Abstract
To inform proposed changes in hepatitis C virus (HCV) screening guidelines in the United States, we assessed the cost-effectiveness of HCV antenatal rescreening for women without evidence of HCV during a prior pregnancy, using a previously published model. Universal HCV rescreening among pregnant women was cost-effective (incremental cost-effectiveness ratio, $6000 per quality-adjusted life-year) and should be recommended nationally.
Keywords: testing, hepatitis C virus, economic, treatment, pregnancy
A consequence of the opioid epidemic in the United States (US) is the rise of hepatitis C virus (HCV) among people of reproductive age. HCV among pregnant women doubled nationally from 2009 to 2014, to approximately 0.7% [1, 2]. Highly effective HCV direct-acting antiviral treatments are available, yet many remain undiagnosed.
Prenatal HCV testing presents an opportunity to increase diagnosis among women in the US. For many women, pregnancy is a rare time in contact with healthcare and insurance coverage, providing a critical opportunity for reaching this population. Two recent studies found HCV screening in pregnant women to be cost-effective, yet neither examined rescreening at subsequent pregnancies [3, 4]. New US Preventive Services Task Force guidelines recommend a one-time screen for all adults aged 18–79 including pregnant women, but note there is limited information on how pregnancy changes the need for additional screening, calling for additional research in this area [5]. Proposed draft Centers for Disease Control and Prevention (CDC) guidelines recommend universal HCV screening (including rescreening) for pregnant women [6]. The Society for Maternal-Fetal Medicine and the American College of Obstetricians and Gynecologists guidelines only recommend risk-based screening for HCV among pregnant women [7].
To inform HCV screening policy and practice, we assessed the cost-effectiveness of offering HCV antenatal rescreening for women in the US previously screened during a prior pregnancy and without evidence of past HCV exposure, followed by treatment after pregnancy.
METHODS
Overview
We used a previously published [4] economic model of HCV screening among pregnant women to assess the cost-effectiveness of HCV antenatal rescreening among pregnant women previously screened during a prior pregnancy and without evidence of past HCV exposure in the US, followed by treatment after pregnancy, compared to background community risk-based screening. We take a public sector healthcare payer perspective and include long-term health benefits among pregnant women only. We assume no treatment restrictions by fibrosis stage at baseline.
Model
We utilized a previously published natural history cohort Markov model of HCV progression and treatment among pregnant women attending antenatal clinics [4]. We simulated a closed cohort of women previously screened during a prior pregnancy who showed no evidence of past HCV exposure and who present for rescreening at a subsequent pregnancy. We assumed all individuals become diagnosed upon progression to decompensated cirrhosis or hepatocellular carcinoma due to clinical severity. We incorporated loss to follow-up; individuals lost to follow-up were eligible for community retesting. Individuals whose treatment failed were not re-treated.
Cost-effectiveness Methods
Cost (in 2019 US dollars [$]) and health utilities (in quality-adjusted life-years [QALYs]) were attached to each health state, discounted 3% per year. Due to parameter uncertainty, probabilistic uncertainty analysis was performed with parameters randomly sampled from distributions (Supplementary Table 1), generating 10 000 parameter sets. We calculated mean incremental cost-effectiveness ratios (ICERs, $/QALY gained, mean incremental costs divided by mean incremental QALYs), assessing cost-effectiveness under a willingness to pay (WTP) threshold of $50 000 per QALY gained.
Model Parameterization
All parameters, sampling distributions, and references are shown in Supplementary Table 1. We assumed a starting population of women at their second pregnancy, given the average total fertility rate of 1.7 births per women in 2018 [8]. Therefore, the baseline population included pregnant women with an average age of 30 (mean age of 27 at first pregnancy [8], plus 3 years’ average interpregnancy interval [9]). No data are available on HCV prevalence among pregnant women who had a previous negative HCV antibody screen at their first pregnancy. We therefore estimated prevalence by multiplying the estimated proportion of pregnant women with injecting drug use (IDU) by the estimated HCV chronic prevalence among women who had screened anti-HCV negative in their prior pregnancy but remained at ongoing risk of HCV (derived using a static incidence model; see Supplementary Materials). At baseline, we assumed a mean 1.25% of pregnant women with IDU risk [10], 17 per 100 person-years HCV primary incidence among female people who inject drugs (PWID), 38% spontaneous clearance rate among women, and a 3-year interpregnancy interval, generating a mean chronic HCV prevalence among women at rescreening of 0.36%. As all screened HCV antibody-negative at their previous pregnancy, we conservatively assume all infected women were at Metavir F0 stage. We assumed a 75% per year loss to follow-up after diagnosis based on data from pregnant women on opiate substitution therapy [11]. Female stage-specific transition rates were obtained from published studies (Supplementary Table 1). Individuals who achieved sustained virological response (SVR) with F0–F3 fibrosis had no further progression, while those with more advanced disease progressed at a reduced rate. We assumed an SVR rate of 95% and $25 000 per treatment course plus treatment delivery costs (Supplementary Table 1).
Sensitivity Analyses
Due to uncertainty, we performed 2-way sensitivity analyses varying the prevalence of IDU among pregnant women at a subsequent pregnancy who had no evidence of HCV exposure at a prior pregnancy (from 0.25% to 1.5%) and interpregnancy interval (1–4 years). This resulted in chronic HCV prevalences between 0.03% and 0.5% (Supplementary Table 2). We additionally explored 1-way sensitivity analyses on fibrosis progression rates (0.95/year vs 0.11/year at baseline) and treatment eligibility based on state-based Medicaid reimbursement policies (restricted until Metavir stage F1 or beyond [F1+] and F2 or beyond [F2+], vs no restrictions at baseline). In the treatment restriction scenarios, individuals were eligible for treatment upon progression if remaining linked to care. We additionally assessed cost-effectiveness of risk-based rescreening during pregnancy, assuming 34%–64% of women with IDU will not be screened with this strategy [12, 13].
RESULTS
Universal HCV rescreening for pregnant women was associated with incremental costs of $47 (95% confidence interval [CI], $10–$91) and an incremental increase in QALYs of 0.008 (95% CI, .001–.015) per pregnant woman screened compared to background risk-based screening (Supplementary Table 3), assuming a mean 1.25% prevalence of IDU and 3-year interpregnancy interval (leading to a chronic HCV prevalence of 0.036%). Rescreening was cost-effective compared to background screening, with a mean ICER of $6000 per QALY gained, falling below the WTP threshold of $50 000 per QALY gained for 100% of simulations.
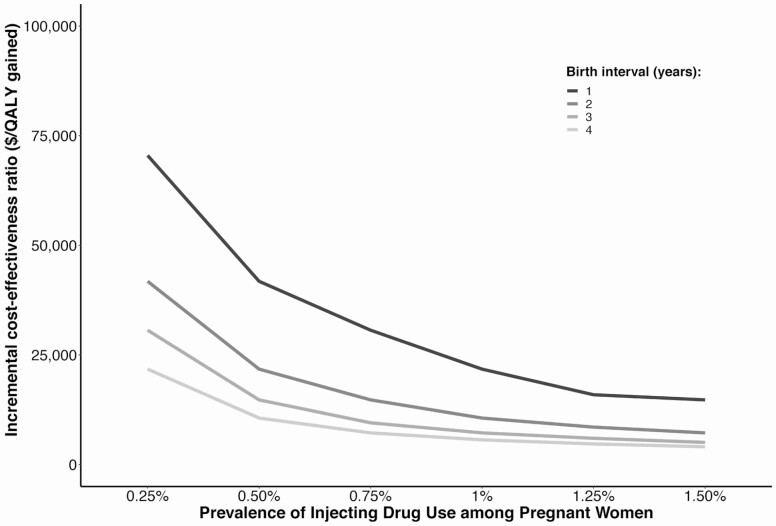
Due to uncertainty and heterogeneity regarding prevalence of injecting and interpregnancy interval (which leads to uncertainty in chronic HCV prevalence at rescreening), Figure 1 shows how cost-effectiveness varies both by prevalence of IDU and interpregnancy interval. Screening remained cost-effective for the lowest prevalence of IDU (0.25%) and interpregnancy interval (1 year) examined. Results were additionally robust to lower fibrosis progression rates (Supplementary Table 4) and treatment restrictions by fibrosis status. In settings with F1+ fibrosis restrictions (Supplementary Figure 1A), rescreening was cost-effective under a $50 000 WTP with intepregnancy interval ≤ 3 years and IDU prevalence ≥ 0.75%, or interpregnancy interval ≤ 2 years and IDU prevalence ≥ 0.5%. All scenarios fell under a $100 000 WTP except the lowest birth interval (1 year) and lowest IDU prevalence (0.25%). In settings with F2+ fibrosis restrictions (Supplementary Figure 1B), rescreening was cost-effective under a $50 000 WTP with interpregnancy interval ≤ 3 and IDU prevalence ≥ 1%, or interpregnancy interval ≤ 2 years and IDU prevalence ≥ 0.75%. Finally, a strategy of risk-based rescreening during pregnancy was more cost-effective, but less effective because of the substantial proportion of high-risk women not tested through current practice (Supplementary Table 5).
Figure 1.
Incremental cost-effectiveness ratio of universal rescreening of pregnant women compared to background community risk-based screening with varied prevalence of injecting drug use among pregnant women and various interpregnancy intervals. Abbreviation: QALY, quality-adjusted life-year.
Discussion
Our analysis indicates that universal HCV rescreening among pregnant women in the US without evidence of past HCV exposure during a prior pregnancy is cost-effective. As HCV prevalence among women with evidence of past HCV exposure during a prior pregnancy will likely be much higher than among those without evidence of past exposure (due to ongoing untreated chronic infection and potential reinfection), rescreening of all pregnant women during subsequent pregnancies is likely cost-effective.
Our results were robust to variations in prevalence of ongoing risk, as well as interpregnancy interval. As such, our analysis supports the proposed draft CDC guidelines, which recommend universal HCV screening of pregnant women at every pregnancy, regardless of ongoing risk [6]. This would reduce logistical complications as providers would not need to determine screening history. Our study is the first to assess cost-effectiveness of HCV rescreening among pregnant women, supporting studies showing that HCV screening in pregnant women is cost-effective [3, 4]. While our sensitivity analysis indicates that risk-based rescreening is more cost-effective than universal rescreening, it is much less effective (50% fewer QALYs gained) as a large proportion (34%–64%) [12, 13] of high-risk women are not screened during pregnancy, leading to many missed diagnoses.
Our study has limitations. First, there is uncertainty in the prevalence of IDU (and relatedly, HCV prevalence) among pregnant women without past evidence of HCV, yet our analyses indicated that results were robust to these uncertainties. Epidemiological studies are warranted to determine HCV prevalence among this group. Second, we do not simulate changing insurance eligibility over time. In some non–Medicaid expansion states, women can lose their insurance 30 days after giving birth, which could limit uptake of treatment, unless treatment during pregnancy is safe, which could reduce loss to follow-up. Third, we incorporate benefits among pregnant women only, neglecting potential benefits to children, which could increase cost-effectiveness. Finally, we neglect the risk of reinfection and treatment as prevention benefits. However, our modeling shows that in settings with 50% chronic prevalence among PWID, like the US, early treatment of people with ongoing IDU prevents 0.2–0.8 infections per treatment, despite reinfection [14]. As such, including reinfection and prevention benefits would increase cost-effectiveness.
In conclusion, universal HCV screening of pregnant women at each pregnancy (including rescreening) in the US is cost-effective and should be recommended nationally.
Supplementary Data
Supplementary materials are available at Clinical Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Notes
Disclaimer. The funder had no influence on the design, analysis, or content of the study.
Financial support. This study was supported through a research grant from Gilead Sciences. N. K. M. acknowledges funding from the National Institute of Allergy and Infectious Diseases/National Institute on Drug Abuse (grant number R01 AI147490) and the University of San Diego Center for AIDS Research, a National Institutes of Health–funded program (P30 AI036214).
Potential conflicts of interest. N. K. M. has received unrestricted research grants from Gilead and Merck. N. R. has received grants from Gilead and honoraria from Gilead and AbbVie. T. K. has received advisory board fees from Gilead. All other authors report no potential conflicts of interest. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1. Ly KN, Jiles RB, Teshale EH, Foster MA, Pesano RL, Holmberg SD. Hepatitis C virus infection among reproductive-aged women and children in the United States, 2006 to 2014. Ann Intern Med 2017; 166:775–82. [DOI] [PubMed] [Google Scholar]
- 2. Patrick SW, Bauer AM, Warren MD, Jones TF, Wester C. Hepatitis C virus infection among women giving birth—Tennessee and United States, 2009–2014. MMWR Morb Mortal Wkly Rep 2017; 66:470–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Tasillo A, Eftekhari Yazdi G, Nolen S, et al. . Short-term effects and long-term cost-effectiveness of universal hepatitis C testing in prenatal care. Obstet Gynecol 2019; 133:289–300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Chaillon A, Rand EB, Reau N, Martin NK. Cost-effectiveness of universal hepatitis C virus screening of pregnant women in the United States. Clin Infect Dis 2019; 69:1888–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Owens DK, Davidson KW, Krist AH, et al. . Screening for hepatitis C virus infection in adolescents and adults: US Preventive Services Task Force recommendation statement. JAMA 2020. doi: 10.1001/jama.2020.1123. [DOI] [PubMed] [Google Scholar]
- 6. Centers for Disease Control and Prevention. Peer review plan for recommendations for hepatitis C screening among adults 2019. Available at: https://www.cdc.gov/hepatitis/policy/isireview/HepCScreeningAmongAdults.htm. Accessed 15 December 2019.
- 7. Society for Maternal-Fetal Medicine. Hepatitis C in pregnancy: screening, treatment, and management 2018. Available at: https://www.smfm.org/publications/248-smfm-consult-series-43-hepatitis-c-in-pregnancy-screening-treatment-and-management. Accessed 15 December 2019.
- 8. Martin J, Hamilton B, Osterman M, Driscoll A. Births: final data for 2018. Natl Vital Stat Rep 2019; 68:1–47. [PubMed] [Google Scholar]
- 9. Ahrens KA, Hutcheon JA. Birth spacing in the United States—towards evidence-based recommendations. Paediatr Perinat Epidemiol 2019; 33:O1–4. [DOI] [PubMed] [Google Scholar]
- 10. Salihu HM, Salemi JL, Aggarwal A, et al. . Opioid drug use and acute cardiac events among pregnant women in the United States. Am J Med 2018; 131:64–71.e1. [DOI] [PubMed] [Google Scholar]
- 11. Krans EE, Zickmund SL, Rustgi VK, Park SY, Dunn SL, Schwarz EB. Screening and evaluation of hepatitis C virus infection in pregnant women on opioid maintenance therapy: a retrospective cohort study. Subst Abus 2016; 37:88–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Nolen LD, Gustin C, Seeman S, et al. . Risk-based prenatal hepatitis C testing practices and results, Alaska 2013–2016. Can J Gastroenterol Hepatol 2019; 2019:8654741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Boudova S, Mark K, El-Kamary SS. Risk-based hepatitis C screening in pregnancy is less reliable than universal screening: a retrospective chart review. Open Forum Infect Dis 2018; 5:ofy043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Martin NK, Vickerman P, Dore GJ, et al. . STOP-HCV Consortium . Prioritization of HCV treatment in the direct-acting antiviral era: an economic evaluation. J Hepatol 2016; 65:17–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.