Abstract
Background
Pregnancy is accompanied by immune suppression. We hypothesized that Mycobacterium tuberculosis-specific inflammatory responses used to identify latent tuberculosis infection (LTBI) lose positivity during pregnancy. We also hypothesized that isoniazid preventive therapy (IPT) may revert LTBI diagnoses because of its sterilizing activity.
Methods
944 women with human immunodeficiency virus infection (HIV) participating in a randomized, double-blind, placebo-controlled study comparing 28 weeks of IPT antepartum versus postpartum, were tested by QuantiFERON-gold-in-tube (QGIT) antepartum and by QGIT and tuberculin skin test (TST) at delivery and postpartum. Serial QGIT positivity was assessed by logistic regression using generalized estimating equations.
Results
From entry to delivery, 68 (24%) of 284 QGIT-positive women reverted to QGIT-negative or indeterminate. Of these, 42 (62%) recovered QGIT positivity postpartum. The loss of QGIT positivity during pregnancy was explained by decreased interferon gamma (IFNγ) production in response to TB antigen and/or mitogen. At delivery, LTBI was identified by QGIT in 205 women and by TST in 113 women. Corresponding numbers postpartum were 229 and 122 women. QGIT and TST kappa agreement coefficients were 0.4 and 0.5, respectively. Among QGIT-positive women antepartum or at delivery, 34 (12%) reverted to QGIT-negative after IPT. There were no differences between women who initiated IPT antepartum or postpartum.
Conclusions
Decreased IFNγ responses in pregnancy reduced QGIT positivity, suggesting that this test cannot reliably rule out LTBI during pregnancy. TST was less affected by pregnancy, but had lower positivity compared to QGIT at all time points. IPT was associated with loss of QGIT positivity, the potential clinical consequences of which need to be investigated.
Keywords: latent tuberculosis infection, interferon gamma response assays, pregnancy, HIV infection, isoniazid preventive therapy
Adriana Weinberg, Lisa Aaron, Grace Montepiedra, Timothy R. Sterling, Renee Browning, Blandina Mmbaga, Tichaona Vhembo, Shilpa Naik, Enid Kabugho, Gaerolwe Masheto, Savita Pahwa, Jyoti S. Mathad, Sylvia M. LaCourse, Katie McCarthy, Sarah Bradford, Gerhard Theron, Diane Costello, Bonnie Zimmer, Marie F. Pierre, Kamunkhwala Gausi, Paolo Denti, David W. Haas, and Amita Gupta; for the IMPAACT P1078 study team
The sensitivity of QuantiFERON-gold-in-tube decreased during pregnancy. QuantiFERON-gold-in-tube and tuberculin skin testing had modest agreement, with QuantiFERON-gold-in-tube more likely to detect latent TB infection. 12% of participants positive by QuantiFERON-goldin-tube during pregnancy became negative after completion of isoniazid treatment.
Tuberculosis (TB) is the most common opportunistic infection and the most important cause of morbidity and mortality among people with human immunodeficiency virus (HIV) in low-income settings. This prompted the World Health Organization (WHO) to recommend administration of isoniazid preventive therapy (IPT) to all people with HIV living in TB endemic areas. TB predominantly affects young adults, including women of childbearing potential [1]. Nevertheless, very few TB-related studies have been conducted in pregnant women with or without HIV.
IMPAACT P1078 was a phase IV randomized double-blind placebo-controlled trial that evaluated the safety of immediate (antepartum-initiated) versus deferred (postpartum-initiated) IPT among women with HIV in high TB prevalence settings [2]. This was the first large study to investigate the use of IPT in pregnancy. It enrolled 956 pregnant women on combination antiretroviral therapy (ART) from sub-Saharan Africa, India, Thailand, and Haiti. In this report, we present a substudy of IMPAACT P1078, in which we investigated the effect of pregnancy on the positivity of tests used to identify latent TB infection (LTBI).
The diagnosis of LTBI relies on tuberculin skin test (TST) and IFNγ release assay (IGRA) results [3]. IGRA is preferred for several reasons: 1) higher specificity in the general population compared to TST; 2) higher sensitivity in people with HIV; 3) reduced variability of the manufacturing process; 4) reduced dependence on the skills of health care workers placing and/or reading the TST; and 5) ability to obtain the results after a single visit [4–11]. However, IGRA also has limitations, including variability of test results related to specimen handling, cost, need for laboratory infrastructure, and restricted availability in areas of high TB incidence [6, 12].
The goal of this substudy was to test the hypothesis that pregnancy decreases the sensitivity of IGRA and TST due to maternal immune suppression, which is an adaptation to prevent alloreactive rejection of the fetus [13]. Since IFNγ responses are primarily effector responses, we also hypothesized that IPT may decrease the sensitivity of IGRA by reducing the exposure of the immune system to TB antigens. The main objectives of this study were: 1) to determine the effect of pregnancy and IPT on IGRA and TST; 2) to compare the results of IGRA with TST at delivery and postpartum; and 3) to identify factors associated with the diagnosis of LTBI in pregnancy in women with HIV residing in areas of high TB prevalence.
METHODS
Study Design
P1078 was a prospective, randomized, double-blind, placebo-controlled trial conducted at 13 sites in 8 countries with high TB prevalence (≥60 per 100 000 population) [2]. Participants were randomized to initiate isoniazid during pregnancy (immediate arm) or 12 weeks postpartum (deferred arm). Main eligibility criteria included pregnancy between 14 and 34 weeks, HIV infection; and no suspected TB, recent known TB exposure, or TB treatment >30 days in the previous year. The study was approved by the local ethics committees, and all women provided written informed consent. Randomization was stratified according to the gestational age at entry (>=14 weeks to <24 weeks, or >=24 weeks to <=34 weeks) and was balanced at each site.
IGRA and TST
The IGRA used QuantiFERON-Gold-in-tube (QGIT) performed at certified local laboratories at entry, delivery (+ <12 weeks), and at 44 weeks postpartum, using kits provided by the study. The test was performed and interpreted as per manufacturer’s instructions. TST was placed by trained nurses at delivery and 44 weeks postpartum using a tuberculin product available at the clinical site and read 2–3 (<7) days later without knowledge of the QGIT results. Positive results were defined by ≥5 mm induration.
Assessment of TB Incident Disease
At every scheduled visit, we performed a standardized TB exposure history since the previous visit; a WHO-recommended TB symptom screen; other symptom assessment; and targeted physical examination. Women were educated about the signs and symptoms of TB disease and were asked to come to the clinic should any of the symptoms develop during the course of the study.
NAT2 Genotyping
Four NAT2 polymorphisms, rs1801279 (NAT2*14), rs1801280 (NAT2*5), rs1799930 (NAT2*6), and rs1799931 (NAT2*7) were genotyped as previously described and categorized as slow acetylator, homozygous for a variant allele at any locus (ie, AA, CC, AA, AA, respectively), or heterozygous at 2 or more loci; intermediate acetylator, heterozygous at a single locus; or rapid acetylator, no variant allele at any locus (ie, GG, TT, GG, GG, respectively) [14].
Statistical Analysis
Generalized estimating equation models were fit to assess the trend over time in diagnosis of LTBI using QGIT at entry, delivery, and week 44 postpartum and the association of QGIT results with study arm and important baseline characteristics, including maternal age, nutritional status measured by mid upper arm circumference (MUAC), CD4 count, plasma HIV RNA, duration of ART, and gestational age at entry. Indeterminate QGIT results were considered negative for this analysis. Linear mixed modeling was performed on the quantitative IFNγ values of Nil, TB-antigen, and PHA-mitogen.
Logistic regression models were used to investigate the association of study arm and of baseline characteristics, including NAT2 acetylator status, with QGIT conversion from negative to positive, reversion from positive to negative, or indeterminate. All multivariable models included study arm and factors with P ≤ .15 in univariate analysis.
Concordance between QGIT and TST was assessed using the Kappa measure of agreement and conditional logistic regression. The Kappa statistic was interpreted as follows: < 0 as poor, 0–0.20 as slight, 0.21–0.40 as fair, 0.41–0.60 as moderate, 0.61–0.80 as substantial, and 0.81–1.00 as almost perfect.
RESULTS
Characteristics of the Study Population
The analysis included 944 women with QGIT results at entry. The median CD4 count at entry was 521 cells/µL and 63% of participants had undetectable HIV plasma RNA (Table 1). All women received ART consisting of 3 drugs before or at study entry, which was reflected in the increase in CD4 count over time (Supplementary Table S1). One-third of participants entered the study at 14–24 weeks gestational age. There were no differences in demographic or HIV disease characteristics between study arms. Of 944 women, 781 (83%) completed the week 44 postpartum visit.
Table 1.
Demographic and HIV Disease Characteristics of the Participants at Study Entry
| Immediate INH (N = 471) | Deferred INH (N = 473) | |
|---|---|---|
| Age—years | ||
| Median | 29 | 29 |
| IQR | 25–33 | 24–33 |
| Country—no. (%) | ||
| Botswana | 59 (12.5) | 60 (12.7) |
| Haiti | 5 (1.1) | 10 (2.1) |
| India | 17 (3.6) | 15 (3.2) |
| South Africa | 90 (19.1) | 91 (19.2) |
| Tanzania | 41 (8.7) | 39 (8.2) |
| Thailand | 15 (3.2) | 18 (3.8) |
| Uganda | 82 (17.4) | 83 (17.5) |
| Zimbabwe | 162 (34.4) | 157 (33.2) |
| Gestational age, weeks—no. (%) | ||
| 14–<24 | 159 (33.8) | 157 (33.2) |
| 24–34 | 312 (66.2) | 316 (66.8) |
| CD4 count—cells/mm3 | ||
| Median | 491 | 498 |
| IQR | 351–668 | 351–676 |
| HIV RNA < Lower Limit of Quantification | 299/470 (63.6) | 295/472 (62.5) |
| Time on Current ARV Regimen— months | ||
| Median | 3 | 3 |
| IQR | 1–14 | 1–17 |
| Mid Upper Arm Circumference—cm | ||
| Median | 29 | 28 |
| IQR | 26–31 | 26–31 |
| Mid Upper Arm Circumference—no. (%) | ||
| Moderate/Severe Malnutrition: <21 | 2/471 (0.4) | 1/471 (0.2) |
| Mild Malnutrition: 21–23 | 15/471 (3.2) | 22/471 (4.7) |
| Normal: >23 | 454/471 (96.4) | 448/471 (95.1) |
| Maximum BMI during pregnancy– kg/m2 | ||
| Median | 28 | 28 |
| IQR | 25–31 | 24–31 |
Changes in QGIT Diagnostic Characteristics During Pregnancy and Postpartum
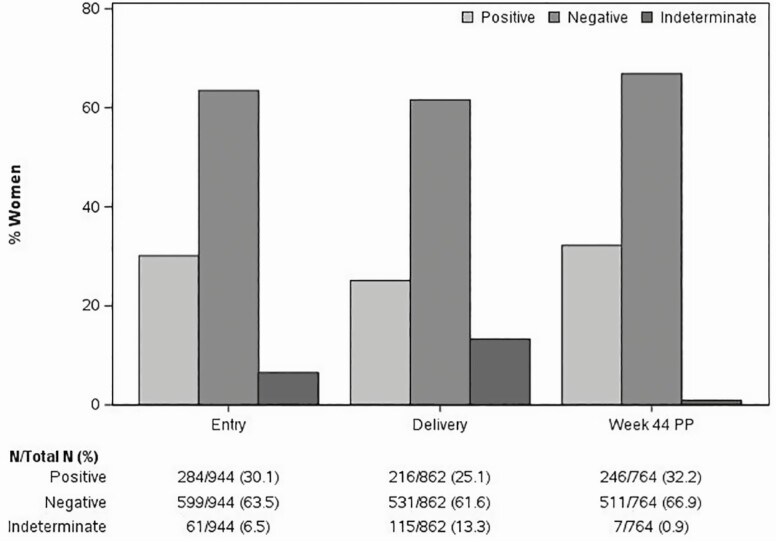
At entry, 284 of 944 participants (30%) had a positive QGIT and 61 (7%) were indeterminate due to low IFNγ responses to PHA-positive control (Figure 1). At delivery, only 25% had positive and 13% indeterminate QGIT results. Postpartum, there was a rebound in QGIT-positive results to 32% and a decrease in indeterminate results to <1%. Moreover, among 68 women who reverted QGIT from entry to delivery, 42 recovered QGIT positivity postpartum.
Figure 1.
Changes in the proportions of QGIT-positive, negative, and indeterminate results during pregnancy and postpartum in women with HIV. Data were derived from 944 women enrolled during the 2nd or 3rd trimester of pregnancy, and longitudinally followed up. The bars indicate the proportions of QGIT-positive, negative, and indeterminate at the time points indicated on the abscissa. Absolute numbers are reported under the graph. Among women with indeterminate results, 61 out of 61 at entry, 115 out of 115 at delivery, and 5 out of 6 at week 44 PP had IFNγ responses to TB antigen below the threshold considered positive. Differences across time points were significant with P < .001 using generalized estimating equations.
The likelihood of QGIT-positive result varied significantly over time (P < .001) with odds ratios of 0.8 at delivery and 1.1 postpartum compared to entry (Supplementary Table S2). Higher CD4 cell count at entry was associated with higher likelihood of QGIT-positive result, while study arm, plasma HIV RNA, duration of ART, and gestational age at entry were not (Supplementary Table S2).
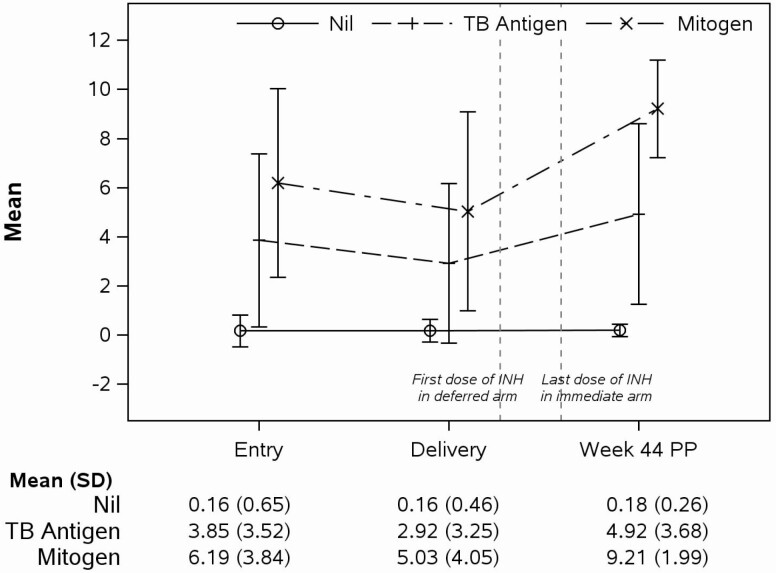
The quantitative analysis of the IFNγ production in response to Nil-negative control, TB-antigen, and PHA-positive control showed significantly lower IFNγ responses to TB-antigen and PHA at delivery and higher responses to TB-antigen, Nil and PHA postpartum compared with entry (Figure 2). In women QGIT-positive at entry, the change from entry to delivery in IFNγ responses to TB-antigen and PHA were significantly correlated (slope point estimate = 0.177, P < .001). Multivariable analyses showed that responses to TB-antigen significantly increased with higher CD4 count (P < .001) and higher MUAC (P = .045) at entry; to PHA with higher CD4 count and longer duration of ART (P < .001 for both); and there were no significant predictors of responses to Nil (Supplementary Tables S3–S5).
Figure 2.
Changes in IFNγ production measured by QGIT in women with positive QGIT results postpartum. Data were derived from 246 women who were QGIT-positive at 44 weeks postpartum. The vertical interrupted lines indicate the times when participants in the deferred arm started IPT and when the last dose of IPT was administered to participants in the immediate arm. The graph shows mean and SD at the time points indicated on the abscissa. Numbers are also reported under the graph. IFNγ production to TB and mitogen responses was significantly different between any time points (P < .001; linear mixed models). Responses to Nil were significantly different from entry and delivery to postpartum only (P < .001). Abbreviation: SD, standard deviation.
QGIT Conversions From Negative at Entry to Positive During The Study
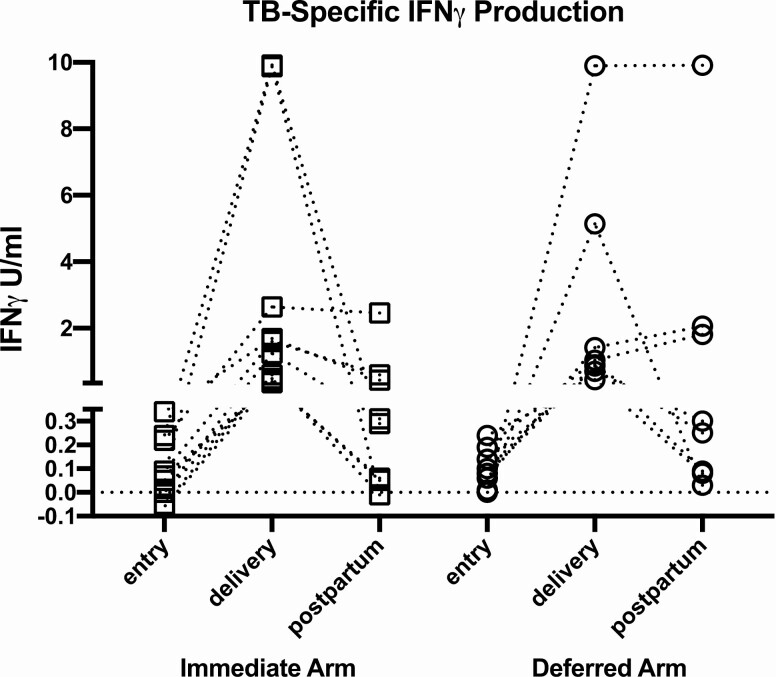
Among women QGIT-negative at entry who underwent retesting, 19 of 553 (3.4%) converted to QGIT-positive at delivery and 33 of 484 (6.8%) postpartum, with no significant differences between treatment arms. Converters had median (range) CD4 cells/µL of 513 (203–778) and nonconverters 483 (7-1332). Conversions from entry to delivery, which were not confounded by the pregnancy-associated immunosuppression, were further investigated. Among 19 women who converted from entry to delivery, 3 participants in the immediate and 3 participants in the deferred arm sustained QGIT positivity postpartum; 11 reverted; and 2 lacked postpartum data (Figure 3). The TB-specific IFNγ responses before and after conversion varied, but very few were close to the cut-off of 0.35 U/mL, such that most conversions and reversions were unambiguous. None of the QGIT converters and, in fact, none of the participants reported exposures to TB during the study. A multivariable logistic regression analysis of the risk of QGIT conversion from entry to delivery, including demographics, HIV disease characteristics, and treatment arm failed to identify any significant associations (Supplementary Table S6).
Figure 3.
Quantitative TB antigen responses in QGIT converters from negative at entry to positive at delivery. Data were derived from 19 participants, including 11 in the immediate and 8 in the deferred IPT arms. Each point indicates TB-nil responses at the time point designated on the abscissa. The lines connect data from individual participants. The ordinate was organized in segments separating QGIT-negative results <0.35 IFNγ U/mL from positive results. Three participants in each arm maintained QGIT positivity postpartum; 6 participants in the immediate arm, and 5 in the deferred arm reverted to QGIT-negative postpartum; and 2 participants in the immediate arm lacked postpartum data.
QGIT conversions during IPT were further investigated. Women had >93% adherence to the study medication by self-report and/or pill count in both arms. The distribution of NAT2 genotypes did not significantly differ between QGIT converters and nonconverters across both immediate and deferred arms, but in the immediate arm, there was a statistically nonsignificant increase in conversions among NAT2 rapid acetylators (n = 32), who had lower levels of plasma isoniazid, compared with intermediate or slow acetylators (n = 180; 9% vs. 3%, P = .14, Supplementary Table S7).
QGIT Reversion From Positive at Entry to Negative During The Study
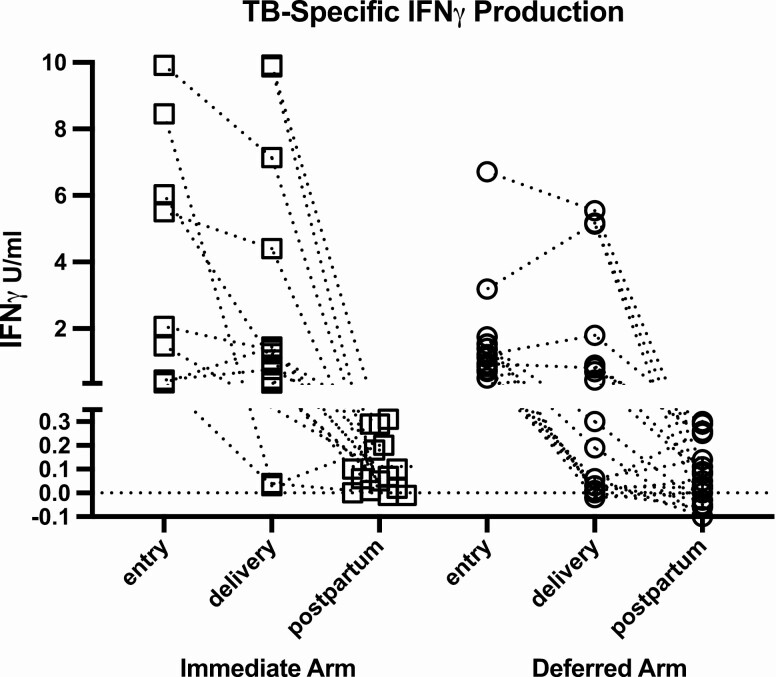
Among women who were QGIT-positive at entry or delivery, 58 of 251 subsequently tested (23%) reverted to negative at delivery, 24 of 229 (10%) reverted from entry to postpartum, and 24 of 187 (13%) from delivery to postpartum. The analysis of reversions from entry or delivery to postpartum, which were not confounded by pregnancy-associated immunosuppression, revealed a range of TB-specific IFNγ responses at entry or delivery, but only few results close to the positivity threshold of 0.35 U/mL (Figure 4). A logistic regression analysis of the factors associated with QGIT reversions identified low TB-specific IFNγ responses and low gestational age at entry to have statistically significant effects (P < .001; Supplementary Table S8).
Figure 4.
Quantitative TB antigen responses in QGIT reverters from positive at entry or delivery to negative postpartum. Data were derived from 15 participants in the immediate arm and from 19 participants in the deferred IPT arms. The points represent TB-nil responses at the designated visit. The lines connect data from individual participants. The ordinate was organized in segments separating QGIT-negative results <0.35 IFNγ U/ml from positive results.
We hypothesized that QGIT reversions from either antepartum or delivery to postpartum might be associated with elimination of M. tuberculosis antigens due to isoniazid. Women with the deferred arm were on isoniazid at the time of reversion by study design. To verify our hypothesis in women in the immediate IPT arm, we reviewed the treatment timeline in those who reverted between delivery and postpartum and determined that all 14 women were still on isoniazid at delivery and finished treatment 30 to 196 days after delivery.
Concordance Between QGIT and TST Results at Delivery and Postpartum
TST was positive in 127 of 858 women tested at delivery (15%) and 126 of 735 postpartum (17%). Among 713 women with both QGIT and TST results at delivery and 714 postpartum, the agreement between tests was moderate with Kappa coefficients of 0.41 and 0.46 (Table 2A and 2B). When the tests were discordant, results were more likely to be positive by QGIT than TST with odds ratios (95% CI) of 4.3 (2.8–6.8) at delivery and 6.4 (3.9–10.7) at postpartum (P < .001).
Table 2A.
Agreementa between QGIT and TST Results at Delivery
| TST Result | ||||
|---|---|---|---|---|
| Positive | Negative | Total | ||
| QGIT Result | Positive | 85 | 121 | 206 |
| Negative | 28 | 480 | 508 | |
| Total | 113 | 601 | 714 |
a Kappa coefficient (95% CI) = 0.41 (.34–.49).
Table 2B.
Agreementa between QGIT and TST Results Postpartum
| TST Result | ||||
|---|---|---|---|---|
| Positive | Negative | Total | ||
| QGIT Result | Positive | 102 | 127 | 229 |
| Negative | 20 | 465 | 485 | |
| Total | 122 | 592 | 714 |
a Kappa coefficient (95% CI) = 0.46 (.39–.53).
QGIT and TST in Women Who Developed TB Disease on Study
Six women, 3 in the immediate and 3 in the deferred arm, developed TB disease, all at a median of 32 weeks postpartum (range 10 to 44 weeks). There were no known exposures (Table 3). Four women were QGIT-positive at diagnosis, including 1 woman who was QGIT-negative at entry and converted during the study, and 2 who were negative or indeterminate. Among the 6 women who developed TB disease, 3 were TST-positive and 3 were TST-negative prior to diagnosis.
Table 3.
QGIT and TST Results in Women Who Developed TB Disease during the Study
| Participant | Treatment group | Week of onset relative to delivery | Type of MTB | INH susceptibility profile | CD4 at entry | QGIT result at entry | Last QGIT result before diagnosis | Last TST result before diagnosis |
|---|---|---|---|---|---|---|---|---|
| 1 | Immediate INH | 48PP | Confirmed pulmonary MTB | INH-Resistant | 254 | Positive | Positive | Positive |
| 2 | Immediate INH | 28PP | Confirmed pulmonary MTB | INH-Sensitive | 289 | Negative | Positive | Positive |
| 3 | Immediate INH | 10PP | Probable pulmonary MTB | Not available | 678 | Positive | Positive | Positive |
| 4 | Deferred INH | 27PP | Probable pulmonary MTB | Not available | 261 | Negative | Negative | Negative |
| 5 | Deferred INH | 40PP | Confirmed pulmonary MTB | INH-Sensitive | 79 | Indeterminate | Indeterminate | Negative |
| 6 | Deferred INH | 36PP | Confirmed pulmonary MTB | INH-Sensitive | 504 | Positive | Positive | Negative |
DISCUSSION
This 944-participant longitudinal study confirmed our hypothesis that pregnancy-associated immune suppression decreases the sensitivity of QGIT for the diagnosis of LTBI. This is also in agreement with findings of smaller studies conducted after the inception of P1078 [7, 15, 16]. Pregnancy increases regulatory T cells, which prevent rejection of the fetus by attenuating conventional T cell function [13]. Here we show that a consequence of this effect is a decrease in the negative predictive value of QGIT during pregnancy. To mitigate the risk of false-negative results in pregnancy, other investigators proposed using QGIT-Plus and lowering the TB-specific IFNγ positivity threshold [7]. However, our data do not support this approach, because neither of these interventions would decrease the large number of indeterminate QGIT results, which predominantly account for the QGIT positivity loss during pregnancy.
Decreased TB-specific IFNγ responses during pregnancy did not seem to increase the risk of TB disease, since none of the women in the deferred arm with QGIT reversion from antepartum to delivery became symptomatic. Moreover, TB disease developed exclusively in the postpartum with a median of 32 weeks and upper limit of 48 weeks, including participants QGIT-positive antepartum, which is in agreement with previous studies in pregnant women without HIV [17]. Patients with drug-induced immune suppression develop symptoms of TB in the first 3 to 6 months of immune suppression [18–20]. Hence, if pregnancy-associated immune suppression were the underlying mechanism of TB reactivation, symptoms might have developed ≤ 24 weeks postpartum in most women. The absence of increased rates of TB disease antepartum or early postpartum challenges the notion that TB-specific IFNγ responses confer protection against TB disease [21]. This is further supported by studies of BCG vaccine in adults, which significantly increases IFNγ responses, but does not confer protection against TB disease [22, 23]. An alternative explanation that deserves to be further investigated is that IFNγ responses represent a nonmechanistic correlate of protection against disease, in which case their loss may not affect the risk of developing TB disease as long as the truly protective mechanism/s is/are maintained [24].
In addition to pregnancy, we considered other factors that might affect the diagnostic accuracy of QGIT [25]. The following biological and technical issues leading to QGIT variability have been previously reported: 1) a unique lot of QGIT was withdrawn from the market due to excess false-positive results; 2) shaking the collection tubes and increasing the incubation time generated false-positive results, while low blood volumes and delays in placing the tubes at 37ºC after blood collection generated false-negative results; 3) malnutrition and, specific to HIV infection, low CD4 count have been associated with false-negative QGIT and TST results; 4) TST placement increased the probability of QGIT-positive results ≥7 days after, but not within 3 days of placement [26, 27]. In our study, potential technical and collection problems were minimized by training and rigorous proficiency evaluations of the clinical sites. In agreement with previous studies, we found higher QGIT positivity in association with higher CD4 count, good nutritional status, and longer duration of ART.
Several participants lost TB-specific IFNγ responses from antepartum to postpartum after completing IPT. This finding is concordant with previous reports showing a correlation of TB-specific IFNγ responses with the bacillary load and a loss of IGRA and/or TST positivity after treatment of TB disease or IPT [28–31]. Other investigators, however, ascribed IGRA reversions to assay variability [32, 33]. These studies did not report on treatment, including studies in US healthcare workers, in whom the CDC recommends IPT. We propose a mechanism that may explain treatment-associated reversions that must be further investigated. Using sophisticated techniques, several studies have demonstrated active M. tuberculosis replication in 10–25% LTBI-positive individuals [34–37]. Eradication of M. tuberculosis replication in response to IPT might lead to the loss of TB-specific IFNγ responses by eliminating the immune system antigen exposure. The clinical significance of losing TB-specific IFNγ responses is currently unknown.
In opposition to the general trend of QGIT reversion from entry to delivery, 3% of participants in each of treatment arm converted to QGIT-positive from entry to delivery. These women had ≥203 CD4 cells/µL, suggesting that new acquisition of TB infection was more likely than ART-associated immune reconstitution. This finding was surprising in women taking isoniazid (immediate arm), but concordant with the similar rates of TB disease in both arms [2]. Only1 of the 6 participants who developed active TB infection on study had isoniazid-resistant M. tuberculosis, suggesting that isoniazidresistance did not play an important role in the acquisition or progression of TB infection during IPT. Adherence to study medication was reported >93% in both arms, although it had the usual limitations of self-reports and pill counts. Our group showed that the isoniazid plasma area under the concentration-time curve over 24 h is lower antepartum compared with postpartum, particularly in rapid acetylators [38]. The number of LTBI conversions during pregnancy in our study was too small to draw significant conclusions. However, our data indicate that there might be an interaction between the increased clearance of isoniazid during pregnancy and the prophylactic efficacy of the drug, which requires further investigation.
QGIT and TST had only moderate agreement in our study. When the results were discordant, QGIT was more likely to be positive than TST, which is in agreement with previous studies in people with HIV [4, 15, 16]. Moreover, 1 of the 6 women who developed TB disease during the study had positive QGIT, but negative TST prior to the diagnosis. Previous reports also showed that QGIT converted before TST in individuals exposed to TB who eventually developed active TB disease, supporting the notion that QGIT is more sensitive than TST for detecting M. tuberculosis infection [6, 9]. TST can also generate false-positive results after intense exposure to nontuberculous mycobacteria and in the first 5 years after infant BCG vaccination or indefinitely after adult vaccination, such that TST does not seem to offer any diagnostic advantages compared to QGIT [4, 6, 10].
Our study was limited by a 17% rate of early withdrawal. This was largely due to a notification sent to participants midway through the study alerting them of 2 study deaths and possible hepatotoxicity. In addition, because the number of QGIT conversions from entry to delivery and reversions from entry to postpartum was relatively small, we cannot completely rule out some assay or biologic variability. Therefore, our interpretations need to be re-evaluated in future studies.
In conclusion, QGIT detected a significantly higher proportion of LTBI in women with HIV compared with TST, confirming its previously demonstrated superior sensitivity in people with HIV. However, we showed that antepartum, QGIT may miss up to 20% of LTBI. This is highly relevant to countries with low incidence of TB, such as the United States, where IPT in people with HIV may be guided by the diagnosis of LTBI. Our study quantifies the conversion, reversion of QGIT in HIV-infected pregnant women, and provides some insights into the impact of IPT on IFNγ responses and optimal timing of performing IGRA-based tests.
Supplementary Data
Supplementary materials are available at Clinical Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Notes
Acknowledgments. The following P1078 clinical site investigators receive credit for this publication: Alisa Shao, KCMC, Tanzania; Mandisa Nyati, Soweto, South Africa; Jeanne Louw, FAM-CRU, South Africa; Tebogo J. Kakhu, Gaborone, Botswana; Tsungai Chipato, St. Mary’s, Zimbabwe; Lynda Stranix-Chibanda, Seke North, Zimbabwe; Nishi Suryavanshi, BJMC, India; Fuanglada Tongprasert, Chiang Mai University, Thailand; and Celeste de Vaal, DTTC, South Africa. The authors are thankful to the IMPAACT Network and operations staff for their support and to the numerous community advisory boards, international site investigators and research teams, and to the women and families who participated in the trial. They also acknowledge Vandana Kulkarni who served as the protocol laboratory technologist, Joan Coetzee who was a field representative, Rebecca LeBlanc who coordinated sample transfer and central laboratory data management, Vivian Rexroad who assisted in pharmacy training, and Renee Browning who was a protocol medical officer. They also thank the independent endpoint review committee members. Timothy R. Sterling represented scientific inputs on behalf of the US CDC funded Tuberculosis Trials Consortium.
Financial support. Overall support for the International Maternal Pediatric Adolescent AIDS Clinical Trials Network (IMPAACT) was provided by the National Institute of Allergy and Infectious Diseases (NIAID) with co-funding from the Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD) and the National Institute of Mental Health (NIMH), all components of the National Institutes of Health (NIH), under Award Numbers UM1AI068632 (IMPAACT LOC), UM1AI068616 (IMPAACT SDMC) and UM1AI106716 (IMPAACT LC), and by NICHD contract number HHSN275201800001I. Additional support included NIH grants AI110527 and AI077505 (to D. W. H.), UM1AI069465 and R01HD081929 (to A. G.). The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH.
Potential conflicts of interest. A.W. reports grants from Merck and GSK, outside the submitted work. All other authors report no potential conflicts. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
Contributor Information
IMPAACT P1078 study team:
Alisa Shao, Mandisa Nyati, Jeanne Louw, Tebogo J Kakhu, Tsungai Chipato, Lynda Stranix-Chibanda, Nishi Suryavanshi, Fuanglada Tongprasert, and Celeste de Vaal
References
- 1. Mathad JS, Gupta A. Tuberculosis in pregnant and postpartum women: epidemiology, management, and research gaps. Clin Infect Dis 2012; 55:1532–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Gupta A, Montepiedra G, Aaron L, et al. ; IMPAACT P1078 TB APPRISE Study Team . Isoniazid preventive therapy in HIV-infected pregnant and postpartum women. N Engl J Med 2019; 381:1333–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Mazurek GH, Jereb J, Vernon A, LoBue P, Goldberg S, Castro K; IGRA Expert Committee; Centers for Disease Control and Prevention (CDC) . Updated guidelines for using interferon gamma release assays to detect Mycobacterium tuberculosis infection—United States, 2010. MMWR Recomm Rep 2010; 59:1–25. [PubMed] [Google Scholar]
- 4. Diel R, Goletti D, Ferrara G, et al. Interferon-γ release assays for the diagnosis of latent Mycobacterium tuberculosis infection: a systematic review and meta-analysis. Eur Respir J 2011; 37:88–99. [DOI] [PubMed] [Google Scholar]
- 5. Menzies D, Pai M, Comstock G. Meta-analysis: new tests for the diagnosis of latent tuberculosis infection: areas of uncertainty and recommendations for research. Ann Intern Med 2007; 146:340–54. [DOI] [PubMed] [Google Scholar]
- 6. Ferrara G, Losi M, D’Amico R, et al. Use in routine clinical practice of two commercial blood tests for diagnosis of infection with Mycobacterium tuberculosis: a prospective study. Lancet 2006; 367:1328–34. [DOI] [PubMed] [Google Scholar]
- 7. König Walles J, Tesfaye F, Jansson M, et al. Performance of QuantiFERON-TB Gold Plus for detection of latent tuberculosis infection in pregnant women living in a tuberculosis- and HIV-endemic setting. PLoS One 2018; 13:e0193589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Stephan C, Wolf T, Goetsch U, et al. Comparing QuantiFERON-tuberculosis gold, T-SPOT tuberculosis and tuberculin skin test in HIV-infected individuals from a low prevalence tuberculosis country. AIDS 2008; 22:2471–9. [DOI] [PubMed] [Google Scholar]
- 9. Buchwald UK, Adetifa IM, Bottomley C, et al. Broad adaptive immune responses to M. tuberculosis antigens precede TST conversion in tuberculosis exposed household contacts in a TB-endemic setting. PLoS One 2014; 9:e116268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Farhat M, Greenaway C, Pai M, Menzies D. False-positive tuberculin skin tests: what is the absolute effect of BCG and non-tuberculous mycobacteria? Int J Tuberc Lung Dis 2006; 10:1192–204. [PubMed] [Google Scholar]
- 11. Rangaka MX, Wilkinson KA, Seldon R, et al. Effect of HIV-1 infection on T-cell-based and skin test detection of tuberculosis infection. Am J Respir Crit Care Med 2007; 175:514–20. [DOI] [PubMed] [Google Scholar]
- 12. Andrews JR, Nemes E, Tameris M, et al. Serial QuantiFERON testing and tuberculosis disease risk among young children: an observational cohort study. Lancet Respir Med 2017; 5:282–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Aluvihare VR, Kallikourdis M, Betz AG. Regulatory T cells mediate maternal tolerance to the fetus. Nat Immunol 2004; 5:266–71. [DOI] [PubMed] [Google Scholar]
- 14. Luetkemeyer AF, Rosenkranz SL, Lu D, et al. Combined effect of CYP2B6 and NAT2 genotype on plasma efavirenz exposure during rifampin-based antituberculosis therapy in the STRIDE study. Clin Infect Dis 2015; 60:1860–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. LaCourse SM, Cranmer LM, Matemo D, et al. Effect of pregnancy on interferon gamma release assay and tuberculin skin test detection of latent TB infection among HIV-infected women in a high burden setting. JAIDS 2017; 75:128–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Mathad JS, Bhosale R, Balasubramanian U, et al. Quantitative IFN-γ and IL-2 response associated with latent tuberculosis test discordance in HIV-infected pregnant women. Am J Respir Crit Care Med 2016; 193:1421–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Zenner D, Kruijshaar ME, Andrews N, Abubakar I. Risk of tuberculosis in pregnancy: a national, primary care-based cohort and self-controlled case series study. Am J Respir Crit Care Med 2012; 185:779–84. [DOI] [PubMed] [Google Scholar]
- 18. Keane J, Gershon S, Wise RP, et al. Tuberculosis associated with infliximab, a tumor necrosis factor alpha-neutralizing agent. N Engl J Med 2001; 345:1098–104. [DOI] [PubMed] [Google Scholar]
- 19. Singh N, Paterson DL. Mycobacterium tuberculosis infection in solid-organ transplant recipients: impact and implications for management. Clin Infect Dis 1998; 27:1266–77. [DOI] [PubMed] [Google Scholar]
- 20. Shah NM, Imami N, Johnson MR. Progesterone modulation of pregnancy-related immune responses. Front Immunol 2018; 9:1293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Lahey T, Sheth S, Matee M, et al. Interferon γ responses to mycobacterial antigens protect against subsequent HIV-associated tuberculosis. J Infect Dis 2010; 202:1265–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Mangtani P, Abubakar I, Ariti C, et al. Protection by BCG vaccine against tuberculosis: a systematic review of randomized controlled trials. Clin Infect Dis 2014; 58:470–80. [DOI] [PubMed] [Google Scholar]
- 23. Suliman S, Geldenhuys H, Johnson JL, et al. Bacillus Calmette-Guérin (BCG) revaccination of adults with latent Mycobacterium tuberculosis infection induces long-lived BCG-reactive NK cell responses. J Immunol 2016; 197:1100–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Lu LL, Smith MT, Yu KKQ, et al. IFN-γ-independent immune markers of Mycobacterium tuberculosis exposure. Nat Med 2019; 25:977–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. van Zyl-Smit RN, Pai M, Peprah K, et al. Within-subject variability and boosting of T-cell interferon-gamma responses after tuberculin skin testing. Am J Respir Crit Care Med 2009; 180:49–58. [DOI] [PubMed] [Google Scholar]
- 26. Leyten EM, Prins C, Bossink AW, et al. Effect of tuberculin skin testing on a Mycobacterium tuberculosis-specific interferon-gamma assay. Eur Respir J 2007; 29:1212–6. [DOI] [PubMed] [Google Scholar]
- 27. Richeldi L, Bergamini BM, Vaienti F. Prior tuberculin skin testing does not boost QuantiFERON-TB results in paediatric contacts. Eur Respir J 2008; 32:524–5. [DOI] [PubMed] [Google Scholar]
- 28. Johnson DF, Malone LL, Zalwango S, et al. ; Tuberculosis Research Unit . Tuberculin skin test reversion following isoniazid preventive therapy reflects diversity of immune response to primary Mycobacterium tuberculosis infection. PLoS One 2014; 9:e96613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Adekambi T, Ibegbu CC, Kalokhe AS, Yu T, Ray SM, Rengarajan J. Distinct effector memory CD4+ T cell signatures in latent Mycobacterium tuberculosis infection, BCG vaccination and clinically resolved tuberculosis. PLoS One 2012; 7:e36046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. O’Shea MK, Fletcher TE, Beeching NJ, et al. Tuberculin skin testing and treatment modulates interferon-gamma release assay results for latent tuberculosis in migrants. PLoS One 2014; 9:e97366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Connell TG, Davies MA, Johannisen C, et al. Reversion and conversion of Mycobacterium tuberculosis IFN-gamma ELISpot results during anti-tuberculous treatment in HIV-infected children. BMC Infect Dis 2010; 10:138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Joshi M, Monson TP, Joshi A, Woods GL. IFN-γ release assay conversions and reversions. Challenges with serial testing in U.S. health care workers. Ann Am Thorac Soc 2014; 11:296–302. [DOI] [PubMed] [Google Scholar]
- 33. Dorman SE, Belknap R, Graviss EA, et al. ; Tuberculosis Epidemiologic Studies Consortium . Interferon-γ release assays and tuberculin skin testing for diagnosis of latent tuberculosis infection in healthcare workers in the United States. Am J Respir Crit Care Med 2014; 189:77–87. [DOI] [PubMed] [Google Scholar]
- 34. Walzl G, Ronacher K, Hanekom W, Scriba TJ, Zumla A. Immunological biomarkers of tuberculosis. Nat Rev Immunol 2011; 11: 343–54. [DOI] [PubMed] [Google Scholar]
- 35. Achkar JM, Jenny-Avital ER. Incipient and subclinical tuberculosis: defining early disease states in the context of host immune response. J Infect Dis 2011; 204 Suppl 4:S1179–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Cardona PJ. Revisiting the natural history of tuberculosis. The inclusion of constant reinfection, host tolerance, and damage-response frameworks leads to a better understanding of latent infection and its evolution towards active disease. Arch Immunol Ther Exp (Warsz) 2010; 58:7–14. [DOI] [PubMed] [Google Scholar]
- 37. Esmail H, Lai RP, Lesosky M, et al. Characterization of progressive HIV-associated tuberculosis using 2-deoxy-2-[18F]fluoro-D-glucose positron emission and computed tomography. Nat Med 2016; 22:1090–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Gausi K, Denti P, Wiesner L, et al. Pregnancy is associated with decreased serum isoniazid levels in women living with HIV. Available at: https://www.croiconference.org/sessions/pregnancy-associated-decreased-serum-isoniazid-levels-women-living-hiv. Accessed 28 August 2020.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.