . The risk of tuberculosis in children after close exposure: a systematic review and individual-participant meta-analysis. Lancet 2020; 395:973–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Crampin AC, Glynn JR, Traore H, et al. Tuberculosis transmission attributable to close contacts and HIV status, Malawi. Emerg Infect Dis 2006; 12:729–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Middelkoop K, Mathema B, Myer L, et al. Transmission of tuberculosis in a South African community with a high prevalence of HIV infection. J Infect Dis 2015; 211:53–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.