Abstract
Background
The relative costs of preemptive therapy (PET) or prophylaxis for the prevention of cytomegalovirus (CMV) disease in high-risk donor CMV-seropositive/recipient-seronegative (D+/R−) liver transplant recipients have not been assessed in the context of a randomized trial.
Methods
A decision tree model was constructed based on the probability of outcomes in a randomized controlled trial that compared valganciclovir as PET or prophylaxis for 100 days in 205 D+/R− liver transplant recipients. Itemized costs for each site were obtained from a federal cost transparency database. Total costs included costs of implementation of the strategy and CMV disease treatment-related costs. Net cost per patient was estimated from the decision tree for each strategy.
Results
PET was associated with a 10% lower absolute rate of CMV disease (9% vs 19%). The cost of treating a case of CMV disease in our patients was $88 190. Considering cost of implementation of strategy and treatment-related cost for CMV disease, the net cost-savings per patient associated with PET was $8707 compared to prophylaxis. PET remained cost-effective across a range of assumptions (varying costs of monitoring and treatment, and rates of disease).
Conclusions
PET is the dominant CMV prevention strategy in that it was associated with lower rates of CMV disease and lower overall costs compared to prophylaxis in D+/R− liver transplant recipients. Costs were driven primarily by more hospitalizations and higher CMV disease–associated costs due to delayed onset postprophylaxis disease in the prophylaxis group.
Keywords: cytomegalovirus, CMV, transplant, cost-effectiveness, preemptive therapy
Preemptive therapy is more cost-effective than prophylaxis for the prevention of CMV disease in CMV-seronegative liver transplant recipients with seropositive donors. Costs were driven by higher CMV disease-associated costs due to delayed-onset postprophylaxis CMV disease.
Cytomegalovirus (CMV) is a major opportunistic pathogen in organ transplant recipients [1, 2]. Transplantation from a seropositive donor to seronegative recipient (D+/R−) confers the highest risk of CMV infection and disease. CMV infection (detection of viral proteins or nucleic acid) occurs in the majority of D+/R− patients. CMV disease manifests as a viral syndrome or as end-organ disease and is associated with significant morbidity and mortality [3].
CMV disease is also a contributor to increased resource utilization, higher 1-year hospitalizations, and overall posttransplant costs [4–6]. Prevention of CMV is accomplished by administering antiviral therapy either prophylactically from the time of transplant to all at-risk patients or preemptively upon detection of CMV viremia to prevent its progression to CMV disease [7, 8]. Valganciclovir has emerged as the preferred agent and the standard of care for the prevention of CMV in organ transplant populations [8, 9].
An optimal intervention should confer benefits beyond improvement in outcomes, such as resources expended in the management and its implementation. Sparse data exist on cost-effectiveness of CMV prevention strategies in transplant recipients in the current era. In addition, available studies have had 1 or more major limitations, including nonrandomized design, use of older or no longer used diagnostic tests (eg, shell vial culture or non–polymerase chain reaction [PCR]–based assays), use of noncontemporary antivirals (oral ganciclovir), or limited duration of follow-up with failure to assess for CMV disease occurring after the intervention period [10–12]. To address these limitations in prior studies, we performed an in-depth cost-effectiveness assessment using data from a randomized multicenter trial that directly compared preemptive therapy (PET) with prophylaxis for the prevention of CMV disease in donor CMV-seropositive/recipient CMV-seronegative (D+/R−) liver transplant recipients [13].
METHODS
Study Design and Participants
The study population included D+/R− liver transplant recipients >18 years of age who had undergone first orthotopic liver transplant. The study was conducted at transplant centers in the Northeastern, Atlantic, Midwestern, Southern, and Western regions of the United States. Patients who met eligibility criteria were randomized 1:1 in a computerized allocation schema within 10 days of transplantation. Patients in the prophylaxis group received valganciclovir 900 mg orally once daily for 100 days. Patients in the PET group underwent weekly testing for 100 days using a previously described highly sensitive real-time plasma CMV-DNA PCR assay performed at the central laboratory [14]. Upon detection of viremia at any level, valganciclovir 900 mg orally twice daily was administered until 2 consecutive negative tests performed 1 week apart. Recurrent viremia within 100 days was treated similarly to the initial episode. All drug dosages were adjusted for renal dysfunction according to the manufacturer’s product label. The National Institute of Allergy and Infectious Diseases, the institutional review boards of all participating sites, and an independent data and safety monitoring board approved the study. Written informed consent was obtained from all patients or their legally authorized representatives.
Assessments and Cost Sources
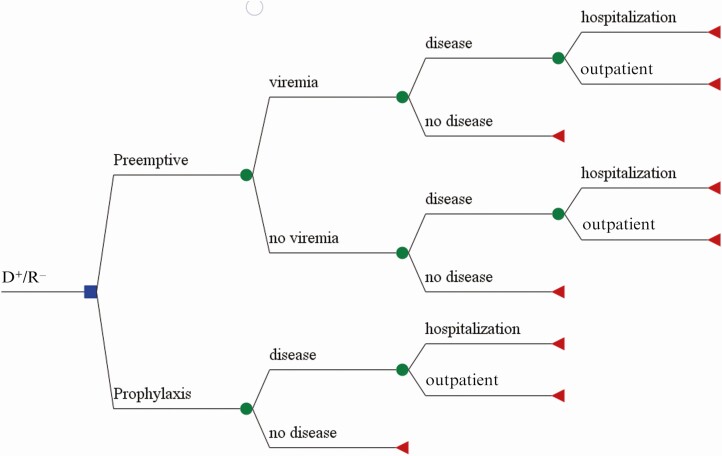
A decision tree analysis model was constructed using TreeAge Pro software (TreeAge Inc, Williamstown, Massachusetts). The model is similar to those previously used for cost-efficacy analysis for the prevention of CMV disease [15–17]. The transitional states for the model were viremia/no viremia (PET group only); CMV disease with inpatient/outpatient management; and no CMV disease in both groups (Figure 1). The probabilities of outcomes at each transition were generated from the trial data (Table 1). Outcomes considered were CMV disease–related inpatient and outpatient medical encounters and included duration of hospitalization (length of stay in total days, and duration by ward and/or intensive care unit [ICU]), number and types of diagnostic procedures performed, and treatment of CMV disease. All costs were based on posttransplantation outcomes by 12 months in all randomized patients. To examine the impact of changes in a parameter on the model’s results, sensitivity analyses were performed across a range of plausible assumptions by systematically varying the costs of PCR monitoring, drug treatment, and CMV disease–associated hospitalizations, as well as the rate of CMV disease. The cost data were from the perspective of the healthcare payor.
Figure 1.
Decision tree for the comparison of costs associated with 2 approaches for the prevention of cytomegalovirus (CMV) disease in high-risk donor CMV-seropositive and recipient CMV-seronegative (D+/R−) liver transplant patients.
Table 1.
Model Parameters Used for the Decision Tree, Derived From Data From the Clinical Trial
| Variable | Probability of the Event |
|---|---|
| PET group only | |
| Probability of viremia | 0.85 |
| CMV disease in patients with viremia | 0.105 |
| CMV disease without viremiaa | 0.001 |
| Prophylaxis group only | |
| Probability of CMV disease | 0.19 |
| Both groups | |
| Inpatient care for CMV disease | 0.79 |
| Outpatient care for CMV disease | 0.21 |
Abbreviations: CMV, cytomegalovirus; PET, preemptive therapy.
aNo CMV disease was documented in this population.
Charges during the study drug administration period (100 days) included cost of average study drug usage per patient in each group, safety laboratory monitoring (complete blood count with differential), and treatment of study drug–related adverse events (AEs). For this analysis, only neutropenia (absolute neutrophil count <500 cells/µL) was considered a drug-related AE, and cost of granulocyte colony-stimulating factor (G-CSF) for the management of neutropenia was assessed. Additional charges in the PET group included costs for weekly testing for real-time plasma CMV-DNA PCR for 100 days.
For cost assessment until 12 months posttransplantation, direct costs associated with the diagnosis, management, and hospitalizations for CMV disease were considered and included diagnostic CMV-DNA PCR testing; diagnostic procedures including liver biopsies, endoscopies, and colonoscopies; hospitalization and ICU utilization; and cost of antiviral treatment for the duration of use for the management of CMV disease.
As of January 2019, the United States (US) Department of Health and Human Services and the Centers for Medicare and Medicaid Services have mandated online reporting of itemized costs and pricing for services provided by all US facilities [18]. The costs of laboratory tests (including costs for CMV-DNA PCR), procedures, and hospitalizations were obtained from this price transparency database as reported by each of the participating centers and were averaged to yield a representative cost (Table 2). No discounting was used in the cost evaluations. Additional laboratory costs were obtained from 2 large national reference laboratories (LabCorp and Quest) [19, 20]. An incremental cost-effectiveness, which represents the cost per CMV disease event prevented, was calculated for both study groups by dividing the difference in cost of strategy implementation by the difference in outcome (CMV disease) [21].
Table 2.
Average Costs Within 12 Months Posttransplant in the Study Population
| Study Drug Administration Period | Cost in 2019 US Dollarsa |
|---|---|
| Valganciclovir 450-mg tabletb | $32.69 |
| Prophylaxis (total) | $6538 |
| PET (total) | $7715 |
| Complete blood count with differential (15 tests) | $1050 |
| G-CSF (filgrastim) for neutropenia/day | $1187 |
| CMV PCR quantitative/test (PET group) (14 weekly tests) | $2044 |
| CMV disease management | |
| ICU, per diem | $10 685 |
| Hospitalization (non-ICU) per diem | $4270 |
| Diagnostic procedures and imaging studies | |
| Colonoscopy with biopsy | $3410 |
| Liver biopsy | $3090 |
| Endoscopy with biopsy | $1902 |
| Abdominal ultrasound | $839 |
| Abdominal CT | $2787 |
| Antiviral therapy for CMV disease | |
| Intravenous ganciclovir/valganciclovir/day | $2641 |
| Other antiviral therapyc | $1446 |
| CMV IVIGc | $2599 |
Abbreviations: CMV, cytomegalovirus; CT, computed tomography; G-CSF, granulocyte colony-stimulating factor; ICU, intensive care unit; IVIG, intravenous immunoglobulin; PCR, polymerase chain reaction; PET, preemptive therapy.
aCosts were averaged over the costs of participating sites, which consist of a geographically diverse set of tertiary care systems.
bCost for prophylaxis based on 900 mg/day for 100 days and cost for PET based on 1800 mg/day for duration of viremia.
cThree patients received foscarnet and 2 patients received CMV IVIG as adjunctive therapy.
RESULTS
Of 205 D+/R− patients, 100 were randomized to PET and 105 to the prophylaxis group. The groups were well-balanced for all baseline characteristics [13]. CMV viremia developed in 81% (81/100) of the PET patients; these patients had a total of 129 episodes of viremia, including 81 initial and 48 recurrent episodes. CMV disease developed in 9% (9/100) of the patients in the PET group and 19% (20/105) in the prophylaxis group (P = .039) and was due primarily to a lower rate of delayed-onset CMV disease (beyond day 100) in the PET group (6/100 [6%] vs 18/105 [17.1%]; P = .014). There was no recurrent CMV disease observed in 1 year of follow-up. Rejection, graft loss, and all-cause mortality were not significantly different between the 2 groups [13].
Costs During Study Drug Administration Period
The cost of CMV-DNA PCR monitoring tests in the PET group was $2044 per patient. PET patients received a median of 57 days (interquartile range [IQR], 39–66.5 days) of valganciclovir compared with 97 days (IQR, 90–99 days) in the prophylaxis group. The study was designed to treat CMV viremia with a treatment dose (1800 mg/day) vs a prophylaxis dose (900 mg/day) of valganciclovir. As such, the total amount of valganciclovir used for the PET group was 7023 g for 100 patients (70.2 g/patient) for PET and 7068 g for 105 patients (67.3 g/patient) for antiviral prophylaxis. The average cost of valganciclovir was higher in the PET group (Table 3). Neutropenia developed in 13% of the PET and 10% of the prophylaxis group patients. The number of patients with neutropenia who received treatment with G-CSF during study drug administration period (6/100 [6%] and 7/105 [6.7%]) and the duration of G-CSF use (median, 3.5 vs 3.1 days) was not significantly different for the PET vs prophylaxis group, respectively (P > .05).
Table 3.
Costs Associated With Cytomegalovirus Prevention Strategies
| Prevention Strategy | CMV Disease Rate | Net Reduction in Disease | Net Costa | Cost of Implementation of Strategy per Patient |
|---|---|---|---|---|
| Treatment of CMV disease | $88 190b | … | ||
| No prevention | 44%–65%c | … | 0 | $38 000–$57 323 |
| Prophylaxis | 19% | 25%–46%d (average 35.5%) | $8706 | $24 756e |
| PET | 9% | 35%–56%d (average 45.5%) | $11 887 | $16 035e |
| PET compared to prophylaxis | 10%f | $3181g | −$8700g |
Abbreviations: CMV, cytomegalovirus; PET, preemptive therapy.
aAverage costs associated with implementation of each strategy (monitoring for viremia, antiviral drug, and treatment of neutropenia).
bCost of treating CMV disease in a patient in this study.
cHistorically reported rates of CMV disease (44%–65%) in D+/R− transplant recipients in the absence of antiviral prevention [22].
dReduction in disease in comparison to historic CMV disease rates without antiviral prevention.
eOverall cost per patient including implementation of strategy and treatment-related costs (antivirals, diagnostic procedures, and hospitalization; hospitalization usage was averaged per strategy).
fReduction in CMV disease rate with preemptive therapy compared to prophylaxis.
gDifference in cost per patient associated with preemptive therapy when compared to prophylaxis.
The total costs during study drug administration period per patient was $11 887 in the PET group and $8706 in the prophylaxis group (Table 3). Compared to prophylaxis, PET therefore cost $3181 more per patient in the first 100 days, related to costs of monitoring (CMV PCR) and drug costs for treating viremia.
CMV Disease–Related Costs Within 12 Months
Hospitalization was required in 23 of 29 (79%) cases of CMV disease; the median length of stay was 8 days (IQR, 3–18 days). ICU management with mechanical ventilation was required in 2 of 29 (6.9%) cases with CMV disease (both in the prophylaxis group) with ICU stay of 14 days in 1 patient and 48 days in another patient. A post hoc evaluation showed that only 3 cases met previously reported criteria criteria for severe CMV disease [19], all cases of severe disease were in the prophylaxis group (P = .2). Diagnostic procedures required for the management of CMV disease in hospitalized patients did not differ for the 2 groups; each case of disease required an average of 2.5 procedures (range, 1–6), the most common being a liver biopsy, followed by colonoscopy and endoscopy with biopsy. The average number of days of antiviral treatment per case of CMV disease was similar (44 and 46 days) for the PET group and prophylaxis group, respectively.
Cost per Disease Case Prevented
Total cost (averaged over both groups) to treat CMV disease was $88 191 per case (Table 3). Average cost per patient that included cost of implementation of strategy and treatment-related cost (antiviral therapy, diagnostic procedures, and hospitalization) was $16 035 for PET and $24 756 for prophylaxis, respectively, with net cost-savings of $8707 per patient with PET (Table 3). Considering historic rates of CMV disease (44%–65%) without the use of any CMV preventive strategy [22], both PET and prophylaxis strategies dominate the option of no prophylaxis (Table 3).
Sensitivity Analyses
Table 4 shows the cost-effectiveness of PET vs prophylaxis with a range of assumptions by varying the cost of CMV PCR testing, study drug, and treatment-related charges for CMV disease. PET was more cost-effective over a range of CMV disease–associated hospitalization days and per diem hospital costs or when the cost per CMV disease case was estimated to be the same for each strategy (Table 4). Only if the incidence of CMV disease in the prophylaxis group decreased to <12%, while assuming that the cost of treatment of CMV disease remained equal regardless of strategy, did prophylaxis become more cost-effective than PET.
Table 4.
Sensitivity Analysis for Cost-effectivenessa
| Parameter | Range of Assumptions | Estimated Cost Saving per Patient Using Preemptive Therapy vs Prophylaxis, 2019 US Dollars |
|---|---|---|
| CMV PCR test costb | $45–$409 | $6300–$11 000 |
| Per diem hospitalizationc | $2500–$6000 | $6700–$10 600 |
| Per diem ICU utilizationc | $8000–$19 000 | $7700–$12 600 |
| Duration of hospitalizationd | 5–21 d | $7600–$10 200 |
| Duration of ICU stayd | 0–7 d | $7000–$11 000 |
| Percentage of CMV disease cases hospitalizede | 58–93 | $5500–$10 600 |
| Percentage with viremia in preemptive therapy groupf | 67–85 | $8500–$10 100 |
| Average cost for treatment of CMV disease, regardless of strategyg | $72 000–$106 000 | $3600–$8300 |
| Rate of CMV disease in prophylaxis groupf | .12–.30 | −$1100h to $11 700 |
| Rate of CMV disease in PET groupf | .02–.15 | $1200–$9900 |
Abbreviations: CMV, cytomegalovirus; ICU, intensive care unit; PCR, polymerase chain reaction; PET, preemptive therapy.
aData depict 1-way sensitivity analysis where 1 parameter/assumption at a time was changed across the range, while holding the other parameters constant at the estimate obtained in the clinical trial (base model). The base model takes into account the average cost of monitoring, cost of drugs given during prophylaxis, cost of drugs for preemptive therapy, antiviral treatment for CMV disease, disease rate for each strategy, and duration/cost of hospitalization and ICU utilization associated with disease in each strategy.
bCost ranges include the highest and lowest cost in geographically diverse study sites as well as reference laboratories.
cCost ranges include the highest and lowest costs in geographically diverse study sites.
dDays are the interquartile range for duration of hospitalizations and ICU utilization for CMV disease in patients in this clinical trial.
eConfidence intervals on proportion of disease subjects requiring hospitalization in the clinical trial.
gInterquartile range for cost of treatment of disease, a composite of hospital utilization, drug costs, and diagnostic procedures averaged regardless of strategy.
hProphylaxis was the dominant strategy when the rate of disease in that group was <12% with disease costs averaged across both groups.
DISCUSSION
This cost analysis from our randomized controlled trial of PET vs prophylaxis in D+/R− liver transplant recipients showed that PET incurred lower overall costs than prophylaxis and that this difference was due primarily to the higher costs associated with hospitalization and management of delayed-onset postprophylaxis CMV disease in the postintervention period. Considering historic rates of CMV disease prior to the routine employment of antiviral agents for the prevention of CMV in D+/R− patients [22], both strategies are cost-effective (Table 3). The higher rate of CMV disease in the prophylaxis group is comparable to that seen in other randomized clinical trials [23–25]. The overall cost of treating an average case of CMV disease in our study was $88 191. Given the high rate of hospitalization associated with CMV disease and the resultant healthcare costs, PET, even with the higher implementation costs, resulted in an overall savings compared to prophylaxis.
Previous comparisons of PET vs prophylaxis in organ transplant recipients have been mainly in kidney transplant recipients. A retrospective study in CMV-seropositive kidney transplant recipients showed that prophylaxis was the more cost-effective of the 2 strategies [17]. However, the primary endpoint was CMV infection and not CMV disease, and assumptions in the PET group were based on historic data, most published over a decade ago [17]. Another study also concluded that prophylaxis was more cost-effective than PET [11]. Probabilities of outcomes in this report were extrapolated from older studies where shell-vial culture was used for CMV monitoring, PET comprised intravenous ganciclovir, and follow-up was limited to 6 months [11]. A more recent study in kidney transplant recipients found that both strategies were equally effective in preventing CMV disease with similar overall costs that remained comparable even with varying costs of monitoring and drug costs in sensitivity analysis [26]. However, the study included a small number of D+/R− patients and therefore the number of patients with primary CMV disease was limited [26]. The characteristics of our study—that is, randomized controlled design with parallel groups, use of valganciclovir as study drug for prevention, and use of PCR-based monitoring for CMV—address important limitations in prior studies and provide the most precise estimates for cost analyses in the current era.
To our knowledge, only 1 previous study from Europe has assessed cost-effectiveness of CMV preventive strategies in liver transplant recipients [12]. However, the outcomes data were based not on a specific trial but rather on expert opinions and published reports [12]. As such, the antiviral agent and regimens, duration of therapy, and CMV detection assays employed varied considerably [12]. It was concluded that drug costs were the primary cost driver. In contrast, drug therapy did not account for the difference in the in costs in our study. Instead, hospitalization-associated costs for the treatment of CMV disease in the postintervention period were the key determinants of overall cost differences between the 2 groups. Although the numbers were small, it is also plausible that PET patients with CMV disease were less ill than those in the prophylaxis group. This was supported by fewer cases of severe CMV disease in the PET group and that the 2 cases with CMV disease who required intensive care with mechanical ventilation were in the prophylaxis group. Greater impairment in CMV-specific immune responses could also account for more severe disease after prophylaxis than PET.
There are several potential limitations of the study. Although the charges are as close an approximation of actual costs as possible, it is plausible that the reported cost estimations over- or understate the actual costs. The participating sites represent a cross-section of transplant centers in diverse geographic locations, and it is possible that each center incurred unique costs that may not be generalizable. However, since randomization was stratified by site, these affects should theoretically have been controlled for in the analyses. The cost of personnel effort devoted for the implementation of PET was not assessed. However, savings from just 1–2 cases of CMV disease prevented (exceeding $88 000) could reasonably cover the effort of a coordinator in most US centers. Additionally, indirect costs such as lost work time, incidental transportation, and family caretaker costs were not evaluated. Based on the primary goal of the study (prevention of CMV disease), costs associated with all CMV disease–related hospitalizations were systematically analyzed. However, assessment for costs of medical encounters other than CMV disease was beyond the scope of this trial and cost-effectiveness analysis. Nevertheless, we note that the rates of opportunistic bacterial and fungal infections, rejection, graft loss, and retransplantation were similar for the 2 groups [13]. Thus, it seems unlikely that non–CMV disease–related costs differed significantly for the study groups. The study did not translate CMV disease episodes into quality-adjusted life-years (QALYs) and did not compute cost per QALY. Nevertheless, this is an acceptable approach for the study at hand given that in the base-case (yielded by the model) and almost every scenario in the sensitivity analyses (Table 4), PET was the dominant strategy. We used any detectable viremia as the trigger for the initiation and discontinuation of PET. Current guidelines acknowledge that optimal viral load thresholds for the initiation of therapy have not been determined [7]. Once established, this may change the duration of antiviral therapy and resultant costs. Nevertheless, given that detection of any level of viremia indicates primary infection in CMV-seronegative patients, and because of known rapid viral kinetics in this setting [27], PET was initiated upon first detection of viremia. Last, 1-way sensitivity analysis was performed to clearly demonstrate the impact of the particular parameter on model’s results. It is possible that >1 parameter when changed simultaneously could yield different results.
Strengths of our study include use of federally mandated and maintained resources, and a transparent database for cost information for each of the participating hospitals in geographically diverse areas. The modeling was based on complete patient-level outcomes data from a randomized clinical trial in a homogenous population where the 2 study groups received the same local standard of care and differed only in the CMV prevention approach used. This study represents the only formal cost-effectiveness analysis derived from data from a randomized multicenter trial in high-risk D+/R− liver transplant recipients.
In summary, PET dominates prophylaxis (and the historic alternative of no preventive intervention) in that it reduces both CMV disease and is associated with lower overall costs in high-risk D+/R− liver transplant recipients. Similar trials of PET vs prophylaxis should be considered for other high-risk organ transplant recipients.
Notes
Presented in part: American Transplant Congress, Boston, Massachusetts, 3 June 2019. Abstract 2855.
Financial support. This work was supported by the National Institutes of Health (contract number HHSN272201100041C to the University of Pittsburgh).
Potential conflicts of interest. D. J. W. has received research support from Merck, Chimerix, Shire, Gilead, and Oxford Immnotech. G. M. L. has received research support from Takeda and Shire Pharmaceuticals. F. P. S. has received research support from Shire, Qiagen, Whiscon, Novartis, and Ansun. A. P. L. has received research support from Merck and Astellas, and consulting support from Helocyte, Amplyx, Novartis, Merck, and AlloVir. R. R. R. reports grants to his institution from Roche and Regeneron, outside the submitted work. All other authors report no potential conflicts of interest. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1. Ho M. The history of cytomegalovirus and its diseases. Med Microbiol Immunol 2008; 197:65–73. [DOI] [PubMed] [Google Scholar]
- 2. Lumbreras C, Manuel O, Len O, ten Berge IJ, Sgarabotto D, Hirsch HH. Cytomegalovirus infection in solid organ transplant recipients. Clin Microbiol Infect 2014; 20:19–26. [DOI] [PubMed] [Google Scholar]
- 3. Ljungman P, Boeckh M, Hirsch HH, et al. Disease Definitions Working Group of the Cytomegalovirus Drug Development Forum . Definitions of cytomegalovirus infection and disease in transplant patients for use in clinical trials. Clin Infect Dis 2017; 64:87–91. [DOI] [PubMed] [Google Scholar]
- 4. Falagas ME, Arbo M, Ruthazer R, et al. Cytomegalovirus disease is associated with increased cost and hospital length of stay among orthotopic liver transplant recipients. Transplantation 1997; 63:1595–601. [DOI] [PubMed] [Google Scholar]
- 5. Kim WR, Badley AD, Wiesner RH, et al. The economic impact of cytomegalovirus infection after liver transplantation. Transplantation 2000; 69:357–61. [DOI] [PubMed] [Google Scholar]
- 6. McCarthy JM, Karim MA, Krueger H, Keown PA. The cost impact of cytomegalovirus disease in renal transplant recipients. Transplantation 1993; 55:1277–82. [DOI] [PubMed] [Google Scholar]
- 7. Kotton CN, Kumar D, Caliendo AM, et al. The Transplantation Society International CMV Consensus Group . The third international consensus guidelines on the management of cytomegalovirus in solid-organ transplantation. Transplantation 2018; 102:900–31. [DOI] [PubMed] [Google Scholar]
- 8. Andrews PA, Emery VC, Newstead C. Summary of the British Transplantation Society Guidelines for the prevention and management of CMV disease after solid organ transplantation. Transplantation 2011; 92:1181–7. [DOI] [PubMed] [Google Scholar]
- 9. Levitsky J, Singh N, Wagener MM, Stosor V, Abecassis M, Ison MG. A survey of CMV prevention strategies after liver transplantation. Am J Transplant 2008; 8:158–61. [DOI] [PubMed] [Google Scholar]
- 10. Das A. Cost-effectiveness of different strategies of cytomegalovirus prophylaxis in orthotopic liver transplant recipients. Hepatology 2000; 31:311–7. [DOI] [PubMed] [Google Scholar]
- 11. Mauskopf JA, Richter A, Annemans L, Maclaine G. Cost-effectiveness model of cytomegalovirus management strategies in renal transplantation. Comparing valaciclovir prophylaxis with current practice. Pharmacoeconomics 2000; 18:239–51. [DOI] [PubMed] [Google Scholar]
- 12. Annemans L, Moeremans K, Mutimer D, Schneeberger H, Milligan D, Kubin M. Modeling costs and cost-effectiveness of different CMV management strategies in liver transplant recipients as a support for current and future decision making. Value Health 2002; 5:347–58. [DOI] [PubMed] [Google Scholar]
- 13. Singh N, Winston DJ, Razonable RR, et al. Effect of preemptive therapy vs antiviral prophylaxis on cytomegalovirus disease in seronegative liver transplant recipients with seropositive donors: a randomized clinical trial. JAMA 2020; 323:1378–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Limaye AP, Green ML, Edmison BC, et al. Prospective assessment of cytomegalovirus immunity in high-risk donor-seropositive/recipient-seronegative liver transplant recipients receiving either preemptive therapy or antiviral prophylaxis. J Infect Dis 2019; 220:752–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Luan FL, Stuckey LJ, Park JM, Kaul D, Cibrik D, Ojo A. Six-month prophylaxis is cost effective in transplant patients at high risk for cytomegalovirus infection. J Am Soc Nephrol 2009; 20:2449–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Rose DN, Sacks HS. Cost-effectiveness of cytomegalovirus (CMV) disease prevention in patients with AIDS: oral ganciclovir and CMV polymerase chain reaction testing. AIDS 1997; 11:883–7. [DOI] [PubMed] [Google Scholar]
- 17. Luan FL, Kommareddi M, Ojo AO. Universal prophylaxis is cost effective in cytomegalovirus serology-positive kidney transplant patients. Transplantation 2011; 91:237–44. [DOI] [PubMed] [Google Scholar]
- 18. Centers for Medicare and Medicaid Services. Medicare.gov procedure price lookup. Available at: https://www.medicare.gov/procedure-price-lookup/. Accessed 15 October 2019. [Google Scholar]
- 19. Quest Diagnostics. Test directory. Available at: https://testdirectory.questdiagnostics.com/test/test-detail/10601/cytomegalovirus-dna-qualitative-real-time-pcrcc. Accessed 31 March 2020. [Google Scholar]
- 20. LabCorp Net Fee Schedule. Available at: http://www.dbhds.virginia.gov/library/administration/ADM%20-%2004093%20LabCorp%20Pricing%20Sheet%202015-2017.pdf. Accessed 31 March 2020. [Google Scholar]
- 21. Generics and Biosimilars Initiative. What is the incremental cost-effectiveness ratio (ICER)? Available at: http://www.gabionline.net/Generics/General/What-is-the-incremental-cost-effectiveness-ratio-ICER. Accessed 31 March 2020. [Google Scholar]
- 22. Razonable RR. Cytomegalovirus infection after liver transplantation: current concepts and challenges. World J Gastroenterol 2008; 14:4849–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Gardiner BJ, Chow JK, Price LL, Nierenberg NE, Kent DM, Snydman DR. Role of secondary prophylaxis with valganciclovir in the prevention of recurrent cytomegalovirus disease in solid organ transplant recipients. Clin Infect Dis 2017; 65:2000–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Paya C, Humar A, Dominguez E, et al. Valganciclovir Solid Organ Transplant Study Group . Efficacy and safety of valganciclovir vs. oral ganciclovir for prevention of cytomegalovirus disease in solid organ transplant recipients. Am J Transplant 2004; 4:611–20. [DOI] [PubMed] [Google Scholar]
- 25. Liu AW, Jutivorakool K, Fisher CE, et al. Comparison of preemptive therapy and antiviral prophylaxis for prevention of cytomegalovirus in seropositive liver transplant recipients. Transplantation 2018; 102:632–9. [DOI] [PubMed] [Google Scholar]
- 26. Khoury JA, Storch GA, Bohl DL, et al. Prophylactic versus preemptive oral valganciclovir for the management of cytomegalovirus infection in adult renal transplant recipients. Am J Transplant 2006; 6:2134–43. [DOI] [PubMed] [Google Scholar]
- 27. Emery VC, Sabin CA, Cope AV, Gor D, Hassan-Walker AF, Griffiths PD. Application of viral-load kinetics to identify patients who develop cytomegalovirus disease after transplantation. Lancet 2000; 355:2032–6. [DOI] [PubMed] [Google Scholar]
- 28. Kalil AC, Mindru C, Botha JF, et al. Risk of cytomegalovirus disease in high-risk liver transplant recipients on valganciclovir prophylaxis: a systematic review and meta-analysis. Liver Transpl 2012; 18:1440–7. [DOI] [PubMed] [Google Scholar]
- 29. Limaye AP, Green ML, Edmison BC, et al. Prospective assessment of cytomegalovirus immunity in high-risk donor-seropositive/recipient-seronegative liver transplant recipients receiving either preemptive therapy or antiviral prophylaxis. J Infect Dis 2019; 220:752–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Sun HY, Cacciarelli TV, Wagener MM, Singh N. Preemptive therapy for cytomegalovirus based on real-time measurement of viral load in liver transplant recipients. Transpl Immunol 2010; 23:166–9. [DOI] [PubMed] [Google Scholar]
- 31. Atabani SF, Smith C, Atkinson C, et al. Cytomegalovirus replication kinetics in solid organ transplant recipients managed by preemptive therapy. Am J Transplant 2012; 12:2457–64. [DOI] [PMC free article] [PubMed] [Google Scholar]